Abstract
Mood disorders such as depression are among the most prevalent psychiatric disorders in the US, but are inadequately treated in a substantial proportion of patients. Accordingly, neuropsychiatric research has pivoted from investigation of monoaminergic mechanisms to exploration of novel mediators including the role of inflammatory processes. Subsets of mood disorder patients exhibit immune-related abnormalities, including elevated levels of pro-inflammatory cytokines, monocytes, and neutrophils in the peripheral circulation, dysregulation of neuroglia and blood-brain barrier function, and disruption of gut microbiota. The field of psychoneuroimmunology is one of great therapeutic opportunity, yielding experimental therapeutics for mood disorders such as peripheral cytokine targeting antibodies, microglia and astrocyte targeting therapies, and probiotic treatments for gut dysbiosis, and producing findings that identify therapeutic targets for future development.
Keywords: Mood disorders, Major Depressive Disorder, Inflammation, Microbiome, Therapeutics
Introduction
Mood disorders are highly prevalent, afflicting 1 in 5 Americans at some point during their lives (1). Major depressive disorder (MDD), with lifetime prevalence just below 17%, is the most common mood disorder (1). MDD diagnosis is largely based on behavioral symptomatology and rarely accounts for biological abnormalities (2). Symptoms of depression include, but are not limited to, depressed mood, anhedonia, altered sleeping and eating patterns, irritability, and cognitive impairments (2). Approved treatments for MDD are unsuccessful in 30–50% of patients (3), and consequently, depression is the leading cause of worldwide non-fatal disability (4). The global disease burden of MDD and its high rate of treatment resistance highlight the need for increased focus by medical and research communities on developing alternative therapies and exploring novel avenues of inquiry into disease pathogenesis.
To this end, depression researchers have increasingly focused on inflammation as a pathological mechanism and as a potential therapeutic target. MDD is often comorbid with chronic inflammatory conditions, and patients with pre-existing inflammatory diseases can be several times more likely to develop depression than otherwise healthy individuals (5). Furthermore, depressed patients often display alterations in peripheral and/or central inflammatory status (3). In this review, we will summarize the current state of knowledge regarding inflammatory symptoms and mechanisms of mood disorders, evaluate available and experimental immune targeting therapies, and highlight research areas of particular therapeutic opportunity. We present both preclinical and clinical evidence wherever possible, and focus our discussion on peripheral and central immune processes and the interaction of these processes with the gut microbiotic environment.
Peripheral immune targets for antidepressant drug development
Over the past three decades, affective disorders research has increasingly focused on peripheral systems, particularly the peripheral immune system, as potential mediators of mood disorder pathophysiology. The first observation of peripheral immune involvement, published in the late ‘80s, reported psychiatric complications in a subset of patients suffering from chronic viral hepatitis who were treated with human Interferon-α (6). In the decades since, multiple clinical and preclinical studies have associated high levels of circulating leukocytes and pro-inflammatory cytokines, such as Interleukin-1 Beta (IL-1β), Interleukin-6 (IL-6), and tumor necrosis factor alpha (TNFα), with depressive symptoms in humans and depression-like behaviors in animal models, informing the “macrophage theory of depression” (for comprehensive reviews, see (3; 5; 7–9)). Polymorphisms in immune-related genes, including those encoding IL-1β, IL-6, TNFα, C-reactive protein (CRP), and monocyte chemoattractant protein-1 (MCP-1 also known as chemokine ligand 2, CCL2), have recently been linked to depression risk, severity, and treatment responsiveness (10). Further supporting a role for peripheral immune processes in mood, treatment of inflammatory diseases with drugs that lower cytokine levels improved depressive symptoms in patients with comorbid mood disorders (11). However, the effect of commonly prescribed antidepressants on inflammatory cytokine levels is not yet well established in humans, as studies have reported decreased levels, no effect, or even increased levels of various cytokines (for review, see (5)). These discrepancies may be related to the heterogeneity of mood disorders or gender differences in treatment response. Indeed, selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs) affect cytokine levels in men but not in women (12). Collectively, these findings highlight the need for further studies that elucidate the complex interactions of the peripheral immune system and brain reward circuitry to ultimately improve antidepressant treatment strategies (13; 14). In this section, we overview stress effects on the peripheral immune system and discuss recent findings in both humans and animal models that are relevant to antidepressant drug development.
The peripheral immune system is composed of several cell types traditionally assigned to either the innate or adaptive immune systems. Innate immune cells, which include monocytes, granulocytes, macrophages, dendritic cells, and innate lymphocytes, are involved in rapid and effective responses against pathogens or sterile injury (3; 5). Tissue-resident dendritic cells and macrophages act through pattern recognition receptors – pathogen associated molecular patterns, PAMPs, or danger associated molecular patterns, DAMPs - activating signaling cascades that lead to release of inflammatory cytokines and recruitment of monocytes and neutrophils (3; 5). Recruited monocytes play a key role in inflammation and can further differentiate into phagocytic macrophages or dendritic cells, whereas tissue-resident macrophages are involved in the resolution of inflammation and tissue homeostasis (15; 16). Granulopoiesis and monocytosis, characterized by a rapid increase in the number of neutrophils and monocytes, respectively, are generally associated with trauma-related injury or antimicrobial defense. Both neutrophilia and monocytosis is observed in a subset of depressed patients (possibly more pronounced in men (8)), suggesting that a prolonged state of exacerbated activation of the innate immune system may contribute to the development of mood disorders. Adaptive immune cells (T and B lymphocytes) mediate immunological memory of previous encounters, enabling the body to mount enhanced, targeted responses in the spleen and lymph nodes to subsequent insults (3; 5). During this process, a subpopulation of circulating effector memory T cells is activated upon presentation of a specific antigen by an antigen-presenting cell. This immune interaction triggers cytokine release and recruitment of dendritic and natural killer cells (17). It has been hypothesized that stress can be stored as an immunological memory (18), raising the exciting possibility of adding a vaccine to the pharmaceutical arsenal that prevents mood disorders (19).
Intriguingly, evidence from rodent studies suggests that it may be possible to inoculate an individual against repeated stressors. T-cell-dependent vaccination with a weak, CNS-specific, myelin-derived peptide agonist before initial exposure to chronic mild stress prevented the establishment of depression-like behaviors in rats (20). Similarly, T-cell-deficient mice showed enhanced vulnerability to stress, while mice overexpressing autoreactive T cells were resistant to depression-like behavior when confronted with a predator odor challenge (21). Inoculation with T cells promoted resilience in T-cell-deficient mice, suggesting that mild activation of the adaptive immune system may confer a relative protection against stress-related mood disorders (21) (Figure 1). In line with this idea, transplantation of lymph node cell suspensions from stressed mice into naïve mice lacking mature lymphocytes not only produced antidepressant effects but also reduced circulating inflammatory cytokine levels (22). Altogether, these studies suggest that it may be possible to counteract stress-induced immune responses by manipulating adaptive T cells (23). Interestingly, treatment with the antidepressant fluoxetine can rescue stress-induced reductions in T-cell reactivity and proliferation (24), and the anti-inflammatory effects of imipramine are associated with activation of T lymphocytes in mice (25). Increased numbers of T cells have also been reported in the blood of patients following successful antidepressant treatment with imipramine (26). Nevertheless, as the effects of antidepressants on the adaptive immune system are still poorly understood (for review, please refer to (27)), far more basic research is needed to determine safety of an efficacy of vaccine-based therapies for treatment of mood disorder (Figure 1).
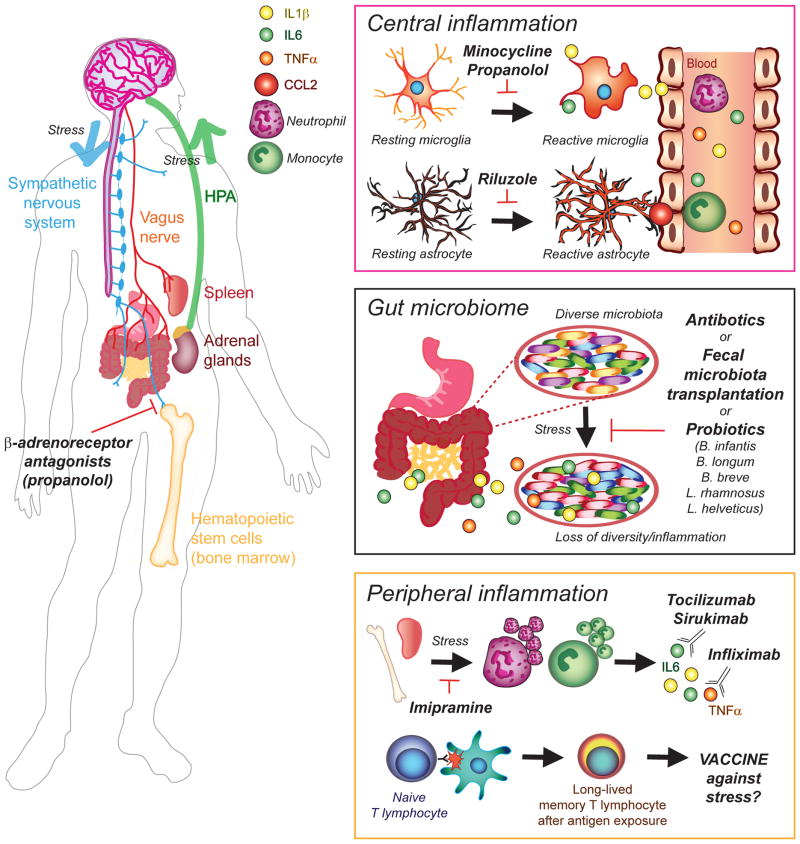
Figure 1. Inflammation-related therapeutic opportunities to treat mood disorders.
