Abstract
BACKGROUND
Reducing breast cancer incidence and achieving equity in breast cancer outcomes remains a priority for public health practitioners, health care providers, policy makers, and health advocates. Monitoring breast cancer survival can help evaluate the effectiveness of health services, quantify inequities in outcomes between states or population subgroups, and inform efforts to improve the effectiveness of cancer management and treatment.
METHODS
We analyzed breast cancer survival using individual patient records from 37 statewide registries that participated in the CONCORD-2 study, covering approximately 80% of the US population. Females were diagnosed between 2001 and 2009 and were followed through December 31, 2009. Age-standardized net survival at 1 year, 3 years, and 5 years after diagnosis was estimated by state, race (white, black), stage at diagnosis, and calendar period (2001–2003 and 2004–2009).
RESULTS
Overall, 5-year breast cancer net survival was very high (88.2%). Survival remained remarkably high from 2001 through 2009. Between 2001 and 2003, survival was 89.1% for white females and 76.9% for black females. Between 2004 and 2009, survival was 89.6% for white females and 78.4% for black females.
CONCLUSIONS
Breast cancer survival was more than 10 percentage points lower for black females than for white females, and this difference persisted over time. Reducing racial disparities in survival remains a challenge that requires broad, coordinated efforts at the federal, state, and local levels. Monitoring trends in breast cancer survival can highlight populations in need of improved cancer management and treatment.
Keywords: breast cancer, health disparities, population-based survival, trends
INTRODUCTION
The burden of breast cancer has been a persistent concern among public health practitioners, health care providers, policy makers, and health advocates. Worldwide, breast cancer is the second most common cancer and the fifth leading cause of cancer deaths among females.1 In the United States, breast cancer is the most commonly diagnosed cancer (excluding skin cancer) and the second leading cause of cancer deaths among females.2 Since early 2004, breast cancer incidence has been stable, whereas breast cancer mortality has been decreasing.3 However, these trends have not been equal among all populations.3
The elimination of health disparities is a goal of many federal agencies. For example, the US. Department of Health and Human Services, the Agency for Healthcare Research and Quality, and the Centers for Disease Control and Prevention (CDC) have developed action plans to reduce health disparities.4–7 Unequal incidence of advanced-stage breast cancer, receipt of high-quality treatment, and breast cancer death rates have not changed much over time.5,8 Breast cancer incidence rates have historically been higher among white females, while mortality rates have been higher among black females. The annual average percentage change in incidence from 1999 through 2013 varied by race, with a 0.8% decrease per year among white females and a 0.4% increase per year among black females.9 Breast cancer mortality rates have fallen since the mid-1990s to 2012 among both black and white females, but the rate of decline has been faster among white females (annual percentage change, −1.9% vs −1.5%).10–12 Although there have been great advancements in treatment options, racial inequalities in breast cancer survival in the United States still persist.
An evaluation of population-based survival trends can help to inform cancer-control efforts by identifying opportunities for improvement in early detection, diagnosis, and treatment. Population-based survival is a key measure of the effectiveness of cancer management and treatment.13 Black females are more often diagnosed with regional or late-stage disease and have higher death rates than white females, regardless of stage.14 In 2008, the CONCORD study reported 5-year population-based survival in 31 countries, including 22 registries in 16 US states.15 Five-year survival in North America was among the highest in the world at 84%, with large survival differences between white (84.7%) and black (70.9%) females in the United States, suggesting that black females may not have the same access to and quality of care as white females. The second cycle of the CONCORD program (CONDORD-2) examined survival from 10 common cancers in 67 countries.16 CONCORD-2 is the largest study of population-based cancer survival trends to date, evaluating trends in 5-year survival for over 25 million individual patients diagnosed during the 15 years between 1995 and 2009. Age-standardized, 5-year net survival from breast cancer increased in many countries worldwide. In the United States, it rose from 86% between 1995 and 1999 to 88.6% between 2005 and 2009.
This report describes trends in net survival in a population-based setting (ie, the survival for all females with breast cancer) after controlling for competing risks of death. Net survival allows us to estimate and compare survival for females with breast cancer between countries or states without using the cause of death, which is often unreliable or not comparable between different states. By using the US data from CONCORD-2, we examined the distribution of breast cancer stage at diagnosis by race, state, and over time. We also analyzed survival trends by race, stage, and state. This study provides information on the effectiveness of the US health system in managing females with breast cancer from diagnosis to the final outcome. This analysis is part of the largest population-based study of cancer survival trends in the United States, including over 10 million patients with cancer.
