Abstract
Adult hippocampal neurogenesis occurs throughout life and supports healthy brain functions. The production of new neurons decreases with age and deficiencies in adult neurogenesis are associated with neurodevelopmental and degenerative disease. The rate of neurogenesis is dynamically sensitive to one’s environmental conditions and experiences, and certain stimuli are known to robustly enhance neurogenesis in rodent models, including voluntary exercise, enriched environment, and electroconvulsive shock. In these models, information about an organism’s environment and physiological state are relayed to neurogenic cell types within the hippocampus through a series of tissue and cellular interfaces, ultimately eliciting a neurogenic response from neural stem cells and newborn neurons. Therefore, understanding how novel genes and proteins act in specific cell types within this circuit-level context is of scientific and therapeutic value. Several well-studied neurotrophic factors have been implicated in environmentally-enhanced neurogenesis, and this review will serve to highlight recently-discovered, novel molecular mediators of neurogenesis in response to environmental cues. Furthermore, the contribution of advancing large-scale gene expression and function assessment technology to past, present, and future efforts to elucidate cell type-specific molecular mediators of environmentally-enhanced neurogenesis will be summarized.
Introduction
Neurogenesis, the process by which new neurons are created from stem cells, is the driving force in sculpting the central nervous system during prenatal and juvenile periods of development. In mammals, neurogenesis is known to persist under basal conditions in restricted areas of the brain throughout life; the subventricular zone (SVZ) near the lateral ventricle, and the dentate gyrus (DG) of the hippocampus (Zhao, et al., 2008). The SVZ generates inhibitory neurons that migrate through the rostral migratory stream to the olfactory bulb, while the DG yields excitatory granule cells that integrate into the existing hippocampal circuitry. SVZ neurogenesis has not been confirmed to occur in humans, but hippocampal neurogenesis is thought to play a critical role in supporting normal brain function throughout life in both human and rodents (Aimone, et al., 2010, Aimone, et al., 2011, Akers, et al., 2014, Clelland, et al., 2009, Deng, et al., 2010, Frankland, et al., 2013, Imayoshi, et al., 2008, Opendak and Gould, 2015). Deficits in adult hippocampal neurogenesis are associated with both neurodevelopmental and degenerative diseases, including autism and Alzheimer’s disorders (Balu and Lucki, 2009, Christian, et al., 2014, Donovan, et al., 2006, Kempermann, et al., 2008, Mu and Gage, 2011, Sahay and Hen, 2007, Wegiel, et al., 2010). During adult hippocampal neurogenesis, new neurons are developed from a largely quiescent pool of adult neural stem cells (aNSCs) in a tightly-regulated, multi-step process that includes aNSC activation, lineage determination, proliferation of intermediate progenitors, maturation and survival of newborn neurons, and integration with mature neural networks (Kempermann, et al., 2015).
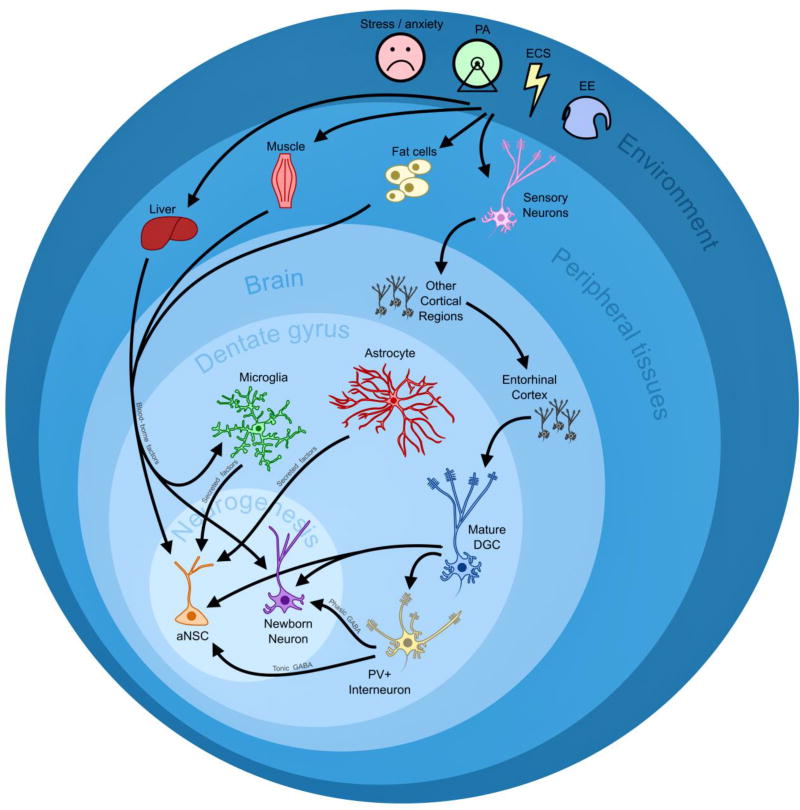
Neurogenesis can be influenced locally by growth factors and neurotransmitters exchanged by neurons and glia in the neurogenic niche, but also by broader physiological and environmental factors that an animal experiences. The interaction between environment and neurogenesis can be thought of as a series of interfaces through which information is relayed from peripheral sources to the proximal cell types involved directly in neurogenesis within the hippocampus, with the final interface regulating activity of aNSCs and newborn neurons themselves (Figure 1). Certain experiences, such as isolation, aging, and stress, are known to have a negative effect on neurogenesis (Czeh, et al., 2002, Dranovsky and Hen, 2006, Fowler, et al., 2002, Gould and Tanapat, 1999, Gould, et al., 2000, Jin, et al., 2003, Klempin and Kempermann, 2007, Kuhn, et al., 1996, Lieberwirth, et al., 2012, Stranahan, et al., 2006, Warner-Schmidt and Duman, 2006, Xu, et al., 2007), while enriched environment, voluntary exercise, and diet can enhance neurogenesis (Boitard, et al., 2012, Brown, et al., 2003, Clark, et al., 2010, Garrett, et al., 2012, Kobilo, et al., 2011, Lee, et al., 2002a, Lee, et al., 2002b, Lindqvist, et al., 2006, Stangl and Thuret, 2009, Tozuka, et al., 2009, van Praag, 2008, van Praag, et al., 1999, Vivar, et al., 2013, Yu, et al., 2014). There is a rich literature documenting the neurogenic phenotypes that result from these treatments, and there is great interest in understanding what specific molecular pathways and mechanisms are responsible for facilitating the interaction between environment and neurogenesis. Numerous peripheral growth factors and metabolic regulators have been well-studied with respect to their response to physical activity and their roles in adult neurogenesis, and several pathways within the DG have been strongly implicated in environmentally-induced neurogenesis. However, probing molecular changes that occur within aNSCs and newborn neurons themselves has proven to be a major hurdle because these neurogenic cell types are relatively rare and intricately interwoven with more abundant mature neurons and glia in the mixed tissue environment of the DG.
Figure 1.
A schematic of how environmental stimuli and experience can modulate neurogenesis through multiple levels of information relay and interfacing tissue and cell types. Environmental experiences, including stress/anxiety, physical exercise (PA), electroconvulsive shock (ECS), and environmental enrichment (EE) are depicted in the outermost circle. Going inward, concentric circles represent peripheral tissues, brain, dentate gyrus, and the neurogenesis itself. Arrows passing from cell types depicted in outer circles to cell types within inner circles indicate information being relayed across tissue and cell interphases from distal sources to the proximal cell types involved in neurogenesis. Peripheral tissues communicate with the brain via blood-borne factors and neuronal connections, and cells within the dentate gyrus communicate with aNSCs and newborn neurons via secreted factors and neurotransmitters, such as GABA.
Studying the molecular mechanisms underlying environmentally-enhanced neurogenesis has profound implications for understanding disease mechanisms. Because the progression of most neurodevelopmental and degenerative disorders is the result of both genetic and environmental components, investigating the molecular biology underlying the contribution of environment to adult neurogenesis at proximal levels will help researchers understand the etiology of these diseases and identify effective therapeutic strategies that may act with high specificity in neurogenic cell types. This review will serve to highlight recently discovered molecular mediators of environmentally-enhanced neurogenesis and describe ongoing efforts and current challenges in identifying novel genes and proteins through advancing large-scale, genome-wide technologies.
Models of environmental influence on neurogenesis
In the context of the environment-neurogenesis interaction, “environment” necessarily refers to both the conditions of an animal’s environment, and how the animal behaves within that environment. For example, the presence of toys and running wheels are conditions of an enriched environment, and an animal experiences those conditions through the actions of exploration and running. Therefore, it becomes impossible to completely dissociate environment from physiology, and these collectively represent an animal’s experiences. Various experimental paradigms have been developed to model aspects of environmental influence on neurogenesis. This review will consider three such models; physical activity (PA), enriched environment (EE), and electroconvulsive shock (ECS).
i. Environmental Enrichment
Environmental enrichment (EE) is defined as housing conditions that provide enhanced sensory, cognitive, or motor stimulation (Zhou, et al., 2017). Such housing conditions may include cardboard rolls, toys, textured ribbons, and running wheels (Monteiro, et al., 2014). EE can also include a component of social enrichment when animals are group housed, or dietary enrichment through the use of non-standard food. In some EE paradigms, conditions are changed every few days to maintain novelty, or exposure to EE is restricted. The contribution of each component of the enriched environment cannot be disentangled post hoc, and EE must therefore be considered as a mixture of effects. EE has been shown to promote neurogenesis, angiogenesis, spatial navigation, and pattern separation, as reviewed by Clemenson et al. (Clemenson, 2015).
ii. Physical activity
PA can be considered as a specific, physiological component of EE, and is usually modeled in rats and mice by providing voluntary access to running wheels or by forced running on a treadmill. Recently, it was shown that the presence of a locked wheel alone has an effect on neurogenesis that is distinct from that of running per se (Dostes, et al., 2016). Some groups therefore house control animals with a locked wheel to control for the novelty of the object. PA is known to increase proliferation within the DG (Ables, et al., 2010, Olson, et al., 2006, Stranahan, Khalil and Gould, 2006, van Praag, Kempermann and Gage, 1999), as well as dendritic spine density of newborn neurons (Leuner and Gould, 2010, Redila and Christie, 2006, Stranahan, et al., 2007). PA causes a transient increase in DG cell proliferation that returns to basal levels after approximately two weeks, followed by a sustained effect on new neuron production that is attributed primarily to enhanced survival (Kronenberg, et al., 2006). Several cellular mechanisms have been proposed to explain PA-induced proliferation, but none can fully account for all experimental observations at this time (Overall, et al., 2016). In addition to changes in neurogenesis, PA enhances spatial memory in the Morris water maze, Y-maze, T-maze, and radial arm maze tests (Fordyce and Farrar, 1991, van Praag, 2008). For a comprehensive review of the neurogenic, neurophysiological, and behavioral phenotypes associated with PA, see Vivar et al. (Vivar, Potter and van Praag, 2013).
iii. Electroconvulsive shock
Electroconvulsive therapy (ECT) is a highly effective treatment option for specific forms of depression, mania, and schizophrenia (Aksay, et al., 2016). Compared with PA and EE treatments, ECS is decidedly less natural, and is more analogous to the physiological medical intervention of ECT. In humans, ECT is performed under general anesthesia with muscle relaxants. In early rodent experiments, ECS was performed in awake animals, but modern protocols typically include anesthesia via an inhaled agent (Schloesser, et al., 2015). ECS can be performed as a single, acute shock, or as a chronic course of repeated daily shocks. In 2000, multiple groups discovered that tonic-clonic seizures induced by ECS robustly trigger adult hippocampal neurogenesis (Madsen, et al., 2000, Malberg, et al., 2000, Scott, et al., 2000). More recently, it has been suggested that neurogenesis is required for the antidepressant-like effects of ECS (Schloesser, Orvoen, Jimenez, Hardy, Maynard, Sukumar, Manji, Gardier, David and Martinowich, 2015).
