Abstract
BACKGROUND
Stomach cancer was a leading cause of cancer-related deaths early in the 20th century and has steadily declined over the last century in the United States. Although incidence and death rates are now low, stomach cancer remains an important cause of morbidity and mortality in black, Asian and Pacific Islander, and American Indian/Alaska Native populations.
METHODS
Data from the CONCORD-2 study were used to analyze stomach cancer survival among males and females aged 15 to 99 years who were diagnosed in 37 states covering 80% of the US population. Survival analyses were corrected for background mortality using state-specific and race-specific (white and black) life tables and age-standardized using the International Cancer Survival Standard weights. Net survival is presented up to 5 years after diagnosis by race (all, black, and white) for 2001 through 2003 and 2004 through 2009 to account for changes in collecting Surveillance, Epidemiology, and End Results Summary Stage 2000 data from 2004.
RESULTS
Almost one-third of stomach cancers were diagnosed at a distant stage among both whites and blacks. Age-standardized 5-year net survival increased between 2001 to 2003 and 2004 to 2009 (26.1% and 29%, respectively), and no differences were observed by race. The 1-year, 3-year, and 5-year survival estimates were 53.1%, 33.8%, and 29%, respectively. Survival improved in most states. Survival by stage was 64% (local), 28.2% (regional), and 5.3% (distant).
CONCLUSIONS
The current results indicate high fatality for stomach cancer, especially soon after diagnosis. Although improvements in stomach cancer survival were observed, survival remained relatively low for both blacks and whites. Primary prevention through the control of well-established risk factors would be expected to have the greatest impact on further reducing deaths from stomach cancer.
Keywords: cancer registries, CONCORD study, gastric cancer, National Program of Cancer Registries (NPCR), population-based cancer survival, stomach cancer, Surveillance, Epidemiology, End Results (SEER)
INTRODUCTION
Stomach cancer was a leading cancer cause of death early in the 20th century in the United States.1 Both incidence and death rates for the disease declined steadily over the course of the last century in many high-income countries worldwide, including the United States.2,3 The causes of the decline are not fully understood, but it has been postulated that improved living conditions, the widespread use of refrigerators, the decreased consumption of salt-preserved and smoked food, and the use of antibiotics, which may eradicate Helicobacter pylori from the stomach mucosa, account for part of this decline.4 Although incidence rates are now low in the United States, stomach cancer remains an important cause of morbidity and mortality in black, Asian and Pacific Islander, and American Indian/Alaska Native populations, with approximately 23,148 new cases and 11,261 new deaths from the disease expected in the United States in 2013, representing approximately 1.5% of all malignant neoplasms.3
The molecular biology of stomach cancer is complex and varies by site and histology. It is classified into several subsites within the stomach: cardia (roughly the top inch of the stomach), fundus, body, antrum, pylorus, and lesser or greater curvature.5 Most gastric tumors may be classified into 2 major histologic types: diffuse and intestinal.4 The incidence of gastric cardia cancer has remained stable or increased and is significantly higher among whites than blacks and among males, especially among whites, where the male-to-female ratio was 5:1.5,6 Cardia carcinomas are most frequent among white males and lowest among females of all racial groups,5 and they have a poorer prognosis.7,8 Non-cardia carcinoma incidence rates among blacks, Asians and Pacific Islanders, and American Indians/Alaska Natives were twice and triple those among whites, respectively,5 but the incidence has been dropping.6,9
Disease stage at diagnosis is an important predictor of prognosis.10 As for most cancers, stomach cancers diagnosed at an early stage have more favorable survival than disease identified at distant stage.11 However, stomach cancer symptoms are often non-specific, and the vast majority of incident stomach cancers in the United States are diagnosed at distant stage.12
Racial and ethnic differences in stomach cancer incidence and mortality rates have been well documented.5,13,14 Previous research has highlighted racial differences in survival within the United States, with higher survival probabilities reported among whites than among blacks during 1988 through 2006.15 Differences in gastric cancer survival by country have also been observed.10,15–17 The CONCORD-2 study established worldwide surveillance of cancer survival using data for over 25 million individuals diagnosed during the 15-year period 1995–2009, supplied by 279 population-based cancer registries in 67 countries.17 The CONCORD-2 study demonstrated that the 5-year, age-standardized net survival from stomach cancer in the United States was 29% between 2005 through 2009, having increased by 7% since the period 1995–1999.17 These estimates combined data for patients of all races and diagnosed at all stages. The objective of the current report was to use CONCORD-2 data to characterize differences in stomach cancer survival between whites and blacks in the United States by state and by disease stage at diagnosis. To our knowledge, this is the largest study to date, covering 80% of the US population, thus providing a baseline set of survival estimates just before implementation of the Affordable Care Act from 2010.