MATERIALS AND METHODS
Data
We used data from 37 state cancer registries funded by either the CDC’s National Program of Cancer Registries (NPCR), the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) program, or both. These registries participated in the CONCORD-2 study,17 which covered approximately 80% of the US population. Registries consented to the inclusion of their data in the more detailed analyses reported here. We analyzed individual tumor records for 1,372,377 females (ages 15–99 years) who were diagnosed with breast cancer (International Classification of Diseases for Oncology, third edition,18 codes C50.0-C50.6 and C50.8-C50.9) between 2001 and 2009 and were followed until December 31, 2009. We included primary invasive cancers of the breast, regardless of whether the woman had a previous cancer. If a woman was diagnosed with ≥2 cancers of the breast between 2001 and 2009, then only the first was considered in the survival analyses. All races were included in the study, but we only reported separate survival for blacks and whites because of the small numbers for the other races in many states. Females who were excluded from the data were those who were registered from a death certificate only, were lost to follow-up, had inconsistent site-morphology combinations, or had invalid dates.16
Patients were grouped by year of diagnosis into 2 calendar periods (2001–2003 and 2004–2009) to reflect changes in the methods used by US registries to collect data on stage at diagnosis. From 2001, most registries coded stage directly from the source data to SEER Summary Stage 2000 (SS2000).19 From 2004, all registries began to derive SS2000 using the Collaborative Stage unified data collection system.20
Survival Analyses
We estimated net survival up to 5 years after diagnosis and 95% confidence intervals (CIs) using the Pohar Perme estimator.21 We analyzed survival by race, stage at diagnosis, calendar period of diagnosis, and US state. Net survival is the probability of surviving up to a given time since diagnosis, after controlling for other causes of death (background mortality). In other words, it is the probability that females with breast cancer will survive their cancer. The certified cause of death and the cause coded as the underlying cause of death are often unreliable or not comparable between different states. The risk of death from causes other than the breast cancer (competing risks of death, or background mortality) also varies widely between populations and over time. To control for the wide differences in background mortality among participating states while estimating net survival, we constructed life tables of all-cause mortality in the general population of each state from the number of deaths and the population, by single year of age, sex, calendar year, and, where possible, by race (black, white), using a flexible Poisson model.22 Methods for constructing the life tables have been published.23
We estimated net survival using the cohort approach for females diagnosed between 2001 and 2003, because they all had been followed for at least 5 years by December 31, 2009. We used the complete approach to estimate net survival for females diagnosed between 2004 and 2009, because 5 years of follow-up data were not available for all females. Net survival was estimated for 5 age-groups (ages 15–44, 45–54, 55–64, 65–74, and 75–99 years). We obtained age-standardized survival estimates using the International Cancer Survival Standard weights.24 If 2 or more of the 5 age-specific estimates could not be obtained, then we present only the pooled, unstandardized survival estimate for all ages combined. Trends, geographic variations, and differences in age-standardized survival by race are presented graphically in bar charts and funnel plots.25 More details on data and methods are provided in an accompanying article.26
RESULTS
There were 1,372,377 US females (85.1% white females and 10.4% black females) diagnosed with breast cancer in participating registries between 2001 and 2009. Overall, there were no substantial changes in SEER Summary Stage distribution between the 2001 through 2003 and 2004 through 2009 periods (Table 1). Between 2001 and 2003, most breast cancers were diagnosed at local stage (60%), approximately 30% were regional stage, and <5% were distant stage. The proportion of females with unknown stage was low at 7%. The distribution remained similar between 2004 and 2009, exhibiting a slight reduction in the percentage of cancers of unknown stage cancer. The stage distribution varied slightly between states (Supporting Table 1). During both calendar periods of diagnosis, a lower proportion of black females were diagnosed at local stage, and the proportion of distant stage was about 1.6 times that of white females.
TABLE 1.
Breast Cancer: Number of Cases for Females (Ages 15–99 Years) Diagnosed 2001–2009 With Distribution (%) According to SEER Summary Stage 2000 at Diagnosis, by Race and Calendar Period of Diagnosis
| 2001–2003
|
2004–2009
|
|||||
|---|---|---|---|---|---|---|
| SEER Summary Stage 2000 | All Races | White | Black | All Races | White | Black |
| No. of patients | 446,106 | 385,888 | 42,401 | 926,271 | 782,220 | 100,611 |
| Local, % | 59.1 | 60.5 | 47.5 | 59.1 | 60.5 | 48.5 |
| Regional, % | 29.8 | 29.1 | 35.3 | 30.2 | 29.6 | 35.2 |
| Distant, % | 4.4 | 4.2 | 6.8 | 5.2 | 4.9 | 7.8 |
| Unknown, % | 6.7 | 6.2 | 10.3 | 5.4 | 5.0 | 8.4 |
Abbreviation: SEER, Surveillance, Epidemiology, and End Results.
Survival decreased with time after diagnosis. Survival at 3 years was lower than at 1 year after diagnosis, and survival at 5 years was lower than at 3 years after diagnosis in both time periods (Table 2). Between 2001 and 2003, breast cancer 5-year net survival for all races combined was high at 88.2%, and it remained high at 88.6% between 2004 and 2009. Overall, the difference in 5-year survival between black females and white females was greater than 10 percentage points between 2001 and 2003, and this disparity did not decrease over time. Between 2004 and 2009, survival varied widely between states (range, 85.4%–92.4%), and it was systematically lower for black females (Supporting Table 2).