Novel mediators of environmentally-induced neurogenesis
The act of running causes the release of molecules in peripheral tissues that promote structural regeneration of muscle and vasculature, regulate body fluids, and manage glucose and protein metabolism. Many of these compounds are secreted into the blood and cross the blood-brain barrier, where they can modulate pathways involved in hippocampal neurogenesis. Most famous among these proteins are insulin-like growth factor 1 (IGF-1), vascular endothelial growth factor (VEGF), and the hormone adiponectin. These proteins are upregulated in response to PA and can enter the brain to promote proliferation, differentiation, maturation, and survival. These and additional peripheral factors that mediate effects of PA on hippocampal neurogenesis are reviewed by Bolijn and Lucassen (Bolijn, 2015), including leptin, angiotensin II, glucocorticoids, adrenaline, reactive oxygen species, AMP-kinase, peroxisome proliferator-activated receptor (PPAR), PPAR γ co-activator 1α (PGC-1 α), ciliary neurotrophic factor (CNTF), and proinflammatory cytokines.
Quiescent aNSCs residing in the subgranular zone (SGZ) of the DG are maintained through slow, controlled self-renewal. Once activated, aNSCs give rise to proliferating adult neural progenitor cells (aNPCs), which differentiate into nascent granule cell neurons that migrate through the DG, mature, and form synapses with established neuronal networks. Pathways within aNSC/aNPCs and newborn neurons that receive information from exogenous sources represent the final interface between environmental influences and increases in activation, proliferation, differentiation, survival, and maturation. Well-established factors that mediate effects of EE and PA on adult neurogenesis include brain-derived neurotrophic factor (BDNF) (Fang, et al., 2013, Farmer, et al., 2004, Gibbons, et al., 2014, Griffin, et al., 2009, Ji, et al., 2014, Johnson and Mitchell, 2003, Johnson, et al., 2003, Molteni, et al., 2002, Russo-Neustadt, et al., 1999, Tong, et al., 2001) and nerve growth factor (NGF) (Chae and Kim, 2009, Molteni, Ying and Gomez-Pinilla, 2002, Neeper, et al., 1996, Tong, Shen, Perreau, Balazs and Cotman, 2001, Zaben and Gray, 2013). The following section will highlight recently-discovered, novel mediators of environmentally-induced neurogenesis, which are summarized in Table 1.
Table 1.
Novel mediators of environmentally-induced neurogenesis.
| Gene | Protein | Origin | Stimuli/ Experience |
Effect on neurogenesis: |
Downstream effectors |
Reference |
|---|---|---|---|---|---|---|
| Adipoq | Adiponectin | Adipose cell | PA | Promotes cell proliferation and new neuron production, but does not influence differentiation per se | ADNR1/AMPK signaling | Yau et al., 2014; Nicolas et al., 2015; Chabry et al., 2015 |
| Ctsb | Cathepsin B | Muscle cell | PA | Enhances number of DCX+ cells | BDNF | Moon et al., 2016 |
| Cx3cl1 | C-X3-C Motif Chemokine Ligand 1 | Microglia in DG | PA | Enhances NPC neurospheres formed in vitro | CX3CR1, BDNF | Vukovic et al., 2012 |
| Cx3cl1 Dbi | C-X3-C Motif Chemokine Ligand 1 Diazepam binding inhibitor | Microgli a in DG aNSCs | PA PA | Enhances NPC neurospheres formed in vitro Permits proliferation of GFAP+SOX2+ NSCs | CX3CR1, BDNF Tonic GABA | Reshef et al., 2014 |
| Dumitru et al., 2017 | ||||||
| Klk8 | Neuropsin; Kallikrein Related Peptidase 8 | PV+ interneurons in DG | EE | Modulates PV immunoreactivity in DG; known to regulate LTP and synaptic plasticity; direct role in EE-enhanced neurogenesis not yet shown | NRG-1, ErbB4, GABA signaling | Suzuki et al., 2014 |
| Grasp | Tamalin; General Receptor for phosphoino sitides 1 Associated Scaffold Protein | Neurons or immature neurons in DG | ECS | Permits proliferation of BrdU+ cells; permits LTP formation | Synaptic scaffolding proteins | Yanpallewar et al., 2012 |
| Gadd45b | Growth Arrest and DNA Damage inducible Beta | DG neurons | ECS | Required for ECS-induced (but not basal) proliferation of MCM2+Nestin+ aNSCs and non-radial MCM2+ cells | Demethylates promoter of Bdnf and Fgf1 | Ma et al., 2009 |
| Gadd45b Gsk3b | Growth Arrest and DNA Damage inducible Beta Glycogen Synthase Kinase 3 Beta | DG neurons Immature DG neurons | ECS PA | Required for ECS-induced (but not basal) proliferation of MCM2+Nestin+ aNSCs and non-radial MCM2+ cells Inhibits measures of total and primary apical dendritic length, and number of dendritic branches | Demethylates promoter of Bdnf and Fgf1 Triggers destruction of β-catenin in Wnt pathway | Jun et al., 2015 |
| Okamoto et al., 2011 | ||||||
| Gsk3b Dnm1l | Glycogen Synthase Kinase 3 Beta Dynamin-related protein 1 (DRP1); Dynamin 1 Like | Immature DG neurons Ubiquitously expressed | PA PA | Inhibits measures of total and primary apical dendritic length, and number of dendritic branches Supports survival of labeled newborn neurons | Triggers destruction of β-catenin in Wnt pathway Fundamental in mitochondrial fission | Llorens-Martin et al., 2016 |
| Steib et al., 2014 | ||||||
| Rasgrf2 | Ras Protein Specific Guanine Nucleotide Releasing Factor 2 | Immature and mature DG neurons | EE | Permits enhanced survival of BrdU+ newborn neurons (4 weeks after injection) | Erk MAP kinase | Darcy et al., 2014 |
This table summarizes information from section 3. Columns include gene symbol, protein, where it is produced, where it exerts its effect, what aspect of neurogenesis it influences, and what downstream molecules it may act through.
i. Adiponectin
Adiponectin is a hormone produced by fat cells that influences numerous physiological processes, including glucose metabolism and inflammation. Although studies dating back to 2002 have investigated adiponectin levels in response to PA due to interest in its role in regulating insulin activity (Ferguson, et al., 2004, Hulver, et al., 2002), it was only recently proven that adiponectin crosses the blood-brain barrier to mediate the pro-neurogenic and anti-depressive effects of PA in mice (Yau, et al., 2014). In this work, Yau and colleagues demonstrated that PA-induced increases in cell proliferation (as measured by BrDU and Ki67) and DCX+ new neuron production was completely ablated in adiponectin knockout animals. The ratio of BrdU/DCX co-labeling was unchanged in the knockout mouse, indicating that adiponectin did not influence the process of neuronal differentiation itself. Because BDNF levels were enhanced by PA in both WT and knockout mice, it is likely that adiponectin acts downstream of BDNF in the response to PA. Finally, application of adiponectin to cultured N2a cells and NPCs concurrently with knockdown of either one of the adiponectin receptors (ADNRs) revealed that adiponectin’s interaction with ADNR1, rather than ADNR2, was responsible for adiponectin-elicited cell proliferation. In 2015, Nicolas and colleagues reported that the antidepressant-like effects of EE were mediated by adiponectin through a neurogenesis-independent mechanism (Nicolas, et al., 2015). The same group went on to show that this EE-induced adiponectin pathway reverses the pro-inflammatory phenotype of depressive mice by reducing the inflammatory action of microglia (Chabry, et al., 2015). Therefore, there are likely to be multiple avenues by which adiponectin can influence neurogenesis and brain plasticity in response to both PA and EE.
ii. Cathepsin B
A novel myokine, Cathepsin B (CTSB), was recently shown to be elevated in plasma after exercise, and it was demonstrated that PA-induced neurogenesis and enhanced memory were ablated in CTSB knockout mice (Moon, et al., 2016). The same study suggests that CTSB may exert its effects by positively regulating BDNF within aNPCs; when exogenous CTSB was applied to hippocampal progenitor cells, Bdnf transcript levels increased, and inhibition of CTSB conversely reduced Bdnf. CTSB has also been implicated in angiogenesis in a meningioma cell line (Tummalapalli, et al., 2007), which could be an indirect mechanism by which CTSB activity may improve nutrient and oxygen delivery to neurogenic cells through enhanced vasculature. In an Alzheimer’s disease (AD) model mouse, injections of adeno-associated virus expressing Ctsb reduced amyloid-β levels (Embury, et al., 2016). Because exercise is known to counteract cognitive decline in aging and AD (Cotman and Berchtold, 2002, Intlekofer and Cotman, 2013), CTSB may promote brain health through neurogenic and non-neurogenic mechanisms. Considering these recent findings, CTSB represents an interesting potential therapy for brain health, but it remains to be seen whether peripherally administered CTSB can enhance neurogenesis or promote cognitive function.
iii. CX3Cl1
Microglia are the resident immune cells of the brain, and play important roles in regulating aNSC activity and neurogenesis (De Lucia, et al., 2016, Sierra, et al., 2014, Sierra, et al., 2010). Therefore, genes and proteins in microglia that respond to environmental stimuli may bridge environment and neurogenesis through immune pathways. For example, the chemokine CX3CL1 (also known as fractalkine or neurotactin) promotes a preponderance of neuroprotective microglia over pro-inflammatory microglia (Cardona, et al., 2006). Vukovic et al. found that wheel running increased hippocampal CX3CL1 levels, particularly in aged animals (Vukovic, et al., 2012). Furthermore, they demonstrated that co-culturing NPCs from running mice with microglia from sedentary mice abolished the effect of running on enhanced aNPC activity, suggesting that PA-induced CX3CL1 acts within microglia, which can in turn regulate aNSCs in neurogenesis. The role of CX3CL1 as an environmentally malleable regulator of the microglia-aNSC interaction was further confirmed when Reshef et al. found that functional deletion of CX3CL1’s receptor, CX3CR1, which is expressed exclusively in microglia, prevented the effect of EE on neurogenesis (Reshef, et al., 2014). Recently, Littlefield et al. used lipopolysaccharide (LPS) as an immune challenge to reduce neurogenesis in aged mice and found that wheel running prevented the LPS-induced loss of new neurons in a manner that correlated strongly with the proportion of microglia that expressed BDNF (Littlefield, et al., 2015). This indicates that BDNF may have an indirect mechanism of promoting neurogenesis by influencing microglia activity, in addition to its well-studied activity in aNSCs themselves.
