Abstract
Purpose of review
Previous concepts regarding the pathways involved in the generation of angiotensin II (Ang II) are been challenged by studies showing the existence of a peptide acting as an endogenous antagonist of Ang II. The discovery that angiotensin-(1-7) [Ang-(1-7)] opposes the pressor, proliferative, profibrotic, and prothrombotic actions mediated by Ang II has contributed to the realization that the renin angiotensin system is comprised by two opposing arms: the pressor arm constituted by the enzyme angiotensin converting enzyme (ACE), Ang II as the product, and the Ang II type 1 receptor (AT1) as the main protein mediating the biological actions of Ang II; the second arm is composed by the mono carboxypeptidase –angiotensin converting enzyme 2 (ACE2)–, Ang-(1-7) produced through hydrolysis of Ang II, and the Mas receptor as the protein conveying the vasodilator, antiproliferative, anti-fibrotic, and anti-thrombotic effects of Ang-(1-7).
Recent findings
Experimental and clinical studies demonstrate a role for the Ang-(1-7)/ACE2/Mas-axis in the evolution of hypertension, the regulation of renal function, and the progression of renal disease including diabetic nephropathy. Additional evidence suggests that reduction in the expression and activity of this vasodepressor component may be a critical factor in mediating the progression of cardiovascular disease.
Summary
Further research on the contribution of the Ang-(1-7)/ACE2/Mas-axis to cardiovascular pathology will lead to the development of new pharmacological approaches resulting in the design of molecular or genetic means to increase the expression of ACE2, allow for increased tissue levels of Ang-(1-7), or both.
Keywords: Angiotensin peptides, angiotensin converting enzyme 2, renal disease, essential hypertension
Introduction
Remarkable progress continues to be made in unraveling the contribution of the renin angiotensin system to cardiovascular pathology and, specifically the role of the vasodepressor pathway composed by the triad of the enzyme angiotensin converting enzyme 2 (ACE2), the heptapeptide product angiotensin-(1-7) [Ang-(1-7)], and the mas receptor. Work originating from our research program established the basis and initial mechanisms for the inclusion of the Ang-(1-7)/ACE2/Mas-axis as a critical counter balancing component of the pressor pathway composed by the angiotensin converting enzyme (ACE), the octapeptide angiotensin II (Ang II) and its signaling action following binding to the subtype I Ang II receptor. Over the last decade data continues to support the hypothesis that the pathological actions of Ang II on cardiovascular regulatory activity may in part result from a diminished expression or activity of the components of the Ang-(1-7)/ACE2/mas-R axis. This review provides a birds-eye-view on the progress made over the last two years. The fact that 2010 publications to date tally 48 in PubMed for the term Ang-(1-7) documents how significant progress is been made on this topic.
Angiotensin-(1-7) Formation and Signaling Mechanisms
There are two biochemical pathways that account for the formation of Ang-(1-7) (Figure 1). The first entails the hydrolysis of angiotensin I (Ang I) by the tissue endopeptidases: prolyl-endopeptidase (EC 3.4.21.26), neutral endopeptidase 24.11 (neprilysin; EC 3.4.24.11), and oligopeptidase 24.15 (thimet oligopeptidase EC 3.4.24.15) [1;2]. The second pathway entails the cleavage of the Pro7-Phe8 bond of Ang II by angiotensin converting enzyme 2 (E.C. 3.4.15.1; ACE2) [3;4], an exopeptidase which has also been shown to cleave Ang I into angiotensin-(1-9) [Ang-(1-9)] [3]. Among the Ang-(1-7) forming enzymes, the kcat/km of ACE2 is 500-fold higher for Ang II compared to the kcat/km for Ang I. A third pathway leading to the ultimate generation of Ang-(1-7) may result from the discovery of an extended form of Ang I, the dodecapeptide angiotensin-(1-12) [Ang-(1-12)] [5]. The studies reported by Nagata et al. [5] showed that this novel angiotensinogen derived peptide was capable of generating Ang II and possessed vasoconstrictor activity that were blocked by prior administration of the ACE inhibitor, captopril or the type 1 Ang II receptor (AT1) candesartan. In confirming their findings, we showed in the isolated heart preparation, a late formation of Ang-(1-7) after administration of Ang-(1-12) [**6]. More detailed information regarding the biochemical pathways leading to Ang-(1-7) formation and metabolism are published [7–9].
Figure 1.
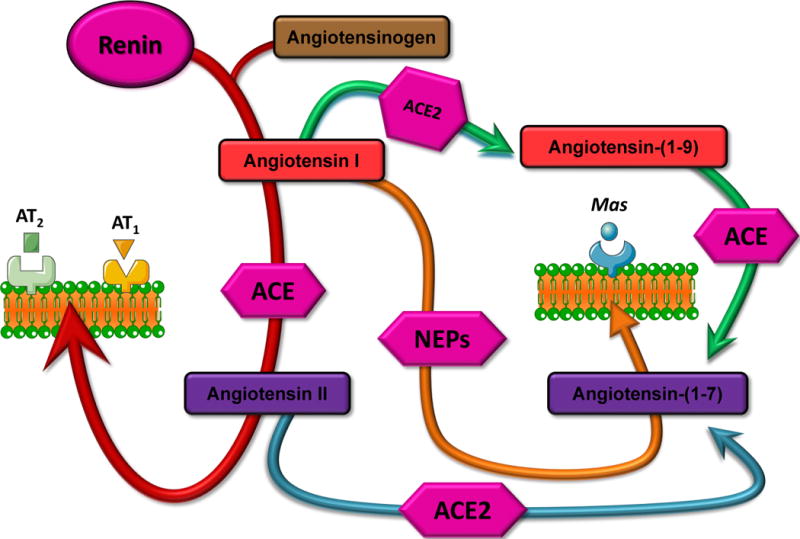
Schematic representation of the components of the renin angiotensin system.
