Abstract
Planar Cell Polarity (PCP) is an essential feature of animal tissues, whereby distinct polarity is established within the plane of a cell sheet. Tissue-wide establishment of PCP is driven by multiple global cues, including gradients of gene expression, gradients of secreted Wnt ligands, and anisotropic tissue strain. These cues guide the dynamic, subcellular enrichment of PCP proteins, which can self-assemble into mutually exclusive complexes at opposite sides of a cell. Endocytosis, endosomal trafficking, and degradation dynamics of PCP components further regulate planar tissue patterning. This polarization propagates throughout the whole tissue, providing a polarity axis that governs collective morphogenetic events such as the orientation of subcellular structures and cell rearrangements. Reflecting the necessity of polarized cellular behaviours for proper development and function of diverse organs, defects in PCP have been implicated in human pathologies, most notably in severe birth defects.
Introduction
Cell polarization refers to the organized establishment of asymmetries within cells. Just as intracellular functions are compartmentalized in organelles, many cellular functions are made more effective by partitioning them along an axis of polarization. Cell polarization typically involves the localization of specific molecular determinants to specific cellular domains, and the coordination of polarity across tissues is essential for the development of specialized form and function in multicellular organisms. Cell polarity is best understood in the context of epithelia, and epithelial cells are generally considered to display two key forms of polarity: apical—basal polarity, which refers to polarized distribution of cellular components and specialized functions between the opposing surfaces of an epithelial sheet, and, the focus of this Review, planar polarity, which refers to organization along the perpendicular axis, in the plane of the epithelial sheet (for comparison between the two polarity axes see Supplementary information S1 (Box)).
Planar tissue patterning is governed by two major signalling pathways: the “core” planar cell polarity (PCP) and “Fat, Dachsous, and Four-Jointed” (Ft-Ds-Fj) modules. These signalling pathways were initially identified in Drosophila melanogaster screens for regulators of the coordinated orientation of external bristles and hairs1–5 (Fig.1a). A key feature of planar polarization through these pathways is the complementary and mutually exclusive distribution of transmembrane signalling complexes, resulting in their asymmetric enrichment in distinct cell compartments within each cell of a patterned tissue (Fig.1b). This in turn directs the orientation of subcellular structures and cell behaviours through the regulation of cytoskeletal elements and cellular adhesions. Proper establishment and maintenance of planar polarity is essential during development and involved in tissue homeostasis and repair, and defects in PCP signalling are associated with diverse human pathologies (Box 1).
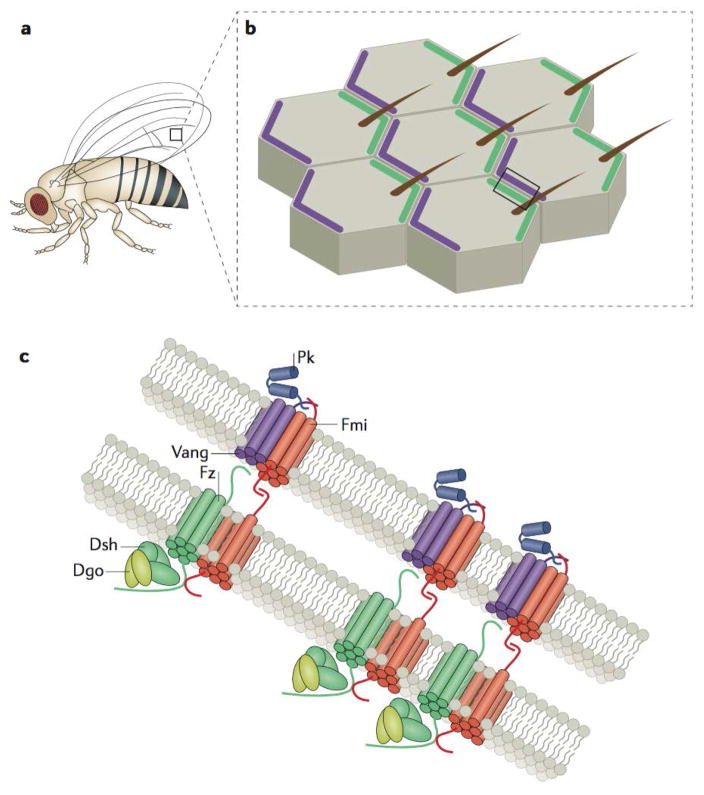
Figure 1. Asymmetric signalling complexes pattern planar polarity.
a | Drosophila melanogaster has many planar-polarized external features, including actin-based, distally or posteriorly oriented hairs and bristles that cover the legs, wings, and notum, which serve as a robust readout of planar cell polarity signalling. The box drawn on the wing blade demarcates the region illustrated in (b). b | Cells of the D. melanogaster wing blade are asymmetrically patterned by proximal accumulations of Van Gogh (Vang) and Prickle (Pk) (both shown in purple) that are complementary to distal accumulations of Frizzled (Fz), Dishevelled (Dsh), and Diego (Dgo) (shown in in green). These patterns govern the distal positioning and orientation of a single actin-based trichome in each cell of the wing epithelium. The box drawn on the tissue demarcates the region illustrated in (c). c | Asymmetric PCP signalling components form junctional signalling complexes that are physically linked from cell to cell. Both Fz and Vang are transmembrane proteins that associate with physically linked Flamingo (Fmi) homodimers established between the opposing membranes at cell–cell junctions. Distal accumulations of Fmi and Fz are asymmetrically clustered through the activity of Dsh and Dgo, while proximal Fmi and Vang complexes are enriched by the activity of Pk. Note that the proteins as they are portrayed are not necessarily to scale and merely approximations as there is no high-resolution protein structural data available to support them.
Box 1. Planar cell polarity genes and human birth defects.
Birth defects, associated with developmental abnormalities, are the leading cause of infant mortality in the United States and are among the leading causes of death for children of all ages120. As planar cell polarity (PCP) is an important organizer of tissues during morphogenesis, studies of the mechanisms of PCP signalling in embryonic development provide an opportunity to shed light on an important public health issue. For example, failure of convergent extension following PCP disruption in model animals results in neural tube defects (NTDs), and PCP genes are now among the most well-defined genetic risk factors for NTDs in humans – a common birth defect121. A flurry of papers has emerged implicating PCP genes in human NTDs. The first identified mutations were in Vang-like protein 1 (VANGL1)116, but since then, mutations in essentially all the core PCP genes have now been identified in human NTD patients. These individual findings are too numerous to list here but have been comprehensively delineated elsewhere122,123. Several studies also provide mechanistic insights, revealing that NTD-associated mutations disrupt known interactions among PCP proteins and/or disrupt their subcellular localization116,124–126.
In addition to NTDs, PCP gene mutations also contribute to the etiology of Robinow Syndrome, a severe skeletal dysplasia characterized by short limbs and craniofacial anomalies. Studies revealed mutations in ROR2 kinase, which now has an established role in the PCP-mediated elongation of the murine limb in response to Wnt5a44, as a genetic background for this syndrome 127,128. More recently, Robinow Syndrome has been associated with genetic variants in the core PCP Dishevelled genes DVL1129,130 and DVL3131, as well as in WNT5a itself132. Importantly, these gene-association studies are supported by functional assays in model animals, providing mechanistic insights to supplement genotype–phenotype correlations.
Lastly, there is evidence to suggest that PCP signalling functions in the proper formation of the spine and that PCP mutations could lead to the onset of idiopathic scoliosis phenotypes. This link was additionally uncovered in the study of zebrafish ptk7 mutants, which exhibit late-onset severe spinal curvature abnormalities that are likely due to defective cerebrospinal fluid flow, mediated by multiciliated cells133,134. A recent survey of a patient cohort with adolescent idiopathic scoliosis (AIS), which screened for mutations in VANGL1, identified two missense mutations of interest were identified as potentially contributing to the AIS phenotypes135.
This review will focus primarily on discussing the mechanisms underlying the establishment and tissue-wide coordination of PCP patterns. We will also outline specific examples to illustrate how cells can exploit these patterns to execute polarized cell behaviours, thereby shedding light on the molecular etiology of PCP-related human birth defects.
Establishment of core PCP patterns
The establishment of planar polarity involves several fundamental processes, including sensing cues that govern the global orientation of polarity, establishing molecular asymmetries according to these cues, refining polarity patterns throughout neighboring cells, and executing respective polarized cell shape changes and behaviours .
Core PCP signalling components
When considering the roster of molecular players at work in PCP, it is useful to understand that distinct molecules participate in these diverse processes. The transmembrane components of this system allow for exchange of polarity information between cells. In D, melanogaster these include Flamingo, ((Fmi, also known as Starry Night (Stan); CELSR in vertebrates), Frizzled (Fz; FZD in vertebrates), and Van Gogh, (Vang also known as Strabismus or Stbm; VANGL in vertebrates). By contrast, the cytoplasmic components encoded by Dishevelled (Dsh; DVL in vertebrates), Prickle (Pk), and Diego (Dgo; ANKRD6 in vertebrates) are involved in amplifying intracellular asymmetries and translating polarity cues into cell behavioral changes1–5.
The basis of PCP establishment is the localization of mutually exclusive subsets of the core PCP proteins to opposing domains along the cell cortex, forming a pattern that propagates throughout the tissue. PCP protein patterns develop from an initially symmetric distribution that gradually sorts out into two complementary domains, with Fz, Dsh, and Dgo complexes typically accumulating on one side of each cell, and Vang and Pk enriched on the other; Fmi is present on both sides1–5 (Fig.1c). Asymmetric sorting and symmetry breaking is driven by dynamic trafficking, binding, and signalling interactions that differentially control stable localization of PCP components at discrete regions of the cell, and as detailed in following sections, the resulting stable accumulations ultimately coordinate planar-polarized cell behaviours.
Cell–cell communication and propagation of PCP asymmetry
The asymmetric localization of a molecular cue can be sufficient to polarize a single cell’s behaviour, but for groups of cells in an epithelium to collectively polarize, polarity information must be transmitted between neighboring cells. Such cell-cell communication of planar polarity is primarily facilitated through the physical interactions of PCP signalling complexes at cellular junctions, of which the seven-pass atypical cadherin Fmi is an essential component6–9. Extracellular interactions of cadherin repeat domains promotes the formation of Fmi homodimers across apical cell–cell junctions, which in result become increasingly stable8–11. These stable transmembrane accumulations link PCP signalling complexes in neighbouring cells, propagating a uniform, cooperative pattern of polarity information (Fig. 1c).
In addition to serving as the molecular bridges that link intercellular polarity, Fmi also promotes the assembly of intracellular asymmetric directional signalling complexes. Junctional Fmi helps recruit PCP components Fz (a seven-pass transmembrane Wnt receptor) or Vang (four-pass transmembrane protein) to lateral regions of the apical cell surface6,12,13. These interactions help stabilize PCP components at cellular junctions but also mutually influence Fmi localization; Fmi is diffusively apically localized rather than apicolaterally enriched in a Vang, fz double mutant background, similar to Vang and Fz localizations in fmi mutant cells6,8,12,13. Once recruited to apicolateral junctions, directional Fmi–Fz and Fmi–Vang signalling complexes can be progressively assembled in a coordinated, collective fashion across the tissue. Fmi molecules seem to preferentially bind Fz over Vang, and a Fmi–Fz complex is more likely to stably associate with a junctional Fmi molecule of an adjacent neighboring cell that is not bound to Fz – that is, it is either bound to Vang or bound to neither Fz nor Vang7–9,14. This cell-cell interaction appears to help maintain the junctional localization of these signalling components in both cells, and the association of Vang with such a complex, specifically by binding a Fmi molecule linked to a Fmi-Fz complex in an adjacent cell, appears to further increase the combined apicolateral stability of all signalling components involved8,11. This occurs by bolstering resistance to the endocytic flux that these molecules are subject to in the absence of such an assemblage (Fig.2).
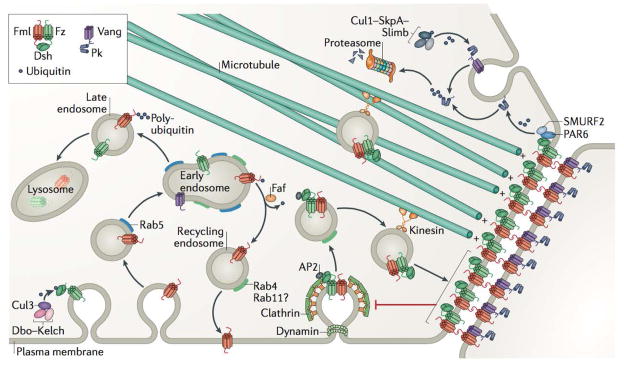
Figure 2. Graphical summary of endocytosis, trafficking, and degradation events that facilitate the dynamic patterning of planar cell polarity.
