Abstract
In the fruit fly Drosophila melanogaster, there are eight insulin-like peptides (DILPs) with DILPs 1-7 interacting with a sole insulin-like receptor tyrosine kinase (DInR) while DILP8 interacts with a single G protein-coupled receptor (GPCR), Lgr3. Loss-of-function dilp mutation studies show that the neuropeptide DILP2 has a key role in carbohydrate and lipid metabolism as well as longevity and reproduction. A better understanding of the processes whereby DILP2 mediates its specific actions is required. Consequently we undertook to prepare DILP2 as part of a larger, detailed structure-function relationship study. Use of our well-established insulin-like peptide synthesis protocol that entails separate solid phase assembly of each of the A- and B-chains with selective cysteine S-protection followed by sequential S-deprotection and simultaneous disulfide bond formation produced DILP2 in good overall yield and high purity. The synthetic DILP2 was shown to induce significant DInR phosphorylation and downstream signalling, with it being more potent than human insulin. This peptide will be a valuable tool to provide further insights into its binding to the insulin receptor, the subsequent cell signalling and role in insect metabolism.
Keywords: Drosophila insulin-like peptide 2, insulin receptor, regioselective disulfide bond formation, solid phase peptide synthesis
TOC image
The fruit fly insulin-like peptide 2, DILP2, is one of seven closely related peptides that each act upon the same insulin-like receptor. It was successfully chemically assembled by regioselective disulfide bond formation within its two synthetic chains and shown to potently stimulate receptor phosphorylation and downstream signalling.
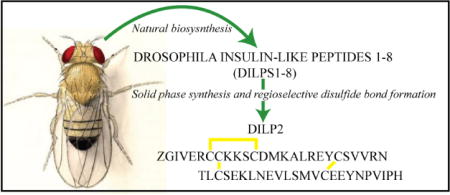
Introduction
The insulin superfamily is an ancient family of structurally similar but functionally diverse peptides that regulate metabolism, growth development, reproduction, stress and ageing[1–3]. Insulin or insulin-like peptides (INSLs) have been described in not only vertebrates but also in invertebrates including insects, nematodes and molluscs[4–6]. They are generally characterised by a heterodimeric (A and B) peptide structure that is linked by three highly conserved cystines in a distinctive arrangement that defines the insulin family. Most members of the insulin family of peptides are synthesized on the ribosome as prohormones in which the B- and A-chains are linked by a C-peptide that is excised upon translation[7].
In insects, the number of insulin-like peptides that has been identified varies from just one in locusts to a remarkable 38 in the silk moth Bombyx mori[8,9]. Their biological activities are generally elicited through interaction with a single insulin receptor tyrosine kinase (InR) and subsequent insulin/IGF-type signalling (IIS). In the fruitfly Drosophila melanogaster, IIS is mediated by eight Drosophila insulin-like peptides (DILPs). DILPs 1-7 bind to a single Drosophila insulin/IGF receptor, DInR[10], and have redundant functions in growth, metabolism, stress resistance, reproduction and longevity[11,12]. The DILPs 1-7 are homologous to both mammalian insulin and IGFs, and have various spatiotemporal expression patterns and functions that are also dependent upon nutrient status[12–14]. In addition, DILP8 was recently discovered and shown to coordinate the growth status of tissues with developmental timing[15]. DILP8 was shown, unlike DILPs 1-7, to bind to Lgr3[16], a GPCR of the relaxin receptor family (RXFPs)[17], and thus is closer to human relaxin than to insulin and IGFs. So far, the only DILP to have been produced as a protein, after cDNA cloning and expression in yeast, is DILP5[18]. Its crystal structure was solved and showed the canonical structure of the insulin-like peptide family, and its binding to the human and Drosophila insulin receptors characterized, as well as some in vitro and in vivo biological properties[18]. It also bound with substantial affinity to the insulin/IGF Drosophila binding protein, IMPL2[18]. A synthetic insulin-like peptide (ILP3) from the mosquito Aedes Aegypti has also been shown to bind with high affinity to the mosquito insulin receptor[19].
