Abstract
Objective
We used CD obese prone (OP) and obese resistant (OR) rats to examine how weight gain and fat accretion relate to fermentation levels and microbiota composition after feeding resistant starch (RS).
Methods
After feeding OP rats and OR rats a high fat (HF) diet for 4 weeks, rats were stratified into three groups: fed either a HF diet (HF), switched to a low fat (LF) diet or a LF diet supplemented with RS (LF-RS, 20% wt) for 4 weeks. Energy intake, body weight, fermentation variables and microbiota composition were determined.
Results
In OP rats, RS elicited robust fermentation (increased cecal contents, short chain fatty acids and serum GLP-1). Total bacteria, species of the Bacteroidales family S24-7 and the archaea Methanobrevibacter smithii increased. The robust fermentation did not elicit higher weight or fat accretion compared to control rats fed the same isocaloric diets (HFLF±RS). In OR rats, body weight and fat accretion were also not different between HFLF±RS diets, but RS elicited minimal changes in fermentation and microbiota composition.
Conclusions
Robust fermentation did not contribute to greater weight. Fermentation levels and changes in microbiota composition in response to dietary RS differ by obese phenotype.
Keywords: Animal Models, Dietary Fibre, Microbiome
Introduction
Gut microbiota composition affects energy balance [1–4]. Individuals predisposed to obesity may have microbial species that promote more fermentation than lean for more efficient extraction from diet [1, 2]. Studies show fermentation of resistant starch (RS) reduces abdominal fat accretion in rodents fed isocaloric diets [5–9] as RS fermentation increased fat oxidation without affecting physical activity [5].
We used outbred colonies of rats: CD obese-resistant (OR) and CD obese-prone (OP) (Charles River Company, Houston, TX). They differ in respect to their disposition to develop obesity on high fat diet [10] with both having a fully functioning leptin receptor [10–12]. Their polygenic pattern of inheritance is similar to most human obese phenotypes [11]. We investigated effects of diet on obesity phenotype and relation to gut microbiota. Belobrajdic et al. [13] reported Sprague Dawley rats fed high fat (HF) diets for 4 weeks resulted in OP and OR rats with reduced body fat when fed RS at different doses. Their study did not use isocaloric diets with RS compared to control group as diets were matched for carbohydrate content. They observed effects of diet energy dilution and fermentation of RS on weight gain. In the current study, our main comparisons had isocaloric diets in two phases of the study and focused on the effects of fermentation by gut bacteria without confounding effects of energy dilution.
We measured Methanobrevibacter smithii (M. smithii), which constitutes up to 10% of anaerobic gut microorganisms [14]. A mutual relationship exists between M. smithii and bacterial species that ferment carbohydrates to produce H2 and formate. Hydrogen accumulation reduces fermentation rate by inhibiting bacterial NADH dehydrogenases and ATP yield [14,15]. By consuming hydrogen in methane production, M. smithii improves fermentation efficacy [14]. Although this function suggests a role for methanogens in greater caloric harvest and weight gain, role of methanogens in the pathogenesis of obesity is unclear. Since fermentation is beneficial to host health [16], higher levels of M. smithii may be beneficial so we sought to determine how their abundance relates to the obese phenotype.
Materials and methods
Animals
The rat study was approved by the LSU IACUC. Three- and four-week-old male OP (N=32) and OR (N=32) (Charles River Laboratory) rats arrived and after one-week quarantine were singly housed in a temperature controlled vivarium with 12:12-h light/dark cycle. Rats began the study at five- and six-weeks old.
Study plan
Our basic design (Figure 1 and Table 1) was to feed either HF or LF diet to OP and OR rats for 4 weeks (phase 1). In phase 1, 10 OP and 10 OR rats were fed LF, and 22 OP and 22 OR rats were fed HF. At end of phase 1, 4 OP and 4 OR rats were euthanized from both diet groups. For phase 2, 6 OP and 6 OR rats continued on LF for 4 weeks. The remaining 18 OP and 18 OR rats from HF in phase 1 were placed into 3 groups for each rat type. One group continued on HF, a second was switched to LF, and the third was switched to LF with 20% by diet weight as RS.
Figure 1.
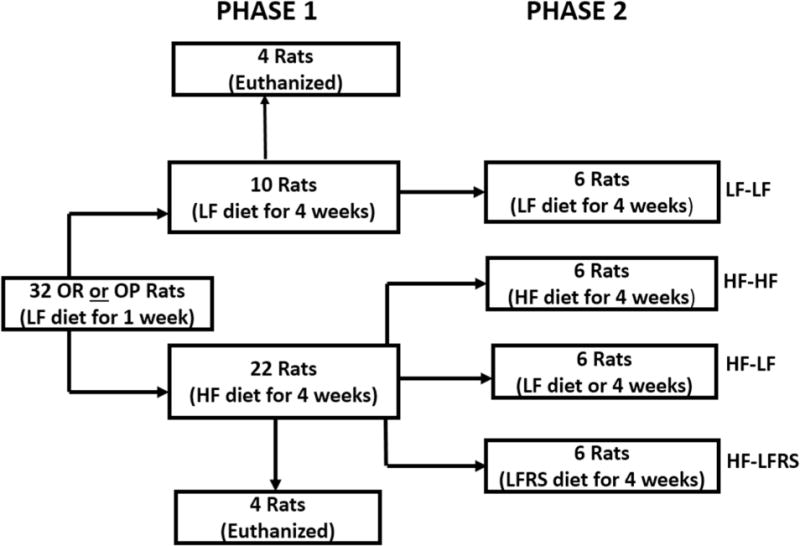
The study design showing the study with two phases with both OP and OR rats.
Table 1.
