Abstract
Objective
To evaluate the efficacy of a home-based lifestyle intervention delivered through Parents as Teachers (PAT) to reduce excessive GWG.
Methods
This was a single-blinded, randomized controlled trial conducted as part of the LIFE-Moms consortium at a single university-based tertiary care institution, from October 2012 to March 2016. There were 267 SED African-American women with overweight or obesity (BMI 25.0–45.0 kg/m2) before pregnancy. Participants were randomized to therapy with standard PAT alone (n=134) or PAT+ a lifestyle intervention program embedded within the standard PAT program (PAT+, n=133). Both interventions were delivered in 10-biweekly home visits during pregnancy. The primary outcome was percentage of women whose GWG exceeded IOM guidelines, and secondary outcomes included both weekly and total GWG.
Results
Compared with the standard PAT group in the intent-to-treat analysis, the PAT+ group gained less weekly (0.4 kg v. 0.5 kg per week, P=0.04) and total (8.0 kg v. 9.6 kg, P=0.02) weight during gestation. Fewer participants in the PAT+ group had excessive total GWG (36.1% vs 45.9%), but the difference between groups was not statistically significant (P=0.11).
Conclusions
PAT+ reduced weekly and total GWG in SED African-American women with overweight or obesity at the start of pregnancy.
Keywords: Gestational weight gain, pregnancy, obesity, lifestyle modifications
Introduction
More than 50% of women who are pregnant in the United States with overweight or obesity, and approximately half of these women exceed the recommended guidelines for gestational weight gain (GWG)(1). Excess body weight and excessive GWG during pregnancy are associated with adverse pregnancy outcomes, including increased rates of preeclampsia, gestational diabetes, fetal growth disorders, stillbirth, preterm, cesarean delivery and post-partum weight retention (2). Overweight and obesity disproportionately affect African American, socioeconomically disadvantaged (SED) women (3). Moreover, this population is at increased risk for adverse pregnancy outcomes, which is further exacerbated by excessive GWG (4).
A series of randomized controlled trials have evaluated the efficacy of lifestyle interventions (diet and physical activity) during pregnancy on GWG. The results from the most recent meta-analyses of randomized controlled trials found lifestyle intervention decreased GWG and lowered the odds of caesarean section, but did not affect neonatal outcomes (5). However, few randomized trials have evaluated the effect of lifestyle interventions for African American, SED, pregnant women, despite their increased disease burden and risk for adverse maternal, fetal, and infant outcomes (6, 7). These women often face multiple barriers, such as time constraints associated with low-income jobs with minimal flexibility, parenting responsibilities, lack of transportation, and related social stressors, which prevent engagement in interventions that promote healthy lifestyle behaviors (8). Most GWG interventions require repeated visits to clinics or other locations, which can increase attrition and decrease treatment efficacy (9). Interventions that are sensitive to individual educational needs, are readily accessible, and offer strategies to reduce participant burden facilitate engagement and ability to comply with therapeutic recommendations. Partnering with community organizations that reach women at home can enhance convenience, accessibility, and availability of lifestyle interventions for SED African American women (10).
The purpose of the present randomized controlled trial was to evaluate whether an optimized home-based lifestyle weight management program could affect GWG and improve maternal and neonatal outcomes in SED African American pregnant women with overweight or obesity at the beginning of pregnancy. We collaborated with Parents As Teachers (PAT), a national home visiting organization that provides an evidence-based curriculum to promote positive child development and school readiness; this curriculum is delivered by parent educators through multiple home visits free-of-charge to SED pregnant women (11). The lifestyle intervention program was embedded within the existing standard PAT curriculum provided during the prenatal home visits.