MATERIALS AND METHODS
Data Source
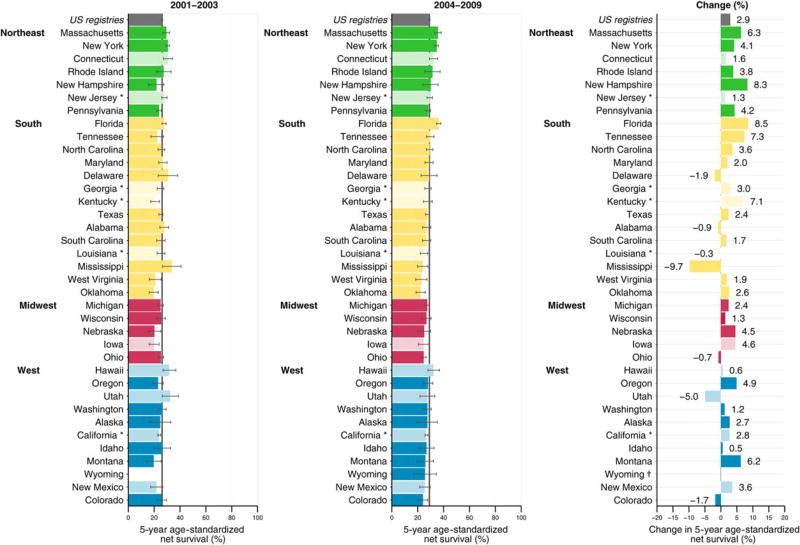
We used data from 37 state-wide cancer registries funded either by the Centers for Disease Control and Prevention’s National Program of Cancer Registries (NPCR) (Fig. 1, dark-colored bars) and/or the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) program (Fig. 1, pale-colored bars), or both (Fig. 1, asterisk).18 These registries participated in the CONCORD-2 study,17 and consented to the inclusion of their data in the more detailed analyses reported here. We analyzed individual tumor records for adults (ages 15–99 years) who were diagnosed with a primary, invasive cancer of the stomach (International Classification of Diseases for Oncology, third revision (ICD-O-3)19 codes C16.0–C16.6 and C16.8–C16.9) between 2001 through 2009 and followed up to December 31, 2009. We included all stomach cancers, regardless of whether the patient had had a previous cancer. If an individual was diagnosed with 2 or more cancers of the stomach between 2001 through 2009, however, then only the first was considered in the survival analyses.
Figure 1.
Stomach cancer: age-standardized 5-year net survival (%) for males and females (ages 15–99 years) who were diagnosed from 2001 to 2003 and from 2004 to 2009, with absolute changes (%). Survival estimates for each state are ranked within US Census Region by the survival estimate for 2004 to 2009. Dark-colored bars denote states affiliated with the National Program of Cancer Registries; pale-colored bars denote states affiliated with the Surveillance, Epidemiology, and End Results Program. An asterisk denotes states affiliated with both federal surveillance programs. †indicates the percentage change was not plotted if a survival estimate was not available for 1 calendar period or if 1 or more estimates were not age-standardized.
Patients were grouped by year of diagnosis into 2 calendar periods (2001–2003 and 2004–2009) to reflect changes in the methods used by US registries to collect data on stage at diagnosis. From 2001, most cancer registries coded stage directly from the source data to SEER Summary Stage 2000.20 From 2004, all registries began to derive SEER Summary Stage 2000 data using the Collaborative Stage System.21
Statistical Analyses
We estimated net survival up to 5 years after diagnosis with 95% confidence intervals (CIs) using the Pohar Perme estimator.22We analyzed survival by black and white race, stage at diagnosis, calendar period of diagnosis, and state.Net survival is interpreted as the probability of survival up to a given time since diagnosis, after controlling for other causes of death (background mortality). To control for wide differences in background mortality among participating states, we constructed life tables of all-cause mortality in the general population for each state from the number of deaths and the population, by single year of age, calendar year, and race (black, white), using a flexible Poisson model.23 The life tables have been published.24
We estimated net survival using the cohort approach for patients diagnosed between 2001 through 2003, because all patients had been followed for at least 5 years by December 31, 2009. We used the complete approach to estimate net survival for patients diagnosed between 2004 through 2009, because 5 years of follow-up data were not available for all patients. Net survival was estimated for 5 age groups (ages 15–44, 45–54, 55–64, 65–74, and 75–99 years). We obtained age-standardized survival estimates using the International Cancer Survival Standard weights.25 If 2 or more of the 5 age-specific estimates could not be obtained, then we present only the pooled, unstandardized net survival estimate for all ages combined.
