Abstract
Sepsis results in a deluge of both pro- and anti-inflammatory cytokines leading to lymphopenia and chronic immunoparalysis. Sepsis induced long-lasting immunoparalysis is, in part, defined by impaired CD4 and CD8 αβ T cell responses in the post-septic environment. The dysfunction in T cell immunity affects naïve, effector, and memory T cells, and is not restricted to classical αβ T cells. While the sepsis-induced severe and transient lymphopenia is a contributory factor to diminished T cell immunity, T cell-intrinsic and -extrinsic factors/mechanisms also contribute to impaired T cell function. In this review, we summarize the current knowledge of how sepsis quantitatively and qualitatively impairs CD4 and CD8 T cell immunity of both classical and non-classical T cell subsets and discuss current therapeutic approaches being developed to boost the recovery of T cell immunity post sepsis induction.
Introduction
Sepsis is characterized by an exaggerated host response, involving both pro- and anti-inflammatory cytokines, to a disseminated infection followed by severe transient lymphopenia and immunological dysregulation. Sepsis is the most expensive clinical condition treated in the United States (>$20B/year) and affects 1.5 million Americans annually. Additionally, one third of the patients who die in the hospital have sepsis (1). Advances in medical technology and practice have resulted in increased survival from the sepsis-induced cytokine storm as the mortality rate is currently ~25% (compared to ~45% in 1993) (2, 3). However, long after the cytokine storm has resolved patients continue to demonstrate increased susceptibility to secondary infection, increased viral reactivation, and decreased 5-year survival compared to control cohorts (4–6). This inability to mount/support effective immune responses is termed immunoparalysis, and while this immunoparalysis affects multiple aspects of innate and adaptive immunity, its effect on αβ T cells is particularly pronounced.
The combination of sepsis-induced quantitative and qualitative impairments to the T cell compartment and our in-depth understanding of T cell biology make these cells prime candidates to assess the overall fitness of the immune system in experimental model(s) and/or clinical setting of sepsis. Animal models present an invaluable array of tools, including a priori knowledge of MHC restriction of T cells, for performing directed hypothesis interrogation. However, recent work has established that the genetically inbred aspects of many mouse models do not always accurately recapitulate what is observed in genetically outbred patients (7). As such validating results in outbred animals, such as Swiss Webster mice, and utilization of ‘reverse translational’ approaches becomes necessary as the field progresses (8–10). In addition, the immunological status of the host can have a big impact on the responsiveness to inflammatory events. Specifically, conventionally housed specific-pathogen-free (SPF) mice have an immune system resembling that of newborn infants, due to limited history of pathogen exposures (11–13). In contrast, use of ‘dirty mice’ (i.e., mice purchased from pet stores or inbred mice co-housed with or exposed to the bedding of feral mice) allows for analysis of animals with an immune system that more closely recapitulates the immune system of an adult human because of multiple pathogen exposures (11, 13). While ‘dirty mice’ have yet to be used in sepsis research, they could represent a model with the capacity to further bridge animal and human research.
Sepsis has been modeled in multiple fashions to encompass the broad etiology of the disease. These models include, but are not limited to: TLR agonist (e.g., LPS) injection, IV bacterial injection, pneumonia, fecal slurry injection, colon ascendens stent peritonitis (CASP), and cecal ligation and puncture (CLP) to induce polymicrobial sepsis (14–20). TLR agonist models elicit different inflammatory profiles between mice and human; however, they do elicit cell loss similar to other sepsis models (7, 21). Additionally, ‘two-hit’ models have been approached in an effort to recapitulate septic outcomes as a result of secondary nosocomial infection. Often the first ‘hit’ involves an injury related induction, such as CLP or burn wound, followed by a secondary infection model, typically pneumonia – a common secondary infection of immunosuppressed septic patients (22–26).
While there is debate regarding the utility of each animal model, the clinical parameters of lymphopenia (including diminished T cell numbers) and induction of immunoparalysis are found (to varying degrees) in each of these models effectively enabling a ‘reverse translational approach’ to connect clinical and experimental research (15, 27–31). Here, we will synthesize our current understanding of how sepsis, across model systems, impairs primary and secondary T cell responses. The major focus will be on naïve, effector, and memory αβ T cells (defined in Figure 1) with a brief discussion of non-classical T cell subsets (i.e. γδ, NKT, MAIT, and IEL), and a description of current therapeutic strategies being evaluated for accelerating the numerical and/or functional recovery of T cells in the survivors of sepsis.
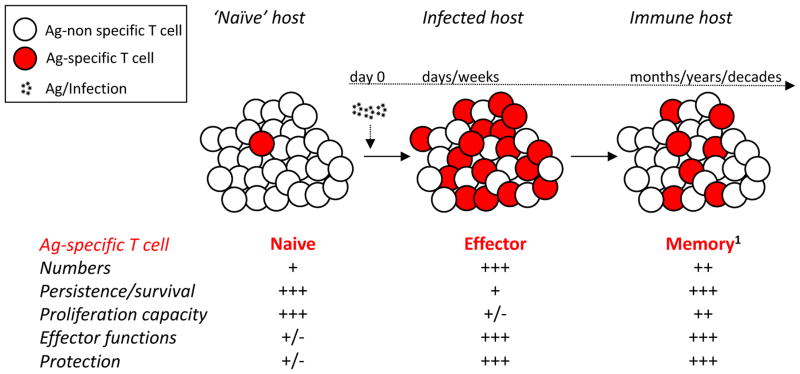
Figure 1. Naïve, effector, and memory T cells generated after acute infection/vaccination.
Naïve T cells, of a given Ag-specificity, exist at low numbers with minimal on-per-cell basis functionality and protective capacity. They are long-lived cells able to vigorously proliferate upon cognate Ag-stimulation, generating a sizable effector pool with ample functionality (cytotoxicity and cytokine production) and protective capacity. However, the vast majority of effector T cells have a limited life-span with diminished Ag-driven proliferative capacity. Those effector T cells that survive the contraction phase will form a long-lived memory T cell pool maintaining their effector functionality and protective capability.
1Of note - memory T cells represent a heterogeneous population of cells with defined phenotype, function, and localization that constantly changes with time after initial antigen encounter (93, 94). It is interesting to posit that memory T cell subsets might have differential susceptibility to sepsis-induced apoptosis and ability to recover in numbers and function in post-septic environment.
Sepsis and naïve T cells: The Mandela Effect and a “hole” other repertoire
Sepsis-induced lymphopenia invariably affects the naïve, ‘antigen (Ag)-inexperienced’ T cell pool in humans and experimental mouse models. In SPF mice, naïve T cells remaining in the periphery after the septic lymphopenia undergo homeostatic proliferation to compensate for the imposed numerical reduction and acquire memory-like characteristics, including memory phenotype marker expression (e.g., CD8 T cells: CD8αloCD11ahi; CD4 T cells: CD44hiCD11ahiCD49dhi) and even effector functionality (Figure 2A), in a potentially Ag-independent manner (32, 33). Although numerical recovery of T cells in sepsis survivors can occur in thymectimized animals, sepsis also reduces the number of newly generated naïve T cells by affecting thymic output (32, 34–36). In addition, homeostatic proliferation in the lymphopenic environment likely selects those T cell specificities with the highest precursor frequencies resulting in “holes” in the naïve T cell compartment and an inability to mount effective primary T cell responses to particular Ag/pathogens (Figure 2A) (33, 37). With these issues in mind, the likelihood of fully regenerating a diverse naïve T cell pool becomes doubtful. This is especially true for elderly septic patients whose naïve T cell pool represent only a small portion of their total T cell repertoire (35, 38). In addition to a changing composition of the T cell compartment, sepsis increases inhibitory receptor (e.g. 2B4 and PD-1) expression on surviving naïve T cells (in both human and animal models), which can be associated with increased mortality (39, 40). Invariably, this change in repertoire/composition and expression of inhibitory receptors expression contributes to the increased susceptibility of the host to unrelated, secondary infection(s) (32, 33). The full extent of the intrinsic impairments in naïve T cells that occur as a result of sepsis, however, is not known or well defined. This could include reduced responsiveness to TCR stimulation, changes in co-stimulatory molecule expression, cytokine responsiveness, and even metabolic functionality. As such, sepsis-induced changes within the naïve T cell repertoire have the potential to lead to lackluster effector and memory T cell generation or even inappropriately tolerizing to some Ag.
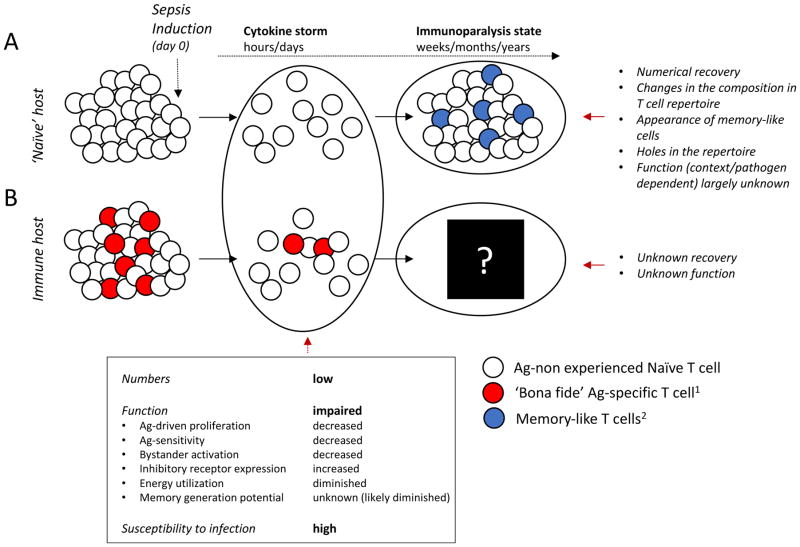
Figure 2. Sepsis-induced changes in naïve and memory T cells.
Sepsis induces rapid and vigorous apoptosis of A) naïve (Ag-non experienced CD11alow/CD8αhigh CD8 or CD11alow/CD49dlow CD4 T cells) T cells creating a lymphopenic environment supporting homeostatic proliferation (HP) of T cells that survive early ‘cytokine storm’ phase of sepsis. As a consequence of HP and in response to microbes that evoke sepsis, numerical recovery of T cell compartment is accompanied by phenotypic/functional changes (memory-like T cells) on a sizeable fraction of T cells. Sepsis can induce ‘holes’ in the T cell repertoire further contributing to overall changes in the composition of T cell pools, making their subsequent T cell responses to newly encountered pathogens potentially impaired. Similarly, B) pre-existing memory T cells (here, we are considering circulatory memory CD8 T cells) are also susceptible to sepsis-induced apoptosis leaving the host susceptible to pathogen re-encounter. The extent to which ‘bona fide’ memory T cell responses recover numerically and/or functionally is currently unknown but critical for our understanding of the sepsis-induced long-lasting immunoparalysis state. Of note, naïve and pre-existing memory T cell responses were modeled separately in A and B for clarity; however, the T cell compartment in any host experiencing sepsis will have both populations of CD4 and CD8 T cells simultaneously present.
