Abstract
The brain does not retain all the information it encodes in a day. Much is forgotten, and of those memories retained, their subsequent “evolution” can follow any of a number of pathways. Emerging data makes clear that sleep is a compelling candidate for performing many of these operations. But how does the sleeping brain know which information to preserve and which to forget? What should sleep do with that information it chooses to keep? For information that is retained, sleep can integrate it into existing memory networks, look for common patterns and distill overarching rules, or simply stabilize and strengthen the memory exactly as it was learned. We suggest such “memory triage” lies at the heart of a sleep-dependent memory processing system that selects new information, in a discriminatory manner, and assimilates it into the brain’s vast armamentarium of evolving knowledge, helping guide each organism through its own, unique life.
In this perspective piece, we outline evidence supporting the concept of evolving knowledge through a process of “memory triage” that first identifies which memories should go through sleep-dependent memory processing, and then determines the form that this processing should take. The model adds to earlier conceptions of sleep-dependent consolidation in several ways. First, it shifts the description away from one of uniform memory consolidation toward a complex offline process that is remarkably selective in terms of which memories are consolidated by sleep, leading to the discriminatory incorporation of initially pluripotent memories. Second, it describes a further stage in this triage process, which directs a given memory through one of several pathways of memory evolution. Such evolution leads to multiple forms of integrated knowledge, potentially governed by a range of neural systems, and facilitated by varied stages of sleep. Finally, it favors a view of consolidation not as an end-goal, but as a stepping-stone along a path that leads to the building and updating of generalized knowledge and beliefs about the world in which we live and act.
We start from a position that assumes the existence of sleep-dependent memory processing. Not withstanding the limitations of some experimental paradigms that require careful controls (e.g. for time of day or non-specific effects of sleep deprivation), when taken as a whole, evidence to date from (i) nap studies (that match circadian time concerns), (ii) sleep deprivation studies (with delayed retests), (iii) studies finding correlations of offline memory improvement with sleep-stages and associated sleep physiology (e.g., sleep spindles and slow wave activity), and with regional brain activity measured during and after sleep with PET and fMRI, (iv) studies using direct current brain stimulation to modify sleep physiology and memory, and (v) studies of cellular firing patterns in rodents, along with (vi) synaptic and intracellular measures of plasticity across a range of phylogeny, together offer, in our opinion, incontrovertible converging evidence for the existence of sleep-dependent memory processing1-5.
In using the term “sleep-dependent memory processing”, we are not implying that all offline memory processing is sleep-dependent, rather that there are such processes that occur only during sleep. For example, some forms of procedural motor skill learning6-8 and of more complex rule extraction9 can develop in the absence of sleep. Yet these forms of learning also show additional processing in sleep7, 9, 10 that does not seem to occur in waking. In this perspective piece, we focus specifically on memory processing that occurs during sleep, and paradigms for which, in most cases, there is evidence of sleep-dependent physiological correlates of improvement. This evidence argues against models of both passive protection from interference and ideas of “opportunistic” consolidation11, the latter proposed to occur when memory-specific neural structures are not encoding new information. Instead, such data favors the existence of proactive and sleep-dependent memory processing, rather than passive, brain-state independent memory processing. This is not to suggest that consolidation cannot occur during the wake state, as described above. Moreover, as with consolidation during wake, we are not arguing that passive protection and opportunistic consolidation cannot also occur during sleep.
Sleep-dependent consolidation: A process of discriminatory selection
The concept of memory “consolidation” dates back to 1900, when Müller and Pilzecker12 first proposed that item-memories were not encoded in a permanent form, but rather required a process of “Konsolidierung” (consolidation) that occurred over time. Jenkins and Dallenbach13 subsequently discovered the superior preservation of item-memories across a night of sleep relative to equivalent time awake across the day, ultimately leading in the 1970s to the notion of an active process of consolidation during sleep14. This notion of sleep-dependent stabilization of declarative memory is now established15, and has been extended to include the offline enhancement (beyond preservation) of procedural memories, such as visual and motor skill learning16 (and see17). However, a newly emerging concept in sleep-dependent memory processing is now becoming clear: that of selectivity18, 19.
Counter to earlier assumptions, memory consolidation during sleep does not lead to the uniform preservation of all recently encoded memories. Instead, emerging evidence favors a more discerning form of sleep-dependent memory processing; one that preferentially determines what information is (and is not) ultimately retained (Fig. 1A), as well as the form in which it is retained (Fig. 2). Moreover, this initial selection appears to be governed by salience tags attached to memories during or shortly after encoding and subsequently utilized during sleep (Fig. 1A &1B). We suggest that this selective gating of relevant and irrelevant memories is a fundamental necessity, accomplishing discriminatory and arguably optimal retention and forgetting. This selective information gating provides the organism with the ability to adapt to environmental change rapidly and effectively, guided by the most relevant information from its own autobiographical history, which has been optimally integrated into memory networks by sleep-dependent processes discussed in the second half of this paper.
Figure 1. Selective memory consolidation.
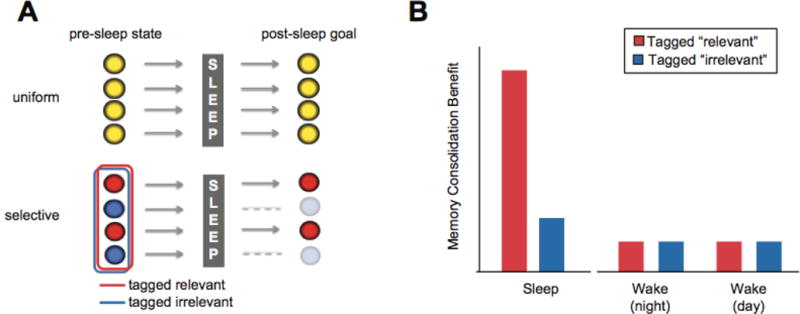
(A) Conceptual difference between uniform consolidation (top row), and selective consolidation (bottom row). In the latter, sleep returns discriminative offline memory retention, the selection of which is governed by instructional cues of relevance (red) and non-relevance (blue) created in the peri-encoding wake period. (B) Conceptual outcome of selective consolidation following sleep and an equivalent time wake (across the night or day) following differential tagged relevance at initial encoding.
Figure 2. Forms of memory evolution.