TABLE 2.
Breast Cancer: Age-Standardized Net Survival (%) at 1 Year, 3 Years, and 5 Years for Females (Ages 15–99 Years) Diagnosed Between 2001 and 2009, by Race and Calendar Period of Diagnosis
| 2001–2003
|
2004–2009
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All Races
|
White
|
Black
|
All Races
|
White
|
Black
|
|||||||
| Years | NS (%) | 95% CI | NS (%) | 95% CI | NS (%) | 95% CI | NS (%) | 95% CI | NS (%) | 95% CI | NS (%) | 95% CI |
| 1 | 96.5 | 96.4–96.6 | 96.8 | 96.7–96.9 | 92.6 | 92.2–93.0 | 96.7 | 96.7–96.8 | 97.0 | 97.0–97.1 | 93.6 | 93.3–93.8 |
| 3 | 91.8 | 91.6–91.9 | 92.5 | 92.4–92.7 | 83.1 | 82.5–83.7 | 92.1 | 92.0–92.2 | 92.9 | 92.7–93.0 | 84.3 | 83.9–84.8 |
| 5 | 88.2 | 88.0–88.3 | 89.1 | 88.9–89.3 | 76.9 | 76.2–77.6 | 88.6 | 88.4–88.8 | 89.6 | 89.4–89.8 | 78.4 | 77.7–79.1 |
Abbreviations: CI, confidence interval; NS, net survival.
Five-year net survival was high for females who were diagnosed with local stage cancer between 2001 and 2003 at 98% and remained high for those who were diagnosed between 2004 and 2009 (Table 3). In both periods, the difference in survival between white females and black females was highest for those with regional tumors (82.3% and 83.5%, respectively, for white females vs 69.9% and 71.8%, respectively, for black females) and distant tumors (22.5% and 25.7%, respectively, for white females vs 15.2% and 17.1%, respectively, for black females). This difference was noticed in all states and did not decrease over time (Supporting Table 3). For local stage, the racial disparity persisted but to a smaller extent (98.2% and 98.6%, respectively, for white females vs 92.8% and 94.3%, respectively, for black females).
TABLE 3.
Breast Cancer: 5-Year Age-Standardized Net Survival (%) for Females (Ages 15–99 Years) Diagnosed Between 2001–2009, by SEER Summary Stage 2000 at Diagnosis, Race, and Calendar Period of Diagnosis
| 2001–2003
|
2004–2009
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All Races
|
White
|
Black
|
All Races
|
White
|
Black
|
|||||||
| SEER Summary Stage 2000 | NS (%) | 95% CI | NS (%) | 95% CI | NS (%) | 95% CI | NS (%) | 95% CI | NS (%) | 95% CI | NS (%) | 95% CI |
| All stages | 88.2 | 88.0–88.3 | 89.1 | 88.9–89.3 | 76.9 | 76.2–77.6 | 88.6 | 88.4–88.8 | 89.6 | 89.4–89.8 | 78.4 | 77.7–79.1 |
| Local | 98.0 | 97.8–98.2 | 98.2 | 98.0–98.4 | 92.8 | 91.9–93.7 | 98.3 | 98.1–98.6 | 98.6 | 98.3–98.8 | 94.3 | 93.4–95.2 |
| Regional | 81.2 | 80.8–81.6 | 82.3 | 82.0–82.7 | 69.9 | 68.5–71.2 | 82.3 | 81.9–82.7 | 83.5 | 83.0–83.9 | 71.8 | 70.4–73.2 |
| Distant | 21.5 | 20.9–22.2 | 22.5 | 21.7–23.2 | 15.2 | 13.6–16.9 | 24.5 | 23.7–25.2 | 25.7 | 24.9–26.6 | 17.1 | 15.4–18.8 |
| Unknown | 72.5 | 71.8–73.2 | 73.1 | 72.4–73.9 | 65.9 | 63.8–67.9 | 72.6 | 71.9–73.4 | 73.2 | 72.4–74.1 | 68.3 | 66.3–70.4 |
Abbreviations: CI, confidence interval; NS, net survival; SEER, Surveillance, Epidemiology, and End Results.
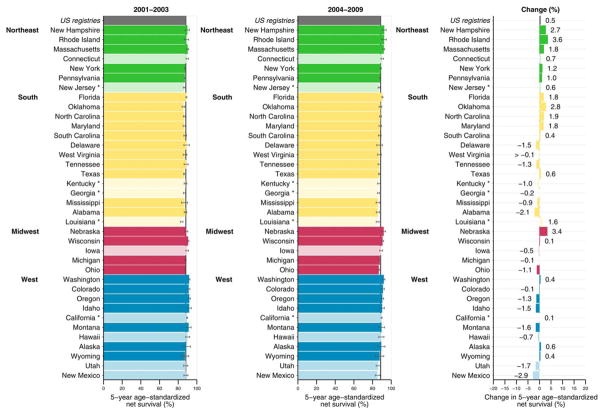
Figure 1 illustrates 5-year age-standardized net survival by state. Between 2001 and 2003, 5-year survival for breast cancer was very high at approximately 90%. Survival remained high between 2004 and 2009, with slight variation between states and regions. There was a 0.5% increase in net survival across the United States. The change in survival among states ranged from −2.9% to 3.6%.