The Mas receptor has been identified as the protein transducing the vasodilator and antiproliferative actions of Ang-(1-7) [10–12]. The Mas protein is a G-protein couple receptor originally linked to modulation of growth regulating pathways involved in oncogenic effects and high expression in testis, hypothalamus and amygdala [13;14]. In a detailed study of the function of the Mas receptor Kostenis et al. [15] showed that in transfected Mas cells this G-protein coupled receptor antagonized Ang II actions at the Ang II type 1 receptor (AT1) owing to formation of a hetero-oligomeric complex. This important study explains previous findings of a weak direct vasodilator effect of Ang-(1-7) [16] that contrasts with effects of the heptapeptide in reducing pressor actions of Ang II both in vitro and in vivo [*17–29]. See also annotated references 20, 24, 26, 27 and 28. Although a search of the current literature did not reveal the existence of a detailed analysis of renal mas distribution, a picture of the mas receptor localization in rat kidney suggest that it may reside in proximal and distal tubules (Alomone Labs; http://www.alomone.com/p_postcards/database/510.htm). On the other hand, Metzger et al. [14] reported the presence of mas mRNA in renal tissues from mice and rats.
The signaling mechanisms by which Ang-(1-7) antagonizes the pleiotropic actions of Ang II remain under investigation. Earlier studies implicated a stimulatory action of the heptapeptide on release of endothelial derived nitric oxide (NO) and vasodilator prostaglandins [30–40] as well as potentiation of the vasodilator and metabolic actions of bradykinin [41–46]. Intracellular mediators accounting for the antagonistic actions of Ang-(1-7) on Ang II responses may include phosphorylation of both Akt (adipose tissue, skeletal muscle, and liver) and GSK-3β in liver and skeletal muscle of the rat [*47]. In keeping with this finding, Sampaio et al. [38] reported that Ang-(1-7) stimulates AKt kinase from human endothelial cells. In vascular smooth muscle cells Ang-(1-7) inhibited Ang II stimulation of mitogen-activated protein kinase activities (ERK1/2) [48]. A preliminary observation by Tallant and Gallagher, reported as abstracts [49;50] only, suggests that Ang-(1-7) may upregulate the mitogen-activated DUSP-1 in vascular smooth muscle cells.
As progress continues to be made in the understanding of the molecular mechanisms of Ang-(1-7) signaling, other new information is accumulating as to the actions of Ang II and Ang-(1-7) in the regulation of ACE2 gene and activity. Ishiyama et al. [51] first reported that blockade of AT1 receptors was accompanied by increase cardiac ACE2 transcripts, a finding that was interpreted to indicate that Ang II may exert a negative influence in the regulation of ACE2. These findings were corroborated in further studies in astrocytes in culture [52], and later in neonatal cardiac myocytes and fibroblasts [53]. In these experiments, the observation that Ang-(1-7) by itself had no effect on ACE2 mRNA while it blocked the reduction in ACE2 transcripts produced by Ang II or endothelin-1 through activation of the extracellular signal-regulated kinase ERK1/ERK2 is reminiscent of the conclusions made by Kostenis et al [15] showing that Ang-(1-7) antagonizes AT1 receptors.
A conflicting report in primary human cardiac fibroblast suggests that Ang II stimulates rather than suppress ACE2 gene expression [*54]. As recognized by the authors [54], their findings may be restricted to the type of cell employed in their experiments and the state of cell differentiation, as the work showing increased cardiac ACE2 mRNA following chronic blockade of Ang II production or activity by lisinopril or losartan [55] is in support of the view of a negative Ang II effect on ACE2 transcripts. Our studies [56;57] showing upregulation of ACE2 mRNA in the aorta of SHR given the AT1 receptor blocker olmesartan or in the neointima of olmesartan-treated SHR are also at variance with the idea that Ang II exerts a positive regulatory action on ACE2.
The Ang-(1-7)/ACE2/Mas-axis in Hypertension. Focus on the Kidney
Since we first proposed that a deficit in the counter regulatory effects of Ang-(1-7) may directly contribute to the pathogenesis of human essential hypertension [58] evidence for this possibility continues to gain support mostly from data obtained in experimental models of the disease. As commented by us elsewhere [59], the paucity of clinical studies directed to address this possibility are rooted in the belief that the antihypertensive actions resulting from inhibition of Ang II synthesis or activity suffice to explain their cardio-renal protective effects. Advances in the understanding of the molecular and physiological mechanisms of ACE2 has began to reverse this tide but the task is a difficult one as it entails the design of agents that will increase the activity of ACE2, augment tissue levels of Ang-(1-7), or both in combination [60–62].
Clinical studies supporting the view of hemodynamic and end-organ effects of Ang-(1-7) are summarized elsewhere [59]. A first glimpse to the possible favorable actions of Ang-(1-7) in contributing to the antihypertensive actions of ACE inhibition was gained from the observation of an increased excretion of Ang-(1-7) in the urine of essential hypertensives whose blood pressure was controlled by a 6-month treatment with captopril [63]. Two years later, a more detailed study was performed in 31 normal healthy volunteers and 18 untreated essential hypertensive subjects [64]. This study showed that urinary excretion rates of Ang-(1-7) in the essential hypertensive subjects averaged almost one-half of those measured in the normotensive controls [64]. Additional studies showed that administration of the Ang II receptor blocker (ARB) irbesartan was associated with increases in plasma Ang-(1-7) concentrations [64;65]. Similar findings were reported in salt-sensitive hypertensive subjects medicated with omapatrilat, a potent dual inhibitor of ACE and neprilysin [66]. Changes in the sodium status may regulate the influence of Ang-(1-7) on the action of ACE inhibitors as in healthy normal volunteers, the increases in Ang-(1-7) produced by the combination of enalapril and a low salt diet were much greater than those observed in the same subjects when exposed to the low salt diet alone [67].