DILP2, like DILPs 3 and 5, is produced by a set of neurosecretory cells, insulin-producing cells (IPCs), in the brain although each has distinct but overlapping functional roles[11,12]. Interestingly, as a dilp2 mutant fly has a prolonged lifespan, DILP2 is the only DILP to be directly implicated in ageing[11,13]. Given the increasing burden ageing plays both in society and in health care costs, an understanding of the structural and functional features associated with the interaction of DILP2 with DInR is of considerable importance, more so when it is unclear how the DILPs mediate various organismal phenotypes when they activate the same receptor. As a preliminary step towards addressing this issue, we undertook to chemically synthesize DILP2, characterise it and to measure its DInR stimulation using Drosophila S2 cells.
Results and Discussion
Chemical synthesis of DILP2
The chemical synthesis of heterodimeric, insulin-like peptides has long been a significant challenge given the complexity of its two-chain, three-disulfide structure[20]. Our laboratory has developed efficient protocols that exploit the availability of cysteine S-thiol protecting groups that are orthogonal to one another and which allows the sequential, directed disulfide bond formation of separately assembled A- and B-chains (Scheme 1)[21]. These have enabled the successful preparation of many INSLs ranging from relaxins of different species to human INSLs3-5 and their analogues[22–25]. The expressed form of DILP2 has not been identified but its predicted structure consists of a 26 residue A-chain and a 24 residue B-chain[26] (Figure 1), the latter being unusually truncated at its N-terminus compared to many other INSLs. Each of the two chains were readily assembled by microwave-assisted solid phase peptide synthesis after which cleavage from the solid support and side chain deprotection was followed by preliminary RP-HPLC purification. After intra-chain disulfide bond formation within the A-chain by 2,2′-dipyridyl disulfide (DPDS)-mediated oxidation, the Cys8 residue had its S-tert-butyl protecting group displaced with the pyridinyl group via DPDS in strong acid. The resulting functionalized A-chain intermediate was combined with an equimolar amount of the mono-Cys3 thiol B-chain in pH 8.5 buffer to form the first inter-chain disulfide bond. The final, inter-chain, disulfide bond was formed by iodolysis to simultaneously remove the two S-acetamidomethyl groups on the A-chain Cys22 and B-chain Cys15 residues (Scheme 1). The resulting synthetic DILP2 was purified by RP-HPLC in overall yield of approximately 7% relative to the starting crude B-chain peptide. Both analytical RP-HPLC and MALDI-TOF MS confirmed the high purity of the peptide (Figure 2). RP-HPLC-monitored tryptic mapping and MALDI-TOF MS identification methods[27] confirmed the expected disulfide bond pairings and absence of disulfide exchange (data not shown).
Scheme 1.
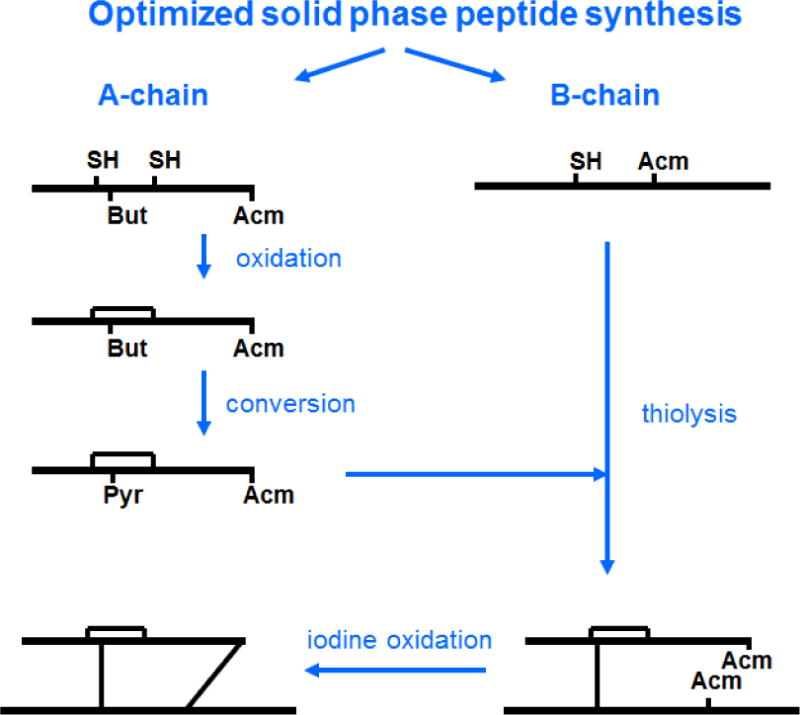
General scheme for the chemical synthesis and regioselective disulfide bond formation of insulin-like peptides as applied to DILP2.