iv. Diazepam binding inhibitor
In the adult hippocampus, neurons projecting from the DG to CA3 are recurrently connected with local inhibitory interneurons, and these inhibitory neurons are responsible for determining levels of ambient GABA in the DG. NPCs and their progeny express functional GABA receptors, and GABA signaling is crucially involved in both embryonic and adult hippocampal neurogenesis, influencing progenitor proliferation, fate specification, migration of new neurons, and synaptic integration (Ge, et al., 2007). Pioneering studies by Overstreet-Wadiche and colleagues discovered that adult-born new neurons initially receive GABAergic inputs exclusively (Overstreet Wadiche, et al., 2005), and later revealed the importance of GABA signaling in granule cell maturation and experience-dependent synapse un-silencing (Chancey, et al., 2013, Dieni, et al., 2012). In particular, parvalbumin (PV)-expressing interneurons have been shown to synapse directly on proliferating newborn progeny and couple circuit activity to neurogenesis (Song, et al., 2013). Song et al. describe a diametric model in which PV+ neurons simultaneously promote aNSC quiescence through tonic, ambient GABA and survival/maturation of newborn progeny synaptically (Song, et al., 2014). This occurs because, during the first two weeks after a granule cell is born, intracellular Cl− concentrations are relatively high, and the opening of GABAARs results in a net efflux of Cl− ions and depolarization of the newborn neuron (Owens and Kriegstein, 2002). In contrast, aNSCs with lower intracellular concentrations of Cl− are inhibited by GABAAR activity. However, it was unclear how PA-induced GABA signaling could result in an increase in proliferation if such signaling would be expected to maintain aNSC quiescence. This was recently resolved by Dumitru et al. (Dumitru, et al., 2017), who demonstrated that diazepam binding inhibitor (DBI), which binds to the benzodiazepine binding site of GABAARs, is expressed in aNSCs and serves to reduce GABA currents in these cells, counteracting the negative effect of increased GABA on aNSC proliferation. Furthermore, knockdown of DBI abolished EE-induced increases in the production of DCX+ newborn neurons, the number of dividing Ki67+ cells, and the percentage of proliferating aNSCs.
v. Neuropsin
Suzuki et al. investigated the secretory serine protease neuropsin for a potential role in regulating PV+ interneurons in response to EE and PA (Suzuki, et al., 2014). Neuropsin cleaves its target, neuregulin-1 (NRG-1), allowing NRG-1 to interact with p185 (ErbB4) in PV+ interneurons in a neural activity-dependent manner (Tamura, et al., 2012). In neuropsin-deficient mice, the intensity of PV+ terminals was reduced in DG, indicating that neuropsin controls PV activity and GABA signaling in PV-neurons. EE triggered enhanced PV immunoreactivity and elevated hippocampal neuropsin mRNA and protein, suggesting that neuropsin may link environmental stimuli to GABA signaling in PV+ neurons. However, induction of PV by EE was not abolished by deletion of neuropsin, which shows that EE-induced PV induction occurs, at least in part, through a neuropsin-independent mechanism. Whether neuropsin is required for EE-induced neurogenesis remains to be tested.
vi. Tamalin
Yanpallewar et al. (Yanpallewar, et al., 2012) noted that the scaffold protein tamalin (also known as General Receptor for phosphoinositides 1 Associated Scaffold Protein, encoded by the Grasp gene) modulated morphine and cocaine sensitivity by altering adaptive neural plasticity involved with addiction, and asked whether Tamalin might influence plasticity in the context of ECS. To this end, they observed a dramatic increase in Grasp mRNA within the DG as measured by in situ hybridization, which was strongest at 1–3 hours after ECS and returned to basal levels by 24 hours. Interestingly, the tamalin knockout mouse exhibited no defects in basal proliferation and survival of aNPCs, and tamalin is not required for normal development. However, tamalin was required for ECS-induced proliferation, neurogenesis, mossy fiber sprouting, granule cell dendrite arborization, and LTP. Therefore, tamalin may act specifically to facilitate neural plasticity in response to hippocampal neural activity, but no follow-up studies have pursued this intriguing protein as of yet.
vii. Gadd45b
Upregulation of Growth Arrest and DNA Damage inducible Beta (Gadd45b) after ECS was first detected in a microarray experiment of rat DG tissue in 2006 (Ploski, et al., 2006). Three years later, it was identified as an immediate early gene in mature hippocampal neurons that is transiently and robustly upregulated after ECS (Ma, et al., 2009). In that study, Gadd45b knockout mice did not have significantly altered neurogenesis, but showed reduced levels of proliferation and dendritic development in response to both ECS and 7 days of wheel running. Because Gadd45b is known to be an epigenetic modifier, the authors measured methylation levels of key neurogenic genes in WT and KO animals. They found that ECS induced demethylation of regulatory elements within Bdnf and Fgf1 in WT animals, but failed to do so in KO littermates. In this way, it is thought that transient increases in Gadd45b activity may result in long-lasting changes in DNA methylation and neurodevelopment. More recently, the same group expanded upon their work by showing that Gadd45b is essential for ECS-induced proliferation of both quiescent aNSCs and proliferating progenitors (Jun, et al., 2015).
viii. Glycogen synthase kinase-3 β
In addition to activation and proliferation of aNSCs, altering the maturation and survival of newborn neurons can affect the output of neurogenesis. Wnt3 is produced by astrocytes and induces NeuroD1, a proneuronal transcription factor, through the canonical Wnt/β-catenin signaling pathway (Kuwabara, et al., 2009). Wnt3 was observed to increase in DG astrocytes after PA, which was accompanied by enhanced production of neurons in both young and aged mice (Okamoto, et al., 2011). Inhibition of glycogen synthase kinase-3 (Gsk3)-mediated phosphorylation of β-catenin is considered to be the key event in the Wnt/β-catenin pathway (Wu and Pan, 2010). Using a retroviral strategy, it was recently shown by Llorenz-Martin et al. (Llorens-Martin, et al., 2016) that over-expressing Gsk3β specifically in newborn granule neurons resulted in a cell-autonomous impairment of PA-induced maturation, as measured by the number of dendritic spines, the percentage of mushroom spines, and the head diameter of mushroom spines. In this study, however, Gsk3β over-expression also impaired these neuronal development parameters under basal conditions; therefore, it is possible that the action of Gsk3β opposes neuronal maturation generally, and not specifically in the context of PA. Because of its diverse functions, Gsk3 has long been of interest as a therapeutic target (Pandey and DeGrado, 2016). Although over-expression of Gsk3β can restrict the efficacy of PA-induced neuronal maturation, it has not yet been shown that inhibiting Gsk3β can enhance the same processes.
ix. Dynamin-related protein 1
Maturation of new neurons from aNSCs in adult animals requires that energy and metabolic demands be met sufficiently by the developing cell. Dynamin-related protein 1 (DRP1) facilitates mitochondrial fission, and was recently found to be upregulated in the cortex of aged mice in a treadmill running paradigm (Gusdon, et al., 2017). Steib et al. (Steib, et al., 2014) observed that developing neurons undergo dramatic changes in mitochondrial distribution and shape. This group genetically manipulated Drp1 in the context of PA and discovered that inhibition of Drp1 impairs neurogenesis, while over-expression enhances PA-induced acceleration of neuronal maturation. Because DRP1 is a critical regulator of mitochondrial function, the effects seen in these experiments may not be attributable exclusively to DRP1, but may instead reflect the broader contribution of mitochondria to PA-induced neurodevelopment. Furthermore, inhibition of Drp1 also diminished neurogenesis under basal conditions, indicating that mitochondria activity is fundamentally coupled to neurogenesis. This notion is supported by experiments in which inhibition of mitochondria activity through ablation of the mitochondrial transcription factor A (Tfam) impaired neurogenesis at the intermediate progenitor cell (IPC) level (Beckervordersandforth, et al., 2017). Furthermore, enhanced mitochondrial function ameliorated aging-associated defects in neuronal maturation and in a mouse model of AD (Richetin, et al., 2017). Therefore, it can be concluded that mitochondria are essential for basal and PA-induced neuronal development, and DRP1 is one protein that can be targeted to impact mitochondrial function.
iv. Grf2
Ras-GRF1 (GRF1) and Ras-GRF2 (GRF2) are members of a family of calcium-activated guanine nucleotide exchange factors that can activate Ras and Rac GTP-ases (Feig, 2011). GRF1 was observed to promote survival of new DG neurons (Darcy, et al., 2014a), and EE-enhanced survival of new neurons was ablated in GRF2 KO mice (Darcy, et al., 2014b). GRF2 knockout also impaired LTP through its downstream effector, Erk MAP kinase. Retroviral shRNA knockdown of GRF2 in newborn neurons showed that it promotes survival in a cell-autonomous manner under basal conditions. However, whether GRF2 expressed in aNSCs is critical for EE-enhanced neurogenesis and how GRF2 may exert such a function remain to be explored.
Genome-wide studies of environmentally-enhanced gene regulation
The genes and proteins described so far have been shown to facilitate the environmental impact on neurogenesis through experimental validation. However, many of them were chosen as candidates due to their close relationship to well-established neurogenic pathways. Genome-wide screening techniques allow researchers to cast a broader net when searching for genes that may be involved in the neurogenic response to environmental stimuli, and can offer a big-picture understanding of gene and protein networks acting in concert to coordinate cellular changes. The following sections will discuss unbiased, large-scale studies of gene expression associated with hippocampal neurogenesis in response to PA, ECS, and EE, which are summarized in Table 2. From the historical context of early experiments, progress of the field will be summarized, and recent advancements will be highlighted.
Table 2.