Planar cell polarity (PCP) signalling components are resistant to endocytic flux when stably linked across junctions (red inhibitory arrow)8,11. The internalization of PCP components is partially dependent on dynamin and Clathrin-mediated endocytosis27, and the interaction of membrane-associated Dishevelled (Dsh)150 with Clathrin-associated adaptor protein AP-2 is essential for PCP signalling29. PCP protein internalization and trafficking are regulated through ubiquitylation. For example, the activity of a Cullin-3–Diablo–Kelch (Cul3–Dbo–Kelch) ubiquitin ligase complex promotes ubiquitylation and internalization – but not degradation – of Dsh, while fat facets (faf) deubiquitinase prevents the accumulation of Flamingo (Fmi) in Rab5-positive endosomes and promotes Fmi recycling to the membrane48 – a process that appears to be mediated by Rab4 and potentially Rab11 for PCP proteins8. Although faf loss of function does not lead to a reduction in the overall levels of Fmi, inhibition of lysosomal maturation leads to the overaccumulation of Fmi as well as Frizzled (Fz) in endosomes, indicating that cellular levels of these components are, at least in part, regulated through lysosomal degradation8. Kinesin-dependent trafficking of Fmi, Fz and Dsh towards microtubule plus ends appears to direct these complexes to sites of junctional enrichment36. Prickle (Pk) appears to have a dual role in both clustering and stable Fmi–Van Gogh (Vang) complexes at junctions as well as in mediating the internalization of unstable Vang28. Proteasomal degradation also plays a role in regulating PCP components. For example, Vang promotes the proteasomal degradation of farnesylated (membrane associated) Pk49, likely through poly-ubiquitylation mediated by a Cullin-1 (Cul1)–SkpA–Supernumerary limbs (Slimb) E3 ubiquitin ligase complex28. Similarly, vertebrate DVL2 (when phosphorylated in response to WNT5a stimulation) can associate with PAR6 and ubiquitin ligase SMURF2 resulting in poly-ubiquitylation of PK1, which leads to proteasomal degradation of PK1, thereby controlling PK1 localization and protein levels43.
Binding of Fmi to Fz or Vang induces differential Fmi signalling behaviours across cellular junctions that promote the propagation of a uniform PCP pattern7–9,14. Because signalling through Fmi occurs also when the extracellular domains of Fz and Vang are not intact, this indicates that Fmi signalling can be modulated by the binding to its cytoplasmic partners – with Fz, Vang or neither Fz nor Vang being bound to the C-terminal region of Fmi. Thus, it seems that even though Fmi is localized to both sides of a PCP-patterned epithelial cell, it can exhibit asymmetry in behaviour that will influence the potential interactions between PCP signalling complexes of adjacent cells. Demonstrating this, fz mutant cells (over a distance of several cells) can be polarized quite remarkably by a neighbouring clone of cells, as long as both cell populations retain Fmi function and the neighbouring clone expresses fz 14. Vang function does not appear to be required for a cell to be polarized by signals from a nearby clone expressing fz, though the effects of this polarizing activity are less strong than when Vang is present in the signal receiving cells (the distance over which the polarization induced by the fz expressing clone is maintained is less for fz and Vang double mutants as compared to fz single mutants)14. Lastly, fz mutant cells can be polarized by Fz positive cells that do not express Vang14. Cumulatively, this analysis shows that to ensure directional non-autonomous cell polarization, Fmi must be present in both sending and receiving cells, and Fz must be present in at least one of the two cell populations. Vang activity may not be absolutely essential to initiate polarization, but it appears to facilitate the polarization of the receiving cells. Thus, Fmi is essential for the intercellular communication of axial planar polarity and appears to assume one of two signalling states: one – with Fz – required for establishing a stable, vectorial planar polarity signal, and another – either unbound or bound to Vang – that associates with Fmi–Fz in the neighbouring cell across the cell–cell junction7,8,14.
Feedback amplification of asymmetry
The recruitment of junctional PCP complexes by Fmi is necessary for intercellular communication of polarity, but not sufficient for the establishment of robust PCP patterns within planar-polarized cells. The differential signalling states of Fmi with either Fz or Vang, which promote stability of the oppositely associated complexes across junctions, can set the stage for such patterns8,9. However, the concomitant sorting and enrichment of PCP signalling complexes is mediated by positive and negative interactions between the asymmetric PCP components. While some of these interactions may be driven by the membrane components, such as the extracellular domain of Fz potentially acting as a ligand for Vang8,10, much of the feedback amplification process occurs through activity of the cytoplasmic components Dsh, Dgo, and Pk. These cytoplasmic components act by clustering transmembrane signalling complexes into stable PCP enrichments11,15–19, which are not necessarily required for intercellular PCP signalling, but rather for locally accumulating these signalling complexes to amplify the asymmetric pattern of PCP signalling complexes19 (Fig. 1c). Thus, these interactions that promote PCP patterning are often collectively referred to as the feedback amplification of asymmetry3,20. Notably, for robust amplification to occur, the activies of all the core PCP components seem to be essential. Significant perturbation of any single core PCP protein is often sufficient to disrupt the asymmetric patterning of them all, adversely affecting tissue structure and collective cell behaviour19.
In a polarized epithelial cell, one half of the hallmark PCP pattern consists of Fmi, Fz, Dsh and Dgo assembled into asymmetric junctional enrichments (Fig 1c). As Fmi homodimers recruit Fz to apicolateral regions of the cell, Dsh can be recruited as well in a Fz-dependent manner through the binding of the DEP domain of Dsh to the Fz cytoplasmic tail12,15,16. In addition, the ankyrin repeat domain of Dgo can bind the PDZ domain of Dsh, serving as an additional interaction that promotes the unilateral clustering of the Fmi–Fz–Dsh–Dgo complex18. The asymmetric enrichment of Fz and overall junctional levels of Fmi appear greatly diminished in dsh mutant cells6,8,12,13, likely due to a reduction in junctional stability11.
Complementary to the Fz, Dsh and Dgo accumulations that decorate one side of a planar-polarized cell, Fmi, Vang and Pk assemble along the opposite half of the apical membrane, generating the characteristic PCP pattern13,20 (Fig. 1c). Vang and Pk accumulate through interactions of their respective C-terminal regions, mediating the heterotypic binding of Vang with Pk, as well as homotypic Pk and Vang binding17. These binding interactions, similarly to with Fz–Dsh11, facilitate the clustering of Vang and Pk and increase the stability of their junctional localization21.
The positive interactions that promote the clustering of PCP signalling components appear to be sufficient to drive the enrichment of these complexes in any given junctional region of the cell, yet there must be additional negative interactions in order to develop and maintain the mutually exclusive enrichment of Fmi–Fz–Dsh–Dgo and Fmi–Vang–Pk complexes to the opposing cell membranes. One simple mechanism would be for PCP signalling components to “exclude” the complementary set from localizing in the same region. Indeed, interactions between members of oppositely localizing complexes, namely between Vang, Pk and Dsh and Dgo, were observed17,18,20,22,23. Interestingly, it is the C-terminal regions of Pk and Vang (through which they cluster with each other to accumulate on the one side of the cell) that have been demonstrated to associate with both the PDZ domain of Dsh and ankyrin repeats of Dgo18,23. Utilizing the same binding region to interact with members of both asymmetric domains could establish a framework for negative feedback mechanisms, and it has been proposed that Pk inhibits the association of Dsh with Fmi–Vang by competing for the same binding region of Vang, while Dgo competes with Pk for binding to Dsh17,18,23. Along with the positive interactions that promote clustering PCP complexes, these negative interactions appear to allow for the self-sorting of PCP asymmetry and short-range propagation of polarity patterns, likely in the absence of any additional molecular inputs or guidance cues19,24,25.
Endocytosis and endosomal trafficking facilitates asymmetric sorting
It stands to reason that in order to generate and maintain PCP patterns, mislocalized or unstable components need to be removed from the membrane, and endocytosis, endosomal trafficking and degradation can indeed influence core PCP localization and signalling (Fig. 2). Both Rab GTPase Rab5 and Dynamin have been shown to play a role in the internalization of unstable PCP signalling components8, 26,27. Inhibition of endocytosis leads to the overaccumulation of Fmi at the plasma membrane, and owing to the apparent role of Fz and Vang in stabilizing junctional Fmi, there appears to be an increased rate at which Fmi is internalized in fz and Vang mutant wing blade cells8,11. Along with Fmi, both Fz and Vang also appear to be subject to membrane turnover, and Fz may facilitate the feedback amplification of asymmetry by promoting removal of Fmi–Vang–Pk complexes where Fz is accumulated8,11,28. Ubiquitylation of Pk by an E3 ubiquitin ligase complex consisting of Cullin-1, SkpA and Supernumerary limbs can promote the interalization of Fmi–Vang–Pk complexes, and the Pk-dependent internalization of Fmi in this manner is significantly reduced in fz mutants28 (Fig. 2).
Both Dsh-dependent and -independent mechanisms contribute to control of Fmi internalization8, and the former is likely to require Fz bound to Fmi due to known Fz-Dsh binding interactions30. Interfering with the ability of DVL2 (one of the vertebrate Dsh homologues) to interact with the Clathrin adaptor AP-2 leads to a reduction in internalized FZD4, which in turn results in PCP-defective gastrulation movements in Xenopus laevis29. As described before, Pk has similarly been shown to mediate the internalization of Fmi and Vang, which may be related to its function in clustering Fmi–Vang–Pk accumulations28. Together, the cytoplasmic PCP signalling components appear to have a dual regulatory role on PCP, clustering their associated transmembrane components in some regions and mediating their internalization in others (Fig. 2).
While internalization of PCP proteins is important for patterning of the PCP axis, this is only the first step in the intracellular sorting of these signalling components. Following endocytosis, PCP proteins are subject to one of several downstream processes, including recycling back to the membrane, trafficking to another region of the cell, or degradation. Inhibition of lysosomal maturation leads to the intracellular accumulation of internalized Fmi, suggesting some fraction of Fmi is normally turned over via lysosomal degradation8,11. Internalized Fmi colocalizes with endosomal makers such as Rab4, Rab5, Rab7 and Rab11, and both Rab4 and Rab11 appear to be capable of mediating the recycling of Fmi back to the membrane8,11,27. As unstable components are likely continually degraded, it would seem that newly synthesized proteins must be continually added to the plasma membrane to help generate stable PCP patterns. Indeed, factors such as Clathrin adaptor protein AP-1 and trafficking GTPase ARF1, which are thought to facilitate trafficking from the Golgi and endosomes to the plasma membrane, have been identified as important factors in PCP establishment, and their perturbation results in strong PCP phenotypes31. These findings further underline the importance of membrane trafficking in the regulation of PCP complex distribution.
Endocytosis is essential not only for establishing PCP patterns, but also for adjusting patterns during cell rearrangements and in the face of frequent mitotic events in proliferative tissues27. In the developing mammalian epidermis, PCP components are internalized and redistributed during mitoses, which appears to be essential for the proper patterning of the skin and planar polarization of hair follicles32. The events of internalization and redistribution are synced with cell division through the control of CELSR phosphorylation by Polo-like kinase -1 (PLK1), and phosphorylation promotes the internalization of CELSR1 and associated FZD proteins33. Thus, the endocytic control of PCP components is an important part of maintaining uniform polarity, particularly during dynamic cellular behaviours.
Polarized microtubule trafficking facilitates PCP protein anisotropy
Feedback amplification and endosomal trafficking mechanisms facilitate the asymmetric sorting of PCP proteins, but these rely upon an initial anisotropic bias in order to become enriched and stable. This initial bias can be subtle, or random even, and with amplification can result in locally aligned asymmetry19,24,25. However, in order to uniformly align PCP asymmetry across a given tissue, there must be an additional, reliable input that influences PCP patterning. Microtubules and microtubule-based trafficking are used throughout development to introduce biases that break symmetry and specify axes, and arrays of planar-polarized microtubules have been visualized in various PCP-patterned tissues24,34,35. As it turns out, these polarized microtubule arrays can bias the transport of core PCP particles consisting of Dsh, Fz and Fmi towards one side of the cell, aiding the PCP patterning process36–39. These Fmi–Fz–Dsh accumulations are preferentially trafficked towards the plus ends of microtubules, showing a bias in directionality similar to plus-end tip tracking proteins such as EB138 (Fig. 2). However, while this process has been visualized in cells of the wing blade and likely is working similarly in the abdomen of D. melanogaster, the distal bias of microtubule plus ends is diminished in the most distal regions of the wing, demonstrating that this is just one potential mechanism for globally aligning PCP asymmetry.
Interestingly, while polarized microtubules are essential for establishing some PCP asymmetries, they may be dispensable for maintaining these patterns once they have been stably generated37,40, and this supports the notion that the key role of microtubules in PCP patterning is to introduce an initial directional bias. It has separately been shown that intact PCP patterns are necessary for the polarization of microtubule arrays24,38,41, demonstrating that intricate and mutual relationships exist between microtubule polarity and planar polarity patterning.