1Memory T cell responses of defined Ag-specificity generated after primary infection and/or vaccination that exist prior to septic insult;
2Memory-like cells (defined as CD11ahigh/CD8αlow CD8 or CD11ahigh/CD49dhigh CD4 T cells) are those which acquire memory characteristics as a result of the septic event and potentially include both Ag-independent (HP) and Ag-dependent (pathogens that induce sepsis) T cell responses.
Sepsis and effector T cells: Too hot or too cold but nothing just right
Effector T cell function in the post-septic environment can be viewed in response to the pathogen(s) that precipitated the septic event or in response to a newly introduced secondary infectious pathogen. With widespread inflammation and bacterial translocation occurring in most sepsis models, it is reasonable to posit that multiple Ag-specific responses to the polymicrobial infection occur, even with the concurrent sepsis-induced lymphopenia. The acquisition of Ag-specific effector CD4 T cell responses to microbes present in the gut can indeed be detected, further contributing to the changes in composition of T cell compartment, in a host recovering from the CLP-induced sepsis (Figure 2A). Using inbred C57Bl/6 (B6) mice obtained from different vendors naturally colonized or devoid of commensal segmented filamentous bacteria (SFB), Cabrera-Perez et al. demonstrated acquisition of a memory phenotype and proliferative expansion of SFB-specific CD4 T cells in mice undergoing CLP with SFB as a part of gut microbiome (41). Intriguingly, no such Ag-specific responses have been observed for CD8 T cells. Whether Ag-specific responses to microbial commensals are restricted to CD4 T cells or not, a response to gut-resident commensals may skew host immunity long-term as a result of immune-mediated dysbiosis at epithelial surfaces (42, 43). This immune-mediated dysbiosis may in part result in the sepsis associated ‘pathobiome’ which develops in patients (44–46), which is subsequently associated with an elevated inflammatory state of the host and has the capacity to impact T cell response to Ag (47). In the case of intestinal dysbiosis, this can affect nutrient acquisition and an individual’s long-term health (48). Additionally, chronically elevated local inflammation, because of inappropriate responses to gut commensals, can lead to increased barrier permeability that may potentiate another septic event as a result of bacterial translocation (43, 48–52). This represents a seemingly paradoxical outcome where functional normalcy of a T cell response may be detrimental or have long-lasting effects in modulating the composition of CD4 and CD8 T cells in the post-septic environment.
Effector T cell responses to newly introduced pathogens will be influenced by the status of the host post-sepsis. The reduced number of naïve Ag-specific T cell precursors early after sepsis induction will contribute to suboptimal generation of primary effector T cells; however, the extent to which T cell intrinsic impairments further compromise effective T cell immunity (even in scenarios when numerical recovery is achieved) are currently ill defined. Interestingly, Markwart et al. did not observe TCR signaling defects in naïve T cells following CD3/CD28 stimulation in a TLR agonist injection mouse model (53). In fact, Borken et al. observed enhanced proliferation of T cells from septic patients after CD3/CD28 cross-linking, but they were unable to identify any changes in proximal TCR signaling events to account for this difference (54). These data suggest impairments in T cells from sepsis patients can be overcome with strong TCR stimulation. However, impairment may still be relevant at a lower stimulation threshold achieved in vivo during T cell stimulation by Ag-presenting dendritic cells (DC) (55). Additionally, changes in the metabolic state of T cells impair their capacity to expand and perform effector function (56–58), and sepsis affects the metabolism of a variety of cells, including T cells (59–62). These metabolic changes likely have a direct association with the impaired accumulation and decreased functionality of T cells in vivo in the post-septic environment and will require further interrogation (Figure 2). The extent to which those intrinsic impairments are transient and recover with time, or are reversible and could be sped-up with intervention to enable recovery are likely to be a focus of future studies.
Under normal conditions, the priming of naïve CD8 T cells is done in a highly controlled manner – largely to prevent the generation of responses to normal healthy tissues – under the assumption that a mixture of cell intrinsic and extrinsic factors is needed for the proper expansion and functionality of naïve CD8 T cells. Among the various extrinsic factors, CD4 T cell ‘help’ is a key feature in the formation of a primary CD8 T cell response (63–65). The instructional programming that occurs within CD8 T cells helped by CD4 T cells prevents TNF-related apoptosis-inducing ligand (TRAIL)-mediated activation-induced cell death of the CD8 T cells (66, 67). The numerical and functional deficits in CD4 T cells during sepsis creates the potential for a number of CD8 T cell responses to proceed without the necessary CD4 T cell help. The combination of these facts led to data suggesting sepsis impairs T cell effector responses during early immunoparalysis state (in part) in a TRAIL-dependent manner (68–70). The importance of TRAIL in sepsis-induced immunosuppression was exemplified with the therapeutic use of a blocking anti-TRAIL mAb, which restored CD8 T cell responses and improved the control of a secondary bacterial infection following a CLP model (69).
Sepsis-induced numerical loss and compositional changes within the DC compartment were also recently found to directly contribute to the impaired pathogen-specific primary CD8 T cell responses (71), which even extended to an impairment in naïve CD8 T cells from non-septic mice transferred into CLP-treated recipients. Interestingly, post-sepsis Flt3 ligand (Flt3L) treatment increased the number of DCs and improved DC function, including the ability to sense inflammation and produce cytokine IL-12, leading to improved primary CD8 T cell responses to newly introduced Ag. Thus, a direct link between sepsis-induced deficiencies in T cell intrinsic and extrinsic factors has been established and therapeutic approaches designed to target both T cells and supporting innate cells (such as DC) at the same time might further benefit the host recovering from the septic incident.
Sepsis and memory T cells: Retrograde and Anterograde Amnesia
Alterations to existing memory T cells
As humans and mice age their pool of memory T cells expands to become the major population in the total T cell repertoire due (in part) to well-defined, age-related changes and a history of pathogen encounters and/or vaccinations (72–75). Although memory CD8 T cells are more resistant to radiation-induced apoptosis than their naïve counterparts, a sepsis-induced decline in existing circulatory memory (TCIRCM) CD8 T cell numbers are equal to those observed for the naïve CD8 T cell pool (Figure 2B) (76–80). Interestingly, some data suggest different subsets of CD8 TCIRCM (e.g. CD62L−CCR7− ‘effector’ and CD62L+CCR7+ ‘central memory’) are similarly susceptible to sepsis-induced apoptosis suggesting a stochastic and/or non-discriminatory nature of CD8 TCIRCM decline in septic hosts (VPB unpublished data and (76)). Similarly, memory CD4 T cells experience a numerical loss following sepsis (36, 81, 82). Proportionally, however, CD4 T cell subsets shift to a higher frequency of FoxP3+ regulatory T (Treg) cells, due to preferential loss of other subpopulations (e.g. TH1, TH2, TH17, and TFH) (36, 82–84). In mouse models this population shift can be abrogated by the transfer of BMDC and is associated with decreased PD-1 expression by CD4 T cells (40, 85, 86). Additionally, recent data suggest IL-33 plays a role in promoting Treg expansion and immunoparalysis up to 15 d post-infection (87). The relevance of this population shift continues to be debated as contrasting associations have been made based on timing of analyses, among other considerations (88–92). But the potential for this increased prevalence to impair immunity to new or re-encountered infection remains open.
In both CD4 and CD8 T cells, existing memory shows impaired Ag-specific expansion and effector functionality in the post-septic environment (Figure 2) (69, 71, 76, 77, 81). For CD8 T cells this includes decreased Ag-sensitivity (functional avidity) and Ag-driven secondary expansion – directly contributing to the diminished memory CD8 T cell-mediated immunity (“retrograde amnesia”) to bacterial or viral re-infections (77). Moreover, inflammation induced Ag-independent bystander activation of memory CD8 T cells in response to heterologous infection is also significantly impaired in vivo early after sepsis induction. When analyzed on a per-cell-basis, the sensitivity of pre-existing memory CD8 T cells to respond (as measured by IFN-γ production) to heterologous infection/cytokine stimulation are mainly intact (77). Moreover, memory CD8 T cells obtained from a septic animal can respond to secondary Ag-stimulation when transferred to a normal (non-septic) host. Together, these findings suggest the functional impairments observed in memory CD8 T cell responses are also influenced by the post-septic environment. The nature of the extrinsic factors controlling T cell immunity, the extent to which CD8 TCIRCM numerically recover and their ability to differentiate into long-term memory CD8 with defined phenotype and function (93, 94) are, though, a metaphoric black box (Figure 2B), but critical for our understanding of sepsis-induced long-lasting impairments observed in sepsis survivors.
In contrast to CD8 TCIRCM, tissue resident memory CD8 (TRM) T cells are necessary and sufficient (in some cases) to provide robust protection to localized pathogen re-encounter (95–98). Interestingly, in direct contrast to CD8 TCIRCM of the same Ag-specificity, CD8 TRM remain numerically intact after moderate CLP sepsis (76). Moreover, the sensing and alarming functions (e.g., production of IFN-γ in response to cognate Ag injection or pathogen re-infection) of CD8 TRM are maintained after sepsis induction (Figure 3A–B) (76, 96). Sepsis does, however, dramatically change the ability of the host to recruit bystander immune cells (i.e., B cells, Ag-experienced T cells) to sites of localized Ag-encounter in response to the CD8 TRM–derived sensing and alarming signals, resulting in increased susceptibility to re-infection (Figure 3C–D) (76). In this setting, local endothelial cells cannot detect TRM-produced IFN-γ and subsequently upregulate CXCL9/10 and VCAM to permit entrance of recruited cells into the infected tissue (76). Thus, sepsis has the capacity to influence the host response to pathogen re-infection either by directly influencing memory CD8 T cell populations (e.g. number and function of CD8 TCIRCM) and/or by preventing other cell types from properly recognizing localized pathogen-induced alarming signals delivered by CD8 TRM. It is yet to be determined to what extent CD4 TRM (compared to CD4 TCIRCM) are affected by sepsis. Given their differential localization within some tissues (e.g., CD8 T cells reside predominately in the epidermis while CD4 T cells are preferentially in the dermis), CD4 TRM may be more affected by sepsis (99, 100).