Rat and Diet Acronyms for Phases 1 and 2 of Study
| Acronym | Description |
|---|---|
| OP | CD obese-prone rat (Charles River Company) |
| OR | CD obese-resistant rat (Charles River Company) |
| LF | Low-fat diet |
| HF | High-fat diet |
| LF-LF | Low-fat diet in phases 1 and 2 of study |
| HF-HF | High-fat diet in phases 1 and 2 of study |
| HF-LF | High-fat diet in phase 1 with switch to low-fat diet for phase 2 |
| HF-LFRS | High-fat diet in phase 1 with switch to low-fat diet with 20% resistant starch by weight in phase 2 |
Our hypothesis, based on a previous study in which HF reduced fermentation of RS compared to LF when HF and RS were fed at the same time [7], was that HF would promote dysbiosis in rats and may affect subsequent bacterial types with feeding RS with LF. Thus, the need for a control group also fed HF in phase 1 and switched to LF without RS in phase 2. Without effect of dietary energy dilution for this comparison, OP rats may not respond as well as in Belobrajdic et al. [13] study. For phase 2, we thought OP rats may have greater accretion of abdominal fat because of greater energy harvest not offset by dietary energy dilution.
Diets and feeding
The composition of the modified AIN-93M diets are in Table 2. Weights and food intake were determined twice a week. Food and water were provided ad libitum. After one-week quarantine on chow there was one week acclimation period with LF diet. Rats were then fasted (~6 hr) to determine serum glucose and insulin after retro-orbital bleeding. The homeostatic model of assessment-insulin resistance (HOMA-IR) index was calculated [17]. Rats were stratified based on weight and HOMA-IR.
Table 2.
Diets for CD obese-prone and obese-resistant rats
| Diet components | Low fat diet | High fat diet | ||
|---|---|---|---|---|
|
| ||||
| Ingredients (g) | Energy value Kcal/g | LF (g) | LF-RS (g) | HF (g) |
| 1 100% amylopectin cornstarch | 3.5 | 521.1 | 147.1 | 405.7 |
| 2 HM260 (containing RS at 20% of diet3) | 2.8 | 0 | 472.4 | 0 |
| Sucrose | 4.0 | 100 | 100 | 100 |
| Casein | 3.50 | 140 | 140 | 140 |
| Cellulose | 0 | 150.8 | 52.4 | 106.2 |
| Corn oil | 8.84 | 40 | 40 | 100 |
| Lard | 9.00 | 0 | 0 | 100 |
| Mineral mix (AIN -93M) | 0.88 | 35 | 35 | 35 |
| Vitamin mix (AIN 93) | 3.87 | 10 | 10 | 10 |
| Choline chloride | 0 | 1.3 | 1.3 | 1.3 |
| L-Cystine | 4 | 1.8 | 1.8 | 1.8 |
|
| ||||
| Total weight (Kcal) | 1000 g (3160.2) | 1000 g (3160.1) | 1000 g (4182.5) | |
100% amylopectin cornstarch is the AMIOCA® cornstarch product from Ingredion Incorporated (Bridgewater, NJ).
HM260 is high-amylose cornstarch (HI-MAIZE® 260 resistant starch) from Ingredion Incorporated (Bridgewater, NJ).
HM260 lot was 42.3% RS based on wet weight as used in diet.
Serum and GI tract collections and analyses
At euthanasia, abdominal fat pads, epididymal, perirenal, and retroperitoneal, were excised. Serum was collected by heart puncture. Weight of GI tract without contents was added to the disemboweled body weight for emboweled body weight (EBW). Cecal contents were frozen in liquid nitrogen and stored at −80°C. Fermentation (cecum weights, cecal contents pH and short chain fatty acids, and serum GLP-1 active) was measured as described [7, 18–19].
Bacterial DNA extraction
DNA was extracted from 200 mg cecal contents using QIAamp DNA Fast Stool kit (Qiagen, Valencia, CA) according to manufacturer’s protocol with slight modifications. In third step, zirconium beads (200 mg) and InhibitEX buffer were added and mixture subjected to bead beating (FastPrep®-24, MP Biomedicals, Santa Ana, CA) for 1 min at 6.5 m/sec (2 times). After centrifugation (20°C, 20,000 g), 500 ul of supernatant was collected. Purified DNA was quantified by NanoDrop spectrophotometry and stored at −80°C.
Primer selection
Primers (16S rRNA) for M. smithii were from Dridi et al. [20] and produced by Integrated DNA Technologies- (Coralville, IA) were F:5′CCGGGTATCTAATCCGGTTC3′ and R:5′CTCCCAGGGTAGAGGTGAAA3′. Primers for total bacteria (16S rRNA gene) were F: 5′ACGTCRTCCMCNCCTTCCTC 3′ and R: 5′GTGSTGCAYGGYYGTCGTCA3′ reported by Belenguer et al. [21]. BLAST tool (https://blast.ncbi.nlm.nih.gov/Blast.cgi, accessed 06/22/2017) was used to verify in silico that primers for M. smithii ATCC 35061 were specific (100% identity with 123 base pair double-stranded amplicon) to this species and 3 others (M. Ruminantium, M. millerae, and M. oralis in genus Methanobrevibacter and Methanosphaera stadtmanae DSM 3091). Dridi et al. [20] found M. smithii in human stool samples, but also found Methanosphaera stadtmanae in some samples with a different set of primers. They sequenced the amplicon for their primers for M. smithii and found 99–100% similarity to M. smithii ATCC 35061 so we concluded our results with these primers reflect M.smithii. In silico results for universal 16S rRNA gene matched a broad range of bacteria.