Methods
Study participants
A total of 267 women participated in this study. Written informed consent was obtained from all participants before they participated in this study, which was approved by the Washington University Institutional Review Board. Eligibility criteria included: 1) African-American ancestry; 2) age 18–45 years; 3) body mass index [BMI] 25.0–45.0 kg/m2) measured at the initial visit during the first trimester; 4) singleton viable gestation at or before 15 0/7 weeks (established by date of last menstrual period if it was within 5 days of first trimester ultrasound dating, or by ultrasound itself); and 5) disadvantaged socioeconomic status (Medicaid recipient, or home zip code associated with a median household income below the poverty level). Key exclusion criteria were: 1) diabetes; 2) history of gestational diabetes or macrosomia; 3) glycosylated hemoglobin ≥6.5%; 4) any contraindication to exercise during pregnancy (12); 5) substance abuse; and 6) non-English speaker. A complete list of inclusion and exclusion criteria are listed in Table S1.
Study design
We conducted a randomized controlled trial at a single university-based tertiary care institution (Barnes-Jewish Hospital and Washington University School of Medicine, St. Louis, MO) from October 2012 to March 2016. This trial was part of the LIFE-Moms consortium (https://lifemoms.bsc.gwu.edu/), a collaborative group evaluating the effect of lifestyle therapies on maternal GWG and maternal, fetal and infant health in pregnant women with overweight or obesity (13).
Participants were randomly assigned in a 1:1 allocation to treatment with the standard Parents as Teachers (PAT) education curriculum or PAT education curriculum plus lifestyle (diet and activity) weight management counselling (PAT+). The randomization sequence was determined by using a random number generator and a fixed allocation block strategy; investigators and study staff were blind to group assignment.
Participants in both groups were seen by the parent educators in 1-hour home visits, every other week during pregnancy. Participants assigned to the standard PAT curriculum had home visits focused on development-centered parenting support and education, and parent-child interaction using a family strength-based approach. Participants assigned to PAT+ received the standard PAT curriculum plus a lifestyle curriculum based on cognitive behavior change theory, which included participant goals for achieving appropriate GWG, regular self-assessment of weight, education and reinforcement of positive eating and physical activity behaviors, observational learning through role play, and environmental changes in the home. The lifestyle intervention was developed in partnership with PAT to assure consistency with organizational mission, format, practice, and funding requirements, and addressed barriers and facilitators for healthy GWG identified by African American women during the development of the program. Specific topics embedded within each home visit are outlined in Table S2. To ensure the home intervention was delivered as designed, parent educators audiotaped the visits and completed lesson plan checklists documenting delivery of content, which were reviewed by study staff. Study staff also randomly observed two home visits each year for each parent educator, an approach consistent with PAT standards of practice.
Participants were seen in the Washington University Clinical Research Unit at 15 weeks and 35 weeks of gestation to determine body weight, body composition (fat mass and fat-free mass assessed by using air displacement plethysmography; Bod Pod, Life Measurements Inc., Concord, CA), plasma glucose, insulin and lipid concentrations, and the plasma glucose and insulin response to an oral glucose load (blood samples obtained before and at 30, 60, 90, and 120 minutes after ingesting a 75 g glucose drink). Infant plasma glucose and insulin concentrations were assessed from cord blood obtained at time of delivery, and infant length, weight and body composition (fat mass and fat-free mass assessed by using air displacement plethysmography; Pea Pod, Life Measurements Inc., Concord, CA) were determined after delivery, before hospital discharge. All research and clinical visits were conducted by staff that were blinded as to the participants’ treatment assignment.
Study outcomes
The primary study outcome was percentage of participants with GWG that exceeded the goal range set by the Institute of Medicine for women with overweight or obesity (1). Maternal secondary outcomes were weekly GWG, and changes from 15 weeks to 35 weeks in: 1) total GWG; 2) body fat and fat-free masses; 3) indices of glycemic control (fasting plasma glucose and insulin concentrations, homeostasis model assessment of insulin resistance [HOMA-IR], oral glucose insulin sensitivity [OGIS], and both glucose and insulin areas under the curve [AUC] during the oral glucose tolerance test [OGTT]); 4) plasma lipid profile (total cholesterol, low-density lipoprotein [LDL]-cholesterol, high-density lipoprotein [HDL]-cholesterol, and triglycerides); and 5) systolic and diastolic blood pressures. Obstetric secondary outcomes included the following complications: 1) gestational diabetes (diagnosed at 24–28 weeks, based on a fasting plasma glucose ≥92 mg/dL or plasma glucose ≥180 mg/dL or ≥153 mg/dL 1 and 2 hours after ingesting a 75 g glucose drink, respectively); 2) hypertensive disease of pregnancy (14); 3) preterm birth (delivery before 37 0/7 weeks); 4) cesarean delivery; and 5) fetal death (after 20 0/7 weeks). Neonatal secondary outcomes included: 1) birth weight and length; 2) body composition (fat-free mass and percent body fat); 3) percent who were large for gestational age (LGA); 4) percent who were small for gestational age (SGA); 5) umbilical cord plasma glucose and insulin concentrations; and 6) medical complications (neonatal intensive care unit admission within 24 hours of life, respiratory distress syndrome, hypoglycemia [plasma glucose <30 mg/dL at any time], and neonatal death within the first 28 days of life.