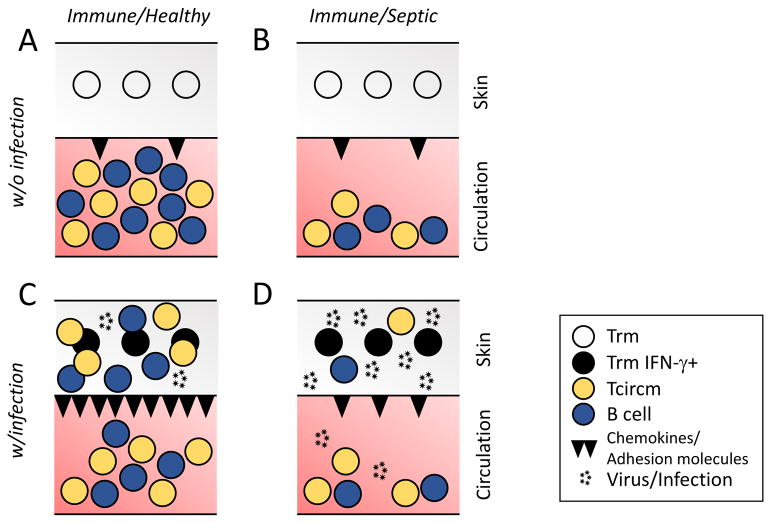
Figure 3. CD8 T cell-mediated immunity to localized re-infection diminished after sepsis in a multifactorial manner.
A) Tissue resident memory CD8 T cells (TRM) and circulating memory (TCIRCM) CD8 T and B cells are evoked upon primary infection/immunization. B) ‘Moderate’ sepsis (that leads to 90%+ long-term survival) induces dramatic numerical loss of circulating but not resident CD8 T cell populations. C) Localized pathogen re-infection (or cognate Ag-encounter) of the healthy host induces the ‘sensing and alarm’ function of TRM. As a consequence, the IFN-γ produced by TRM acts on the local endothelium to upregulate chemokines and adhesion molecules (e.g., CXCL9 and VCAM1, respectively) promoting the influx of memory T and B cells from circulation and facilitating clearance of the pathogen in situ. D) ‘Moderate’ sepsis does not significantly impact the number and/or function of pre-existing TRM responding to pathogen re-infection. However, endothelial cells are unable to respond to the IFN-γ signal and upregulate chemokines and adhesion molecules. Consequently, there is a dramatically reduced number of effector cells recruited from the circulation and pathogen clearance is significantly impaired.
Memory T cell formation post-sepsis
Post-sepsis primary memory T cell formation faces the same environmental conditions that impair the naïve T cell pool and existing memory T cell responses, which potentially culminates in T cells exhibiting a type of “anterograde amnesia” – the impaired ability to generate ‘new’ CD4 and CD8 T cell memory (76). The extent to which sepsis influences naïve to memory CD4 and CD8 T cell differentiation in response to acute infections/vaccinations is unknown and critical for defining immunity in post-septic environment. However, not all infections encountered will be acute in nature as chronic/latent infection may exist prior to the initiation of sepsis or established in the post-septic environment.
When considering chronic infection, memory T cell responses acquire functional defects over time as a result of constant stimulation (i.e., T cell “exhaustion”) (5, 6, 101–104). LCMV clone 13 infection of the septic hosts results in exacerbated exhaustion of CD8 T cells (based on increased PD-1 and LAG-3 expression and decreased Ag-driven cytokine production) and increased viral burden compared to non-septic controls (105). Similarly, recent clinical data show reduced poly-functionality of T cells from patients with CMV reactivation after sepsis (106). CD8 T cells from these patients also exhibited enhanced PD-1 expression, highlighting the relevance of animal models for studying sepsis-induced impairments (105, 106). In contrast to these data, Choi et al. did not observe increased PD-1 and 2B4 on CD4 T cells and the effect of sepsis on CD4 T cell exhaustion has yet to be evaluated in mouse models (106), nor has the effect of sepsis on previously established chronic viruses been modeled. Taken together, this information highlights how sepsis impairs T cell immunity at multiple junctures. However, additional investigation is required to address several biologically relevant questions regarding how sepsis affects existing memory T cells long-term (Figure 2B).
Sepsis and non-conventional T cells: In need of an unconventional perspective
We have focused our discussion on the consequences of sepsis on conventional αβ T cells to this point, but we recognize other T cell populations, both variant and invariant in nature, exist in humans and mice (107–110). Unfortunately, very little is known about these non-conventional T cell subsets in the post-septic environment. Clinically, circulating γδ T cells numerically decline in the post-septic environment (111); however, in contrast to their αβ counterparts, murine γδ T cells (especially Vγ4) accumulate and have increased intracellular IL-17 in the lungs post-sepsis (112). Additionally, FoxP3+ Vδ1 T cells are increased in frequency in patients after sepsis (113). The parallel with conventional CD4+ Treg reveals an important aspect of non-conventional T cells in sepsis that remains to be studied. Intriguingly, γδ T cells from septic patients stimulated with PMA and Ionomycin show reduced capacity to upregulate CD69 and produce IFN-γ (114), suggesting cell intrinsic impairment. Thus, it is pertinent to understand this impairment as it is likely distinct from any changes occurring in αβ T cells and may require different therapeutic strategies to resolve.
Interestingly, NKT cells, a T cell population (often expressing a semi-invariant TCR Vα14i which recognize lipids and glycolipids presented by CD1d) with characteristics of both natural killer and T cells, have shown conflicting results when using different models of sepsis (36, 115, 116). There was no numerical loss of NKT cells in the liver in a burn wound model (116), but a loss in both the number and frequency of NKT cells was noted after CLP (36). The timing of these observations may be a determining factor in the data, as the CLP assessment occurred 20 h post-surgery while the burn wound observation occurred 4 days after burn induction. Our own data show a numerical reduction of NKT cells two days after CLP, but they also represented a larger proportion of lymphocytes in the liver (VPB unpublished observation). An additional factor to consider is the proximity of the site of evaluation to the nidus of the septic event, as the numerical loss of NKT cells in the liver occurred during CLP – an event proximal to the liver, while the burn wound – an event distal to the liver – did not. The differences in NKT cell frequency in the liver between the two CLP experiments indicates that NKT cell redistribution of these cells may occur following the 20 h time point (36). This would be consistent with the results of Heffernan et al., who clinically observed an increased frequency of circulating NKT cells after sepsis (115). As such it is important to clarify how sepsis may be affecting the distribution of NKT cells and how this affects host immunity. The recognition of distinct Ag repertoires by αβ T cells and NKT cells/γδ T cells, proteins and glycoproteins/lipids, respectively, present distinct aspects of immunity whose impairment by sepsis has yet to be understood.
Mucosal associated invariant T cells (MAITs) and intraepithelial lymphocytes (IELs) represent the most understudied T cell populations in sepsis. Circulating MAITs numerically decline in patients early after sepsis, though it remains to be determined to what extent this is apoptosis-induced reduction or relocation as a result of infection (117, 118). IELs have a reduced frequency in the small intestine after CLP, coinciding with an increased frequency of apoptotic IELs (119). The commonality among these subsets is that they largely exist at epithelial surfaces which are often the site of sepsis initiation (118, 120–124). Given the unique distribution and distinct Ag repertoires of these cell subsets a more thorough numerical and functional evaluation in the post-septic environment should be approached.
Sepsis and immune targeting therapies: Making more and making them better
Several commonalities have arisen across all T cell subsets during sepsis, and each in turn have been targeted by therapeutic interventions to alleviate sepsis-induced immunoparalysis. These strategies include limiting cell death, expanding the surviving cells, expanding DC populations, and blocking inhibitory ligand expression [e.g. PD-1/PD-L1, CTLA-4, B- and T-lymphocyte Attenuator (BTLA), T cell Membrane Protein-3 (TIM-3), Lymphocyte Activation-Gene-3 (LAG-3), and 2B4] to allow for cell proper activation (125–128). Limiting cell death by blocking apoptotic pathways was originally approached as a method of reducing the severity of the cytokine storm, induced by various sepsis models, by preventing the release of additional danger-associated molecular patterns (33, 66, 68–70, 129–131). Among the proteins targeted in the apoptosis signaling pathway, caspase inhibition seemed to have great promise when initially investigated. Caspases are involved in the apoptotic process responsible for the loss of lymphoid cells (among the many dying cells found during a septic event), but are also necessary in the response to endotoxin and processing of cytokines (e.g., IL-1β) into their mature forms (132). As such, a number of approaches have been tested in preclinical models to block apoptosis as a means of ameliorating the progression of sepsis – including, the administration of caspase inhibitors to block caspase activation or siRNA to inhibit the caspase production (133–135). Unfortunately, the idea of targeting caspases as a sepsis treatment failed to gain traction because of the importance of caspases in a number of other physiological events and the difficulties in delivering inhibitors in sufficient amounts and timeframes to have a clinical benefit.
The next strategy, and most common for T cell impairment, is to drive the expansion of the remaining cells by administration of cytokines that promote T cell survival, proliferation, and/or function – namely, IL-2, IL-7, and IL-15 (79, 116, 136–139). Additionally, treatment with these cytokines promotes mTOR activation, which is an aspect of oxidative phosphorylation, and an important metabolic aspect in the maintenance of memory T cells (62). As a result, treatment with IL-2/7/15 may have the additional benefit of resolving the metabolic deficits of memory T cells imposed by sepsis (57, 62, 140, 141). However, T cell expansion induced by these cytokines in a post-septic host is generally reduced relative to non-septic mice (62, 79, 137), suggesting a cell intrinsic impairment(s) exists and cytokine administration may only result in expansion of a functionally impaired population. Additionally, lymphocyte numbers typically drop after therapy is halted (142). Of the candidate cytokines tested to date, IL-7 seems to be the best tolerated and importantly improved host immunity and survival when given to CLP-treated mice that also received a secondary heterologous infection (137, 142). The therapeutic benefit of exogenous IL-7 administration has also been evaluated in parallel clinical trials in the U.S. (NCT02640807) and France (NCT02797431). The purpose of these double-blinded, placebo-controlled trials was to evaluate the ability of recombinant IL-7 (CYT107) to restore absolute lymphocyte counts in sepsis patients. These U.S. and French trials are active, but not accruing patients, or have been terminated, respectively. Data describing the outcomes of these studies will likely be published in the near future. Another therapeutic strategy using IL-2/7/15 has been to administer them in tandem with therapies which address other aspects of sepsis induced impairment. Shindo et al. recently demonstrated the combination IL-7 and anti-PD-1 mAb, following the two-hit model, yielded improved functionality, IFN-γ production, over monotherapy (127). In addition to therapies directly targeting T cells, additional supportive therapies boosting the recovery of T cell extrinsic factors should be considered. For example, the administration of Flt3L to expand DC populations would have the twofold effect of promoting more effective T cell priming and re-establishing a population of immune cells normally responsible for the production of “signal 3” cytokines (IL-12 and IFN–γ) needed for optimal T cell activation (71). Flt3L therapy has been tested in a number of clinical settings, but has yet been evaluated in sepsis patients. Other potential therapies include administration of chemokines following re-admittance with secondary infection to assist in the recruitment and migration of T cells to sites of infection in the post-septic environment, such as the CXCL9/10 used by Danahy et al. (76). The use of chemokines like CXCL9/10 during secondary infection is meant to overcome impairments as a result of the septic environment and is unlikely to resolve cell intrinsic defects. In contrast, production of other chemokines during sepsis may be detrimental during sepsis. Ramonell and colleagues recently showed CXCR4 antagonism, which prevented the binding of CXCL12, lead to a decrease in sepsis-induced mortality (143).