Figure 1.
Breast cancer 5-year age-standardized net survival (%) is illustrated for females (ages 15–99 years) diagnosed 2001–2003 and 2004–2009 along with absolute changes (%). States are grouped by US Census region. Note that data from 37 statewide cancer registries (covering 80.6% of the population) are ranked within US Census region by the survival estimate for 2004–2009. Dark bars denote states funded by the Centers for Disease Control and Prevention National Program of Cancer Registries; pale bars, states funded by the National Cancer Institute’s Surveillance, Epidemiology and End Results Program. Asterisks indicate states that are funded by both federal surveillance programs. Change (%) was not plotted if a survival estimate was not available for 1 calendar period or if 1 or more estimates were not age standardized.
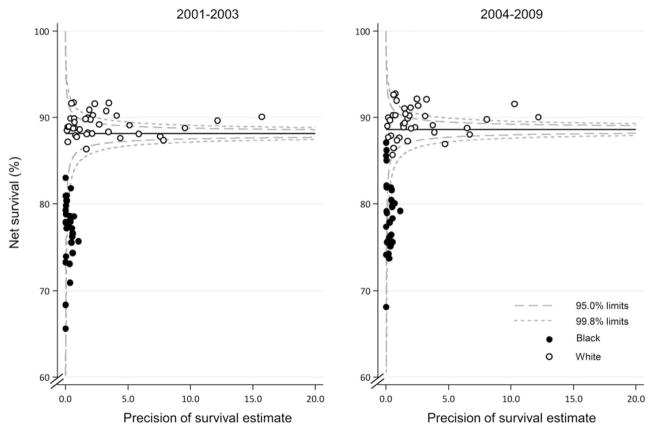
Figure 2 provides funnel plots of net survival for the periods 2001 through 2003 and 2004 through 2009 to provide further insight into the variability of breast cancer survival in the United States by race and state. The plots reveal how much a particular survival estimate deviates from the pooled US value (the “target,” represented in the plot by the horizontal line) given the precision of each estimate.24,25 These plots indicate striking geographic and racial variation in survival. Between 2001 and 2003, net survival was lower for black females in all states. Although survival for black females increased slightly between 2004 and 2009, it remained lower for black females compared with white females in most states. Also in most states, survival for white females was above the pooled US estimate, whereas survival for all black females was below the pooled US estimate.
Figure 2.
Breast cancer 5-year age-standardized net survival (%) is illustrated for females (ages 15–99 years) by state, race, and calendar period of diagnosis. Note that the pooled US survival estimate for each calendar period is indicated by the solid horizontal line with corresponding 95.0% and 99.8% control limits (dashed lines).
DISCUSSION
The current study is the largest study to date on trends in population-based breast cancer survival in the United States. It is also unique, in that the data are analyzed by race, stage at diagnosis, and state. This expands the US results from CONCORD-2 by providing a detailed analysis of net survival by race and stage at diagnosis. Population-based survival is a key measure of the overall effectiveness of the health system in dealing with cancer and thus is a useful measure for evaluating access to and quality of care and appropriate treatment. These results can be used to plan future cancer-control strategies.
Overall survival for breast cancer was strikingly high between 2001 and 2003 and remained high between 2004 and 2009. There was wide variability in survival between states, with lower survival for black than for white females in all 37 states included in the analyses. Disappointingly, the greater than 10% difference in survival between white and black females did not improve over time. More efforts need to be initiated to understand the reasons for this persistent racial disparity in breast cancer survival and to help black females in accessing early diagnosis and appropriate treatment.
Black/white inequalities in survival suggest differences in access to care and in tumor morphology between the groups. Within the same stage of disease, net survival is lower among black females. However, black/white differences in mammography rates by state are not consistent with stage of disease or survival. Indeed, there were no significant state-specific differences between black and white females who reported having had a mammogram within the past 2 years during the study periods.27
Clinical Perspective
Having access to diagnostic studies and high-quality treatment is important for improving breast cancer survival. Black females are more likely than white females to experience delays in treatment and not to receive appropriate treatment for breast cancer.28 Delays in care and inappropriate treatment can result in larger tumors and poorer outcomes. Timely follow-up of abnormal test results and receipt of treatment after diagnosis could improve outcomes of all females with breast cancer.29 Access to quality care is a key factor to reducing disparities in breast cancer survival.