Calculations and analyses
Plasma glucose concentration was determined by using an automated glucose analyzer (Yellow Springs Instruments, Yellow Springs, OH). Plasma insulin concentration was determined by using a chemiluminescent immunometric method (Immulite; Siemens, Los Angeles, CA). HOMA-IR was calculated by dividing the product of the plasma glucose (mmol/L) and plasma insulin (mU/L) concentrations by 22.5 (15), glucose and insulin AUC during the OGTT was calculated by the trapezoidal rule, and OGIS was calculated as described previously (16).
Statistical analyses
Preliminary data obtained from our clinic records demonstrated that 69.2% and 75.8% SED, African American women with overweight or obesity respectively, gained more weight during pregnancy than that recommended by the IOM. Assuming a 10% attrition rate by delivery, we estimated 133 women in each group would be needed to detect a 30% reduction in GWG that exceeded the IOM recommendations, with a power of 0.9 and an alpha value of 0.05.
Primary analyses were performed according to the intention-to-treat (ITT) principle, using the last recorded maternal weight. A modified ITT (mITT) analysis was also performed that excluded participants with fetal deaths, miscarriages, absence of maternal weight measured between 33 and 37 weeks, and those who were lost to follow-up. Continuous variables were compared by using the Student’s t-test or Mann Whitney U test, as appropriate. Normality was tested with the Kolmogorov-Smirnov test. Categorical variables were compared by using the χ2 or Fisher’s exact test, as appropriate. No interim analyses were planned or performed. A P < .05 was considered statistically significant.
Results
Participant flow
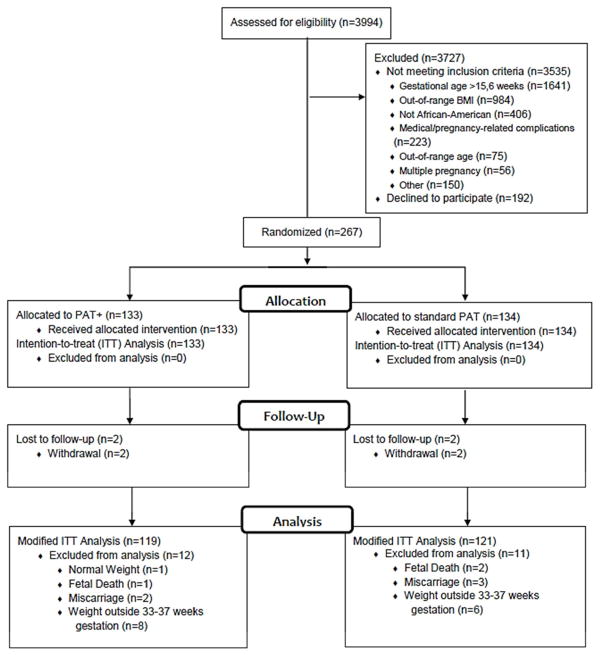
A total of 3994 women were screened for participation in this study and 3535 (88.5%) were deemed ineligible (Figure 1). The two most common reasons for ineligibility were gestational age ≥16 weeks and an out-of-range BMI. Of the 459 eligible women, 267 (58.2%) consented to participate and were randomized and included in the ITT analysis; 133 were assigned to the PAT+ group and 134 were assigned to the standard PAT group. The modified ITT analysis included 240 participants: 119 in the PAT+ group and 121 in the standard PAT group (14 from the PAT+ and 13 from the standard PAT groups were excluded). Baseline characteristics between participants included in the modified ITT analysis compared with those who were excluded were similar (Table S3). Participants in the PAT+ group had more home visits than did standard PAT participants (PAT+: 9 visits (7, 10) vs. standard PAT: 8 visits (5, 10), P = 0.04). Participants in the PAT+ group also had longer visits than did standard PAT participants (PAT+: 53 min (44, 60) vs. standard PAT: 32 min (21, 49), P < .01). The increased duration of home visits in the PAT+ group was expected due to the increased content delivered during these visits.