The explosion in the past 15–20 years in the use of biologics targeting components of the immune system has given researchers and clinicians another set of powerful reagents to treat a variety of diseases. Among these, immune checkpoint inhibitors have revolutionized the way cancer is treated, and checkpoint blockade is also proving to be a means to remove some of the sepsis-imposed limitations on the immune system. A number of publications have reported the increased expression of PD-1, CTLA-4, BTLA, TIM-3, LAG-3, and 2B4 on T cells or in the plasma from septic hosts (39, 125, 144–146). Generally speaking, interaction among these immune cell checkpoint receptors with their cognate ligands inhibits T cell function, and it has been hypothesized such interactions contribute to the immune dysfunction seen during sepsis. Data showing T cell function is improved (mostly in in vitro assays) with inclusion of mAb to these inhibitory receptors support this hypothesis. For example, disruption of the PD-1/PD-L1 pathway has demonstrated some effect in correcting septic impairment of T cells, including increased CD28 expression and IFN-γ production by both CD4 and CD8 T cells – especially when used in combination with immunostimulatory cytokines (40, 127, 139). One important benefit when considering checkpoint blockade in the treatment of sepsis is that a number of mAb targeting these molecules (i.e., PD-1, PD-L1, and CTLA-4) have been and are currently being evaluated in other clinical (primarily oncology) settings, thus providing a large information base in regard to safety and efficacy. As such, the safety, tolerability, and PK/PD of an anti-PD-L1 mAb was recently evaluated in a Phase 1b/2a trial in patients with severe sepsis (NCT02576457). This was a randomized, double-blinded placebo-controlled study measuring a variety of clinical and immunological parameters in these patients, but it also gave the investigators the opportunity to determine the therapeutic potential of ameliorating mortality and restoring immune function in these patients after blocking PD-1 signaling. The study was completed in early 2017, but the results of the trial are yet to be made public. Positive findings (i.e., improved survival and/or immune function) could provide an important new means of treating patients with sepsis.
The therapies described here have demonstrated success in various preclinical sepsis models, but their clinical potentials are only beginning to be evaluated. Most (if not all) sepsis therapeutics targeting the immune system have generally not been as effective in the clinical setting as in preclinical models, but the positive preliminary reports coming from trials testing IL-7 and checkpoint inhibitors may be reversing this negative trend (125, 147). A number of reasons for the limited clinical effect in the past have been posited, but the complex etiology of sepsis and range of immunological impairments observed suggest the possibility that monotherapy targeting a single cell type or pathway is unlikely to be effective. Rather integrative therapeutic strategies that engage multiple aspects of T cell biology are more likely to benefit most patients. However, understanding how sepsis affects other arms of the immune system, for both their distinct and T cell supportive roles, is crucial to developing strategies to reverse immunoparalysis.
Conclusions
The massive attrition of lymphocytes during sepsis has detrimental effects on multiple aspects of T cell immunity. In addition to the sepsis-induced T cell apoptosis, most of the remaining T cells also exhibit prolonged functional impairment. This impairment is multifactorial driven by both cell intrinsic and extrinsic factors. Many of the impairments highlighted throughout this review indicate T cell-extrinsic impairments being a major factor in impaired T cell immunity. Additionally, while several intrinsic changes occur, including altered TCR repertoires and increased inhibitory receptor expression, much remains to be understood about the extent to which/how sepsis affects TCR signaling. Further, an understanding of the effect of sepsis on T cell metabolic activity is likely to reveal important aspects about how functional impairment manifests in these cells.
Other questions regarding the effect of sepsis on T cell immunity have been partially answered in either CD4 or CD8 T cells but not both. The distinctions between CD4 and CD8 T cells are important as impairment of functional and interdependent mechanisms of these T cells will shape our understanding of how sepsis affects T cell immunity. To compound this further many of these evaluations are completely lacking for non-conventional T cell subsets. Finally, the question of resolving the sepsis-induced quantitative and/or qualitative changes in T cells is becoming more investigated as the mechanisms responsible for suboptimal T cell immunity in the septic host are being better defined. Given the variety and nature of the impairments observed in the post-septic environment, it would seem the therapeutic strategies that bolster multiple aspects of T cell immunity would best alleviate the sepsis-induced immunoparalysis. Yet, some underlying T cell-intrinsic impairments may remain. As such further interrogation into how sepsis affects the inherent functionality of T cells is required if this is to be overcome. Improved knowledge of T cell biology is driving the development of new therapies for clinical settings where number and/or function of T cells is abnormal, and many of these new drugs have the potential for use across multiple disease platforms (e.g., use of checkpoint inhibitors for improved T cell activity in cancer or sepsis patients). We can only hope the exciting advances being made now in regard to immune system knowledge and manipulation will only be the beginning of a wave of future findings to expand our arsenal of weapons used in our fight against sepsis.
Footnotes
Supported by National Institutes of Health Grants GM113961, AI119160, AI114543 (V.P.B.), and GM115462 (T.S.G.), 5 T32 AI007485 (I.J.J), 5 T32 CA009138 (F.V.S.) and U.S. Department of Veterans Affairs Merit Review Award (T.S.G.)
References
- 1.CDC. Sepsis: Data & Reports. Center for Disease Control and Prevention; 2017. https://www.cdc.gov/sepsis/datareports/index.html. [Google Scholar]
- 2.Dombrovskiy VY, Martin AA, Sunderram J, Paz HL. Rapid increase in hospitalization and mortality rates for severe sepsis in the United States: A trend analysis from 1993 to 2003*. Crit Care Med. 2007;35:1244–50. doi: 10.1097/01.CCM.0000261890.41311.E9. [DOI] [PubMed] [Google Scholar]
- 3.Gaieski DF, Edwards JM, Kallan MJ, Carr BG. Benchmarking the Incidence and Mortality of Severe Sepsis in the United States*. Crit Care Med. 2013;41:1167–74. doi: 10.1097/CCM.0b013e31827c09f8. [DOI] [PubMed] [Google Scholar]
- 4.Donnelly JP, Hohmann SF, Wang HE. Unplanned Readmissions After Hospitalization for Severe Sepsis at Academic Medical Center–Affiliated Hospitals*. Crit Care Med. 2015;43:1916–27. doi: 10.1097/CCM.0000000000001147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kutza AST, Muhl E, Hackstein H, Kirchner H, Bein G. High Incidence of Active Cytomegalovirus Infection Among Septic Patients. Clin Infect Dis. 1998;26:1076–82. doi: 10.1086/520307. [DOI] [PubMed] [Google Scholar]
- 6.Walton AH, Muenzer JT, Rasche D, Boomer JS, Sato B, Brownstein BH, Pachot A, Brooks TL, Deych E, Shannon WD, Green JM, Storch GA, Hotchkiss RS. Reactivation of Multiple Viruses in Patients with Sepsis. PLoS ONE. 2014;9:e98819. doi: 10.1371/journal.pone.0098819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seok J, Warren HS, Cuenca AG, Mindrinos MN, Baker HV, Xu W, Richards DR, McDonald-Smith GP, Gao H, Hennessy L, Finnerty CC, López CM, Honari S, Moore EE, Minei JP, Cuschieri J, Bankey PE, Johnson JL, Sperry J, Nathens AB, Billiar TR, West MA, Jeschke MG, Klein MB, Gamelli RL, Gibran NS, Brownstein BH, Miller-Graziano C, Calvano SE, Mason PH, Cobb JP, Rahme LG, Lowry SF, Maier RV, Moldawer LL, Herndon DN, Davis RW, Xiao W, Tompkins RG Inflammation t, Host Response to Injury LSCRP. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proceedings of the National Academy of Sciences. 2013;110:3507–12. doi: 10.1073/pnas.1222878110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martin MD, Danahy DB, Hartwig SM, Harty JT, Badovinac VP. Revealing the Complexity in CD8 T Cell Responses to Infection in Inbred C57B/6 versus Outbred Swiss Mice. Frontiers in Immunology. 2017;8:1527. doi: 10.3389/fimmu.2017.01527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rice MC, O’Brien SJ. Genetic variance of laboratory outbred Swiss mice. Nature. 1980;283:157. doi: 10.1038/283157a0. [DOI] [PubMed] [Google Scholar]
- 10.Danahy DB, Strother RK, Badovinac VP, Griffith TS. Clinical and Experimental Sepsis Impairs CD8 T-Cell-Mediated Immunity. Critical reviews in immunology. 2016;36:57–74. doi: 10.1615/CritRevImmunol.2016017098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beura LK, Hamilton SE, Bi K, Schenkel JM, Odumade OA, Casey KA, Thompson EA, Fraser KA, Rosato PC, Filali-Mouhim A, Sekaly RP, Jenkins MK, Vezys V, Haining WN, Jameson SC, Masopust D. Normalizing the environment recapitulates adult human immune traits in laboratory mice. Nature. 2016;532:512. doi: 10.1038/nature17655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ford ML. Of (Dirty) Mice, Men and Memory. American Journal of Transplantation. 2016;16:2243. [Google Scholar]
- 13.Masopust D, Sivula CP, Jameson SC. Of Mice, Dirty Mice, and Men: Using Mice To Understand Human Immunology. The Journal of Immunology. 2017;199:383–8. doi: 10.4049/jimmunol.1700453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brealey D, Karyampudi S, Jacques TS, Novelli M, Stidwill R, Taylor V, Smolenski RT, Singer M. Mitochondrial dysfunction in a long-term rodent model of sepsis and organ failure. Am J Physiol Regul Integr Comp Physiol. 2004;286:R491–R7. doi: 10.1152/ajpregu.00432.2003. [DOI] [PubMed] [Google Scholar]