PCR amplification and amplicon DNA standards
Using Qiagen Taq PCR master mix kit (Qiagen, Valencia, CA), genomic DNA from pooled samples from each treatment in phase 1 was used as template to prepare amplicon DNA with specific gene sequences and lengths (123 bp M. smithii and 147 bp universal 16S rRNA genes). Amplicon DNA size was verified by 2% agarose gel electrophoresis. Briefly, 100 ul reactions containing 5 ul (0.5 uM) each of the forward and reverse primers, 1000 ng of template DNA and 50 ul of the PCR mix were used. Cycling conditions were: initial denaturation at 94° for 3 min, 35 cycles of denaturation at 94°C for 1 min, annealing for 1 min, extension at 72°C for 1 min and final extension at 72°C for 10 min. Annealing temperatures were 52°C for M. smithii and 60°C for 16S rRNA gene. After completion of PCR, DNA amounts increased by 314 ng and 1218 ng for M. smithii and universal primers, respectively. These amounts were used in the formula below to calculate amplicon amounts for standard curves. Post-PCR DNA was cleaned by QIAquick PCR purification kit (Qiagen, Valencia, CA), and quantified by NanoDrop spectrophotometer. Molar concentrations of amplicon standard DNA were converted into gene copies/ul using the formula shown by Oldham and Duncan [22] assuming average molecular mass of dsDNA bp is 6.6 × 1011 ng/mol and the Avogadro’s number of copies per mole is 6.022 × 1023.
Standard curves ranging from 108–102 copies/ul were generated by serial 10-fold dilutions of amplicon DNA with nuclease free water.
Quantitative Real-time PCR
The SYBR® Green qPCR assay was used to quantify total bacteria and M. smithii using ABI Prism 7900HT Sequence Detection System (Life Technologies, Foster City, CA). Cycling conditions were 50°C for 2 min, 95°C for 10 min, 40 cycles of 95°C for 15 seconds, followed by primer annealing at 52°C for M. smithii and 60°C for the 16S rRNA gene, then 78°C for 30 seconds. No-template controls and amplicon standards were included in each plate. Amplicon quantities (gene copies/ul) vs. cycles to threshold (Cts) standard curves were used to determine M. smithii and total bacteria quantities.
Microbiota analysis by next generation sequencing using Illumina Mi-Seq
Amplification, sequencing and bioinformatics of bacterial DNA from phase 2 were performed at the LSU Microbial Genomics Resource Group using V3 2X300 bp kits as described previously [19, 23–24]. Analysis using the UPARSE process was with 97% identity for Operational Taxonomic Units (OTUs) [19, 24].
Statistical analyses
Phase 1 data were analyzed as 2X2 factorial (OP and OR) and (LF and HF). Phase 2 data were analyzed as a 2X4 factorial (OP and OR) and (LF-LF, HF-HF, HF-LF and HF-LFRS). We used MIXED procedure of SAS 9.4 (SAS Institute, Cary, NC). Main and interactive effects were considered significant at P<0.05 and expressed as means ± SE. Further a priori comparisons used alpha level divided by number of comparisons. Next-generation sequencing data were analyzed by linear discriminant analysis (LDA) as screening tool for differences in relative abundance of OTUs among treatments (https://huttenhower.sph.harvard.edu/galaxy/). The Benjamini-Hochberg procedure [25] was applied to P values for dependent variables to decrease false discovery.
Results
Fermentation
After phase 1, no differences in weight of full and empty ceca were observed. In phase 2, OP rats fermented RS better than OR rats (P<0.0001, Figure 2A and 2D) as there were increases in weights of full and empty ceca of rats fed RS (diet P<0.001, Figure 2B and 2E). Interaction of diet and phenotype was significant (P<0.001, Figure 2C and 2F). There was a 65fold increase of full cecum weight and 25fold increase in empty cecum weight for OPHF-LFRS vs. OPLF-LF and OPHF-LF groups. In OR rats, the cecum weights in rats in HF-LFRS group were 30% larger than for rats fed HF-LF (P<0.0167).
Figure 2. Weight and size of ceca.
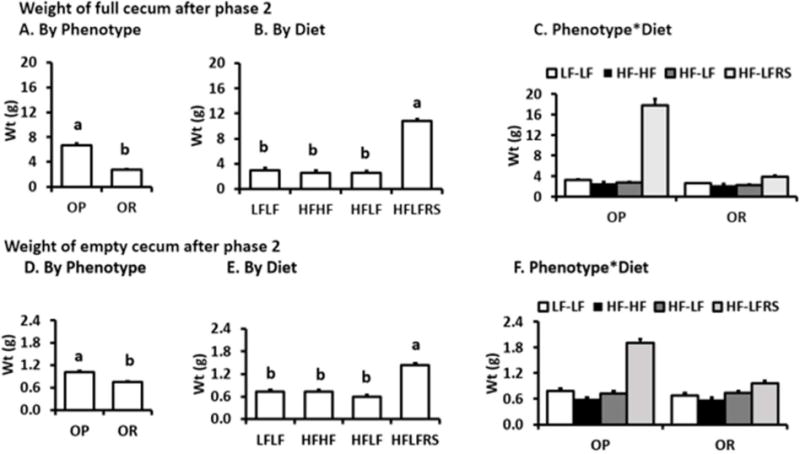
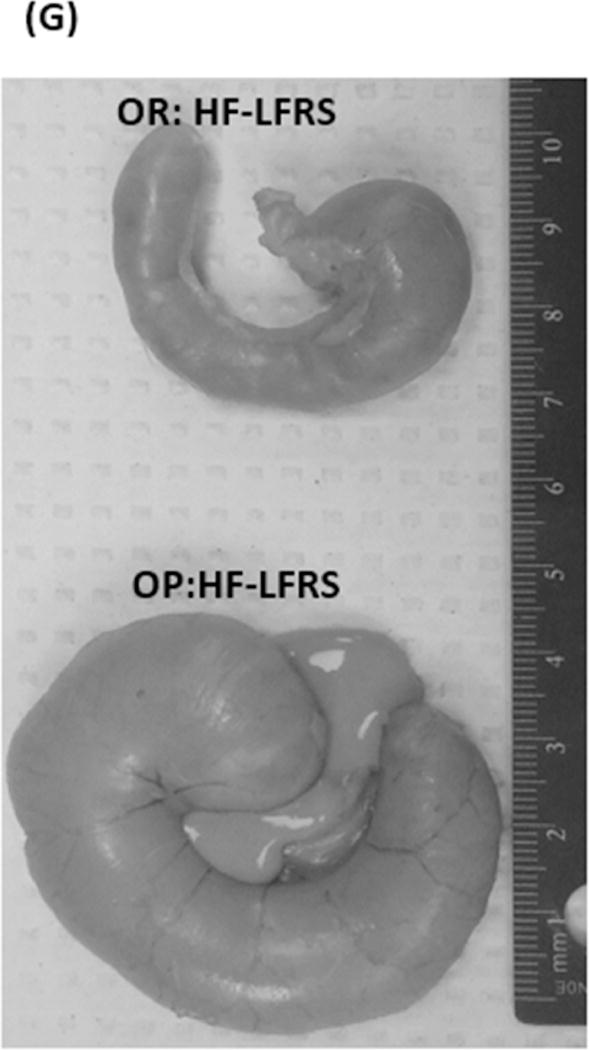