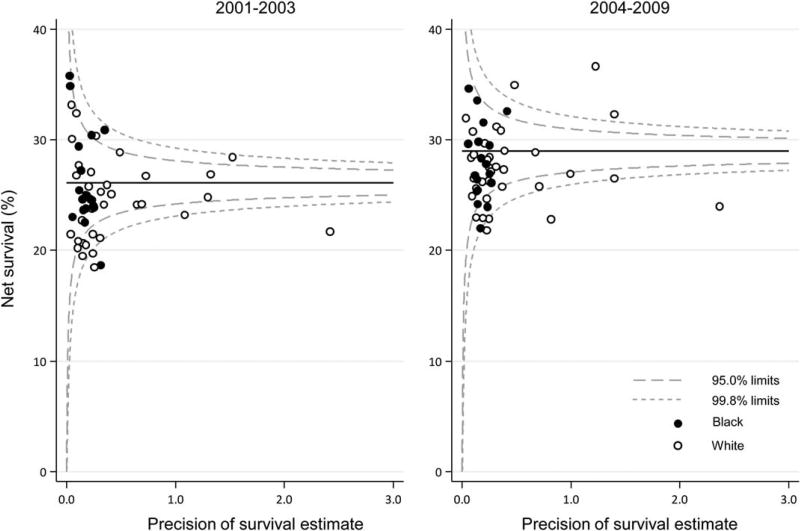
Trends, geographic variations, and differences in age-standardized net survival by race are presented graphically in bar charts and funnel plots.26 Funnel plots are graphic representations designed to detect excessive variation in performance indicators by simple visual inspection of the data.27 A funnel plot comprises 4 elements26: the target (or reference) value for the outcome, a set of control limits (the funnel), data points for the outcome variable (indicator), and the associated precision parameter for each data point. Data points outside the control limits (the funnel) indicate variation in the indicator beyond what would be expected by chance while taking account of precision.27 Funnel plots of net survival for the periods 2001 through 2003 and 2004 through 2009 provide insight into the variability of cancer survival in the United States by race and state. They show how much a particular survival estimate deviates from the pooled estimate of US registries (horizontal line) given the precision of each estimate. More details on data and methods are provided in the accompanying article by Allemani et al.28
RESULTS
We present the results for all patients diagnosed with stomach cancer in the areas covered by the 37 registries in Tables 1, 2 and 3. Results by state are presented in Supporting Tables 1, 2, and 3. In total, 150,700 stomach cancers were diagnosed in the study population between 2001 and 2009: 76.2% were diagnosed in whites, and 15.4% were diagnosed in blacks (Table 1). Overall, for 2004 through 2009, 30.8% of these cancers were diagnosed at a distant stage, 28.8%at regional, 24.9%were localized, and the remaining cancers (15.4%) were of unknown stage. Differences in the proportions of each stage by race between 2004 through 2009 were ≤1%. Case counts by state over the 9-year period as indicated in Supporting Table 1, ranged from a minimum of 227 (Wyoming) to a maximum of 23,247 (California).
TABLE 1.
Stomach Cancer: The Number of Males and Females (Ages 15–99 Years) Diagnosed Between 2001 to 2009 and Distribution (Percentage) by SEER Summary Stage 2000 at Diagnosis, by Race and Calendar Period of Diagnosis
| 2001–2003 | 2004–2009 | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| SEER Summary Stage 2000 |
All Races | White | Black | All Races | White | Black |
| No. of patients | 49,225 | 38,036 | 7332 | 101,475 | 76,800 | 15,906 |
| Localized, % | 22.1 | 22.2 | 21.2 | 24.9 | 24.8 | 24.6 |
| Regional, % | 31.5 | 31.4 | 30.2 | 28.8 | 28.7 | 27.6 |
| Distant, % | 28.9 | 39.1 | 28.5 | 30.8 | 31.4 | 30.1 |
| Unknown, % | 17.5 | 17.3 | 20.1 | 15.4 | 15.1 | 17.7 |
Abbreviations: SEER: Surveillance, Epidemiology, and End Results.
TABLE 2.
Stomach Cancer: Age-Standardized Net Survival at 1, 3, and 5 Years for Males and Females (Ages 15–99 Years) Diagnosed Between 2001 to 2009, by Race and Calendar Period of Diagnosis
| 2001–2003 | 2004–2009 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||||
| All Races | White | Black | All Races | White | Black | |||||||
|
|
|
|
|
|
|
|||||||
| Year | NS (%) | 95% CI | NS (%) | 95% CI | NS (%) | 95% CI | NS (%) | 95% CI | NS (%) | 95% CI | NS (%) | 95% CI |
| 1 | 49.1 | 48.7–49.6 | 48.6 | 48.1–49.1 | 46.9 | 45.7–48.1 | 53.1 | 52.7–53.4 | 52.5 | 52.1–52.9 | 50.9 | 50.1–51.8 |
| 3 | 30.5 | 30.1–31.0 | 29.4 | 28.9–29.9 | 30.3 | 29.1–31.4 | 33.8 | 33.4–34.2 | 32.9 | 32.4–33.3 | 32.4 | 31.5–33.3 |
| 5 | 26.1 | 25.7–26.6 | 24.9 | 24.4–25.4 | 26.1 | 25.0–27.3 | 29.0 | 28.6–29.5 | 28.0 | 27.5–28.5 | 28.3 | 27.1–29.4 |
Abbreviations: CI, confidence interval; NS, net survival.
TABLE 3.