Chronic stress activates the sympathetic nervous system promoting release of monocytes and neutrophils from the spleen and bone marrow. Exacerbated stress-induced hematopoietic monocytosis can be blocked by antagonists of β-adrenergic receptors, such as propranolol, reducing peripheral inflammation. Under normal conditions, stimulation of the sympathetic nervous system triggers the hypothalamic-pituitary-adrenal (HPA) axis and glucocorticoid release from the adrenal glands which act as a feedback loop. However, leukocytes of chronically stressed mice are resistant to the anti-inflammatory effects of glucocorticoids maintaining a prolonged inflammatory state and high levels of circulating leukocytes (neutrophils, monocytes) and pro-inflammatory cytokines - Interleukin-1Beta (IL-1β), IL-6, tumor necrosis factor alpha (TNFα) in the blood. These immune signals are then recruited into the brain possibly because of chemokine ligand 2 (CCL2) release by reactive astrocytes. Activation of microglia, the resident immune cells of the brain, is also exacerbated in the context of affective disorders. Central inflammation mediated by these glial cells can be modulated by minocycline or propranolol for microglia, and riluzole for astrocytes, and these compounds have been recently tested in clinical trials as adjunctive therapies to commonly prescribed antidepressants. The gut microbiota can influence the central nervous system and subsequent behavioral responses to stress through activation of the vagus nerve and cytokine release. Loss of microbiota diversity has been reported in stressed mice and depressed patients and associated with increased inflammation and possibly a leaky gut. Thus, administration of antibiotics or probiotics represents novel therapeutic opportunities to treat mood disorders. Fecal microbiotal transplants from patients suffering from major depressive disorder (MDD) recapitulate deleterious behaviors in naive mice raising the possibility to eventually transplant microbiota from healthy donors to treat affective disorders. Recent and ongoing clinical trials are also testing humanized antibodies against inflammatory cytokines – Tocilizumab and Sirukimab for IL-6 and Infliximab for IL-1β - to reduce peripheral inflammation and treat MDD and bipolar disorders. This strategy could be very effective in subsets of patients characterized by pro-inflammatory transcriptional profiles. Finally, preclinical studies suggest that stress could be stored as an immunological memory in T lymphocytes raising the possibility to develop a vaccine against stress-related affective disorders
Another promising avenue of research involves manipulation of stress-induced innate immune priming to prevent enhanced proliferation and release of inflammatory monocytes and neutrophils as well as their recruitment to the brain (28). In an elegant study, Powell et al. (29) showed that social stress induced monocytosis and granulopoiesis in mice and up-regulated inflammatory gene expression in the leukocyte transcriptome. The authors confirmed these findings in peripheral blood mononuclear cells of human subjects of low socioeconomic status, which is considered a form of chronic social stress that increases risk of mood disorders. They further found that pharmacological modulation of the sympathetic nervous system with antagonists of β-adrenergic receptors blocked bone marrow myelopoiesis and stress-induced monocytosis in mice (Figure 1), demonstrating that manipulation of the sympathetic nervous system can alter downstream peripheral innate immune processes characteristic of mood disorders. In steady state conditions, stimulation of the sympathetic nervous system activates the hypothalamic-pituitary-adrenal axis, promoting the release of glucocorticoids that act as a feedback loop to modulate peripheral immune responses, including IL-6 release by monocytes (3). However, under chronically stressful conditions, sympathetic nerve fiber signals are altered, leading to increased hematopoietic stem cell proliferation and exacerbated release of inflammatory monocytes and neutrophils into the bloodstream (30). Furthermore, leukocytes of chronically stressed rodents display resistance to the anti-inflammatory effects of glucocorticoids (31–33), indicating that negative feedback mechanisms to reign in aberrant inflammation can become dysfunctional in conditions of prolonged stress. Future therapeutic strategies that limit stress-induced leukocytosis and prevent leukocyte glucocorticoid resistance may prove advantageous in human patients. The existing antidepressant imipramine has been shown to attenuate corticosterone and IL-6 responses and decrease the number of circulating monocytes in mice (34). However, imipramine does not affect the stress-related increased frequency of granulocytes in spleen and it may be these cells that prime the organism for increased stress-induced inflammation (34).
Subsets of patients suffering from mood disorders have circulating immune cells characterized by pro-inflammatory transcriptional profiles (29) and potential priming to stress-induced signals. Leukocytes from stressed mice display an exacerbated response to an immune challenge with higher release of pro-inflammatory cytokines (IL-6, TNFα) (31; 35) and are less responsive to inhibitory feedback (32). Higher concentrations of pro-inflammatory cytokines have been consistently reported in the blood of patients suffering from major depression (7) and bipolar disorder (36) according to meta-analyses. To date, more than 20 clinical trials evaluating therapies that reduce circulating pro-inflammatory cytokine levels for the treatment of chronic inflammatory conditions have measured depressive symptoms as a secondary outcome (37). These studies included those evaluating humanized antibodies, such as infliximab and tocilizumab, and TNF inhibitors including etanercept and adalimumab (37). Many have reported significant improvements in depressive symptoms motivating a new series of clinical trials specifically designed to evaluate safety and efficacy of these treatments for mood disorders (5). For example, a clinical trial is currently recruiting participants to evaluate the potential antidepressant effect of tocilizumab, a humanized monoclonal antibody against the IL-6 receptor currently used as an immunosuppressive drug in the treatment of arthritis. Tocilizumab has also been proposed to treat bipolar disorder as increased levels of IL-6 have been reported during manic and depressive episodes (38). In addition, a new clinical trial of Sirukumab, a humanized monoclonal antibody against IL-6, in the treatment of unipolar depression is also underway (NCT02473289). Early results suggest that Sirukumab and Siltuximab, a chimeric monoclonal against IL-6, produces antidepressant effects in patients with rheumatoid arthritis and Castleman disease. Further, a double-blind, placebo-controlled, randomized clinical trial recently reported beneficial effects of the TNFα-related chimeric monoclonal antibody infliximab on depressive symptoms in treatment-resistant patients presenting a high baseline level of inflammation (39). Furthermore, a meta-analysis of randomized clinical trials recently reviewed the potential of pharmacological anti-inflammatory treatment with nonsteroidal anti-inflammatory drugs (NSAIDs) for treatment of mood disorders (40). Ten publications including over 6,000 participants revealed positive outcomes when compared to placebo in studies including patients with depression or reporting depressive symptoms (40). These findings align with the observation that antidepressants exert anti-inflammatory activity. Nevertheless, future therapeutic strategies should use caution when adding on NSAIDs, as NSAIDs can impact the absorption of SSRIs, and as such, the antidepressant effects of SSRIs are attenuated by anti-inflammatory drugs in mice and humans (41).
Central inflammatory targets for antidepressant drug development
Alterations in number or reactivity of the main CNS cell types responsive to peripheral inflammatory signals—microglia, astrocytes, and endothelial cells—are characteristic of depression (3). PET imaging studies in MDD patients experiencing a major depressive episode revealed enhanced microglial activation in depression-associated brain regions (prefrontal cortex, anterior cingulate cortex (ACC), and insula) compared to healthy controls, and microglial activation in ACC positively correlated with depressive episode severity (42). Furthermore, immunohistochemical analyses of subgenual ACC and anterior midcingulate cortex in depressed suicide completers revealed increased levels of microglial cells positive for quinolinic acid, a metabolite thought to be released by microglia in response to cytokine signaling (43). Additional postmortem studies indicate that MDD patients display reduced glial density in ACC (44), orbitofrontal cortex (45), subgenual PFC (46), amygdala (47), and dorsolateral PFC (48). The most numerous glia in brain are astrocytes, and astrocytic abnormalities have been well documented in depressed patients (49). Depressed suicide completers exhibit altered morphology of fibrous astrocytes in ACC white matter, including larger astrocytic cell bodies, longer projections, and more branching points (50). Depression has further been associated with reduced expression of astrocyte markers in brain, including glial fibrillary acidic protein (GFAP), connexin gap junction proteins, aquaporin-4 (a water channel on astrocytic endfeet), S100β calcium binding protein, EAAT1 and EAAT2 glutamate transporters, and glutamine synthetase (49). Collectively, astrocyte-associated changes in MDD suggest impairment of plasticity rather than cell loss, which may enhance damage to the blood-brain barrier (BBB) and promote signaling of peripheral inflammatory signals to the brain (3; 49). This hypothesis is supported by findings of reduced coverage of blood vessels by astrocytic endfeet in postmortem orbitofrontal cortex of depressed patients (51). Endothelial cell-mediated neurovascular dysfunction also contributes to BBB hyperpermeability in numerous neurological disorders, and as such, endothelial cells are an increasing focus of depression research (52). Patients with chronic vascular conditions including diabetes, cardiovascular disease, and stroke have an increased risk of developing depression (53). MDD patients display reduced brachial artery relative uptake ratio (an indicator of endothelial function) (52), and blood serum derived from MDD patients has enhanced pro-apoptotic activity on cultured endothelial cells (54). These vascular impairments likely have consequences for BBB permeability to inflammatory factors. Indeed, increased CSF to serum ratios of albumin and/or urate—measures of BBB permeability—have been associated with MDD in elderly women and suicidality in unipolar MDD patients (52).