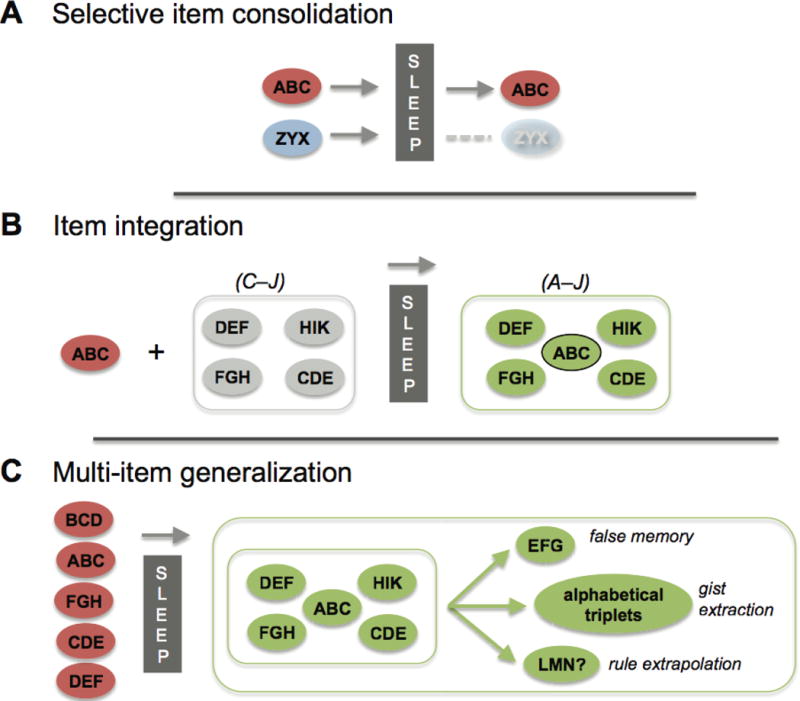
Categories of offline memory processing. All of these forms of offline memory processing have been shown to occur preferentially during sleep. (a) Item consolidation. Individual item-memories can be stabilized and/or enhanced, or they can be forgotten. (b) Item integration. Individual new item memories can be integrated into existing associative memory networks, extending the range of the network and enriching the information associated with the new item memory. (c) Multi-item generalization. Related item-memories encoded over a brief time interval can generate a new memory network and conceptual schema.
One example of such discriminatory processing is the selective consolidation of affective experiences. There is evidence that emotional memories can undergo preferential preservation during sleep, and especially during REM sleep20, 21,22, 23 (but also see24, 25). Furthermore, even individual emotional elements from an affective experience can be selectively retained. By experimentally varying the foreground and background elements of photographs, sleep has been shown to target the retention of emotional foreground objects within scenes, relative to either non-emotional foreground items or the peripheral background elements of the same scenes26. Thus, sleep (and not wake26) can separate affectively relevant from irrelevant components of a single episodic memory for selective consolidation.
Beyond emotional memories, sleep can selectively retain memories based on waking knowledge of potential monetary reward27, even when such knowledge is only provided after learning. Moreover, merely being told of a future test after encoding new information enhances sleep-dependent consolidation of that material19. This effect can be observed in tests of episodic item-memory, spatial memory and procedural skill memory19, all resulting in differential and selective post-sleep memory recall (conceptualized in Fig. 1B). All of these scenarios potentially involve the retrospective salience tagging of previously encoded memory representations. In a similar investigation, participants were informed after encoding that they would later be tested on only one of two sets of items studied (even though all studied information was ultimately tested)28. Following a period of sleep but not after an equivalent period of awake, subjects showed superior retention of those items previously designated for future testing, once again demonstrating selective sleep-dependent memory consolidation.
Explicit instructions to either “remember” or “forget” individual items immediately after their encoding can significantly modulate the course of their consolidation during sleep. Such studies further suggest that sleep can specifically, and perhaps actively, control forgetting as well as remembering. A recent study examined offline influences of wake and sleep using a directed forgetting paradigm in which presentation of each item was followed by a cue to either “remember” or “forget” that item29. Such explicit item-memory manipulation has previously been used to establish differential remembering and forgetting when tested immediately after encoding30. However, when recall is measured following longer delays, significant differences emerge based on the intervening brain-state. Sleep produced significant enhancement of memories for words cued to be remembered, but not for others cued for forgetting. The difference in recall between words cued to be remembered and those cued for forgetting was significantly greater after sleep than following an equivalent time awake. Importantly, there were no differences between the wake and sleep groups in immediate recall of either words cued for remembering or words cued for forgetting, indicating that the strength of encoding for each class of words was equivalent between conditions. Only after offline processing, during sleep or wake, did differences in selective remembering and forgetting develop between groups. Curiously, being instructed to either think about or to suppress thoughts of individual items after their initial encoding did not result in differential offline consolidation across sleep relative to wake31. Thus, it was the specific awareness of future relevance (in the form of a future test) that appears to have led to this sleep-dependent discriminatory processing.
The effectiveness of explicit waking instructional cues is time-sensitive, requiring the occurrence of sleep within 24hr of encoding. Participants not allowed to sleep the night after item learning, and tested after two subsequent nights of recovery sleep, showed reduced differences in recall between to-be-remembered and to-be-forgotten items, with more non-specific memory retention32. These findings not only establish that explicit waking tags utilized for sleep-dependent processing degrade over 24 hr, but that they do so faster than the item-memories themselves32, suggesting that the tags may be distinct from the memories themselves.
The precise neural mechanisms that initially create waking instructional tags, as well as the mechanisms controlling selective “gating” of consolidation during sleep, remain largely uncharacterized, and represent a unique opportunity to provide unifying insights into the reciprocity between wake-dependent learning and sleep-dependent consolidation. Neural mechanisms of tagging that support the conversion of early-phase LTP (long-term potentiation) into late-phase LTP have been identified33, but their relevance to sleep-related tagging is unknown. Nevertheless, early evidence offers some clues. The degree of hippocampal activity measured during initial encoding of items cued for remembering and forgetting accurately predicts the subsequent magnitude of differential offline sleep-dependent consolidation32. This hippocampal signal during wake may therefore reflect the tagging of items for subsequent consideration by sleep. This is consistent with evidence of initial wake-dependent hippocampal replay or persistent encoding-like (re)activity seen shortly after learning in both humans and rodents (e.g.,34, 35), which may also provide a neural tag that can be utilized during subsequent sleep. Future work will be required to determine whether such tags can be produced in diverse anatomical networks (e.g., the amygdala for selective emotional memory consolidation20, 22, 36-38, and the dopaminergic brainstem and/or striatum for reward cued memory39), or whether a single mechanism orchestrates tagging across memory systems.
It is similarly unknown whether these same tags determine the form that memory processing takes during sleep, or whether the form of processing that occurs is determined by events during sleep, itself. Finally, it remains unclear whether tagging is absolutely required for subsequent sleep-dependent consolidation to occur, or whether some forms of consolidation (e.g., stabilization without enhancement) can occur in the absence of any tagging.