Full ceca (A–C) and empty ceca (D–F). In A and D, the letters designate differences between OP and OR rats (P<0.0001). For B and E the letters denote a priori differences between HF-LFRS and the three other diets (P<0.0167). For panels C and F there were differences (P<0.0125) between a priori comparisons: OPHF-LFRS vs OPLF-LF, OPHF-LFRS vs. OPHF-LF, OPHF-LFRS vs. ORHF-LFRS and ORHF-LFRS vs. ORHF-LF. G shows a representative picture of ceca of OP and OR rats fed RS.
Amounts of short-chain fatty acids (SCFAs) in cecal contents and cecal contents pH were not different after phase 1 and pH values were above pH 7 (Table S1). In phase 2, there were significant phenotype, diet and interaction effects that reflected greater amounts of fermentation in OP rats fed HF-LFRS (Table 3). A priori comparisons of groups with diets containing RS vs. no RS resulted in higher amounts of SCFAs (may also reflect absorption/utilization) and lower pH of cecal contents.
Table 3.
Fermentation variables: SCFAs, pH of cecal contents and GLP-1 active in serum1
| Variable | Diet | OP | OR | P value (Phenotype) | P value (Diet) | P value (Phenotype*Diet) | Pooled SEM |
|---|---|---|---|---|---|---|---|
| Acetate (mmol/cecum) | LF-LF | 0.081 | 0.045 | <0.0001 | <0.0001 | <0.0001 | 0.007 |
| HF-HF | 0.056 | 0.038 | |||||
| HF-LF | 0.061 | 0.032 | |||||
| HF-LFRS | 0.401 | 0.095 | |||||
|
| |||||||
| Propionate (mmol/cecum) | LF-LF | 0.015 | 0.009 | <0.001 | <0.001 | <0.0001 | 0.001 |
| HF-HF | 0.010 | 0.006 | |||||
| HF-LF | 0.030 | 0.004 | |||||
| HF-LFRS | 0.049 | 0.008 | |||||
|
| |||||||
| Butyrate (mmol/cecum) | LF-LF | 0.020 | 0.010 | <0.001 | <0.001 | <0.0001 | 0.0017 |
| HF-HF | 0.014 | 0.008 | |||||
| HF-LF | 0.016 | 0.007 | |||||
| HF-LFRS | 0.127 | 0.022 | |||||
|
| |||||||
| pH | LF-LF | 7.46 | 7.62 | <0.001 | <0.001 | <0.0001 | 0.973 |
| HF-HF | 7.56 | 7.57 | |||||
| HF-LF | 7.56 | 7.30 | |||||
| HF-LFRS | 5.62 | 6.90 | |||||
|
| |||||||
| GLP-1 (pmol/L) | LF-LF | 0.97 | 0.89 | 0.20 | 0.0083 | 0.839 | 0.073 |
| HF-HF | 1.15 | 1.13 | |||||
| HF-LF | 0.88 | 0.99 | |||||
| HF-LFRS | 1.66 | 1.06 | |||||
For all Table 2 variables there were differences (P<0.0125) for four a priori comparisons (OPHF-LFRS vs. OPLF-LF, OPHF-LFRS vs. OPHF-LF, OPHF-LFRS vs. ORHF-LFRS and ORHF-LFRS vs. ORHF-LF).
GLP-1 active concentrations were not different at the end of phase 1 (Table S1). After phase 2, there were increased values for GLP-1 active for LFRS diet (P<0.0083) (Table 3).
Energy intake, body weight and fat accretion
Energy intake in OP rats was higher than in OR rats in both phases (Figure S1). Most weight gain occurred in phase 1 (Figure 3). After phase 1, EBW was significantly higher (P<0.0065) in OP rats compared to OR rats while a priori diet comparisons showed no significant effects within rat type (P=0.64). After phase 2, OP rats had higher EBW (P<0.0001) than OR rats. Diet also had an effect and OP rats fed HF had highest increase in EBW, as there was a diet effect (P<0.008). The interaction of diet and phenotype was not significant (P=0.43) indicating relationship of weight gain among diets with two phenotypes was similar. Among a priori comparisons, OPHF-LFRS group had greater EBW than ORHF-LFRS group (P<0.0125) (Figure 3A). Percent increase in EBW during phase 2 was higher in OP rats compared to OR rats (P<0.005) and diet effect observed (P<0.03) because OP rats fed the HF gained the most weight. Among a priori comparisons, percent increase in weight in rats switched from a HF to LFRS was not different from those switched to LF without RS (Figure 3B). There was an effect of both phenotype (P<0.001) and diet (P<0.001) on total abdominal fat after phase 2. Interaction was not significant (P=0.064). Total abdominal fat and percent abdominal fat in rats switched from HF to LFRS were not different from those switched to LF (Figure 3C and 3D). Although feeding RS to OP rats increased fermentation compared to other groups, it did not contribute to greater energy storage.