Preclinical evidence indicates that microglia, astrocytes, and endothelial cells serve indispensable functions in the adult CNS and they both produce and respond to inflammatory signals. Of these three cell types, microglia are the most studied with relation to central immune processes contributing to affective disorders and will therefore be the primary focus of our discussion. Microglia are self renewing, tissue-resident macrophages of the CNS that actively survey their local environment, participate in synaptic pruning, promote tissue repair, and recruit peripheral leukocytes to sites of inflammation (55; 56). Microglia respond to neuronal inflammatory signals through the fractalkine receptor CX3CR1 and release cytokines and prostaglandins that in turn regulate neuronal function (56). Microglia are highly responsive to stress, and stress-induced morphological changes in microglia have been observed in brain regions including the hippocampus, amygdala, and prefrontal cortex (55). Microglial activation and subsequent cytokine release is mediated through stress regulation of neuroendocrine, neurotransmitter, and danger-associated molecular pattern (DAMP) signaling (56). Inhibition of β-adrenergic receptor signaling via systemic propranolol injection reduces social defeat-induced microglial hypertrophy, IL-1β mRNA expression, and microglial pro-inflammatory cell surface marker expression (57), and prevents stress induction of IL-1β protein in hypothalamus (58; 59), hippocampus (58), and pituitary (58) (Figure 1). Treatment with β-adrenergic receptor agonists produces the opposite effect on central cytokine signaling (58). Glucocorticoid (GC) signaling also mediates microglial response to stress. Pretreatment of rats with the GC receptor antagonist RU486 or adrenalectomy before stress exposure prevented subsequent microglial pro-inflammatory IL-1β response to LPS stimulation ex vivo (60). DAMP binding to pattern recognition receptors (PRRs) promotes microglial cleavage and release of IL-1β through activation of the NACHT, LRR and PYD domains-containing protein 3 (NLRP3) inflammasome (56). Nlrp3−/− mice exhibit reduced activation of microglia in hippocampus and PFC following chronic restraint stress (61). Furthermore, inhibition of the NLRP3 inflammasome in mice treated with a P2X7R (adenosine triphosphate (ATP)/purinergic type 2X7 receptor, a receptor for the cellular danger signal ATP) antagonist prevented stress-induced elevations of IL-1β and TNF-α in hippocampus (62). This group also determined that the source of the stress-induced ATP danger signal is most likely astrocytes. Astrocytes reside in gray and white matter, where they form contacts with both neurons and blood vessels, playing an essential role in glutamate reuptake and recycling, glucose metabolism, and blood-brain barrier integrity (49). Cultured human astrocytes stimulated with TNF-α or IL-1β display a characteristic transcriptional response to the stressor, including induction of chemokine (Ccl2, Ccl5, Cxcl8) and growth factor (Bdnf) genes (63), indicating that astrocytes may serve as important communicators of peripheral inflammatory status to the CNS. Astrocyte production of CCL2 may also be important for the recruitment of peripheral leukocytes to the BBB (55) (Figure 1). Astrocyte endfeet, together with endothelial cell tight junctions and the blood vessel basal lamina, compose the BBB (51). As mentioned earlier, BBB disruption is not well studied in the context of mood disorders, but existing studies suggest some level of dysregulation. Restraint stress in rats reduced expression of GFAP and endothelial tight junction proteins Claudin-5 and occludin, and increased expression of endothelial glucose transporter-1 (GLUT-1) in frontal cortex and/or hippocampus, suggesting a more permissive BBB and alterations in glucose metabolism (64). Stress also produced capillary and astrocyte abnormalities in both regions, including endothelial tight junction damage and swelling of astrocytic processes. Endothelial cells may also play an active role in recruiting peripheral cells to the BBB and transducing stress-induced inflammatory signals to microglia, as endothelial cell specific IL-1R1 knockdown in mice prevented recruitment of peripheral myeloid cells to CNS tissue and reduced mRNA expression of IL-1β, TNF-α, IL-6, and CCL2 in brain CD11b+ cells (65).
Preclinical studies further highlight the potential therapeutic opportunity of targeting central inflammatory processes, particularly those mediated by microglia. Minocycline is a second-generation tetracycline antibiotic that inhibits microglia somewhat selectively, although it also has reported effects on peripheral macrophages, T cells, and cultured neurons (66). Systemic minocycline administration prevents microglial activation and IL-1β induction in hippocampus and PFC (Figure 1) as well as depression-like behavior in the forced swim test in mice exposed to chronic restraint stress (61). Another study found that minocycline treatment of mice during social defeat reduced microglial activation and IL-1β mRNA expression in hippocampus and prevented stress-induced spatial memory deficits in the Barnes maze, suggesting that minocycline may be of particular interest in mediating the cognitive and memory symptoms associated with affective disorders (67). The authors did not observe a prevention or reduction of depression-like social avoidance behavior, however. Interestingly, Blandino et al. (59) reported that systemic injection of minocycline prevented repeated foot shock-induced elevations in hypothalamic IL-1β levels but had no effect on stress-induced elevation of splenic IL-1β, indicating that minocycline may preferentially target central immune processes. Perhaps attenuation of central, but not peripheral, inflammation is insufficient to prevent social stress-induced emotional symptoms. Other studies have used the β-adrenergic receptor antagonist propranolol as a strategy to prevent microglial activation. Wohleb et al. (57) reported that daily pretreatment with propranolol throughout social defeat exposure prevented stress-induced anxiety-like behavior in the light/dark test. Targeting the microglial NLRP3 inflammasome is also antidepressant in rodents. Inhibition of the microglial NLRP3 inflammasome through genetic Nlrp3 deletion (61; 62), systemic administration of the caspase-1 inhibitor VX-765 (68), or administration of P2X7R antagonists (62) prevented anxiety and depression-like behavior in mice exposed to chronic mild (62; 68) or chronic restraint (61) stress. NLRP3 inhibition also prevented stress induction of IL-1β in hippocampus (61; 62; 68), PFC (61), and blood serum (68). Furthermore, at baseline, Nlrp3−/− mice show reduced anxiety in the elevated plus maze and open field tests (62). Another microglial therapeutic target is CX3CR1-mediated signaling between neurons and microglia, as Cx3cr1−/− mice display enhanced behavioral resilience to social defeat (69) and repeated swim stress (70). Astrocyte-targeting therapeutic strategies have also been effective in rodents. Riluzole, an approved drug originally indicated for the treatment of ALS, exerts glutamate modulating effects through numerous mechanisms, one of which involves promoting the uptake of glutamate by astrocytes (71; 72). Chronic, systemic treatment with riluzole of rats exposed to chronic unpredictable stress prevented stress-induced behavioral despair and reversed anhedonic behavior in the sucrose preference test (72) (Figure 1). Riluzole also rescued stress-induced impairments in astrocyte metabolism, GFAP expression, and cycling of glutamate and GABA in PFC (72).
Some of these therapies have proved efficacious in human patients, and others are promising targets for future clinical investigation. An open-label study in patients with unipolar psychotic depression indicated that minocycline is a safe and effective adjunctive therapy to SSRIs for alleviation of depressive and psychotic symptoms (73). The only randomized, double-blind, placebo-controlled trial of minocycline to date was conducted in HIV patients with mild-to-moderate comorbid depression, and resulted in a significant reduction in both depression symptoms and time to symptom alleviation (74). Interestingly, a follow-up study failed to demonstrate an effect of minocycline on cognitive symptoms in this patient population (75). Therefore, although minocycline appears to prevent stress-induced cognitive dysfunction in animal models, its cognitive enhancing effects in human patients require further investigation. Additional placebo-controlled studies for evaluating the safety and efficacy of minocycline as an adjunctive therapy in treatment resistant MDD (76; 77) and bipolar depression (78) are planned or ongoing. Open-label studies and case reports have demonstrated safety and efficacy of riluzole as a stand-alone or add-on therapy for treatment-resistant depression and generalized anxiety disorder (79–82). A recent parallel, randomized, double-blind, placebo-controlled study reports that riluzole in combination with citalopram produced a greater reduction of depression symptoms and reduced time to symptom alleviation compared to citalopram alone (83). Targeting the NLRP3 inflammasome may also be an effective therapeutic strategy to mitigate depression. MDD patients exhibit increased mRNA expression of inflammasome components NLRP3 and Caspase-1 as well as increased NLRP3 protein levels in peripheral blood mononuclear cells (84). The type 2 diabetes drug glyburide inhibits the NLRP3 inflammasome, effectively preventing LPS and ATP-induced caspase-1 activation and release of IL-1β and IL-18 from cultured human and mouse bone marrow derived macrophages (85; 86). However, it should be noted that data out of the Mayo clinic suggests that depression and anxiety are side effects of glyburide) (http://www.mayoclinic.org/drugs-supplements/glyburide-oral-route/side-effects/drg-20072094. Thus, further research is clearly needed to understand whether NLRP3 targeting therapies are effective in treating depression. Another potential therapeutic opportunity lies in modulating BBB function to protect the CNS from excess stress-induced peripheral inflammatory signals. BBB-modulating interventions are untested in human affective disorders, but as the BBB is an emerging focus of neuropsychiatric research, we anticipate further preclinical and clinical insights on this important interface of the peripheral immune and central nervous systems.