Figure 1.
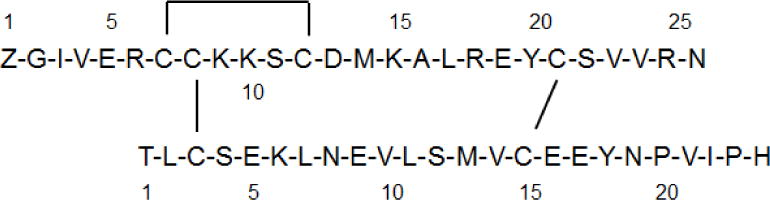
Predicted primary structure of Drosophila melanogaster insulin-like peptide 2 (DILP2).
Figure 2.
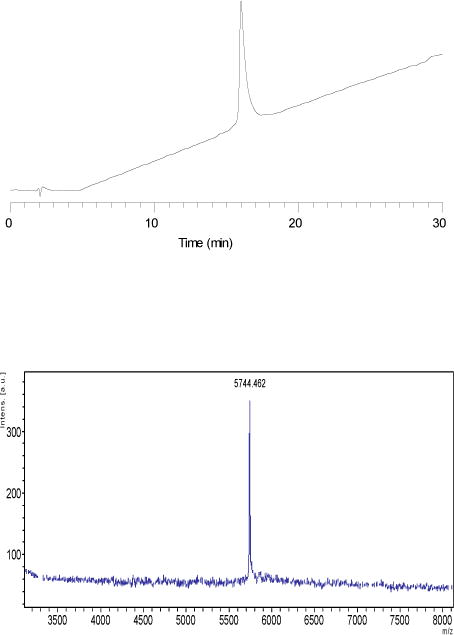
Chemical characterization of synthetic DILP2. Upper. RP-HPLC. Conditions: Buffer A: 0.1% aq. TFA; buffer B: 0.1% TFA/CH3CN. Column: Vydac C4 (4.6 × 250 mm). Flow rate: 1.5 ml/min. Gradient: 15-45% B over 30min. Detection wavelength: 214 nm. Lower. MALDI-TOF MS. Theoretical MH+ 5744.89; found 5, 744.46
Downstream signaling by synthetic DILP2 in Drosophila S2 cells
The synthetic DILP2 at 100 nM was shown to induce autophosphorylation of the DInR in Drosophila S2 cells (Figure 3), demonstrating its functional activity to be similar to that of human insulin. Although not quantified, the relative intensity of the bands showed that DILP2 was clearly more potent than equimolar human insulin in this assay. Synthetic DILP2 also stimulated downstream signalling, increasing phosphorylation of Akt at both previously reported phosphosites, Ser505 and Thr342[28–30], of ERK at Tyr202/Thr204 and of the TOR pathway target S6K at Thr398 (Figure 4, left). The effect on Akt was seen whether the cells were adherent or in suspension (data not shown), showing that the signaling specificity is independent of cell morphology. Furthermore, DILP2 stimulation repressed FOXO activity, as gene expression of two FOXO transcriptional targets (4eBP and DInR) was decreased about 50% after one hour of DILP2 stimulation compared to control stimulation (Figure 4, right).
Figure 3.