Mechanistic insights into the role of physiological oscillations during sleep in governing differential consolidation of tagged memories are also beginning to emerge. The selective sleep benefit for recall of items cued-to-be-remembered over items cued-to-be-forgotten is predicted by fast sleep spindles (13.5–15.0 Hz) over left parietal cortex29. Importantly, subjects with more spindles not only recalled more items previously cued for remembering, but also recalled fewer items cued for forgetting. Such findings do not appear to fit a simple decay theory, in which items tagged as irrelevant or non-salient simply decay passively over time. Instead, it suggests sleep-dependent mechanisms that not only actively promote remembering, but also actively lead to forgetting. In addition EEG source analysis of these data revealed, during these spindles, a loop of recurring activity in a network of brain regions previously implicated in differential remembering and forgetting40 – medial temporal cortex, prefrontal cortex and posterior parietal cortex. Such a network has been proposed to enact differential memory consolidation29, with “top-down” cues of instructed intent (prefrontal cortex: remember, forget) utilized during the offline processing of “bottom-up” item-memories (medial temporal lobe: individual item-memories), leading to their differential consolidation and integration within association cortices (parietal cortex)41. Similar sleep-oscillation relationships have been reported for other forms of selective consolidation. In one study described above19, participants expecting a future memory test showed enhanced overnight consolidation. But they also showed correlations between task performance and either slow wave activity (SWA) or sleep spindles, depending on the task. Moreover, post-encoding SWA and sleep spindles correlations with overnight memory retention were only observed in those participants that received the test-expectation cue before sleep, and not in those who did not expect the future memory test.
While a full behavioral and neurophysiological characterization of selective sleep-dependent memory processing remains to be established, mounting evidence encourages a revised account of overnight consolidation, one in which sleep is ecologically guided by qualitative features evoked or presented in the peri-encoding period (e.g., emotionality, reward potential motivation, or explicit cue instructions and intentions). The result is selective offline memory consolidation, potentially mediated by specific sleep oscillations. We now turn to how the sleeping brain further processes these chosen memories.
Memory evolution: Selecting the right path
Consolidation, including discriminatory consolidation (Fig. 2A), and which stabilizes and enhances memories, is just one possible form of offline information processing that occurs during sleep. Others, processes of sleep-dependent memory integration, can additionally generate new knowledge, beyond that found in individual item-memories. Whether consolidation necessarily precedes these integrative processes (serial processing) or whether they can occur independently (asynchronous processing) is not yet known, but no clear cases of integration without consolidation have been observed to date. We use the term “memory evolution” to reflect both the qualitative changes that can occur during such integrative processing as well as the extended time course over which they occur42. Two overarching categories encompass the majority of these memory evolution processes: Item Integration and Multi-Item Generalization.
Item Integration assimilates newly learned memory representations into preexisting schemas43 (Fig. 2B). The general characteristics of the schema are expanded when a new item is absorbed, increasing the schema’s potential utility and applicability, while the meaning attributed to the new item is enhanced as well. In Fig. 2B, a new item-memory, “ABC” is added to an existing network, expanding the knowledge in the network, while providing a valuable informational context for the new item “ABC”.
Multi-item Generalization combines multiple new item-memories, creating an entirely new schema (Fig. 2C). In doing so, it can identify shared statistical regularities, thereby extracting the gist from the set of experiences (e.g., the knowledge that “ABC is one of many alphabetical triplets”). But as we describe below, it can also lead to a false memory (“I saw EFG”). Multi-item generalization can also promote identification of a rule or set of rules governing the information set and, as a result, can allow extrapolation of never before seen items (“LMN fits the same pattern”).
These categories of memory evolution are not unique to sleep. Piaget’s work on schema development in children43 and, more recently, work from the laboratories of Morris44, Fernandez45, and McGaugh46, provide important frameworks for our model. Interestingly, while these more recent works sometimes note the possible role of sleep in schema development44, 45, they do not pursue the possibility further.
Below, we review evidence that sleep contributes to each of these forms of memory evolution, doing so in a manner superior to wake.
Item integration
One example of sleep-dependent integration of new memories (Fig. 2B) is the incorporation of novel words into one’s pre-existing mental lexicon. In a pair of studies, subjects learned 30 invented novel words (e.g., CATHEDRUKE). When tested immediately after learning, they showed no evidence that these novel words had been effectively integrated into subjects’ mental lexicons. In the first study, integration was observed only after a night of sleep, and not after an equivalent period of daytime wake47. Moreover, in a second, sleep laboratory study48, the number of sleep spindles during the post-training night predicted the degree of lexical integration the next morning, although, in this study, integration was also observed across periods of wake, suggesting that it was not absolutely dependent on sleep.
Sleep-dependent item integration is also seen with the Remote Associates Task, in which subjects are presented with word triplets (e.g., HEART, SIXTEEN, COOKIES), and must identify the word associatively linked with all three (SWEET). Subjects retested on triplets that they initially failed to solve were more successful following a period of sleep than after an equivalent period of wake49. Moreover, participants in the sleep group who obtained REM sleep demonstrated significantly more benefit than those who did not49. Sleep therefore integrated new memories into existing networks, producing new associations and relationships, reflected in superior task performance.
Multi-item generalization
Sleep-dependent memory evolution can also combine sets of new item-memories to form novel schemas, producing new knowledge (Fig. 2C). Here we give examples of various forms that such new knowledge can take.
Gist extraction
Sleep can combine information from a collection of new items to identify commonalities, even while individual item-memories are forgotten. In one example, subjects learn to find the exit from a virtual three-dimensional maze, starting from a variety of widely separated locations. Following sleep, relative to an equivalent time period awake, subjects demonstrated an improved understanding of the overall layout of the maze, reaching the exit more rapidly, and in fewer steps (Fig. 3A)50, 51. In another example using a “false memory” task52, subjects were exposed to lists of related words, but not to the common linking “gist” words (e.g., BED, REST, AWAKE, TIRED, DREAM, etc., but not “SLEEP”). After a night of sleep, or even a daytime nap, subjects were more likely to falsely remember encountering the common link words (e.g., “SLEEP”) than after an equivalent time awake38. While recall of studied words decreased across wake and sleep, as did false recall of these “gist” words across wake, memory for the gist words was undiminished across sleep (Fig. 3B). In this case, multi-item gist extraction involved referencing existing networks to identify gist words. But the extraction of the gist word SLEEP could not have been done based on individual items, such as REST or AWAKE, instead requiring multi-item generalization.