Figure 1.
Flow of Study Participants
Participant characteristics
Advanced maternal age, BMI, body weight, fat-free mass, percent body fat, insurance carrier, maternal education, household income level, gravidity, and parity were not different between treatment groups (Table 1). Mean age was about 1 year younger and history of previous cesarean delivery was more common in the PAT+ group than the standard PAT group. Fifty-one percent of the participants changed their address once during the ~20-week prenatal intervention and 12% moved two or more times.
Table 1.
Baseline Characteristics of Standard PAT and PAT+ Groups
| Standard PAT (N=134) | PAT+ (N=133) | P value | |
|---|---|---|---|
| N (%) | N (%) | ||
| Maternal age (yrs)a | 26.0 ± 4.9 | 24.7 ± 4.9 | 0.04 |
| Advanced maternal age (n, %) | 9 (6.7) | 7 (5.3) | 0.62 |
| BMI at randomization (kg/m2)a | 31.9 ± 4.9 | 32.8 ± 5.1 | 0.16 |
| Body weight (kg) | 86.1 ± 15.2 | 87.3 ± 16.1 | 0.52 |
| Fat-free mass (kg) | 57.4 ± 8.1 | 56.8 ± 7.7 | 0.55 |
| Body fat (%) | 66.7 ± 5.2 | 65.6 ± 5.4 | 0.08 |
| Overweight (n, %) | 52 (38.8) | 42 (31.6) | 0.22 |
| Obese (n, %) | 82 (61.2) | 90 (67.7) | 0.27 |
| Medicaid (n, %) | 119 (92.2) | 119 (90.8) | 0.42 |
| Maternal education | 0.47 | ||
| Less than high school (n, %) | 25 (18.7) | 29 (21.8) | |
| High school graduate (n, %) | 51 (38.1) | 59 (44.4) | |
| Some college (n, %) | 47 (35.1) | 36 (27.1) | |
| College graduate (n, %) | 11 (8.2) | 9 (6.8) | |
| Household income level | 0.69 | ||
| <$25,000 | 120 (89.5) | 121 (91.0) | |
| ≥$25,000 | 14 (10.4) | 12 (9.0) | |
| Marital status | 0.59 | ||
| Married | 18 (13.) | 11 (8.3) | |
| Not married and living with significant other | 42 (31.4) | 43 (32.3) | |
| Separated/widowed/divorced | 4 (3.0) | 5 (3.8) | |
| Not married | 70 (52.2) | 74 (55.6) | |
| Gravidityb | 2.0 (1.0–3.0) | 1.0 (0.0–3.0) | 0.16 |
| Nulliparous (n, %) | 25 (18.7) | 38 (28.6) | 0.06 |
| Prior cesarean (n, %) | 25 (50.0) | 38 (69.1) | 0.04 |
BMI, body mass index
Data are means ± SD
Data are median (IQR)
Maternal body weight and composition
In both the ITT and mITT analyses, total GWG was 1.6–1.7 kg less in the PAT+ group than the standard PAT group (ITT P = 0.02; mITT P = .01) (Table 2 and Figure S1). Fewer participants in the PAT+ group than in the standard PAT group had total GWG that exceeded the recommended IOM guidelines, but the difference between groups was not statistically significant (ITT: 36.1% vs. 45.9%, P = .11; mITT: 36.1% vs. 47.9%, P = 0.06). In both the ITT and mITT analyses, the PAT+ group gained 0.08 kg less weight per week than did the standard PAT group (P = 0.04 and P= 0.01, respectively). Fewer participants in the PAT+ group than in the standard PAT group had weekly GWG that exceeded the recommended IOM guidelines (ITT: 62.4% vs. 77.4%, P = 0.01; mITT: 60.5% vs. 78.5%, P < 0.01). In addition, the PAT+ group gained less body fat than did the standard PAT group (ITT: 0.25 ± 5.2 kg vs 2.18 ± 5.8 kg, P < 0.01; mITT: 0.16 ± 5.1 kg vs 2.18 ± 5.8 kg, P < 0.01) (Table 2).
