Abstract
Ongoing drug use during opioid agonist treatment (OAT) negatively affects treatment and health outcomes, and increases treatment dropout. This study aimed to examine correlates of concurrent illicit drug use among OAT patients in Ukraine. A random sample of 434 patients currently on OAT receiving buprenorphine (BMT) or methadone maintenance treatment (MMT) from five cities in Ukraine were assessed for factors associated with self-reported concurrent illicit drug use during OAT using a multivariable logistic regression. Among 434 OAT patients, 100 (23%) reported concurrent drug injecting in the previous 30 days; 28% of these were injecting ≥20 days. While 100 (100%) of these injected opioids, 24 (24%) injected stimulants; 40 (40%) met criteria for polysubstance use disorder that included opioids, stimulants and alcohol. Independent correlates of concurrent drug injection included: being on MMT vs. BMT (aOR=2.8, 95%CI=1.4–5.8), lower OAT dosage (aOR=1.7, 95%CI=1.1–2.7), more severe addiction severity (aOR=2.3, 95%CI=1.4–3.8), younger age of injection initiation (aOR=2.3, 95%CI=1.3–3.9), and presence of alcohol use disorder (aOR=2.1, 95%CI=1.3–3.5); participants living with parents were negatively associated with concurrent drug injection. Concurrent drug use was prevalent among OAT patients in Ukraine indicating the urgent needs for tailored interventions and changes in OAT program design and implementation. Results highlight the importance of prescribing an adequate OAT dosage, and discrepancies between MMT and BMT programs in Ukraine addressing needs of PWID with specific characteristics such as severe opioid and alcohol dependence.
Keywords: concurrent drug injecting, opioid agonist therapy, Ukraine
1. Introduction
Opioid agonist therapy (OAT) using methadone or buprenorphine is the most cost-effective strategy to reduce HIV infections and one of the most effective treatments for PWID with opioid use disorders (Connock et al., 2007). OAT benefits include 54% reduction in risk of HIV infection (MacArthur et al., 2012), 46% reduction in opioid use (Mattick, Breen, Kimber, & Davoli, 2009), and overdose (L. R. Gowing, Hickman, & Degenhardt, 2013), and 29–36% reduction in criminal activity (Holloway, Bennett, & Farrington, 2006). In people living with HIV, OAT improves HIV treatment outcomes along the HIV care continuum (Low et al., 2016).
According to the available estimates, Ukraine has the highest HIV burden in Europe (Degenhardt et al., 2014; Joint United Nations Programme on HIV/AIDS (UNAIDS), 2013) mainly concentrated in people who inject drugs (PWID)(United Nations Office on Drugs and Crime (UNODC), 2016). In addition to unsafe injecting practices, PWID in Ukraine are also engaged in risky sexual behavior that put their partners at a greater risk of HIV infection (Mazhnaya et al., 2014; Taran, Johnston, Pohorila, & Saliuk, 2011).
Unlike much of the world where OAT is used to treat opioid use disorders, OAT was introduced in Ukraine to prevent HIV in PWID (Bruce, Dvoryak, Sylla, & Altice, 2007). Buprenorphine was introduced in Ukraine in 2004 (Bruce et al., 2007) followed by methadone as a more cost-effective option in 2008 (Schaub, Chtenguelov, Subata, Weiler, & Uchtenhagen, 2010). Despite considerable clinical promise, there are numerous barriers to OAT scale-up in Ukraine (Bojko et al., 2015; Bojko et al., 2016; Makarenko et al., 2016; Mazhnaya et al., 2016). Coverage with OAT remains limited – among the estimated 340,000 PWID in Ukraine (Alliance for Public Health, 2017) less than 3% of PWIDs receive OAT within the national program (Ukrainian Center for Disease Control (UCDC), 2016). Mathematical modeling for Ukraine suggests that at least 25% coverage with OAT is needed to effectively reduce the HIV epidemic (Alistar, Owens, & Brandeau, 2011). Besides structural-level factors, a number of individual-level barriers such as negative attitudes towards OAT mostly based on myths and beliefs also decrease willingness of PWID to enroll into the treatment and constrain OAT expansion in Ukraine (Makarenko et al., 2016).
Scaling up OAT also involves a focus on retention in treatment. Opioid use disorder is a chronic disease and may require life-long management (McLellan, Lewis, O’Brien, & Kleber, 2000). Consequently, treatments like OAT requires long-term or even life-long adherence to methadone or buprenorphine prescribed at an adequate dosage. (Bojko et al., 2015; Bojko et al., 2016; Joseph, Stancliff, & Langrod, 2000; Mattick et al., 2009). Dosing adequacy, a relatively new concept, has emerged as a strategy to promote retention (Reimer et al., 2014) and improve treatment outcomes. Dosing adequacy balances the amount needed to alleviate withdrawal symptoms, eliminate ongoing illicit opioid use, and markedly reduce craving while avoiding signs of opioid excess that can be measured objectively with validated instruments. (Gardini, Poehlke, Reimer, Walcher, & Weber, 2010; González-Saiz et al., 2008) Longer retention in treatment is critical to achieving optimal outcomes in OAT (Fareed, Casarella, Amar, Vayalapalli, & Drexler, 2009; L. Gowing, Ali, & White, 2009; Hser, Evans, Huang, & Anglin, 2004).
Concurrent drug use is often cited as a major factor contributing to dropout from OAT (Lin et al., 2010; E. W. Liu et al., 2008; Raffa et al., 2007). The precise relationship of concurrent drug use, retention, OAT dosage and other intervening factors is complex (Raffa et al., 2007) and not examined in the Ukrainian context. In other settings, concurrent drug use while on OAT is common (Li, Lin, Wan, Zhang, & Lai, 2012; Luo et al., 2016; Tran et al., 2012). Elsewhere, other factors are associated with concomitant drug use, including female sex (Cao et al., 2010; Chen, Xia, Hong, Hall, & Ling, 2013; Kamal et al., 2007; Li et al., 2012), mental health problems (Ilgen, Jain, Kim, & Trafton, 2008), social networks of PWID (Li et al., 2012; Tuten & Jones, 2003), and program-related factors like suboptimal OAT dosage (Bao et al., 2009; E. Liu et al., 2009), and lack of available psychological counseling or comprehensive services (Joe, Simpson, Dansereau, & Rowan-Szal, 2001; Lin et al., 2010).
To better understand the Ukrainian context, we examined data on concurrent drug use from a random sample of OAT patients in five major cities in Ukraine to explore the complex inter-relationship on concurrent drug injection and other factors that might assist clinicians and policy makers to help guide OAT scale-up in a region where HIV incidence and mortality is increasing (Joint United Nations Programme on HIV/AIDS (UNAIDS), 2016a, 2016b) in the absences of sub-optimally scaled HIV prevention.