Genome-wide studies of environmentally-induced gene regulation.
| Year | Species | Type of expression analysis |
Stimuli/Experience | Duration | Tissue | Reference |
|---|---|---|---|---|---|---|
| 2001 | Rat | Microarray | Running wheel | 3 weeks | Hippocampus | Tong et al., 2001 |
| 2002 | Rat | Microarray | Resistance running wheel (100 g) | 3 days, 1 week, 4 weeks | Hippocampus | Molteni et al., 2002 |
| 2008 | Mouse | Microarray | Running wheel | 16 months | Hippocampus | Stranahan et al., 2008 |
| 2011 | Rat | Microarray | Running wheel | 2 weeks | Hippocampus | Funk et al., 2011 |
| 2011 | Mouse | Microarray | Running wheel | 8 weeks | Hippocampus | Kohman et al., 2011 |
| 2013 | Mouse | Microarray | Running wheel 3 days per week | 6 months | Hippocampus | Alvarez-Lopez et al., 2012 |
| 2014 | Rat | Microarray | Resistance running wheel (30% body weight) | 4 weeks | Hippocampus | Lee et al., 2014 |
| Running wheel (no resistance | ||||||
| 2015 | Rat | Microarray | Forced running on treadmill (mild exercise) 1 hour per day | 6 weeks | Hippocampus | Inoue et al., 2015 |
| Forced running on treadmill (intense exercise) 1 hour per day | ||||||
| 2015 | Mouse | Microarray | Running wheel | 3 days | Dentate gyrus | Guerrieri et al., 2015 |
| 1 week | ||||||
| 2 weeks | ||||||
| 2003 | Rat | Growth factor chip | ECS: 1 shock | Hippocampus | Newton et al., 2003 | |
| 2004 | Rat | Microarray | ECS: 1 shock per day | 10 days | Hippocampus | Altar et al., 2004 |
| 2006 | Rat | Microarray | ECS: 1 shock per day | 10 days | DG granule cell layer | Ploski et al., 2006 |
| 2007 | Rat | Microarray | ECS: 4 shocks per day | 2 days | Prefrontal cortex | Conti et al., 2007 |
| Frontal cortex | ||||||
| Amygdala | ||||||
| Hypothalamus | ||||||
| Hippocampus | ||||||
| Dorsal raphe | ||||||
| Locus coeruleus | ||||||
| 2008 | Rat | CREB ChIP + microarray | ECS: 1 shock | 1 day | Frontal cortex, hippocampus, striatum | Tanis et al., 2008 |
| 2011 | Mouse | Methyl-sensitive cut counting + sequencing | ECS: 1 shock | 1 day | DG tissue | Guo et al., 2011 |
| 2013 | Rat | miRNA microarray | ECS: 1 shock per day | 10 days | Hippocampus | O’Connor et al., 2013 |
| 2000 | Mouse | Microarray | 6 hours daily exposure; various toys, wooden blocks, running wheel, and small houses; rearranged every half-day | 3–6 hours | Cortex | Rampon et al., 2000 |
| 2–14 days | ||||||
| 2004 | Rat | Microarray | 24 hours daily; spacious 2-level glass terraria with nesting material, plastic stairs, hemp ropes, pipes, box of soil, wooden blocks, other colored objects; rearranged twice per week | 2 weeks | Hippocampus | Keyvani et al., 2004 |
| 2005 | Mouse | Microarray | 3 hours per day for first month, 3 sessions per week for next 4 months; large cages with running wheels, colored tunnels, toys, and chewable material | 5 months | Hippocampus | Lazarov et al., 2005 |
| 2016 | Mouse | Single-nuclei RNA-seq | One 15-minute session; large cages with huts and tunnels | One time | Individual DG neurons | Lacar et al., 2016 |
This table summarizes published genome-wide studies discussed in section 4.
1. Physical activity
Researchers began using microarray technology to measure gene expression in the rat hippocampus in response to PA in the early 2000’s. Tong et al. (Tong, Shen, Perreau, Balazs and Cotman, 2001) applied a t-test analysis to microarray data and detected 88 differentially expressed genes (DEGs), equally split between up- and down-regulation after three weeks of wheel running. They observed increases in Bdnf, Vgf, and genes related to neuronal signaling, plasticity, extracellular matrix, protein trafficking, biosynthetic processes, and immune response. Molteni et al. (Molteni, Ying and Gomez-Pinilla, 2002) normalized array signal to five housekeeping genes and considered expression ratios ≥ 1.8 or ≤ 0.5 to be significantly different after three days, seven days, or 28 days of running, resulting in 15 up-regulated genes and two down-regulated genes. DEGs included key neurotrophins (Bdnf, Ngfb, Fgf2, Trkb), synapse-related genes, neurotransmitters, and signal transduction-related molecules. These pioneering studies provided the first glimpse into the global impact of wheel running on hippocampal gene expression. However, these early experiments yielded relatively few individual gene results overall, representing the small but robust tip of a much larger iceberg.
As microarray platforms advanced and granted more power in discerning expression changes, researchers were able to detect hundreds of DEGs in hippocampal tissue after eight weeks of wheel running (Kohman, et al., 2011). In addition to hallmark neurotrophins, such as Bdnf, novel regulatory gene networks emerged from this richer dataset. Gene ontology (GO) analysis implicated chromatin remodeling as a mechanism by which PA facilitates changes in cellular activity within the hippocampus. Lee et al. (Lee, et al., 2014) compared the transcriptional effects of wheel running on rat hippocampus to that of a resistance wheel running paradigm in which wheels were loaded with 30% of the subject’s body weight. They determined that intensity of PA has a significant influence on dynamic gene expression, and specifically that the addition of resistance causes a greater number of genes to be down-regulated relative to sedentary controls. Non-resistance wheel running induced changes in metabolism, endocrine system, and molecular transport, and genes altered specifically by resistance wheel running were related to inflammatory/immune response, protein synthesis, and cellular movement. This finding suggests that mild and intense exercise may exert distinct effects on the adult hippocampus. Inoue et al. (Inoue, et al., 2015) used a treadmill running paradigm and concluded that mild exercise below the lactate threshold is more effective than intense exercise in promoting adult neurogenesis as measured by DG cell proliferation. Mild exercise in these experiments led to a greater number of expression changes measured by microarray, but hallmark factors Bdnf, Igf1, and Vegf were unchanged by either treatment. These discrepancies may be due to the timing of tissue collection, as samples were collected two days after the cessation of running, which could allow for running-induced elevation of neurotrophins to subside. Furthermore, the treadmill running design is a forced exercise model that employs mild electric shocks to motivate running, which could introduce an additional stress component to transcriptional perturbations. DEGs in this study were enriched for lipid metabolism, protein synthesis, and inflammatory/immune response, demonstrating a degree of consistency across experiments in biological processes associated with PA despite methodological differences.
Whole hippocampus was used for all the microarray experiments described thus far. One limitation of this approach is that the hippocampal tissue is composed of numerous cell types that are likely to act in transcriptionally distinct ways in response to PA. It would therefore be expected that cell type-specific gene networks would be lost in the bulk output of a such a system. Additionally, a readout of mixed tissue would be biased towards mature neurons, glia, and secreted factors, and biased against rarer NSCs and newborn neurons. One strategy to tailor a microarray experiment more towards neurogenic cell types is to restrict tissue collection to the DG itself, where neurogenesis occurs. Guerrieri and van Praag (Guerrieri and van Praag, 2015) measured gene expression specifically from DG in response to 3 days, 7 days, and 14 days of wheel running and identified novel candidate genes that had not been previously implicated in the brain’s response to PA, including upregulation of Wdr37, Armc8, Phactr1, Zmpste24, and Pfkp. Armc8 has been linked to carcinogenesis (Fan, et al., 2014), while Phactr1 is involved in capillary tube formation in endothelial cells (Allain, et al., 2012). It is therefore possible that these developmental genes may contribute to the cellular division processes of neurogenesis and angiogenesis in response to PA.
2. Electroconvulsive shock
Large-scale gene expression studies of ECS began around the same time as those of PA; the first experiment using a custom chip targeting 645 genes in the rat hippocampus in 2003 identified increases in Bdnf, Wnt2, Ngf, Fgf2, Fgfr1, Vegf, Npy, Sst, Egr1/2/3, and Fos (Newton, et al., 2003). The following year, Altar et al. (Altar, et al., 2004) used a genome-wide chip to discern hippocampal gene changes resulting from both acute and chronic ECS. They observed 26 genes specifically changed by chronic ECS, which may be more relevant to the clinical effects of the sustained course of treatment as used in human patients. The same study included cortical tissue, in which only three genes were changed by ECS. The authors interpreted this finding to indicate that the hippocampus is primarily responsible for mediating the chronic effects of ECS. In 2007, Conti et al. performed a microarray study of ECS generated region-specific transcriptional profiles from hippocampus, prefrontal cortex (PFC), frontal cortex (FC), amygdala, hypothalamus, dorsal raphe (DR), and locus coeruleus (LC) (Conti, et al., 2007); in this dataset, hundreds of DEGs were detected in hippocampus, but even more were observed in PFC, FC, amygdala, and especially LC. The large discrepancy between studies of specific brain regions may be due primarily to differences in ECS protocol; Altar et al. utilized one shock per day for 10 consecutive days and collected tissue four hours later, while Conti et al. applied four shocks per day for two days and collected tissue one hour later. The neurogenic niche was targeted by Ploski et al. (Ploski, Newton and Duman, 2006), who used laser capture microdissection to isolate the granule cell layer of the rat DG after ECS treatment. Their microarray data revealed ECS-induced upregulation of Gadd45b, Slc1a1, and Ell2, for which dynamic expression could not previously be detected in whole hippocampus.
In addition to directly measuring gene expression, various techniques have been used to study other mechanisms of gene regulation in response to ECS. By combining chromatin immunoprecipitation (ChIP) with microarray technology, Tanis et al. (Tanis, et al., 2008) profiled promoters bound by the transcription factor CREB. It was observed that hippocampal CREB occupancy and phosphorylation was rapidly enhanced by ECS in promoters of gene networks involved in angiogenesis, neurogenesis, plasticity, and trophic support, supporting a role for CREB as a high-level coordinator of gene transcription in the neurogenic response to ECS. Guo et al. (Guo, et al., 2011) used methyl-sensitive cut counting (MSCC) to quantify CpG methylation in DG tissue at single-nucleotide resolution after ECS, implicating epigenetics as a general mechanism by which ECS-induced neurogenic gene networks are regulated by external stimuli. MicroRNAs (miRNAs), considered to be another form of epigenetic regulation of transcription, were investigated using a microarray containing probes for 350 miRNAs in hippocampus after ECS (O’Connor, et al., 2013). 10 miRNAs were found to change in mice raised under normal conditions, but the effect was much more pronounced in mice exposed to early life stress, as demonstrated by significant changes in 86 miRNAs. These changes exhibited convergence with miRNAs altered by ketamine, suggesting that ECS may exert its anti-depressive effects through epigenetic control by miRNAs. However, functional validation of identified genes is largely lacking.
3. Environmental enrichment
The first genome-wide experiment to investigate the transcriptional effects of EE was carried out in mouse cortex (Rampon, et al., 2000) and showed that EE influences gene expression related to neuronal structure, plasticity, and neurotransmission, although not directly related to neurogenesis per se. Early microarray data from the hippocampus of rats housed in an enriched environment for two weeks implicated kinase/phosphatase networks, synapse-related molecules, transcription factors, metabolic enzymes, and the immune system among 58 EE-modulated genes in the neurogenic niche (Keyvani, et al., 2004). A mouse study using five months of EE detected 41 altered genes (Lazarov, et al., 2005), indicating that EE has long-lasting effects on gene expression. Gadd45b was among these genes, which may therefore act as a convergent epigenetic regulator of neurogenesis in response to both EE and ECS.
