INTRODUCTION
The goal of successful treatment of thumb basal joint arthritis is relief of pain with retention of motion and stability. Fusion by various techniques1,2 achieves relief of pain but at the cost of lost motion. Since 1947,3 arthritis of the thumb has been treated with various types of arthroplasty in an attempt to relieve pain and preserve motion, but often with resulting instability and/or weakness. Arthroplasties involving implants have been associated with various problems including particulate synovitis, subsidence, and/or dislocation.
Arthroplasty for the base of the thumb has gone through many refinements over the years. In the past, advanced disease at the carpometacarpal (CMC) joint of the thumb at presentation was the rule. Tackling this disease necessitated the development of techniques that allowed for the treatment of a wide array of problems. The treatment options evolved to deal with various stages of the disease.
There is no consensus on the critical elements of arthroplasty for the base of the thumb, as surgeons seem to rely mostly on their experience to pursue critical elements and variations in arthroplasty technique to ensure a pain-free and strong thumb long after surgery.
HISTORY
The ideal arthroplasty relieves pain, provides stability, and retains or improves strength. Arthroplasty pursues one of two general schemes. The first is to resurface the opposing joint surfaces with artificial noninnervated alternatives; the second is to resect the joint surfaces and to reconstruct attenuated or missing ligaments, and interpose material or tissue in between the bone ends. These arthroplasty methods contrast with simple resection of the joint as seen in trapeziectomy, or fusion, both of which are classic treatments for painful CMC joint arthritis.1–3
The evolution of resection and interpositional arthroplasty has led to surgical principles that underlie a variety of surgical procedures. Some of the concepts in the surgical treatment of basal joint osteoarthritis originate from the treatment of inflammatory rheumatoid arthritis (RA) in the era before disease-modifying antirheumatic drugs. In that setting, with the long-term shortcomings of silicone arthroplasty still unknown, many RA patients had very successful implant arthroplasty for basal joint arthritis. These procedures were based on the principle that a soft artificial spacer that maintained the distance between painful bone ends could decrease pain by interposing noninnervated material while retaining strength because of maintained thumb length. In this way, implant arthroplasty was preferable to arthrodesis, which offers pain relief in exchange for motion.
Unfortunately, the success with silicone basal joint arthroplasty for the rheumatoid patient did not prove to be the situation with the higher demand hand seen in osteoarthritis. Over the decades between the introduction of the silicone arthroplasty for basal joint in RA and its large-scale abandonment in the treatment of osteoarthritis of the basal joint, the consequences of silicone interposition in small joints began to appear: (1) fragmentation, (2) instability, (3) particulate synovitis, and (4) cold flow deformation.4 Failure of silicone interposition arthroplasty when applied to the osteoarthritic thumb basal joint often led to revision surgery whereby salvage fusion was either difficult or impossible to achieve in the setting of a foreign-body reaction and decreased bone stock. The failure of a reliable interposition using foreign materials fueled the search for a reliable biological interpositional and stabilizing procedure free from the problems seen with the prosthetic implants.
At the same time, there evolved a nuanced understanding of the pathophysiology of basal joint osteoarthritis. At its earliest stage, shear forces at the trapeziometacarpal joint cause subluxation of the joint and incompetency of the palmar oblique ligament. Ligamentous reconstruction in early-stage, unstable trapeziometacarpal joint was also meeting with success regarding prevention of instability and subluxation.5,6
It was the combination of these 2 seemingly separate treatments, (1) ligament reconstruction for early instability with (2) biological interposition, which led to successful treatment of basal joint arthritis without foreign material. This approach combined the stabilizing effect of the ligament reconstruction with the standard arthroplasty principles of interposition with autologous tissue.7–9
Since the development of this combined approach, much has evolved both in the presurgical treatment and surgical variations on treatment. Some surgical treatments exclude one or another portion of the technique described herein in an effort to distill the minimal, critical elements of surgical treatment. What follows is a description of the authors’ surgical technique, which aims to provide a single reliable basal joint arthroplasty procedure that can be applied to a wide range of pathologic states in this joint.