Along the animal and human nephron, multiple studies have documented the presence of renin angiotensin system components underscoring the concept that the kidney is a site at which an intrarenal system participates in the regulation of glomerular-tubular balance in health and disease [9;68;69]. Increase expression of cortical and medullary Ang II and cortical ACE2 activity was found in a model of hypertension with increased tissue renin [70]. And the blood pressure lowering action of administering lisinopril or losartan to normotensive rats were associated with increased urinary excretion of Ang-(1-7), augmented expression of cortical renin and angiotensinogen gene transcripts, and higher cortical ACE2 activity in renal membranes from the same treated animals [71]. In an experimental model of renal hypertension, the administration of a selective Ang-(1-7) receptor blocker or an ACE2 inhibitor was associated with worsening of hypertension and renal function [17].
The experimental evidence demonstrating a palette of actions of Ang-(1-7) and ACE2 in the regulation of nephron function [72;73] correlates with newer studies showing that altered ACE2 expression or activity contributes to the progression of renal disease and diabetic nephropathy [74]. Lely et al. [74;75] showed neo-expression of ACE2 in the glomerular and peritubular capillary endothelium of biopsied kidneys of patients with primary and secondary forms of renal disease as well as renal transplants. In patients with diabetes and overt nephropathy, an increase in the ACE/ACE2 ratio resulted from a decreased expression of tubulointerstitium and glomeruli ACE2 [76]. Similar findings were also reported in patients with diabetic nephropathy [*77]. These findings are in keeping with the observation of increased deposition of collagen I, collagen III and fibronectin in the glomeruli and increased urinary albumin excretion compared to age-matched control mice in mutant male ACE (−/−) mice [78]. Of additional interest is the recent report demonstrating the presence of serum ACE2 autoantibodies in patients with connective tissue diseases to constrictive vasculopathy, pulmonary arterial hypertension (PAH), or persistent digital ischemia [77;79]. These findings are in keeping with the observation of reduced plasma Ang I, Ang II, and Ang-(1-7) levels in patients with systemic sclerosis compare to controls [80]. Since the ratio of Ang II/Ang-(1-7) indicated a prevalence of Ang II over the countervailing Ang-(1-7), these data provide additional support for an important interaction among ACE2 and Ang-(1-7) in multiple disease states in which the actions of Ang II favor proliferative, profibrotic, and thrombotic effects.
Conclusion
The question posed on the title as to whether more of Ang-(1-7) or less of Ang II may participate in the pathogenesis of hypertensive vascular disease cannot be answered with certainty but the research done to-date continues to support the hypothesis that a decrease in the expression or activity of Ang-(1-7) renders the cardiovascular system more susceptible to the pathological actions of Ang II. In the kidney, the opposing effects of low and high doses of Ang-(1-7) on tubular sodium reabsorption needs further study, as binding of Ang-(1-7) to mas may alter expression or activity of AT1 or AT2 receptors [9,15]. In addition, accumulating evidence suggest that ACE2 may play a critical role in modulating the relative contributions of Ang II and Ang-(1-7) to blood pressure regulation and that changes in the relative tissue expression of ACE and ACE2 activities may be deterministic in diseases of the heart, the blood vessels, and the kidneys. While the experimental evidence is rather appealing, there is a real need to extend these findings to human’s conditions such as essential hypertension, chronic renal disease, and diabetes.
Acknowledgments
Support for studies described in this review from our laboratory was achieved from a grant provided by the National Heart, Blood, and Lung Institute of the National Institutes of Health (PO1 HL051952). The author gratefully acknowledges additional grant support provided by the Farley-Hudson Foundation, Jacksonville, NC.
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
* of special interest
** of outstanding interest
- 1.Chappell MC, Welches WR, Brosnihan KB, Ferrario CM. Inhibition of angiotensin converting enzyme by the metalloendopeptidase 3.4.24.15 inhibitor c-phenylpropyl-alanyl-alanyl-phenylalanyl-p-aminobenzoate. Peptides. 1992;13:943–946. doi: 10.1016/0196-9781(92)90053-6. [DOI] [PubMed] [Google Scholar]