Figure 3. Body weight and body fat.
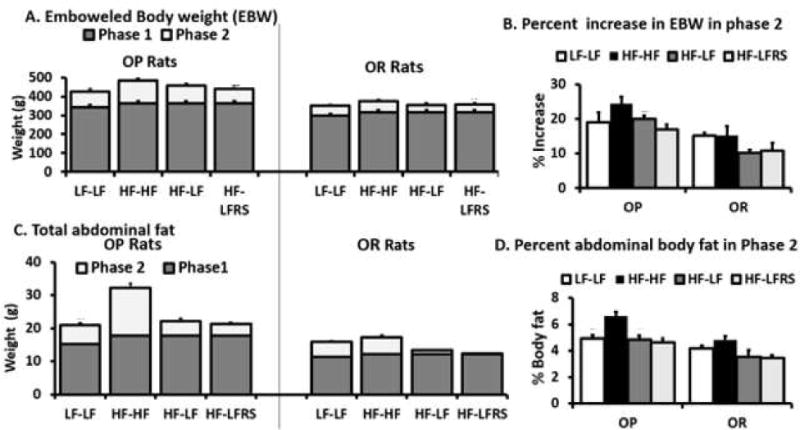
A. Weight of the GI tract contents was subtracted from total body weight to determine emboweled body weight (EBW) for OP and OR rats. B. Percent increases in EBW as phase 2/phase 1*100. C. Total abdominal fat. D. Percent abdominal fat (abdominal fat/EBW*100) after phase 2. In A and C data for the lower darker bars was determined from the 4 animals per LF and HF diet groups euthanized after phase 1. Data for the upper lighter bars was determined from the 6 animals per group after phase 2. The only a priori difference after phase 2 for A-D was between OPHF-LFRS vs. ORHF-LFRS (P<0.0125). There were no significant differences for OPHF-LFRS vs OPHF-LF, OPHF-LFRS vs OPLF-LF and ORHF-LFRS vs ORHF-LF.
Total bacteria and Methanobrevibacter smithii levels
In phase 1, OR rats had more total bacteria compared to OP rats (P<0.014), but diet (P=0.86) and interaction (P=0.69) were not significant. In phase 2 (Figure 4A–C), RS increased total bacteria more than 3-fold (P< 0.005) only in OP rats fed LFRS. Dietary manipulation did not change total bacteria in OR rats.
Figure 4. Abundance of total bacteria and Methanobrevibacter smithii after phase 2 (qPCR).
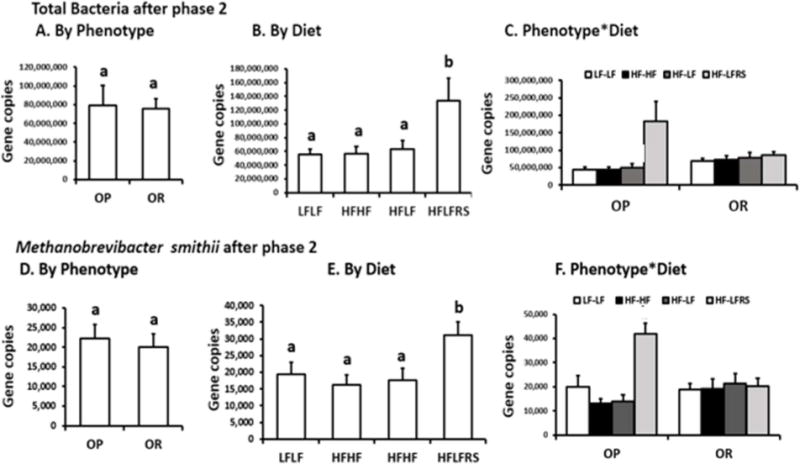
A–C. Total bacteria. D-F. M. smithii. For A and D, there were no significant differences in both total bacteria and M. smithii for OP and OR rats. The letters a and b in B and E indicate three a priori differences P<0.0167) between HF-LFRS diet and all other diets. In C and F for OP rats there were a priori differences (P<0.0125) between OPHF-LFRS vs. OPHF-LF, ORHF-LFRS vs. OPLF-LF, and OPHF-LFRS vs. ORHF-LFRS. No significant difference was observed between ORHF-LFRS and ORHF-LF.
Diet and phenotype had no effect on abundance of M. smithii in phase 1 and HF did not change M. smithii levels in either OP or OR rats. In phase 2 (Figure 4D–F), there was a diet effect (P<0.02) because M. smithii abundance with greater fermentation increased in LFRS group by 3-fold in OP rats (P<0.005, LFRS vs. other 3 groups). There was interaction between diet and phenotype (P<0.007) because there was not a similar increase in M. smithii in OR rats fed RS.
Proportions of bacteria taxa
Most changes in bacterial composition occurred in OP rats fed RS (Figures 5, S2–S4). Dietary RS increased bacteria in phylum Bacteroidetes and reduced bacteria in Firmicutes in OP rats compared to OR rats (Figures S2–S4). The abundance of Bacteroidales family S24-7 was highest in OP rats fed RS (P<0.001), while Firmicutes families Lactobacillaceae and Lachnospiraceae were more abundant in OR rats fed RS (LDA effect size, figure S4, P<0.05, Figures 5, S3–S4).