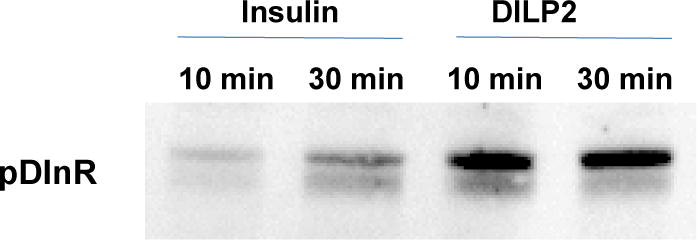
Stimulation of phosphorylation of cell surface DInR in S2 cells by DILP2 and human insulin at 100 nM.
Figure 4.
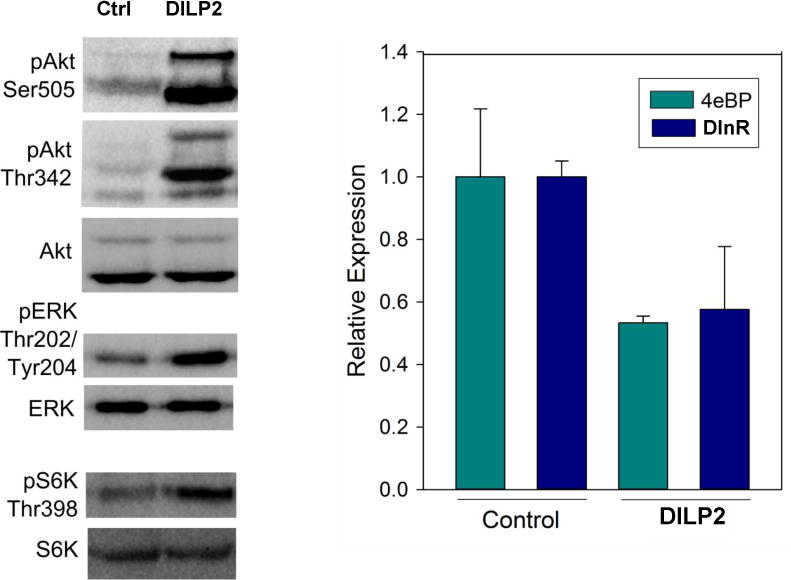
Stimulation of downstream insulin/IGF signalling by synthetic DILP 2 in S2 cells. Left panel. Three minutes DILP2 stimulation at 100 nM activates the Akt pathway, ERK pathway and TOR pathway (S6K). Blots represent results of at least three independent experiments. Right panel. One hour of DILP2 stimulation at 100 nM represses gene expression of two dFOXO transcriptional targets, 4eBP and DInR (n=3, two-tailed t-test p=0.02 for 4eBP and p=0.05 for DInR).
Conclusions
The 50-residue insect peptide, DILP2, was successfully assembled by a previously well-established process of combination of solid phase synthesis and directed, selective disulfide bond formation. The resulting peptide was shown to be highly purified and to possess the biological activity (DInR and Akt phosphorylation) and immunological properties expected of native DILP2. The availability of this peptide will enable detailed analysis of both its functional properties as well as the molecular basis of its binding to the dInR. A similar synthesis strategy can be applied in order to provide chemically well-characterized INSLs for studying the in vitro and in vivo biology of various members of this complex peptide family.
Experimental Section
Materials and Methods
9-Fluorenylmethoxycarbonyl (Fmoc) protected L-α-amino acids and 1-[bis(dimethylamino)methylene]-1H-benzotriazolium hexafluorophosphate 3-oxide (HBTU) were purchased from GL Biochem (Shanghai, China). Piperidine (PPD) and trifluoroacetic acid (TFA) were purchased from Auspep (Melbourne, Australia). Fmoc-PAL-PEG-PS resins with substitution of 0.18 mmol/g were purchased from Applied Biosystems Inc. (Melbourne, Australia). Dimethylformamide (DMF), methanol, diethyl ether, and dichloromethane (DCM) were purchased from Merck (Melbourne, Australia). 3,6-Dioxa-1,8-octanedithiol (DODT), triisopropylsilane (TIPS), diisopropylethylamine (DIPEA) and trifluoromethanesulfonic acid (TFMSA) were purchased from Sigma-Aldrich (Sydney, Australia). 2,2-Dipyridyl disulfide (DPDS) was purchased from Fluka (Switzerland). Acetonitrile was purchased from BDH Laboratory Supplies, (Poole, UK). All other reagents were from Sigma-Aldrich (Sydney, Australia).