Breast cancer is a very heterogeneous disease. There are several different types of breast cancer with multiple subtypes based on biomarkers, such as estrogen receptor, progesterone receptor, and human epidermal growth factor receptor status. Differences in tumor morphology not only dictate treatment but also are prognostic. For example, triple-negative breast cancer (a breast cancer subtype that does not have estrogen receptor, progesterone receptor, or HER2 protein) is a more aggressive tumor with lower survival and is diagnosed more often among black females.30,31 There may be other tumor characteristics or underlying biologic differences between black and white females impacting tumor progression or therapy response that could be contributing to the observed survival differences by race.
Research is continually identifying new tumor bio-markers that have potential for use in a clinical setting. As treatment becomes more individualized, not all options are always available to all patients. Therefore, as newer treatments improve survival, it may not improve survival equally among all females. If minority or low-income females do not have access to newer treatment options, then survival improvements may not be as apparent among these groups in population-based surveillance systems. This may be 1 reason why we see that, at the same stage of diagnosis, net survival is lower among black than among white females.
Cancer-Control Perspective
In addition to addressing potential racial differences in the clinical care for breast cancer to reduce racial breast cancer survival disparities, there are several health behavior factors that can reduce the risk for breast cancer.32–35 Modifying risk factors, such as obesity, lack of physical activity, alcohol consumption, exposure to environmental toxins, and use of hormone therapy, can reduce a woman’s risk of getting breast cancer. Black females often have more comorbid conditions, which put them at greater risk for poorer survival outcomes.36 Women who may be at high risk for breast cancer because of family history or genetic reasons may need enhanced screening and risk-reduction procedures, such as taking antiestrogens or undergoing prophylactic mastectomy. Minority and low-income females more often have limited access to these options,37,38 which ultimately may put them at increased risk of dying from breast cancer.
Once diagnosed with breast cancer, females need to be educated and empowered to take part in treatment decisions and to receive timely, guideline-concordant, and complete treatment. Females who have lack of trust in the health care system or who are characterized by cultural differences from their health care providers may not receive the needed treatment and supportive care.39 Studies that analyzed the availability of equal treatment among all patients demonstrated that patients have similar outcomes when they receive equal treatment.40–43 This suggests that the large and persistent survival deficit in black females between 2001 and 2009 may be attributable to the receipt of less effective treatment. Supportive care is also an area that needs more attention. Specifically, interventions to address geographic isolation, cultural differences, financial burden, and fear may be needed to improve access to quality care. These issues may be addressed along with clinical care.
Strengths and Limitations
This is the largest study comparing breast cancer survival by state and race using data from CONCORD-2, which include high-quality data covering greater than 80% of the US population. In addition, for females diagnosed with breast cancer, 98.9% of cases were morphologically verified, and the percentage of cases with unknown stage was low.44 All participating registries were certified by the North American Association of Central Cancer Registries as having met data quality and completeness standards (available at: http://www.naaccr.org/Certification/Criteria.aspx, Accessed June 28, 2017).
The current study had a few limitations. First, the follow-up procedures in the United States differed according to the federal funding source.45 All SEER registries are required to conduct follow-up of all cases to ascertain vital status. NPCR registries are only funded to ascertain deaths through linkage with the state vital records and the National Death Index. Some state cancer registries assumed that a patient was alive if the case was not included on a state death certificate database or on the National Death Index. Second, the manner in which SEER SS2000 data were collected and reported changed for all registries in 2004, as described above (see Methods). This resulted in a decrease in the percentage of cases with unknown stage when stage was derived rather than manually coded. Next, the states in the Western Region (Mountain and Pacific Divisions) had too few black females to reliably assess racial differences, and some states did not report stage data, which could result in the data not being generalizable to the entire United States. Further, we were only able to report on black and white women due to low numbers of other races in most states. Finally, vital status follow-up data were only available through December 31, 2009; therefore, we did not have 5-year follow-up for each patient. Because the remarkable differences between black and white females have persisted for more than a decade in this study, they are unlikely to simply disappear with the inclusion of more recent years of follow-up data. The third cycle of the CONCORD program is ongoing, and the data set is currently being updated. This will include patients who were diagnosed and followed until the end of 2014.
Conclusion
There is increasing awareness and effort to achieve health equity among racial, ethnic, geographic, socioeconomic, and other groups. With the persistent disparity in breast cancer survival between black females and white females, more national, local, and individual efforts are needed. This study provides accurate, useful information on incidence and survival of breast cancer patients in various US subpopulations and is supported through a nation-wide system of central cancer registries funded by the CDC’s NPCR or the National Cancer Institute’s SEER program.45
The CDC and its partners are dedicated to identifying and addressing the factors that lead to health disparities among racial, ethnic, geographic, socioeconomic, and other groups so that barriers to health equity can be removed. The CDC’s National Breast and Cervical Cancer Early Detection Program works with the states, tribes/tribal organizations, and territories to provide assistance to low-income females to ensure that they have access to appropriate breast and cervical cancer screening, diagnostic, and treatment services.45 The CDC’s Comprehensive Cancer Control Program works with states, tribes/tribal programs, and territories to develop cancer-control plans that focus on prevention, screening, and survivorship issues.45 Working with partner organizations, such as the National African American Tobacco Prevention Network and the National Alliance for Hispanic Health, these programs target specific populations who are at risk of developing or dying from cancer.