Post-translational modifications fine-tune PCP protein function
Protein localization and function is often regulated through post-translational modifications. Similar to the PLK1-1 mediated internalization of CELSR1 during murine epidermal divisions mentioned previously, other PCP component phosphorylations are known to occur, and this has been demonstrated to impact PCP signalling in several contexts. VANGL and DVL proteins have multiple phosphorylation sites, modification of which can regulate protein localization and behaviour5,42. For example, mouse DVL2 is phosphorylated in response to WNT5a stimulation and can associate with the ubiquitin ligase SMURF2 and apical polarity protein PAR6 as a result of this phosphorylation, and this complex regulates PK1 protein stability by ubiquitylating PK1 and thereby promoting its proteasomal degradation43. WNT5a can also promote VANGL2 phosphorylation through tyrosine-protein kinase receptor ROR2, which is essential for governing planar-polarized cellular behaviours in the mouse limb bud44 (see also below). In addition, serine/threonine kinase misshapen-like kinase 1 (MINK1) phosphorylates a conserved residue of PK1, which promotes membrane localization with VANGL2 and apical enrichment of PK1–VANGL2 complexes45. The localization of FZD3 may also be regulated through its phosphorylation, which is disrupted upon loss of DVL1 in murine axon growth cones46. On the other hand, VANGL2 promotes the dephosphorylation and internalization of FZD3 in this same context46.
Transmembrane proteins are often sorted into lysosomes following ubiquitylation, and because both Fmi and Fz accumulate intracellularly upon the prevention of lysosomal maturation, these proteins are likely subject to lysosomal turnover8 (Fig. 2). Along these lines, a deubiquitylating enzyme, fat facets (faf), was identified in a D. melanogaster genetic screen for enhancers of a hypomorphic fz allele47. It was shown that the presence of faf is important for proper junctional localization of Fmi, but faf knockdown does not affect total protein levels, suggesting that the faf-mediated deubiquitylation of Fmi serves to regulate Fmi recycling back to membranes48. A related mechanism influences Dsh localization, with a Cullin-3–Diablo–Kelch ubiquitin ligase complex mediating the removal of Dsh from junctions without impacting overall Dsh levels48.
Following ubiquitylation, PCP components can also be subject to proteasomal degradation (Fig. 2). For example, ubiquitylation and degradation of PK1 mediated by the before-mentioned DVL2, PAR6, and SMURF complex is essential for normal PCP signalling during mouse development43. In D. melanogaster, Vang appears to additionally play role in the ubiquitin-mediated proteosomal degradation of Pk49. Here, as well as in X. laevis, Pk overexpression results in an increase in junctional Vang, yet Vang overexpression counter intuitively results in diminished levels of junctional Pk13,21,49. Similarly, loss of Vang function can lead to higher overall Pk protein levels, which is likely the result of decreased Pk degradation49. This Vang-dependent degradation of farnesylated (that is, membrane associated) Pk is likely mediated through the ubiquitin ligase Cullin-128.
Global Alignment of PCP Patterning
While interactions between core PCP proteins and the regulated dynamics of their localization can coordinate PCP asymmetries between neighbouring cells and can propagate them throughout a tissue, global inputs into these patterns must govern their overall directionality with respect to the axis of an organ. Recent work has helped identify multiple, potentially overlapping influences on the global orientation of PCP, which apparently include expression gradients of fj and ds – the components of the “global” Ft–Ds–Fj PCP pathway, Wnt ligand gradients, and anisotropic tissue strain (Fig. 3).
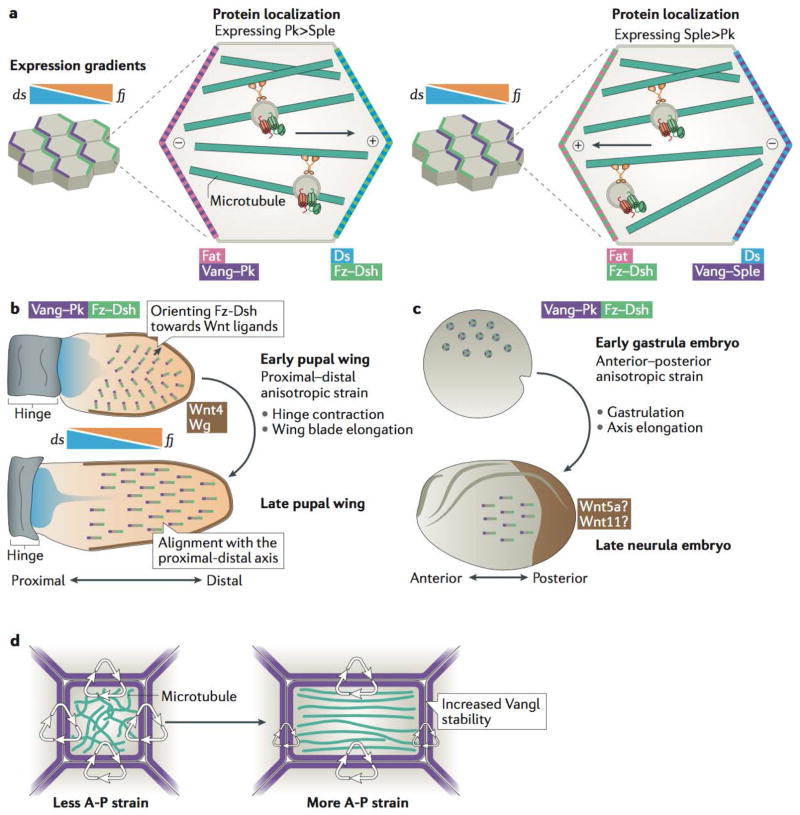
Figure 3. Multiple global inputs can collectively influence planar cell polarity orientation and stability across developing tissues.
a | In Drosophila melanogaster core planar cell polarity (PCP) components organize according to Fat (Ft)–Dachsous (Ds) axis, established as a result of expression gradient of dachsous (ds) and the gradient of expression of kinase four-jointed (fj), which regulates the interactions between Ft and Ds and thereby contributes to the asymmetric distribution of these two proteins in the cell. Localization of PCP components with respect to Ft–Ds axis depends on the levels of expression of two Prickle isoforms: Prickle (Pk) and Spiny Legs (Sple)38. In cells expressing predominantly Pk, Vang–Pk localizes on the same side of the cell as Ft, but in cells expressing mostly Sple, Vang–Sple localizes together with Ds. This then regulates the directionality of microtubule-based transport in cells, as microtubule plus ends orient towards the Fz–Dsh-containing membranes. b | Top: The D. melanogaster wing blade exhibits a narrow source of Wnt ligands along the distal margin (brown) that may serve to orient PCP complex vectors (grey lines with Frizzled (Fz) and Dishevelled (Dsh) (green) localizing towards the wing margin opposite Vang Gogh (Vang) and Prickle (Pk) (purple) early in development (around 5-15 hours after puparium formation (hAPF))60. Broad and shallow expression gradients of ds and fj oppose one another along the proximal–distal axis of the developing wing and may influence the directionality of early PCP pattern39,56,57 so that PCP is oriented in a radial fashion towards the edge of the wing. Bottom: Later in development (16–32 hAPF), during hinge contraction and rapid wing blade elongation, cell shape changes and cell rearrangement reorient microtubule polarity, leading to the coordinated alignment of asymmetric PCP patterns along the proximal–distal axis of the tissue (with Fz–Dsh oriented towards the distal edge of the tissue)65. Note the increased length of PCP vectors at the later stage that signifies an increase in the amount and stability of core PCP complexes at junctions c | Top: Prior to gastrulation in Xenopus laevis, no detectable PCP vector is observed (rings). Bottom: PCP axes (line vectors) are established in the embryonic ectoderm during gastrulation62, as forces along the axis of elongation increase the cortical stability of Vang-like protein 2 (Vangl2)41 (purple)(see also panel d). A posterior source of Wnt ligands (such as Wnt5a and Wnt11;brown) is likely involved by directing the anterior localization of Vangl261. d | Anisotropic tissue strain orients microtubules along the axis of strain. Concomitantly, Vangl2 (purple) becomes more stable at cell–cell junctions along the axis of tissue strain, in a manner that has been shown to depend on microtubules41.
Ft-Ds-Fj signalling
Ft and Ds are atypical protocadherins that hetereotypically interact across cellular junctions, and Fj is a transmembrane kinase that modulates Ft and Ds interactions50. These proteins have been shown to influence planar polarity (Box 2), and while it has remained contentious whether Ft-Ds-Fj and core PCP signalling are acting in parallel or in consecution51,52, an intersect between these two pathways is becoming increasingly clear (at least in the fly). Gene expression gradients are a common mechanism of tissue patterning during development, and opposing gradients of ds and fj expression were observed along the axis of planar polarity in D. melanogaster, making these gradients attractive candidates for a global PCP-orienting cue53,54. In addition, these expression gradients result in the asymmetric localization of Ft and Ds to opposite sides of the cell, oriented similarly to core PCP asymmetry (Box 2). Indeed, while the core PCP pathway appears to retain function in mutant clones of Ft-Ds-Fj signalling components, the locally coordinated cells are not properly aligned along the global tissue axis53,54. The differential alignment of core PCP patterns in respect to the ds-fj gradient in different tissues challenged this notion. However, this could be explained by the fact that Pk exists in two isoforms, Prickle (Pk) and Spiny Legs (Sple), that only differ in the most N-terminal region of protein55 and differentially interpret the Ft-Ds-Fj cues39,56; cells predominantly expressing Pk, such as those in the fly wing, asymmetrically localize Fmi–Vang–Pk on the same side of the cell as Ft, but in cells predominantly expressing Sple, Fmi-Vang-Sple localizes in the opposite orientation due to Sple interacting with Ds and Dachs56,57 (Fig. 3a). Thus, as Fmi–Vang–Sple complexes localize with Ds and Dachs, Fmi–Fz–Dsh complexes then localize on the same side of the cell as Ft.
Box 2. Role of Fat, Dachsous, and Four-Jointed in planar polarity signalling.
In addition to having roles in controlling tissue growth through the Hippo pathway, Fat (Ft)–Dachsous (Ds)–Four-jointed (Fj) signalling components participate in the planar polarization of the ommatidia in the eye, trichomes in the wing, and abdominal bristles of Drosophila melanogaster, similarly to the core planar cell polarity (PCP) pathway4,136. A vertebrate ortholog of Fj (Fjx1) and multiple homologs of Fat (Fat1-4) and Ds (Dchs1 and Dchs2) exist in vertebrates137, and Fat4 and Dchs1, which have the highest homology to D. melanogaster Ft and Ds, are clearly important for PCP-mediated processes in mice, such as directionality of cell elongation and oriented cell division138–141.However, the molecular functions of Ft–Ds–Fj signalling components in vertebrates and how well they are conserved with their D. melanogaster counterparts are currently poorly understood.
In D. melanogaster, planar asymmetries in Ft and Ds localization can arise, likely owing to the opposing expression gradients of ds and fj51,54,142. Despite the fact that expression of ft appears uniform, the kinase activity of Fj (that forms a gradient), which may phosphorylate cadherin repeats of both Ft and Ds, can seemingly promote Ft binding to Ds143–146 and inhibit Ds binding to Ft146. Controlling Ds binding to Ft and Ft binding to Ds is thought to lead to a graded distribution of binding affinity between these two proteins along the axis of differential ds and fj expression, whereby a cell with more Fj activity than its neighbor is going to have “stronger” Ft and “weaker” Ds in the sense of binding capacity, promoting Ft in the cell with higher Fj to bind with Ds in the cell with lower Fj (and thus “stronger” Ds). In the D. melanogaster wing, for example, the result is a higher proximal level of ds expression stabilizing Ds protein on the distal side of the cell, down the ds gradient144,147 (Fig. 3a and 3b). Similar to the fashion in which core PCP proteins localize then, components of the Ft–Ds–Fj signalling pathway become asymmetrically distributed in cells of many planar-polarized tissues105,147,148. In more complex scenarios, such as in the D. melanogaster epidermis at later larval stages where large single cells can have multiple polarized structures and exchange neighbours, two sides of the same cell can be differentially polarized according the localization of Ft–Ds–Fj signalling components in the neighbours at any given time149. These findings suggest that although an uniform expression gradient has the potential to form a uniformly asymmetric distribution of Ft–Ds, it is the local, cell-to-cell sensing of asymmetries that in the end governs a distinct regional polarity. Whether expression gradients, localization asymmetries, or similar molecular functions for these proteins exist in vertebrates has yet to be determined.
One of the functional consequences of the differential interpretation of Ft–Ds–Fj through Pk and Sple is the orientation of microtubule polarity along Ft–Ds activity gradients. Microtubule plus ends are often oriented towards Ds in cells predominately expressing Pk and towards Ft in cells predominantly expressing Sple38. Using Ft–Ds–Fj polarity cues to orient microtubules results in a bias in the directional transport of core PCP signalling components36,38, thereby providing a means of coupling these two signalling pathways39,58. However, detailed molecular mechanism through which these microtubules are oriented in response to the Ft–Ds gradient and whether this mode of orienting PCP is conserved in tissues other than the fly wing, as well as in other species, remains unclear.