Table 2.
Gestational Weight Gain and Body Composition in Standard PAT and PAT+ Groups at 35 weeks gestation
| Standard PAT | PAT+ | P value | |
|---|---|---|---|
| ITT Analysis | N=134 | N=133 | |
| Total GWG (kg)a | 9.64 ± 5.4 | 8.05 ± 5.6 | 0.02 |
| Total GWG above IOM guidelines (n, %) | 61 (45.9) | 48 (36.1) | 0.11 |
| Weekly GWG (kg/week) a | 0.48 ± 0.3 | 0.40 ± 0.3 | 0.04 |
| Weekly GWG above IOM guidelines (n, %) | 103 (77.4) | 83 (62.4) | 0.01 |
| Increase in fat mass (kg) | 2.18 ± 5.8 | 0.25 ± 5.2 | 0.01 |
| Increase in fat-free mass (kg) | 7.83 ± 4.6 | 7.52 ± 4.4 | 0.61 |
| Modified ITT Analysis | N=121 | N=119 | |
| Total GWG (kg)a | 9.93 ± 5.3 | 8.11 ± 5.6 | 0.01 |
| Total GWG above IOM guidelines (n, %) | 58 (47.9) | 43 (36.1) | 0.06 |
| Weekly GWG (kg/week)a | 0.46 ± 0.2 | 0.38 ± 0.3 | 0.01 |
| Weekly GWG above IOM guidelines (n, %) | 95 (78.5) | 72 (60.5) | <0.01 |
| Increase in fat mass (kg) | 2.18 ± 5.8 | 0.16 ± 5.1 | <0.01 |
| Change in fat-free mass (kg) | 7.83 ± 4.6 | 7.54 ± 4.5 | 0.64 |
Abbreviations: GWG, gestational weight gain; kg, kilogram; IOM, Institute of Medicine
Data are means ± SD
Maternal outcomes
Cardiometabolic outcomes
Advancing gestation from 15 to 35 weeks was associated with expected increases in fasting insulin, HOMA-IR, total cholesterol, LDL-cholesterol, HDL-cholesterol, and triglyceride concentrations that occur during pregnancy, but did not differ between groups (Table 3 and Table S4). Changes in insulin AUC during the OGTT and systolic blood pressure between 15 and 35 weeks gestation were significantly different between groups. The relative increase in insulin AUC was greater in the standard PAT group than in the PAT+ group (ITT: 25.1% vs. 8.8% increase, P = 0.01; mITT: 28.3% vs. 8.9% P = .01), and the relative increase in systolic blood pressure was greater in in the standard PAT group than in the PAT+ group (ITT: 1.6% vs. −1.7%, P = 0.02; mITT: 1.6% vs. −1.6%, P = 0.03).
Table 3.