Future directions and perspective
While genome-wide studies have been performed in hippocampal tissue and specifically within the neurogenic DG, our knowledge of how molecular pathways act across the series of interfaces that relay information to neurogenic cells from an animal’s environment remains primitive. To understand how neurogenesis is orchestrated by an interacting network of diverse CNS cell populations, we must be able to attribute gene expression and protein activity triggered by environmental stimuli to their respective cell types. Modern technologies, including translating ribosome affinity purification (TRAP), RiboTag, and fluorescence-activated cell sorting (FACS), have allowed researchers to isolate and purify specific cell types (Okaty, et al., 2011). Single-cell RNA sequencing is also emerging as a powerful tool to achieve unprecedented specificity in gene expression (Poirion, et al., 2016). Researchers can use these approaches coupled with models of environmentally-induced neurogenesis to deduce the transcriptional activity of individual cells in the DG as they respond to environmental stimuli. A recent publication by Lacar et al. (Lacar, et al., 2016) demonstrates such a design with dentate granule neurons. In this study, mice were exposed to 15 minutes of EE and sacrificed one hour later. Single nuclei were dissociated from DG tissue and immunostained for PROX1 and FOS to identify dentate granule neurons that had been activated by EE, and RNA-seq data were generated from both active (PROX1+FOS+) and inactive (PROX1+FOS-) cells. Over 3,000 DEGs were identified, which highlights the degree to which increasing tissue/cell specificity improves the power of gene expression analysis. Up-regulated DEGs were enriched for downstream targets of FOS as expected, and bore other hallmarks of neuronal activity, including genes related to the MAPK pathway, postsynaptic density, and potassium ion transport. Genes more highly expressed in quiescent neurons represented mitochondria activity and DNA repair. This study serves as a proof of concept that transcriptional effects of environmental stimuli can be observed in cell type-specific expression data. This type of data has recently been generated and analyzed from NSCs (Shin, et al., 2015) and newborn neurons (Gao, et al., 2017). Therefore, an effective strategy for studying environmentally-induced neurogenesis will involve combining models of environmental stimuli with transgenic animals and new technology to capture RNA and protein activity within specific neurogenic cell types as they respond to external influences. For a single cell type, information on epigenetic changes can be collected and layered with expression data to gain mechanistic insight of gene regulation. By assessing the parallel activity of the various cell types interacting within DG tissue to regulate neurogenesis, circuit-like models of activity can be constructed to understand the molecular biology at work at the cellular interfaces that transduce an animal’s experience into a neurogenic response. Finally, gain- and loss-of-function analysis of identified genes and pathways will validate the roles of novel regulatory factors. Identifying and characterizing key molecules in this process will inform efforts to develop targeted therapies with specific effects on brain health.
Supplementary Material
Supplementary Table 1 – Genome-wide studies of environmentally-induced gene regulation. This supplementary table contains a more detailed and comprehensive version of Table 2 with hyperlinks to reference materials.
Acknowledgments
Funding
This work was supported by grants from the NIH to X. Z. (RO1MH080434, RO1MH07897, R21NS095632), grants from the NIH to the Waisman Center (P30HD03352; U54 HD090256), and NIH NRSA to B.E.E. (F32NS094120)
Footnotes
Conflict of Interest
The authors declare no competing financial interests.
References
- Ables JL, Decarolis NA, Johnson MA, Rivera PD, Gao Z, Cooper DC, Radtke F, Hsieh J, Eisch AJ. Notch1 is required for maintenance of the reservoir of adult hippocampal stem cells. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:10484–10492. doi: 10.1523/JNEUROSCI.4721-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aimone JB, Deng W, Gage FH. Adult neurogenesis: integrating theories and separating functions. Trends in cognitive sciences. 2010;14:325–337. doi: 10.1016/j.tics.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aimone JB, Deng W, Gage FH. Resolving new memories: a critical look at the dentate gyrus, adult neurogenesis, and pattern separation. Neuron. 2011;70:589–596. doi: 10.1016/j.neuron.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akers KG, Martinez-Canabal A, Restivo L, Yiu AP, De Cristofaro A, Hsiang HL, Wheeler AL, Guskjolen A, Niibori Y, Shoji H, Ohira K, Richards BA, Miyakawa T, Josselyn SA, Frankland PW. Hippocampal neurogenesis regulates forgetting during adulthood and infancy. Science. 2014;344:598–602. doi: 10.1126/science.1248903. [DOI] [PubMed] [Google Scholar]
- Aksay SS, Bumb JM, Janke C, Biemann R, Borucki K, Lederbogen F, Deuschle M, Sartorius A, Kranaster L. Serum lipid profile changes after successful treatment with electroconvulsive therapy in major depression: A prospective pilot trial. Journal of affective disorders. 2016;189:85–88. doi: 10.1016/j.jad.2015.09.037. [DOI] [PubMed] [Google Scholar]
- Allain B, Jarray R, Borriello L, Leforban B, Dufour S, Liu WQ, Pamonsinlapatham P, Bianco S, Larghero J, Hadj-Slimane R, Garbay C, Raynaud F, Lepelletier Y. Neuropilin-1 regulates a new VEGF-induced gene, Phactr-1, which controls tubulogenesis and modulates lamellipodial dynamics in human endothelial cells. Cellular signalling. 2012;24:214–223. doi: 10.1016/j.cellsig.2011.09.003. [DOI] [PubMed] [Google Scholar]
- Altar CA, Laeng P, Jurata LW, Brockman JA, Lemire A, Bullard J, Bukhman YV, Young TA, Charles V, Palfreyman MG. Electroconvulsive seizures regulate gene expression of distinct neurotrophic signaling pathways. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24:2667–2677. doi: 10.1523/JNEUROSCI.5377-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balu DT, Lucki I. Adult hippocampal neurogenesis: regulation, functional implications, and contribution to disease pathology. Neuroscience and biobehavioral reviews. 2009;33:232–252. doi: 10.1016/j.neubiorev.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckervordersandforth R, Ebert B, Schaffner I, Moss J, Fiebig C, Shin J, Moore DL, Ghosh L, Trinchero MF, Stockburger C, Friedland K, Steib K, von Wittgenstein J, Keiner S, Redecker C, Holter SM, Xiang W, Wurst W, Jagasia R, Schinder AF, Ming GL, Toni N, Jessberger S, Song H, Lie DC. Role of Mitochondrial Metabolism in the Control of Early Lineage Progression and Aging Phenotypes in Adult Hippocampal Neurogenesis. Neuron. 2017;93:1518. doi: 10.1016/j.neuron.2017.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boitard C, Etchamendy N, Sauvant J, Aubert A, Tronel S, Marighetto A, Laye S, Ferreira G. Juvenile, but not adult exposure to high-fat diet impairs relational memory and hippocampal neurogenesis in mice. Hippocampus. 2012;22:2095–2100. doi: 10.1002/hipo.22032. [DOI] [PubMed] [Google Scholar]
- Bolijn SL, Paul J. How the Body Talks to the Brain; Peripheral Mediators of Physical Activity-Induced Proliferation in the Adult Hippocampus. Brain Plasticity. 2015;1:5–27. doi: 10.3233/BPL-150020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J, Cooper-Kuhn CM, Kempermann G, Van Praag H, Winkler J, Gage FH, Kuhn HG. Enriched environment and physical activity stimulate hippocampal but not olfactory bulb neurogenesis. The European journal of neuroscience. 2003;17:2042–2046. doi: 10.1046/j.1460-9568.2003.02647.x. [DOI] [PubMed] [Google Scholar]
- Cardona AE, Pioro EP, Sasse ME, Kostenko V, Cardona SM, Dijkstra IM, Huang D, Kidd G, Dombrowski S, Dutta R, Lee JC, Cook DN, Jung S, Lira SA, Littman DR, Ransohoff RM. Control of microglial neurotoxicity by the fractalkine receptor. Nature neuroscience. 2006;9:917–924. doi: 10.1038/nn1715. [DOI] [PubMed] [Google Scholar]
- Chabry J, Nicolas S, Cazareth J, Murris E, Guyon A, Glaichenhaus N, Heurteaux C, Petit-Paitel A. Enriched environment decreases microglia and brain macrophages inflammatory phenotypes through adiponectin-dependent mechanisms: Relevance to depressive-like behavior. Brain Behav Immun. 2015;50:275–287. doi: 10.1016/j.bbi.2015.07.018. [DOI] [PubMed] [Google Scholar]
- Chae CH, Kim HT. Forced, moderate-intensity treadmill exercise suppresses apoptosis by increasing the level of NGF and stimulating phosphatidylinositol 3-kinase signaling in the hippocampus of induced aging rats. Neurochemistry international. 2009;55:208–213. doi: 10.1016/j.neuint.2009.02.024. [DOI] [PubMed] [Google Scholar]
- Chancey JH, Adlaf EW, Sapp MC, Pugh PC, Wadiche JI, Overstreet-Wadiche LS. GABA depolarization is required for experience-dependent synapse unsilencing in adult-born neurons. J Neurosci. 2013;33:6614–6622. doi: 10.1523/JNEUROSCI.0781-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian KM, Song H, Ming GL. Functions and dysfunctions of adult hippocampal neurogenesis. Annual review of neuroscience. 2014;37:243–262. doi: 10.1146/annurev-neuro-071013-014134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark PJ, Kohman RA, Miller DS, Bhattacharya TK, Haferkamp EH, Rhodes JS. Adult hippocampal neurogenesis and c-Fos induction during escalation of voluntary wheel running in C57BL/6J mice. Behavioural brain research. 2010;213:246–252. doi: 10.1016/j.bbr.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clelland CD, Choi M, Romberg C, Clemenson GD, Jr, Fragniere A, Tyers P, Jessberger S, Saksida LM, Barker RA, Gage FH, Bussey TJ. A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science. 2009;325:210–213. doi: 10.1126/science.1173215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemenson GDD, Wei Gage, Fred H. Environmental enrichment and neurogenesis: from mice to humans. Current Opinion in Behavioral Sciences. 2015;4:56–62. [Google Scholar]
- Conti B, Maier R, Barr AM, Morale MC, Lu X, Sanna PP, Bilbe G, Hoyer D, Bartfai T. Region-specific transcriptional changes following the three antidepressant treatments electro convulsive therapy, sleep deprivation and fluoxetine. Molecular psychiatry. 2007;12:167–189. doi: 10.1038/sj.mp.4001897. [DOI] [PubMed] [Google Scholar]