Figure 5. Relative abundance of the family S24–7 gene copies (qPCR) data after phase 2 (S24-7/total bacteria).
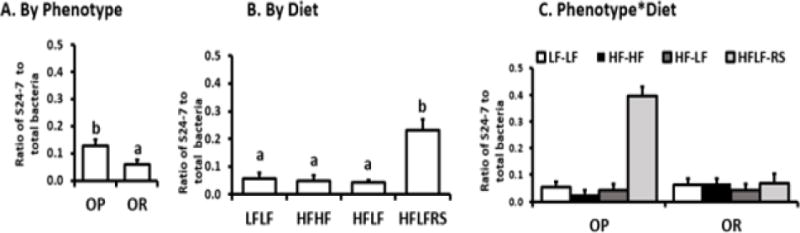
A. OP rats had higher abundance of S24–7 compared to OR rats (P<0.003) indicated by different letters. B. The HF-LFRS diet had increased S24-7 abundance compared to all other diets (P<0.0005). The letters a and b indicate a priori differences between OPHF-LFRS and OPLF-LF, OPHF-LF and ORHF-LFRS (P<0.0125) C. Interaction between diet and phenotype influenced S24-7 abundance (P<0.0001). There were a priori differences (P<0.0125) between OPHF-LFRS vs. OPLF-LF, ORHF-LFRS vs. OPLF-LF, and OPHF-LFRS vs. ORHF-LFRS. No difference was observed between ORHF-LFRS and ORHF-LF.
Discussion
Based on prior findings that RS fermentation reduces accretion of abdominal fat [5–9], we hypothesized OR rats would retain a lean phenotype with HF diet followed by LFRS diet compared to HFLF diets by a mechanism that involves fermentation of dietary components despite a presumably higher energy harvest with RS. Our results rejected this because OR rats exhibited modest fermentation of RS and OP rats robustly fermented RS.
The obesity trait in CD rats mimics characteristics of human obese phenotype, where not all individuals who consume increased calories develop obesity [11]; and allows delineating effects of microbiota composition on obesity phenotype and fermentation of RS. The finding that the OP rats gain greater weight and fat even on LF had not been shown before (Figure 3B, D). Previous studies have shown they gain more weight on HF diet [10,11]. In phase 2, diet components had no effect on body weight and had a minimal effect on fermentation in OR rats. In OP rats, RS elicited very high fermentation as demonstrated by fermentation variables measured (Figure 2 and Table 2). High fermentation presumably ensures more energy harvest and has been proposed to be one of the causes of obesity [1, 2]. Studies using germ-free and knockout mice have indicated that the extracted energy is stored in adipocytes through a pathway that involves microbial down-regulation of intestinal epithelial expression of fasting-induced adipocyte factor (Fiaf), a circulating inhibitor of lipoprotein lipase (LPL). Suppression of Fiaf increases LPL activity from adipocytes, and enhances storage of liver-derived triglycerides in fat cells [1]. In the current study, dramatically increased fermentation in OP rats did not result in higher energy storage because switching rats to a LF diet or a LF diet with RS did not lead to significant differences in weight and fat accretion in OP rats (Figure 3). The absence of robust fermentation in OR rats fed RS was unexpected, given that this type of rat strain is reported to remain insulin sensitive and maintains leaner phenotype when fed increased calories [10–11]. Neither OP nor OR rats responded to fermentation with decreased body fat accretion. Our past study observed increased fatty acid oxidation [5] and this may be the result of stimulation by increased SCFAs reaching liver via the portal blood [26] causing reduced accretion of abdominal fat in several rat models fed RS [5–9]. The results with OP and OR rats may be explained by their development from breeding of heavier Sprague Dawley rats (Charles River Company). However, it is surprising that both OR and OP models were developed. OR and OP rats may harvest enough energy from fermentation to balance any increase in fat oxidation and do not reduce fat accretion or have a reduced metabolic response to fermentation. It is also possible that feeding RS without prior feeding of HF, might have produced a different result.
Our study had different results than Belobrajdic et al. [13] possibly because we focused on the effects of fermentation with isocaloric diets for our comparisons of HF-LFRS groups (OP or OR) to HF-LF groups rather than combined effects of fermentation and dilution of energy density. With resistance to digestion, RS has a lower metabolizable energy than control starches [27]. Belobrajdic et al. [13] used RS to replace control starches, and RS diets in their study had lower amounts of energy. Secondly, our study used commercially established CD-OP and CD-OR rat strains, but Belobrajdic et al. [13] used Sprague Dawley rats. We discovered an OR rat model that is a low-fermenter of RS. Such a model could serve as a model for humans that are reported to be low-fermenters [28–30].
Amounts of total bacteria and the archaeon M. smithii determined by qPCR were not different between the HF and LF diets (phase 1). OR rats had significantly higher amounts of total bacteria than OP rats, but the amount of M. smithii was not significantly different. Including RS in the diet (phase 2) increased both total bacteria and M. smithii in OP rats, but not in OR rats (Figure 4). In absence of dietary RS, the role of methanogenic archaea and particularly M. smithii in the pathogenesis of obesity is not clear. Mathur et al. [15, 31] showed that methanogenic archaea contribute to altered metabolism and weight gain in the host. They observed higher proportions of M. smithii in humans with obesity compared to lean counterparts and genetically obese mice when compared to lean littermates. Gut colonization with M. smithii correlated with and predicted the degree of weight gain [15]. In contrast, our results showed that M. smithii levels were not affected by the obese phenotype but were affected by fermentation of RS. Furthermore, the increase in M. smithii levels was beneficial to the host as it appeared to contribute to enhanced fermentation with no extra weight gain or fat accretion. Mathur et al. [15] and Mathur and Barlow [31] did not elaborate on components of diet, particularly on amounts of fermentable fiber. The observed higher proportions of M. smithii in humans with obesity and genetically obese mice may have been errantly attributed to the obese phenotype rather than to greater amounts of fermentable fiber.