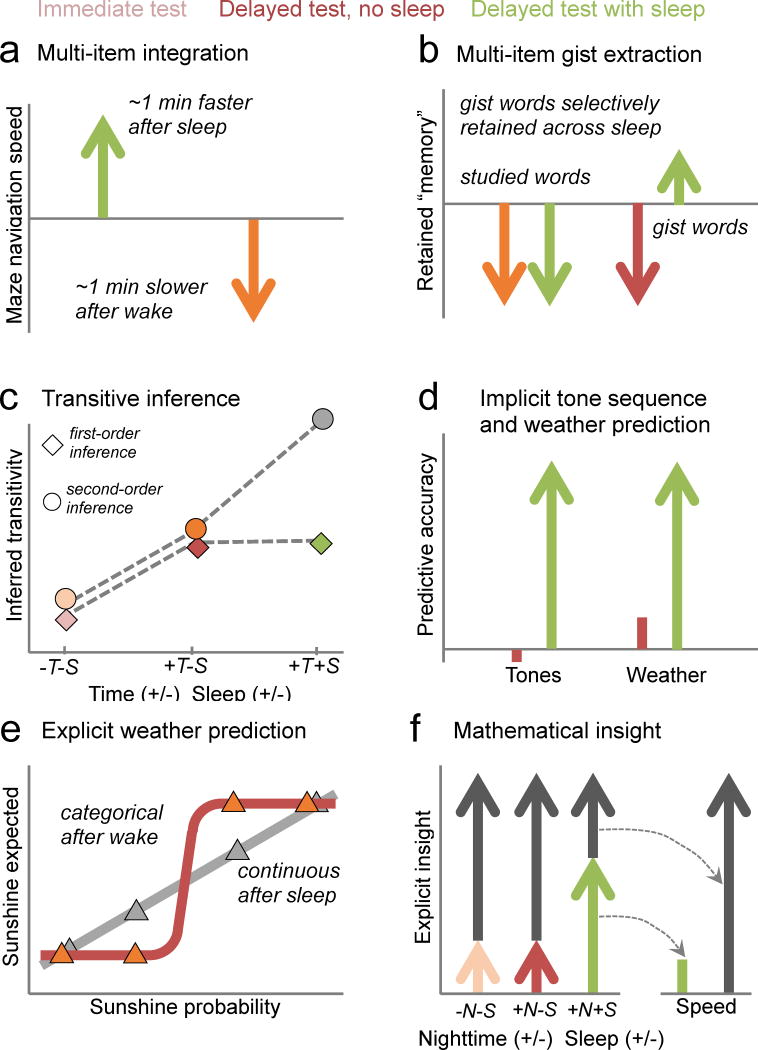
Figure 3. Examples of memory evolution.
The integration of memory is often enhanced by sleep (green) compared to equivalent periods of wake (red): (A) Spatial learning: Exploration of a virtual maze produces complex episodic memories of the experience. Sleep facilitates the extraction of a generalized spatial map of the maze, resulting in enhanced maze navigation speed, while an equivalent time spent awake leads to degraded maze navigation (from51). (B) False memories: Extraction of the gist of a set of recent item-memories leads to the false belief that the gist was part of the original memory set. Sleep shows both of these consequences of gist extraction, including preservation of the gist memory while actual studied items are forgotten, and while memory for both item-memories and gist decrease across wake (from38). (C) Transitive inference: Transitive inference was absent 20min after training (−T−S), but was seen after 12hr of wake (+T−S). After 12hr including a night of sleep (+T+S), performance on second-order inferences was significantly further enhanced (from9). (D) Probabilistic Learning: Statistical sequence learning (left) was enhanced after a 12hr period containing a night of sleep, but not after equivalent periods without sleep (orange bar; from59). Similarly, probabilistic category learning, studied in the weather prediction task (right), showed significant improvement following a night of sleep, and significantly more than after an equal period of daytime wake, when no significant improvement was seen. (E) Following wake, subjects rated the probabilities of four card stimuli predicting sunshine into pairs of high and low probabilities (red triangles), while following sleep, they more accurately described the cards’ individually varying probabilities (green triangles) (from62). (F) Mathematical insight: LEFT – Subjects trained on a rote mathematical task were significantly more likely to discover a shortcut during retesting after a night of sleep (+N+S), as compared to after equivalent periods of wake across the day (−N−S) or night (+N−S). RIGHT – Those who failed to gain this insight instead showed significant improvement in the speed with which they performed the rote procedure (from63).
Other studies, using the same false memory task, reveal an even richer and more complex process of gist extraction. Diekelmann et al53 also found enhanced gist recall after sleep, although only in the more poorly performing half of subjects. In contrast, Fenn et al54, measuring gist memory with word recognition rather than word recall, found no sleep benefit. Perhaps explaining these differences, Darsaud et al55, used a recognition test that additionally distinguished between memory recognition based on recollection of actually seeing a word, as opposed to simply having a sense of familiarity. They found a sleep-dependent benefit for gist words based on recollection memory, while exhibiting reduced memory based on familiarity. When recognition based on recollection and on familiarity were summed no sleep-wake difference was seen. Thus, sleep does appear to benefit gist memory in this paradigm, but not when the measure of memory includes recognition based on familiarity. While still preliminary, these results, taken as a whole, suggest that sleep may benefit gist extraction from relationships in particular memory networks, considering the potentially different contributions of medial temporal lobe subdivisions to familiarity and recollection56.
Rule extrapolation
Several reports have demonstrated that sleep can promote the extraction of overarching rules that govern recently studied sets of information. In each case, implicit knowledge of patterns and rules was gained during sleep, and led to improved performance upon awakening, often without explicit awareness. The distinction between gist extraction and rule extrapolation may seem arbitrary. But it is, in fact, reflective of the foundational distinction between sets and relations described by Whitehead and Russell57 – while gist is extracted from sets, rules are extracted from relations, such as those seen in the four examples below.
(i) Artificial grammars
The ability of sleep to support rule extraction can be seen in children as early as 15 months. In a pair of studies, 15-month old infants were exposed to an artificial grammar in which the first syllable of a four-syllable nonsense word (e.g., “PEL”) predicted the last syllable (e.g., PELwadimRUD and PELchilaRUD; but VOTwadimJIC and VOTchilaJIC). When tested four hours later, infants who had napped before testing displayed knowledge of this grammar, while infants who happened not to nap did not13. Grammatical knowledge was seen in infants tested 24hr after training, but again only if they napped within 4 hours of training14. These studies demonstrate that early-life rule learning can be sleep-dependent. This may explain not only the high demand for sleep during formative developmental years, when cognitive schemas are constantly being built, but the demand for frequent sleep (i.e., the canonical polyphasic sleep of infants) as well43.