- Cotman CW, Berchtold NC. Exercise: a behavioral intervention to enhance brain health and plasticity. Trends in neurosciences. 2002;25:295–301. doi: 10.1016/s0166-2236(02)02143-4. [DOI] [PubMed] [Google Scholar]
- Czeh B, Welt T, Fischer AK, Erhardt A, Schmitt W, Muller MB, Toschi N, Fuchs E, Keck ME. Chronic psychosocial stress and concomitant repetitive transcranial magnetic stimulation: effects on stress hormone levels and adult hippocampal neurogenesis. Biological psychiatry. 2002;52:1057–1065. doi: 10.1016/s0006-3223(02)01457-9. [DOI] [PubMed] [Google Scholar]
- Darcy MJ, Trouche S, Jin SX, Feig LA. Age-dependent role for Ras-GRF1 in the late stages of adult neurogenesis in the dentate gyrus. Hippocampus. 2014a;24:315–325. doi: 10.1002/hipo.22225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darcy MJ, Trouche S, Jin SX, Feig LA. Ras-GRF2 mediates long-term potentiation, survival, and response to an enriched environment of newborn neurons in the hippocampus. Hippocampus. 2014b;24:1317–1329. doi: 10.1002/hipo.22313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Lucia C, Rinchon A, Olmos-Alonso A, Riecken K, Fehse B, Boche D, Perry VH, Gomez-Nicola D. Microglia regulate hippocampal neurogenesis during chronic neurodegeneration. Brain, behavior, and immunity. 2016;55:179–190. doi: 10.1016/j.bbi.2015.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng W, Aimone JB, Gage FH. New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory? Nature reviews Neuroscience. 2010;11:339–350. doi: 10.1038/nrn2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieni CV, Chancey JH, Overstreet-Wadiche LS. Dynamic functions of GABA signaling during granule cell maturation. Front Neural Circuits. 2012;6:113. doi: 10.3389/fncir.2012.00113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donovan MH, Yazdani U, Norris RD, Games D, German DC, Eisch AJ. Decreased adult hippocampal neurogenesis in the PDAPP mouse model of Alzheimer’s disease. The Journal of comparative neurology. 2006;495:70–83. doi: 10.1002/cne.20840. [DOI] [PubMed] [Google Scholar]
- Dostes S, Dubreucq S, Ladeveze E, Marsicano G, Abrous DN, Chaouloff F, Koehl M. Running per se stimulates the dendritic arbor of newborn dentate granule cells in mouse hippocampus in a duration-dependent manner. Hippocampus. 2016;26:282–288. doi: 10.1002/hipo.22551. [DOI] [PubMed] [Google Scholar]
- Dranovsky A, Hen R. Hippocampal neurogenesis: regulation by stress and antidepressants. Biological psychiatry. 2006;59:1136–1143. doi: 10.1016/j.biopsych.2006.03.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumitru I, Neitz A, Alfonso J, Monyer H. Diazepam Binding Inhibitor Promotes Stem Cell Expansion Controlling Environment-Dependent Neurogenesis. Neuron. 2017;94:125–137. doi: 10.1016/j.neuron.2017.03.003. e125. [DOI] [PubMed] [Google Scholar]
- Embury CM, Dyavarshetty B, Lu Y, Wiederin JL, Ciborowski P, Gendelman HE, Kiyota T. Cathepsin B Improves ss-Amyloidosis and Learning and Memory in Models of Alzheimer’s Disease. Journal of neuroimmune pharmacology : the official journal of the Society on NeuroImmune Pharmacology. 2016 doi: 10.1007/s11481-016-9721-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan C, Zhao Y, Mao X, Miao Y, Lin X, Jiang G, Zhang X, Han Q, Luan L, Wang E. Armc8 expression was elevated during atypia-to-carcinoma progression and associated with cancer development of breast carcinoma. Tumour biology : the journal of the International Society for Oncodevelopmental Biology and Medicine. 2014;35:11337–11343. doi: 10.1007/s13277-014-2473-0. [DOI] [PubMed] [Google Scholar]
- Fang ZH, Lee CH, Seo MK, Cho H, Lee JG, Lee BJ, Park SW, Kim YH. Effect of treadmill exercise on the BDNF-mediated pathway in the hippocampus of stressed rats. Neuroscience research. 2013;76:187–194. doi: 10.1016/j.neures.2013.04.005. [DOI] [PubMed] [Google Scholar]
- Farmer J, Zhao X, van Praag H, Wodtke K, Gage FH, Christie BR. Effects of voluntary exercise on synaptic plasticity and gene expression in the dentate gyrus of adult male Sprague-Dawley rats in vivo. Neuroscience. 2004;124:71–79. doi: 10.1016/j.neuroscience.2003.09.029. [DOI] [PubMed] [Google Scholar]
- Feig LA. Regulation of Neuronal Function by Ras-GRF Exchange Factors. Genes & cancer. 2011;2:306–319. doi: 10.1177/1947601911408077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson MA, White LJ, McCoy S, Kim HW, Petty T, Wilsey J. Plasma adiponectin response to acute exercise in healthy subjects. Eur J Appl Physiol. 2004;91:324–329. doi: 10.1007/s00421-003-0985-1. [DOI] [PubMed] [Google Scholar]
- Fordyce DE, Farrar RP. Enhancement of spatial learning in F344 rats by physical activity and related learning-associated alterations in hippocampal and cortical cholinergic functioning. Behavioural brain research. 1991;46:123–133. doi: 10.1016/s0166-4328(05)80105-6. [DOI] [PubMed] [Google Scholar]
- Fowler CD, Liu Y, Ouimet C, Wang Z. The effects of social environment on adult neurogenesis in the female prairie vole. Journal of neurobiology. 2002;51:115–128. doi: 10.1002/neu.10042. [DOI] [PubMed] [Google Scholar]
- Frankland PW, Kohler S, Josselyn SA. Hippocampal neurogenesis and forgetting. Trends in neurosciences. 2013;36:497–503. doi: 10.1016/j.tins.2013.05.002. [DOI] [PubMed] [Google Scholar]
- Gao Y, Wang F, Eisinger BE, Kelnhofer LE, Jobe EM, Zhao X. Integrative Single-Cell Transcriptomics Reveals Molecular Networks Defining Neuronal Maturation During Postnatal Neurogenesis. Cerebral cortex. 2017;27:2064–2077. doi: 10.1093/cercor/bhw040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett L, Lie DC, Hrabe de Angelis M, Wurst W, Holter SM. Voluntary wheel running in mice increases the rate of neurogenesis without affecting anxiety-related behaviour in single tests. BMC neuroscience. 2012;13:61. doi: 10.1186/1471-2202-13-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge S, Pradhan DA, Ming GL, Song H. GABA sets the tempo for activity-dependent adult neurogenesis. Trends in neurosciences. 2007;30:1–8. doi: 10.1016/j.tins.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Gibbons TE, Pence BD, Petr G, Ossyra JM, Mach HC, Bhattacharya TK, Perez S, Martin SA, McCusker RH, Kelley KW, Rhodes JS, Johnson RW, Woods JA. Voluntary wheel running, but not a diet containing (-)-epigallocatechin-3-gallate and beta-alanine, improves learning, memory and hippocampal neurogenesis in aged mice. Behavioural brain research. 2014;272:131–140. doi: 10.1016/j.bbr.2014.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E, Tanapat P. Stress and hippocampal neurogenesis. Biological psychiatry. 1999;46:1472–1479. doi: 10.1016/s0006-3223(99)00247-4. [DOI] [PubMed] [Google Scholar]
- Gould E, Tanapat P, Rydel T, Hastings N. Regulation of hippocampal neurogenesis in adulthood. Biological psychiatry. 2000;48:715–720. doi: 10.1016/s0006-3223(00)01021-0. [DOI] [PubMed] [Google Scholar]
- Griffin EW, Bechara RG, Birch AM, Kelly AM. Exercise enhances hippocampal-dependent learning in the rat: evidence for a BDNF-related mechanism. Hippocampus. 2009;19:973–980. doi: 10.1002/hipo.20631. [DOI] [PubMed] [Google Scholar]
- Guerrieri D, van Praag H. Exercise-mimetic AICAR transiently benefits brain function. Oncotarget. 2015;6:18293–18313. doi: 10.18632/oncotarget.4715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo JU, Ma DK, Mo H, Ball MP, Jang MH, Bonaguidi MA, Balazer JA, Eaves HL, Xie B, Ford E, Zhang K, Ming GL, Gao Y, Song H. Neuronal activity modifies the DNA methylation landscape in the adult brain. Nature neuroscience. 2011;14:1345–1351. doi: 10.1038/nn.2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusdon AM, Callio J, Distefano G, O’Doherty RM, Goodpaster BH, Coen PM, Chu CT. Exercise increases mitochondrial complex I activity and DRP1 expression in the brains of aged mice. Experimental gerontology. 2017;90:1–13. doi: 10.1016/j.exger.2017.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulver MW, Zheng D, Tanner CJ, Houmard JA, Kraus WE, Slentz CA, Sinha MK, Pories WJ, MacDonald KG, Dohm GL. Adiponectin is not altered with exercise training despite enhanced insulin action. Am J Physiol Endocrinol Metab. 2002;283:E861–865. doi: 10.1152/ajpendo.00150.2002. [DOI] [PubMed] [Google Scholar]
- Imayoshi I, Sakamoto M, Ohtsuka T, Takao K, Miyakawa T, Yamaguchi M, Mori K, Ikeda T, Itohara S, Kageyama R. Roles of continuous neurogenesis in the structural and functional integrity of the adult forebrain. Nature neuroscience. 2008;11:1153–1161. doi: 10.1038/nn.2185. [DOI] [PubMed] [Google Scholar]
- Inoue K, Okamoto M, Shibato J, Lee MC, Matsui T, Rakwal R, Soya H. Long-Term Mild, rather than Intense, Exercise Enhances Adult Hippocampal Neurogenesis and Greatly Changes the Transcriptomic Profile of the Hippocampus. PloS one. 2015;10:e0128720. doi: 10.1371/journal.pone.0128720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Intlekofer KA, Cotman CW. Exercise counteracts declining hippocampal function in aging and Alzheimer’s disease. Neurobiology of disease. 2013;57:47–55. doi: 10.1016/j.nbd.2012.06.011. [DOI] [PubMed] [Google Scholar]
- Ji JF, Ji SJ, Sun R, Li K, Zhang Y, Zhang LY, Tian Y. Forced running exercise attenuates hippocampal neurogenesis impairment and the neurocognitive deficits induced by whole-brain irradiation via the BDNF-mediated pathway. Biochemical and biophysical research communications. 2014;443:646–651. doi: 10.1016/j.bbrc.2013.12.031. [DOI] [PubMed] [Google Scholar]
- Jin K, Sun Y, Xie L, Batteur S, Mao XO, Smelick C, Logvinova A, Greenberg DA. Neurogenesis and aging: FGF-2 and HB-EGF restore neurogenesis in hippocampus and subventricular zone of aged mice. Aging cell. 2003;2:175–183. doi: 10.1046/j.1474-9728.2003.00046.x. [DOI] [PubMed] [Google Scholar]
- Johnson RA, Mitchell GS. Exercise-induced changes in hippocampal brain-derived neurotrophic factor and neurotrophin-3: effects of rat strain. Brain research. 2003;983:108–114. doi: 10.1016/s0006-8993(03)03039-7. [DOI] [PubMed] [Google Scholar]
- Johnson RA, Rhodes JS, Jeffrey SL, Garland T, Jr, Mitchell GS. Hippocampal brain-derived neurotrophic factor but not neurotrophin-3 increases more in mice selected for increased voluntary wheel running. Neuroscience. 2003;121:1–7. doi: 10.1016/s0306-4522(03)00422-6. [DOI] [PubMed] [Google Scholar]