2. Methods
2.1. Study Setting
At the time of the study, Order 200, the major governmental policy governing OAT delivery greatly influenced OAT expansion. This policy required that patients interested in OAT must be officially “registered” as a drug dependent person, which resulted in revocation of a driver’s license and restrictions for many types of employment. Patients receiving treatment also must clear numerous administrative hurdles to initiate OAT, and once started, must have their medication administration supervised 7 days per week. OAT dosing in most settings is highly restricted to a few hours each morning and no take-home doses were allowed. Each region of the country had autonomy to interpret Order 200 with high variability. Integrated care for PWID to receive OAT, HIV and tuberculosis services is recommended and implemented in many settings (Bachireddy et al., 2014).
2.2. Study Sample
Data for this analysis included 434 PWID currently receiving OAT from a cross-sectional study of 1,613 opioid dependent PWID (currently, previously or never on OAT) from 5 regions in Ukraine. A detailed description of the study methods is presented elsewhere (Makarenko et al., 2016). The study participants were recruited using random sampling from pre-existing lists of OAT patients in five cities (Kyiv, Mykolaiv, Odesa, Dnipro and Lviv) from January 2014 – March 2015. The eligibility criteria included: ≥18 years; met ICD-10 criteria for opioid dependence; lived, worked or studied in the city where the survey was conducted; and provided informed consent for survey completion, including rapid HIV and HCV testing.
Measures
Participants completed a computer-assisted, self-administered instrument (CASI) survey using a Qualtrics® web-based platform. The questionnaire included sections related to socio-demographic characteristics, drug use and addiction severity (Skinner, 1982), experience and attitude towards OAT, injection and sexual risk behaviors, and alcohol use disorder using the Alcohol Use Disorder Identification Test-AUDIT (Babor, Higgins-Biddle, Saunders, & Monteiro, 2001), HIV testing and treatment experience and health-related quality of life (J. Ware, Jr., Kosinski, & Keller, 1996).
The primary outcome for this study was defined as self-report of injecting any drug in the past 30 days while receiving OAT. Additionally, frequent drug injection was defined as injecting any kind of illicit drugs ≥20 days in the last 30 days. Participants’ age and age of injection drug use initiation were stratified using the lowest quartiles for the sample (<32 years old or ≥32 years old and <16 or ≥16 years old, respectively). Income was categorized based on the average monthly wage (3500 UAH/~440 USD) (State Statistics Service of Ukraine, 2014) in Ukraine at the time of the data collection. Duration on OAT was analyzed as a continuous variable. Using standardized cut-offs, having an alcohol use disorder was defined as ≥8 for men and ≥4 for women on the AUDIT scale (Babor et al., 2001; Caviness et al., 2009). Moderate to severe depression was defined as ≥10 on the CES-D scale (Andresen, Malmgren, Carter, & Patrick, 1994; Zhang et al., 2012), and addiction severity was coded as severe if scores were ≥ 9 on the DAST-10 (Gavin, Ross, & Skinner, 1989). Health-related quality of life (HRQoL) was assessed using both physical and mental health summary scales from SF12v2 score (J. Ware, Kosinski, & Keller, 1998) and analyzed as continuous variable. OAT dosages were dichotomized by the median dose for methadone (<75 mg or ≥75 mg) or buprenorphine (<10 mg or ≥10 mg). In addition, we created two composite variables reflecting patients’ access to psychosocial and medical services at the OAT site. Receiving psychological counseling was defined if study participants reported at least one of the following services obtained on OAT site: help in finding a job or a place to live, help in getting social benefit payments, psychological counseling, or help with referral to medical services. Receiving other medical services at the OAT site was defined if patients received: HIV testing, CD4 monitoring, prescription of HIV medications, hepatitis B and/or C virus testing, tuberculosis testing, prescription of tuberculosis medications, testing and treatment for sexually transmitted infections, or antibiotics to treat cellulitis or abscesses.
2.3. Statistical Analysis
We examined a number of potentially important independent correlates of concurrent drug injection for this sample, which was guided by the literature as well as findings from other studies in the Ukrainian context. Bivariate associations between potential independent factors and the dependent variable were tested using Fisher’s exact tests with two-sided p-values generated at the 95% significance level. Bivariate and multivariable logistic regressions were used to identify factors associated with concurrent drug injection while being maintained on OAT. The multivariable regression model was adjusted for all covariates associated with the outcome in the bivariate analysis at p<0.1. The best fit model was identified using backward elimination and forward selection strategies, with variables retained in the model if they were independently associated with the outcome at p<0.05. Both selection strategies produced the same results. Model fit was assessed using a Chi-square goodness-of-fit.
Ethical approval
Institutional review boards at Yale University, New Haven, USA and the Gromashevskiy Institute at the National Academy of Medical Sciences in Ukraine approved the study protocol.
3. Results
The characteristics of the 434 PWID in the sample are summarized in Table 1. Participants were mostly men (78%) in their mid-thirties (median=36.0; IQR=32–43 years) with over half (53%) being unemployed; 86% earned wages below the national average. Prevalence of HIV and HCV infections were high among OAT patients enrolled in the study (45% and 70%, respectively). More than half (53%) of participants met criteria for severe addiction severity and 26.5% met screening criteria for having an alcohol use disorder. Overall, 100 (23%) of 434 participants reported injecting drugs at least once in the previous 30 days (Figure 1). Of these, however, 28 (6%) injected drugs frequently (≥20 days) during the past month. For individuals who recently injected drugs, all 100 injected opioids, but 24 of them also injected stimulants (amphetamine-type substances). Additionally, 40 (40%) of concurrent injectors injected more than one substance per day, including various types of opioids available in Ukraine (Table 2).
Table 1.