Maternal Cardiometabolic Outcomes in Standard PAT and PAT+ Groups, ITT analysis
| Standard PAT (N=134) median (IQR) |
PAT+ (N=133) Median (IQR) |
|||
|---|---|---|---|---|
|
| ||||
| ~15 Weeks | ~35 Weeks | ~15 Weeks | ~35 Weeks | |
| Fasting glucose | 80.7 | 78.1 | 81.1 | 77.7 |
| (mg/dL) | (76.7, 85.1) | (75.4, 83.2) | (76.0, 85.3) | (73.1, 84.4) |
| Fasting insulin | 9.6 | 14.2 | 10.7 | 15.5 |
| (μU/mL) | (6.6, 14.8) | (8.8, 19.0) | (7.0, 17.0) | (9.6, 25.0) |
| OGIS a | 406.5 ± 68.2 | 388.8 ± 70.4 | 390.9 ± 73.9 | 373.2 ± 80.5 |
| Glucose AUC | 15047 | 14488 | 14946 | 14604 |
| (mg/dL x 120 min) | (13667, 16295) | (13524, 16389) | (13918, 16269) | (13349, 15798) |
| Insulin AUC | 10733 | 13149 | 13109 | 13421* |
| (μU/mL x 120 min) | (8212, 15503) | (10625, 17563) | (9333, 19339) | (8848, 19590) |
| HOMA-IR | 2.0 | 2.6 | 2.1 | 2.9 |
| (1.3, 3.0) | (1.6, 3.7) | (1.5, 3.4) | (1.8, 4.4) | |
| Total cholesterol | 158.0 | 205.0 | 160.0 | 198.5 |
| (mg/dL) | (142.0, 178.0) | (177.0, 225.0) | (140.0, 186.0) | (179.0, 225.0) |
| LDL-cholesterol | ||||
| (mg/dL)a | 81.0 ± 23.6 | 103.4 ± 32.4 | 83.34 ± 25.63 | 108.5 ± 36.0 |
| HDL-cholesterol | ||||
| (mg/dL)a | 63.0 ± 13.7 | 68.0 ± 16.3 | 63.1 ± 14.0 | 65.9 ± 15.7 |
| Triglycerides | 75.0 | 145.0 | 76.0 | 143.0 |
| (mg/dL) | (60.0, 97.0) | (125.0, 187.0) | (60.0, 98.0) | (122.0, 175.0) |
| SBP (mmHg) | 109.0 | 111.5 | 112.0 | 109.7** |
| (104.0, 119.0) | (105.0, 120.0) | (105.7, 120.0) | (105.0, 117.0) | |
| DBP (mmHg)a | 67.0 ± 8.0 | 69.7 ± 8.9 | 67.2 ± 8.7 | 67.5 ± 8.8 |
OGIS, Oral Glucose Insulin Sensitivity; AUC, area under the curve; HOMA-IR, Homeostasis Model Assessment of Insulin Resistance; LDL, low density lipoprotein; HDL, high density lipoprotein; SBP, systolic blood pressure; DBP, diastolic blood pressure; kg, kilogram SI conversion factors: To convert glucose from mg/dL to mmol/L, multiply by 0.0555; for insulin from μU/mL to pmol/L, multiply by 6.945; for cholesterol from mg/dL to mmol/L, multiply by 0.0259; for triglycerides from mg/dL to mmol/L, multiply value by 0.0113
Data are means ± SD
Change in value during pregnancy significantly different from Standard PAT group, *P =0.01, **P = 0.02
Obstetric outcomes
There were no significant differences between the treatment group in obstetric complications, including development of gestational diabetes or hypertensive disorders, preterm birth, or cesarean delivery (Table 4 and Table S5).
Table 4.