- Jun H, Hussaini SM, Cho CH, Welby J, Jang MH. Gadd45b Mediates Electroconvulsive Shock Induced Proliferation of Hippocampal Neural Stem Cells. Brain stimulation. 2015;8:1021–1024. doi: 10.1016/j.brs.2015.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempermann G, Krebs J, Fabel K. The contribution of failing adult hippocampal neurogenesis to psychiatric disorders. Current opinion in psychiatry. 2008;21:290–295. doi: 10.1097/YCO.0b013e3282fad375. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Song H, Gage FH. Neurogenesis in the Adult Hippocampus. Cold Spring Harbor perspectives in biology. 2015;7:a018812. doi: 10.1101/cshperspect.a018812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyvani K, Sachser N, Witte OW, Paulus W. Gene expression profiling in the intact and injured brain following environmental enrichment. Journal of neuropathology and experimental neurology. 2004;63:598–609. doi: 10.1093/jnen/63.6.598. [DOI] [PubMed] [Google Scholar]
- Klempin F, Kempermann G. Adult hippocampal neurogenesis and aging. European archives of psychiatry and clinical neuroscience. 2007;257:271–280. doi: 10.1007/s00406-007-0731-5. [DOI] [PubMed] [Google Scholar]
- Kobilo T, Liu QR, Gandhi K, Mughal M, Shaham Y, van Praag H. Running is the neurogenic and neurotrophic stimulus in environmental enrichment. Learning & memory. 2011;18:605–609. doi: 10.1101/lm.2283011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohman RA, Rodriguez-Zas SL, Southey BR, Kelley KW, Dantzer R, Rhodes JS. Voluntary wheel running reverses age-induced changes in hippocampal gene expression. PloS one. 2011;6:e22654. doi: 10.1371/journal.pone.0022654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronenberg G, Bick-Sander A, Bunk E, Wolf C, Ehninger D, Kempermann G. Physical exercise prevents age-related decline in precursor cell activity in the mouse dentate gyrus. Neurobiology of aging. 2006;27:1505–1513. doi: 10.1016/j.neurobiolaging.2005.09.016. [DOI] [PubMed] [Google Scholar]
- Kuhn HG, Dickinson-Anson H, Gage FH. Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1996;16:2027–2033. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwabara T, Hsieh J, Muotri A, Yeo G, Warashina M, Lie DC, Moore L, Nakashima K, Asashima M, Gage FH. Wnt-mediated activation of NeuroD1 and retro-elements during adult neurogenesis. Nature neuroscience. 2009;12:1097–1105. doi: 10.1038/nn.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacar B, Linker SB, Jaeger BN, Krishnaswami S, Barron J, Kelder M, Parylak S, Paquola A, Venepally P, Novotny M, O’Connor C, Fitzpatrick C, Erwin J, Hsu JY, Husband D, McConnell MJ, Lasken R, Gage FH. Nuclear RNA-seq of single neurons reveals molecular signatures of activation. Nature communications. 2016;7:11022. doi: 10.1038/ncomms11022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarov O, Robinson J, Tang YP, Hairston IS, Korade-Mirnics Z, Lee VM, Hersh LB, Sapolsky RM, Mirnics K, Sisodia SS. Environmental enrichment reduces Abeta levels and amyloid deposition in transgenic mice. Cell. 2005;120:701–713. doi: 10.1016/j.cell.2005.01.015. [DOI] [PubMed] [Google Scholar]
- Lee J, Duan W, Mattson MP. Evidence that brain-derived neurotrophic factor is required for basal neurogenesis and mediates, in part, the enhancement of neurogenesis by dietary restriction in the hippocampus of adult mice. Journal of neurochemistry. 2002a;82:1367–1375. doi: 10.1046/j.1471-4159.2002.01085.x. [DOI] [PubMed] [Google Scholar]
- Lee J, Seroogy KB, Mattson MP. Dietary restriction enhances neurotrophin expression and neurogenesis in the hippocampus of adult mice. Journal of neurochemistry. 2002b;80:539–547. doi: 10.1046/j.0022-3042.2001.00747.x. [DOI] [PubMed] [Google Scholar]
- Lee MC, Rakwal R, Shibato J, Inoue K, Chang H, Soya H. DNA microarray-based analysis of voluntary resistance wheel running reveals novel transcriptome leading robust hippocampal plasticity. Physiological reports. 2014;2 doi: 10.14814/phy2.12206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuner B, Gould E. Structural plasticity and hippocampal function. Annual review of psychology. 2010;61:111–140. doi: 10.1146/annurev.psych.093008.100359. C111-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberwirth C, Liu Y, Jia X, Wang Z. Social isolation impairs adult neurogenesis in the limbic system and alters behaviors in female prairie voles. Hormones and behavior. 2012;62:357–366. doi: 10.1016/j.yhbeh.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindqvist A, Mohapel P, Bouter B, Frielingsdorf H, Pizzo D, Brundin P, Erlanson-Albertsson C. High-fat diet impairs hippocampal neurogenesis in male rats. European journal of neurology. 2006;13:1385–1388. doi: 10.1111/j.1468-1331.2006.01500.x. [DOI] [PubMed] [Google Scholar]
- Littlefield AM, Setti SE, Priester C, Kohman RA. Voluntary exercise attenuates LPS-induced reductions in neurogenesis and increases microglia expression of a proneurogenic phenotype in aged mice. Journal of neuroinflammation. 2015;12:138. doi: 10.1186/s12974-015-0362-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llorens-Martin M, Teixeira CM, Jurado-Arjona J, Rakwal R, Shibato J, Soya H, Avila J. Retroviral induction of GSK-3beta expression blocks the stimulatory action of physical exercise on the maturation of newborn neurons. Cellular and molecular life sciences : CMLS. 2016;73:3569–3582. doi: 10.1007/s00018-016-2181-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma DK, Jang MH, Guo JU, Kitabatake Y, Chang ML, Pow-Anpongkul N, Flavell RA, Lu B, Ming GL, Song H. Neuronal activity-induced Gadd45b promotes epigenetic DNA demethylation and adult neurogenesis. Science. 2009;323:1074–1077. doi: 10.1126/science.1166859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen TM, Treschow A, Bengzon J, Bolwig TG, Lindvall O, Tingstrom A. Increased neurogenesis in a model of electroconvulsive therapy. Biological psychiatry. 2000;47:1043–1049. doi: 10.1016/s0006-3223(00)00228-6. [DOI] [PubMed] [Google Scholar]
- Malberg JE, Eisch AJ, Nestler EJ, Duman RS. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2000;20:9104–9110. doi: 10.1523/JNEUROSCI.20-24-09104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molteni R, Ying Z, Gomez-Pinilla F. Differential effects of acute and chronic exercise on plasticity-related genes in the rat hippocampus revealed by microarray. The European journal of neuroscience. 2002;16:1107–1116. doi: 10.1046/j.1460-9568.2002.02158.x. [DOI] [PubMed] [Google Scholar]
- Monteiro BM, Moreira FA, Massensini AR, Moraes MF, Pereira GS. Enriched environment increases neurogenesis and improves social memory persistence in socially isolated adult mice. Hippocampus. 2014;24:239–248. doi: 10.1002/hipo.22218. [DOI] [PubMed] [Google Scholar]
- Moon HY, Becke A, Berron D, Becker B, Sah N, Benoni G, Janke E, Lubejko ST, Greig NH, Mattison JA, Duzel E, van Praag H. Running-Induced Systemic Cathepsin B Secretion Is Associated with Memory Function. Cell metabolism. 2016;24:332–340. doi: 10.1016/j.cmet.2016.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu Y, Gage FH. Adult hippocampal neurogenesis and its role in Alzheimer’s disease. Molecular neurodegeneration. 2011;6:85. doi: 10.1186/1750-1326-6-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neeper SA, Gomez-Pinilla F, Choi J, Cotman CW. Physical activity increases mRNA for brain-derived neurotrophic factor and nerve growth factor in rat brain. Brain research. 1996;726:49–56. [PubMed] [Google Scholar]
- Newton SS, Collier EF, Hunsberger J, Adams D, Terwilliger R, Selvanayagam E, Duman RS. Gene profile of electroconvulsive seizures: induction of neurotrophic and angiogenic factors. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2003;23:10841–10851. doi: 10.1523/JNEUROSCI.23-34-10841.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolas S, Veyssiere J, Gandin C, Zsurger N, Pietri M, Heurteaux C, Glaichenhaus N, Petit-Paitel A, Chabry J. Neurogenesis-independent antidepressant-like effects of enriched environment is dependent on adiponectin. Psychoneuroendocrinology. 2015;57:72–83. doi: 10.1016/j.psyneuen.2015.03.017. [DOI] [PubMed] [Google Scholar]
- O’Connor RM, Grenham S, Dinan TG, Cryan JF. microRNAs as novel antidepressant targets: converging effects of ketamine and electroconvulsive shock therapy in the rat hippocampus. The international journal of neuropsychopharmacology. 2013;16:1885–1892. doi: 10.1017/S1461145713000448. [DOI] [PubMed] [Google Scholar]
- Okamoto M, Inoue K, Iwamura H, Terashima K, Soya H, Asashima M, Kuwabara T. Reduction in paracrine Wnt3 factors during aging causes impaired adult neurogenesis. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2011;25:3570–3582. doi: 10.1096/fj.11-184697. [DOI] [PubMed] [Google Scholar]
- Okaty BW, Sugino K, Nelson SB. Cell type-specific transcriptomics in the brain. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:6939–6943. doi: 10.1523/JNEUROSCI.0626-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson AK, Eadie BD, Ernst C, Christie BR. Environmental enrichment and voluntary exercise massively increase neurogenesis in the adult hippocampus via dissociable pathways. Hippocampus. 2006;16:250–260. doi: 10.1002/hipo.20157. [DOI] [PubMed] [Google Scholar]
- Opendak M, Gould E. Adult neurogenesis: a substrate for experience-dependent change. Trends in cognitive sciences. 2015;19:151–161. doi: 10.1016/j.tics.2015.01.001. [DOI] [PubMed] [Google Scholar]
- Overall RW, Walker TL, Fischer TJ, Brandt MD, Kempermann G. Different Mechanisms Must Be Considered to Explain the Increase in Hippocampal Neural Precursor Cell Proliferation by Physical Activity. Frontiers in neuroscience. 2016;10:362. doi: 10.3389/fnins.2016.00362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overstreet Wadiche L, Bromberg DA, Bensen AL, Westbrook GL. GABAergic signaling to newborn neurons in dentate gyrus. J Neurophysiol. 2005;94:4528–4532. doi: 10.1152/jn.00633.2005. [DOI] [PubMed] [Google Scholar]
- Owens DF, Kriegstein AR. Is there more to GABA than synaptic inhibition? Nature reviews Neuroscience. 2002;3:715–727. doi: 10.1038/nrn919. [DOI] [PubMed] [Google Scholar]
- Pandey MK, DeGrado TR. Glycogen Synthase Kinase-3 (GSK-3)-Targeted Therapy and Imaging. Theranostics. 2016;6:571–593. doi: 10.7150/thno.14334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ploski JE, Newton SS, Duman RS. Electroconvulsive seizure-induced gene expression profile of the hippocampus dentate gyrus granule cell layer. Journal of neurochemistry. 2006;99:1122–1132. doi: 10.1111/j.1471-4159.2006.04156.x. [DOI] [PubMed] [Google Scholar]