(ii) Transitive inference
The transitive inference paradigm57, 58 reflects the building of inferential relationships based on individual item premises. For example, imagine that the symbol > means “choose over”, so that A>B means “choose A over B”, and B>C means “choose B over C”. The transitive inference from these two premises is A>C, “choose A over C”. Both humans and rodents routinely make such inferences57, 58, although normally only after a post-training delay. In one human study, subjects were taught five premise pairs (A>B, B>C, C>D, D>E, E>F), which embedded the implicit hierarchy A>B>C>D>E>F. Knowledge of this hierarchy was demonstrated when subjects made first order inferences (B>D and C>E), as well as the more distant, second order inference (B>E), none of which were presented during training. When tested just 20 min after training, subjects showed no evidence of having extracted any inferences (Fig. 3C). But after 12hr of daytime wake subjects displayed moderate knowledge of both first and second order inferences (70-75%; Fig. 3C). Yet, after 12hr (or 24hr) including a night of sleep, subjects developed markedly superior second order inference ability (94%; Fig. 3C). Thus, the sleeping brain extracted the second order inference more effectively than the wake brain, and did so selectively; performance on the first order inference was similar to after 12 hr of wake (Fig. 3C). Thus, sleep facilitated the inferring of transitivity rules, potentially those of greatest associative distance, and enhanced subsequent decision-making.
(iii) Probabilistic learning
Two very different studies of probabilistic learning have shown that sleep enhances this form of multi-item generalization. In the first, subjects listened to five tones played in a probabilistically determined sequence of 1,800 tones, and were then tested on their ability to identify short, 18-tone sequences similar to this pattern. In two separate experiments (Fig. 3D) subjects who slept between training and testing showed better test performance than others who remained awake59. The second study used a “weather prediction” task”60 in which subjects were shown one, two, or three of four cards with geometrical patterns, along with the “outcome”, either sunshine or rain. Subjects were told they would be tested on their ability to predict this weather outcome based on similar presentations of cards alone. Unbeknownst to the subjects, each card had a specific probability of predicting sunshine (26%, 42%, 58%, and 76%;61). After 200 training trials, subjects were immediately given 100 test trials and, 12 hr later, were given the same 100 test trials a second time. No feedback was given during these tests. At immediate testing subjects demonstrated significant knowledge of the probabilistic structure of the task, on average scoring 76 of the 100 trials optimally (p=10−6). Twelve hours later, only the overnight sleep group showed significant improvement (Fig. 3D)62. In this case, the greater improvement in the sleep group was accompanied by more accurate knowledge – both explicit and implicit – of the graded probabilities across the four cards (Fig. 3E)62.
(iv) Insight
Sleep-dependent processing can also lead to explicit declarative awareness of rules and associations. Sleep has been shown to prime the brain for explicit discovery both of a shortcut for a mathematical task63 (Fig 3F) and, in the Remote Associates Task described earlier64, of words linking triplets of otherwise unrelated words49. In the mathematical number reduction task, subjects were taught a rote method for solving a class of mathematical problems for which there was also a shortcut, the existence of which subjects were not told. Following a night of post-training sleep, subjects were 2.6 times more likely to discover this short cut than after an equal period of time awake (59.1 vs. 22.7% of subjects)63, a topic we will return to in the next section.
Selective memory evolution
The examples above demonstrate the rich variety of forms that sleep-dependent memory evolution can take. However, a more detailed examination makes clear that these chosen paths of integration are applied neither universally nor uniformly. Just like with selective item-memory consolidation described earlier, there appears to be evidence of a discriminatory selection of integration processes. For example, while sleep can integrate word triplets into preexisting associative networks49, and newly invented words into one’s mental lexicon48, it can also generalize lists of words, creating a gist representation of each list, along with a false memory from that representation38. In all cases, the selection of one form of integrative processing over another is the one that optimizes the behavior required for that task. Thus, when subjects heard novel words, such as CATHEDRUKE, the sleeping brain integrated the words into its existing lexicon47, 48, rather than, for example, just forming a new schema of novel words. Conversely, when learning to use sets of cards with weather outcomes, the sleeping brain enhanced generalized rules of card-weather correlations62, rather than, for example, just integrating the novel cards into existing networks of playing cards, or trying to integrate the card–weather correlations into pre-existing schemas for real-word weather prediction.
Interestingly, in circumstances where no one form of integrative processing is clearly preferable, a selection from multiple possibilities appears to be made during sleep, as in the case of insight discovery in the number reduction task, described above. Although a night of sleep led to subsequent insight into the shortcut in 59% of participants (Fig 3F), 41% of sleep subjects failed to discover the insight. However, this 41% still benefited from sleep, just in a different way. Instead, and unlike the 59% of insight discoverers, these subjects improved the speed with which they performed the original rote method of solving the problems, improving it three fold more than either those who discovered the insight or those who remained awake (Fig 3F)63. In the absence of explicit knowledge that the is a short cut, it becomes unclear which form of processing was more optimal or “intelligent”. Under these conditions, sleep appeared to either prime subjects for the subsequent discovery of an insight, or to simply enhance their ability to use the rote algorithm practiced during the training session, with an approximate 60:40 split among subjects.
Mechanisms of Memory Evolution
As with the selection of memories for item-consolidation, the physiological sleep mechanisms regulating associative memory evolution are unclear, and represent another important knowledge gap in the field. Different sleep stages clearly play different roles in these varied memory evolution processes. However, the limited sleep-physiology studies performed to date do not yet allow us to predict sleep-stage dependencies based on any proposed categorizations of integration, including our own classification scheme described above (Fig. 2). For item integration, REM sleep has been implicated in promoting the discovery of associative solutions on the Remote Associates Task49. Congruent with this form of associative inter-item memory processing, solving anagrams10 and identifying weak (relative to strong) semantic priming65 is consistently superior when subjects are woken and tested from REM sleep than when woken from NREM sleep. Such relationships are also compatible with biologically informed computational models of hippocampal-neocortical functioning that identify REM sleep as a state favorable for associative learning66. However, the integration of novel words into an existing mental lexicon, also a form of item integration, is correlated with sleep spindles, an oscillation of NREM sleep48. Similarly, examples of multi-item integration, such as the Tower of Hanoi task67 and the categorical probabilistic learning task62, have demonstrated relationships with REM sleep, while probabilistic tone sequence learning demonstrates an association with NREM slow wave sleep59.