- 2.Welches WR, Brosnihan KB, Ferrario CM. A comparison of the properties and enzymatic activities of three angiotensin processing enzymes: angiotensin converting enzyme, prolyl endopeptidase and neutral endopeptidase 24.11. Life Sci. 1993;52:1461–1480. doi: 10.1016/0024-3205(93)90108-f. [DOI] [PubMed] [Google Scholar]
- 3.Donoghue M, Hsieh F, Baronas E, Godbout K, Gosselin M, Stagliano N, Donovan M, Woolf B, Robison K, Jeyaseelan R, Breitbart RE, Acton S. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1-9. Circ Res. 2000;87:E1–E9. doi: 10.1161/01.res.87.5.e1. [DOI] [PubMed] [Google Scholar]
- 4.Turner AJ, Tipnis SR, Guy JL, Rice G, Hooper NM. ACEH/ACE2 is a novel mammalian metallocarboxypeptidase and a homologue of angiotensin-converting enzyme insensitive to ACE inhibitors. Can J Physiol Pharmacol. 2002;80:346–353. doi: 10.1139/y02-021. [DOI] [PubMed] [Google Scholar]
- 5.Nagata S, Kato J, Sasaki K, Minamino N, Eto T, Kitamura K. Isolation and identification of proangiotensin-12, a possible component of the renin-angiotensin system. Biochem Biophys Res Commun. 2006;350:1026–1031. doi: 10.1016/j.bbrc.2006.09.146. [DOI] [PubMed] [Google Scholar]
- 6**.Trask AJ, Jessup JA, Chappell MC, Ferrario CM. Angiotensin-(1-12) is an alternate substrate for angiotensin peptide production in the heart. Am J Physiol Heart Circ Physiol. 2008;294:H2242–H2247. doi: 10.1152/ajpheart.00175.2008. The assessment of the effluent of isolated perfused hearts mounted on a Langendorff apparatus in three normotensive and two hypertensive strains (Sprague-Dawley, Lewis, congenic mRen2.Lewis, Wistar-Kyoto, and spontaneously hypertensive rats) showed that Ang-(1-12) is processed into biologically active angiotensin peptides by a non-renin dependent mechanism. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferrario CM, Trask AJ, Jessup JA. Advances in biochemical and functional roles of angiotensin-converting enzyme 2 and angiotensin-(1-7) in regulation of cardiovascular function. Am J Physiol Heart Circ Physiol. 2005;289:H2281–H2290. doi: 10.1152/ajpheart.00618.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferrario CM, Varagic J, Habibi J, Nagata S, Kato J, Chappell MC, Trask AJ, Kitamura K, Whaley-Connell A, Sowers JR. Differential regulation of angiotensin-(1-12) in plasma and cardiac tissue in response to bilateral nephrectomy. Am J Physiol Heart Circ Physiol. 2009;296:H1184–H1192. doi: 10.1152/ajpheart.01114.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferrario CM, Varagic J. The ANG-(1-7)/ACE2/mas axis in the regulation of nephron function. Am J Physiol Renal Physiol. 2010;298:F1297–F1305. doi: 10.1152/ajprenal.00110.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Santos RA. Angiotensin-(1-7) is an endogenous ligand for the G protein-coupled receptor Mas. Proc Natl Acad Sci USA. 2003;100:8258–8263. doi: 10.1073/pnas.1432869100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Santos RA, Castro CH, Gava E, Pinheiro SV, Almeida AP, Paula RD, Cruz JS, Ramos AS, Rosa KT, Irigoyen MC, Bader M, Alenina N, Kitten GT, Ferreira AJ. Impairment of in vitro and in vivo heart function in angiotensin-(1-7) receptor MAS knockout mice. Hypertension. 2006;47:996–1002. doi: 10.1161/01.HYP.0000215289.51180.5c. [DOI] [PubMed] [Google Scholar]
- 12.Tallant EA, Ferrario CM, Gallagher PE. Angiotensin-(1-7) inhibits growth of cardiac myocytes through activation of the mas receptor. Am J Physiol Heart Circ Physiol. 2005;289:H1560–H1566. doi: 10.1152/ajpheart.00941.2004. [DOI] [PubMed] [Google Scholar]
- 13.Bunnemann B, Fuxe K, Metzger R, Mullins J, Jackson TR, Hanley MR, Ganten D. Autoradiographic localization of mas proto-oncogene mRNA in adult rat brain using in situ hybridization. Neurosci Lett. 1990;114:147–153. doi: 10.1016/0304-3940(90)90063-f. [DOI] [PubMed] [Google Scholar]
- 14.Metzger R, Bader M, Ludwig T, Berberich C, Bunnemann B, Ganten D. Expression of the mouse and rat mas proto-oncogene in the brain and peripheral tissues. FEBS Lett. 1995;357:27–32. doi: 10.1016/0014-5793(94)01292-9. [DOI] [PubMed] [Google Scholar]
- 15.Kostenis E, Milligan G, Christopoulos A, Sanchez-Ferrer CF, Heringer-Walther S, Sexton PM, Gembardt F, Kellett E, Martini L, Vanderheyden P, Schultheiss HP, Walther T. G-protein-coupled receptor Mas is a physiological antagonist of the angiotensin II type 1 receptor. Circulation. 2005;111:1806–1813. doi: 10.1161/01.CIR.0000160867.23556.7D. [DOI] [PubMed] [Google Scholar]
- 16.Benter IF, Diz DI, Ferrario CM. Cardiovascular actions of angiotensin(1-7) Peptides. 1993;14:679–684. doi: 10.1016/0196-9781(93)90097-z. [DOI] [PubMed] [Google Scholar]
- 17*.Burgelova M, Vanourkova Z, Thumova M, Dvorak P, Opocensky M, Kramer HJ, Zelizko M, Maly J, Bader M, Cervenka L. Impairment of the angiotensin-converting enzyme 2-angiotensin-(1-7)-Mas axis contributes to the acceleration of two-kidney, one-clip Goldblatt hypertension. J Hypertens. 2009;27:1988–2000. doi: 10.1097/HJH.0b013e32832f0d06. The authors demonstrate worsening of experimental renal hypertension following chronic inhibition of ACE2 in the rat. [DOI] [PubMed] [Google Scholar]