INDICATIONS FOR LIGAMENT RECONSTRUCTION WITH TENDON INTERPOSITION
Ligament reconstruction with tendon interposition was first introduced in the 1970s in the population of patients older than 50 years with very symptomatic and severe basal joint arthritis.8,10 However, in the decades since the introduction of this procedure, the indications have extended to younger age groups.11,12
Mild cases of basal joint arthritis with relative preservation of the joint can be treated with other procedures such as the Eaton ligament reconstruction,5 metacarpal extension osteotomy,13 and more recently, carpometacarpal arthroscopic debridement.14,15 Alternatively, if basal joint arthroplasty is not an option because of high demands placed as part of athletic or work-related needs, arthrodesis is still the treatment of choice with the preferred technique described by Eaton and Littler.1 One particularly attractive advantage of fusion with the Eaton-Littler technique is the relative ease with which it can be revised to a tendon interposition with ligament reconstruction, should the need arise, because of subsequent scaphotrapeziotrapezoidal arthritis or symptoms at the thumb metacarpophalangeal (MP) joint.
PREOPERATIVE CONSIDERATIONS AND COEXISTING CONDITIONS
Multiple conditions may coexist with basal joint arthritis, including DeQuervain tenosynovitis16 and carpal tunnel syndrome.17 A careful history and physical examination can often reveal if either of these conditions contributes to pain on the radial side of the wrist and weakness in the thenar musculature. The existence of these conditions should be considered in formulating an operative plan for the patient who has failed nonoperative treatment.
Preoperatively, the evaluation should also assess the MP joint of the thumb. If more than 30° of MP extension or valgus instability is found, the operative plan should also address this instability. The standard approach to such a patient includes arthrodesis of the MP joint in 15° to 20° of flexion, with 15° to 20° of pronation, or one of a variety of soft-tissue procedures.
TECHNIQUE
Anesthesia
The preferred form of anesthetic is the regional axillary block. Advantages include continued postoperative pain relief in addition to safety. Patients typically awaken from sedation without postoperative nausea and are able to return home shortly after the procedure.
Incision
Many approaches have been used to successfully expose the trapeziometacarpal joint.18,19 Although the anterior and anterolateral approaches have led to successful results, the posterolateral triradiate approach described here allows for concomitant surgical treatment of the common copresenting DeQuervain tenosynovitis. The senior author has used the posterolateral triradiate approach to perform thousands of basal joint arthroplasty procedures.
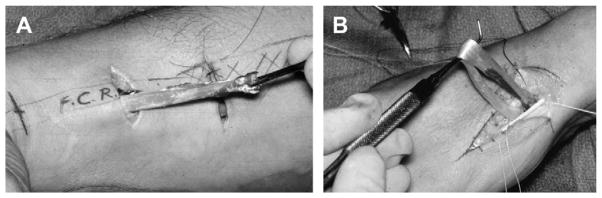
For this approach, anatomic observations can be used to very accurately define the preferred location of the incision. When the patient is anesthetized, the basal joint grind test is used to mark the exact location of the CMC joint on the radial surface of the hand. The radial artery is palpated as it traverses the anatomic snuffbox and accurately locates the scaphotrapezial (ST) joint. With these landmarks (Fig. 1A), a longitudinal incision is made along the proximal shaft of the metacarpal and is continued to the level of the ST joint, at which point 2 separate limbs of the triradiate incision are extended in palmar and dorsal proximal directions. One planned incision will be made later in the procedure to harvest the entire proximal flexor carpi radialis (FCR) tendon (Fig. 1B).
Fig. 1.
(A) Putative plan for incision for main surgical procedure. (B) Plan for flexor carpi radialis (FCR) harvest. (C) Note the branch of the radial sensory nerve.