Another factor that may determine integrative process selection is the extent and nature of simultaneous memory reactivation during sleep, which has been shown to occur not only in NREM sleep (e.g.,68, 69), but also in REM sleep70. When reactivation of a recently formed memory is accompanied by the parallel activation of a larger set of recently formed memories, multi-item integration may occur; when a new single-item memory is reactivated in conjunction with an entire or even select components of an existing associative memory network, item integration may occur; and if neither occurs, then sleep-dependent processing might be limited to the comparatively straightforward consolidation and enhancement of the recently encoded item-memory itself. Clearly the questions of selective memory reactivation, sleep-stage dependencies, possible pre-sleep tagging, and underlying neural mechanism(s) of memory evolution remain important unresolved issues of research in this area.
Summary
The encoding of a memory is just the first step in a long and complex process of memory evolution. Such processing is neither universal nor uniform, but appears to be preferentially, and in many cases exclusively, dependent on sleep. This apparently intelligent sleep-dependent memory triage promotes the offline discriminatory selection of which item-memories to retain, and which to forget, based on prior waking salience tags. It can also lead to memory integration, creating de novo knowledge beyond that available from individual item-memories. This can include the integration of item-memories into already existing memory networks, enriching both the network and the new item-memory, or the generalization of multiple new item-memories, extracting common rules, gist, novel schemas or even singular insights. The underlying neurophysiological mechanisms governing these memory triage processes are beginning to be uncovered, but more work is needed. This will require not only the matching of different forms of memory processing with different stages or neurophysiological phenomena during sleep, but embracing the importance of the repeating cycles of sleep stages across the night71, 72, or even the alteration of wake and sleep across multiple days73, 74. These features, which have been mostly ignored to date, may be important determinants of memory evolution, as sleep-dependent memory processing is unlikely to be complete after just a single night. What is clear, however, is that a true understanding of how learning optimally governs the rich collection of unique behavioral repertoires of any individual organism75, 76 will require an appreciation of the equally rich collection of mechanisms occurring during sleep-dependent memory processing.
Acknowledgments
Support provided by NIH grants MH48832 (RS) and AG031164, MH093537, DA031939, (MPW). We thank Edwin Robertson, Jon Chamberlain, Matthew Tucker, Erin Wamsley, Jared Saletin and Ingrid Nieuwenhuis for helpful discussions on these concepts, and comments on this manuscript.
References
- 1.Stickgold R. Sleep-dependent memory consolidation. Nature. 2005;437:1272–1278. doi: 10.1038/nature04286. [DOI] [PubMed] [Google Scholar]
- 2.Diekelmann S, Born J. The memory function of sleep. Nat Rev Neurosci. 2010;11:114–126. doi: 10.1038/nrn2762. [DOI] [PubMed] [Google Scholar]
- 3.Maquet P. The role of sleep in learning and memory. Science. 2001;294:1048–1052. doi: 10.1126/science.1062856. [DOI] [PubMed] [Google Scholar]
- 4.Walker MP, Stickgold R. Sleep-dependent learning and memory consolidation. Neuron. 2004;44:121–133. doi: 10.1016/j.neuron.2004.08.031. [DOI] [PubMed] [Google Scholar]
- 5.Wang G, Grone B, Colas D, Appelbaum L, Mourrain P. Synaptic plasticity in sleep: learning, homeostasis and disease. Trends Neurosci. 2011;34:452–463. doi: 10.1016/j.tins.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Born J, Wagner U. Awareness in memory: being explicit about the role of sleep. Trends Cogn Sci. 2004;8:242–244. doi: 10.1016/j.tics.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 7.Walker MP, et al. Sleep and the time course of motor skill learning. Learn Mem. 2003;10:275–284. doi: 10.1101/lm.58503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shadmehr R, Brashers-Krug T. Functional stages in the formation of human long-term motor memory. Journal of Neuroscience. 1997;17:409–419. doi: 10.1523/JNEUROSCI.17-01-00409.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ellenbogen JM, Hu PT, Payne JD, Titone D, Walker MP. Human relational memory requires time and sleep. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:7723–7728. doi: 10.1073/pnas.0700094104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walker MP, Liston C, Hobson JA, Stickgold R. Cognitive flexibility across the sleep-wake cycle: REM-sleep enhancement of anagram problem solving. Brain research Cognitive brain research. 2002;14:317–324. doi: 10.1016/s0926-6410(02)00134-9. [DOI] [PubMed] [Google Scholar]
- 11.Mednick SC, Cai DJ, Shuman T, Anagnostaras S, Wixted JT. An opportunistic theory of cellular and systems consolidation. Trends in neurosciences. 2011;34:504–514. doi: 10.1016/j.tins.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Muller GE, Pilzecker A. Experimentelle Beitrage zur Lehre von Gedachtnis. Z Psychol. 1900;1:1–300. [Google Scholar]
- 13.Jenkins JG, Dallenbach KM. Obliviscence during sleep and waking. American Journal of Psychology. 1924;35:605–612. [Google Scholar]
- 14.Smith C, Kitahama K, Valatx JL, Jouvet M. Increased paradoxical sleep in mice during acquisition of a shock avoidance task. Brain Research. 1974;77:221–230. doi: 10.1016/0006-8993(74)90786-0. [DOI] [PubMed] [Google Scholar]
- 15.Diekelmann S, Born J. The memory function of sleep. Nature Reviews Neuroscience. 2010;11:114–126. doi: 10.1038/nrn2762. [DOI] [PubMed] [Google Scholar]
- 16.Walker MP, Stickgold R. Sleep-dependent learning and memory consolidation. Neuron. 2004;44:121–133. doi: 10.1016/j.neuron.2004.08.031. [DOI] [PubMed] [Google Scholar]