- 18.Dhaunsi GS, Yousif MH, Akhtar S, Chappell MC, Diz DI, Benter IF. Angiotensin-(1-7) prevents diabetes-induced attenuation in PPAR-gamma and catalase activities. Eur J Pharmacol. 2010;638:108–114. doi: 10.1016/j.ejphar.2010.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Durand MJ, Raffai G, Weinberg BD, Lombard JH. Angiotensin 1-7 and low dose angiotensin II infusion reverse salt-induced endothelial dysfunction via different mechanisms in rat middle cerebral arteries. Am J Physiol Heart Circ Physiol. 2010 doi: 10.1152/ajpheart.00328.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20*.Giani JF, Munoz MC, Mayer MA, Veiras LC, Arranz C, Taira CA, Turyn D, Toblli JE, Dominici FP. Angiotensin-(1-7) improves cardiac remodeling and inhibits growth-promoting pathways in the heart of fructose-fed rats. Am J Physiol Heart Circ Physiol. 2010;298:H1003–H1013. doi: 10.1152/ajpheart.00803.2009. In a rat experimental model of insulin resistance, a 2 week infusion of angiotensin-(1-7) blunts the elevation in blood pressure induced by fructose feeding and reduced cardiac phosphorylation levels of ERK1/2, JNK1/2, and p38MAPK. [DOI] [PubMed] [Google Scholar]
- 21.Grobe JL, Mecca AP, Lingis M, Shenoy V, Bolton TA, Machado JM, Speth RC, Raizada MK, Katovich MJ. Prevention of angiotensin II-induced cardiac remodeling by angiotensin-(1-7) Am J Physiol Heart Circ Physiol. 2007;292:H736–H742. doi: 10.1152/ajpheart.00937.2006. [DOI] [PubMed] [Google Scholar]
- 22.Iyer SN, Ferrario CM, Chappell MC. Angiotensin-(1-7) contributes to the antihypertensive effects of blockade of the renin-angiotensin system. Hypertension. 1998;31:356–361. doi: 10.1161/01.hyp.31.1.356. [DOI] [PubMed] [Google Scholar]
- 23.Iyer SN, Averill DB, Chappell MC, Yamada K, Allred AJ, Ferrario CM. Contribution of angiotensin-(1-7) to blood pressure regulation in salt-depleted hypertensive rats. Hypertension. 2000;36:417–422. doi: 10.1161/01.hyp.36.3.417. [DOI] [PubMed] [Google Scholar]
- 24*.Mercure C, Yogi A, Callera GE, Aranha AB, Bader M, Ferreira AJ, Santos RA, Walther T, Touyz RM, Reudelhuber TL. Angiotensin(1-7) blunts hypertensive cardiac remodeling by a direct effect on the heart. Circ Res. 2008;103:1319–1326. doi: 10.1161/CIRCRESAHA.108.184911. Chronic overproduction of either Ang II or Ang-(1-7) in the heart of transgenic mice was tested on age-related contractility and on cardiac remodeling in response to a hypertensive challenge. The results indicate reduction in ventricular hypertrophy and fibrosis as a result of an 8-fold increase in cardiac Ang-(1-7). [DOI] [PubMed] [Google Scholar]
- 25.Nie W, Yan H, Li S, Zhang Y, Yu F, Zhu W, Fan F, Zhu J. Angiotensin-(1-7) enhances angiotensin II induced phosphorylation of ERK1/2 in mouse bone marrow-derived dendritic cells. Mol Immunol. 2009;46:355–361. doi: 10.1016/j.molimm.2008.10.022. [DOI] [PubMed] [Google Scholar]
- 26**.Wang Y, Qian C, Roks AJ, Westermann D, Schumacher SM, Escher F, Schoemaker RG, Reudelhuber TL, van Gilst WH, Schultheiss HP, Tschope C, Walther T. Circulating rather than cardiac angiotensin-(1-7) stimulates cardioprotection after myocardial infarction. Circ Heart Fail. 2010;3:286–293. doi: 10.1161/CIRCHEARTFAILURE.109.905968. This important study shows that Ang-(1-7) stimulates the proliferation of endothelial progenitor cells. [DOI] [PubMed] [Google Scholar]
- 27*.Wysocki J, Ye M, Rodriguez E, Gonzalez-Pacheco FR, Barrios C, Evora K, Schuster M, Loibner H, Brosnihan KB, Ferrario CM, Penninger JM, Batlle D. Targeting the degradation of angiotensin II with recombinant angiotensin-converting enzyme 2: prevention of angiotensin II-dependent hypertension. Hypertension. 2010;55:90–98. doi: 10.1161/HYPERTENSIONAHA.109.138420. Soluble human recombinant ACE2 (hrACE2) infusion normalizes the systolic blood pressure and plasma angiotensin II of conscious mice by a mechanism that appears to be independent of blockade of Mas receptor as interpreted from the results obtained by co-administration of the angiotensin-(1-7) antagonist, A779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28*.Zhang J, Noble NA, Border WA, Huang Y. Infusion of angiotensin-(1-7) reduces glomerulosclerosis through counteracting angiotensin II in experimental glomerulonephritis. Am J Physiol Renal Physiol. 2010;298:F579–F588. doi: 10.1152/ajprenal.00548.2009. An experimental form of glomerulonephritis induced by administration of the monoclonal anti-Thy-1 antibody, OX-7 provided a model to study the anti-fibrotic actions of infused angiotensin-(1-7). The authors showed that this treatment reduced disease-induced increases in proteinuria, glomerular periodic acid Schiff staining, collagen I, and fibronectin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zimpelmann J, Burns KD. Angiotensin-(1-7) activates growth-stimulatory pathways in human mesangial cells. Am J Physiol Renal Physiol. 2009;296:F337–F346. doi: 10.1152/ajprenal.90437.2008. [DOI] [PubMed] [Google Scholar]