Characteristics of Patients Prescribed Opioid Agonist Therapies by Concurrent Drug Injection in the Previous 30 days (N=434)
| Characteristic | Total* | Injected any drug at least once in the previous 30 days** | ||
|---|---|---|---|---|
|
| ||||
| N=434 | Yes (N=100) |
No (N=334) |
P-value | |
|
| ||||
| N (%) | N (%) | N (%) | ||
|
| ||||
| City | <0.0001 | |||
| Kyiv | 140 (32.3) | 26 (18.6) | 114 (81.4) | |
| Odesa | 47 (10.8) | 13 (27.7) | 34 (72.3) | |
| Mykolaiv | 105 (24.2) | 43 (41.0) | 62 (59.0) | |
| Dnipro | 102 (23.5) | 11 (10.8) | 91 (89.2) | |
| Lviv | 40 (9.2) | 7 (17.5) | 33 (82.5) | |
|
| ||||
| Sex | 0.7106 | |||
| Male | 340 (78.3) | 77 (22.6) | 263 (77.4) | |
| Female | 94 (21.7) | 23 (24.5) | 71 (75.5) | |
|
| ||||
| Age | 0.0098 | |||
| <32 years | 100 (23.0) | 33 (33.0) | 67 (67.0) | |
| ≥32 years | 334 (77.03) | 67 (20.1) | 267 (79.9) | |
|
| ||||
| Living with husband/wife or permanent sexual partner | 0.7627 | |||
| Yes | 168 (38.7) | 40 (23.8) | 128 (76.2) | |
| No | 266 (61.3) | 60 (22.6) | 206 (77.4) | |
|
| ||||
| Have dependent children | 0.9403 | |||
| Yes | 175 (40.3) | 40 (22.9) | 135 (77.1) | |
| No | 259 (59.7) | 60 (23.2) | 199 (76.8) | |
|
| ||||
| Living with parents | 0.0346 | |||
| Yes | 183 (42.2) | 33 (18.0) | 150 (82.0) | |
| No | 251 (57.8) | 67 (26.7) | 184 (73.3) | |
|
| ||||
| Employment | 0.2284 | |||
| Full-time/part-time | 204 (47.0) | 41 (20.1) | 163 (79.9) | |
| Seasonal/day laborer | 70 (16.1) | 21 (30.0) | 49 (70.0) | |
| Not-employed | 160 (36.9) | 38 (23.7) | 122 (76.3) | |
|
| ||||
| Income | 0.0227 | |||
| >3500 UAH | 61 (14.1) | 21 (34.4) | 40 (65.6) | |
| ≤3500 UAH | 373 (85.9) | 79 (21.2) | 294 (78.8) | |
|
| ||||
| Age of drug injection initiation | 0.0023 | |||
| <16 years | 94 (21.7) | 33 (35.1) | 61 (64.9) | |
| ≥ 16 years | 340 (78.3) | 67 (19.7) | 273 (80.3) | |
|
| ||||
| High addiction severity | 0.0003 | |||
| Yes | 230 (53.0) | 69 (30.0) | 161 (70.0) | |
| No | 204 (47.0) | 31 (15.2) | 173 (84.9) | |
|
| ||||
| Moderate to severe depression | 0.2689 | |||
| Yes | 222 (51.1) | 56 (25.2) | 166 (74.8) | |
| No | 212 (48.9) | 44 (20.7) | 168 (79.3) | |
|
| ||||
| Alcohol use disorder | 0.0002 | |||
| Yes | 115 (26.5) | 41 (35.6) | 74 (64.3) | |
| No | 319 (73.5) | 59 (18.5) | 260 (81.5) | |
|
| ||||
| Tested HIV positive (N=433) | 0.2136 | |||
| Yes | 193 (44.6) | 50 (25.9) | 143 (74.1) | |
| No | 240 (55.4) | 50 (20.8) | 190 (79.2) | |
|
| ||||
| Tested hepatitis C positive (N=420) | 0.0411 | |||
| Yes | 288 (68.6) | 74 (25.7) | 214 (74.4) | |
| No | 132 (31.4) | 22 (16.7) | 110 (83.3) | |
|
| ||||
| Quality of life – median (IQR) | ||||
| Physical composite score | 17 (15–20) | 16 (15–19) | 17 (15–20) | 0.2976 |
| Mental composite score | 20 (17–23) | 19 (17–22) | 20 (17–23) | 0.0880 |
|
| ||||
| Experienced police harassment | ||||
| Yes | 319 (73.5) | 81 (25.4) | 238 (74.6) | 0.0539 |
| No | 115 (26.5) | 19 (16.5) | 96 (83.5) | |
Percentages are presented within the column.
Percentages for drug injection (Yes/No) are presented within the rows.
Figure 1. Any Recent Injection Drug Use and Frequency of Injection (N=434).
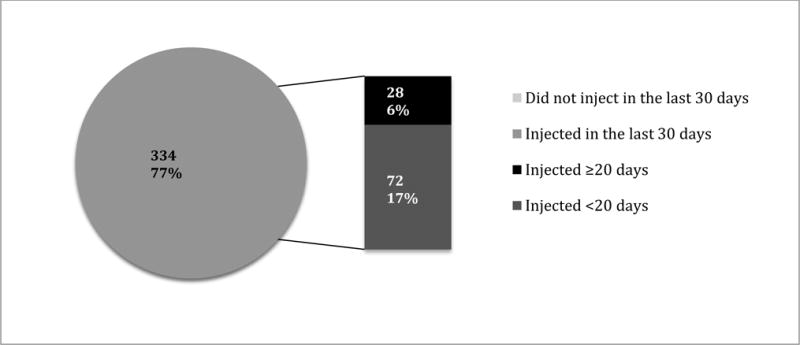
Source: Random sample of 434 PWID from a cross-sectional study conducted in 5 regions in Ukraine, 2014–2015 (Makarenko et al., 2016).
Table 2.
Types of illegal drugs used during OAT
| Type of drug injected | Injected in the last 30 days (N=100) | |
|---|---|---|
| N (%) | Median number of days when injected (IQR) | |
| Any opiates | 100 (100%) | – |
| Homemade opioids | 61 (61%) | 5 (2–15) |
| Poly-substance use (including alcohol) | 40 (40%) | 3 (1–7) |
| Medical opiates | 24 (25%) | 5 (2–18) |
| Stimulants | 24 (24%) | 3 (2–7.5) |
| Heroin | 17 (17%) | 3 (1–5) |
| Methadone | 16 (16%) | 4.5 (2–10) |
| Buprenorphine | 4 (4%) | 2.5 (1–17) |
The bivariate comparisons of those reporting recent concurrent drug injection is further described in Table 1. Of note, there were significant differences based on the city of recruitment, which is explained in Figure 2. For example in Lviv where only 7% of participants injected concurrently, 85% of participants were on higher OAT doses. In Mykolaiv where 41% concurrently injected, only 41% were on higher OAT doses. Other factors in bivariate analysis associated with concurrent drug injection were younger age, higher income, younger injection debut, higher levels of addiction severity, having an alcohol use disorder and having a reactive test for HCV infection. Additionally, those participants who were living with their parents were significantly less likely to concurrently inject drugs.
Figure 2. OAT Dose and Concurrent Drug use during OAT by Survey Site.
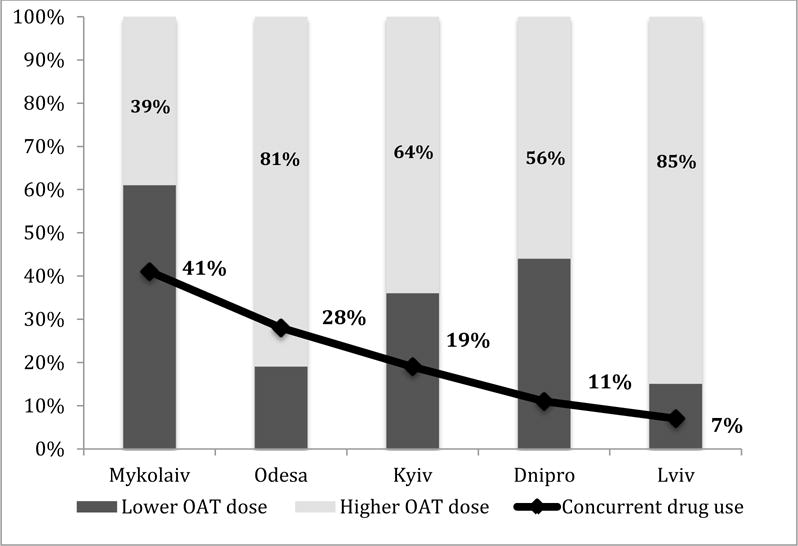
Source: Random sample of 434 PWID from a cross-sectional study conducted in 5 regions in Ukraine, 2014–2015 (Makarenko et al., 2016).