Eliminating or reducing disparities in breast cancer survival will require broad, coordinated population efforts at various federal, state, and local levels. The persistent black/white disparities in breast cancer survival reported here demonstrate that increased focus on individual, provider, community, organizational, and policy levels are warranted. Public health has an important opportunity to expand outreach to medically underserved females, provide education about breast cancer risk reduction, and increase awareness about post-treatment strategies to improve outcomes. Evidence-based interventions to further improve provider awareness, knowledge, and practice also need to be identified. Finally, public health professionals can champion community-level engagement and partnerships to leverage organizational infrastructure and provide evidence to inform organizational policies that ensure equal cancer care among all females and a reduction in breast cancer survival disparities. Focusing on education and informed decisions, cultural sensitivities, personalized care, and equal access to care are critical steps.
Supplementary Material
Acknowledgments
FUNDING SUPPORT
Funding support for Claudia Allemani: US Centers for Disease Control and Prevention (CDC:12FED03123, ACO12036).
Footnotes
The findings and conclusions in this report are those of the authors and do not necessarily reflect the official position of the CDC.
This Supplement edition of Cancer has been sponsored by the U.S. Centers for Disease Control and Prevention (CDC), an Agency of the Department of Health and Human Services.
The CONCORD-2 study was approved by the Ethics and Confidentiality Committee of the UK’s statutory National Information Governance Board (now the Health Research Authority) (ref ECC 3-04(i)/2011) and by the National Health Service Research Ethics Service (Southeast; 11/LO/0331).
CONFLICT OF INTEREST DISCLOSURES
The authors made no disclosures.
AUTHOR CONTRIBUTIONS
Jacqueline W. Miller: Writing–original draft and supervision. Judith Lee Smith: Writing–review and editing. A. Blythe Ryerson: Writing–review and editing. Thomas C. Tucker: Writing–review and editing. Claudia Allemani: Conceptualization, data validation, analysis, visualization, writing, and funding acquisition.
References
- 1.International Agency for Research on Cancer (IARC) GLOBOCAN 2012: Estimated cancer incidence, mortality and prevalence worldwide in 2012. Lyon, France: IARC; 2013. [Accessed April 12, 2016]. Available at: http://globo-can.iarc.fr/Pages/fact_sheets_cancer.aspx. [Google Scholar]
- 2.US Cancer Statistics Working Group. US Cancer Statistics: 1999–2013 Incidence and Mortality Web-based Report. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, and National Cancer Institute; 2016. [Accessed December 9, 2016]. Available at: www.cdc.gov/uscs. [Google Scholar]
- 3.Ryerson AB, Eheman CR, Altekruse SF, et al. Annual Report to the Nation on the Status of Cancer, 1975–2012, featuring the increasing incidence of liver cancer. Cancer. 2016;122:1312–1337. doi: 10.1002/cncr.29936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.US Department of Health and Human Services (HHS) HHS Action Plan to Reduce Racial and Ethnic Health Disparities: A Nation Free of Disparities in Health and Health Care. Washington, DC: HHS; 2015. [Accessed April 10, 2016]. Available at: http://minorityhealth.hhs.gov/npa/files/plans/hhs/hhs_plan_complete.pdf. [Google Scholar]
- 5.Agency for Healthcare Research and Quality (AHRQ) National Healthcare Quality & Disparities Report Chartbooks. Rockville, MD: AHRQ, S Department of Health and Human Services; 2016. [Accessed April 13, 2016]. Available at: http://www.ahrq.gov/research/findings/nhqrdr/chart-books/index.html. [Google Scholar]
- 6.Centers for Disease Control and Prevention (CDC) Strategies for reducing health disparities—selected CDC-sponsored interventions, United States, 2016. MMWR Suppl. 2016;65:1–69. [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention (CDC) [Accessed April 13, 2016];About CDC’s Office of Minority Health and Health Equity (OMHHE) Available at: https://www.cdc.gov/healthequity/about/index.html.