Wnt ligand gradients
Along with gradients in gene expression, gradients of secreted ligands are also commonly employed in tissue patterning. There are a number of different Wnt proteins which can act as secreted ligands for Frizzled receptors, and the tendency for Wnts to be found in graded distributions along an axis of planar polarity makes them attractive candidates for global PCP orientation cues59. Indeed, a potentially instructive role for Wnt gradients in PCP pattern orientation is becoming increasingly apparent, with recent breakthroughs having highlighted that various Wnt ligands can influence PCP patterning in different capacities and that some may act redundantly60,61. For example, both Wg and Wnt4 may be acting in the orientation of PCP complexes in the fly wing, where during early wing morphogenesis, the distal Fmi–Fz–Dsh complexes are oriented towards the expression gradients of these ligands emanating from the wing margin and the proximal Fmi-Vang-Pk complexes are oriented more towards the central regions of the wing blade60 (Fig. 3b, top). Additionally, in the X. laevis ectoderm, Wnt5a, Wnt11, and Wnt11b have the capacity to orient Pk and Vang accumulations away from the source of ligand expression (Fig. 3c), whereas a closely related ligand, Wnt3a, elicits no such behaviour61.
With this recently ascribed role for global PCP orientation, the mechanisms through which Wnt gradients could promote global orientation of PCP complexes remain not fully understood. Wnt4 and Wg seemingly inhibit Fz–Vang interactions between cultured fly S2 cells and can attenuate fz overexpression defects in vivo, which may indicate a role for Wnts in locally competing with Vang for Fz binding60. In mice, Wnt5a can promote the association of PCP-specific component VANGL2 into a signalling complex with ROR2, promoting VANGL2 phosphorylation, which has been shown to be required for planar-polarized rearrangements of osteoblasts that drive elongation of the limbs44.
Mechanical Forces
When tissues change shape during growth and morphogenesis, or when fluid flows across a tissue surface, the anisotropic strains that are introduced can influence the development of PCP asymmetry and orientation of PCP patterns. Early reports of this phenomenon demonstrated the direct impact of fluid flow on the polarization of ciliary beating patterns in multicilated epithelia62–64. In addition, forces exerted throughout the embryonic epidermis of X.laevis during gastrulation, which is thought to initially establish the PCP axis63, were found to measurably increase the polarized stability of core PCP complexes at cellular junctions, and these effects were shown to depend on microtubules, which can rearrange in response to high tissue strain41 (Fig. 3d). This increase in the stability of Vangl2 at anteroposterior junctions can be measured even prior to the establishment of a clear PCP asymmetry41, and it is interesting that the subsequent emergence of clear PCP asymmetry coincides with both rapid embryonic elongation and increasing strength of fluid flow21 – events associated with directional mechanical strain.
Tissues that already exhibit PCP patterning can also shift their axis of localization orientation in response to morphogenetic forces. For example, in early pupal D. melanogaster wings, complexes of PCP proteins are oriented initially towards the wing margin (towards the source of Wg/Wnt4) as discussed previously; however, as hinge contraction drives rapid wing elongation, promoting large-scale cell shape changes and rearrangements, PCP complex orientation also seems to shift into alignment with proximal-distal axis of the developing wing65 (Fig. 3a, bottom). This shift in patterning also coincides with an increase in the amount of protein that appears to be asymmetrically enrichment into planar-polarized accumulations seen at later pupal stages, indicative of larger and more stable complexes11 (Fig. 3b bottom, longer vectors compared to Fig. 3b top). Similar behaviours are observed in the developing mouse epidermis, where again, PCP is aligned according to tissue strains that are coincident with the remodelling of junctions and tissue shape changes25. Actin regulators such as WDR1 and CFL1 are important for maintaining cortical tension during these rearrangements, which when disrupted, result in the inability to establish robust PCP patterning66 and in the impediment of coordinated cell movements67 (see also Supplementary information S2 (Box)).
Directionally biased strain can clearly promote polarized patterning events, but molecular mechanisms of the force-influenced changes in PCP orientation and magnitude remain poorly characterized. Rab11-mediated trafficking has been shown to be involved in the delivery and recycling of PCP components to apicolateral junctions and can utilize some of the same actin regulators necessary to maintain cortical tension11,66. Interestingly, Rab11 and Ankrd6 appear to be planar-polarized together towards the central fold of the neural plate during neural tube closure and wound margins during wound healing, thus presumably having asymmetries that are oriented along the axis of tissue strain in these contexts68. Perturbing PCP signalling disrupts the localization of Rab11 towards the neural fold, whereas dominant-negative Rab11 disrupts actomyosin-driven cell shape changes and rearrangements, which are required for proper wound healing68.
From molecular to functional asymmetry
The first description of PCP probably comes from Robert Hooke himself who discussed in his Micrographia69 the “conical bristles, all whose ends pointed backwards” present on the body of insects. The robust readout of these bristles provided the foundation for genetic screens identifying PCP proteins that direct assembly of these structures through control of the actin cytoskeleton2. These structures also facilitated the use of mutant clones to explore signalling mechanisms, for example in the discovery of directional non-autonomy, which refers to directional non-autonomous effects of specific PCP mutations70,71. PCP proteins also control both polarized organization and asymmetric cell fate choices in D. melanogaster photoreceptors72.
Following the work in flies, evolutionary conservation of PCP signalling in vertebrates was first revealed in the context of coordinated cell movements during gastrulation in X. laevis and zebrafish73–75. So-called convergent extension cell movements are most conspicuous for driving elongation of the embryonic body axis76, but importantly, they also drive tissue elongation during organogenesis, for example in the kidney tubules77. While the asymmetric localization of PCP components is well-established in D. melanogaster wing, the control of core PCP localization during convergent extension remains poorly defined. However, predominant features of the system appear to be conserved, as PK and VANGL were described to localize anteriorly in cells undergoing convergent extension, while DVL was shown to localize posteriorly78–82.
Another setting in which vertebrate PCP is essential is the oriented positioning of cilia within cells. First reported in the mouse inner ear, planar polarization of hair cells is organized by the asymmetric kinocilium positioning, which is preceded by asymmetric PCP patterning83. More recently, PCP signalling was also revealed to control planar polarization of motile ciliary beating in diverse ciliated cell types, and has been implicated in a wide array of developmental events, including the orientation of cell division, the migration of facial motorneurons, and the planar orientation of hair follicles in the mammalian epidermis84,85.
The mechanisms linking PCP to cell behaviour are most well defined in the context of wing hair positioning in D. melanogaster, and several reviews have been dedicated to this issue; we refer readers to that work1–4. Here, we will focus instead on two PCP-dependent processes in vertebrates, better understanding of which could have major clinical implications. We note here that our understanding of these mechanisms is only now beginning to emerge, so the following passages focus as much on what is not known as what is known.
Directional beating in multiciliated cells
Multiciliated cells (MCCs) bear dozens of directionally beating cilia that act in concert to drive fluid flow across the tissue (Fig. 4A). Such cells are critical for development and homeostasis of the airway, the central nervous system (CNS), and reproductive tracts84 (Fig. 4B). Directional beating of MCCs has been studied on the amphibian epidermis for nearly 200 years86, and not surprisingly, the first description of a role for PCP genes in these cells was in this context63,87. PCP signalling is now known to control polarized beating in all MCCs examined to date, including in the mammalian brain64,88–90, airway24, and oviduct40,91. Core PCP localization examined in these tissues reflects that seen in the fly wing; PK and VANGL occupy a domain complementary to that of DVL and FZ (Fig. 4A, C); however several additional cilia-associated proteins not found in D. melanogaster are required for normal core PCP protein localization in MCCs, including the myosin motor Myo1d92, the novel protein Spag6 (Sperm-associated antigen 6), which localizes to the ciliary axoneme and is essential for rotational polarity93, and c21orf59/Kurly, a novel cytoplasmic protein that physically interacts with the core PCP protein Dishevelled94.
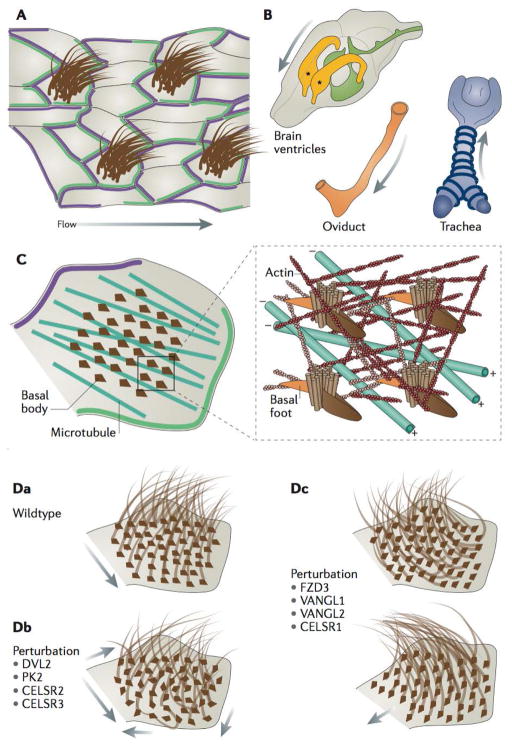
Figure 4. Planar cell polarity patterns direct the cytoskeletal organization and ciliary polarity of multiciliated cells.
a | Illustration of a multiciliated epithelium with asymmetric localization of planar cell polarity (PCP) complexes (green and purple), which direct polarized fluid flow across the surface of the tissue along the axis of PCP asymmetry (arrow). b | PCP patterning directs the fluid flow in various vertebrate tissues: the rostral flow of cerebrospinal fluid in the lateral ventricles64 (asterisks) of the brain, rostral mucociliary flow in the trachea24, and medial flow in the oviduct of mice91. c | PCP signalling is essential for the organization of the apical cytoskeletal elements responsible for ciliary orientation in multiciliated cells (MCCs)87,90. An apical actin network (red) spaces basal bodies, whereas a subapical actin network (pink) links basal feet of one basal body to the neighboring basal body. These linkages to actin control rotational polarity, that ensures the uniform orientation of basal bodies, and thereby cilia in individual MCCs, whereas polarized apical microtubules orient groups of basal bodies along the axis of planar polarity24,90, with the microtubule plus-ends oriented towards Frizzled and Dishevelled (FZD–DVL) accumulations (green)24. d | Conserved rotational polarity observed in wild-type MCCs (a), can be disorganized by perturbation of DVL2, PK2, and CELSR2 and 321,87,89,90, leading to largely disorganized cilia arrangements and disorganized ciliary beating and fluid flow (arrows) (b). Tissue-level polarity, the conservation of the uniform orientation of cilia across multiple MCCs, is disrupted upon perturbation of FZD3, Vang-like 1 (VANGL1) and VANGL2, and CELSR121,63,90; in this case different cells in the tissue can feature largely different orientation of their cilia. These defects can have considerable implications for the functions of MCCs in regulating fluid flow over tissues surfaces.
MCCs display multiple levels of planar polarization (Fig. 4D), a “rotational polarity” (organization of ciliary basal bodies within each cell) and distinct “tissue-level polarity (coordination of polarity between all cells across a tissue)84,85. Cell-autonomous defects in rotational polarity result from disruption of DVL or PK21,87,89, but mild rotational with more striking non-autonomous, tissue-level polarity defects result from disruption of the transmembrane proteins VANGL, FZ, or CELSR21,63,64,90. Interestingly, in mouse ependymal cells, CELSR1 appears to coordinate tissue-level polarity by influencing microtubule patterning, whereas CELSR2 and CELSR3 appear instead to be required for the rotational polarity and organization of individual basal body patches through the control of actin90.
Exactly how polarity information is communicated between PCP proteins and ciliary basal bodies is not yet known, but the cytoskeleton clearly plays a key role (see also Supplementary information S2 (Box)). Actin and microtubules comprise the cortical cytoskeleton and at the same time interact with accessory structures of the basal body, and both cytoskeletal elements are implicated in polarizing beating84,85,90 (Fig. 4C). Indeed, planar-polarized apical microtubules are a conserved feature of MCCs in the mouse airway24, and this is reminiscent of the microtubule arrangement observed in the D. melanogaster wing cells discussed above34–36. It is important, then, that PCP proteins localize both to the cell cortex, where they are known to control cell polarity, and to the basal body, where they could act to control basal body polarity21,24,64,87,89. There is a growing roster of additional proteins asymmetrically localized to basal bodies, including ODF2, non-canonical tubulins, and Centrin2, that may additionally be involved95–97, and among these, ODF2 is perhaps the most interesting, as mice lacking ODF2 display overt defects in the apical microtubule lattice95. Thus, these proteins provide excellent entry points for future studies exploring the interplay between PCP proteins, polarization of the cytoskeleton, and polarization of ciliary beating.