Harnessing the antidepressant and immunomodulatory potential of gut microbiota
Recent evidence suggests a role for another peripheral system—the gut microbiome—in immunomodulation and depression. Healthy adults possess a diverse and large gut microbiotic environment that varies considerably from person to person and is dependent upon the interplay of genetics and environmental influences such as diet and medication status (i.e., antibiotics, probiotics) (87). Depressed patients exhibit dysbiosis, or altered fecal counts of several types of gut bacteria, including those belonging to the predominant phyla colonizing the adult human gut—Bacteriodetes and Firmicutes (87–90). Recent evidence suggests that this dysbiosis results in a reduction in both the diversity and richness of gut bacterial populations (90; 91) (Figure 1). Further supporting a role for gut processes in depression, irritable bowel syndrome (IBS) is more prevalent in patients with depression than in otherwise healthy individuals (88). Studies in healthy volunteers indicate that acute stress can promote a “leaky gut,” although changes in intestinal permeability have not been evaluated in MDD patients (92). Subjects exposed to acute stress in the form of public speaking exhibited increased small intestinal permeability, an effect recapitulated in unstressed subjects through exogenous corticotropin-releasing hormone (CRH) administration (93). Women may be especially vulnerable to stress effects on gut permeability. Adult women, but not men, exposed to acute cold pain stress, which activates the HPA axis, exhibited increased small intestinal permeability during and immediately after the stressor (94) (Figure 1).
The gastrointestinal tract (GIT) and the brain communicate in a bidirectional manner through the brain-gut-enteric axis, an intricate network made up of the CNS, gut microbiota, neuroendocrine system, peripheral and central immune systems, sympathetic and parasympathetic divisions of the autonomic nervous system, and the enteric nervous system (95). Stress can initiate CNS signaling in reward and mood-related nuclei that communicate with the GIT through neuroendocrine (HPA Axis) and autonomic processes, leading to modulation of intestinal motility and permeability (96). Gut microbiota, in turn, can utilize several mechanisms to influence CNS processes and subsequent behavioral response to stress including local production of neurotransmitters such as serotonin and oxytocin, and activation of neural (vagus nerve stimulation) and humoral (cytokine-mediated) immune signaling pathways (87; 95) (Figure 1). The gut microbiome is tightly linked to inflammatory processes, and gut microbiota mediate both the development and function of central and peripheral immune cells. Centrally, rodent studies have revealed the necessity of a diverse and complete gut bacterial colonization for normal microglial maturation, morphology, and response to immune challenge (89). Peripherally, gut microbiota are essential for the normal maturation of both the innate and adaptive immune systems. Reduced exposure to and colonization by diverse microbes in industrialized western societies is thought to underlie the increased prevalence of autoimmune and allergic disorders such as IBS and asthma within the past three decades (97).
Preclinical studies have employed probiotic administration, fecal microbiota transplantation, and germ free (GF) mice to both investigate the functional importance of gut bacteria in behavior and to evaluate the therapeutic utility of modulating the gut microbiome. GF mice are reared in sterile environments and therefore completely lack bacterial colonization (89; 90). Compared to conventionally raised, specific pathogen-free (SPF) mice, GF mice at baseline show increased motor activity (98) and reduced anxiety and behavioral despair (90; 98). Bacterial colonization promotes a partial shift toward SPF-like baseline anxiety behavior, but only when performed in early life (98). GF mice also exhibit numerous neurochemical and synaptic abnormalities, including increased turnover of dopamine, serotonin, and noradrenaline in striatum (98), increased concentration of serotonin in hippocampus (99), altered BDNF and NGF-1A mRNA expression in various mood-related brain regions (98–100), and elevated striatal synaptophysin and PSD-95 protein expression in striatum (98). Various studies indicate that GF mice are more susceptible to stress than SPF mice. They exhibit an exaggerated HPA response to stress (87), displaying elevated circulating corticosterone and/or ACTH levels following stressors including novel environment exposure (99) and acute restraint stress (100). In addition, chronic social defeat stress in mice disrupts microbiota diversity leading to a heightened inflammatory state (101) (Figure 1). Thus, in line with deficits in microbiota diversity displayed by MDD patients, findings of rodent studies suggest that a healthy microbiome is essential for optimal neuroendocrine, neurochemical, and behavioral response to stress. Further supporting the functional role of microbiota in the expression of depression-like behavior, fecal microbiotal transplant (FMT) experiments in rodents indicate that transplanting an MDD-like microbiotic environment can functionally drive behavior. In the absence of stress, antibiotic-treated adult rats (91) and adult GF mice (90) that received FMT from human MDD donors displayed increased anxiety and depression-like behavior compared to rodents transplanted with microbiota from healthy control donors. Importantly, both of these studies demonstrated a recapitulation and maintenance of the donor microbiotic environment in host mice following FMT. Numerous preclinical studies highlight probiotics as an area of untapped therapeutic opportunity (89). For instance, chronic Bifidobacterim (B.) infantis administration has been shown to reverse maternal separation-induced immobility in the forced swim test in rats (102) (Figure 1). Furthermore, chronic treatment with B. longum, Lactobaciullus (L.) rhamnosus, or B. breve exerted anxiolytic effects (as well as antidepressant effects for B. longum and L. rhamnosus) in the BALB/c anxious mouse strain (103; 104) (Figure 1). L. rhamnosus administration also attenuated acute stress-induced corticosterone release in BALB/c mice (104). When given chronically to C57BL/6 mice prior to and during chronic social defeat exposure, L. rhamnosus partially prevented defeat-induced impairments in social interaction (although only to a conspecific C57BL/6 mouse, not to a novel aggressor) and anxiety-like behavior (105). Chronic administration of L. helveticus and B. longum to rats undergoing experimental myocardial infarction (MI), which produces peripheral monocytosis and symptoms of depression in 65% of patients, prevented MI-induced deficits in social interaction, forced swim, and passive avoidance step down tests (106) (Figure 1). The probiotic treatment also prevented the MI-induced increase in intestinal permeability.
Probiotics may exert their anxiolytic and antidepressant effects through immune mechanisms. Cultured peripheral blood cells derived from naïve rats given two weeks of B. infantis supplementation released less IL-6, IFN-γ, and TNF-α in response to incubation with LPS (TNF-α) or Concanavalin A (IL-6, IFN-γ), a plant mitogen used to stimulate T lymphocytes, suggesting an attenuation of peripheral inflammatory response to immune challenge (107). The anxiolytic and antidepressant effects of L. rhamnosus treatment are dependent upon the vagus nerve, an important mediator of neuroimmune communication between the gut and CNS, as vagotomy prevented these effects (104). Furthermore, findings in the mouse experimental autoimmune encephalitis (EAE) model of multiple sclerosis suggest that Lactobacillus probiotics in part mediate anti-inflammatory effects by stimulating release of the anti-inflammatory cytokine IL-10 into the peripheral circulation, leading to an induction of immunosuppressive Tregs in the spleen and mesenteric lymph nodes (108). In the CSDS mouse model, L. rhamnosus induced a baseline increase in splenic Tregs, but CSDS itself produced an increase in these cells that did not differ between L. rhamnosus and vehicle-treated mice (105). In addition, immunization of mice prior to social stress with Mycobacterium vaccae, an immunoregulatory environmental microorganism, reduced inflammatory signaling and prevented development of depression-like behaviors (101). Therefore, the immunomodulatory properties of probiotics represent an exciting therapeutic avenue requiring further investigation.