Obstetric and Infant Complications in Standard PAT and PAT+ Groups, ITT analysis
| Standard PAT | PAT+ | P value | |
|---|---|---|---|
| N (%) | N (%) | ||
| Obstetrics Complications | N=134 | N=133 | |
| Gestational diabetes | 12 (9.0) | 11 (8.3) | 0.83 |
| Hypertensive disorder | 27 (21.1) | 32 (24.8) | 0.48 |
| Preterm birth | 12 (9.5) | 18 (14.1) | 0.26 |
| Cesarean delivery | 47 (36.7) | 53 (41.1) | 0.47 |
| Infant Complications | N=133 | N=133 | |
| Birth weight (g)a | 3130 (2810, 3463) | 3155 (2875, 3540) | 0.77 |
| Birth length (cm)a | 50.0 (48.3, 51.5) | 50.0 (48.20, 51.50) | 0.86 |
| Body fat mass (%)b | 12.2 ± 4.3 | 12.5 ± 3.8 | 0.57 |
| LGA | 6 (4.8) | 11 (8.6) | 0.22 |
| SGA | 13 (10.3) | 13 (10.2) | 0.97 |
| Hypoglycemia | 2 (1.6) | 3 (2.3) | >0.99 |
| Cord blood glucose (mg/dL)a | 86.0 (76.0, 95.0) | 86.0 (76.0, 96.5) | 0.72 |
| Cord blood insulin (μU/mL)a | 5.7 (3.3, 10.0) | 6.0 (4.0, 12.0) | 0.19 |
| Respiratory distress | 11 (8.2) | 16 (12.0) | 0.30 |
| NICU admission | 19 (14.3) | 25 (18.8) | 0.32 |
| Neonatal death | 0 (0.0) | 1 (0.8) | >0.99 |
Abbreviations: g, grams; cm, centimeters; LGA, large for gestational age; SGA, small for gestational age; NICU, neonatal intensive care unit SI conversion factors: To convert glucose from mg/dL to mmol/L, multiply by 0.0555; for insulin from μU/mL to pmol/L, multiply by 6.945
Data are median (IQR)
Data are means ± SD
Neonatal outcomes
There were no significant differences in birth weight, birth length, body fat mass, large for gestational age, small for gestational age, hypoglycemia, cord blood glucose, cord blood insulin, respiratory distress, neonatal intensive care unit admissions, or neonatal deaths between the two treatment groups (Table 4 and Table S6).
Discussion
This randomized controlled trial was conducted to evaluate the effect of PAT+, a home-based GWG lifestyle intervention embedded within an existing national parent education program (PAT), and delivered to SED African-American women with overweight or obesity at the start of their pregnancy. Our data demonstrate that women who received PAT+ gained less weekly and total weight during pregnancy than those who received standard PAT home visits without the GWG intervention. In addition, fewer women in the PAT+ group than in the standard PAT group exceeded the IOM guidelines for weekly weight gain, but the difference between groups was not statistically significant. These data demonstrate the potential of a home-based lifestyle intervention in modulating GWG in an underserved SED population with extensive barriers to treatment engagement. Moreover, use of the existing infrastructure of a national home visiting program allows for widespread dissemination of this therapeutic intervention at minimal additional cost.
It is likely that our PAT+ participants would have shown even greater efficacy in reducing GWG if the comparator group received usual care rather than standard PAT. Standard PAT involves intensive parent support through regular home visits, and is not representative of usual prenatal clinical care. GWG in the standard PAT group was approximately 40% less than what we have previously observed in a similar patient population in our obstetric clinic (data obtained from clinic records from March, 2008 to February, 2010), where 76% of our SED African American patients with obesity and 69% with overweight gained more total weight than that recommended by the IOM guidelines. This observation suggests the social support and contact provided by standard PAT alone helped prevent excessive GWG, which decreased our ability to detect the therapeutic effect of PAT+.
Although our PAT+ intervention had a significant effect on GWG, the clinical importance of this effect was modest. PAT+ resulted in a lower maternal systolic blood pressure, less of an increase in plasma insulin during the oral glucose tolerance test, and less gestational gain in body fat. However, there was no difference in other maternal cardiometabolic outcomes, pregnancy complications, neonatal birth weight and body composition, or neonatal complications.
We are aware of two previous randomized controlled trials that evaluated the effect of lifestyle intervention on GWG in SED African-American women (6, 7). The results from one study conducted in 66 African American women who were overweight or obese found that a lifestyle intervention delivered through text messaging and health coach telephone calls was able to decrease GWG, demonstrating the importance of using interventions that are convenient to help patients overcome barriers to participation (6). The other randomized trial was conducted in 59 women (~ two-thirds of the participants were overweight or obese), and used PAT to deliver a lifestyle (diet and physical activity) weight management curriculum through monthly home visits, beginning in the early second trimester of pregnancy (7). The percentage of women who had excessive GWG in the group treated with PAT plus lifestyle education was not different than the group who were treated with PAT alone. However, the attrition rate was much higher in participants randomized to PAT plus lifestyle education than PAT, possibly because of the long, 90-minute duration of each home visit, which could have influenced weight outcomes. In contrast, in our study, attrition was lower in participants treated with PAT+ compared to those treated with standard PAT, possibly because we embedded the lifestyle content within more frequent, biweekly home visits and made sure home visits were completed within 60 minutes.