Convergent extension
Convergent extension was the first context in which vertebrate PCP signalling was studied73–75, so it is surprising that it remains among the most poorly understood processes with respect to the instructive role of PCP. Convergent extension is driven by the mediolateral intercalation of cells, resulting in the subsequent narrowing and consequent elongation of the tissue in the anteroposterior axis (Fig. 5a, b). Two mechanisms have been evoked to explain force generation for cell movement during vertebrate mesenchymal convergent extension, though the two are not mutually exclusive. In one model, cellular protrusions at the mediolateral cell faces exert traction force on cell neighbors, driving intercalation by cell crawling98. In the second model, junctions between apposed anterior and posterior cell faces are actively contracted, drawing mediolaterally-attached neighbours toward one another99 (Fig. 5a). Interestingly, PCP proteins can be implicated in both paradigms, as their manipulation disrupts both the stability and the polarity of mediolateral protrusions and the shrinkage of cell junctions to be biased towards those anteroposterior cell faces75,99,100. PCP-dependent stable cell protrusions and polarized junctional shrinking are also characteristics of cells undergoing convergent extension in vertebrate neural plate epithelia (Fig. 5b)101,102, with slight differences arising due to differing tissue architecture and context.
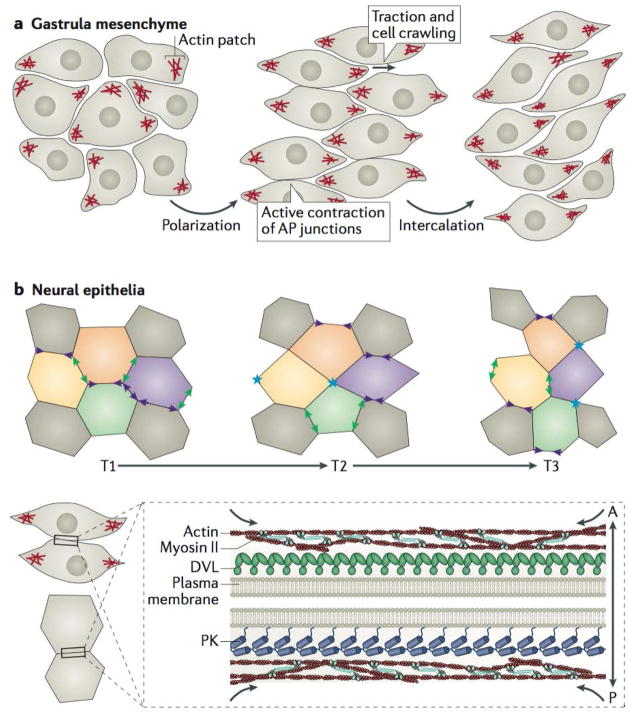
Figure 5. Planar cell polarity signalling directs polarized cell rearrangements during convergent extension.
a | Mesenchymal cells during vertebrate gastrulation collectively elongate along the mediolateral axis, stabilize mediolateral actin-based protrusions, and mediolaterally intercalate to narrow and lengthen the tissue; all these processes require intact planar cell polarity (PCP) signalling75,110. b | Neural ectodermal cells mediolaterally intercalate by preferentially shrinking antero-posterior (AP) junctions (purple arrowheads), which first resolve (blue asterisks) and then elongate to form new medio-lateral (ML) junctions (green arrowheads). The stages of this intercalation behaviour are often referred to as “T” transitions, where T1 to T2 transitions involve the shrinking of the AP junction, whereas T2 to T3 transitions involve the lengthening of a new ML junction between two cells previously separated along the ML axis at T1. c | Core PCP components can be found asymmetrically enriched along AP junctions of cells undergoing convergent extension in vertebrates, with Prickle (PK) typically localizing to the anterior cell faces and Dishevelled (DVL) along the posterior cell faces79–82. In the context of convergent extension, localization of PCP components at AP junctions coincides with the localization of filamentous actin enrichments and phosphorylated non-muscle Myosin II, which comprises the contractile actomyosin machinery that shrinks AP junctions81,99,101,102; there is evidence that PCP components are involved in regulating this contractility108,109.
Our understanding of the molecular biology of PCP during vertebrate convergent extension emerged from prior work in D. melanogaster, where PCP genes were shown to directly control actomyosin machinery via Rho GTPases and Rho Kinase103,104. Indeed, PCP-mediated convergent extension requires RhoA and Rho Kinase, as well as a host of other actin regulators such as the formin Daam1 and Rho GTPase Cdc4284. This conserved role for PCP in regulating actomyosin contraction makes it possible to assemble a preliminary model of how planar-polarized PCP protein localization directs collective cell movements (see also Supplementary information S2 (Box)).
Most evidence suggests that the PCP proteins form complementary asymmetric zones of localization on the anterior and posterior cell faces during convergent extension78–82 (Fig. 5c). There is precedent for PCP asymmetries in the mechanical control of cell intercalation, as the atypical myosin Dachs, asymmetrically localized by Ds, has been shown to drive anisotropic junctional tension in the developing D. melanogaster thorax, promoting planar-polarized cell rearrangements105. With this in mind, it seems feasible that PCP proteins could directly recruit contractile actomyosin regulators and thereby drive junction shrinkage. Indeed, live imaging has demonstrated pulses of actin, phosphorylation of myosin II, and enrichment of myosin regulators at sites of shrinking junctions in X. laevis gastrula mesenchyme and chick neural epithelial cells99,101 (Fig. 5c).
Additional recent insights have come from studies of PTK7, which is a vertebrate-specific regulator of core PCP function106. PTK7 is required for convergent extension-associated cell behaviours during gastrulation and neurulation100,106,107, but more recent work in the inner ear has revealed a mechanism linking PCP and PTK7 to Myosin II activation through Src kinase phosphorylation of myosin regulatory light chains108,109. While this preliminary model provides a parsimonious explanation of asymmetric PCP protein function during junction shrinking, it does not explain the effect of PCP proteins on formation of polarized membrane protrusions during convergent extension, which are also PTK7-dependent102. Among the possible mediators of this effect are septins, which have conserved functions in compartmentalizing cortical actomyosin during cytokinesis and are required for proper PCP function during convergent extension99,110.
While significant strides have been made in our understanding of PCP in more static epithelial tissues (in particular in the fly), the mechanism of PCP signalling during convergent extension is only now coming into focus. This slow progress likely reflects the dynamic nature of PCP processes, which are more easily visualized in stable tissues. Unlike other epithelia, cells undergoing convergent extension constantly exchange their neighbors. Dynamic analyses of epithelial PCP protein localization with time-lapse imaging and probing localization stability utilizing photobleaching and photoconversion techniques are now providing deeper mechanistic insights into PCP signalling11,21,40,41; studies of PCP involvement in convergent extension should also benefit from the application of similar techniques.
Finally, mutations of Vangl2 and Celsr2 were found to elicit neural tube closure defects in mice111,112. Furthermore, time-lapse imaging in X. laevis revealed that PCP-related neural tube closure defects arise from a failure of convergent extension75,113, and similar mechanisms were also documented in mice114,115. Therefore, better understanding of how PCP proteins direct cell behaviours during convergent extension can shed more light on the role of PCP gene mutations in human birth defects (Box 1).
Conclusions
PCP signalling is a ubiquitous mechanism of tissue patterning, with particular importance for animal development. Although significant strides have been made in understanding the establishment of PCP patterns and how they influence collective cell behaviours, much work remains to fully understand the instructive roles of PCP in morphogenesis and tissue organization. Importantly, many PCP effectors appear to be context-specific, especially in vertebrates, and the potential outcomes of establishing a directional PCP signal become increasingly complex in multifaceted tissues comprising multiple layers and cell types. Indeed, given the broad roles ascribed to PCP signalling in development in model animals, it is perhaps surprising that so few human disorders have yet been linked directly to mutation in PCP genes (Box 1). For example, despite their role in airway MCCs, no PCP genes have yet been associated with diseases stemming from defective motile cilia (such as ciliary dyskinesias). Similarly, PCP dysfunction has been associated with cystic kidney disease in animal models, but there has been little evidence for PCP genes being involved in this condition in humans. One potential explanation is that more severe birth defects mask the subtler phenotypes. For example, some patients with neural tube closure defects associated with PCP gene mutations present with hydrocephalus116–118, which could be a secondary consequence of neural tube defects or, alternatively, might be the manifestation of defects in MCC planar polarity in the brain88. Moreover, re-examination of patients with spina bifida with underlying PCP gene mutations recently revealed a high prevalence of congenital kidney defects that reflects those seen in PCP mutant mice119. These data highlight the complex nature of human birth defects and the potential role of PCP genes in their etiology. We consider this to be an essential area for future work.
Supplementary Material
Key Points.
Planar Cell Polarity (PCP) is a polarity axis that organizes cells throughout the plane of the tissue. It is conserved in metazoans and is essential for proper development and tissue homeostasis.
Asymmetric and mutually exclusive subcellular enrichments of key PCP proteins pattern cells in planar-polarized tissues. They also coordinate planar polarity between cells and control polarized behaviours through modulation of the cytoskeleton.
PCP patterns develop gradually from an initially disordered state through dynamic trafficking and various feedback interactions that can influence protein localization and stability.
PCP patterns seem to be globally oriented along a pre-defined axis in a given tissue . Notably, multiple mechanistic inputs may have differential influences on PCP patterning depending on developmental timing and tissue context, and may only partially overlap in different contexts.
The morphogenetic events governed by PCP signalling are best understood in Drosophila melanogaster, where in particular the orientation of hairs and bristles on the fly body has served to unravel basic principles of PCP-dependent processes. Work in this model has helped to better understand equivalent mechanisms in vertebrates, particularly in the context of the orientation of fluid flow mediated by multiciliated cells and cell rearrangements during convergent extension.
Mutations in PCP genes have been implicated in diverse human pathologies, and the body of evidence supporting involvement of PCP defects in human birth defects continues to grow at an increasing rate.
Acknowledgments
This work was supported by grants from the NHLBI (R01HL117164), NIGMS (R01 GM104853-02), and NICHD (1R01HD085901, 5R21HD084072) to J.B.W.
Glossary
- Endocytic Flux
The constant endocytosis and subsequent recycling of unstable protein components associated with the plasma membrane.
- Clathrin
Vesicular coating component that plays an important structural role in mediating endocytosis of membrane-associated proteins.
- Rab GTPase
Large family of small GTPases central to membrane trafficking regulation.
- Dynamin
GTPase protein critical for driving membrane fission that helps facilitate endocytic events.
- Polo-like kinase-1 (PLK1)
Serine/Threonine kinase that serves as an important cell cycle regulator, playing a notable role during the G2/M transition.
- Signalling Protocadherins
Largest subgroup of cadherins with variable extracelluar domains and diverse cytoplasmic domains that distinguish their variety of potential functions from those of classical cadherins.
- Mutant Clones
Groups of cells in a tissue that have perturbed gene expression and are surrounded by or interspersed between otherwise wild-type cells.
- Neural plate
Sheet of neuroepithelial cells that undergo convergent extension, apical constriction, and regional folding to form the vertebrate neural tube.
- Central fold
Midline of the neural plate that divides the left and right half, comprised of cells that apically constrict to facilitate neural plate folding and neural tube closure.
- Kinocilium
Microtubule-based protrusion on the apical surface of hair cells in tissues of the inner ear that guides the polarized orientation of an actin-based stereociliary bundle.
- Ependymal cells
Multiciliated cells the line the interface between the central nervous system and cerebrospinal fluid, which propel cerebrospinal flow through the brain ventricles and central spinal canal.
- Basal body
Modified centriole and accessory proteins that serves as a specialized microtubule organizing center at the base of the cilium, which they nucleate.
- Non-canonical tubulins
Tubulin superfamily members that are not conserved in all eukaryotes as alpha-, beta-, and gamma-tubulin are and that often function as centriole accessory structures.
- Traction force
The forces exerted across the junction between two cells that help facilitate cell rearrangements and tissue morphogenesis.
- Septins
Cytoskeletal components that upon binding to GTP can polymerize into ordered structures such as rings and filaments, which can function as scaffolds or diffusion barriers, for example.
- Spina bifida
Neural tube defect associated with a regional, incomplete closure of the spinal chord and associated tissues.
- Ommatidia
Optical units of insect compound eyes containing groups of polarized photoreceptive cells
- Trichomes
Actin-based hairs found on each cell of the fly wing blade that emanate from the apical, distal side of the cells and point distally as well – properties which are controlled by PCP signalling.
- Notum
The dorsal region of the thorax of a mature insect.
Biographies
Mitchell Butler is a graduate student under the advisement of John Wallingford at UT Austin. His doctoral research reflects his profound interest in cell polarity and tissue morphogenesis during development and is primarily focused on studying various planar-polarized protein dynamics and cellular behaviours in the Xenopus embryo.