Legend: OAT: opioid agonist therapy; OAT dose: lower: <75 mg – methadone; <10 mg – buprenorphine, higher: ≥75 mg – methadone; ≥10 mg – buprenorphine.
To further explore factors associated with concurrent drug injection, we examined a number of OAT program-level characteristics (Table 3) associated with the primary outcome. Overall, most (79%) participants were prescribed MMT and their median duration on OAT was 36 months (IQR=17–62 months). Factors for this analysis associated with concurrent drug injection included being prescribed MMT rather than BMT (25% vs 11%), prescribed lower OAT doses (28% vs 19%), and experience of short interruptions during treatment (33% vs 21%).
Table 3.
Program-Level Characteristics of Patients Prescribed Opioid Agonist Therapies, by Drug Injection in the Previous 30 days (N=434)
| Characteristic | Total* | Injected any drug at least once in the last 30 days** | ||
|---|---|---|---|---|
|
| ||||
| N=434 | Yes (N=100) |
No (N=334) |
P-value | |
|
| ||||
| N (%) | N (%) | N (%) | ||
|
| ||||
| OAT type | 0.0019 | |||
| MMT | 334 (79.3) | 90 (26.2) | 254 (73.8) | |
| BMT | 90 (20.7) | 10 (11.1) | 80 (88.9) | |
|
| ||||
| Duration on OAT (months) – median (IQR) | 35.9 (17.2–61.8) | 33.4 (13.2–54.0) | 37.2 (18.1–63.0) | 0.0787 |
|
| ||||
| OAT dosage | 0.0383 | |||
| Lower | 174 (40.1) | 49 (28.2) | 125 (71.8) | |
| Higher | 260 (59.9) | 51 (19.6) | 209 (80.4) | |
|
| ||||
| Short interruptions in OAT (<10 days) | 0.0126 | |||
| Yes | 84 (19.3) | 28 (33.3) | 56 (66.7) | |
| No | 350 (80.7) | 72 (20.6) | 278 (79.4) | |
|
| ||||
| Any side effect after taking OAT | 0.3682 | |||
| Yes | 76 (17.5) | 14 (18.4) | 62 (81.6) | |
| No | 358 (82.5) | 86 (24.0) | 272 (76.0) | |
|
| ||||
| Family/friends support OAT | 0.0634 | |||
| Yes | 277 (63.8) | 56 (20.2) | 221 (79.8) | |
| No | 157 (36.2) | 44 (28.0) | 113 (72.0) | |
|
| ||||
| Receive psychosocial counseling on OAT site | 0.4842 | |||
| Yes | 278 (64.1) | 67 (24.1) | 211 (75.9) | |
| No | 156 (35.9) | 33 (21.1) | 123 (78.9) | |
|
| ||||
| Receive medical services on OAT site | 0.5754 | |||
| Yes | 171 (39.4) | 37 (21.6) | 134 (78.4) | |
| No | 263 (60.6) | 63 (24.0) | 200 (76.0) | |
Percentages are presented within the column.
Percentages for drug injection (Yes/No) are presented within the rows.
Legend: OAT: opioid agonist therapy; IQR: interquartile range; OAT dose: lower: <75 mg – methadone; <10 mg – buprenorphine, higher: ≥75 mg – methadone; ≥10 mg – buprenorphine; BMT: buprenorphine maintenance treatment; MMT: methadone maintenance treatment.
Table 4 shows the results of bivariate and multivariable logistic regressions. The bivariate associations are presented only for variables that were included in the final multivariable model. In the final multivariable model, factors significantly and independently associated with concurrent drug injection while prescribed OAT included: being prescribed MMT (adjusted odds ratio (aOR)=2.8, 95% confidence interval (CI) =1.4–5.8, p<0.001), lower OAT dosage (aOR=1.7, 95%CI=1.1–2.7, p<0.05), high addiction severity (aOR=2.3, 95%CI=1.4–3.8, p<0.01), younger age of injection drug use initiation (aOR=2.3, 95%CI=1.3–3.9, p<0.01), having an alcohol use disorder (aOR=2.1, 95%CI=1.3–3.5, p<0.01), and living with parents (aOR=0.5, 95%CI=0.3–0.9, p<0.05).
Table 4.
Independent factors associated with concurrent drug injection while prescribed opioid agonist therapies (N=434)
| Characteristic | Bivariate logistic regression | Multivariable logistic regression | ||
|---|---|---|---|---|
| uOR (95% CI) | P-value | aOR (95% CI) | P-value | |
| Prescribed MMT (referent: BMT) | 2.8 (1.4–5.7) | 0.0035 | 2.8 (1.4–5.8) | <0.0001 |
| Lower daily OAT dose* (referent: higher doses) | 1.6 (1.02–2.5) | 0.0391 | 1.7 (1.1–2.7) | 0.0314 |
| High addiction severity (referent: low to moderate addiction severity) | 2.4 (1.5–3.8) | 0.0003 | 2.3 (1.4–3.8) | 0.0030 |
| Age of drug injection initiation (<16 years) | 2.2 (1.3–3.6) | 0.0020 | 2.3 (1.3–3.9) | 0.0040 |
| Alcohol use disorder | 2.4 (1.5–3.9) | 0.0002 | 2.1 (1.3–3.5) | 0.0047 |
| Living with parents | 0.6 (0.4–0.9) | 0.0354 | 0.5 (0.3–0.9) | 0.0117 |
Legend: OAT: opioid agonist therapy; MMT: methadone maintenance treatment; BMT: buprenorphine maintenance; OR: odds ratio; uOR: unadjusted odds ratio; aOR: adjusted odds ratio; CI: confidence interval.
Lower OAT doses included <75 mg for MMT and <10 mg for BMT
4. Discussion
Concurrent injecting of illicit drugs among OAT patients is common and broadly recognized phenomenon worldwide (Li et al., 2012; Luo et al., 2016; Tran et al., 2012). Concurrent illicit drug use can negatively impact OAT adherence and retention (Raffa et al., 2007) that result in suboptimal treatment outcomes (Magura, Nwakeze, & Demsky, 1998; Rowan-Szal, Chatham, & Simpson, 2000; Sofuoglu, Gonzalez, Poling, & Kosten, 2003; Strain, Stitzer, Liebson, & Bigelow, 1998). To our knowledge, this is the first study of concurrent drug injection in patients on OAT in the Eastern European and Central Asian region, the only region globally where HIV incidence and morbidity continue to increase (Joint United Nations Programme on HIV/AIDS (UNAIDS), 2016a). Given that Ukraine is experiencing major economic challenges during the ongoing conflict with Russia, strategies that facilitate OAT scale-up are crucial since OAT is the most cost-effective HIV prevention strategy for Ukraine (Alistar et al., 2011).