Stomach Cancer: 5-Year Age-Standardized Net Survival for Males and Females (Ages 15–99 Years) Diagnosed Between 2001 to 2009 by SEER Summary Stage 2000 at Diagnosis, by Race and Calendar
| 2001–2003 | 2004–2009 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||||
| All Races | White | Black | All Races | White | Black | |||||||
|
|
|
|
|
|
|
|||||||
| SEER Summary Stage 2000 |
NS (%) | 95% CI | NS (%) | 95% CI | NS (%) | 95% CI | NS (%) | 95% CI | NS (%) | 95% CI | NS (%) | 95% CI |
| All stages | 26.1 | 25.7–26.6 | 24.9 | 24.4–25.4 | 26.1 | 25.0–27.3 | 29.0 | 28.6–29.5 | 28.0 | 27.5–28.5 | 28.3 | 27.1–29.4 |
| Localized | 60.9 | 59.8–62.0 | 60.0 | 58.7–61.3 | 58.1 | 55.1–61.1 | 64.0 | 62.9–65.0 | 63.0 | 61.8–64.2 | 61.1 | 58.4–63.8 |
| Regional | 25.2 | 24.4–26.0 | 23.5 | 22.6–24.4 | 27.0 | 24.9–29.1 | 28.2 | 27.4–29.0 | 26.9 | 26.0–27.9 | 28.3 | 26.0–30.5 |
| Distant | 4.8 | 4.4–5.1 | 4.5 | 4.1–4.9 | 4.8 | 3.8–5.8 | 5.3 | 4.9–5.7 | 5.0 | 4.6–5.5 | 5.8 | 4.8–6.7 |
| Unknown | 24.4 | 23.2–25.5 | 22.7 | 21.4–24.0 | 24.0 | 21.4–26.7 | 27.9 | 26.8–29.0 | 27.2 | 25.9–28.6 | 25.3 | 22.9–27.6 |
Abbreviations: CI, confidence interval; NS, net survival; SEER: Surveillance, Epidemiology, and End Results.
Absolute changes in 5-year age-standardized net survival between 2001 through 2003 and 2004 through 2009 by US geographic region and state are illustrated in Figure 1. For all states combined, there was an increase of 2.9% in 5-year age-standardized net survival between these 2 periods. Although age-standardized 5-year net survival increased in 29 of the 37 states, the pooled survival estimate for all 37 states combined was low for both periods (26.1% and 29%, respectively) (Fig. 1).
The 1-year, 3-year, and 5-year survival estimates for patients diagnosed between 2004 through 2009 were 53.1%, 33.8%, and 29%, respectively (Table 2). There were no meaningful differences in survival between blacks and whites when all 37 states were combined. Some states exhibited substantial in-state differences between blacks and whites; however, there were no discernible geographic patterns to the differences (Supporting Table 2). The 5-year age-standardized net survival improvement by race from the first to the second period was 3.1% for whites and 2.2% for blacks, resulting in a slight divergence in survival between blacks and whites over time. The differences in 5-year net survival between whites and blacks indicate that there were no differences in short-term survival but that differences between races could widen over time.
Five-year age-standardized net survival by disease stage (Table 3) for 2004 through 2009 was 64%, 28.2%, and 5.3% for patients with localized, regional, and distant disease, respectively. Improvements in 5-year net survival by stage at diagnosis were observed between the 2 periods for both whites and blacks. At the localized stage, there were no racial differences in survival, and increases of 3% were observed for both races. At the regional stage, survival increased by 3.4% among whites but by only 1.3% among blacks. At the distant stage, the increase between the 2 periods was 0.5% for whites and 1% for blacks. Whereas blacks experienced lower survival than whites when diagnosed at the regional stage, this was not the case at the distant stage, in which whites experienced lower survival than blacks. Five-year age-standardized survival was uniformly low across all states (Supporting Table 3).
Funnel plots were used to illustrate state-specific and race-specific 5-year age-standardized stomach cancer net survival in relation to the precision of the survival estimate. The pooled (US) survival estimates for each calendar period (2001–2003 and 2004–2009, 26.1% and 29%, respectively) are indicated by the solid lines in Figure 2. Five-year, age-standardized net survival was similar for whites (Fig. 2, open circles) and blacks (Fig. 2, closed circles) from 2001 through 2003. For the period from 2004 through 2009, similar patterns were observed, although the aggregates tended to be less clustered, with larger survival differences, especially among white populations in more populous states (Fig. 2, right). The pooled net survival did improve slightly between the 2 periods, as indicated by the solid lines in Figure 2.
Figure 2.
Stomach cancer: age-standardized 5-year net survival (%) for males and females (ages 15–99 years) by state, race, and calendar period of diagnosis. The pooled survival estimate for all 37 states combined in each calendar period is indicated by a solid horizontal line, and corresponding 95% and 99.8% control limits are indicated by dashed lines.
DISCUSSION
Stomach cancer incidence and mortality have been falling in the United States for decades.2 However, despite these favorable trends, survival has remained poor.4,11,29 The current study provides the most comprehensive and current profile of stomach cancer survival patterns in the United States, which may be useful to public health and medical communities focused on stomach cancer research and control efforts. Our findings suggest that there has been only a very modest improvement in stomach cancer survival in recent years. Furthermore, unlike other cancers that were included in the CONCORD-2 study, we did not observe a large difference in survival or disease stage at diagnosis between whites and blacks. Examination of survival by sex, anatomic sub-site and histology was outside the scope of this report, and this may partly explain the lack of survival disparity observed between blacks and whites.