Solid-phase peptide synthesis (SPPS)
Regioselectively S-protected A- and B-chains of DILP2 (Figure 1) were separately synthesized by the Fmoc solid-phase method by using microwave-assisted synthesis on a Liberty system (CEM Corporation, NC, USA). The following side chain protecting groups were used: Arg, Pbf; Asn, Trt; Asp and Glu, O-But; His, Trt; Lys, Boc; Ser, Thr and Tyr, tBu. Selective cysteine S-protection was also employed: Cys(A21, B15), acetamidomethyl; Cys(A8), tert-butyl, and Cys(A7, A12, B3), trityl. A small amount of resin bound peptide was cleaved by TFA and analyzed by RP-HPLC and MALDI-TOF MS which confirmed successful synthesis of linear A- and B-chains.
Regioselective disulfide bond formation
Cleavage of the tBu from the side chain of Cys to free thiol and its subsequent activation
The first inter-molecular regioselective disulfide bond was formed as previously reported[22]. Briefly, the A–chain and 2,2-dipyridyl disulfide were added to TFA in an ice bath. Thioanisole and ice-cold trifluoromethanesulfonic acid in TFA (1:4 v/v) were added and the mixture stirred for 1 h on ice. The peptide was then precipitated with ice-cold diethyl ether, and the pellet collected by centrifugation, washed 3 times with ice-cold diethyl ether, air-dried and subjected to RP-HPLC purification. The MALDI TOF MS analysis confirmed the formation of the desired SPyr-activated A- chain.
Formation of first inter-chain disulfide bond by thiolysis (chain combination)
The A-chain peptide was dissolved in GnHCl solution (pH 8.5). The B-chain (dissolved separately in GnHCl, pH 5) was added to this solution drop by drop. The mixture was stirred vigorously at room temperature and the reaction was monitored by analytical RP-HPLC. The MALDI-TOF MS analysis showed the formation of the desired combination products. After 30 min, the reaction was terminated by addition of neat TFA, and the target product was isolated by preparative RP-HPLC.
Formation of second inter-molecular disulfide bond in DILP2 via iodination
The combination product was dissolved in glacial acetic acid and to this was added 20 mM iodine/acetic acid and 60 mM HCl. After 1 h, the reaction was stopped by addition of ice cold ether, further cooled on dry ice for 3 min, and the pellet was collected by centrifugation employing an anti-explosive centrifuge (Spintron, GT-175FR), and purified by RP-HPLC. From a 0.2 mmol scale assembly of both A- and B-chains, a total of 2.26 mgs (net adjusted for peptide content by amino acid analysis) was obtained.
Chemical characterization
The purity of each synthetic intermediate and the final DILP2 was assessed by analytical RP-HPLC and MALDI-TOF mass spectrometry using a Bruker Autoflex II instrument (Bremen, Germany) in the linear mode at 19.5 kV. Peptides were also quantitated by amino acid analysis of a 24 hour vapour phase acid hydrolyzate followed by derivatization with AccuTag chemistry and resolution of the labelled residues using a Shimadzu microbore RP-HPLC system (Melbourne, Australia).
Biological assays
Human insulin was from obtained from Sigma Aldrich (USA).
DInR phosphorylation
Autophosphorylated DInR expressed at the S2 cell surface was isolated using Cell Surface Protein Isolation Kit (ThermoFisher Scientific, USA) according to manufacturer instructions. Briefly, exposed proteins of intact S2 cells (DILP2 or human insulin stimulated, and control) were labelled with biotinylation agent. Then cells were collected and lysed, and biotinylated proteins were affinity purified with NeutrAvidin Agarose. Total isolated proteins were separated by SDS-PAGE and immunoblotted using Phospho-IGF-I-Receptor beta (Tyr1135/1136)/Insulin Receptor beta (Tyr1150/1151)(19H7) Rabbit mAb (Cell Signaling Technology, USA).