Key findings important for clinicians and policy makers from this research are that relative to other settings where OAT was introduced recently, concurrent drug injection is low. Among the 28% who continued to inject, one important clinical consideration is the need to achieve therapeutic methadone doses, especially since all of those who injected drugs did so with opioids. This finding supports data from other research which suggested that an appropriate OAT dose was the most effective in retaining patients in treatment and suppressing drug use (Amato et al., 2005; Strain, Bigelow, Liebson, & Stitzer, 1999). Compared to one study of concurrent drug use in China by Li et al., the majority (~60%) of patients were prescribed <60 mg of daily methadone dose and 45% of them reported concurrent heroin use (Li et al., 2012). Another study from China showed even higher levels of concurrent drug use (75%), but also reported that 70% were prescribed average daily methadone doses of <60 mg (Luo et al., 2016). In a longitudinal study from Vietnam, however, concurrent drug use was reported in only 14% after being stabilized on methadone for 9 months. The Vietnam study differed from the studies in China and Ukraine in that only patients with HIV were included and no baseline information on addiction severity was provided (Tran et al., 2012).
Previous research has documented that longer OAT retention produces optimal outcomes, including less concurrent illicit opiate use (Gossop, Marsden, Stewart, & Treacy, 2001; Liu et al., 2008). Though our study did not find an association between duration in OAT and concurrent drug injection, the majority of the OAT patients enrolled in the study survey had been receiving MMT or BMT for a substantial period of time. Consequently, the sample size of OAT patients who had been on OAT for 6 months or less could be insufficient to capture an association between treatment duration and the study outcome.
Of interest is that concurrent drug use was higher for MMT patients rather than for BMT patients. This is contrary to previous studies which showed that less opioid use was associated with both buprenorphine and methadone treatment compared to no treatment, however no difference was found between the two treatments (Hser et al., 2016; Otiashvili et al., 2013). Despite no direct explanation for this finding, a number of potential reasons may exist. Methadone differs from buprenorphine in that methadone’s pure opioid agonist properties require a longer induction and stabilization period to achieve a therapeutic dose. Moreover, patients on methadone are more likely to experience opioid excess symptoms (i.e., drowsiness, nodding, constipation, etc.) and may avoid higher doses. Patients may also prefer to keep OAT doses lower so that they don’t experience opioid withdrawal symptoms, but want the ability to inject to continue to feel euphoria. Alternatively, addiction specialists who might believe that OAT is effective HIV prevention may not support its use solely for addiction treatment. This is especially true in this region where OAT introduction and scale-up has been more influenced by myths than by evidence (Polonsky et al., 2016; Sarang, Stuikyte, & Bykov, 2007; Schwartz, Kelly, O’Grady, Mitchell, & Brown, 2011; Torrens, Fonseca, Castillo, & Domingo-Salvany, 2013). Additionally, in Ukraine, unlike elsewhere, OAT started with buprenorphine but was supplanted by MMT as the primary addiction treatment in 2008 due to cost constraints (Bruce et al., 2007). Consequently, patients valued the buprenorphine due to its limited availability (Bojko et al., 2016) Similarly, in a large sample of PWID, buprenorphine was preferred over methadone (Makarenko et al., 2017; Makarenko et al., 2016). When patients receive the treatment they prefer, satisfaction and adherence are improved (Makarenko et al., 2017), An alternative explanation is that due to buprenorphine’s pharmacological properties, it may be dosed every other day, thus allowing patients more autonomy and higher satisfaction (Substance Abuse and Mental Health Services Administration, 2016).
Aside from OAT dosing and type of OAT, the findings from Ukraine showed that younger PWID and those with higher addiction severity were more likely to concurrently inject drugs is consistent with literature elsewhere (Banta-Green, Maynard, Koepsell, Wells, & Donovan, 2009; Lawrinson et al., 2008; E. Liu et al., 2009). Additionally, patients with alcohol use disorders were more likely to concurrently use drugs. There may be several explanations for these findings, including the use of stimulants such as amphetamine-type substances to reverse the sedative properties of methadone and alcohol or, alternatively, that having an alcohol use disorders is yet another contributor to addiction severity.
The finding that living with parents was protective of concurrent drug injection is intriguing. While strengthening family relationships often plays a crucial role in improving outcomes for addiction treatment, several explanations might be considered. First, the social support provided by parents may serve as a positive influence on patient behavior and foster treatment participation and compliance (H. Liu, Li, Lu, Liu, & Zhang, 2010; Luo et al., 2016). Further, younger PWID in Russia who were treated with oral naltrexone markedly reduced their opioid injection, yet when older PWID were treated, there were no reductions in opioid use. The findings were explained because the younger PWID were accompanied to treatment by their parents, but older PWID were emancipated from family and did not remain in treatment (Krupitsky et al., 2004). On the other hand, the influence of drug-using friends, sexual partners or family members might substantially attribute to initiation or continuation of drug use in OAT patients (Li et al., 2012; Tran et al., 2012). This finding underlines the importance of the social context and family support to the provision and success of addiction treatment (Sullivan, Wu, Cao, Liu, & Detels, 2014; Tran et al., 2012).
Limitations
Though this study is the first to provide insights into concurrent drug injection in the Eastern European and Central Asian context, it is not without limitations. First, the cross-sectional design limits our ability to determine causality and thus, only associations can be made. Second, use of self-report may result in inaccurate reporting, including recall bias and social-desirability bias that could lead to underreporting of concurrent drug injection among the study participants. Third, some important factors may not have been included in the survey such as interpersonal and community factors that have been found to be associated with concurrent drug use in previous studies including peer pressure, having sexual partner who also inject drugs, or stigma towards injection drug use among healthcare practitioners (Li et al., 2012; Tran et al., 2012). Finally, though the study participants were randomly selected from five heterogeneous Ukrainian cities, the study results may not be generalizable for other regions including more rural areas in the country.
Conclusion
Findings from this study provide important implications for future harm reduction programs targeting injection drug use among OAT patients in Ukraine. Given that our data provide evidence on the association between higher OAT dose and reduction in concurrent drug use during OAT, strategies that optimize OAT treatment dynamics are crucial for the region. It is recommended that future interventions address continuous and efficient OAT consulting and health education to reduce ongoing drug use during the treatment, especially for those with severe drug dependence, alcohol use problems and lack of family support. Findings here support more adequate dosing efforts, including education for patients about the benefits of adequate dosing, as the first strategy to reduce concurrent drug injection. The present study also highlights the need for further examination of the influence of social networks on concurrent drug use behavior for OAT patients.
Highlights.
Relative to other settings where OAT was introduced recently, concurrent drug injection is low.