During dissection, multiple branches of the radial sensory nerve are encountered and must be protected (Fig. 1C). Experience has taught that these nerves are best left in their protective fatty envelope of soft tissue and not finely skeletonized. These nerve branches are protected by gentle retraction with moistened Ragnell retractors (self-retaining retractors are never used here). Later, the placement of capsular sutures will allow the gentle retraction of these deep soft-tissue flaps, which hold these delicate nerves out of the way.
Radial Artery
The radial artery resides in the interval between the extensor pollicis brevis (EPB) and abductor pollicis longus (APL) tendons, as it courses from palmar to dorsal. It is this distal route that brings it closest to the deep ulnar beak of the trapezium as the artery enters into the first web space. The artery is mobilized away from the ST joint and distal scaphoid, and carefully retracted, with care taken to coagulate small branches of the artery located at the ST joint in the anatomic snuffbox (Fig. 2).
Fig. 2.
(A) Native position of radial artery. (B) Mobilized and proximally displaced artery revealing the capsule of the carpometacarpal joint.
Trapezial Excision
Beneath the former position of the radial artery is the capsule of the ST joint. The capsule of the CMC joint, the periosteum of the trapezium, and the ST capsule are incised longitudinally, with care taken to ensure the further trapezial dissection remains subperiosteal in the palmar and dorsal directions. This action ensures safety to the dorsal structures already mentioned in addition to the thenar muscles and FCR in the deep portion of the wound. The landmarks for the dissection are the dorsal extent of the trapeziotrapezoid (TT) joint and palmar extent of the trapezium to the ridge overlying the FCR, well beneath the thenar muscles. At this point, capsular sutures are placed to facilitate later repair of the capsule. Gentle traction on these sutures allows the protection of the radial sensory nerves as already noted. Once this has been done, small Homan retractors are placed: 1 palmar, 1 dorsal, and 1 in the ST. The trapezium is now fully visualized and isolated (Fig. 3).
Fig. 3.

Retention sutures hold the capsule open and allow for later tight closure over the interposition bolus.
The trapezium is then scored with a sagittal saw to 75% depth of the trapezial bone in cruciate configuration so as to ensure safety to the FCR tendon, which courses deep to the bone (Fig. 3). It is important to orient the longitudinal cut parallel to the course of the FCR tendon so as to decrease the likelihood of tendon injury should the cut be inadvertently made too deep. An osteotome is used to lever in the saw cuts using a twisting motion which “pops” the trapezium into 4 pieces, and the bone is removed in 4 quadrants. An effort is made to ensure complete excision of the trapezium from the valley occupied by the medial beak of the bone. Large and small osteophytes and loose bodies are removed at this time. Special attention should be paid to the deep cul-de-sac at the beak of the metacarpal between the base of the first and second metacarpals. This area often harbors loose bodies hidden from view.
Preparation for the Ligament Reconstruction
Subperiosteal dissection is done along the base of the metacarpal to a level 1 cm distal to the metacarpal base; this will be the planned exit point of the new ligament reconstruction. The entry point will be on the deep side of the metacarpal base at the former attachment site of the old palmar-beak ligament. A channel through the proximal metacarpal bone will have to connect these 2 points. The channel is created with gouges and curettes, with appropriate retraction to protect the extensor tendon and sensory nerves (Fig. 4).
Fig. 4.
(A) Schematic of channel in the metacarpal base. (B) Gouge in the channel as it is being formed. (C) Completed channel.
Flexor Carpi Radialis Harvest
The FCR is now visualized in the defect left by the trapezial excision. Dissection and slight tension on it in this space can free it from scarring often seen at the distal scaphoid and attachment to the palmar and deep capsule. It must be freed to the level of its insertion on the second metacarpal.
The musculotendinous junction of the FCR is next identified by palpation through intact skin in the mid-proximal forearm. A single transverse incision is made over this site with dissection to the level of the deep fascia. The tendon is identified and a curved hemostat is passed beneath the tendon. The tendon is transected, and adjacent muscle fibers (usually deep to the tendon) need to be sharply freed from the tendon.