Western blots
S2 cells at a density of about 1×106 cells/ml were serum-depleted overnight and stimulated with 100 nM DILP2 for 3 minutes. Cells were washed very briefly with sterile, cold PBS, resuspended NP40 lysis buffer (Thermo Fisher Scientific, USA) supplemented with 1 mM PMSF, PhosSTOP phosphatase inhibitor cocktail (Roche, USA) and Protease Inhibitor Cocktail (Invitrogen, USA). Cells were incubated on ice for 30 minutes, vortexing at 10 minute intervals. Cell lysates were spun down for 10 minutes at 13K rpm and the supernatant incubated at 70°C with gel loading buffer and reducing reagent. Samples loaded onto SDS-PAGE (Invitrogen, USA) and ran at 200V according to the user manual. Gels were transferred to nitrocellulose membrane (Whatman, GE Healthcare Life Sciences, USA) for one hour at 30V and blocked with 5% BSA in TBS-T for one hour. Membranes incubated with antibody diluted 1:1000 in 5% BSA overnight at 4°C with gentle rocking. Antibodies were from from Cell Signaling Technology (USA): Drosophila phospho-Akt Ser505, Pan-Akt, Pan-phospho-ERK, Pan-ERK, Drosophila phospho-S6K, and Pan-S6K. Drosophila phospho-Akt Thr342 antibody was from PhosphoSolutions (USA). Blots were washed for 5 minutes 3 times in TBS-T and incubated in horseradish peroxidase conjugated anti-rabbit secondary antibody (Jackson Immunolabs, USA) diluted 1:5000 in 1% BSA for one hour at room temperature. Blots were also washed for 5 minutes 3 times in TBS-T and incubated with ECL reagent (Perkin Elmer, USA). Blots were imaged in ImageLab (BioRad Inc., USA).
Quantitative RT-PCR
S2 cells at a density of about 1×106 cells/ml were serum-depleted overnight and stimulated with 100 nM DILP2 for one hour at the given concentration. Cells were washed with sterile, cold PBS, resuspended in Trizol (Invitrogen, USA) and lysed by mechanical force with two 3.2 mm steel beads in a tissuelyser. RNA was Trizol extracted, treated with Turbo DNase (Invitrogen) and quantified on a NanoDrop ND-1000 (Thermo Fisher Scientific Inc., USA). RNA was reverse transcribed using an iScript cDNA synthesis kit (Bio-Rad Laboratories, Inc., USA). Quantitative RT-PCR was conducted on an ABI Prism 7300 Sequence Detection System (Applied Biosystems Inc., USA) using SYBR green PCR master mix (Applied Biosystems, Inc.). Relative mRNA levels were calculated relative to actin expression by the comparative Ct method and normalized to control (solvent) stimulation.
Acknowledgments
We thank the NHMRC (Australia) for financial support for the peptide chemistry studies. JDW is an NHMRC Principal Research Fellow. Research at The Florey Institute of Neuroscience and Mental Health is supported by the Victorian Government Operational Infrastructure Support Program.