John Wallingford is a Professor of Molecular Biosciences at The University of Texas at Austin. He received his Ph.D. from UT Austin in 1998 and performed postdoctoral work at UC Berkeley with Richard Harland and at Caltech with Scott Fraser. His lab seeks to understand the mechanisms linking systems-level programs of gene expression and protein function to discrete cell biological processes in developing embryos with the ultimate aim of understanding the etiology of human developmental disorders. When not in the lab, he enjoys a fine gin, climbing large rocks, and shooting birds.
References
- 1.Goodrich LV, Strutt D. Principles of planar polarity in animal development. Development. 2011;138:1877–1892. doi: 10.1242/dev.054080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adler PN. The frizzled/stan pathway and planar cell polarity in the Drosophila wing. Curr Top Dev Biol. 2012;101:1–31. doi: 10.1016/B978-0-12-394592-1.00001-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peng Y, Axelrod JD. Asymmetric protein localization in planar cell polarity: Mechanisms, puzzles and challenges. Curr Top Dev Biol. 2012;101:33. doi: 10.1016/B978-0-12-394592-1.00002-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lawrence PA, Casal J. The mechanisms of planar cell polarity, growth and the Hippo pathway: some known unknowns. Developmental Biology. 2013;377:1–8. doi: 10.1016/j.ydbio.2013.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang Y, Mlodzik M. Wnt-Frizzled/Planar Cell Polarity Signalling: Cellular Orientation by Facing the Wind (Wnt) Annu Rev Cell Dev Biol. 2015;31:623–646. doi: 10.1146/annurev-cellbio-100814-125315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Usui T, et al. Flamingo, a seven-pass transmembrane cadherin, regulates planar cell polarity under the control of Frizzled. Cell. 1999;98:585–595. doi: 10.1016/s0092-8674(00)80046-x. [DOI] [PubMed] [Google Scholar]
- 7.Lawrence PA, Casal J, Struhl G. Cell interactions and planar polarity in the abdominal epidermis of Drosophila. Development. 2004;131:4651–4664. doi: 10.1242/dev.01351. Analysis of multiple mutant clones and backgrounds identified Fmi as the essential component for intercellular PCP signalling and provided a more detailed description of the establishment of the global PCP patterns. [DOI] [PubMed] [Google Scholar]
- 8.Strutt H, Strutt D. Differential Stability of Flamingo Protein Complexes Underlies the Establishment of Planar Polarity. Current Biology. 2008;18:1555–1564. doi: 10.1016/j.cub.2008.08.063. An examination of Fmi binding preferences and dynamics under different conditions provided a fresh perspective on how PCP complexes assemble. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen W-S, et al. Asymmetric Homotypic Interactions of the Atypical Cadherin Flamingo Mediate Intercellular Polarity Signaling. Cell. 2008;133:1093–1105. doi: 10.1016/j.cell.2008.04.048. A detailed analysis of cell-cell signalling through Fmi provided evidence for two different signalling states of Fmi when bound with Fz or when bound with Vang or neither. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu J, Mlodzik M. The Frizzled Extracellular Domain Is a Ligand for Van Gogh/Stbm during Nonautonomous Planar Cell Polarity Signaling. Developmental Cell. 2008;15:462–469. doi: 10.1016/j.devcel.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Strutt H, Warrington SJ, Strutt D. Dynamics of Core Planar Polarity Protein Turnover and Stable Assembly into Discrete Membrane Subdomains. Developmental Cell. 2011;20:511–525. doi: 10.1016/j.devcel.2011.03.018. The first highly dynamic analyses of PCP localization contributed greatly to how we understand and study PCP signalling complex assembly. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Strutt DI. Asymmetric localization of frizzled and the establishment of cell polarity in the Drosophila wing. Molecular Cell. 2001;7:367–375. doi: 10.1016/s1097-2765(01)00184-8. [DOI] [PubMed] [Google Scholar]
- 13.Bastock R, Strutt H, Strutt D. Strabismus is asymmetrically localised and binds to Prickle and Dishevelled during Drosophila planar polarity patterning. Development. 2003;130:3007–3014. doi: 10.1242/dev.00526. [DOI] [PubMed] [Google Scholar]
- 14.Struhl G, Casal J, Lawrence PA. Dissecting the molecular bridges that mediate the function of Frizzled in planar cell polarity. Development. 2012;139:3665–3674. doi: 10.1242/dev.083550. Comprehensive study of transmembrane core PCP components provided multiple insights into the intricacies of intercellular PCP signalling. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Axelrod JD, Miller JR, Shulman JM, Moon RT, Perrimon N. Differential recruitment of Dishevelled provides signaling specificity in the planar cell polarity and Wingless signaling pathways. Genes & Development. 1998;12:2610–2622. doi: 10.1101/gad.12.16.2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Axelrod JD. Unipolar membrane association of Dishevelled mediates Frizzled planar cell polarity signaling. Genes & Development. 2001;15:1182–1187. doi: 10.1101/gad.890501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jenny A, Darken RS, Wilson PA, Mlodzik M. Prickle and Strabismus form a functional complex to generate a correct axis during planar cell polarity signaling. The EMBO Journal. 2003;22:4409–4420. doi: 10.1093/emboj/cdg424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jenny A, Reynolds-Kenneally J, Das G, Burnett M, Mlodzik M. Diego and Prickle regulate Frizzled planar cell polarity signalling by competing for Dishevelled binding. Nature Cell Biology. 2005;7:691–697. doi: 10.1038/ncb1271. [DOI] [PubMed] [Google Scholar]
- 19.Strutt D, Strutt H. Differential activities of the core planar polarity proteins during Drosophila wing patterning. Developmental Biology. 2007;302:181–194. doi: 10.1016/j.ydbio.2006.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tree DRP, et al. Prickle mediates feedback amplification to generate asymmetric planar cell polarity signaling. Cell. 2002;109:371–381. doi: 10.1016/s0092-8674(02)00715-8. [DOI] [PubMed] [Google Scholar]
- 21.Butler MT, Wallingford JB. Control of vertebrate core planar cell polarity protein localization and dynamics by Prickle 2. Development. 2015;142:3429–3439. doi: 10.1242/dev.121384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park M, Moon RT. The planar cell-polarity gene stbm regulates cell behaviour and cell fate in vertebrate embryos. Nat Cell Biol. 2002;4:20–25. doi: 10.1038/ncb716. [DOI] [PubMed] [Google Scholar]
- 23.Das G, Jenny A, Klein TJ, Eaton S, Mlodzik M. Diego interacts with Prickle and Strabismus/Van Gogh to localize planar cell polarity complexes. Development. 2004;131:4467–4476. doi: 10.1242/dev.01317. [DOI] [PubMed] [Google Scholar]
- 24.Vladar EK, Bayly RD, Sangoram AM, Scott MP, Axelrod JD. Microtubules Enable the Planar Cell Polarity of Airway Cilia. Current Biology. 2012:1–10. doi: 10.1016/j.cub.2012.09.046. Exhaustive analysis revealed cell- and tissue-specific localizations of different vertebrate PCP family members and strong links between PCP signalling and microtubule polarity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aw WY, Heck BW, Joyce B, Devenport D. Transient Tissue-Scale Deformation Coordinates Alignment of Planar Cell Polarity Junctions in the Mammalian Skin. Curr Biol. 2016:1–12. doi: 10.1016/j.cub.2016.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mottola G, Classen AK, Gonzalez-Gaitan M, Eaton S, Zerial M. A novel function for the Rab5 effector Rabenosyn-5 in planar cell polarity. Development. 2010;137:2353–2364. doi: 10.1242/dev.048413. [DOI] [PubMed] [Google Scholar]
- 27.Classen AK, Anderson KI, Marois E, Eaton S. Hexagonal Packing of Drosophila Wing Epithelial Cells by the Planar Cell Polarity Pathway. Developmental Cell. 2005;9:805–817. doi: 10.1016/j.devcel.2005.10.016. [DOI] [PubMed] [Google Scholar]
- 28.Cho B, Pierre-Louis G, Sagner A, Eaton S, Axelrod JD. Clustering and Negative Feedback by Endocytosis in Planar Cell Polarity Signaling Is Modulated by Ubiquitinylation of Prickle. PLoS Genet. 2015;11:e1005259. doi: 10.1371/journal.pgen.1005259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yu A, et al. Association of Dishevelled with the Clathrin AP-2 Adaptor Is Required for Frizzled Endocytosis and Planar Cell Polarity Signaling. Developmental Cell. 2007;12:129–141. doi: 10.1016/j.devcel.2006.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wong HC, et al. Direct binding of the PDZ domain of Dishevelled to a conserved internal sequence in the C-terminal region of Frizzled. Molecular Cell. 2003;12:1251–1260. doi: 10.1016/s1097-2765(03)00427-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carvajal-Gonzalez JM, et al. The clathrin adaptor AP-1 complex and Arf1 regulate planar cell polarity in vivo. Nature Communications. 2015;6:6751–15. doi: 10.1038/ncomms7751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Devenport D, Oristian D, Heller E, Fuchs E. Mitotic internalization of planar cell polarity proteins preserves tissue polarity. Nat Cell Biol. 2011;13:893–902. doi: 10.1038/ncb2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shrestha R, et al. Mitotic Control of Planar Cell Polarity by Polo-like Kinase 1. Developmental Cell. 2015;33:522–534. doi: 10.1016/j.devcel.2015.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eaton S, Wepf R, Simons K. Roles for Rac1 and Cdc42 in planar polarization and hair outgrowth in the wing of Drosophila. The Journal of Cell Biology. 1996;135:1277–1289. doi: 10.1083/jcb.135.5.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hannus M, Feiguin F, Heisenberg CP, Eaton S. Planar cell polarization requires Widerborst, a B' regulatory subunit of protein phosphatase 2A. Development. 2002;129:3493–3503. doi: 10.1242/dev.129.14.3493. [DOI] [PubMed] [Google Scholar]
- 36.Shimada Y, Yonemura S, Ohkura H, Strutt D, Uemura T. Polarized Transport of Frizzled along the Planar Microtubule Arrays in Drosophila Wing Epithelium. Developmental Cell. 2006;10:209–222. doi: 10.1016/j.devcel.2005.11.016. [DOI] [PubMed] [Google Scholar]
- 37.Sepich DS, Usmani M, Pawlicki S, Solnica-Krezel L. Wnt/PCP signaling controls intracellular position of MTOCs during gastrulation convergence and extension movements. Development. 2011;138:543–552. doi: 10.1242/dev.053959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Olofsson J, Sharp KA, Matis M, Cho B, Axelrod JD. Prickle/spiny-legs isoforms control the polarity of the apical microtubule network in planar cell polarity. Development. 2014;141:2866–2874. doi: 10.1242/dev.105932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matis M, Russler-Germain DA, Hu Q, Tomlin CJ, Axelrod JD. Microtubules provide directional information for core PCP function. eLife. 2014;3:e02893. doi: 10.7554/eLife.02893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shi D, et al. Dynamics of planar cell polarity protein Vangl2 in the mouse oviduct epithelium. MOD. 2016:1–52. doi: 10.1016/j.mod.2016.05.002. [DOI] [PubMed] [Google Scholar]
- 41.Chien Y-H, Keller R, Kintner C, Shook DR. Mechanical Strain Determines the Axis of Planar Polarity in Ciliated Epithelia. Curr Biol. 2015:1–12. doi: 10.1016/j.cub.2015.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cervenka I, et al. Dishevelled is a NEK2 kinase substrate controlling dynamics of centrosomal linker proteins. Proceedings of the National Academy of Sciences. 2016 doi: 10.1073/pnas.1608783113. 201608783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Narimatsu M, et al. Regulation of Planar Cell Polarity by Smurf Ubiquitin Ligases. Cell. 2009;137:295–307. doi: 10.1016/j.cell.2009.02.025. Ubiquitylation and degradation were revealed as a PCP signalling feedback mechanisms. [DOI] [PubMed] [Google Scholar]
- 44.Gao B, et al. Wnt Signaling Gradients Establish Planar Cell Polarity by Inducing Vangl2 Phosphorylation through Ror2. Developmental Cell. 2011;20:163–176. doi: 10.1016/j.devcel.2011.01.001. Examination of Wnt and its directional influence on cell behaviour provided insight into the etiology of Robinow syndrome and related phenotypes that can result from mutations in PCP components. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Daulat AM, et al. Mink1 Regulates β-catenin-Independent Wnt signalling via Prickle Phosphorylation. Molecular and Cellular Biology. 2011;32:173–185. doi: 10.1128/MCB.06320-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shafer B, Onishi K, Lo C, Colakoglu G, Zou Y. Vangl2 Promotes Wnt/Planar Cell Polarity-like Signaling by Antagonizing Dvl1-Mediated Feedback Inhibition in Growth Cone Guidance. Developmental Cell. 2011;20:177–191. doi: 10.1016/j.devcel.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Strutt H, Strutt D. EGF Signaling and Ommatidial Rotation in the Drosophila Eye. Current Biology. 2003;13:1451–1457. doi: 10.1016/s0960-9822(03)00545-1. [DOI] [PubMed] [Google Scholar]