Another bacterial composition increase in OP rats in response to RS was in abundance of S24–7, an uncultured gram-negative family of order Bacteroidales (Figure 5). S24–7 is involved in host-microbe interactions that impact gut function and health and abundance is altered with different conditions [32] and in the current study in response to feeding of RS. Analysis of 16S rRNA gene databases from metagenomics by Ormerod et al. [32] showed that members of the family S24-7 are fermentative but have alternative modes of energy production as they encode elements of an electron transport chain. Like other families in Bacteroidales order, carbohydrate-active enzymes constitute about 6% of S24-7 coding sequences. Based on enzyme abundance, S24-7 encode glycoside hydrolases; largely ɑ-amylases suggesting starch as key substrate, with ability to ferment several other carbohydrate moieties [32].
We conclude that increases in M. smithii and S24-7 are markers of bacterial fermentation of RS because both rat phenotypes had similar amounts of these potential markers in phase 1 when no RS was fed. We previously observed increased S24-7 in obese ZDF rats that robustly fermented RS [19], and hypothesize we would have observed an increase in M. smithii if these were measured in other studies. Increases in S24-7 and M. smithii may be under a homeostatic mechanism of host dynamics that regulates the gut community responses to promote host fermentation. The robust fermentation in OP rats did not elicit reduced food intake and did not result in extra weight or fat accretion, which demonstrated that fermentation does not appear to be the cause of obesity in OP rats. OR rats may be a good model for humans reported to be low fermenters of RS and the cause of their low response to RS is unknown. It may be related to their microbiota and inability to increase M. smithii and S24-7 levels. In OP rats, host environment favored RS fermentation and M. smithii and S24-7 were then beneficial to the host for increased fermentation.
Supplementary Material
Study Importance Questions.
-
What is already known about this subject?
CD obese-prone rats gain more weight and fat than CD obese-resistant rats when fed a high-calorie diet.
-
What does your study add?
Robust fermentation of dietary resistant starch in CD obese-prone rats does not increase body weight and abdominal fat accretion.
CD obese-resistant rats poorly ferment dietary resistant starch and may serve as a model for humans that poorly ferment resistant starch.
Archaeon M. smithii amounts were similar in CD obese-prone and obese-resistant rats when not fed resistant starch, but increased with robust fermentation in CD obese-prone rats.
Acknowledgments
Research funded by Ingredion Incorporated and LSU AgCenter (NIFA 94268). Starches were gifts from Ingredion Incorporated. Research partially supported by NIH NORC Center Grant #P30DK072476 entitled “Nutritional Programming: Environmental and Molecular Interactions.”
Funding: Research was funded by Ingredion Incorporated and LSU AgCenter. The starches were gifts from Ingredion Incorporated. This research was also partially supported by a NIH NORC Center Grant #P30DK072476 entitled “Nutritional Programming: Environmental and Molecular Interactions.”
Footnotes
Disclosure: Michael J Keenan has received research funding and gifts of starch products from Ingredion Incorporated
References
- 1.Bäckhed F, Ding H, Wang T, et al. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci USA. 2004;101:15718–15723. doi: 10.1073/pnas.0407076101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ley RE, Backhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI. Obesity alters gut microbial ecology. Proc Natl Acad Sci USA. 2005;102:11070–11075. doi: 10.1073/pnas.0504978102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Turnbaugh PJ, Backhed F, Fulton L, et al. Diet-Induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome. Cell Host and Microbe. 2008;3:213–223. doi: 10.1016/j.chom.2008.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.David LA, Maurice CF, Gootenberg DB, Button JE. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505:559–563. doi: 10.1038/nature12820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou J, Martin RJ, Tulley RT, et al. Failure to ferment dietary resistant starch in specific mouse models of obesity results in no body fat loss. J Agric Food Chem. 2009;57:8844–8851. doi: 10.1021/jf901548e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Keenan MJ, Zhou J, McCutcheon KL, et al. Effects of resistant starch, a non-digestible fermentable fiber, on reducing body fat. Obesity. 2006;14:1523–34. doi: 10.1038/oby.2006.176. [DOI] [PubMed] [Google Scholar]
- 7.Charrier JA, Martin RJ, McCutcheon KL, et al. High fat diet partially attenuates fermentation responses in rats fed resistant starch from high-amylose maize. Obesity. 2013;21:2350–2355. doi: 10.1002/oby.20362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shen L, Keenan MJ, Raggio A, Williams C, Martin RJ. Dietary-resistant starch improves maternal glycemic control in Goto-Kakazaki rat. Mol Nutr Food Res. 2011;55:1499–1508. doi: 10.1002/mnfr.201000605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Keenan MJ, Janes M, Robert J, et al. Resistant starch from high amylose maize (HAM-RS2) reduces body fat and increases gut bacteria in ovariectomized (OVX) rats. Obesity. 2013;21:981–984. doi: 10.1002/oby.20109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levin BE, Dunn-Meynell AA, Balkan B, Keesey RE. Selective breeding for diet-induced obesity and resistance in Sprague-Dawley rats. Am J Physiol. 1997;273(2Pt2):R725–730. doi: 10.1152/ajpregu.1997.273.2.R725. [DOI] [PubMed] [Google Scholar]