References
- 1.Tatar M, Bartke A, Antebi A. The endocrine regulation of aging by insulin-like signals. Science. 2003;299:1346. doi: 10.1126/science.1081447. [DOI] [PubMed] [Google Scholar]
- 2.Blumenthal S. From insulin and insulin-like activity to the insulin superfamily of growth-promoting peptides: a 20th century odyssey. Perspect Biol Med. 2010;53:491. doi: 10.1353/pbm.2010.0001. [DOI] [PubMed] [Google Scholar]
- 3.Shabanpoor F, Separovic F, Wade JD. The human insulin superfamily of polypeptide hormones. Vitam Horm. 2009;80:1. doi: 10.1016/S0083-6729(08)00601-8. [DOI] [PubMed] [Google Scholar]
- 4.Piñero-González J, Gonzalez-Perez A. The ubiquity of the insulin superfamily across the eukaryotes detected using a bioinformatics approach. OMICS. 2011;15:439. doi: 10.1089/omi.2010.0141. [DOI] [PubMed] [Google Scholar]
- 5.Wu Q, Brown MR. Signaling and function of insulin-like peptides in insects. Annu Rev Entomol. 2006;51:1. doi: 10.1146/annurev.ento.51.110104.151011. [DOI] [PubMed] [Google Scholar]
- 6.De Meyts P. Insulin and its receptor: structure, function and evolution. BioEssays. 2004;26:1351. doi: 10.1002/bies.20151. [DOI] [PubMed] [Google Scholar]
- 7.Guo ZY, Qiao ZS, Feng YM. The in vitro oxidative folding of the insulin superfamily. Antiox Redox Signal. 2008;10:127. doi: 10.1089/ars.2007.1860. [DOI] [PubMed] [Google Scholar]
- 8.Badisco L, Claeys I, Van Hiel M, Clynen E, Huybrechts J, Vandersmissen T, Van Soest S, Vanden Bosch L, Simonet G, Vanden Broek J. Purification and characterization of an insulin-related peptide in the desert locust, Schistercerca gregaria: immunolocalization, cDNA cloning, transcript profiling and interaction with neuroparsin. J Mol Endocrinol. 2008;40:137. doi: 10.1677/JME-07-0161. [DOI] [PubMed] [Google Scholar]
- 9.Mizoguchi A, Okamoto N. Insulin-like and IGF-like peptides in the silk moth Bombyx mori: discovery, structure, secretion, and function. Front Physiol. 2013;4:217. doi: 10.3389/fphys.2013.00217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kannan K, Fridell YW. Functional implications of Drosophila insulin-like peptides in metabolism, aging and dietary restriction. Front Physiol. 2013;4:1. doi: 10.3389/fphys.2013.00288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grönke S, Clarke DF, Broughton S, Andrews TD, Partridge L. Molecular evolution and functional characterization of Drosophila insulin-like peptides. PLOS Genetics. 2010;6:e1000857. doi: 10.1371/journal.pgen.1000857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nassel DR, Vanden Broek J. Insulin/IGF signalling in Drosophila and other insects: factors that regulate production, release and post-release action of the insulin-like peptides. Cell Mol Life Sci. 2016;73:271. doi: 10.1007/s00018-015-2063-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nassel DR, Kubrak OI, Liu Y, Luo J, Lushchak OV. Factors that regulate insulin producing cells and their output in Drosophila. Front Physiol. 2013;4:252. doi: 10.3389/fphys.2013.00252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Post S, Tatar M. Nutritional geometric profiles of insulin/IGF expression in Drosophila Melanogaster. PLOS One. 2016 doi: 10.1371/journal.pone.0155628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Colombani J, Andersen DS, Leopold P. Secreted peptide DILP8 coordinates Drosophila tissue growth with developmental timing. Science. 2012;336:582. doi: 10.1126/science.1216689. [DOI] [PubMed] [Google Scholar]
- 16.Colombani J, Andersen DS, Boulan L, Boone E, Romero N, Virolle V, Texada M, Leopold P. Drosophila Lgr3 couples organ growth with maturation and ensures developmental stability. Current Biol. 2015;25:2723. doi: 10.1016/j.cub.2015.09.020. [DOI] [PubMed] [Google Scholar]