- Poirion OB, Zhu X, Ching T, Garmire L. Single-Cell Transcriptomics Bioinformatics and Computational Challenges. Frontiers in genetics. 2016;7:163. doi: 10.3389/fgene.2016.00163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rampon C, Jiang CH, Dong H, Tang YP, Lockhart DJ, Schultz PG, Tsien JZ, Hu Y. Effects of environmental enrichment on gene expression in the brain. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:12880–12884. doi: 10.1073/pnas.97.23.12880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redila VA, Christie BR. Exercise-induced changes in dendritic structure and complexity in the adult hippocampal dentate gyrus. Neuroscience. 2006;137:1299–1307. doi: 10.1016/j.neuroscience.2005.10.050. [DOI] [PubMed] [Google Scholar]
- Reshef R, Kreisel T, Beroukhim Kay D, Yirmiya R. Microglia and their CX3CR1 signaling are involved in hippocampal- but not olfactory bulb-related memory and neurogenesis. Brain, behavior, and immunity. 2014;41:239–250. doi: 10.1016/j.bbi.2014.04.009. [DOI] [PubMed] [Google Scholar]
- Richetin K, Moulis M, Millet A, Arrazola MS, Andraini T, Hua J, Davezac N, Roybon L, Belenguer P, Miquel MC, Rampon C. Amplifying mitochondrial function rescues adult neurogenesis in a mouse model of Alzheimer’s disease. Neurobiology of disease. 2017;102:113–124. doi: 10.1016/j.nbd.2017.03.002. [DOI] [PubMed] [Google Scholar]
- Russo-Neustadt A, Beard RC, Cotman CW. Exercise, antidepressant medications, and enhanced brain derived neurotrophic factor expression. Neuropsychopharmacology. 1999;21:679–682. doi: 10.1016/S0893-133X(99)00059-7. [DOI] [PubMed] [Google Scholar]
- Sahay A, Hen R. Adult hippocampal neurogenesis in depression. Nature neuroscience. 2007;10:1110–1115. doi: 10.1038/nn1969. [DOI] [PubMed] [Google Scholar]
- Schloesser RJ, Orvoen S, Jimenez DV, Hardy NF, Maynard KR, Sukumar M, Manji HK, Gardier AM, David DJ, Martinowich K. Antidepressant-like Effects of Electroconvulsive Seizures Require Adult Neurogenesis in a Neuroendocrine Model of Depression. Brain stimulation. 2015;8:862–867. doi: 10.1016/j.brs.2015.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott BW, Wojtowicz JM, Burnham WM. Neurogenesis in the dentate gyrus of the rat following electroconvulsive shock seizures. Experimental neurology. 2000;165:231–236. doi: 10.1006/exnr.2000.7458. [DOI] [PubMed] [Google Scholar]
- Shin J, Berg DA, Zhu Y, Shin JY, Song J, Bonaguidi MA, Enikolopov G, Nauen DW, Christian KM, Ming GL, Song H. Single-Cell RNA-Seq with Waterfall Reveals Molecular Cascades underlying Adult Neurogenesis. Cell stem cell. 2015;17:360–372. doi: 10.1016/j.stem.2015.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra A, Beccari S, Diaz-Aparicio I, Encinas JM, Comeau S, Tremblay ME. Surveillance, phagocytosis, and inflammation: how never-resting microglia influence adult hippocampal neurogenesis. Neural plasticity. 2014;2014:610343. doi: 10.1155/2014/610343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra A, Encinas JM, Deudero JJ, Chancey JH, Enikolopov G, Overstreet-Wadiche LS, Tsirka SE, Maletic-Savatic M. Microglia shape adult hippocampal neurogenesis through apoptosis-coupled phagocytosis. Cell stem cell. 2010;7:483–495. doi: 10.1016/j.stem.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J, Crowther AJ, Olsen RHJ, Song H, Ming G-l. A diametric mode of neuronal circuitry-neurogenesis coupling in the adult hippocampus via parvalbumin interneurons. Neurogenesis. 2014;1:e29949. doi: 10.1038/nn.3572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J, Sun J, Moss J, Wen Z, Sun GJ, Hsu D, Zhong C, Davoudi H, Christian KM, Toni N, Ming GL, Song H. Parvalbumin interneurons mediate neuronal circuitry-neurogenesis coupling in the adult hippocampus. Nature neuroscience. 2013;16:1728–1730. doi: 10.1038/nn.3572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stangl D, Thuret S. Impact of diet on adult hippocampal neurogenesis. Genes & nutrition. 2009;4:271–282. doi: 10.1007/s12263-009-0134-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steib K, Schaffner I, Jagasia R, Ebert B, Lie DC. Mitochondria modify exercise-induced development of stem cell-derived neurons in the adult brain. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2014;34:6624–6633. doi: 10.1523/JNEUROSCI.4972-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stranahan AM, Khalil D, Gould E. Social isolation delays the positive effects of running on adult neurogenesis. Nature neuroscience. 2006;9:526–533. doi: 10.1038/nn1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stranahan AM, Khalil D, Gould E. Running induces widespread structural alterations in the hippocampus and entorhinal cortex. Hippocampus. 2007;17:1017–1022. doi: 10.1002/hipo.20348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H, Kanagawa D, Nakazawa H, Tawara-Hirata Y, Kogure Y, Shimizu-Okabe C, Takayama C, Ishikawa Y, Shiosaka S. Role of neuropsin in parvalbumin immunoreactivity changes in hippocampal basket terminals of mice reared in various environments. Frontiers in cellular neuroscience. 2014;8:420. doi: 10.3389/fncel.2014.00420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura H, Kawata M, Hamaguchi S, Ishikawa Y, Shiosaka S. Processing of neuregulin-1 by neuropsin regulates GABAergic neuron to control neural plasticity of the mouse hippocampus. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:12657–12672. doi: 10.1523/JNEUROSCI.2542-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanis KQ, Duman RS, Newton SS. CREB binding and activity in brain: regional specificity and induction by electroconvulsive seizure. Biological psychiatry. 2008;63:710–720. doi: 10.1016/j.biopsych.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong L, Shen H, Perreau VM, Balazs R, Cotman CW. Effects of exercise on gene-expression profile in the rat hippocampus. Neurobiology of disease. 2001;8:1046–1056. doi: 10.1006/nbdi.2001.0427. [DOI] [PubMed] [Google Scholar]
- Tozuka Y, Wada E, Wada K. Diet-induced obesity in female mice leads to peroxidized lipid accumulations and impairment of hippocampal neurogenesis during the early life of their offspring. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2009;23:1920–1934. doi: 10.1096/fj.08-124784. [DOI] [PubMed] [Google Scholar]
- Tummalapalli P, Spomar D, Gondi CS, Olivero WC, Gujrati M, Dinh DH, Rao JS. RNAi-mediated abrogation of cathepsin B and MMP-9 gene expression in a malignant meningioma cell line leads to decreased tumor growth, invasion and angiogenesis. International journal of oncology. 2007;31:1039–1050. [PMC free article] [PubMed] [Google Scholar]
- van Praag H. Neurogenesis and exercise: past and future directions. Neuromolecular medicine. 2008;10:128–140. doi: 10.1007/s12017-008-8028-z. [DOI] [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nature neuroscience. 1999;2:266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- Vivar C, Potter MC, van Praag H. All about running: synaptic plasticity, growth factors and adult hippocampal neurogenesis. Current topics in behavioral neurosciences. 2013;15:189–210. doi: 10.1007/7854_2012_220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vukovic J, Colditz MJ, Blackmore DG, Ruitenberg MJ, Bartlett PF. Microglia modulate hippocampal neural precursor activity in response to exercise and aging. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:6435–6443. doi: 10.1523/JNEUROSCI.5925-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner-Schmidt JL, Duman RS. Hippocampal neurogenesis: opposing effects of stress and antidepressant treatment. Hippocampus. 2006;16:239–249. doi: 10.1002/hipo.20156. [DOI] [PubMed] [Google Scholar]
- Wegiel J, Kuchna I, Nowicki K, Imaki H, Wegiel J, Marchi E, Ma SY, Chauhan A, Chauhan V, Bobrowicz TW, de Leon M, Louis LA, Cohen IL, London E, Brown WT, Wisniewski T. The neuropathology of autism: defects of neurogenesis and neuronal migration, and dysplastic changes. Acta neuropathologica. 2010;119:755–770. doi: 10.1007/s00401-010-0655-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D, Pan W. GSK3: a multifaceted kinase in Wnt signaling. Trends in biochemical sciences. 2010;35:161–168. doi: 10.1016/j.tibs.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Ku B, Cui L, Li X, Barish PA, Foster TC, Ogle WO. Curcumin reverses impaired hippocampal neurogenesis and increases serotonin receptor 1A mRNA and brain-derived neurotrophic factor expression in chronically stressed rats. Brain research. 2007;1162:9–18. doi: 10.1016/j.brainres.2007.05.071. [DOI] [PubMed] [Google Scholar]
- Yanpallewar SU, Barrick CA, Palko ME, Fulgenzi G, Tessarollo L. Tamalin is a critical mediator of electroconvulsive shock-induced adult neuroplasticity. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:2252–2262. doi: 10.1523/JNEUROSCI.5493-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau SY, Li A, Hoo RL, Ching YP, Christie BR, Lee TM, Xu A, So KF. Physical exercise-induced hippocampal neurogenesis and antidepressant effects are mediated by the adipocyte hormone adiponectin. Proc Natl Acad Sci U S A. 2014;111:15810–15815. doi: 10.1073/pnas.1415219111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu JL, Ma L, Ma L, Tao YZ. [Voluntary wheel running enhances cell proliferation and expression levels of BDNF, IGF1 and WNT4 in dentate gyrus of adult mice] Sheng li xue bao : [Acta physiologica Sinica] 2014;66:559–568. [PubMed] [Google Scholar]
- Zaben MJ, Gray WP. Neuropeptides and hippocampal neurogenesis. Neuropeptides. 2013;47:431–438. doi: 10.1016/j.npep.2013.10.002. [DOI] [PubMed] [Google Scholar]
- Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132:645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Liu T, Sun X, Mu X, Zhu G, Xiao T, Zhao M, Zhao C. CXCR4 antagonist AMD3100 reverses the neurogenesis promoted by enriched environment and suppresses long-term seizure activity in adult rats of temporal lobe epilepsy. Behavioural brain research. 2017;322:83–91. doi: 10.1016/j.bbr.2017.01.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1 – Genome-wide studies of environmentally-induced gene regulation. This supplementary table contains a more detailed and comprehensive version of Table 2 with hyperlinks to reference materials.