- 30.Eatman D, Wang M, Socci RR, Thierry-Palmer M, Emmett N, Bayorh MA. Gender differences in the attenuation of salt-induced hypertension by angiotensin (1-7) Peptides. 2001;22:927–933. doi: 10.1016/s0196-9781(01)00404-1. [DOI] [PubMed] [Google Scholar]
- 31.Ebermann L, Spillmann F, Sidiropoulos M, Escher F, Heringer-Walther S, Schultheiss HP, Tschope C, Walther T. The angiotensin-(1-7) receptor agonist AVE0991 is cardioprotective in diabetic rats. Eur J Pharmacol. 2008;590:276–280. doi: 10.1016/j.ejphar.2008.05.024. [DOI] [PubMed] [Google Scholar]
- 32.Fraga-Silva RA, Pinheiro SV, Goncalves AC, Alenina N, Bader M, Santos RA. The antithrombotic effect of angiotensin-(1-7) involves mas-mediated NO release from platelets. Mol Med. 2008;14:28–35. doi: 10.2119/2007-00073.Fraga-Silva. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gironacci MM, Valera MS, Yujnovsky I, Pena C. Angiotensin-(1-7) inhibitory mechanism of norepinephrine release in hypertensive rats. Hypertension. 2004;44:783–787. doi: 10.1161/01.HYP.0000143850.73831.9d. [DOI] [PubMed] [Google Scholar]
- 34.Heitsch H, Brovkovych S, Malinski T, Wiemer G. Angiotensin-(1-7)-Stimulated Nitric Oxide and Superoxide Release From Endothelial Cells. Hypertension. 2001;37:72–76. doi: 10.1161/01.hyp.37.1.72. [DOI] [PubMed] [Google Scholar]
- 35.Kozlovski VI, Lomnicka M, Fedorowicz A, Chlopicki S. On the mechanism of coronary vasodilation induced by angiotensin-(1-7) in the isolated guinea pig heart. Basic Clin Pharmacol Toxicol. 2007;100:361–365. doi: 10.1111/j.1742-7843.2007.00057.x. [DOI] [PubMed] [Google Scholar]
- 36.Porsti I, Bara AT, Busse R, Hecker M. Release of nitric oxide by angiotensin-(1-7) from porcine coronary endothelium: implications for a novel angiotensin receptor. Br J Pharmacol. 1994;111:652–654. doi: 10.1111/j.1476-5381.1994.tb14787.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rajendran S, Chirkov YY, Campbell DJ, Horowitz JD. Angiotensin-(1-7) enhances anti-aggregatory effects of the nitric oxide donor sodium nitroprusside. J Cardiovasc Pharmacol. 2005;46:459–463. doi: 10.1097/01.fjc.0000176729.51819.a6. [DOI] [PubMed] [Google Scholar]
- 38.Sampaio WO, Souza dos Santos RA, Faria-Silva R, da Mata Machado LT, Schiffrin EL, Touyz RM. Angiotensin-(1-7) through receptor Mas mediates endothelial nitric oxide synthase activation via Akt-dependent pathways. Hypertension. 2007;49:185–192. doi: 10.1161/01.HYP.0000251865.35728.2f. [DOI] [PubMed] [Google Scholar]
- 39.Zhang Y, Lu J, Shi J, Lin X, Dong J, Zhang S, Liu Y, Tong Q. Central administration of angiotensin-(1-7) stimulates nitric oxide release and upregulates the endothelial nitric oxide synthase expression following focal cerebral ischemia/reperfusion in rats. Neuropeptides. 2008;42:593–600. doi: 10.1016/j.npep.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 40.Zhi JM, Chen RF, Wang J, Jiao XY, Zhao RR. Comparative studies of vasodilating effects of angiotensin-(1-7) on the different vessels. Sheng Li Xue Bao. 2004;56:730–734. [PubMed] [Google Scholar]
- 41.Almeida AP, Frabregas BC, Madureira MM, Santos RJ, Campagnole-Santos MJ, Santos RA. Angiotensin-(1-7) potentiates the coronary vasodilatatory effect of bradykinin in the isolated rat heart. Braz J Med Biol Res. 2000;33:709–713. doi: 10.1590/s0100-879x2000000600012. [DOI] [PubMed] [Google Scholar]
- 42.Brosnihan KB, Li P, Ferrario CM. Angiotensin-(1-7) dilates canine coronary arteries through kinins and nitric oxide. Hypertension. 1996;27:523–528. doi: 10.1161/01.hyp.27.3.523. [DOI] [PubMed] [Google Scholar]
- 43.Fernandes L, Fortes ZB, Nigro D, Tostes RC, Santos RA, Catelli De Carvalho MH. Potentiation of bradykinin by angiotensin-(1-7) on arterioles of spontaneously hypertensive rats studied in vivo. Hypertension. 2001;37:703–709. doi: 10.1161/01.hyp.37.2.703. [DOI] [PubMed] [Google Scholar]
- 44.Gorelik G, Carbini LA, Scicli AG. Angiotensin 1-7 induces bradykinin-mediated relaxation in porcine coronary artery. J Pharmacol Exp Ther. 1998;286:403–410. [PubMed] [Google Scholar]
- 45.Nakamoto H, Ferrario CM, Fuller SB, Robaczewski DL, Winicov E, Dean RH. Angiotensin-(1-7) and nitric oxide interaction in renovascular hypertension. Hypertension. 1995;25:796–802. doi: 10.1161/01.hyp.25.4.796. [DOI] [PubMed] [Google Scholar]