Traction is then placed either with a dental probe, or umbilical tape preplaced around the tendon in the arthroplasty space, and the tendon is delivered into the distal wound. If difficulty is encountered delivering the free end of the tendon, a second transverse incision may be needed just proximal to the wrist midway between the 2 incisions (Fig. 5). This tendon is then wrapped in a moistened gauze sponge.
Fig. 5.
(A) Harvest of the FCR. (B) Passing of FCR into the arthroplasty space.
Extraneous muscle fibers are removed sharply from the tendon, and a deep number-2 Ethibond suture (Ethicon, Cornelia, GA) on a small needle is placed in the FCR just proximal to its insertion on the second metacarpal at the deepest portion of the arthroplasty space. This suture is placed so that it can be used to secure the FCR tendon deep in the arthroplasty space after the tendon is passed through the metacarpal base boney channel. At this point, the tendon is passed through the channel and allowed to exit the dorsal side of the metacarpal. This passage of the tendon through the bone tunnel is facilitated by tightly tying a 26-gauge wire 2 mm from the cut end of the FCR, passing the 2 ends of this wire from deep to superficial through the tunnel, and then gently delivering the tendon through the tunnel; a soft circular motion with the gentle traction is often helpful.
In the arthroplasty space, the deep capsule is repaired at its depth with at least 2 sutures of 3-0 or 4-0 monofilament nonabsorbable sutures. The needle is removed from the suture, but the tails of each are left long and separately clamped for the subsequent placement of the interposition, as shown in Fig. 6. In addition to these 2 sutures, multiple additional sutures are often necessary for the deep capsular closure. Because of the small deep space that must accept these sutures, small curved needles are useful for the completion of this very important careful deep capsular repair and the placement of the retention sutures. This deep capsular closure, combined with the very tight lateral/superficial capsular closure done after the interposition, ensures the interposition is securely enclosed.
Fig. 6.
(A) The deep capsule is closed and (B) sutures are kept long, emanating from the deep closure to allow for insertion of the interpositional bolus. (C) The FCR is passed through the channel.
Thumb Positioning and Kirschner-Wire Placement
It is essential that the position of the thumb in space, and therefore the size and position of the arthroplasty, is precise. If the projection of the thumb metacarpal from the fixed unit is the same as would be used for a CMC fusion, the maximum of thumb function will occur with the least amount of displacement force on the reconstruction. The following elements should guide the placement of the thumb relative to the fixed unit: (1) the thumb projection should be coaxial with the scaphoid; (2) the thumb metacarpal must project from the fixed unit in the fist position such that the thumb tip rests over the middle phalanx of the index finger with the thumb placed in the fist position; (3) 1 to 2 mm of space should be maintained between the base of the first and second metacarpals; and (4) the base of the thumb metacarpal should be at the same level as the base of the index metacarpal, thereby ensuring no overdistraction or underdistraction of the thumb arthroplasty space.
For these reasons, 1 or 2 Kirschner (K) pins are placed to hold the thumb metacarpal in this proper position relative to the fixed unit. The K-wire(s) must not traverse the boney tunnel or the arthroplasty space, as this would preclude proper tension on the ligament and/or proper placement of the interposition material (Fig. 7A, B). The K-wire will also serve to limit compression of the interpositional arthroplasty under the weight of the dressing and splint in the early postoperative period. With the thumb metacarpal properly positioned and stabilized in this manner, maximum function is ensured with the least stress on the new “ligament.” Firm traction is applied to the FCR tendon, so “it’s as tight as it will go,” with the metacarpal secure in the exact position desired. A stay suture is used to secure the tendon at the exit hole of the channel from the metacarpal bone (Fig. 7C). This stay suture goes from the dorsal metacarpal periosteum at the level of the exit hole in the bone, through the FCR, through the volar metacarpal periosteum, and thence back through the FCR and the dorsal periosteum. It should be placed with simultaneous gentle proximal traction on the capsular sutures to the maximum capsule for adequate lateral closure later.
Fig. 7.