- 48.Strutt H, Searle E, Thomas-MacArthur V, Brookfield R, Strutt D. A Cul-3-BTB ubiquitylation pathway regulates junctional levels and asymmetry of core planar polarity proteins. Development. 2013;140:1693–1702. doi: 10.1242/dev.089656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Strutt H, Thomas-MacArthur V, Strutt D. Strabismus Promotes Recruitment and Degradation of Farnesylated Prickle in Drosophila melanogaster Planar Polarity Specification. PLoS Genet. 2013;9:e1003654. doi: 10.1371/journal.pgen.1003654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thomas C, Strutt D. The roles of the cadherins Fat and Dachsous in planar polarity specification in Drosophila. Dev Dyn. 2011;241:27–39. doi: 10.1002/dvdy.22736. [DOI] [PubMed] [Google Scholar]
- 51.Matakatsu H, Blair SS. Interactions between Fat and Dachsous and the regulation of planar cell polarity in the Drosophila wing. Development. 2004;131:3785–3794. doi: 10.1242/dev.01254. [DOI] [PubMed] [Google Scholar]
- 52.Casal J, Lawrence PA, Struhl G. Two separate molecular systems, Dachsous/Fat and Starry night/Frizzled, act independently to confer planar cell polarity. Development. 2006;133:4561–4572. doi: 10.1242/dev.02641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang CH, Axelrod JD, Simon MA. Regulation of Frizzled by fat-like cadherins during planar polarity signaling in the Drosophila compound eye. Cell. 2002;108:675–688. doi: 10.1016/s0092-8674(02)00658-x. [DOI] [PubMed] [Google Scholar]
- 54.Ma D, Yang CH, McNeill H, Simon MA, Axelrod JD. Fidelity in planar cell polarity signalling. Nature. 2003;421:543–547. doi: 10.1038/nature01366. [DOI] [PubMed] [Google Scholar]
- 55.Gubb D, et al. The balance between isoforms of the prickle LIM domain protein is critical for planar polarity in Drosophila imaginal discs. Genes & Development. 1999;13:2315–2327. doi: 10.1101/gad.13.17.2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ayukawa T, et al. Dachsous-Dependent Asymmetric Localization of Spiny-Legs Determines Planar Cell Polarity Orientation in Drosophila. CELREP. 2014;8:610–621. doi: 10.1016/j.celrep.2014.06.009. [DOI] [PubMed] [Google Scholar]
- 57.Ambegaonkar AA, Irvine KD. Coordination of planar cell polarity pathways through Spiny legs. eLife. 2015;4 doi: 10.7554/eLife.09946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Merkel M, et al. The Balance of Prickle/Spiny-Legs Isoforms Controls the Amount of Coupling between Core and Fat PCP Systems. Curr Biol. 2014:1–13. doi: 10.1016/j.cub.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 59.Sokol SY. Spatial and temporal aspects of Wnt signaling and planar cell polarity during vertebrate embryonic development. Seminars in Cell and Developmental Biology. 2015;42IS:78–85. doi: 10.1016/j.semcdb.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wu J, Roman AC, Carvajal-Gonzalez JM, Mlodzik M. Wg and Wnt4 provide long-range directional input to planar cell polarity orientation in Drosophila. Nature Cell Biology. 2013;15:1–13. doi: 10.1038/ncb2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chu C-W, Sokol SY. Wnt proteins can direct planar cell polarity in vertebrate ectoderm. eLife. 2016;5:e16463–33. doi: 10.7554/eLife.16463. Utilizing dual mosaic embryos provided solid evidence for an instructive role of Wnt proteins in determining PCP pattern orientation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mitchell B, Jacobs R, Li J, Chien S, Kintner C. A positive feedback mechanism governs the polarity and motion of motile cilia. Nature. 2007;447:97–101. doi: 10.1038/nature05771. [DOI] [PubMed] [Google Scholar]
- 63.Mitchell B, et al. The PCP Pathway Instructs the Planar Orientation of Ciliated Cells in the Xenopus Larval Skin. Current Biology. 2009;19:924–929. doi: 10.1016/j.cub.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Guirao B, et al. Coupling between hydrodynamic forces and planar cell polarity orients mammalian motile cilia. Nature Cell Biology. 2010;12:341–350. doi: 10.1038/ncb2040. [DOI] [PubMed] [Google Scholar]
- 65.Aigouy B, et al. Cell Flow Reorients the Axis of Planar Polarity in the Wing Epithelium of Drosophila. Cell. 2010;142:773–786. doi: 10.1016/j.cell.2010.07.042. Tissue-wide analysis of PCP orientation over time showed shifts along the axis of dramatic growth and cell rearrangements, suggesting forces can contribute to PCP alignment. [DOI] [PubMed] [Google Scholar]
- 66.Mahaffey JP, Grego-Bessa J, Liem KF, Anderson KV. Cofilin and Vangl2 cooperate in the initiation of planar cell polarity in the mouse embryo. Development. 2013;140:1262–1271. doi: 10.1242/dev.085316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Luxenburg C, et al. Wdr1-mediated cell shape dynamics and cortical tension are essential for epidermal planar cell polarity. Nature Publishing Group. 2015;17:592–604. doi: 10.1038/ncb3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ossipova O, et al. Role of Rab11 in planar cell polarity and apical constriction during vertebrate neural tube closure. Nature Communications. 2014;5:1–8. doi: 10.1038/ncomms4734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hooke R. In: Micrographia: or some physiological descriptions of minute bodies made by magnifying glasses. Martyn J, Allestry J, editors. pp. 1–323. [Google Scholar]
- 70.Vinson CR, Adler PN. Directional non-cell autonomy and the transmission of polarity information by the frizzled gene of Drosophila. 1987 doi: 10.1038/329549a0. The directional, non-autonomous effects of frizzled perturbation on neighboring cells were uncovered and thus revealed the coordinated nature of planar tissue polarity. [DOI] [PubMed] [Google Scholar]
- 71.Taylor J, Abramova N, Charlton J, Adler PN. Van Gogh: a new Drosophila tissue polarity gene. Genetics. 1998;150:199–210. doi: 10.1093/genetics/150.1.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jenny A. Planar Cell Polarity Signaling in the Drosophila Eye. Curr Top Dev Biol. 2010;93:189. doi: 10.1016/B978-0-12-385044-7.00007-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tada M, Smith JC. Xwnt11 is a target of Xenopus Brachyury: regulation of gastrulation movements via Dishevelled, but not through the canonical Wnt pathway. Development. 2000;127:2227–2238. doi: 10.1242/dev.127.10.2227. [DOI] [PubMed] [Google Scholar]
- 74.Heisenberg CP, et al. Silberblick/Wnt11 mediates convergent extension movements during zebrafish gastrulation. Nature. 2000;405:76–81. doi: 10.1038/35011068. [DOI] [PubMed] [Google Scholar]
- 75.Wallingford JB, et al. Dishevelled controls cell polarity during Xenopus gastrulation. Nature. 2000;405:81–85. doi: 10.1038/35011077. References 73–75 are the initial discovery and study of PCP signalling in vertebrates and provided the foundation for understanding how PCP perturbation might lead to NTDs. [DOI] [PubMed] [Google Scholar]
- 76.Tada M, Heisenberg CP. Convergent extension: using collective cell migration and cell intercalation to shape embryos. Development. 2012;139:3897–3904. doi: 10.1242/dev.073007. [DOI] [PubMed] [Google Scholar]
- 77.Lienkamp SS, et al. Vertebrate kidney tubules elongate using a planar cell polarity–dependent, rosette-based mechanism of convergent extension. Nature Genetics. 2012;44:1382–1387. doi: 10.1038/ng.2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jiang D, Munro EM, Smith WC. Ascidian prickle regulates both mediolateral and anterior-posterior cell polarity of notochord cells. Curr Biol. 2005;15:79–85. doi: 10.1016/j.cub.2004.12.041. [DOI] [PubMed] [Google Scholar]
- 79.Ciruna B, Jenny A, Lee D, Mlodzik M, Schier AF. Planar cell polarity signalling couples cell division and morphogenesis during neurulation. Nature Cell Biology. 2006;439:220–224. doi: 10.1038/nature04375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yin C, Kiskowski M, Pouille PA, Farge E, Solnica-Krezel L. Cooperation of polarized cell intercalations drives convergence and extension of presomitic mesoderm during zebrafish gastrulation. The Journal of Cell Biology. 2008;180:221–232. doi: 10.1083/jcb.200704150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ossipova O, Kim K, Sokol SY. Planar polarization of Vangl2 in the vertebrate neural plate is controlled by Wnt and Myosin II signaling. Biology Open. 2015;4:722–730. doi: 10.1242/bio.201511676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Roszko I, Sepich SD, Jessen JR, Chandrasekhar A, Solnica-Krezel L. A dynamic intracellular distribution of Vangl2 accompanies cell polarization during zebrafish gastrulation. Development. 2015;142:2508–2520. doi: 10.1242/dev.119032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rida PCG, Chen P. Line up and listen: Planar cell polarity regulation in the mammalian inner ear. Seminars in Cell and Developmental Biology. 2009;20:978–985. doi: 10.1016/j.semcdb.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wallingford JB. Planar Cell Polarity and the Developmental Control of Cell Behavior in Vertebrate Embryos. Annu Rev Cell Dev Biol. 2012;28:627–653. doi: 10.1146/annurev-cellbio-092910-154208. [DOI] [PubMed] [Google Scholar]
- 85.Devenport D. Tissue morphodynamics: Translating planar polarity cues into polarized cell behaviors. Seminars in Cell and Developmental Biology. 2016:1–12. doi: 10.1016/j.semcdb.2016.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sharpey W. Cilia. 1836. Marchant, printer. [Google Scholar]
- 87.Park TJ, Mitchell BJ, Abitua PB, Kintner C, Wallingford JB. Dishevelled controls apical docking and planar polarization of basal bodies in ciliated epithelial cells. Nature Genetics. 2008;40:871–879. doi: 10.1038/ng.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tissir F, et al. Lack of cadherins Celsr2 and Celsr3 impairs ependymal ciliogenesis, leading to fatal hydrocephalus. Nat Neurosci. 2010;13:700–707. doi: 10.1038/nn.2555. [DOI] [PubMed] [Google Scholar]
- 89.Ohata S, et al. Loss of Dishevelleds Disrupts Planar Polarity in Ependymal Motile Cilia and Results in Hydrocephalus. Neuron. 2014;83:558–571. doi: 10.1016/j.neuron.2014.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Boutin C, et al. A dual role for planar cell polarity genes in ciliated cells. Proceedings of the National Academy of Sciences. 2014 doi: 10.1073/pnas.1404988111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Shi D, et al. Celsr1 is required for the generation of polarity at multiple levels of the mouse oviduct. Development. 2014;141:4558–4568. doi: 10.1242/dev.115659. [DOI] [PubMed] [Google Scholar]
- 92.Hegan PS, Ostertag E, Geurts AM, Mooseker MS. Myosin Id is required for planar cell polarity in ciliated tracheal and ependymal epithelial cells. Cytoskeleton. 2015;72:n/a–n/a. doi: 10.1002/cm.21259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Teves ME, et al. Sperm-Associated Antigen 6 (SPAG6) Deficiency and Defects in Ciliogenesis and Cilia Function: Polarity, Density, and Beat. PLoS ONE. 2014;9:e107271. doi: 10.1371/journal.pone.0107271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jaffe KM, et al. c21orf59/kurly Controls Both Cilia Motility and Polarization. CELREP. 2016:1–10. doi: 10.1016/j.celrep.2016.01.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kunimoto K, et al. Coordinated ciliary beating requires Odf2-mediated polarization of basal bodies via basal feet. Cell. 2012;148:189–200. doi: 10.1016/j.cell.2011.10.052. [DOI] [PubMed] [Google Scholar]
- 96.Ying G, et al. Centrin 2 Is Required for Mouse Olfactory Ciliary Trafficking and Development of Ependymal Cilia Planar Polarity. Journal of Neuroscience. 2014;34:6377–6388. doi: 10.1523/JNEUROSCI.0067-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Turk E, et al. Zeta-Tubulin Is a Member of a Conserved Tubulin Module and Is a Component of the Centriolar Basal Foot in Multiciliated Cells. Curr Biol. 2015;25:2177–2183. doi: 10.1016/j.cub.2015.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Shih J, Keller R. Cell motility driving mediolateral intercalation in explants of Xenopus laevis. Development. 1992;116:901–914. doi: 10.1242/dev.116.4.901. [DOI] [PubMed] [Google Scholar]