- 15.Danahy DB, Strother RK, Badovinac VP, Griffith TS. Clinical and Experimental Sepsis Impairs CD8 T-Cell-Mediated Immunity. Crit Rev Immunol. 2016;36:57–74. doi: 10.1615/CritRevImmunol.2016017098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dejager L, Pinheiro I, Dejonckheere E, Libert C. Cecal ligation and puncture: the gold standard model for polymicrobial sepsis? Trends Microbiol. 2011;19:198–208. doi: 10.1016/j.tim.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 17.Li CC, Munitic I, Mittelstadt PR, Castro E, Ashwell JD. Suppression of Dendritic Cell-Derived IL-12 by Endogenous Glucocorticoids Is Protective in LPS-Induced Sepsis. PLoS Biol. 2015;13:e1002269. doi: 10.1371/journal.pbio.1002269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muenzer JT, Davis CG, Chang KC, Schmidt RE, Dunne WM, Coopersmith CM, Hotchkiss RS. Characterization and Modulation of the Immunosuppressive Phase of Sepsis. Infect Immun. 2010;78:1582–92. doi: 10.1128/IAI.01213-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Greisman SE, DuBuy JB, Woodward CL. Experimental gram-negative bacterial sepsis: prevention of mortality not preventable by antibiotics alone. Infection and Immunity. 1979;25:538–57. doi: 10.1128/iai.25.2.538-557.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zantl N, Uebe A, Neumann B, Wagner H, Siewert J-R, Holzmann B, Heidecke C-D, Pfeffer K. Essential Role of Gamma Interferon in Survival of Colon Ascendens Stent Peritonitis, a Novel Murine Model of Abdominal Sepsis. Infection and Immunity. 1998;66:2300–9. doi: 10.1128/iai.66.5.2300-2309.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Markwart R, Condotta SA, Requardt RP, Borken F, Schubert K, Weigel C, Bauer M, Griffith TS, Förster M, Brunkhorst FM, Badovinac VP, Rubio I. Immunosuppression after Sepsis: Systemic Inflammation and Sepsis Induce a Loss of Naïve T-Cells but No Enduring Cell-Autonomous Defects in T-Cell Function. PLoS ONE. 2014;9:e115094. doi: 10.1371/journal.pone.0115094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dressler DP, Skornik WA. Pulmonary Bacterial Susceptibility in the Burned Rat. Annals of Surgery. 1974;180:221–7. doi: 10.1097/00000658-197408000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Drechsler S, Weixelbaumer KM, Redl H, van Griensven M, Bahrami S, Osuchowski MF. Experimentally Approaching the ICU: Monitoring Outcome-Based Responses in the Two-Hit Mouse Model of Posttraumatic Sepsis. Journal of Biomedicine and Biotechnology. 2011;2011:357926. doi: 10.1155/2011/357926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morris CFM, Tahir M, Arshid S, Castro MS, Fontes W. Reconciling the IPC and Two-Hit Models: Dissecting the Underlying Cellular and Molecular Mechanisms of Two Seemingly Opposing Frameworks. Journal of Immunology Research. 2015;2015:697193. doi: 10.1155/2015/697193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Griensven M, Kuzu M, Breddin M, Böttcher F, Krettek C, Pape H-C, Tschernig T. Polymicrobial sepsis induces organ changes due to granulocyte adhesion in a murine two hit model of trauma. Experimental and Toxicologic Pathology. 2002;54:203–9. doi: 10.1078/0940-2993-00247. [DOI] [PubMed] [Google Scholar]
- 26.Muenzer JT, Davis CG, Dunne BS, Unsinger J, Dunne WM, Hotchkiss RS. PNEUMONIA AFTER CECAL LIGATION AND PUNCTURE: A CLINICALLY RELEVANT "TWO-HIT" MODEL OF SEPSIS. Shock. 2006;26:565–70. doi: 10.1097/01.shk.0000235130.82363.ed. [DOI] [PubMed] [Google Scholar]
- 27.Efron PA, Mohr AM, Moore FA, Moldawer LL. The future of murine sepsis and trauma research models. J Leukoc Biol. 2015;98:945–52. doi: 10.1189/jlb.5MR0315-127R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hotchkiss RS, McConnell KW, Bullok K, Davis CG, Chang KC, Schwulst SJ, Dunne JC, Dietz GPH, Bähr M, McDunn JE, Karl IE, Wagner TH, Cobb JP, Coopersmith CM, Piwnica-Worms D. TAT-BH4 and TAT-Bcl-xL Peptides Protect against Sepsis-Induced Lymphocyte Apoptosis In Vivo. The Journal of Immunology. 2006;176:5471–7. doi: 10.4049/jimmunol.176.9.5471. [DOI] [PubMed] [Google Scholar]
- 29.Hotchkiss RS, Tinsley KW, Swanson PE, Schmieg RE, Hui JJ, Chang KC, Osborne DF, Freeman BD, Cobb JP, Buchman TG, Karl IE. Sepsis-Induced Apoptosis Causes Progressive Profound Depletion of B and CD4+ T Lymphocytes in Humans. The Journal of Immunology. 2001;166:6952–63. doi: 10.4049/jimmunol.166.11.6952. [DOI] [PubMed] [Google Scholar]
- 30.Peck-Palmer OM, Unsinger J, Chang KC, Davis CG, McDunn JE, Hotchkiss RS. Deletion of MyD88 markedly attenuates sepsis-induced T and B lymphocyte apoptosis but worsens survival. Journal of Leukocyte Biology. 2008;83:1009–18. doi: 10.1189/jlb.0807528. [DOI] [PubMed] [Google Scholar]
- 31.Schwulst SJ, Grayson MH, DiPasco PJ, Davis CG, Brahmbhatt TS, Ferguson TA, Hotchkiss RS. Agonistic Monoclonal Antibody Against CD40 Receptor Decreases Lymphocyte Apoptosis and Improves Survival in Sepsis. The Journal of Immunology. 2006;177:557–65. doi: 10.4049/jimmunol.177.1.557. [DOI] [PubMed] [Google Scholar]
- 32.Cabrera-Perez J, Condotta SA, James BR, Kashem SW, Brincks EL, Rai D, Kucaba TA, Badovinac VP, Griffith TS. Alterations in Antigen-Specific Naive CD4 T Cell Precursors after Sepsis Impairs Their Responsiveness to Pathogen Challenge. J Immunol. 2015;194:1609–20. doi: 10.4049/jimmunol.1401711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Condotta SA, Rai D, James BR, Griffith TS, Badovinac VP. Sustained and incomplete recovery of naïve CD8(+) T-cell precursors after sepsis contributes to impaired CD8(+) T-cell responses to infection. J Immunol. 2013;190:1991–2000. doi: 10.4049/jimmunol.1202379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ayala A, Herdon CD, Lehman DL, DeMaso CM, Ayala CA, Chaudry IH. The induction of accelerated thymic programmed cell death during polymicrobial sepsis: Control by corticosteroids but not tumor necrosis factor. Shock. 1995;3:259–67. doi: 10.1097/00024382-199504000-00003. [DOI] [PubMed] [Google Scholar]
- 35.Netzer C, Knape T, Kuchler L, Weigert A, Zacharowski K, Pfeilschifter W, Sempowski G, Parnham MJ, Brüne B, von Knethen A. Apoptotic Diminution of Immature Single and Double Positive Thymocyte Subpopulations Contributes to Thymus Involution During Murine Polymicrobial Sepsis. Shock. 2017;48:215–26. doi: 10.1097/SHK.0000000000000842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sharma A, Yang W-L, Matsuo S, Wang P. Differential alterations of tissue T-cell subsets after sepsis. Immunol Lett. 2015;168:41–50. doi: 10.1016/j.imlet.2015.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yager EJ, Ahmed M, Lanzer K, Randall TD, Woodland DL, Blackman MA. Age-associated decline in T cell repertoire diversity leads to holes in the repertoire and impaired immunity to influenza virus. J Exp Med. 2008;205:711–23. doi: 10.1084/jem.20071140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.den Braber I, Mugwagwa T, Vrisekoop N, Westera L, Mögling R, Bregje de Boer A, Willems N, Schrijver EHR, Spierenburg, Gaiser K, Mul E, Otto SA, Ruiter AFC, Ackermans MT, Miedema F, Borghans JAM, de Boer RJ, Tesselaar K. Maintenance of Peripheral Naive T Cells Is Sustained by Thymus Output in Mice but Not Humans. Immunity. 2012;36:288–97. doi: 10.1016/j.immuni.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 39.Chen C-w, Mittal R, Klingensmith NJ, Burd EM, Terhorst C, Martin GS, Coopersmith CM, Ford ML. Cutting Edge: 2B4-Mediated Coinhibition of CD4+ T Cells Underlies Mortality in Experimental Sepsis. The Journal of Immunology. 2017;199:1961–6. doi: 10.4049/jimmunol.1700375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Spec A, Shindo Y, Burnham CD, Wilson S, Ablordeppey EA, Beiter ER, Chang K, Drewry AM, Hotchkiss RS. T cells from patients with Candida sepsis display a suppressive immunophenotype. Crit Care. 2016;20:15. doi: 10.1186/s13054-016-1182-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cabrera-Perez J, Babcock JC, Dileepan T, Murphy KA, Kucaba TA, Badovinac VP, Griffith TS. Gut Microbial Membership Modulates CD4 T Cell Reconstitution and Function after Sepsis. J Immunol. 2016;197:1692–8. doi: 10.4049/jimmunol.1600940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cadwell K, Patel KK, Maloney NS, Liu T-C, Ng ACY, Storer CE, Head RD, Xavier R, Stappenbeck TS, Virgin HW. Virus-Plus-Susceptibility Gene Interaction Determines Crohn’s Disease Gene Atg16L1 Phenotypes in Intestine. Cell. 2010;141:1135–45. doi: 10.1016/j.cell.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hand TW, Dos Santos LM, Bouladoux N, Molloy MJ, Pagán AJ, Pepper M, Maynard CL, Elson CO, Belkaid Y. Acute Gastrointestinal Infection Induces Long-Lived Microbiota-Specific T Cell Responses. Science. 2012;337:1553–6. doi: 10.1126/science.1220961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zaborin A, Defazio JR, Kade M, Kaiser BLD, Belogortseva N, Camp DG, Smith RD, Adkins JN, Kim SM, Alverdy A, Goldfeld D, Firestone MA, Collier JH, Jabri B, Tirrell M, Zaborina O, Alverdy JC. Phosphate-Containing Polyethylene Glycol Polymers Prevent Lethal Sepsis by Multidrug-Resistant Pathogens. Antimicrobial Agents and Chemotherapy. 2014;58:966–77. doi: 10.1128/AAC.02183-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Krezalek MA, DeFazio J, Zaborina O, Zaborin A, Alverdy JC. The Shift of an Intestinal “Microbiome” to a “Pathobiome” Governs the Course and Outcome of Sepsis Following Surgical Injury. Shock. 2016;45:475–82. doi: 10.1097/SHK.0000000000000534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zaborin A, Smith D, Garfield K, Quensen J, Shakhsheer B, Kade M, Tirrell M, Tiedje J, Gilbert JA, Zaborina O, Alverdy JC. Membership and Behavior of Ultra-Low-Diversity Pathogen Communities Present in the Gut of Humans during Prolonged Critical Illness. mBio. 2014:5. doi: 10.1128/mBio.01361-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wirth TC, Martin MD, Starbeck-Miller G, Harty JT, Badovinac VP. Secondary CD8(+) T-cell responses are controlled by systemic inflammation. European journal of immunology. 2011;41:1321–33. doi: 10.1002/eji.201040730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Peterson CT, Sharma V, Elmén L, Peterson SN. Immune homeostasis, dysbiosis and therapeutic modulation of the gut microbiota. Clin Exp Immunol. 2015;179:363–77. doi: 10.1111/cei.12474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Calderón-Gómez E, Bassolas-Molina H, Mora-Buch R, Dotti I, Planell N, Esteller M, Gallego M, Martí M, Garcia-Martín C, Martínez-Torró C, Ordás I, Singh S, Panés J, Benítez-Ribas D, Salas A. Commensal-Specific CD4+ Cells From Patients With Crohn’s Disease Have a T-Helper 17 Inflammatory Profile. Gastroenterology. 2016;151:489–500. e3. doi: 10.1053/j.gastro.2016.05.050. [DOI] [PubMed] [Google Scholar]