(A) Schematic depicting the plan of placement of K-wires with the thumb in the functional position. (B) K-wire placement. (C) Suture of the snugly tensioned tendon to periosteal tissue.
The metacarpal base is resurfaced to completely cover the hole in the metacarpal base left by making the channel. This action eliminates the possibility of the interposition bolus from migrating into the metacarpal base itself. The FCR is folded back on itself. After ensuring that the tendon is draped across the surface of the metacarpal base, the tendon is sutured to itself in the depth of the arthroplasty space with the preplaced suture in the FCR, where it inserts into the second metacarpal base (Fig. 8A).
Fig. 8.
(A) The metacarpal base is resurfaced by suturing the FCR to itself at the base of the arthroplasty space. (B) The interpositional bolus is formed by folding the tendon over a Keith needle. (C) The tendon is fully folded in the form of an accordion. (D) The quadrants of the tendon interposition are sutured and the needles threaded, with the deep sutures passed across the bolus.
Interposition
The interposition tendon material should have the characteristics of a firm cushion of appropriate size.
Approximately 3 inches (7.6 cm) of FCR tendon are usually available for the interposition. A Keith needle is passed through the FCR tendon at the level of the skin. Successive 1-cm lengths of the remaining tendon are folded over the needle to form an interposition, which resembles a neatly folded ribbon candy (Fig. 8B). It normally takes 8 to 10 such folds to completely fold the FCR tendon in the form of an accordion (Fig. 8C).
At this point, a second Keith needle is passed through the bolus parallel to the first, and the needles are advanced through the bolus to compress the interposition. Sutures are then placed in each of the 4 quadrants of the interposition to firmly hold the tendon in this configuration. The Keith needles are then threaded with the previously placed sutures from the deep capsular closure so that further advancement of the Keith needles threads these sutures onto the bolus (Fig. 8D). Gentle traction is then placed on the flaps of the superficial capsule to create an open “funnel” into the arthroplasty space, and the bolus of interpositional tendon is then advanced into that space. The sutures threaded through the bolus are then tied to each other to hold the interposition firm to the base of the arthroplasty space. At this point, the configuration of the sutures and the bolus should ensure that (1) the bolus is pulled tightly into the trapezial space and (2) the bolus is stable (Fig. 9A, B).
Fig. 9.
(A) Schematic depicting inserted interpositional bolus with sutures in deep capsule. (B) Bolus inserted into arthroplasty space. (C) Tight capsular closure over bolus.
Closure
The capsule is closed with the preplaced sutures used to open the arthroplasty space and allow the bolus to be inserted. If these lateral capsular structures do not include a firm attachment to the distal scaphoid, a fine K-wire is used to place a drill hole in the distal scaphoid through which 1 or 2 additional capsular closure sutures may be placed. Care is taken to ensure that the tight capsular closure is free of gaps and defects, usually accomplished by tying sutures in opposite flaps of the capsule together in a directly side-to-side configuration.
Transfer of the Extensor Pollicis Brevis
If the MP joint is not fused, a transfer of EPB can serve to decrease the extension force on the MP and increase the abduction power of the metacarpal away from the fixed unit. This transfer is accomplished by first suturing the EPB over the FCR tendon, which exits the bone channel, and then transecting the EPB distal to this point over the metacarpal (Fig. 10A, B). The remaining EPB is then tucked into the channel itself so as to recreate a tendon-to-bone interface when it has healed (Fig. 10C). The opening of the channel is then closed with a 4-0 purse-string nonabsorbable monofilament suture.
Fig. 10.
Transfer of the extensor pollicis brevis (EPB). (A) Schematic of plan of transfer. (B) Suture of EPB to FCR exiting the channel. (C) Tucking and closing of the channel over the transfer of EPB.
Pins are cut 2 to 3 mm superficial to the skin to allow easy removal in the office, and the wound is closed with fine nylon suture with care taken to avoid any branches of the radial sensory nerve in the closure. The skin-to-pin interface is coated with collodion in 2 or 3 applications.