Racial and ethnic differences in the incidence and mortality for stomach cancer exist, with rates greater in males than in females.14,30 US cancer statistics estimates that incidence rates are 1.7 times higher in blacks than whites, and mortality rates are 2.0 times higher in blacks than in whites.3 SEER data indicate that the stomach cancer burden in the population has been decreasing in the United States.14,31 The National Center for Health Statistics mortality trend from 1984 to 2008 consistently declined by 30% to 40% in all racial or race-sex groups, and differences in mortality became smaller.31 SEER incidence trends from 1974 through 1978 to 2004 through 2008 exhibited a steady decline in all racial or race-sex groups that was consistent with the decline in mortality.31 An increased incidence among young white populations in the United States has also been observed in SEER data9 but requires further exploration. The reductions in death rates from stomach cancer parallel a reduction in incidence rates.
Previous studies indicated that the decreases in stomach cancer incidence in the United States may be attributed to declines in distal (noncardia) and intestinal tumors.5,32 The intestinal type at noncardia sites is often identified in males and older adults, and the declining rates could be because of decreases in H. pylori infection during childhood and the use of refrigeration instead of salt to preserve foods.4,7,9,33 The higher incidence rates of noncardia carcinoma in blacks may reflect higher prevalence of H. pylori infection in this population.30 Cardia stomach cancers, which are more often of the diffuse histologic subtype, occur in all age groups and have been increasing in both males and females.32,33 The diffuse type was consistently more common among blacks than among whites.5 The diffuse type is harder to detect early, which results in a poorer prognosis.32 If cardia and/or diffuse tumors increase as a proportion of the total tumors, then it is possible that overall survival will decrease for gastric cancer.
The distribution of tumors by subsite, for all participating states combined, was 33.8% and 46.2%, for cardia and noncardia sites respectively, for whites, and 11.5% and 62.1%, respectively, for blacks (data not shown). On the basis of these distributions, it is likely that racial differences in survival exist but are being masked because survival has not been examined by anatomic subsite.
Although stomach cancer has a poor prognosis, the increases in overall survival for all stages between the 2 periods are positive, especially because there was an increase in the proportion of the types with the worst prognosis—cardia and diffuse—in the total pool of stomach cancers. The improvement in survival for each stage could be because of better therapy regimens or more access to appropriate treatment.10,34,35
Some common risk factors for both cardia and noncardia carcinoma of the stomach include alcohol consumption, foods preserved by salting, older age, male gender, tobacco smoking, race, family history, low physical activity, low fiber intake, and radiation.4,33 Risk factors associated with cardia carcinomas include obesity and gastroesophageal reflux disease.4,33 During 2011 through 2014, the prevalence of obesity was just over 36% in adults and 17% in younger adults, and it was higher among females than males.36 Between 1999–2000 and 2013–2014, a significant increase in obesity was observed in both adults and youth.36 With obesity on the rise and cardia tumors likely to increase with their worse prognosis, the future burden of stomach cancer may increase.37,38 Risk factors associated with noncardia carcinomas include infection with H. pylori, low socioeconomic status, consumption of processed meat, high intake of salty and smoked food, and low consumption of fruits and vegetables.4,33,39–42
Overall, in the current study, we observed a small increase in survival between 2001–2003 and 2004–2009. This may be related to the increase in locally staged cancers; however, most stomach cancers are diagnosed when they are already at a distant stage, and that proportion is increasing (Table 1), at the expense of regional and unknown stages. The increase in locally staged cancer could be related to heightened awareness in the clinical community. More studies are needed to understand why survival is increasing despite the absence of improving stage distribution and the decreasing trend in subcategories with a good prognosis.
Clinical Perspective
Stomach cancer symptoms are often non-specific; consequently, most patients present at an advanced stage. Diagnosis is by endoscopy and biopsy; computed tomography scans of the chest, abdomen, and pelvis are required to determine stage at diagnosis.43 Adjuvant chemotherapy and chemoradiotherapy after resection of stomach cancer offer survival benefits, but the discrepancies between the surgical technique and type of adjuvant chemotherapy used in clinical trials and survival probabilities have resulted in different adjuvant treatment protocols.34 The major challenge for clinicians is treatment sequence.34 New studies are needed to determine the best therapy. More effective therapy is needed, as evidenced by the low survival and only modest improvement during 2001–2009.
Cancer-Control Perspective
Given the low stomach cancer survival observed in all states, cancer-control efforts directed at primary prevention through the control of well established risk factors, such as avoidance of smoking, maintaining a healthy weight, being physically active, eating a healthy diet (avoiding processed meat and limiting salt intake), and limiting alcohol consumption, may have an impact on reducing the burden of stomach cancer.4,33 Currently, there is inadequate evidence to support screening for stomach cancer.