Several clinical studies report psychotropic effects of probiotics, however only one to date has investigated the antidepressant properties of probiotics in MDD patients. Akkasheh et al. (109), in a randomized, double-blind, placebo-controlled trial, administered MDD patients either a placebo or capsules containing a combination of three probiotic strains (L. acidophilus, L. casei, and B. bifidum) daily for a period of 8 weeks. At the end of the treatment period, probiotic-treated patients exhibited a decrease in self-reported symptoms of depression as quantified by the Beck Depression Inventory compared to the placebo-treated group. In blood measures, patients demonstrated increased antioxidant levels and decreased glucocorticoid resistance. Interestingly, the probiotic treatment also seemed to exert an anti-inflammatory effect as probiotic-treated patients exhibited decreased serum levels of CRP, an indicator of peripheral inflammatory status. Although not in MDD patients, other clinical studies report positive effects of probiotics on mood. A trial investigating the effects of 6 weeks of probiotic ingestion on mood in healthy Iranian petrochemical workers, a population in which an increased prevalence of mood disorders has been reported, found an improvement of anxiety and depression symptom scores in both a probiotic yogurt (L. acidophilus and B. lactis) treatment group and a probiotic capsule (Actobacillus casei, L. acidophilus, L. rhamnosus, L. bulgaricus, B. breve, B. longum, Streptococcus thermophilus) treatment group compared to the placebo group (110). Another trial found that in patients with chronic fatigue syndrome, a condition that is comorbid with clinical depression or anxiety in ~50% of all patients, 8 weeks of L. casei (Shirota strain) probiotic treatment increased fecal levels of Lactobacillus and Bifidobacteria (both of which are decreased in gut of MDD patients (88)) and decreased anxiety symptoms (111). Messaoudi et al. (112) report that healthy volunteers administered a capsule containing L. helveticus and B. longum for 30 days experienced an improvement of measures of depression and anxiety across several questionnaires and decreased urinary cortisol levels compared to placebo-treated volunteers. Another study in healthy volunteers found that subjects receiving 4 weeks of daily probiotic supplementation (B. bifidum, B. lactis, L. acidophilus, L. brevis, L. casei, L. salivarius, L. lactis) reported decreased cognitive reactivity to sad mood (a risk factor for depression) specific to rumination and aggressive thoughts (113). An alternative to probiotic treatment is the more aggressive procedure of FMT. FMT has been effectively employed to treat IBS and severe bacterial infections (114). While the therapeutic utility of FMT has not been evaluated in affective disorders, FMT may be merited for cases of intractable depression. Interestingly, a recent open-label trial found that FMT improved both gastrointestinal and behavioral symptoms in autistic children for at least 8 weeks post-FMT (115). As the US clinical trials registry (clinicaltrials.gov) indicates that clinical studies involving prebiotic, probiotic, or oral FMT supplementation are ongoing for neurological and psychiatric conditions including Parkinson’s disease, severe depression, autism, and anxiety, we anticipate further elucidation of the psychotropic effects of various methods of gut microbiota modulation in human patients.
The study of gut microbiota in affective disorders is a relatively young field, and several outstanding questions will need to be addressed to fully realize the therapeutic potential of the gut microbiome in patients. First, is intestinal permeability altered in patients with mood disorders, and does this affect peripheral and central inflammation? Furthermore, do existing antidepressant therapies alter intestinal permeability and gut microbiota composition? Many preclinical studies utilize unstressed animals or rodents exposed to acute stress, and therefore, more research is necessary in chronic stress models that more closely recapitulate human affective disorders. Another question involves the relationship of diet to gut microbiota composition. Depression often involves changes in eating habits and therefore nutritional intake, which can impact gut microbiota diversity (87). Therefore, are changes in microbiota composition a cause or consequence of depression? Should nutritional consultation be a component of standard treatment for affective disorders? Another very interesting question involves the interaction of gut microbiota, intestinal permeability, and bacterial infectious diseases in susceptibility to affective disorders. Epidemiological evidence indicates that individuals who have undergone at least one hospitalization for infection have a 62% increased risk of developing a mood disorder, and this risk rises with the number of infections (116). Interestingly, the increased risk of mood disorders was more pronounced in patients who had an infectious hospitalization than in patients with autoimmune disorders. Much more research is required to understand how infection affects gut and immune processes relevant to depression. Another research area requiring further clarification involves the antidepressant properties of the antibiotic minocycline. As described earlier, minocycline is thought to exert its antidepressant effects by repressing microglia-mediated inflammatory processes, but as it targets gram-negative and gram-positive bacteria, minocycline likely alters the gut microbiome in depressed patients and animal models (87). The nature and therapeutic consequences of this microbiotic remodeling require further elucidation.
Conclusion
Stress and depression have numerous inflammatory consequences in both human patients and animal models. Immune symptoms include enhanced levels of pro-inflammatory cytokines, monocytes, and neutrophils in the peripheral circulation, alterations in morphology and activation of microglia, astrocytes, and endothelial cells in the central nervous system, and dysregulation of commensal bacterial populations in the gut. Experimental therapeutics are successfully targeting peripheral cytokines and central cell populations, and promoting healthy gut microenvironments. While outstanding questions remain, we anticipate continued advancement in both understanding of inflammatory mediators of mood disorders and translation of basic findings to clinical solutions.
Acknowledgments
This research was supported by grants from the National Institutes of Health R01 MH090264 (Role of thalamic vs cortical inputs to nucleus accumbens in stress-related disorders), R01 MH104559 (Peripheral IL-6 from leukocytes controls susceptibility to social defeat stress) and P50 AT008661-01 (Dietary botanicals in the preservation of cognitive and psychological resilience) to SJR and a Brain & Behavior Research Foundation Young Investigator Grant (sponsored by the P&S Fund) to CM.
References
- 1.Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Archives of general psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- 2.Krishnan V, Nestler EJ. The molecular neurobiology of depression. Nature. 2008;455:894–902. doi: 10.1038/nature07455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hodes GE, Kana V, Menard C, Merad M, Russo SJ. Neuroimmune mechanisms of depression. Nat Neurosci. 2015;18:1386–93. doi: 10.1038/nn.4113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lopez AD, Murray CC. The global burden of disease, 1990–2020. Nat Med. 1998;4:1241–3. doi: 10.1038/3218. [DOI] [PubMed] [Google Scholar]
- 5.Menard C, Pfau ML, Hodes GE, Russo SJ. Immune and Neuroendocrine Mechanisms of Stress Vulnerability and Resilience. Neuropsychopharmacology. 2017;42:62–80. doi: 10.1038/npp.2016.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Renault PF, Hoofnagle JH, Park Y, Mullen KD, Peters M, et al. Psychiatric complications of long-term interferon alfa therapy. Arch Intern Med. 1987;147:1577–80. [PubMed] [Google Scholar]
- 7.Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, et al. A meta-analysis of cytokines in major depression. Biological psychiatry. 2010;67:446–57. doi: 10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- 8.Maes M, Van der Planken M, Stevens WJ, Peeters D, DeClerck LS, et al. Leukocytosis, monocytosis and neutrophilia: hallmarks of severe depression. J Psychiatr Res. 1992;26:125–34. doi: 10.1016/0022-3956(92)90004-8. [DOI] [PubMed] [Google Scholar]
- 9.Miller AH, Raison CL. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat Rev Immunol. 2016;16:22–34. doi: 10.1038/nri.2015.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barnes J, Mondelli V, Pariante CM. Genetic Contributions of Inflammation to Depression. Neuropsychopharmacology. 2017;42:81–98. doi: 10.1038/npp.2016.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kulmatycki KM, Jamali F. Therapeutic relevance of altered cytokine expression. Cytokine. 2001;14:1–10. doi: 10.1006/cyto.2000.0827. [DOI] [PubMed] [Google Scholar]