DRESSING AND POSTOPERATIVE CARE
First Month
The position of the wrist in the postoperative dressing is important to the final result. Care is taken to position the wrist in approximately 15° to 25° of extension and in neutral radial-ulnar deviation. Some extension is necessary so that the weight of the dressing and plaster shell while the hand is elevated is borne by the palm and pin and not the thumb; this helps to minimize the postoperative pain. Excessive wrist extension potentially compromises the ability to engage in rehabilitation exercises to achieve active wrist flexion, because of the lack of an active FCR muscle and immobilization in extension for 4 weeks.
Patients can be transferred to a cast at 1 week or can remain in the postoperative splint for an entire month. During the first month, the hand is kept elevated at all times, and the use of a foam wrist elevation pillow or similar device is helpful. Slings are avoided for their dependent positioning of the hand and the immobilization of the shoulder, which can lead to stiffness. Should discomfort arise after 1 or 2 weeks owing to decreased swelling and increased movement in the dressing, the dressing is changed and sutures removed, and a short-arm thumb spica cast is applied for the remainder of the first month after surgery. During the first month, finger range of motion along with range of motion of the thumb interphalangeal (IP) joint is encouraged with a home program several times per day.
Second Month
At 4 weeks, the K-wires are removed along with sutures. The patient is fitted with a forearm-based thumb spica splint. This phase emphasizes range of motion in the entire upper extremity with a focus on flexion and extension of the wrist and thenar muscle isometric strengthening, especially opponens muscle setting, as this muscle with its fibers nearly perpendicular to the metacarpal is especially important for stability of the metacarpal base. Specific elements of the wrist program include forearm wrist and hand being placed on a flat surface in supination with the wrist in 25° to 30° of extension, emphasizing controlled active wrist flexion from 30° of extension through a flexion arc of 45° to 50°.
Three to 6 Months
After 2 months, there is a gradual return to functional use in the activities of daily living. At 2 to 3 months the patient is weaned out of the splint, starting with gentle activities such a daily hygiene, eating, and writing. There is a gradual transition to unprotected full activity. Wrist weights are increased gradually to 6 lb (2.7 kg) and the repetitions are increased. Gripping, pinching, and pulling putty are added to the regimen after about 2 months along with manipulation of clothespins. All of the gripping exercises are performed with the thumb metacarpal in the position of functional activity (thumb positioned between palmar and radial abduction with IP joint free). No attempts are made to pinch to the ring or small fingers, as these motions risk stretching out the ligament reconstruction and have no functional use in activities of daily living. For the same reason, stretching the thumb into the palm-flat position is avoided.
Timing for return to work depends on the occupational manual needs and whether or not the dominant hand is the one operated on. Some executive positions permit return to work within a week. Higher-demand manual situations may require 3 to 6 months before a return to work, depending on the hand involved and rapidity in regaining excellent strength with the exercise program. Therapy exercises are continued for 1 to 2 years postoperatively; these are simple putty exercises that the patient does on his or her own.
Beyond 6 Months
The patient is encouraged to continue using the putty for an indefinite period after the surgery (6 to 36 months postoperatively). When these procedures were first done in the 1970s, the physicians and therapists assumed that maximum strength and function would occur within 3 to 6 months. To the pleasant surprise of all, patients continued to gain in strength for up to 1 to 2 years. Patients with a return of symptoms can reliably be found to have weakness attributable to discontinuation of exercises. Simple resumption of the strengthening program resolves these symptoms.
RESULTS
Although multiple procedures have been described for basal joint arthritis, the heterogeneity of procedures performed prevents the valid comparisons across different surgical series. Multiple studies have documented the short-, medium-, and long-term results of basal joint arthroplasty procedures.12,20 The technique described here has led to very good patient satisfaction and high objective measures of function, including return of strength and dexterity to even the severely affected thumb.12
In the longest-term study to date of the procedure described in this article, Tomaino and colleagues12 examined 24 thumbs of 22 patients at an average of 9 years (range, 8–11 years) after a ligament reconstruction–tendon interposition arthroplasty for osteoarthritis at the base of the thumb. The data included preoperative and sequential long-term follow-up on the same cohort of patients. The same cohort of patients had been followed previous to this longer-term follow-up at 2 and 6 years postoperatively. Three of the thumbs were revisions of failed implant arthroplasty, and the rest were performed as primary procedures.