Strengths and Limitations
Several limitations could impact the interpretation of these findings. First, the NPCR registries that participated in the CONCORD-2 study conducted linkage with their state vital records to obtain information on deaths that occurred within their state and with the Centers for Disease Control and Prevention’s National Death Index to obtain information on deaths that occurred anywhere in the United States. Reliance on deaths reported by state vital records or the National Death Index may miss deaths that occur in patients who leave the United States between the time of their diagnosis and death. Therefore, survival estimates for whites in Figure 2 for the states indicated above the upper limit of the US survival estimate in the second calendar period may need to be interpreted with caution because of issues related to follow-up. Missing deaths, especially for foreign-born blacks as well as for Hispanic whites, can impact the estimates.28,44–46 Second, the manner in which SEER Summary Stage 2000 data were collected and reported changed for all registries in 2004.17 The impact of this change was most evident among NPCR-funded registries, in which the percentage of patients with unknown stage decreased slightly from around 2004. Third, in addition to stage, stomach cancer survival depends on anatomic site and histologic type. Cancers located in the cardia or cancers of the diffuse Lauren histologic type have a worse prognosis than noncardia and intestinal types, respectively. Moreover, the proportion of cardia tumors is higher among whites than among blacks, as indicated in our examination of the distribution of tumors by subsite. In this study, survival was not analyzed by these tumor features; comparisons by race require further study. Fourth, stomach cancer survival was not analyzed by sex for this study, although evidence does indicate that women have better survival from stomach cancer than men.16,47 Finally, analyses of net survival by race were restricted to whites and blacks, the 2major racial groups in the United States, because robust life tables for other races and Hispanics were not available.
Conclusions
Our results indicate that stomach cancer survival is low but appears to be increasing and is similar to the survival pattern reported in Europe.29 We did not observe a large difference in survival between blacks and whites, but the difference widened slightly between 2001–2003 and 2004–2009. Primary prevention through the control of well-established risk factors will be an important public health action for the longer term.
Supplementary Material
Acknowledgments
FUNDING SUPPORT
Funding support for Helena Carreira: US Centers for Disease Control and Prevention (CDC; 12FED03123, ACO12036).
Footnotes
The findings and conclusions in this report are those of the authors and do not necessarily reflect the official position of the CDC.
This Supplement edition of Cancer has been sponsored by the U.S. Centers for Disease Control and Prevention (CDC), an Agency of the Department of Health and Human Services.
The CONCORD-2 study was approved by the Ethics and Confidentiality Committee of the UK’s statutory National Information Governance Board (now the Health Research Authority) (ref ECC 3-04(i)/2011) and by the National Health Service Research Ethics Service (Southeast; 11/LO/0331).
CONFLICT OF INTEREST DISCLOSURES
The authors made no disclosures.
AUTHOR CONTRIBUTIONS
Melissa A. Jim: Writing–original draft, and supervision. Paulo S. Pinheiro: Writing–review and editing. Helena Carreira: Data validation, formal analysis, and visualization. David K. Espey: Writing– review and editing. Charles L. Wiggins: Writing–review and editing. Hannah K Weir: Conceptualization, Writing–review and editing.
References
- 1.Wingo PA, Cardinez CJ, Landis SH, et al. Long-term trends in cancer mortality in the United States, 1930–1998. Cancer. 2003;97:3133–3275. doi: 10.1002/cncr.11380. [DOI] [PubMed] [Google Scholar]
- 2.Howson CP, Hiyama T, Wynder EL. The decline in gastric cancer: epidemiology of an unplanned triumph. Epidemiol Rev. 1986;8:1–27. doi: 10.1093/oxfordjournals.epirev.a036288. [DOI] [PubMed] [Google Scholar]
- 3.US Cancer Statistics Working Group. United States Cancer Statistics: 1999–2013 Incidence and Mortality [web-based report] Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Cancer Institute; 2016. [Accessed August 1, 2017]. Available at: www.cdc.gov/uscs. [Google Scholar]
- 4.Karimi P, Islami F, Anandasabapathy S, Freedman ND, Kamangar F. Gastric cancer: descriptive epidemiology, risk factors, screening, and prevention. Cancer Epidemiol Biomarkers Prev. 2014;23:700–713. doi: 10.1158/1055-9965.EPI-13-1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu H, Rusiecki JA, Zhu K, Potter J, Devesa SS. Stomach carcinoma incidence patterns in the United States by histologic type and anatomic site. Cancer Epidemiol Biomarkers Prev. 2009;18:1945–1952. doi: 10.1158/1055-9965.EPI-09-0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wayman J, Forman D, Griffin SM. Monitoring the changing pattern of esophago-gastric cancer: data from a UK regional cancer registry. Cancer Causes Control. 2001;12:943–949. doi: 10.1023/a:1013756531219. [DOI] [PubMed] [Google Scholar]
- 7.Hohenberger P, Gretschel S. Gastric cancer. Lancet. 2003;362:305–315. doi: 10.1016/s0140-6736(03)13975-x. [DOI] [PubMed] [Google Scholar]
- 8.Mitry E, Rachet B, Quinn MJ, Cooper N, Coleman MP. Survival from cancer of the stomach in England and Wales up to 2001. Br J Cancer. 2008;99(suppl 1):S16–S18. doi: 10.1038/sj.bjc.6604574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anderson WF, Camargo MC, Fraumeni JF, Jr, Correa P, Rosenberg PS, Rabkin CS. Age-specific trends in incidence of noncardia gastric cancer in US adults. JAMA. 2010;303:1723–1728. doi: 10.1001/jama.2010.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Verdecchia A, Mariotto A, Gatta G, Bustamante-Teixeira MT, Ajiki W. Comparison of stomach cancer incidence and survival in 4 continents. Eur J Cancer. 2003;39:1603–1609. doi: 10.1016/s0959-8049(03)00360-5. [DOI] [PubMed] [Google Scholar]