- 12.Vogelzangs N, Duivis HE, Beekman AT, Kluft C, Neuteboom J, et al. Association of depressive disorders, depression characteristics and antidepressant medication with inflammation. Transl Psychiatry. 2012;2:e79. doi: 10.1038/tp.2012.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Russo SJ, Nestler EJ. The brain reward circuitry in mood disorders. Nature reviews Neuroscience. 2013;14:609–25. doi: 10.1038/nrn3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Felger JC, Li Z, Haroon E, Woolwine BJ, Jung MY, et al. Inflammation is associated with decreased functional connectivity within corticostriatal reward circuitry in depression. Mol Psychiatry. 2016;21:1358–65. doi: 10.1038/mp.2015.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosenblat JD, Cha DS, Mansur RB, McIntyre RS. Inflamed moods: a review of the interactions between inflammation and mood disorders. Prog Neuropsychopharmacol Biol Psychiatry. 2014;53:23–34. doi: 10.1016/j.pnpbp.2014.01.013. [DOI] [PubMed] [Google Scholar]
- 16.Shi C, Pamer EG. Monocyte recruitment during infection and inflammation. Nat Rev Immunol. 2011;11:762–74. doi: 10.1038/nri3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mueller SN, Mackay LK. Tissue-resident memory T cells: local specialists in immune defence. Nat Rev Immunol. 2016;16:79–89. doi: 10.1038/nri.2015.3. [DOI] [PubMed] [Google Scholar]
- 18.Lewitus GM, Schwartz M. Behavioral immunization: immunity to self-antigens contributes to psychological stress resilience. Mol Psychiatry. 2009;14:532–6. doi: 10.1038/mp.2008.103. [DOI] [PubMed] [Google Scholar]
- 19.Miller AH. Depression and immunity: a role for T cells? Brain Behav Immun. 2010;24:1–8. doi: 10.1016/j.bbi.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lewitus GM, Wilf-Yarkoni A, Ziv Y, Shabat-Simon M, Gersner R, et al. Vaccination as a novel approach for treating depressive behavior. Biological psychiatry. 2009;65:283–8. doi: 10.1016/j.biopsych.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 21.Cohen H, Ziv Y, Cardon M, Kaplan Z, Matar MA, et al. Maladaptation to mental stress mitigated by the adaptive immune system via depletion of naturally occurring regulatory CD4+CD25+ cells. J Neurobiol. 2006;66:552–63. doi: 10.1002/neu.20249. [DOI] [PubMed] [Google Scholar]
- 22.Brachman RA, Lehmann ML, Maric D, Herkenham M. Lymphocytes from chronically stressed mice confer antidepressant-like effects to naive mice. J Neurosci. 2015;35:1530–8. doi: 10.1523/JNEUROSCI.2278-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Toben C, Baune BT. An Act of Balance Between Adaptive and Maladaptive Immunity in Depression: a Role for T Lymphocytes. J Neuroimmune Pharmacol. 2015;10:595–609. doi: 10.1007/s11481-015-9620-2. [DOI] [PubMed] [Google Scholar]
- 24.Frick LR, Rapanelli M, Cremaschi GA, Genaro AM. Fluoxetine directly counteracts the adverse effects of chronic stress on T cell immunity by compensatory and specific mechanisms. Brain Behav Immun. 2009;23:36–40. doi: 10.1016/j.bbi.2008.06.010. [DOI] [PubMed] [Google Scholar]
- 25.Curzytek K, Kubera M, Majewska-Szczepanik M, Szczepanik M, Ptak W, et al. Inhibitory effect of antidepressant drugs on contact hypersensitivity reaction is connected with their suppressive effect on NKT and CD8(+) T cells but not on TCR delta T cells. Int Immunopharmacol. 2015;28:1091–6. doi: 10.1016/j.intimp.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 26.Himmerich H, Milenovic S, Fulda S, Plumakers B, Sheldrick AJ, et al. Regulatory T cells increased while IL-1beta decreased during antidepressant therapy. J Psychiatr Res. 2010;44:1052–7. doi: 10.1016/j.jpsychires.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 27.Eyre HA, Lavretsky H, Kartika J, Qassim A, Baune BT. Modulatory Effects of Antidepressant Classes on the Innate and Adaptive Immune System in Depression. Pharmacopsychiatry. 2016;49:85–96. doi: 10.1055/s-0042-103159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wohleb ES, McKim DB, Sheridan JF, Godbout JP. Monocyte trafficking to the brain with stress and inflammation: a novel axis of immune-to-brain communication that influences mood and behavior. Front Neurosci. 2014;8:447. doi: 10.3389/fnins.2014.00447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Powell ND, Sloan EK, Bailey MT, Arevalo JM, Miller GE, et al. Social stress up-regulates inflammatory gene expression in the leukocyte transcriptome via beta-adrenergic induction of myelopoiesis. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:16574–9. doi: 10.1073/pnas.1310655110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heidt T, Sager HB, Courties G, Dutta P, Iwamoto Y, et al. Chronic variable stress activates hematopoietic stem cells. Nat Med. 2014 doi: 10.1038/nm.3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Avitsur R, Kavelaars A, Heijnen C, Sheridan JF. Social stress and the regulation of tumor necrosis factor-alpha secretion. Brain Behav Immun. 2005;19:311–7. doi: 10.1016/j.bbi.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 32.Avitsur R, Stark JL, Sheridan JF. Social stress induces glucocorticoid resistance in subordinate animals. Hormones and behavior. 2001;39:247–57. doi: 10.1006/hbeh.2001.1653. [DOI] [PubMed] [Google Scholar]
- 33.Stark JL, Avitsur R, Padgett DA, Campbell KA, Beck FM, Sheridan JF. Social stress induces glucocorticoid resistance in macrophages. American journal of physiology. Regulatory, integrative and comparative physiology. 2001;280:R1799–805. doi: 10.1152/ajpregu.2001.280.6.R1799. [DOI] [PubMed] [Google Scholar]
- 34.Ramirez K, Sheridan JF. Antidepressant imipramine diminishes stress-induced inflammation in the periphery and central nervous system and related anxiety- and depressive- like behaviors. Brain Behav Immun. 2016;57:293–303. doi: 10.1016/j.bbi.2016.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hodes GE, Pfau ML, Leboeuf M, Golden SA, Christoffel DJ, et al. Individual differences in the peripheral immune system promote resilience versus susceptibility to social stress. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:16136–41. doi: 10.1073/pnas.1415191111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Modabbernia A, Taslimi S, Brietzke E, Ashrafi M. Cytokine alterations in bipolar disorder: a meta-analysis of 30 studies. Biological psychiatry. 2013;74:15–25. doi: 10.1016/j.biopsych.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 37.Kappelmann N, Lewis G, Dantzer R, Jones PB, Khandaker GM. Antidepressant activity of anti-cytokine treatment: a systematic review and meta-analysis of clinical trials of chronic inflammatory conditions. Mol Psychiatry. 2016 doi: 10.1038/mp.2016.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brietzke E, Scheinberg M, Lafer B. Therapeutic potential of interleukin-6 antagonism in bipolar disorder. Med Hypotheses. 2011;76:21–3. doi: 10.1016/j.mehy.2010.08.021. [DOI] [PubMed] [Google Scholar]
- 39.Raison CL, Rutherford RE, Woolwine BJ, Shuo C, Schettler P, et al. A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment-resistant depression: the role of baseline inflammatory biomarkers. JAMA Psychiatry. 2013;70:31–41. doi: 10.1001/2013.jamapsychiatry.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kohler O, Benros ME, Nordentoft M, Farkouh ME, Iyengar RL, et al. Effect of anti-inflammatory treatment on depression, depressive symptoms, and adverse effects: a systematic review and meta-analysis of randomized clinical trials. JAMA Psychiatry. 2014;71:1381–91. doi: 10.1001/jamapsychiatry.2014.1611. [DOI] [PubMed] [Google Scholar]
- 41.Warner-Schmidt JL, Vanover KE, Chen EY, Marshall JJ, Greengard P. Antidepressant effects of selective serotonin reuptake inhibitors (SSRIs) are attenuated by antiinflammatory drugs in mice and humans. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:9262–7. doi: 10.1073/pnas.1104836108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Setiawan E, Wilson AA, Mizrahi R, Rusjan PM, Miler L, et al. Role of translocator protein density, a marker of neuroinflammation, in the brain during major depressive episodes. JAMA Psychiatry. 2015;72:268–75. doi: 10.1001/jamapsychiatry.2014.2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Steiner J, Walter M, Gos T, Guillemin GJ, Bernstein HG, et al. Severe depression is associated with increased microglial quinolinic acid in subregions of the anterior cingulate gyrus: evidence for an immune-modulated glutamatergic neurotransmission? J Neuroinflammation. 2011;8:94. doi: 10.1186/1742-2094-8-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cotter D, Mackay D, Landau S, Kerwin R, Everall I. Reduced glial cell density and neuronal size in the anterior cingulate cortex in major depressive disorder. Archives of general psychiatry. 2001;58:545–53. doi: 10.1001/archpsyc.58.6.545. [DOI] [PubMed] [Google Scholar]
- 45.Rajkowska G, Miguel-Hidalgo JJ, Wei J, Dilley G, Pittman SD, et al. Morphometric evidence for neuronal and glial prefrontal cell pathology in major depression. Biological psychiatry. 1999;45:1085–98. doi: 10.1016/s0006-3223(99)00041-4. [DOI] [PubMed] [Google Scholar]
- 46.Ongur D, Drevets WC, Price JL. Glial reduction in the subgenual prefrontal cortex in mood disorders. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:13290–5. doi: 10.1073/pnas.95.22.13290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bowley MP, Drevets WC, Ongur D, Price JL. Low glial numbers in the amygdala in major depressive disorder. Biological psychiatry. 2002;52:404–12. doi: 10.1016/s0006-3223(02)01404-x. [DOI] [PubMed] [Google Scholar]