- 50.Fouts DE, Torralba M, Nelson KE, Brenner DA, Schnabl B. Bacterial translocation and changes in the intestinal microbiome in mouse models of liver disease. J Hepatol. 2012;56:1283–92. doi: 10.1016/j.jhep.2012.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee JS, Tato CM, Joyce-Shaikh B, Gulen MF, Cayatte C, Chen Y, Blumenschein WM, Judo M, Ayanoglu G, McClanahan TK, Li X, Cua DJ. Interleukin-23-Independent IL-17 Production Regulates Intestinal Epithelial Permeability. Immunity. 2015;43:727–38. doi: 10.1016/j.immuni.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yokouchi M, Kubo A, Kawasaki H, Yoshida K, Ishii K, Furuse M, Amagai M. Epidermal tight junction barrier function is altered by skin inflammation, but not by filaggrin-deficient stratum corneum. J Dermatol Sci. 2015;77:28–36. doi: 10.1016/j.jdermsci.2014.11.007. [DOI] [PubMed] [Google Scholar]
- 53.Markwart R, Condotta SA, Requardt RtP, Borken F, Schubert K, Weigel C, Bauer M, Griffith TS, Förster M, Brunkhorst FM, Badovinac VP, Rubio I. Immunosuppression after Sepsis: Systemic Inflammation and Sepsis Induce a Loss of Naïve T-Cells but No Enduring Cell-Autonomous Defects in T-Cell Function. PLoS ONE. 2014;9:e115094. doi: 10.1371/journal.pone.0115094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Borken F, Markwart R, Requardt RP, Schubert K, Spacek M, Verner M, Rückriem S, Scherag A, Oehmichen F, Brunkhorst FM, Rubio I. Chronic Critical Illness from Sepsis Is Associated with an Enhanced TCR Response. J Immunol. 2017;198:4781–91. doi: 10.4049/jimmunol.1700142. [DOI] [PubMed] [Google Scholar]
- 55.Henrickson SE, Mempel TR, Mazo IB, Liu B, Artyomov MN, Zheng H, Peixoto A, Flynn MP, Senman B, Junt T, Wong HC, Chakraborty AK, von Andrian UH. T cell sensing of antigen dose governs interactive behavior with dendritic cells and sets a threshold for T cell activation. Nat Immunol. 2008;9:282–91. doi: 10.1038/ni1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Colombetti S, Basso V, Mueller DL, Mondino A. Prolonged TCR/CD28 Engagement Drives IL-2-Independent T Cell Clonal Expansion through Signaling Mediated by the Mammalian Target of Rapamycin. J Immunol. 2006;176:2730–8. doi: 10.4049/jimmunol.176.5.2730. [DOI] [PubMed] [Google Scholar]
- 57.Delgoffe GM, Kole TP, Zheng Y, Zarek PE, Matthews KL, Xiao B, Worley PF, Kozma SC, Powell JD. mTOR differentially regulates effector and regulatory T cell lineage commitment. Immunity. 2009;30:832–44. doi: 10.1016/j.immuni.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Waickman AT, Powell JD. mTOR, metabolism, and the regulation of T-cell differentiation and function. Immunol Rev. 2012;249:43–58. doi: 10.1111/j.1600-065X.2012.01152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cheng S-C, Quintin J, Cramer RA, Shepardson KM, Saeed S, Kumar V, Giamarellos-Bourboulis EJ, Martens JHA, Rao NA, Aghajanirefah A, Manjeri GR, Li Y, Ifrim DC, Arts RJW, van der Meer BMJW, Deen PMT, Logie C, O’Neill LA, Willems P, van de Veerdonk FL, van der Meer JWM, Ng ACY, Joosten LAB, Wijmenga C, Stunnenberg HG, Xavier RJ, Netea MG. mTOR/HIF1α-mediated aerobic glycolysis as metabolic basis for trained immunity. Science. 2014;345:1250684. doi: 10.1126/science.1250684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Laufenberg LJ, Pruznak AM, Navaratnarajah M, Lang CH. Sepsis-induced changes in amino acid transporters and leucine signaling via mTOR in skeletal muscle. Amino Acids. 2014;46:2787–98. doi: 10.1007/s00726-014-1836-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Morel J, Palao J-C, Castells J, Desgeorges M, Busso T, Molliex S, Jahnke V, Del Carmine P, Gondin J, Arnould D, Cécile Durieux A, Freyssenet D. Regulation of Akt-mTOR, ubiquitin-proteasome and autophagy-lysosome pathways in locomotor and respiratory muscles during experimental sepsis in mice. Sci Rep. 2017;7:10866. doi: 10.1038/s41598-017-11440-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Venet F, Demaret J, Blaise BJ, Rouget C, Girardot T, Idealisoa E, Rimmelé T, Mallet F, Lepape A, Textoris J, Monneret G. IL-7 Restores T Lymphocyte Immunometabolic Failure in Septic Shock Patients through mTOR Activation. J Immunol. 2017;199:1606–15. doi: 10.4049/jimmunol.1700127. [DOI] [PubMed] [Google Scholar]
- 63.Shedlock DJ, Shen H. Requirement for CD4 T cell help in generating functional CD8 T cell memory. Science. 2003;300:337–9. doi: 10.1126/science.1082305. [DOI] [PubMed] [Google Scholar]
- 64.Shedlock DJ, Whitmire JK, Tan J, MacDonald AS, Ahmed R, Shen H. Role of CD4 T cell help and costimulation in CD8 T cell responses during Listeria monocytogenes infection. J Immunol. 2003;170:2053–63. doi: 10.4049/jimmunol.170.4.2053. [DOI] [PubMed] [Google Scholar]
- 65.Sun JC, Bevan MJ. Defective CD8 T cell memory following acute infection without CD4 T cell help. Science. 2003;300:339–42. doi: 10.1126/science.1083317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Janssen EM, Droin NM, Lemmens EE, Pinkoski MJ, Bensinger SJ, Ehst BD, Griffith TS, Green DR, Schoenberger SP. CD4+ T-cell help controls CD8+ T-cell memory via TRAIL-mediated activation-induced cell death. Nature. 2005;434:88–93. doi: 10.1038/nature03337. [DOI] [PubMed] [Google Scholar]
- 67.Wolkers MC, Gerlach C, Arens R, Janssen EM, Fitzgerald P, Schumacher TN, Medema JP, Green DR, Schoenberger SP. Nab2 regulates secondary CD8+ T-cell responses through control of TRAIL expression. Blood. 2012;119:798–804. doi: 10.1182/blood-2011-08-373910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Condotta SA, Cabrera-Perez J, Badovinac VP, Griffith TS. T-cell mediated immunity and the role of TRAIL in sepsis-induced immunosuppression. Crit Rev Immunol. 2013;33:23–40. doi: 10.1615/critrevimmunol.2013006721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gurung P, Rai D, Condotta SA, Babcock JC, Badovinac VP, Griffith TS. Immune unresponsiveness to secondary heterologous bacterial infection after sepsis induction is TRAIL-dependent. J Immunol. 2011;187:2148–54. doi: 10.4049/jimmunol.1101180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Steinwede K, Henken S, Bohling J, Maus R, Ueberberg B, Brumshagen C, Brincks EL, Griffith TS, Welte T, Maus UA. TNF-related apoptosis-inducing ligand (TRAIL) exerts therapeutic efficacy for the treatment of pneumococcal pneumonia in mice. J Exp Med. 2012;209:1937–52. doi: 10.1084/jem.20120983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Strother RK, Danahy DB, Kotov DI, Kucaba TA, Zacharias ZR, Griffith TS, Legge KL, Badovinac VP. Polymicrobial Sepsis Diminishes Dendritic Cell Numbers and Function Directly Contributing to Impaired Primary CD8 T Cell Responses In Vivo. J Immunol. 2016;197:4301–11. doi: 10.4049/jimmunol.1601463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Clénet M-L, Gagnon F, Moratalla AC, Viel EC, Arbour N. Peripheral human CD4(+)CD8(+) T lymphocytes exhibit a memory phenotype and enhanced responses to IL-2, IL-7 and IL-15. Sci Rep. 2017;7:11612. doi: 10.1038/s41598-017-11926-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jiang J, Fisher E, Murasko DM. Intrinsic Defects in CD8 T Cells with Aging Contribute to Impaired Primary Antiviral Responses. Exp Gerontol. 2013;48:579–86. doi: 10.1016/j.exger.2013.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Larbi A, Fulop T. From “truly naïve” to “exhausted senescent” T cells: When markers predict functionality. Cytometry A. 2014;85:25–35. doi: 10.1002/cyto.a.22351. [DOI] [PubMed] [Google Scholar]
- 75.van der Geest KSM, Abdulahad WH, Teteloshvili N, Tete SM, Peters JH, Horst G, Lorencetti PG, Bos NA, Lambeck A, Roozendaal C, Kroesen B-J, Koenen HJPM, Joosten I, Brouwer E, Boots AMH. Low-affinity TCR engagement drives IL-2-dependent post-thymic maintenance of naive CD4+ T cells in aged humans. Aging Cell. 2015;14:744–53. doi: 10.1111/acel.12353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Danahy DB, Anthony SM, Jensen IJ, Hartwig SM, Shan Q, Xue H-H, Harty JT, Griffith TS, Badovinac VP. Polymicrobial sepsis impairs bystander recruitment of effector cells to infected skin despite optimal sensing and alarming function of skin resident memory CD8 T cells. PLoS Pathog. 2017;13:e1006569. doi: 10.1371/journal.ppat.1006569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Duong S, Condotta SA, Rai D, Martin MD, Griffith TS, Badovinac VP. Polymicrobial sepsis alters Ag-dependent and -independent memory CD8 T cell functions. J Immunol. 2014;192:3618–25. doi: 10.4049/jimmunol.1303460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Grayson JM, Harrington LE, Lanier JG, Wherry EJ, Ahmed R. Differential sensitivity of naive and memory CD8+ T cells to apoptosis in vivo. J Immunol. 2002;169:3760–70. doi: 10.4049/jimmunol.169.7.3760. [DOI] [PubMed] [Google Scholar]