- 46.Oliveira MA, Fortes ZB, Santos RA, Kosla MC, de Carvalho MH. Synergistic effect of angiotensin-(1-7) on bradykinin arteriolar dilation in vivo. Peptides. 1999;20:1195–1201. doi: 10.1016/s0196-9781(99)00123-0. [DOI] [PubMed] [Google Scholar]
- 47*.Munoz MC, Giani JF, Dominici FP. Angiotensin-(1-7) stimulates the phosphorylation of Akt in rat extracardiac tissues in vivo via receptor Mas. Regul Pept. 2010;161:1–7. doi: 10.1016/j.regpep.2010.02.001. From studies assesing mediators of insulin sensitivity, the authors conclude that angiotensin-(1-7) has a beneficial positive effect on insulin through phosphorylation of AKt signaling pathways at the level of the Mas receptor. [DOI] [PubMed] [Google Scholar]
- 48.Tallant EA, Clark MA. Molecular mechanisms of inhibition of vascular growth by angiotensin-(1-7) Hypertension. 2003;42:574–579. doi: 10.1161/01.HYP.0000090322.55782.30. [DOI] [PubMed] [Google Scholar]
- 49.Tallant EA, Gallagher PE. Angiotensin-(1-7) upregulates the mitogen-activated DUSP-1 in vascular smooth muscle cells. Hypertension. 2007;50 [Google Scholar]
- 50.Angiotensin-(1-7) increases DUSP-1 and reduces the endothelin-1 mediated increase in COX-2 and PGES-1 in cardiac fibroblasts. Hypertension. 2009 [Google Scholar]
- 51.Ishiyama Y, Gallagher PE, Averill DB, Tallant EA, Brosnihan KB, Ferrario CM. Upregulation of angiotensin-converting enyzme 2 after myocardial infarction by blockade of angiotensin II receptors. Hypertension. 2004;43:970–976. doi: 10.1161/01.HYP.0000124667.34652.1a. [DOI] [PubMed] [Google Scholar]
- 52.Gallagher PE, Chappell MC, Ferrario CM, Tallant EA. Distinct roles for ANG II and ANG-(1-7) in the regulation of angiotensin-converting enzyme 2 in rat astrocytes. Am J Physiol Cell Physiol. 2006;290:C420–C426. doi: 10.1152/ajpcell.00409.2004. [DOI] [PubMed] [Google Scholar]
- 53.Gallagher PE, Ferrario CM, Tallant EA. Regulation of ACE2 in cardiac myocytes and fibroblasts. Am J Physiol Heart Circ Physiol. 2008;295:H2373–H2379. doi: 10.1152/ajpheart.00426.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54*.Lin CS, Pan CH, Wen CH, Yang TH, Kuan TC. Regulation of angiotensin converting enzyme II by angiotensin peptides in human cardiofibroblasts. Peptides. 2010;31:1334–1340. doi: 10.1016/j.peptides.2010.03.026. Contrasting with the findings of other studies, the authors report that both angiotensin II and angiotensin-(1-7)upregulates ACE2 expression in human cardiac fibroblasts. [DOI] [PubMed] [Google Scholar]
- 55.Ferrario CM, Jessup J, Chappell MC, Averill DB, Brosnihan KB, Tallant EA, Diz DI, Gallagher PE. Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation. 2005;111:2605–2610. doi: 10.1161/CIRCULATIONAHA.104.510461. [DOI] [PubMed] [Google Scholar]
- 56.Igase M, Strawn WB, Gallagher PE, Geary RL, Ferrario CM. Angiotensin II AT1 receptors regulate ACE2 and angiotensin-(1-7) expression in the aorta of spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol. 2005;289:H1013–H1019. doi: 10.1152/ajpheart.00068.2005. [DOI] [PubMed] [Google Scholar]
- 57.Igase M, Kohara K, Nagai T, Miki T, Ferrario CM. Increased expression of angiotensin converting enzyme 2 in conjunction with reduction of neointima by angiotensin II type 1 receptor blockade. Hypertens Res. 2008;31:553–559. doi: 10.1291/hypres.31.553. [DOI] [PubMed] [Google Scholar]
- 58.Ferrario CM, Chappell MC, Tallant EA, Brosnihan KB, Diz DI. Counterregulatory actions of angiotensin-(1-7) Hypertension. 1997;30:535–541. doi: 10.1161/01.hyp.30.3.535. [DOI] [PubMed] [Google Scholar]
- 59.Schindler C, Bramlage P, Kirch W, Ferrario CM. Role of the vasodilator peptide angiotensin-(1-7) in cardiovascular drug therapy. Vasc Health Risk Manag. 2007;3:125–137. [PMC free article] [PubMed] [Google Scholar]
- 60.Ferreira AJ, Raizada MK. Are we poised to target ACE2 for the next generation of antihypertensives? J Mol Med. 2008;86:685–690. doi: 10.1007/s00109-008-0339-x. [DOI] [PubMed] [Google Scholar]
- 61.Iusuf D, Henning RH, van Gilst WH, Roks AJ. Angiotensin-(1-7): pharmacological properties and pharmacotherapeutic perspectives. Eur J Pharmacol. 2008;585:303–312. doi: 10.1016/j.ejphar.2008.02.090. [DOI] [PubMed] [Google Scholar]
- 62.Santos RA, Ferreira AJ, Pinheiro SV, Sampaio WO, Touyz R, Campagnole-Santos MJ. Angiotensin-(1-7) and its receptor as a potential targets for new cardiovascular drugs. Expert Opin Investig Drugs. 2005;14:1019–1031. doi: 10.1517/13543784.14.8.1019. [DOI] [PubMed] [Google Scholar]
- 63.Luque M, Martin P, Martell N, Fernandez C, Brosnihan KB, Ferrario CM. Effects of captopril related to increased levels of prostacyclin and angiotensin-(1-7) in essential hypertension. J Hypertens. 1996;14:799–805. doi: 10.1097/00004872-199606000-00017. [DOI] [PubMed] [Google Scholar]