Concurrent drug injecting is associated with lower OAT dosage, being on methadone maintenance therapy vs. buprenorphine, more severe addiction severity, younger age of injection initiation, and alcohol use disorder.
Participants living with parents were negatively associated with concurrent drug injecting.
Results highlight the importance of prescribing an adequate OAT dosage, and discrepancies between MMT and BMT programs in Ukraine addressing needs of PWID with specific characteristics such as severe opioid and alcohol dependence.
Acknowledgments
The authors would like to acknowledge the National Institute on Drug Abuse for funding for research (R01 DA029910 and R01 DA033679) and career development (K24 DA017072) and the Global Health Equity Scholars Program funded by the Fogarty International Center and the National Institute of Allergy and Infectious Diseases (Research Training Grant R25 TW009338) as well as New York State International Training and Research Program through in-country training grant funded by the Fogarty International Center (D43TW000233).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alistar SS, Owens DK, Brandeau ML. Effectiveness and cost effectiveness of expanding harm reduction and antiretroviral therapy in a mixed HIV epidemic: a modeling analysis for Ukraine. PLoS Med. 2011;8(3):e1000423. doi: 10.1371/journal.pmed.1000423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alliance for Public Health. Analitical Report “Results of Sociological Research: Estimation of the size of high risk groups for HIV transmission in Ukraine in 2016”. Kyiv: ICF “Alliance for Public Health”; 2017. [Google Scholar]
- Amato L, Davoli M, Perucci CA, Ferri M, Faggiano F, Mattick RP. An overview of systematic reviews of the effectiveness of opiate maintenance therapies: available evidence to inform clinical practice and research. J Subst Abuse Treat. 2005;28(4):321–329. doi: 10.1016/j.jsat.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Andresen EM, Malmgren JA, Carter WB, Patrick DL. Screening for depression in well older adults: evaluation of a short form of the CES-D (Center for Epidemiologic Studies Depression Scale) Am J Prev Med. 1994;10(2):77–84. [PubMed] [Google Scholar]
- Babor TF, Higgins-Biddle JC, Saunders JB, Monteiro MG. The alcohol use disorders Identification test (AUDIT) World Health Organization, Department of Mental Health and Substance Dependence; 2001. [Google Scholar]
- Bachireddy C, Soule MC, Izenberg JM, Dvoryak S, Dumchev K, Altice FL. Integration of health services improves multiple healthcare outcomes among HIV-infected people who inject drugs in Ukraine. Drug Alcohol Depend. 2014;134:106–114. doi: 10.1016/j.drugalcdep.2013.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banta-Green CJ, Maynard C, Koepsell TD, Wells EA, Donovan DM. Retention in methadone maintenance drug treatment for prescription-type opioid primary users compared to heroin users. Addiction. 2009;104(5):775–783. doi: 10.1111/j.1360-0443.2009.02538.x. [DOI] [PubMed] [Google Scholar]
- Bao YP, Liu ZM, Epstein DH, Du C, Shi J, Lu L. A meta-analysis of retention in methadone maintenance by dose and dosing strategy. Am J Drug Alcohol Abuse. 2009;35(1):28–33. doi: 10.1080/00952990802342899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bojko MJ, Mazhnaya A, Makarenko I, Marcus R, Dvoriak S, Islam Z, Altice FL. “Bureaucracy & Beliefs”: Assessing the barriers to accessing opioid substitution therapy by people who inject drugs in Ukraine. Drugs: Education prevention and policy. 2015 doi: 10.3109/09687637.2015.1016397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bojko MJ, Mazhnaya A, Marcus R, Makarenko I, Islam Z, Filippovych S, Altice FL. The Future of Opioid Agonist Therapies in Ukraine: A Qualitative Assessment of Multilevel Barriers and Ways Forward to Promote Retention in Treatment. J Subst Abuse Treat. 2016;66:37–47. doi: 10.1016/j.jsat.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce RD, Dvoryak S, Sylla L, Altice FL. HIV treatment access and scale-up for delivery of opiate substitution therapy with buprenorphine for IDUs in Ukraine–programme description and policy implications. Int J Drug Policy. 2007;18(4):326–328. doi: 10.1016/j.drugpo.2006.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao XB, Yin WY, Pang L, Zhang CB, Xu JS, Xiao YK, Wu ZY. Risk factors which were associated with heroin use during the methadone maintenance treatment among 1301 patients in 9 cities of China. Zhonghua Liu Xing Bing Xue Za Zhi. 2010;31(3):269–272. [PubMed] [Google Scholar]
- Caviness CM, Hatgis C, Anderson BJ, Rosengard C, Kiene SM, Friedmann PD, Stein MD. Three brief alcohol screens for detecting hazardous drinking in incarcerated women. J Stud Alcohol Drugs. 2009;70(1):50–54. doi: 10.15288/jsad.2009.70.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Xia Y, Hong Y, Hall BJ, Ling L. Predictors of continued HIV-risk behaviors among drug users in methadone maintenance therapy program in China–a prospective study. Harm Reduct J. 2013;10:23. doi: 10.1186/1477-7517-10-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connock M, Juarez-Garcia A, Jowett S, Frew E, Liu Z, Taylor RJ, Taylor RS. Methadone and buprenorphine for the management of opioid dependence: a systematic review and economic evaluation. Health Technol Assess. 2007;11(9):1–171. iii–iv. doi: 10.3310/hta11090. [DOI] [PubMed] [Google Scholar]
- Degenhardt L, Mathers BM, Wirtz AL, Wolfe D, Kamarulzaman A, Carrieri MP, Beyrer C. What has been achieved in HIV prevention, treatment and care for people who inject drugs, 2010–2012? A review of the six highest burden countries. Int J Drug Policy. 2014;25(1):53–60. doi: 10.1016/j.drugpo.2013.08.004. [DOI] [PubMed] [Google Scholar]
- Fareed A, Casarella J, Amar R, Vayalapalli S, Drexler K. Benefits of retention in methadone maintenance and chronic medical conditions as risk factors for premature death among older heroin addicts. J Psychiatr Pract. 2009;15(3):227–234. doi: 10.1097/01.pra.0000351884.83377.e2. [DOI] [PubMed] [Google Scholar]
- Gardini A, Poehlke T, Reimer J, Walcher S, Weber B. Cultural and Linguistic Validation of the questionnaire ODAS (EADO) used to determine the adequacy of the daily dose of methadone as part of the maintenance programme fo rhte treatment of opioid dependence [German] Suchttherapie. 2010;11:138–145. [Google Scholar]