- 48.Si X, Miguel-Hidalgo JJ, O’Dwyer G, Stockmeier CA, Rajkowska G. Age-dependent reductions in the level of glial fibrillary acidic protein in the prefrontal cortex in major depression. Neuropsychopharmacology. 2004;29:2088–96. doi: 10.1038/sj.npp.1300525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rajkowska G, Stockmeier CA. Astrocyte pathology in major depressive disorder: insights from human postmortem brain tissue. Curr Drug Targets. 2013;14:1225–36. doi: 10.2174/13894501113149990156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Torres-Platas SG, Hercher C, Davoli MA, Maussion G, Labonte B, et al. Astrocytic hypertrophy in anterior cingulate white matter of depressed suicides. Neuropsychopharmacology. 2011;36:2650–8. doi: 10.1038/npp.2011.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rajkowska G, Hughes J, Stockmeier CA, Javier Miguel-Hidalgo J, Maciag D. Coverage of blood vessels by astrocytic endfeet is reduced in major depressive disorder. Biological psychiatry. 2013;73:613–21. doi: 10.1016/j.biopsych.2012.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Najjar S, Pearlman DM, Devinsky O, Najjar A, Zagzag D. Neurovascular unit dysfunction with blood-brain barrier hyperpermeability contributes to major depressive disorder: a review of clinical and experimental evidence. J Neuroinflammation. 2013;10:142. doi: 10.1186/1742-2094-10-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Valkanova V, Ebmeier KP. Vascular risk factors and depression in later life: a systematic review and meta-analysis. Biological psychiatry. 2013;73:406–13. doi: 10.1016/j.biopsych.2012.10.028. [DOI] [PubMed] [Google Scholar]
- 54.Politi P, Brondino N, Emanuele E. Increased proapoptotic serum activity in patients with chronic mood disorders. Arch Med Res. 2008;39:242–5. doi: 10.1016/j.arcmed.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 55.Menard C, Pfau ML, Hodes GE, Russo SJ. Immune and Neuroendocrine Mechanisms of Stress Vulnerability and Resilience. Neuropsychopharmacology. 2016 doi: 10.1038/npp.2016.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wohleb ES. Neuron-Microglia Interactions in Mental Health Disorders: “For Better, and For Worse”. Front Immunol. 2016;7:544. doi: 10.3389/fimmu.2016.00544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wohleb ES, Hanke ML, Corona AW, Powell ND, Stiner LM, et al. beta-Adrenergic receptor antagonism prevents anxiety-like behavior and microglial reactivity induced by repeated social defeat. J Neurosci. 2011;31:6277–88. doi: 10.1523/JNEUROSCI.0450-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Johnson JD, Campisi J, Sharkey CM, Kennedy SL, Nickerson M, et al. Catecholamines mediate stress-induced increases in peripheral and central inflammatory cytokines. Neuroscience. 2005;135:1295–307. doi: 10.1016/j.neuroscience.2005.06.090. [DOI] [PubMed] [Google Scholar]
- 59.Blandino P, Jr, Barnum CJ, Deak T. The involvement of norepinephrine and microglia in hypothalamic and splenic IL-1beta responses to stress. J Neuroimmunol. 2006;173:87–95. doi: 10.1016/j.jneuroim.2005.11.021. [DOI] [PubMed] [Google Scholar]
- 60.Frank MG, Thompson BM, Watkins LR, Maier SF. Glucocorticoids mediate stress-induced priming of microglial pro-inflammatory responses. Brain Behav Immun. 2012;26:337–45. doi: 10.1016/j.bbi.2011.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Alcocer-Gomez E, Ulecia-Moron C, Marin-Aguilar F, Rybkina T, Casas-Barquero N, et al. Stress-Induced Depressive Behaviors Require a Functional NLRP3 Inflammasome. Mol Neurobiol. 2016;53:4874–82. doi: 10.1007/s12035-015-9408-7. [DOI] [PubMed] [Google Scholar]
- 62.Iwata M, Ota KT, Li XY, Sakaue F, Li N, et al. Psychological Stress Activates the Inflammasome via Release of Adenosine Triphosphate and Stimulation of the Purinergic Type 2X7 Receptor. Biological psychiatry. 2016;80:12–22. doi: 10.1016/j.biopsych.2015.11.026. [DOI] [PubMed] [Google Scholar]
- 63.Meeuwsen S, Persoon-Deen C, Bsibsi M, Ravid R, van Noort JM. Cytokine, chemokine and growth factor gene profiling of cultured human astrocytes after exposure to proinflammatory stimuli. Glia. 2003;43:243–53. doi: 10.1002/glia.10259. [DOI] [PubMed] [Google Scholar]
- 64.Santha P, Veszelka S, Hoyk Z, Meszaros M, Walter FR, et al. Restraint Stress-Induced Morphological Changes at the Blood-Brain Barrier in Adult Rats. Front Mol Neurosci. 2015;8:88. doi: 10.3389/fnmol.2015.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wohleb ES, Patterson JM, Sharma V, Quan N, Godbout JP, Sheridan JF. Knockdown of interleukin-1 receptor type-1 on endothelial cells attenuated stress-induced neuroinflammation and prevented anxiety-like behavior. J Neurosci. 2014;34:2583–91. doi: 10.1523/JNEUROSCI.3723-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hellwig S, Heinrich A, Biber K. The brain’s best friend: microglial neurotoxicity revisited. Front Cell Neurosci. 2013;7:71. doi: 10.3389/fncel.2013.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.McKim DB, Niraula A, Tarr AJ, Wohleb ES, Sheridan JF, Godbout JP. Neuroinflammatory Dynamics Underlie Memory Impairments after Repeated Social Defeat. J Neurosci. 2016;36:2590–604. doi: 10.1523/JNEUROSCI.2394-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang Y, Liu L, Liu YZ, Shen XL, Wu TY, et al. NLRP3 Inflammasome Mediates Chronic Mild Stress-Induced Depression in Mice via Neuroinflammation. Int J Neuropsychopharmacol. 2015:18. doi: 10.1093/ijnp/pyv006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wohleb ES, Powell ND, Godbout JP, Sheridan JF. Stress-induced recruitment of bone marrow-derived monocytes to the brain promotes anxiety-like behavior. J Neurosci. 2013;33:13820–33. doi: 10.1523/JNEUROSCI.1671-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hellwig S, Brioschi S, Dieni S, Frings L, Masuch A, et al. Altered microglia morphology and higher resilience to stress-induced depression-like behavior in CX3CR1-deficient mice. Brain Behav Immun. 2016;55:126–37. doi: 10.1016/j.bbi.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 71.Ernst C, Nagy C, Kim S, Yang JP, Deng X, et al. Dysfunction of astrocyte connexins 30 and 43 in dorsal lateral prefrontal cortex of suicide completers. Biological psychiatry. 2011;70:312–9. doi: 10.1016/j.biopsych.2011.03.038. [DOI] [PubMed] [Google Scholar]
- 72.Banasr M, Chowdhury GM, Terwilliger R, Newton SS, Duman RS, et al. Glial pathology in an animal model of depression: reversal of stress-induced cellular, metabolic and behavioral deficits by the glutamate-modulating drug riluzole. Mol Psychiatry. 2010;15:501–11. doi: 10.1038/mp.2008.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Miyaoka T, Wake R, Furuya M, Liaury K, Ieda M, et al. Minocycline as adjunctive therapy for patients with unipolar psychotic depression: an open-label study. Prog Neuropsychopharmacol Biol Psychiatry. 2012;37:222–6. doi: 10.1016/j.pnpbp.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 74.Emadi-Kouchak H, Mohammadinejad P, Asadollahi-Amin A, Rasoulinejad M, Zeinoddini A, et al. Therapeutic effects of minocycline on mild-to-moderate depression in HIV patients: a double-blind, placebo-controlled, randomized trial. Int Clin Psychopharmacol. 2016;31:20–6. doi: 10.1097/YIC.0000000000000098. [DOI] [PubMed] [Google Scholar]
- 75.Nakasujja N, Miyahara S, Evans S, Lee A, Musisi S, et al. Randomized trial of minocycline in the treatment of HIV-associated cognitive impairment. Neurology. 2013;80:196–202. doi: 10.1212/WNL.0b013e31827b9121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Husain MI, Chaudhry IB, Rahman RR, Hamirani MM, Qurashi I, et al. Minocycline as an adjunct for treatment-resistant depressive symptoms: study protocol for a pilot randomised controlled trial. Trials. 2015;16:410. doi: 10.1186/s13063-015-0933-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dean OM, Maes M, Ashton M, Berk L, Kanchanatawan B, et al. Protocol and rationale-the efficacy of minocycline as an adjunctive treatment for major depressive disorder: a double blind, randomised, placebo controlled trial. Clin Psychopharmacol Neurosci. 2014;12:180–8. doi: 10.9758/cpn.2014.12.3.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Husain MI, Chaudhry IB, Hamirani MM, Minhas FA, Kazmi A, et al. Minocycline and celecoxib as adjunctive treatments for bipolar depression: a study protocol for a multicenter factorial design randomized controlled trial. Neuropsychiatr Dis Treat. 2017;13:1–8. doi: 10.2147/NDT.S115002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zarate CA, Jr, Payne JL, Quiroz J, Sporn J, Denicoff KK, et al. An open-label trial of riluzole in patients with treatment-resistant major depression. Am J Psychiatry. 2004;161:171–4. doi: 10.1176/appi.ajp.161.1.171. [DOI] [PubMed] [Google Scholar]
- 80.Sanacora G, Kendell SF, Fenton L, Coric V, Krystal JH. Riluzole augmentation for treatment-resistant depression. Am J Psychiatry. 2004;161:2132. doi: 10.1176/appi.ajp.161.11.2132. [DOI] [PubMed] [Google Scholar]
- 81.Sanacora G, Kendell SF, Levin Y, Simen AA, Fenton LR, et al. Preliminary evidence of riluzole efficacy in antidepressant-treated patients with residual depressive symptoms. Biological psychiatry. 2007;61:822–5. doi: 10.1016/j.biopsych.2006.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mathew SJ, Amiel JM, Coplan JD, Fitterling HA, Sackeim HA, Gorman JM. Open-label trial of riluzole in generalized anxiety disorder. Am J Psychiatry. 2005;162:2379–81. doi: 10.1176/appi.ajp.162.12.2379. [DOI] [PubMed] [Google Scholar]