- 17.Brawn TP, Fenn KM, Nusbaum HC, Margoliash D. Consolidating the effects of waking and sleep on motor-sequence learning. Journal of Neuroscience. 2010;30:13977–13982. doi: 10.1523/JNEUROSCI.3295-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saletin JM, Walker MP. Nocturnal mnemonics: sleep and hippocampal memory processing. Frontiers in neurology. 2012;3:59. doi: 10.3389/fneur.2012.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilhelm I, et al. Sleep selectively enhances memory expected to be of future relevance. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:1563–1569. doi: 10.1523/JNEUROSCI.3575-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu P, Stylos-Allan M, Walker MP. Sleep facilitates consolidation of emotional declarative memory. Psychol Sci. 2006;17:891–898. doi: 10.1111/j.1467-9280.2006.01799.x. [DOI] [PubMed] [Google Scholar]
- 21.Atienza M, Cantero JL. Modulatory effects of emotion and sleep on recollection and familiarity. J Sleep Res. 2008;17:285–294. doi: 10.1111/j.1365-2869.2008.00661.x. [DOI] [PubMed] [Google Scholar]
- 22.Nishida M, Pearsall J, Buckner RL, Walker MP. REM sleep, prefrontal theta, and the consolidation of human emotional memory. Cerebral cortex. 2009;19:1158–1166. doi: 10.1093/cercor/bhn155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wagner U, Gais S, Born J. Emotional memory formation is enhanced across sleep intervals with high amounts of rapid eye movement sleep. Learning & memory. 2001;8:112–119. doi: 10.1101/lm.36801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baran B, Pace-Schott EF, Ericson C, Spencer RM. Processing of emotional reactivity and emotional memory over sleep. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:1035–1042. doi: 10.1523/JNEUROSCI.2532-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lewis PA, Cairney S, Manning L, Critchley HD. The impact of overnight consolidation upon memory for emotional and neutral encoding contexts. Neuropsychologia. 2011;49:2619–2629. doi: 10.1016/j.neuropsychologia.2011.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Payne JD, Stickgold R, Swanberg K, Kensinger EA. Sleep preferentially enhances memory for emotional components of scenes. Psychol Sci. 2008;19:781–788. doi: 10.1111/j.1467-9280.2008.02157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fischer S, Born J. Anticipated reward enhances offline learning during sleep. J Exp Psychol Learn Mem Cogn. 2009;35:1586–1593. doi: 10.1037/a0017256. [DOI] [PubMed] [Google Scholar]
- 28.van Dongen EV, Thielen JW, Takashima A, Barth M, Fernandez G. Sleep supports selective retention of associative memories based on relevance for future utilization. PLoS ONE. 2012;7:e43426. doi: 10.1371/journal.pone.0043426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saletin JM, Goldstein AN, Walker MP. The Role of Sleep in Directed Forgetting and Remembering of Human Memories. Cerebral Cortex. 2011;21:2534–2541. doi: 10.1093/cercor/bhr034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anderson MC, Huddleston E. Towards a cognitive and neurobiological model of motivated forgetting. Nebraska Symposium on Motivation Nebraska Symposium on Motivation. 2012;58:53–120. doi: 10.1007/978-1-4614-1195-6_3. [DOI] [PubMed] [Google Scholar]
- 31.Fischer S, Diekelmann S, Born J. Sleep’s role in the processing of unwanted memories. J Sleep Res. 2010;20(2):267–74. doi: 10.1111/j.1365-2869.2010.00881.x. [DOI] [PubMed] [Google Scholar]
- 32.Rauchs G, et al. Sleep contributes to the strengthening of some memories over others, depending on hippocampal activity at learning. Journal of Neuroscience. 2011;31:2563–2568. doi: 10.1523/JNEUROSCI.3972-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Frey S, Morris R, Petrides M. A neuroanatomical method to assess the integrity of fibers of passage following ibotenate-induced damage to the central nervous system. Neurosci Res. 1997;28:285–288. doi: 10.1016/s0168-0102(97)00048-5. [DOI] [PubMed] [Google Scholar]
- 34.Foster DJ, Wilson MA. Reverse replay of behavioural sequences in hippocampal place cells during the awake state. Nature. 2006;440:680–683. doi: 10.1038/nature04587. [DOI] [PubMed] [Google Scholar]
- 35.Tambini A, Ketz N, Davachi L. Enhanced brain correlations during rest are related to memory for recent experiences. Neuron. 2011;65:280–290. doi: 10.1016/j.neuron.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Payne JD, Kensinger EA. Sleep Leads to Changes in the Emotional Memory Trace: Evidence from fMRI. J Cogn Neurosci. 2010 doi: 10.1162/jocn.2010.21526. [DOI] [PubMed] [Google Scholar]
- 37.Yoo SS, Gujar N, Hu P, Jolesz FA, Walker MP. The human emotional brain without sleep–a prefrontal amygdala disconnect. Current biology : CB. 2007;17:R877–878. doi: 10.1016/j.cub.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 38.Payne JD, et al. The role of sleep in false memory formation. Neurobiology of learning and memory. 2009;92:327–334. doi: 10.1016/j.nlm.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Knutson B, Adcock RA. Remembrance of rewards past. Neuron. 2005;45:331–332. doi: 10.1016/j.neuron.2005.01.020. [DOI] [PubMed] [Google Scholar]
- 40.Wylie GR, Foxe JJ, Taylor TL. Forgetting as an active process: an FMRI investigation of item-method-directed forgetting. Cerebral Cortex. 2008;18:670–682. doi: 10.1093/cercor/bhm101. [DOI] [PubMed] [Google Scholar]
- 41.Shimamura AP. Episodic retrieval and the cortical binding of relational activity. Cogn Affect Behav Neurosci. 2011;11:277–291. doi: 10.3758/s13415-011-0031-4. [DOI] [PubMed] [Google Scholar]
- 42.Squire LR, Slater PC, Chace PM. Retrograde amnesia: temporal gradient in very long term memory following electroconvulsive therapy. Science. 1975;187:77–79. doi: 10.1126/science.1109228. [DOI] [PubMed] [Google Scholar]
- 43.Piaget J. The origins of intelligence in children. International Universities Press, Inc.; New York: 1952. [Google Scholar]
- 44.Tse D, et al. Schemas and memory consolidation. Science. 2007;316:76–82. doi: 10.1126/science.1135935. [DOI] [PubMed] [Google Scholar]
- 45.van Kesteren MT, Ruiter DJ, Fernandez G, Henson RN. How schema and novelty augment memory formation. Trends in neurosciences. 2012;35:211–219. doi: 10.1016/j.tins.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 46.McGaugh JL. Memory–a century of consolidation. Science. 2000;287:248–251. doi: 10.1126/science.287.5451.248. [DOI] [PubMed] [Google Scholar]
- 47.Gaskell MG, Dumay N. Lexical competition and the acquisition of novel words. Cognition. 2003;89:105–132. doi: 10.1016/s0010-0277(03)00070-2. [DOI] [PubMed] [Google Scholar]