- Gavin DR, Ross HE, Skinner HA. Diagnostic validity of the drug abuse screening test in the assessment of DSM-III drug disorders. Br J Addict. 1989;84(3):301–307. doi: 10.1111/j.1360-0443.1989.tb03463.x. [DOI] [PubMed] [Google Scholar]
- González-Saiz F, Lozano Rojas O, Ballesta Gómez R, Bilbao Acedos I, Galiana Martínez J, García Collantes MA. Evidence of reliability and validity of the Opiate Dosage Adequacy Scale (ODAS) in a sample of methadone maintenance patients. Heroin Addict Relat Clin Probl. 2008;10(1):25–38. [Google Scholar]
- Gowing L, Ali R, White JM. Opioid antagonists with minimal sedation for opioid withdrawal. Cochrane Database Syst Rev. 2009;(4):Cd002021. doi: 10.1002/14651858.CD002021.pub3. [DOI] [PubMed] [Google Scholar]
- Gowing LR, Hickman M, Degenhardt L. Mitigating the risk of HIV infection with opioid substitution treatment. Bull World Health Organ. 2013;91(2):148–149. doi: 10.2471/BLT.12.109553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holloway KR, Bennett TH, Farrington DP. The effectiveness of drug treatment programs in reducing criminal behavior: a meta-analysis. Psicothema. 2006;18(3):620–629. [PubMed] [Google Scholar]
- Hser YI, Evans E, Huang D, Anglin DM. Relationship between drug treatment services, retention, and outcomes. Psychiatr Serv. 2004;55(7):767–774. doi: 10.1176/appi.ps.55.7.767. [DOI] [PubMed] [Google Scholar]
- Hser YI, Evans E, Huang D, Weiss R, Saxon A, Carroll KM, Ling W. Long-term outcomes after randomization to buprenorphine/naloxone versus methadone in a multi-site trial. Addiction. 2016;111(4):695–705. doi: 10.1111/add.13238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilgen M, Jain A, Kim HM, Trafton JA. The effect of stress on craving for methadone depends on the timing of last methadone dose. Behav Res Ther. 2008;46(10):1170–1175. doi: 10.1016/j.brat.2008.05.013. [DOI] [PubMed] [Google Scholar]
- Joe GW, Simpson DD, Dansereau DF, Rowan-Szal GA. Relationships between counseling rapport and drug abuse treatment outcomes. Psychiatr Serv. 2001;52(9):1223–1229. doi: 10.1176/appi.ps.52.9.1223. [DOI] [PubMed] [Google Scholar]
- Joint United Nations Programme on HIV/AIDS (UNAIDS) Global Report: UNAIDS report on the global AIDS epidemic 2013. Geneva, Switzerland: UNAIDS; 2013. [Google Scholar]
- Joint United Nations Programme on HIV/AIDS (UNAIDS) Global AIDS Update 2016. Geneva, Switzerland: 2016a. [Google Scholar]
- Joint United Nations Programme on HIV/AIDS (UNAIDS) Prevention Gap Report. Geneva, Switzerland: 2016b. [Google Scholar]
- Joseph H, Stancliff S, Langrod J. Methadone maintenance treatment (MMT): a review of historical and clinical issues. Mt Sinai J Med. 2000;67(5–6):347–364. [PubMed] [Google Scholar]
- Kamal F, Flavin S, Campbell F, Behan C, Fagan J, Smyth R. Factors affecting the outcome of methadone maintenance treatment in opiate dependence. Ir Med J. 2007;100(3):393–397. [PubMed] [Google Scholar]
- Krupitsky EM, Zvartau EE, Masalov DV, Tsoi MV, Burakov AM, Egorova VY, Woody GE. Naltrexone for heroin dependence treatment in St. Petersburg, Russia. J Subst Abuse Treat. 2004;26(4):285–294. doi: 10.1016/j.jsat.2004.02.002. [doi] S0740547204000078 [pii] [DOI] [PubMed] [Google Scholar]
- Lawrinson P, Ali R, Buavirat A, Chiamwongpaet S, Dvoryak S, Habrat B, Zhao C. Key findings from the WHO collaborative study on substitution therapy for opioid dependence and HIV/AIDS. Addiction. 2008;103(9):1484–1492. doi: 10.1111/j.1360-0443.2008.02249.x. [DOI] [PubMed] [Google Scholar]
- Li L, Lin C, Wan D, Zhang L, Lai W. Concurrent heroin use among methadone maintenance clients in China. Addict Behav. 2012;37(3):264–268. doi: 10.1016/j.addbeh.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C, Wu Z, Rou K, Yin W, Wang C, Shoptaw S, Detels R. Structural-level factors affecting implementation of the methadone maintenance therapy program in China. J Subst Abuse Treat. 2010;38(2):119–127. doi: 10.1016/j.jsat.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu E, Liang T, Shen L, Zhong H, Wang B, Wu Z, Detels R. Correlates of methadone client retention: a prospective cohort study in Guizhou province, China. Int J Drug Policy. 2009;20(4):304–308. doi: 10.1016/j.drugpo.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu EW, Wu ZY, Liang T, Shen LM, Zhong H, Wang B, Roger D. Risk factors associated with continued heroin use during methadone maintenance treatment in Guizhou province, China. Zhonghua Yu Fang Yi Xue Za Zhi. 2008;42(12):875–878. [PubMed] [Google Scholar]
- Liu H, Li J, Lu Z, Liu W, Zhang Z. Does Chinese culture influence psychosocial factors for heroin use among young adolescents in China? A cross-sectional study. BMC Public Health. 2010;10:563. doi: 10.1186/1471-2458-10-563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low AJ, Mburu G, Welton NJ, May MT, Davies CF, French C, Vickerman P. Impact of Opioid Substitution Therapy on Antiretroviral Therapy Outcomes: A Systematic Review and Meta-Analysis. Clin Infect Dis. 2016 doi: 10.1093/cid/ciw416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo X, Zhao P, Gong X, Zhang L, Tang W, Zou X, Ling L. Concurrent Heroin Use and Correlates among Methadone Maintenance Treatment Clients: A 12-Month Followup Study in Guangdong Province, China. Int J Environ Res Public Health. 2016;13(3) doi: 10.3390/ijerph13030305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacArthur GJ, Minozzi S, Martin N, Vickerman P, Deren S, Bruneau J, Hickman M. Opiate substitution treatment and HIV transmission in people who inject drugs: systematic review and meta-analysis. Bmj. 2012;345:e5945. doi: 10.1136/bmj.e5945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makarenko I, Mazhnaya A, Marcus R, Bojko MJ, Madden L, Filippovich S, Altice FL. Willingness to pay for opioid agonist treatment among opioid dependent people who inject drugs in Ukraine. Int J Drug Policy. 2017;45:56–63. doi: 10.1016/j.drugpo.2017.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makarenko I, Mazhnaya A, Polonsky M, Marcus R, Bojko MJ, Filippovych S, Altice FL. Determinants of willingness to enroll in opioid agonist treatment among opioid dependent people who inject drugs in Ukraine. Drug Alcohol Depend. 2016;165:213–220. doi: 10.1016/j.drugalcdep.2016.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattick RP, Breen C, Kimber J, Davoli M. Methadone maintenance therapy versus no opioid replacement therapy for opioid dependence. Cochrane Database Syst Rev. 2009;(3):Cd002209. doi: 10.1002/14651858.CD002209.pub2. [DOI] [PubMed] [Google Scholar]