- 11.Madsen AN, Hansen G, Paulsen SJ, et al. Long-term characterization of the diet induced obese and diet resistant rat model: a polygenetic rat model mimicking the human obesity syndrome. J of Endocr. 2010;206:287–296. doi: 10.1677/JOE-10-0004. [DOI] [PubMed] [Google Scholar]
- 12.de La Serre CB, Ellis CL, Lee J, Hartman AL, Rutledge JC, Raybould HE. Propensity to high-fat diet-induced obesity in rats is associated with changes in the gut microbiota and gut inflammation. Am J Physiol Gastrointest Liver Physiol. 2010;299:G440–G448. doi: 10.1152/ajpgi.00098.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Belobrajdic DB, King RA, Christopherson CT, Bird AR. Dietary resistant starch dose-dependently reduces adiposity in obesity-prone and obesity-resistant male rats. Nutr Metab. 2012;9:93. doi: 10.1186/1743-7075-9-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Samuel BS, Gordon JI. A humanized gnotobiotic mouse model of host-archaeal-bacterial mutualism. Proc Natl Acad Sci USA. 2006;103:10011–10016. doi: 10.1073/pnas.0602187103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mathur R, Kim G, Morales W, et al. Intestinal Methanobrevibacter smithii but not total bacteria is related to diet-induced weight gain in rats. Obesity. 2013;21:748–754. doi: 10.1002/oby.20277. [DOI] [PubMed] [Google Scholar]
- 16.Desai MS, Seekatz AM, Koropatkin MM, et al. A dietary fiber-deprived gut microbiota degrades the colonic mucus barrier and enhances pathogen susceptibility. Cell. 167:1339–1353. doi: 10.1016/j.cell.2016.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cacho J, Sevillano J, de Castro J, et al. Validation of simple indexes to assess insulin sensitivity during pregnancy in Wistar and Sprague-Dawley rats. Am J Phyiol Endrocrinol Metab. 2008;295:E1269–E1276. doi: 10.1152/ajpendo.90207.2008. [DOI] [PubMed] [Google Scholar]
- 18.Vidrine K, Ye J, Martin RJ, et al. Resistant starch from high amylose maize (HAM-RS2) and dietary butyrate reduce abdominal fat by a different apparent mechanism. Obesity. 2014;22:344–348. doi: 10.1002/oby.20501. [DOI] [PubMed] [Google Scholar]
- 19.Goldsmith F, Guice J, Page R, et al. Obese ZDF rats fermented resistant starch with effects on gut microbiota but no reduction in abdominal fat. Mol Nutr Food Res. 2017;61:1–9. doi: 10.1002/mnfr.201501025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dridi B, Henry M, El Khéchine A, Raoult D, Drancourt M. High prevalence of Methanobrevibacter smithii and Methanosphaera stadtmanae detected in the human gut using an improved DNA detection protocol. PLoS ONE. 2009;4:e7063. doi: 10.1371/journal.pone.0007063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Belenguer A, Duncan SH, Calder AG, et al. Two Routes of Metabolic Cross-Feeding between Bifidobacterium adolescentis and Butyrate-Producing Anaerobes from the Human Gut. Appl and Environ Microbiol. 2006;72:3593–3599. doi: 10.1128/AEM.72.5.3593-3599.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oldham AL, Duncan KE. Similar gene estimates from circular and linear standards in quantitative PCR analyses using the prokaryotic 16S rRNA Gene as a model. PLoS one. 7:e-51931. doi: 10.1371/journal.pone.0051931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kozich JJ, Westcott SL, Baxter NT, et al. Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the MiSeq Illumina sequencing platform. Appl Environ Microbiol. 2013;79:5112–5120. doi: 10.1128/AEM.01043-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Edgar RC. UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat Methods. 2013;10:996–998. doi: 10.1038/nmeth.2604. [DOI] [PubMed] [Google Scholar]
- 25.Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J Royal Stat Soc Series B (methodological) 1995;57:289–300. [Google Scholar]
- 26.Topping DL, Clifton PM. Short chain fatty acids and human colonic function: roles of resistant starch and non-starch polysaccharides. Physio Rev. 2001;81:1031–1064. doi: 10.1152/physrev.2001.81.3.1031. [DOI] [PubMed] [Google Scholar]
- 27.Tulley RT, Appel MJ, Enos TG, et al. Comparative methodologies for measuring metabolizable energy of various types of resistant high amylose corn starch. J Agric Food Chem. 2009;57:8474–8479. doi: 10.1021/jf900971c. [DOI] [PubMed] [Google Scholar]
- 28.Kovatcheva-Datchary P, Nilsson A, Akrami R, et al. Dietary fiber-induced improvement in glucose metabolism is associated with increased abundance of Prevotella. Cell Metab. 2015;22:971–982. doi: 10.1016/j.cmet.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 29.Venkataraman A, Sieber JR, Schmidt AW, Waldron C, Theis KR, Schmidt TM. Variable responses of human microbiomes to dietary supplementation with resistant starch. Microbiome. 2016;4:33. doi: 10.1186/s40168-016-0178-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martinez I, Kim J, Duffy PR, Schlegel VL, Walter J. Resistant starches types 2 and 4 have differential effects on the composition of the fecal microbiota in human subjects. PLoS one. 2010;5:e15046. doi: 10.1371/journal.pone.0015046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mathur R, Barlow GM. Obesity and the microbiome. Expert Rev Gastroenterol Hepatol. 2015;9(8):1087–99. doi: 10.1586/17474124.2015.1051029. [DOI] [PubMed] [Google Scholar]
- 32.Ormerod KL, Wood DL, Lachner N, Gellatly SL, et al. Genomic characterization of the uncultured Bacteroidales family S24-7 inhabiting the guts of homeothermic animals. Microbiome. 2016;4:36. doi: 10.1186/s40168-016-0181-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