- 8.van Ravesteyn NT, Schechter CB, Near AM, et al. Race-specific impact of natural history, mammography screening, and adjuvant treatment on breast cancer mortality rates in the United States. Cancer Epidemiol Biomarkers Prev. 2011;20:112–122. doi: 10.1158/1055-9965.EPI-10-0944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Richardson LC, Henley J, Miller J, Massetti G, Thomas CC. Patterns and trends in black-white differences in breast cancer incidence and mortality—United States, 1999–2013. MMWR Morb Moral Wkly Rep. 2016;65:1093–1098. doi: 10.15585/mmwr.mm6540a1. [DOI] [PubMed] [Google Scholar]
- 10.Howlader N, Noone AM, Krapcho M, et al., editors. SEER Cancer Statistics Review, 1975–2012. Bethesda, MD: National Cancer Institute; 2015. [Accessed December 9, 2016]. Available at: http://seer.cancer.gov/csr/1975_2012/ [Google Scholar]
- 11.Hunt BR, Whitman S, Hurlbert MS. Increasing black:white disparities in breast cancer mortality in the 50 largest cities in the United States. Cancer Epidemiol. 2014;38:118–123. doi: 10.1016/j.canep.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 12.Ward E, Jemal A, Cokkinides V, et al. Cancer disparities by race/ethnicity and socioeconomic status. CA Cancer J Clin. 2004;54:78–93. doi: 10.3322/canjclin.54.2.78. [DOI] [PubMed] [Google Scholar]
- 13.Coleman M. Cancer survival: global surveillance will stimulate health policy and improve equity. Lancet. 2014;383:564–573. doi: 10.1016/S0140-6736(13)62225-4. [DOI] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention (CDC) Vital signs: racial disparities in breast cancer severity—United States, 2005–2009. MMWR Morb Mortal Wkly Rep. 2012;61:922–926. [PubMed] [Google Scholar]
- 15.Coleman MP, Quaresma M, Berrino F, et al. Cancer survival in 5 continents: a worldwide population-based study (CONCORD) Lancet Oncol. 2008;9:730–756. doi: 10.1016/S1470-2045(08)70179-7. [DOI] [PubMed] [Google Scholar]
- 16.Allemani C, Weir HK, Carreira H, et al. Global surveillance of cancer survival 1995–2009: analysis of individual data for 23,676,887 patient from 279 population-based registries in 67 countries (CONCORD-2) Lancet. 2015;385:977–1010. doi: 10.1016/S0140-6736(14)62038-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Allemani C, Weir HK, Carreira H, et al. CONCORD Working Group. Global surveillance of cancer survival 1995–2009: analysis of individual data for 25,676,887 patients from 279 population-based registries in 67 countries (CONCORD-2) Lancet. 2015;385:977–1010. doi: 10.1016/S0140-6736(14)62038-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fritz AG, Percy C, Jack A, et al., editors. International Classification of Diseases for Oncology (ICD-O) 3. Geneva, Switzerland: World Health Organization; 2000. [Google Scholar]
- 19.Young JL, Roffers SD, Ries LAG, Fritz AG, Hurlbut AA. SEER Summary Staging Manual-2000: Codes and Coding Instructions. Bethesda, MD: National Cancer Institute; 2001. NIH Pub. No. 01-4969. [Google Scholar]
- 20.Surveillance, Epidemiology, and End Results (SEER) Program. Collaborative Stage. Bethesda, MD: National Cancer Institute; 2004. [Accessed April 1, 2016]. Available at: http://seer.cancer.gov/tools/collabstaging/ [Google Scholar]
- 21.Pohar Perme M, Stare J, Esteve J. On estimation in relative survival. Biometrics. 2012;68:113–120. doi: 10.1111/j.1541-0420.2011.01640.x. [DOI] [PubMed] [Google Scholar]
- 22.Rachet B, Maringe C, Woods LM, Ellis L, Spika D, Allemani C. Multivariable flexible modelling for estimating complete, smoothed life tables for sub-national populations [serial online] BMC Public Health. 2015;15:1240. doi: 10.1186/s12889-015-2534-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spika D, Bannon F, Bonaventure A, et al. Life tables for global surveillance of cancer survival (the CONCORD programme): data sources and methods [serial online] BMC Cancer. 2017;17:159. doi: 10.1186/s12885-017-3117-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Corazziari I, Quinn MJ, Capocaccia R. Standard cancer patient population for age standardising survival ratios. Eur J Cancer. 2004;40:2307–2316. doi: 10.1016/j.ejca.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 25.Quaresma M, Coleman MP, Rachet B. Funnel plots for population-based cancer survival: principles, methods and applications. Stat Med. 2014;33:1070–1080. doi: 10.1002/sim.5953. [DOI] [PubMed] [Google Scholar]
- 26.Allemani C, Harewood R, Johnson C, et al. Population-based cancer survival in the United States: data, quality control, and statistical methods. Cancer. 2017;123:4982–4993. doi: 10.1002/cncr.31025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Behavioral Risk Factor Surveillance System (BRFSS) Prevalence and trends data. Atlanta, GA: Centers for Disease Control and Prevention; 2016. [Accessed November 4, 2016]. Available at: http://www.cdc.gov/brfss/brfssprevalence/ [Google Scholar]
- 28.Lund MJ, Brawley OP, Ward KC, Young JL, Gabram SS, Eley JW. Parity and disparity in first course treatment of invasive breast cancer. Breast Cancer Res Treat. 2008;109:545–557. doi: 10.1007/s10549-007-9675-8. [DOI] [PubMed] [Google Scholar]
- 29.Taplin SH, Yabroff KR, Zapka J. A multilevel research perspective on cancer care delivery: the example of follow-up to an abnormal mammogram. Cancer Epidemiol Biomarkers Prev. 2012;21:1709–1715. doi: 10.1158/1055-9965.EPI-12-0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stead LA, Lash TL, Sobieraj JE, et al. Triple negative breast cancers are increased in black women regardless of age or body mass index [serial online] Breast Cancer Res. 2009;11:R18. doi: 10.1186/bcr2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brouckaert O, Wildiers H, Floris G, Neven P. Update on triple-negative breast cancer: prognosis and management strategies. Int J Womens Health. 2012;4:511–520. doi: 10.2147/IJWH.S18541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Institute of Medicine (IOM) Breast Cancer and the Environment: A Life Course Approach. Washington, DC: The National Academies Press; 2011. [Accessed June, 21, 2016]. Available at: http://nationalacademies.org/hmd/Reports/2011/Breast-Cancer-and-the-Environment-A-Life-Course-Approach.aspx. [Google Scholar]
- 33.Division of Cancer Prevention and Control, Centers for Disease Control and Prevention. [Accessed June 20, 2016];What can I do to reduce my risk of breast cancer? Available at: http://www.cdc.gov/cancer/breast/basic_info/prevention.htm.