- 79.Unsinger J, McGlynn M, Kasten KR, Hoekzema AS, Watanabe E, Muenzer JT, McDonough JS, Tschoep J, Ferguson TA, McDunn JE, Morre M, Hildeman DA, Caldwell CC, Hotchkiss RS. IL-7 Promotes T Cell Viability, Trafficking, and Functionality and Improves Survival in Sepsis. J Immunol. 2010;184:3768–79. doi: 10.4049/jimmunol.0903151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Serbanescu MA, Ramonell KM, Hadley A, Margoles LM, Mittal R, Lyons JD, Liang Z, Coopersmith CM, Ford ML, McConnell KW. Attrition of memory CD8 T cells during sepsis requires LFA-1. J Leukoc Biol. 2016;100:1167–80. doi: 10.1189/jlb.4A1215-563RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Inoue S, Suzuki K, Komori Y, Morishita Y, Suzuki-Utsunomiya K, Hozumi K, Inokuchi S, Sato T. Persistent inflammation and T cell exhaustion in severe sepsis in the elderly. Crit Care. 2014;18:R130-R. doi: 10.1186/cc13941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Scumpia PO, Delano MJ, Kelly KM, O’Malley KA, Efron PA, McAuliffe PF, Brusko T, Ungaro R, Barker T, Wynn JL, Atkinson MA, Reeves WH, Clare Salzler MJ, Moldawer LL. Increased Natural CD4+CD25+ Regulatory T Cells and Their Suppressor Activity Do Not Contribute to Mortality in Murine Polymicrobial Sepsis. J Immunol. 2006;177:7943–9. doi: 10.4049/jimmunol.177.11.7943. [DOI] [PubMed] [Google Scholar]
- 83.Cavassani KA, Carson WF, Moreira AP, Wen H, Schaller MA, Ishii M, Lindell DM, Dou Y, Lukacs NW, Keshamouni VG, Hogaboam CM, Kunkel SL. The post sepsis-induced expansion and enhanced function of regulatory T cells create an environment to potentiate tumor growth. Blood. 2010;115:4403–11. doi: 10.1182/blood-2009-09-241083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Monneret G, Debard A-L, Venet F, Bohe J, Hequet O, Bienvenu J, Lepape A. Marked elevation of human circulating CD4+CD25+ regulatory T cells in sepsis-induced immunoparalysis. Crit Care Med. 2003;31:2068–71. doi: 10.1097/01.CCM.0000069345.78884.0F. [DOI] [PubMed] [Google Scholar]
- 85.Liu Q, An L, Qi Z, Zhao Y, Li C. Increased Expression of Programmed Cell Death-1 in Regulatory T Cells of Patients with Severe Sepsis and Septic Shock: An Observational Clinical Study. Scand J Immunol. 2017;86:408–17. doi: 10.1111/sji.12612. [DOI] [PubMed] [Google Scholar]
- 86.Wang H-W, Yang W, Gao L, Kang J-R, Qin J-J, Liu Y-P, Lu J-Y. Adoptive transfer of bone marrow-derived dendritic cells decreases inhibitory and regulatory T-cell differentiation and improves survival in murine polymicrobial sepsis. Immunology. 2015;145:50–9. doi: 10.1111/imm.12423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nascimento DC, Melo PH, Piñeros AR, Ferreira RG, Colón DF, Donate PB, Castanheira FV, Gozzi A, Czaikoski PG, Niedbala W, Borges MC, Zamboni DS, Liew FY, Cunha FQ, Alves-Filho JC. IL-33 contributes to sepsis-induced long-term immunosuppression by expanding the regulatory T cell population. Nat Commun. 2017;8:14919. doi: 10.1038/ncomms14919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kühlhorn F, Rath M, Schmoeckel K, Cziupka K, Nguyen HH, Hildebrandt P, Hünig T, Sparwasser T, Huehn J, Pötschke C, Bröker BM. Foxp3(+) Regulatory T Cells Are Required for Recovery from Severe Sepsis. PLoS ONE. 2013;8:e65109. doi: 10.1371/journal.pone.0065109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Okeke EB, Okwor I, Mou Z, Jia P, Uzonna JE. CD4+CD25+ Regulatory T Cells Attenuate Lipopolysaccharide-Induced Systemic Inflammatory Responses and Promotes Survival in Murine Escherichia coli Infection. Shock. 2013;40:65–73. doi: 10.1097/SHK.0b013e318296e65b. [DOI] [PubMed] [Google Scholar]
- 90.Ono S, Kimura A, Hiraki S, Takahata R, Tsujimoto H, Kinoshita M, Miyazaki H, Yamamoto J, Hase K, Saitoh D. Removal of increased circulating CD4+CD25+Foxp3+ regulatory T cells in patients with septic shock using hemoperfusion with polymyxin B-immobilized fibers. Surgery. 2013;153:262–71. doi: 10.1016/j.surg.2012.06.023. [DOI] [PubMed] [Google Scholar]
- 91.Venet F, Chung C-S, Monneret G, Huang X, Horner B, Garber M, Ayala A. Regulatory T cell populations in sepsis and trauma. J Leuk Biol. 2008;83:523–35. doi: 10.1189/jlb.0607371. [DOI] [PubMed] [Google Scholar]
- 92.Zheng Y, Wu Z, Ni H, Ke L, Tong Z, Li W, Li N, Li J. Codonopsis pilosula Polysaccharide Attenuates Cecal Ligation and Puncture Sepsis via Circuiting Regulatory T Cells in Mice. Shock. 2014;41:250–5. doi: 10.1097/SHK.0000000000000091. [DOI] [PubMed] [Google Scholar]
- 93.Martin MD, Badovinac VP. Antigen-dependent and –independent contributions to primary memory CD8 T cell activation and protection following infection. Sci Rep. 2015;5:18022. doi: 10.1038/srep18022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Eberlein J, Davenport B, Nguyen T, Victorino F, Haist K, Jhun K, Karimpour-Fard A, Hunter L, Kedl R, Clambey ET, Homann D. Aging promotes acquisition of naive-like CD8+ memory T cell traits and enhanced functionalities. J Clin Invest. 2016;126:3942–60. doi: 10.1172/JCI88546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Schenkel JM, Fraser KA, Beura LK, Pauken KE, Vezys V, Masopust D. Resident memory CD8 T cells trigger protective innate and adaptive immune responses. Science. 2014;346:98–101. doi: 10.1126/science.1254536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Schenkel JM, Fraser KA, Vezys V, Masopust D. Sensing and alarm function of resident memory CD8(+) T cells. Nat Immunol. 2013;14:509–13. doi: 10.1038/ni.2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Davies B, Prier JE, Jones CM, Gebhardt T, Carbone FR, Mackay LK. Cutting Edge: Tissue-Resident Memory T Cells Generated by Multiple Immunizations or Localized Deposition Provide Enhanced Immunity. J Immunol. 2017;198:2233–7. doi: 10.4049/jimmunol.1601367. [DOI] [PubMed] [Google Scholar]
- 98.Mackay LK, Wynne-Jones E, Freestone D, Pellicci DG, Mielke LA, Newman DM, Braun A, Masson F, Kallies A, Belz GT, Carbone FR. T-box Transcription Factors Combine with the Cytokines TGF-beta and IL-15 to Control Tissue-Resident Memory T Cell Fate. Immunity. 2015;43:1101–11. doi: 10.1016/j.immuni.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 99.Mueller SN, Zaid A, Carbone FR. Tissue-Resident T Cells: Dynamic Players in Skin Immunity. Front Immunol. 2014:5. doi: 10.3389/fimmu.2014.00332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Watanabe R, Gehad A, Yang C, Scott LL, Teague JE, Schlapbach C, Elco CP, Huang V, Matos TR, Kupper TS, Clark RA. Human skin is protected by four functionally and phenotypically discrete populations of resident and recirculating memory T cells. Sci Transl Med. 2015;7:279ra39–ra39. doi: 10.1126/scitranslmed.3010302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–7. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 102.Blackburn SD, Shin H, Haining WN, Zou T, Workman CJ, Polley A, Betts MR, Freeman GJ, Vignali DA, Wherry EJ. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat Immunol. 2009;10:29–37. doi: 10.1038/ni.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gallimore A, Glithero A, Godkin A, Tissot AC, Pluckthun A, Elliott T, Hengartner H, Zinkernagel R. Induction and exhaustion of lymphocytic choriomeningitis virus-specific cytotoxic T lymphocytes visualized using soluble tetrameric major histocompatibility complex class I-peptide complexes. J Exp Med. 1998;187:1383–93. doi: 10.1084/jem.187.9.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zajac AJ, Blattman JN, Murali-Krishna K, Sourdive DJ, Suresh M, Altman JD, Ahmed R. Viral immune evasion due to persistence of activated T cells without effector function. J Exp Med. 1998;188:2205–13. doi: 10.1084/jem.188.12.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Condotta SA, Khan SH, Rai D, Griffith TS, Badovinac VP. Poly-microbial sepsis increases susceptibility to chronic viral infection and exacerbates CD8(+) T cell exhaustion. J Immunol. 2015;195:116–25. doi: 10.4049/jimmunol.1402473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Choi YJ, Kim SB, Kim JH, Park S-H, Park MS, Kim JM, Han SH, Shin E-C. Impaired polyfunctionality of CD8+ T cells in severe sepsis patients with human cytomegalovirus reactivation. Exp Mol Med. 2017;49:e382. doi: 10.1038/emm.2017.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Berkson JD, Prlic M. The MAIT conundrum - how human MAIT cells distinguish bacterial colonization from infection in mucosal barrier tissues. Immunol Lett. 2017;192:7–11. doi: 10.1016/j.imlet.2017.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Carding SR, Egan PJ. Gammadelta T cells: functional plasticity and heterogeneity. Nat Rev Immunol. 2002;2:336–45. doi: 10.1038/nri797. [DOI] [PubMed] [Google Scholar]
- 109.Crosby CM, Kronenberg M. Invariant natural killer T cells: front line fighters in the war against pathogenic microbes. Immunogenetics. 2016;68:639–48. doi: 10.1007/s00251-016-0933-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Konjar S, Ferreira C, Blankenhaus B, Veldhoen M. Intestinal Barrier Interactions with Specialized CD8 T Cells. Front Immunol. 2017;8:1281. doi: 10.3389/fimmu.2017.01281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Andreu-Ballester JC, Tormo-Calandín C, Garcia-Ballesteros C, Pérez-Griera J, Amigó V, Almela-Quilis A, Ruiz del Castillo J, Peñarroja-Otero C, Ballester F. Association of γδ T Cells with Disease Severity and Mortality in Septic Patients. Clin Vaccine Immunol. 2013;20:738–46. doi: 10.1128/CVI.00752-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.de Souza Costa MF, Bastos Trigo de Negreiros C, Ugarte Bornstein V, Hemmi Valente R, Mengel J, Henriques MdG, Farias Benjamim C, Penido C. Murine IL-17(+) Vγ4 T lymphocytes accumulate in the lungs and play a protective role during severe sepsis. BMC Immunol. 2015;16:36. doi: 10.1186/s12865-015-0098-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Galley HF, Lowes DA, Thompson K, Wilson ND, Wallace CA, Webster NR. Characterisation of gamma delta (γδ) T cell populations in patients with sepsis. Cell Biol Int. 2015;39:210–6. doi: 10.1002/cbin.10361. [DOI] [PubMed] [Google Scholar]