- 11.Coleman MP, Quaresma M, Berrino F, et al. Cancer survival in 5 continents: a worldwide population-based study (CONCORD) Lancet Oncol. 2008;9:730–756. doi: 10.1016/S1470-2045(08)70179-7. [DOI] [PubMed] [Google Scholar]
- 12.Howlader N, Noone A, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2013 (based on the November 2015 SEER data submission, posted to the SEER web site April 2016. Bethesda, MD: National Cancer Institute; 2016. [Accessed August 1, 2017]. Available at: http://seer.cancer.gov/csr/1975_2013/ [Google Scholar]
- 13.Wiggins CL, Perdue DG, Henderson JA, et al. Gastric cancer among American Indians and Alaska Natives in the United States, 1999–2004. Cancer. 2008;113:1225–1233. doi: 10.1002/cncr.23732. [DOI] [PubMed] [Google Scholar]
- 14.Howlader N, Noone A, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2012 (based on the November 2014 submission, posted to the SEER web site April 2015) Bethesda, MD: National Cancer Institute; 2015. [Accessed August 1, 2017]. Available at: http://seer.cancer.gov/csr/1975_2012/ [Google Scholar]
- 15.Kim J, Sun CL, Mailey B, et al. Race and ethnicity correlate with survival in patients with gastric adenocarcinoma. Ann Oncol. 2010;21:152–160. doi: 10.1093/annonc/mdp290. [DOI] [PubMed] [Google Scholar]
- 16.Faivre J, Forman D, Estève J, Gatta G. Survival of patients with oesophageal and gastric cancers in Europe. EUROCARE Working Group. Eur J Cancer. 1998;34:2167–2175. doi: 10.1016/s0959-8049(98)00329-3. [DOI] [PubMed] [Google Scholar]
- 17.Allemani C, Weir HK, Carreira H, et al. Global surveillance of cancer survival 1995–2009: analysis of individual data for 25,676,887 patients from 279 population-based registries in 67 countries (CONCORD-2) Lancet. 2015;385:977–1010. doi: 10.1016/S0140-6736(14)62038-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.White MC, Babcock F, Hayes NS, et al. The history and use of cancer registry data by public health cancer control programs in the United States. Cancer. 2017;123:4969–4976. doi: 10.1002/cncr.30905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fritz A, Percy C, Jack A. International Classification of Diseases of Oncology. 3. Geneva, Switzerland: World Health Organization; 2000. [Google Scholar]
- 20.Young JL, Roffers SD, Ries LAG, Fritz AG, Hurlbut AA, editors. SEER Summary Staging Manual-2000: Codes and Coding Instructions. Bethesda, MD: National Cancer Institute; 2001. NIH Pub. No. 01-4969. [Google Scholar]
- 21.Surveillance, Epidemiology, and End Results Program, National Cancer Institute. Collaborative Stage. Bethesda, MD: National Cancer Institute; 2004. [Accessed August 1, 2017]. Available from: http://seer.cancer.gov/tools/collabstaging/ [Google Scholar]
- 22.Perme MP, Stare J, Esteve J. On estimation in relative survival. Biometrics. 2012;68:113–120. doi: 10.1111/j.1541-0420.2011.01640.x. [DOI] [PubMed] [Google Scholar]
- 23.Rachet B, Maringe C, Woods LM, Ellis L, Spika D, Allemani C. Multivariable flexible modelling for estimating complete, smoothed life tables for sub-national populations. BMC Public Health. 2015;15:1240. doi: 10.1186/s12889-015-2534-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spika D, Bannon F, Bonaventure A, et al. Life tables for global surveillance of cancer survival (the CONCORD programme): data sources and methods. BMC Cancer. 2017;17:159. doi: 10.1186/s12885-017-3117-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Corazziari I, Quinn M, Capocaccia R. Standard cancer patient population for age standardising survival ratios. Eur J Cancer. 2004;40:2307–2316. doi: 10.1016/j.ejca.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 26.Quaresma M, Coleman MP, Rachet B. Funnel plots for population-based cancer survival: principles, methods and applications. Stat Med. 2014;33:1070–1080. doi: 10.1002/sim.5953. [DOI] [PubMed] [Google Scholar]
- 27.Spiegelhalter DJ. Funnel plots for comparing institutional performance. Stat Med. 2005;24:1185–1202. doi: 10.1002/sim.1970. [DOI] [PubMed] [Google Scholar]
- 28.Allemani C, Harewood R, Johnson CJ, et al. Population-based cancer survival in the United States: data, quality control, and statistical methods. Cancer. 2017;123:4982–4993. doi: 10.1002/cncr.31025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anderson LA, Tavilla A, Brenner H, et al. Survival for oesophageal, stomach and small intestine cancers in Europe 1999–2007: results from EUROCARE-5. Eur J Cancer. 2015;51:2144–2157. doi: 10.1016/j.ejca.2015.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.DeSantis CE, Siegel RL, Sauer AG, et al. Cancer statistics for African Americans, 2016: progress and opportunities in reducing racial disparities. CA Cancer J Clin. 2016;66:290–308. doi: 10.3322/caac.21340. [DOI] [PubMed] [Google Scholar]
- 31.Fu WJ. Racial-sex disparities—a challenging battle against cancer mortality in the USA. J Racial Ethn Health Disparities. 2015;2:158–166. doi: 10.1007/s40615-014-0059-6. [DOI] [PubMed] [Google Scholar]
- 32.Henson DE, Dittus C, Younes M, Nguyen H, Albores-Saavedra J. Differential trends in the intestinal and diffuse types of gastric carcinoma in the United States, 1973–2000: increase in the signet ring cell type. Arch Pathol Lab Med. 2004;128:765–770. doi: 10.5858/2004-128-765-DTITIA. [DOI] [PubMed] [Google Scholar]
- 33.World Cancer Research Fund International/American Institute for Cancer Research. [Accessed August 1, 2017];Continuous update project report: diet, nutrition, physical activity and stomach cancer. 2016 Available at: wcrf.org/stomach-cancer-2016.