Of this cohort, 21 (95%) were satisfied and had excellent pain relief. Objective improvement in grip strength was 10 kg-force (93% improvement) and tip pinch strength of nearly 2.2 lb (1 kg) (65% improvement). Key-pinch improvement was more modest, with 34% gain over preoperative values. Range of motion of the thumb tip returned to flexion to the base of the small finger in 92% of patients with an average first web angle of 40°.
Stress radiographs showed an average subluxation of the metacarpal base of 11% at 9 years compared with 7% and 8% at 2 and 6 years, respectively. Similarly, these radiographs demonstrated an average loss of height of the arthroplasty space of 13% at 9 years compared with 11% at both of the earlier follow-up examinations. None of the modest changes in radiographic outcome predicted unsatisfactory outcome.
Taken together, these results were interpreted to suggest that the procedure described here led to a stable and functional reconstruction of the thumb, resulting in excellent relief of pain and a significant increase in strength for as long as 11 years after the procedure.12
SUMMARY
The frequency of occurrence and the significant symptoms with functional impairment from basal joint arthritis has motivated many advances in surgical procedures for this condition. Basal joint arthroplasty with ligament reconstruction and tendon interposition is the authors’ treatment of choice, and can lead to reliable results in a variety of situations. It is this reliability, in the face of many variations of arthritis of the thumb, which has led to the popularity of ligament reconstruction and tendon interposition arthroplasty. The procedure is somewhat tedious to execute correctly with all its components being key to a successful outcome. However, the conscientious attention to detail of the surgical procedure as described herein leads to excellent long-term results.
KEY POINTS.
Arthritis at the base of the thumb is common and debilitating. Arthroplasty has evolved over three decades to become a highly refined surgical procedure, with excellent results.
After summarizing the history, method, and expected results of basal joint arthroplasty, the authors describe their method of ligament reconstruction and tendon interposition for thumb basal arthritis.
The goal of successful treatment of thumb basal joint arthritis is relief of pain with retention of motion and stability.
Footnotes
The authors have nothing to disclose for this work.
References
- 1.Eaton RG, Littler JW. A study of the basilar joint of the thumb. Treatment of its disabilities by fusion. J Bone Joint Surg Am. 1969;51:661–8. [PubMed] [Google Scholar]
- 2.Carroll RE, Hill NA. Arthrodesis of the carpometacarpal joint of the thumb. J Bone Joint Surg Br. 1973;55:292–4. [PubMed] [Google Scholar]
- 3.Gervis WH. Excision of the trapezium for osteoarthritis of the trapezio-metacarpal joint. J Bone Joint Surg Br. 1949;31:537–9. [PubMed] [Google Scholar]
- 4.Pellegrini VD, Jr, Burton RI. Surgical management of basilar joint arthritis of the thumb. Part I. Long-term results of silicone implant arthroplasty and Part II. Ligament reconstruction with tendon interposition arthroplasty. J Hand Surg Am. 1986;11:309–24. 324–32. doi: 10.1016/s0363-5023(86)80136-8. [DOI] [PubMed] [Google Scholar]