- 114.Liao X-L, Feng T, Zhang J-Q, Cao X, Wu Q-H, Xie Z-C, Kang Y, Li H. Phenotypic Changes and Impaired Function of Peripheral γδ T Cells in Patients With Sepsis. Shock. 2017;48:321–8. doi: 10.1097/SHK.0000000000000857. [DOI] [PubMed] [Google Scholar]
- 115.Heffernan DS, Monaghan SF, Chung C-S, Cioffi WG, Gravenstein S, Ayala A. A divergent response of innate regulatory T-cells to sepsis in humans: Circulating invariant natural killer T-cells are preserved. Human Immunol. 2014;75:277–82. doi: 10.1016/j.humimm.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 116.Patil NK, Luan L, Bohannon JK, Guo Y, Hernandez A, Fensterheim B, Sherwood ER. IL-15 Superagonist Expands mCD8(+ )T, NK and NKT Cells after Burn Injury but Fails to Improve Outcome during Burn Wound Infection. PLoS ONE. 2016;11:e0148452. doi: 10.1371/journal.pone.0148452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Grimaldi D, Le Bourhis L, Sauneuf B, Dechartres A, Rousseau C, Ouaaz F, Milder M, Louis D, Chiche J-D, Mira J-P, Lantz O, Pène F. Specific MAIT cell behaviour among innate-like T lymphocytes in critically ill patients with severe infections. Intensive Care Med. 2014;40:192–201. doi: 10.1007/s00134-013-3163-x. [DOI] [PubMed] [Google Scholar]
- 118.Szabo PA, Anantha RV, Shaler CR, McCormick JK, Haeryfar SMM. CD1d- and MR1-Restricted T Cells in Sepsis. Front Immunol. 2015;6:401. doi: 10.3389/fimmu.2015.00401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tung J-N, Lee W-Y, Pai M-H, Chen W-J, Yeh C-L, Yeh S-L. Glutamine modulates CD8αα+ TCRαβ+ intestinal intraepithelial lymphocyte expression in mice with polymicrobial sepsis. Nutrition. 2013;29:911–7. doi: 10.1016/j.nut.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 120.Bucy RP, Chen CL, Cooper MD. Tissue localization and CD8 accessory molecule expression of T gamma delta cells in humans. J Immunol. 1989;142:3045–9. [PubMed] [Google Scholar]
- 121.Gibbs A, Leeansyah E, Introini A, Paquin-Proulx D, Hasselrot K, Andersson E, Broliden K, Sandberg JK, Tjernlund A. MAIT cells reside in the female genital mucosa and are biased towards IL-17 and IL-22 production in response to bacterial stimulation. Mucosal Immunol. 2017;10:35–45. doi: 10.1038/mi.2016.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.King IL, Amiel E, Tighe M, Mohrs K, Veerapen N, Besra G, Mohrs M, Leadbetter EA. The mechanism of splenic iNKT cell activation dictates localization in vivo. J Immunol. 2013;191:572–82. doi: 10.4049/jimmunol.1300299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ruscher R, Kummer RL, Lee YJ, Jameson SC, Hogquist KA. CD8[alpha][alpha] intraepithelial lymphocytes arise from two main thymic precursors. Nat Immunol. 2017;18:771–9. doi: 10.1038/ni.3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Slauenwhite D, Johnston B. Regulation of NKT Cell Localization in Homeostasis and Infection. Front Immunol. 2015;6:255. doi: 10.3389/fimmu.2015.00255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Patera AC, Drewry AM, Chang K, Beiter ER, Osborne D, Hotchkiss RS. Frontline Science: Defects in immune function in patients with sepsis are associated with PD-1 or PD-L1 expression and can be restored by antibodies targeting PD-1 or PD-L1. Journal of Leukocyte Biology. 2016;100:1239–54. doi: 10.1189/jlb.4HI0616-255R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Chang KC, Burnham C-A, Compton SM, Rasche DP, Mazuski RJ, SmcDonough J, Unsinger J, Korman AJ, Green JM, Hotchkiss RS. Blockade ofthe negative co-stimulatory molecules PD-1 and CTLA-4 improves survival in primary and secondary fungal sepsis. Crit Care. 2013;17:R85-R. doi: 10.1186/cc12711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Shindo Y, Unsinger J, Burnham C, Green JM, Hotchkiss RS. Interleukin 7 and anti-programmed cell death 1 antibody have differing effects to reverse sepsis-induced immunosuppression. Shock. 2015;43:334–43. doi: 10.1097/SHK.0000000000000317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Patil NK, Guo Y, Luan L, Sherwood ER. Targeting Immune Cell Checkpoints during Sepsis. International Journal of Molecular Sciences. 2017;18:2413. doi: 10.3390/ijms18112413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Badovinac VP, Messingham KAN, Griffith TS, Harty JT. TRAIL Deficiency Delays, but Does Not Prevent, Erosion in the Quality of “Helpless” Memory CD8 T Cells. J Immunol. 2006;177:999–1006. doi: 10.4049/jimmunol.177.2.999. [DOI] [PubMed] [Google Scholar]
- 130.Leng X, Zhang Q, Chen Z, Wang D. Blocking TRAIL-DR5 signaling with soluble DR5 alleviates acute kidney injury in a severely burned mouse model. Int J Clin Exp Pathol. 2014;7:3460–8. [PMC free article] [PubMed] [Google Scholar]
- 131.Sacks JA, Bevan MJ. TRAIL Deficiency Does Not Rescue Impaired CD8+T Cell Memory Generated in the Absence of CD4+ T Cell Help. J Immunol. 2008;180:4570–6. doi: 10.4049/jimmunol.180.7.4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hotchkiss RS, Nicholson DW. Apoptosis and caspases regulate death and inflammation in sepsis. Nat Rev Immunol. 2006;6:813–22. doi: 10.1038/nri1943. [DOI] [PubMed] [Google Scholar]
- 133.Hotchkiss RS, Chang KC, Swanson PE, Tinsley KW, Hui JJ, Klender P, Xanthoudakis S, Roy S, Black C, Grimm E, Aspiotis R, Han Y, Nicholson DW, Karl IE. Caspase inhibitors improve survival in sepsis: a critical role of the lymphocyte. Nat Immunol. 2000;1:496–501. doi: 10.1038/82741. [DOI] [PubMed] [Google Scholar]
- 134.Hotchkiss RS, Tinsley KW, Swanson PE, Chang KC, Cobb JP, Buchman TG, Korsmeyer SJ, Karl IE. Prevention of lymphocyte cell death in sepsis improves survival in mice. Proc Natl Acad Sci U S A. 1999;96:14541–6. doi: 10.1073/pnas.96.25.14541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wesche-Soldato DE, Chung CS, Lomas-Neira J, Doughty LA, Gregory SH, Ayala A. In vivo delivery of caspase-8 or Fas siRNA improves the survival of septic mice. Blood. 2005;106:2295–301. doi: 10.1182/blood-2004-10-4086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Inoue S, Unsinger J, Davis CG, Muenzer JT, Ferguson TA, Chang KC, Osborne DF, Clark AT, Coopersmith CM, McDunn JE, Hotchkiss RS. IL-15 Prevents Apoptosis, Reverses Innate and Adaptive Immune Dysfunction, and Improves Survival in Sepsis. J Immunol. 2010;184:1401–9. doi: 10.4049/jimmunol.0902307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Shindo Y, Fuchs AG, Davis CG, Eitas T, Unsinger J, Burnham CD, Green JM, Morre M, Bochicchio GV, Hotchkiss RS. Interleukin 7 immunotherapy improves host immunity and survival in a two-hit model of Pseudomonas aeruginosa pneumonia. J Leuk Biol. 2017;101:543–54. doi: 10.1189/jlb.4A1215-581R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Sim GC, Radvanyi L. The IL-2 cytokine family in cancer immunotherapy. Cytokine Growth Factor Rev. 2014;25:377–90. doi: 10.1016/j.cytogfr.2014.07.018. [DOI] [PubMed] [Google Scholar]
- 139.West EE, Jin H-T, Rasheed A-U, Penaloza-MacMaster P, Ha S-J, Tan WG, Youngblood B, Freeman GJ, Smith KlA, Ahmed R. PD-L1 blockade synergizes with IL-2 therapy in reinvigorating exhausted T cells. J Clin Invest. 2013;123:2604–15. doi: 10.1172/JCI67008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Pearce EL, Walsh MC, Cejas PJ, Harms GM, Shen H, Wang L-S, Jones RG, Choi Y. Enhancing CD8 T Cell Memory by Modulating Fatty Acid Metabolism. Nature. 2009;460:103–7. doi: 10.1038/nature08097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.van der Windt GJW, Everts B, Chang C-H, Curtis JD, Freitas TC, Amiel E, Pearce EJ, Pearce EL. Mitochondrial Respiratory Capacity Is A Critical Regulator Of CD8(+) T Cell Memory Development. Immunity. 2012;36:68–78. doi: 10.1016/j.immuni.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lee SY, Cho ML, Oh HJ, Ryu JG, Park MJ, Jhun JY, Park MK, Stone JC, Ju JH, Hwang SY, Park SH, Surh CD, Kim HY. Interleukin-2/anti-interleukin-2 monoclonal antibody immune complex suppresses collagen-induced arthritis in mice by fortifying interleukin-2/STAT5 signalling pathways. Immunology. 2012;137:305–16. doi: 10.1111/imm.12008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ramonell KM, Zhang W, Hadley A, Chen C-w, Fay KT, Lyons JD, Klingensmith NJ, McConnell KW, Coopersmith CM, Ford ML. CXCR4 blockade decreases CD4+ T cell exhaustion and improves survival in a murine model of polymicrobial sepsis. PLOS ONE. 2017;12:e0188882. doi: 10.1371/journal.pone.0188882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Lange A, Sundén-Cullberg J, Magnuson A, Hultgren O. Soluble B and T Lymphocyte Attenuator Correlates to Disease Severity in Sepsis and High Levels Are Associated with an Increased Risk of Mortality. PLOS ONE. 2017;12:e0169176. doi: 10.1371/journal.pone.0169176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Inoue S, Bo L, Bian J, Unsinger J, Chang K, Hotchkiss RS. Dose Dependent Effect of Anti-CTLA-4 on Survival in Sepsis. Shock (Augusta, Ga) 2011;36:38–44. doi: 10.1097/SHK.0b013e3182168cce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Boomer JS, Shuherk-Shaffer J, Hotchkiss RS, Green JM. A prospective analysis of lymphocyte phenotype and function over the course of acute sepsis. Critical Care. 2012;16:R112-R. doi: 10.1186/cc11404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Venet F, Foray A-P, Villars-Méchin A, Malcus C, Poitevin-Later F, Lepape A, Monneret G. IL-7 Restores Lymphocyte Functions in Septic Patients. J Immunol. 2012;189:5073–81. doi: 10.4049/jimmunol.1202062. [DOI] [PubMed] [Google Scholar]