- 48.Tamminen J, Payne JD, Stickgold R, Wamsley EJ, Gaskell MG. Sleep spindle activity is associated with the integration of new memories and existing knowledge. The Journal of neuroscience. 2010;30:14356–14360. doi: 10.1523/JNEUROSCI.3028-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cai DJ, Mednick SA, Harrison EM, Kanady JC, Mednick SC. REM, not incubation, improves creativity by priming associative networks. Proc Natl Acad Sci U S A. 2009;106:10130–10134. doi: 10.1073/pnas.0900271106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wamsley EJ, Tucker MA, Payne JD, Stickgold R. A brief nap is beneficial for human route-learning: The role of navigation experience and EEG spectral power. Learn Mem. 2010;17:332–336. doi: 10.1101/lm.1828310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wamsley EJ, Tucker M, Payne JD, Benavides JA, Stickgold R. Dreaming of a learning task is associated with enhanced sleep-dependent memory consolidation. Curr Biol. 2010;20:850–855. doi: 10.1016/j.cub.2010.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Roediger HL, McDermott KB. Creating false memories: Remembering words not presented in lists. Journal of Experimental Psychology: Learning, Memory, & Cognition. 1995;21:803–814. [Google Scholar]
- 53.Diekelmann S, Born J, Wagner U. Sleep enhances false memories depending on general memory performance. Behavioural brain research. 2010;208:425–429. doi: 10.1016/j.bbr.2009.12.021. [DOI] [PubMed] [Google Scholar]
- 54.Fenn KM, Gallo DA, Margoliash D, Roediger HL, 3rd, Nusbaum HC. Reduced false memory after sleep. Learn Mem. 2009;16:509–513. doi: 10.1101/lm.1500808. [DOI] [PubMed] [Google Scholar]
- 55.Darsaud A, et al. Does sleep promote false memories? J Cogn Neurosci. 2011;23:26–40. doi: 10.1162/jocn.2010.21448. [DOI] [PubMed] [Google Scholar]
- 56.Eichenbaum H, Yonelinas AP, Ranganath C. The medial temporal lobe and recognition memory. Annu Rev Neurosci. 2007;30:123–152. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Buckmaster CA, Eichenbaum H, Amaral DG, Suzuki WA, Rapp PR. Entorhinal cortex lesions disrupt the relational organization of memory in monkeys. The Journal of neuroscience. 2004;24:9811–9825. doi: 10.1523/JNEUROSCI.1532-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dusek JA, Eichenbaum H. The hippocampus and memory for orderly stimulus relations. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:7109–7114. doi: 10.1073/pnas.94.13.7109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Durrant SJ, Taylor C, Cairney S, Lewis PA. Sleep-dependent consolidation of statistical learning. Neuropsychologia. 2011;49:1322–1331. doi: 10.1016/j.neuropsychologia.2011.02.015. [DOI] [PubMed] [Google Scholar]
- 60.Knowlton BJ, Squire LR, Gluck MA. Probabilistic classification learning in amnesia. Learning and Memory. 1994;1:106–120. [PubMed] [Google Scholar]
- 61.Gluck M, Shohamy D, Meyers CE. How do people solve the “Weather Prediction”task? Individual variability in strategies for probablistic category learning. Learning & Memory. 2002;9:408–418. doi: 10.1101/lm.45202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Djonlagic I, et al. Sleep enhances category learning. Learn Mem. 2009;16:751–755. doi: 10.1101/lm.1634509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wagner U, Gais S, Haider H, Verleger R, Born J. Sleep inspires insight. Nature. 2004;427:352–355. doi: 10.1038/nature02223. [DOI] [PubMed] [Google Scholar]
- 64.Mednick SA. The associative basis of the creative process. Psychological Review. 1962;69:220–232. doi: 10.1037/h0048850. [DOI] [PubMed] [Google Scholar]
- 65.Stickgold R, Scott L, Rittenhouse C, Hobson JA. Sleep-induced changes in associative memory. J Cogn Neurosci. 1999;11:182–193. doi: 10.1162/089892999563319. [DOI] [PubMed] [Google Scholar]
- 66.Hasselmo ME, Bower JM. Acetylcholine and memory. Trends in neurosciences. 1993;16:218–222. doi: 10.1016/0166-2236(93)90159-j. [DOI] [PubMed] [Google Scholar]
- 67.Smith C, Smith D. Ingestion of ethanol just prior to sleep onset impairs memory for procedural but not declarative tasks. Sleep. 2003;26:185–191. [PubMed] [Google Scholar]
- 68.Lee AK, Wilson MA. Memory of sequential experience in the hippocampus during slow wave sleep. Neuron. 2002;36:1183–1194. doi: 10.1016/s0896-6273(02)01096-6. [DOI] [PubMed] [Google Scholar]
- 69.Wilson MA, McNaughton BL. Reactivation of hippocampal ensemble memories during sleep. Science. 1994;265:676–679. doi: 10.1126/science.8036517. [DOI] [PubMed] [Google Scholar]
- 70.Louie K, Wilson MA. Temporally structured replay of awake hippocampal ensemble activity during rapid eye movement sleep. Neuron. 2001;29:145–156. doi: 10.1016/s0896-6273(01)00186-6. [DOI] [PubMed] [Google Scholar]
- 71.Giuditta A, et al. The sequential hypothesis of the function of sleep. Behav Brain Res. 1995;69:157–166. doi: 10.1016/0166-4328(95)00012-i. [DOI] [PubMed] [Google Scholar]
- 72.Grosmark AD, Mizuseki K, Pastalkova E, Diba K, Buzsaki G. REM Sleep Reorganizes Hippocampal Excitability. Neuron. 2012;75:1001–1007. doi: 10.1016/j.neuron.2012.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Poe GR, Nitz DA, McNaughton BL, Barnes CA. Experience-dependent phase-reversal of hippocampal neuron firing during REM sleep. Brain Res. 2000;855:176–180. doi: 10.1016/s0006-8993(99)02310-0. [DOI] [PubMed] [Google Scholar]
- 74.Stickgold R, James L, Hobson JA. Visual discrimination learning requires sleep after training. Nat Neurosci. 2000;3:1237–1238. doi: 10.1038/81756. [DOI] [PubMed] [Google Scholar]
- 75.Schacter DL, Addis DR, Buckner RL. Remembering the past to imagine the future: the prospective brain. Nature reviews. 2007;8:657–661. doi: 10.1038/nrn2213. [DOI] [PubMed] [Google Scholar]
- 76.Schacter DL, Addis DR. On the nature of medial temporal lobe contributions to the constructive simulation of future events. Philosophical transactions of the Royal Society of London. 2009;364:1245–1253. doi: 10.1098/rstb.2008.0308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Saletin JM, Goldstein AN, Walker MP. The Role of Sleep in Directed Forgetting and Remembering of Human Memories. Cerebr Cortex. 2011;21:2534–2541. doi: 10.1093/cercor/bhr034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hupbach A, Gomez RL, Bootzin RR, Nadel L. Nap-dependent learning in infants. Developmental science. 2009;12:1007–1012. doi: 10.1111/j.1467-7687.2009.00837.x. [DOI] [PubMed] [Google Scholar]