- 99.Shindo A, Wallingford JB. PCP and septins compartmentalize cortical actomyosin to direct collective cell movement. Science. 2014;343:649–652. doi: 10.1126/science.1243126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yen WW, et al. PTK7 is essential for polarized cell motility and convergent extension during mouse gastrulation. Development. 2009;136:2039–2048. doi: 10.1242/dev.030601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nishimura T, Honda H, Takeichi M. Planar Cell Polarity Links Axes of Spatial Dynamics in Neural-Tube Closure. Cell. 2012;149:1084–1097. doi: 10.1016/j.cell.2012.04.021. PCP signalling was shown to control spatially restricted contractile actomyosin machinery, providing a mechanism by which PCP guides neuroepithelial cell rearrangements. [DOI] [PubMed] [Google Scholar]
- 102.Williams M, Yen W, Lu X, Sutherland A. Distinct Apical and Basolateral Mechanisms Drive Planar Cell Polarity-Dependent Convergent Extension of the Mouse Neural Plate. Developmental Cell. 2014:1–13. doi: 10.1016/j.devcel.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Strutt DI, Weber U, Mlodzik M. The role of RhoA in tissue polarity and Frizzled signalling. Nature. 1997;387:292–295. doi: 10.1038/387292a0. [DOI] [PubMed] [Google Scholar]
- 104.Winter CG, et al. Drosophila Rho-associated kinase (Drok) links Frizzled-mediated planar cell polarity signaling to the actin cytoskeleton. Cell. 2001;105:81–91. doi: 10.1016/s0092-8674(01)00298-7. [DOI] [PubMed] [Google Scholar]
- 105.Bosveld F, et al. Mechanical control of morphogenesis by Fat/Dachsous/Four-jointed planar cell polarity pathway. Science. 2012;336:724–727. doi: 10.1126/science.1221071. Asymmetric Dachsous was shown to polarize the myosin Dachs as a mechanism to generate the anisotropic junctional tension essential for proper tissue morphogenesis. [DOI] [PubMed] [Google Scholar]
- 106.Lu X, et al. PTK7/CCK-4 is a novel regulator of planar cell polarity in vertebrates. Nature. 2004;430:93–98. doi: 10.1038/nature02677. [DOI] [PubMed] [Google Scholar]
- 107.Hayes M, Naito M, Daulat A, Angers S, Ciruna B. Ptk7 promotes non-canonical Wnt/PCP-mediated morphogenesis and inhibits Wnt/β-catenin-dependent cell fate decisions during vertebrate development. Development. 2013;140:2245–2245. doi: 10.1242/dev.090183. [DOI] [PubMed] [Google Scholar]
- 108.Lee J, et al. PTK7 regulates myosin II activity to orient planar polarity in the mammalian auditory epithelium. Curr Biol. 2012;22:956–966. doi: 10.1016/j.cub.2012.03.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Andreeva A, et al. PTK7-Src Signaling at Epithelial Cell Contacts Mediates Spatial Organization of Actomyosin and Planar Cell Polarity. Developmental Cell. 2014;29:20–33. doi: 10.1016/j.devcel.2014.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kim SK, et al. Planar Cell Polarity Acts Through Septins to Control Collective Cell Movement and Ciliogenesis. Science. 2010;329:1337–1340. doi: 10.1126/science.1191184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kibar Z, et al. Ltap, a mammalian homolog of Drosophila Strabismus/Van Gogh, is altered in the mouse neural tube mutant Loop-tail. Nature Genetics. 2001;28:251–255. doi: 10.1038/90081. [DOI] [PubMed] [Google Scholar]
- 112.Murdoch JN, Doudney K, Paternotte C, Copp AJ, Stanier P. Severe neural tube defects in the loop-tail mouse result from mutation of Lpp1, a novel gene involved in floor plate specification. Human Molecular Genetics. 2001;10:2593–2601. doi: 10.1093/hmg/10.22.2593. [DOI] [PubMed] [Google Scholar]
- 113.Wallingford JB, Harland RM. Neural tube closure requires Dishevelled-dependent convergent extension of the midline. Development. 2002;129:5815–5825. doi: 10.1242/dev.00123. [DOI] [PubMed] [Google Scholar]
- 114.Wang J, et al. Dishevelled genes mediate a conserved mammalian PCP pathway to regulate convergent extension during neurulation. Development. 2006;133:1767–1778. doi: 10.1242/dev.02347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ybot-Gonzalez P, et al. Convergent extension, planar-cell-polarity signalling and initiation of mouse neural tube closure. Development. 2007;134:789–799. doi: 10.1242/dev.000380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kibar Z, et al. Mutations in VANGL1 Associated with Neural-Tube Defects. N Engl J Med. 2007;356:1432–1437. doi: 10.1056/NEJMoa060651. [DOI] [PubMed] [Google Scholar]
- 117.Bosoi CM, et al. Identification and characterization of novel rare mutations in the planar cell polarity gene PRICKLE1 in human neural tube defects. Hum Mutat. 2011;32:1371–1375. doi: 10.1002/humu.21589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Seo JH, et al. Mutations in the planar cell polarity gene, Fuzzy, are associated with neural tube defects in humans. Human Molecular Genetics. 2011;20:4324–4333. doi: 10.1093/hmg/ddr359. [DOI] [PubMed] [Google Scholar]
- 119.Brzoska HL, et al. Planar cell polarity genes Celsr1 and Vangl2 are necessary for kidney growth, differentiation, and rostrocaudal patterning. Kidney Int. 2016 doi: 10.1016/j.kint.2016.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kochanek KD, Murphy SL, Xu J, Tejada-Vera B. Deaths: Final Data for 2014. Natl Vital Stat Rep. 2016;65:1–122. [PubMed] [Google Scholar]
- 121.Wallingford JB, Niswander LA, Shaw GM, Finnell RH. The Continuing Challenge of Understanding, Preventing, and Treating Neural Tube Defects. Science. 2013;339:1222002–1222002. doi: 10.1126/science.1222002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Juriloff DM, Harris MJ. A consideration of the evidence that genetic defects in planar cell polarity contribute to the etiology of human neural tube defects. Birth Defects Research Part A: Clinical and Molecular Teratology. 2012;94:824–840. doi: 10.1002/bdra.23079. [DOI] [PubMed] [Google Scholar]
- 123.De Marco P, et al. Planar cell polarity gene mutations contribute to the etiology of human neural tube defects in our population. Birth Defects Research Part A: Clinical and Molecular Teratology. 2014;100:n/a–n/a. doi: 10.1002/bdra.23255. [DOI] [PubMed] [Google Scholar]
- 124.Robinson A, et al. Mutations in the planar cell polarity genes CELSR1 and SCRIB are associated with the severe neural tube defect craniorachischisis. Hum Mutat. 2012;33:440–447. doi: 10.1002/humu.21662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Iliescu A, Gravel M, Horth C, Gros P. Independent Mutations at Arg181 and Arg274 of Vangl Proteins That Are Associated with Neural Tube Defects in Humans Decrease Protein Stability and Impair Membrane Targeting. Biochemistry. 2014;53:5356–5364. doi: 10.1021/bi500400g. [DOI] [PubMed] [Google Scholar]
- 126.Lei Y, et al. Identification of novel CELSR1 mutations in spina bifida. PLoS ONE. 2014;9:e92207–8. doi: 10.1371/journal.pone.0092207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Afzal AR, et al. Recessive Robinow syndrome, allelic to dominant brachydactyly type B, is caused by mutation of ROR2. Nature Genetics. 2000;25:419–422. doi: 10.1038/78107. [DOI] [PubMed] [Google Scholar]
- 128.van Bokhoven H, et al. Mutation of the gene encoding the ROR2 tyrosine kinase causes autosomal recessive Robinow syndrome. Nature Genetics. 2000;25:423–426. doi: 10.1038/78113. [DOI] [PubMed] [Google Scholar]
- 129.White J, et al. DVL1 frameshift mutations clustering in the penultimate exon cause autosomal-dominant Robinow syndrome. The American Journal of Human Genetics. 2015;96:612–622. doi: 10.1016/j.ajhg.2015.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Bunn KJ, et al. Mutations in DVL1 Causean Osteosclerotic Form of Robinow Syndrome. The American Journal of Human Genetics. 2015;96:623–630. doi: 10.1016/j.ajhg.2015.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.White JJ, et al. DVL3 alleles resulting in a - 1 frameshift of the last exon mediate autosomal-dominant Robinow syndrome. The American Journal of Human Genetics. 2016;98:553–561. doi: 10.1016/j.ajhg.2016.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Person AD, et al. WNT5A mutations in patients with autosomal dominant Robinow syndrome. Dev Dyn. 2010;239:327–337. doi: 10.1002/dvdy.22156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hayes M, et al. ptk7 mutant zebrafish models of congenital and idiopathic scoliosis implicate dysregulated Wnt signalling in disease. Nature Communications. 2014;5:4777–11. doi: 10.1038/ncomms5777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Grimes DT, et al. Zebrafish models of idiopathic scoliosis link cerebrospinal fluid flow defects to spine curvature. Science. 2016;352:1341–1344. doi: 10.1126/science.aaf6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Andersen MR, et al. Mutation of the planar cell polarity gene VANGL1 in adolescent idiopathic scoliosis. Spine (Phila Pa 1976) 2016 doi: 10.1097/BRS.0000000000001927. [DOI] [PubMed] [Google Scholar]
- 136.Matis M, Axelrod JD. Regulation of PCP by the Fat signaling pathway. Genes & Development. 2013;27:2207–2220. doi: 10.1101/gad.228098.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Rock R, Schrauth S, Gessler M. Expression of mouse dchs1, fjx1, and fat-j suggests conservation of the planar cell polarity pathway identified in drosophila. Dev Dyn. 2005;234:747–755. doi: 10.1002/dvdy.20515. [DOI] [PubMed] [Google Scholar]
- 138.Saburi S, et al. Loss of Fat4 disrupts PCP signaling and oriented cell division and leads to cystic kidney disease. Nature Genetics. 2008;40:1010–1015. doi: 10.1038/ng.179. [DOI] [PubMed] [Google Scholar]
- 139.Mao Y, et al. Characterization of a Dchs1 mutant mouse reveals requirements for Dchs1-Fat4 signaling during mammalian development. Development. 2011;138:947–957. doi: 10.1242/dev.057166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zakaria S, et al. Regulation of Neuronal Migration by Dchs1-Fat4 Planar Cell Polarity. Curr Biol. 2014;24:1620–1627. doi: 10.1016/j.cub.2014.05.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Mao Y, et al. Dchs1-Fat4 regulation of polarized cell behaviours during skeletal morphogenesis. Nat Commun. 2016;7(SP):1–10. doi: 10.1038/ncomms11469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Strutt H, Strutt D. Nonautonomous planar polarity patterning in Drosophila: dishevelled-independent functions of frizzled. Developmental Cell. 2002;3:851–863. doi: 10.1016/s1534-5807(02)00363-5. [DOI] [PubMed] [Google Scholar]
- 143.Ishikawa HO, Takeuchi H, Haltiwanger RS, Irvine KD. Four-jointed is a Golgi kinase that phosphorylates a subset of cadherin domains. Science. 2008;321:401–404. doi: 10.1126/science.1158159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Simon MA, Xu A, Ishikawa HO, Irvine KD. Modulation of Fat:Dachsous Binding by the Cadherin Domain Kinase Four-Jointed. Curr Biol. 2010;20:811–817. doi: 10.1016/j.cub.2010.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Brittle AL, Repiso A, Casal J, Lawrence PA, Strutt D. Four-Jointed Modulates Growth and Planar Polarity by Reducing the Affinity of Dachsous for Fat. Curr Biol. 2010;20:803–810. doi: 10.1016/j.cub.2010.03.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hale R, Brittle AL, Fisher KH, Monk NAM, Strutt D. Cellular interpretation of the long-range gradient of Four-jointed activity in the Drosophila wing. eLife. 2015;4:1–21. doi: 10.7554/eLife.05789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Brittle A, Thomas C, Strutt D. Planar Polarity Specification through Asymmetric Subcellular Localization of Fat and Dachsous. Curr Biol. 2012;22:907–914. doi: 10.1016/j.cub.2012.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ambegaonkar AA, Pan G, Mani M, Feng Y, Irvine KD. Propagation of Dachsous-Fat planar cell polarity. Curr Biol. 2012;22:1302–1308. doi: 10.1016/j.cub.2012.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Rovira M, Saavedra P, Casal J, Lawrence PA. Regions within a single epidermal cell of Drosophila can be planar polarised independently. eLife. 2015;4 doi: 10.7554/eLife.06303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Simons M, et al. Electrochemical cues regulate assembly of the Frizzled/Dishevelled complex at the plasma membrane during planar epithelial polarization. Nature Publishing Group. 2009;11:286–294. doi: 10.1038/ncb1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.