- 5.Eaton RG, Lane LB, Littler JW, et al. Ligament reconstruction for the painful thumb carpometacarpal joint: a long-term assessment. J Hand Surg Am. 1984;9:692–9. doi: 10.1016/s0363-5023(84)80015-5. [DOI] [PubMed] [Google Scholar]
- 6.Glickel SZ, Malerich M, Pearce SM, et al. Ligament replacement for chronic instability of the ulnar collateral ligament of the metacarpophalangeal joint of the thumb. J Hand Surg Am. 1993;18:930–41. doi: 10.1016/0363-5023(93)90068-e. [DOI] [PubMed] [Google Scholar]
- 7.Uriburu IJ, Olazábal AE, Ciaffi M. Trapeziometacarpal osteoarthritis: surgical technique and results of “stabilized resection-arthroplasty”. J Hand Surg Am. 1992;17:598–604. doi: 10.1016/0363-5023(92)90301-5. [DOI] [PubMed] [Google Scholar]
- 8.Burton RI. The arthritic hand: arthritis of the basilar joint. In: McCollister Evarts C, Burton RI, editors. Surgery of the musculoskeletal system. New York: Churchill Livingstone; 1983. [Google Scholar]
- 9.Thompson JS. Surgical treatment of trapeziometacarpal arthrosis. Adv Orthop Surg. 1986;10:105–20. [Google Scholar]
- 10.Burton RI. Master techniques in orthopaedic surgery: the hand. Philadelphia: Lippincott-Raven; 1998. Ligament reconstruction by tendon transfer and soft tissue interposition arthroplasty for basilar joint osteoarthritis of the thumb. (LRTI or Burton procedure) [Google Scholar]
- 11.Burton RI, Pellegrini VD., Jr Basilar joint arthritis of thumb. J Hand Surg Am. 1987;12:645. doi: 10.1016/s0363-5023(87)80232-0. [DOI] [PubMed] [Google Scholar]
- 12.Tomaino MM, Pellegrini VD, Jr, Burton RI. Arthroplasty of the basilar joint of the thumb. Long-term follow-up after ligament reconstruction with tendon interposition. J Bone Joint Surg Am. 1995;77:346–55. doi: 10.2106/00004623-199503000-00003. [DOI] [PubMed] [Google Scholar]
- 13.Uzumcugil A. Long-term outcomes of first metacarpal extension osteotomy in the treatment of carpometacarpal osteoarthritis. J Hand Surg Am. 2009;34:1156. doi: 10.1016/j.jhsa.2009.03.003. author reply: 1156–7. [DOI] [PubMed] [Google Scholar]
- 14.Kapoutsis DV, Dardas A, Day CS. Carpometacarpal and scaphotrapeziotrapezoid arthritis: arthroscopy, arthroplasty, and arthrodesis. J Hand Surg Am. 2011;36:354–66. doi: 10.1016/j.jhsa.2010.11.047. [DOI] [PubMed] [Google Scholar]
- 15.Yao J, Park MJ. Early treatment of degenerative arthritis of the thumb carpometacarpal joint. Hand Clin. 2008;24:251–61. v–vi. doi: 10.1016/j.hcl.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 16.Burton RI. Complications following surgery on the basilar joint of the thumb. Hand Clin. 1986;2:265–9. [PubMed] [Google Scholar]
- 17.Florack TM, Miller RJ, Pellegrini VD, et al. The prevalence of carpal tunnel syndrome in patients with basilar joint arthritis of the thumb. J Hand Surg Am. 1992;17:624–30. doi: 10.1016/0363-5023(92)90305-9. [DOI] [PubMed] [Google Scholar]
- 18.Cassidy C, Glennon PE, Stein AB, et al. Basilar joint arthroplasty and carpal tunnel release through a single incision: an in vitro study. J Hand Surg Am. 2004;29:1085–8. doi: 10.1016/j.jhsa.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 19.Wagner CJ. Method of treatment of Bennett’s fracture dislocation. Am J Surg. 1950;80:230–1. doi: 10.1016/0002-9610(50)90537-x. [DOI] [PubMed] [Google Scholar]
- 20.Kochevar AJ, Adham CN, Adham MN, et al. Thumb basilar joint arthroplasty using abductor pollicis longus tendon: an average 5. 5-year follow-up. J Hand Surg Am. 2011;36:1326–32. doi: 10.1016/j.jhsa.2011.05.026. [DOI] [PubMed] [Google Scholar]