- 17.Bathgate RA, Halls ML, van der Westhuizen ET, Callander GE, Kocan M, Summers RJ. Relaxin family peptides and their receptors. Physiol Rev. 2013;93:405. doi: 10.1152/physrev.00001.2012. [DOI] [PubMed] [Google Scholar]
- 18.Sajid W, Kulahin N, Schlukebier G, Ribel U, Henderson HR, Tatar M, Hansen BF, Svendsen AM, Kiselyov VV, Nørgaard P, Wahlund P-O, Brandt J, Kohanski RA, Andersen AS, Meyts PDe. Structural and Biological properties of the Drosophila insulin-like peptide 5 show evolutionary conservation. J Biol Chem. 2011;286:661. doi: 10.1074/jbc.M110.156018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brown MR, Clark KD, Gulia M, Zhao Z, Gonczynski SF, Crim JW, Suderman RJ, Straud MR. An insulin-like peptide regulates egg maturation and metabolism in the mosquito Aedes Aegypti. Proc Natl Acad Sci USA. 2008;105:5716. doi: 10.1073/pnas.0800478105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu F, Zaykov AN, Levy JJ, DiMarchi RD, Mayer JP. Chemical synthesis of peptides within the insulin superfamily. J Peptide Sci. 2016;22:260. doi: 10.1002/psc.2863. [DOI] [PubMed] [Google Scholar]
- 21.Hossain MA, Wade JD. Synthetic relaxins. Curr Opin Chem Biol. 2014;22:47. doi: 10.1016/j.cbpa.2014.09.014. [DOI] [PubMed] [Google Scholar]
- 22.Rosengren KJ, Zhang S, Lin F, Daly NL, Scott DJ, Hughes RA, Bathgate RAD, Craik DJ, Wade JD. Solution structure and characterization of the receptor binding surface of insulin-like peptide 3. J Biol Chem. 2006;281:28287. doi: 10.1074/jbc.M603829200. [DOI] [PubMed] [Google Scholar]
- 23.Hossain MA, Bathgate RA, Kong C, Shabanpoor F, Zhang S, Haugaard-Jönsson LM, Rosengren KJ, Tregear GW, Wade JD. Synthesis, conformation and receptor binding activity of human insulin-like peptide 5 (INSL5) ChemBioChem. 2008;9:1816. doi: 10.1002/cbic.200800113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hossain MA, Belgi A, Lin F, Zhang S, Shabanpoor F, Belyea C, Truong HT, Blair AR, Andrikopoulos S, Tregear GW, Wade JD. Use of temporary “solubilizing” peptide tag for the Fmoc-solid phase synthesis of human insulin glargine via use of regioselective disulfide bond formation. Bioconj Chem. 2009;20:1390. doi: 10.1021/bc900181a. [DOI] [PubMed] [Google Scholar]
- 25.Chan LJ, Smith CJ, Bathgate RAD, Separovic F, Gundlach AL, Hossain MA, Wade JD. Chemical synthesis and preliminary in vitro and in vivo characterization of fluorescent analogues of relaxin family peptides. Front Chem Biol. 2013;1:30. doi: 10.3389/fchem.2013.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guirao-Rico S, Aguadé M. Molecular evolution of the ligands of the insulin signalling pathway: dilp genes in the genus Drosophila. Mol Biol Evol. 2011;28:1557. doi: 10.1093/molbev/msq353. [DOI] [PubMed] [Google Scholar]
- 27.Canova-Davis E, Kessler TJ, Lee PJ, Fei DTW, Griffin P, Stults JT, Wade JD, Rinderknecht E. Use of recombinant DNA derived human relaxin to probe the structure of the native protein. Biochemistry. 1991;30:6006. doi: 10.1021/bi00238a026. [DOI] [PubMed] [Google Scholar]
- 28.Powell DJ, Turban S, Gray A, Hajduch E, Hindal HS. Intracellular ceramide and protein kinase Czeta activation play an essential role in paalmitate-induced insulin resistance in rat L6 skeletal muscle cells. Biochem J. 2004;382:619. doi: 10.1042/BJ20040139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sopko R, Foos M, Vinayagam A, Zhai B, Binari R, Hu Y, Randiklev S, Perkins LA, Gygi SP, Perrimon N. Developmental Cell. 2014;31:114. doi: 10.1016/j.devcel.2014.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schleich S, Strassburger K, Janiesch PC, Koledachkina T, Miller KK, Haneke K, Cheng YS, Küchler K, Stoecklin G, Duncan KE, Teleman AA. DENR-MCT-1 promotes translation re-initiation of uORFs to control tissue growth. Nature. 2014;512:208. doi: 10.1038/nature13401. [DOI] [PMC free article] [PubMed] [Google Scholar]