- Mazhnaya A, Andreeva TI, Samuels S, DeHovitz J, Salyuk T, McNutt LA. The potential for bridging: HIV status awareness and risky sexual behaviour of injection drug users who have non-injecting permanent partners in Ukraine. J Int AIDS Soc. 2014;17:18825. doi: 10.7448/IAS.17.1.18825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazhnaya A, Bojko MJ, Marcus R, Filippovych S, Islam Z, Dvoriak S, Altice FL. In Their Own Voices: Breaking the Vicious Cycle of Addiction, Treatment and Criminal Justice Among People who Inject Drugs in Ukraine. Drugs: Education, Prevention and Policy. 2016;23(2):163–175. doi: 10.3109/09687637.2015.1127327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLellan AT, Lewis DC, O’Brien CP, Kleber HD. Drug dependence, a chronic medical illness: implications for treatment, insurance, and outcomes evaluation. Jama. 2000;284(13):1689–1695. doi: 10.1001/jama.284.13.1689. [DOI] [PubMed] [Google Scholar]
- Otiashvili D, Piralishvili G, Sikharulidze Z, Kamkamidze G, Poole S, Woody GE. Methadone and buprenorphine-naloxone are effective in reducing illicit buprenorphine and other opioid use, and reducing HIV risk behavior–outcomes of a randomized trial. Drug Alcohol Depend. 2013;133(2):376–382. doi: 10.1016/j.drugalcdep.2013.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polonsky M, Rozanova J, Azbel L, Bachireddy C, Izenberg J, Kiriazova T, Altice FL. Attitudes Toward Addiction, Methadone Treatment, and Recovery Among HIV-Infected Ukrainian Prisoners Who Inject Drugs: Incarceration Effects and Exploration of Mediators. AIDS Behav. 2016 doi: 10.1007/s10461-016-1375-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffa JD, Grebely J, Tossonian H, Wong T, Viljoen M, Khara M, Conway B. The impact of ongoing illicit drug use on methadone adherence in illicit drug users receiving treatment for HIV in a directly observed therapy program. Drug Alcohol Depend. 2007;89:2–3. 306–309. doi: 10.1016/j.drugalcdep.2007.02.007. doi: S0376-8716(07)00073-7 [pii] 10.1016/j.drugalcdep.2007.02.007 [doi] [DOI] [PubMed] [Google Scholar]
- Reimer J, Boniakowski E, Bachner C, Weber B, Tietje W, Verthein U, Walcher S. When higher doses in opioid replacement treatment are still inadequate - association to multidimensional illness severity: a cohort study. Subst Abuse Treat Prev Policy. 2014;9:13. doi: 10.1186/1747-597X-9-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarang A, Stuikyte R, Bykov R. Implementation of harm reduction in Central and Eastern Europe and Central Asia. Int J Drug Policy. 2007;18(2):129–135. doi: 10.1016/j.drugpo.2006.11.007. [DOI] [PubMed] [Google Scholar]
- Schaub M, Chtenguelov V, Subata E, Weiler G, Uchtenhagen A. Feasibility of buprenorphine and methadone maintenance programmes among users of home made opioids in Ukraine. Int J Drug Policy. 2010;21(3):229–233. doi: 10.1016/j.drugpo.2009.10.005. doi: S0955-3959(09)00131-5 [pii] 10.1016/j.drugpo.2009.10.005. [DOI] [PubMed] [Google Scholar]
- Schwartz RP, Kelly SM, O’Grady KE, Mitchell SG, Brown BS. Antecedents and correlates of methadone treatment entry: A comparison of out-of-treatment and in-treatment cohorts. Drug Alcohol Depend. 2011;115(1–2):23–29. doi: 10.1016/j.drugalcdep.2010.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner HA. The drug abuse screening test. Addict Behav. 1982;7(4):363–371. doi: 10.1016/0306-4603(82)90005-3. [DOI] [PubMed] [Google Scholar]
- State Statistics Service of Ukraine. Average wage in Ukraine in 2014. 2014 Retrieved 25 Sep 2015, from https://ukrstat.org/uk/operativ/operativ2014/gdn/reg_zp_m/reg_zpm14_u.htm.
- Strain EC, Bigelow GE, Liebson IA, Stitzer ML. Moderate- vs high-dose methadone in the treatment of opioid dependence: a randomized trial. Jama. 1999;281(11):1000–1005. doi: 10.1001/jama.281.11.1000. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Medication-Assisted Treatment. 2016 Buprenorphine. from https://http://www.samhsa.gov/medication-assisted-treatment/treatment/buprenorphine. [PubMed]
- Sullivan SG, Wu Z, Cao X, Liu E, Detels R. Continued drug use during methadone treatment in China: a retrospective analysis of 19,026 service users. J Subst Abuse Treat. 2014;47(1):86–92. doi: 10.1016/j.jsat.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taran YS, Johnston LG, Pohorila NB, Saliuk TO. Correlates of HIV risk among injecting drug users in sixteen Ukrainian cities. AIDS Behav. 2011;15(1):65–74. doi: 10.1007/s10461-010-9817-6. [DOI] [PubMed] [Google Scholar]
- Torrens M, Fonseca F, Castillo C, Domingo-Salvany A. Methadone maintenance treatment in Spain: the success of a harm reduction approach. Bull World Health Organ. 2013;91(2):136–141. doi: 10.2471/BLT.12.111054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran BX, Ohinmaa A, Mills S, Duong AT, Nguyen LT, Jacobs P, Houston S. Multilevel predictors of concurrent opioid use during methadone maintenance treatment among drug users with HIV/AIDS. PLoS One. 2012;7(12):e51569. doi: 10.1371/journal.pone.0051569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuten M, Jones HE. A partner’s drug-using status impacts women’s drug treatment outcome. Drug Alcohol Depend. 2003;70(3):327–330. doi: 10.1016/s0376-8716(03)00030-9. [DOI] [PubMed] [Google Scholar]
- Ukrainian Center for Disease Control (UCDC) HIV/AIDS Statistics. 2016 from http://ucdc.gov.ua/pages/diseases/hiv_aids/statistics/hiv-aids-treatment.
- United Nations Office on Drugs and Crime (UNODC) Word Drug Report 2016. Vienna, Austria: UNODC; 2016. [Google Scholar]
- Ware J, Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- Ware J, Kosinski MA, Keller SD. SF-12: How to Score the SF-12 Physical and Mental Health Summary Scales. Lincoln RI: Quality Metric Incorporated 1998 [Google Scholar]
- Zhang W, O’Brien N, Forrest JI, Salters KA, Patterson TL, Montaner JS, Lima VD. Validating a shortened depression scale (10 item CES-D) among HIV-positive people in British Columbia, Canada. PLoS One. 2012;7(7):e40793. doi: 10.1371/journal.pone.0040793. [DOI] [PMC free article] [PubMed] [Google Scholar]