- 83.Salardini E, Zeinoddini A, Mohammadinejad P, Khodaie-Ardakani MR, Zahraei N, et al. Riluzole combination therapy for moderate-to-severe major depressive disorder: A randomized, double-blind, placebo-controlled trial. J Psychiatr Res. 2016;75:24–30. doi: 10.1016/j.jpsychires.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 84.Alcocer-Gomez E, de Miguel M, Casas-Barquero N, Nunez-Vasco J, Sanchez-Alcazar JA, et al. NLRP3 inflammasome is activated in mononuclear blood cells from patients with major depressive disorder. Brain Behav Immun. 2014;36:111–7. doi: 10.1016/j.bbi.2013.10.017. [DOI] [PubMed] [Google Scholar]
- 85.Iwata M, Ota KT, Duman RS. The inflammasome: pathways linking psychological stress, depression, and systemic illnesses. Brain Behav Immun. 2013;31:105–14. doi: 10.1016/j.bbi.2012.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lamkanfi M, Mueller JL, Vitari AC, Misaghi S, Fedorova A, et al. Glyburide inhibits the Cryopyrin/Nalp3 inflammasome. J Cell Biol. 2009;187:61–70. doi: 10.1083/jcb.200903124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dinan TG, Cryan JF. Microbes, Immunity, and Behavior: Psychoneuroimmunology Meets the Microbiome. Neuropsychopharmacology. 2017;42:178–92. doi: 10.1038/npp.2016.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Aizawa E, Tsuji H, Asahara T, Takahashi T, Teraishi T, et al. Possible association of Bifidobacterium and Lactobacillus in the gut microbiota of patients with major depressive disorder. J Affect Disord. 2016;202:254–7. doi: 10.1016/j.jad.2016.05.038. [DOI] [PubMed] [Google Scholar]
- 89.Fung TC, Olson CA, Hsiao EY. Interactions between the microbiota, immune and nervous systems in health and disease. Nat Neurosci. 2017;20:145–55. doi: 10.1038/nn.4476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zheng P, Zeng B, Zhou C, Liu M, Fang Z, et al. Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host’s metabolism. Mol Psychiatry. 2016;21:786–96. doi: 10.1038/mp.2016.44. [DOI] [PubMed] [Google Scholar]
- 91.Kelly JR, Borre Y, COB, Patterson E, El Aidy S, et al. Transferring the blues: Depression-associated gut microbiota induces neurobehavioural changes in the rat. J Psychiatr Res. 2016;82:109–18. doi: 10.1016/j.jpsychires.2016.07.019. [DOI] [PubMed] [Google Scholar]
- 92.Kelly JR, Kennedy PJ, Cryan JF, Dinan TG, Clarke G, Hyland NP. Breaking down the barriers: the gut microbiome, intestinal permeability and stress-related psychiatric disorders. Front Cell Neurosci. 2015;9:392. doi: 10.3389/fncel.2015.00392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Vanuytsel T, van Wanrooy S, Vanheel H, Vanormelingen C, Verschueren S, et al. Psychological stress and corticotropin-releasing hormone increase intestinal permeability in humans by a mast cell-dependent mechanism. Gut. 2014;63:1293–9. doi: 10.1136/gutjnl-2013-305690. [DOI] [PubMed] [Google Scholar]
- 94.Alonso C, Guilarte M, Vicario M, Ramos L, Rezzi S, et al. Acute experimental stress evokes a differential gender-determined increase in human intestinal macromolecular permeability. Neurogastroenterol Motil. 2012;24:740–6. e348–9. doi: 10.1111/j.1365-2982.2012.01928.x. [DOI] [PubMed] [Google Scholar]
- 95.Grenham S, Clarke G, Cryan JF, Dinan TG. Brain-gut-microbe communication in health and disease. Front Physiol. 2011;2:94. doi: 10.3389/fphys.2011.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.O’Mahony SM, Hyland NP, Dinan TG, Cryan JF. Maternal separation as a model of brain-gut axis dysfunction. Psychopharmacology (Berl) 2011;214:71–88. doi: 10.1007/s00213-010-2010-9. [DOI] [PubMed] [Google Scholar]
- 97.Torow N, Hornef MW. The Neonatal Window of Opportunity: Setting the Stage for Life-Long Host-Microbial Interaction and Immune Homeostasis. J Immunol. 2017;198:557–63. doi: 10.4049/jimmunol.1601253. [DOI] [PubMed] [Google Scholar]
- 98.Diaz Heijtz R, Wang S, Anuar F, Qian Y, Bjorkholm B, et al. Normal gut microbiota modulates brain development and behavior. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:3047–52. doi: 10.1073/pnas.1010529108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Clarke G, Grenham S, Scully P, Fitzgerald P, Moloney RD, et al. The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Mol Psychiatry. 2013;18:666–73. doi: 10.1038/mp.2012.77. [DOI] [PubMed] [Google Scholar]
- 100.Sudo N, Chida Y, Aiba Y, Sonoda J, Oyama N, et al. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J Physiol. 2004;558:263–75. doi: 10.1113/jphysiol.2004.063388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Reber SO, Siebler PH, Donner NC, Morton JT, Smith DG, et al. Immunization with a heat-killed preparation of the environmental bacterium Mycobacterium vaccae promotes stress resilience in mice. Proceedings of the National Academy of Sciences of the United States of America. 2016;113:E3130–9. doi: 10.1073/pnas.1600324113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Desbonnet L, Garrett L, Clarke G, Kiely B, Cryan JF, Dinan TG. Effects of the probiotic Bifidobacterium infantis in the maternal separation model of depression. Neuroscience. 2010;170:1179–88. doi: 10.1016/j.neuroscience.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 103.Savignac HM, Kiely B, Dinan TG, Cryan JF. Bifidobacteria exert strain-specific effects on stress-related behavior and physiology in BALB/c mice. Neurogastroenterol Motil. 2014;26:1615–27. doi: 10.1111/nmo.12427. [DOI] [PubMed] [Google Scholar]
- 104.Bravo JA, Forsythe P, Chew MV, Escaravage E, Savignac HM, et al. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:16050–5. doi: 10.1073/pnas.1102999108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bharwani A, Mian MF, Surette MG, Bienenstock J, Forsythe P. Oral treatment with Lactobacillus rhamnosus attenuates behavioural deficits and immune changes in chronic social stress. BMC Med. 2017;15:7. doi: 10.1186/s12916-016-0771-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Arseneault-Breard J, Rondeau I, Gilbert K, Girard SA, Tompkins TA, et al. Combination of Lactobacillus helveticus R0052 and Bifidobacterium longum R0175 reduces post-myocardial infarction depression symptoms and restores intestinal permeability in a rat model. Br J Nutr. 2012;107:1793–9. doi: 10.1017/S0007114511005137. [DOI] [PubMed] [Google Scholar]
- 107.Desbonnet L, Garrett L, Clarke G, Bienenstock J, Dinan TG. The probiotic Bifidobacteria infantis: An assessment of potential antidepressant properties in the rat. J Psychiatr Res. 2008;43:164–74. doi: 10.1016/j.jpsychires.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 108.Lavasani S, Dzhambazov B, Nouri M, Fak F, Buske S, et al. A novel probiotic mixture exerts a therapeutic effect on experimental autoimmune encephalomyelitis mediated by IL-10 producing regulatory T cells. PLoS One. 2010;5:e9009. doi: 10.1371/journal.pone.0009009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Akkasheh G, Kashani-Poor Z, Tajabadi-Ebrahimi M, Jafari P, Akbari H, et al. Clinical and metabolic response to probiotic administration in patients with major depressive disorder: A randomized, double-blind, placebo-controlled trial. Nutrition. 2016;32:315–20. doi: 10.1016/j.nut.2015.09.003. [DOI] [PubMed] [Google Scholar]
- 110.Mohammadi AA, Jazayeri S, Khosravi-Darani K, Solati Z, Mohammadpour N, et al. The effects of probiotics on mental health and hypothalamic-pituitary-adrenal axis: A randomized, double-blind, placebo-controlled trial in petrochemical workers. Nutr Neurosci. 2016;19:387–95. doi: 10.1179/1476830515Y.0000000023. [DOI] [PubMed] [Google Scholar]
- 111.Rao AV, Bested AC, Beaulne TM, Katzman MA, Iorio C, et al. A randomized, double-blind, placebo-controlled pilot study of a probiotic in emotional symptoms of chronic fatigue syndrome. Gut Pathog. 2009;1:6. doi: 10.1186/1757-4749-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Messaoudi M, Lalonde R, Violle N, Javelot H, Desor D, et al. Assessment of psychotropic-like properties of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in rats and human subjects. Br J Nutr. 2011;105:755–64. doi: 10.1017/S0007114510004319. [DOI] [PubMed] [Google Scholar]
- 113.Steenbergen L, Sellaro R, van Hemert S, Bosch JA, Colzato LS. A randomized controlled trial to test the effect of multispecies probiotics on cognitive reactivity to sad mood. Brain Behav Immun. 2015;48:258–64. doi: 10.1016/j.bbi.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 114.Vindigni SM, Surawicz CM. Fecal Microbiota Transplantation. Gastroenterol Clin North Am. 2017;46:171–85. doi: 10.1016/j.gtc.2016.09.012. [DOI] [PubMed] [Google Scholar]
- 115.Kang DW, Adams JB, Gregory AC, Borody T, Chittick L, et al. Microbiota Transfer Therapy alters gut ecosystem and improves gastrointestinal and autism symptoms: an open-label study. Microbiome. 2017;5:10. doi: 10.1186/s40168-016-0225-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Benros ME, Waltoft BL, Nordentoft M, Ostergaard SD, Eaton WW, et al. Autoimmune diseases and severe infections as risk factors for mood disorders: a nationwide study. JAMA Psychiatry. 2013;70:812–20. doi: 10.1001/jamapsychiatry.2013.1111. [DOI] [PubMed] [Google Scholar]