- 34.American Cancer Society. [Accessed June 20, 2016];Lifestyle related risk factors for breast cancer. Available at: http://www.cancer.org/cancer/breastcancer/morein-formation/breastcancerearlydetection/breast-cancer-early-detection-risk-lifestyle-related.
- 35.Forman MR, Winn DM, Collman GW, Rizzo J, Birnbaum LS. Environmental exposures, breast development and cancer risk: through the looking glass of breast cancer prevention. Reprod Toxicol. 2015;54:6–10. doi: 10.1016/j.reprotox.2014.10.019. [DOI] [PubMed] [Google Scholar]
- 36.Tammemagi CM, Nerenz D, Neslund-Dudas C, Feldkamp C, Nathanson D. Comorbidity and survival disparities among black and white patients with breast cancer. JAMA. 2005;294:1765–1772. doi: 10.1001/jama.294.14.1765. [DOI] [PubMed] [Google Scholar]
- 37.Zimmerman RK, Tabbarah M, Nowalk MP, et al. Racial differences in beliefs about genetic screening among patients at inner-city neighborhood health centers. J Natl Med Assoc. 2006;98:370–377. [PMC free article] [PubMed] [Google Scholar]
- 38.Armstrong K, Micco E, Carney A, Stopfer J, Putt M. Racial differences in the use of BRCA1/2 testing among women with a family history of breast or ovarian cancer. JAMA. 2005;293:1729–1736. doi: 10.1001/jama.293.14.1729. [DOI] [PubMed] [Google Scholar]
- 39.Peters N, Rose A, Armstrong K. The association between race and attitudes about predictive genetic testing. Cancer Epidemiol Biomarkers Prev. 2004;13:361–365. [PubMed] [Google Scholar]
- 40.Wheeler SB, Reeder-Hayes KE, Carey LA. Disparities in breast cancer treatment and outcomes: biological, social, and health system determinants and opportunities for research. Oncologist. 2013;18:986–993. doi: 10.1634/theoncologist.2013-0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ward E, Halpern M, Schrag N, et al. Association of insurance with cancer care utilization and outcomes. CA Cancer J Clin. 2008;58:9–31. doi: 10.3322/CA.2007.0011. [DOI] [PubMed] [Google Scholar]
- 42.Hershman D, McBride R, Jacobson JS, et al. Racial disparities in treatment and survival among women with early-stage breast cancer. J Clin Oncol. 2005;23:6639–6646. doi: 10.1200/JCO.2005.12.633. [DOI] [PubMed] [Google Scholar]
- 43.Dignam JJ. Differences in breast cancer prognosis among African-American and Caucasian women. CA Cancer J Clin. 2000;50:50–64. doi: 10.3322/canjclin.50.1.50. [DOI] [PubMed] [Google Scholar]
- 44.Suppl to: Allemani C, Weir HK, Carreira H, et al. and the CONCORD Working Group. Global surveillance of cancer survival 1995–2009: analysis of individual data for 25,676,887 patients from 279 population-based registries in 67 countries (CONCORD-2) [Accessed June 28, 2016];Lancet. 2014 doi: 10.1016/S0140-6736(14)62038-9. published online Nov 26. Available at: http://www.the-lancet.com/journals/lancet/article/PIIS0140-673662038-9/supplemental.
- 45.White MC, Babcock F, Hayes NS, et al. The history and use of cancer registry data and public health cancer control programs in the United States. Cancer. 2017;123:4969–4976. doi: 10.1002/cncr.30905. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.