- 64.Ferrario CM, Martell N, Yunis C, Flack JM, Chappell MC, Brosnihan KB, Dean RH, Fernandez A, Novikov SV, Pinillas C, Luque M. Characterization of angiotensin-(1-7) in the urine of normal and essential hypertensive subjects. Am J Hypertens. 1998;11:137–146. doi: 10.1016/s0895-7061(97)00400-7. [DOI] [PubMed] [Google Scholar]
- 65.Schindler C, Brosnihan KB, Ferrario CM, Bramlage P, Maywald U, Koch R, Oertel R, Kirch W. Comparison of inhibitory effects of irbesartan and atorvastatin treatment on the renin angiotensin system (RAS) in veins: a randomized double-blind crossover trial in healthy subjects. J Clin Pharmacol. 2007;47:112–120. doi: 10.1177/0091270006294280. [DOI] [PubMed] [Google Scholar]
- 66.Ferrario CM, Smith RD, Brosnihan B, Chappell MC, Campese VM, Vesterqvist O, Liao WC, Ruddy MC, Grim CE. Effects of omapatrilat on the renin-angiotensin system in salt-sensitive hypertension. Am J Hypertens. 2002;15:557–564. doi: 10.1016/s0895-7061(02)02268-9. [DOI] [PubMed] [Google Scholar]
- 67.Kocks MJ, Lely AT, Boomsma F, de Jong PE, Navis G. Sodium status and angiotensin-converting enzyme inhibition: effects on plasma angiotensin-(1-7) in healthy man. J Hypertens. 2005;23:597–602. doi: 10.1097/01.hjh.0000160217.86597.b6. [DOI] [PubMed] [Google Scholar]
- 68.Kobori H, Nangaku M, Navar LG, Nishiyama A. The intrarenal renin-angiotensin system: from physiology to the pathobiology of hypertension and kidney disease. Pharmacol Rev. 2007;59:251–287. doi: 10.1124/pr.59.3.3. [DOI] [PubMed] [Google Scholar]
- 69.Navar LG. The intrarenal renin-angiotensin system in hypertension. Kidney Int. 2004;65:1522–1532. doi: 10.1111/j.1523-1755.2004.00539.x. [DOI] [PubMed] [Google Scholar]
- 70.Pendergrass KD, Pirro NT, Westwood BM, Ferrario CM, Brosnihan KB, Chappell MC. Sex differences in circulating and renal angiotensins of hypertensive mRen(2). Lewis but not normotensive Lewis rats. Am J Physiol Heart Circ Physiol. 2008;295:H10–H20. doi: 10.1152/ajpheart.01277.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ferrario CM, Jessup J, Gallagher PE, Averill DB, Brosnihan KB, Ann TE, Smith RD, Chappell MC. Effects of renin-angiotensin system blockade on renal angiotensin-(1-7) forming enzymes and receptors. Kidney Int. 2005;68:2189–2196. doi: 10.1111/j.1523-1755.2005.00675.x. [DOI] [PubMed] [Google Scholar]
- 72.Chappel MC, Ferrario CM. ACE and ACE2: their role to balance the expression of angiotensin II and angiotensin-(1-7) Kidney Int. 2006;70:8–10. doi: 10.1038/sj.ki.5000321. [DOI] [PubMed] [Google Scholar]
- 73.Dilauro M, Burns KD. Angiotensin-(1-7) and its effects in the kidney. ScientificWorldJournal. 2009;9:522–535. doi: 10.1100/tsw.2009.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Koitka A, Cooper ME, Thomas MC, Tikellis C. Angiotensin converting enzyme 2 in the kidney. Clin Exp Pharmacol Physiol. 2008;35:420–425. doi: 10.1111/j.1440-1681.2008.04889.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lely AT, Hamming I, van GH, Navis GJ. Renal ACE2 expression in human kidney disease. J Pathol. 2004;204:587–593. doi: 10.1002/path.1670. [DOI] [PubMed] [Google Scholar]
- 76.Mizuiri S, Hemmi H, Arita M, Ohashi Y, Tanaka Y, Miyagi M, Sakai K, Ishikawa Y, Shibuya K, Hase H, Aikawa A. Expression of ACE and ACE2 in individuals with diabetic kidney disease and healthy controls. Am J Kidney Dis. 2008;51:613–623. doi: 10.1053/j.ajkd.2007.11.022. [DOI] [PubMed] [Google Scholar]
- 77*.Reich HN, Oudit GY, Penninger JM, Scholey JW, Herzenberg AM. Decreased glomerular and tubular expression of ACE2 in patients with type 2 diabetes and kidney disease. Kidney Int. 2008;74:1610–1616. doi: 10.1038/ki.2008.497. Renal biopsies document reduced renal expression of ACE2 in type 2 diabetic patients. [DOI] [PubMed] [Google Scholar]
- 78.Oudit GY, Herzenberg AM, Kassiri Z, Wong D, Reich H, Khokha R, Crackower MA, Backx PH, Penninger JM, Scholey JW. Loss of angiotensin-converting enzyme-2 leads to the late development of angiotensin II-dependent glomerulosclerosis. Am J Pathol. 2006;168:1808–1820. doi: 10.2353/ajpath.2006.051091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Takahashi Y, Haga S, Ishizaka Y, Mimori A. Autoantibodies to angiotensin-converting enzyme 2 in patients with connective tissue diseases. Arthritis Res Ther. 2010;12:R85. doi: 10.1186/ar3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pignone A, Rosso AD, Brosnihan KB, Perfetto F, Livi R, Fiori G, Guiducci S, Cinelli M, Rogai V, Tempestini A, Bartoli F, Generini S, Ferrario CM, Cerinic MM. Reduced circulating levels of angiotensin-(1–7) in systemic sclerosis: a new pathway in the dysregulation of endothelial-dependent vascular tone control. Ann Rheum Dis. 2007;66:1305–1310. doi: 10.1136/ard.2006.064493. [DOI] [PMC free article] [PubMed] [Google Scholar]