- 34.Kilic L, Ordu C, Yildiz I, et al. Current adjuvant treatment modalities for gastric cancer: from history to the future. World J Gastrointest Oncol. 2016;8:439–449. doi: 10.4251/wjgo.v8.i5.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim Y, Ejaz A, Spolverato G, et al. Conditional survival after surgical resection of gastric cancer: a multi-institutional analysis of the US gastric cancer collaborative. Ann Surg Oncol. 2015;22:557–564. doi: 10.1245/s10434-014-4116-5. [DOI] [PubMed] [Google Scholar]
- 36.Ogden CL, Carroll MD, Fryar CD, Flegal KM. NCHS Data Brief. Vol. 219. Hyattsville, MD: National Center for Health Statistics; 2015. Prevalence of Obesity Among Adults and Youth: United States, 2011–2014. [PubMed] [Google Scholar]
- 37.Lindblad M, Rodriguez LA, Lagergren J. Body mass, tobacco and alcohol and risk of esophageal, gastric cardia, and gastric non-cardia adenocarcinoma among men and women in a nested case-control study. Cancer Causes Control. 2005;16:285–294. doi: 10.1007/s10552-004-3485-7. [DOI] [PubMed] [Google Scholar]
- 38.Hoyo C, Cook MB, Kamangar F, et al. Body mass index in relation to oesophageal and oesophagogastric junction adenocarcinomas: a pooled analysis from the International BEACON Consortium. Int J Epidemiol. 2012;41:1706–1718. doi: 10.1093/ije/dys176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mendoza D, Herrera P, Gilman RH, et al. Variation in the prevalence of gastric cancer in Peru. Int J Cancer. 2008;123:414–420. doi: 10.1002/ijc.23420. [DOI] [PubMed] [Google Scholar]
- 40.Uthman OA, Jadidi E, Moradi T. Socioeconomic position and incidence of gastric cancer: a systematic review and meta-analysis. J Epidemiol Community Health. 2013;67:854–860. doi: 10.1136/jech-2012-201108. [DOI] [PubMed] [Google Scholar]
- 41.Wiseman M. The second World Cancer Research Fund/American Institute for Cancer Research expert report. Food, nutrition, physical activity, and the prevention of cancer: a global perspective. Proc Nutr Soc. 2008;67:253–256. doi: 10.1017/S002966510800712X. [DOI] [PubMed] [Google Scholar]
- 42.Zhou Y, Zhuang W, Hu W, Liu GJ, Wu TX, Wu XT. Consumption of large amounts of Allium vegetables reduces risk for gastric cancer in a meta-analysis. Gastroenterology. 2011;141:80–89. doi: 10.1053/j.gastro.2011.03.057. [DOI] [PubMed] [Google Scholar]
- 43.Rao S, Cunningham D. Survival from cancer of the stomach in England and Wales up to 2001. Br J Cancer. 2008;99(suppl 1):S19–S20. doi: 10.1038/sj.bjc.6604575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Johnson CJ, Weir HK, Fink AK, et al. The impact of National Death Index linkages on population-based cancer survival rates in the United States. Cancer Epidemiol. 2013;37:20–28. doi: 10.1016/j.canep.2012.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pinheiro PS, Callahan KE, Ragin C, Hage RW, Hylton T, Kobetz EN. Black heterogeneity in cancer mortality: US blacks, Haitians, and Jamaicans. Cancer Control. 2016;23:347–358. doi: 10.1177/107327481602300406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pinheiro PS, Morris CR, Liu L, Bungum TJ, Altekruse SF. The impact of follow-up type and missed deaths on population-based cancer survival studies for Hispanics and Asians. J Natl Cancer Inst Monogr. 2014;2014:210–217. doi: 10.1093/jncimonographs/lgu016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Micheli A, Ciampichini R, Oberaigner W, et al. The advantage of women in cancer survival: an analysis of EUROCARE-4 data. Eur J Cancer. 2009;45:1017–1027. doi: 10.1016/j.ejca.2008.11.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.