Lifestyle weight management interventions that are effective in clinical trials frequently fail in real-world patient care programs, because of challenges related to acceptability and financial costs (9). Our previous work with PAT in African American parents of infants (17), rural parents of young children (18), and teen mothers (19) laid the groundwork for the current intervention by demonstrating PAT has an effective organizational structure for reaching, delivering, and scaling-up interventions that can be successfully used to modify lifestyle behaviors. Moreover, the PAT organization reaches a large number of high-risk, SED women as part of routine home visiting practice, facilitating PAT+ delivery to those who are often unable to participate in multi-visit clinic-based therapies. The PAT program has considerable reach across the United States providing the infrastructure for the scale-up of PAT+ across the country. In 2015–2016, PAT trained 4,999 parent educators located in 1,183 community-based sites in all 50 states, who conducted 1,188,585 home visits involving 116,054 families and 140,740 children, including 20,889 pregnant women (11). Efforts to evaluate the impact of widespread dissemination of PAT+ as part of routine PAT practice in pregnant women deserves further study.
In conclusion, we found that PAT+, a home-based lifestyle intervention for SED African American women with overweight and obesity at the start of pregnancy, resulted in reduced weekly and total GWG. However, the beneficial effect in limiting GWG did not translate into important clinical benefits for pregnancy or infant outcomes. Additional studies with longer duration of follow-up are needed to assess subsequent maternal weight change and child health and development. Nonetheless, our data demonstrate the effectiveness of using a national home visiting program to deliver a lifestyle intervention that reduces GWG in a complex, high-risk population with very limited resources. Moreover, embedding the lifestyle intervention within an existing national infrastructure makes it possible to readily disseminate this approach throughout the United States at minimal cost.
Supplementary Material
Study Importance.
What is already known about this subject?
Socioeconomically disadvantaged (SED) African-American women with overweight or obesity are at increased risk of excessive gestational weight gain (GWG) and associated adverse pregnancy outcomes.
What does your study add?
The program, Parents as Teachers, provides a scalable foundation for delivering an effective lifestyle intervention program that reduces gestational weight gain in a high-risk population with limited resources.
Acknowledgments
Funding: This work was supported by National Institutes of Health grants DK094416, DK56341 (Nutrition Obesity Research Center), DK092950 (Center for Diabetes Translation Research), DK20579 (Diabetes Research Center) and RR024992 (Clinical and Translational Science Award).
LIFE-Moms is supported by the National Institutes of Health through the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK, U01 DK094418, U01 DK094463, U01 DK094416, 5U01 DK094466 (RCU)), the National Heart, Lung, and Blood Institute (NHLBI, U01 HL114344, U01 HL114377), the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD, U01 HD072834), the National Center for Complementary and Integrative Health (NCCIH), the NIH Office of Research in Women’s Health (ORWH), the Office of Behavioral and Social Science Research (OBSSR), the Indian Health Service, and the Intramural Research Program of the NIDDK. We thank the LIFE-Moms consortium members for their contributions to the development and oversight of the common measures and procedures shared across the trials.
Footnotes
Trial Registration: http://www.clinicaltrials.gov; #NCT01768793
Disclosure: The authors declared no conflicts of interest.
Author Contributions: Study concept and design: Drs. Cahill, Klein, Haire-Joshu, Cade, Stein, Mathur, Moley. Data collection: Drs. Cahill, Haire-Joshu, Cade, Stein, Moley, Mathur, Klein. Analysis and interpretation of data: Drs. Cahill, Klein, Woolfolk. Drafting of manuscript: Drs. Cahill, Haire-Joshu, Cade, Stein, Woolfolk, Moley, Mathur, Klein. Critical revision of the manuscript: Drs. Cahill, Haire-Joshu, Cade, Stein, Woolfolk, Moley, Mathur, Klein. Obtain funding: Drs. Cahill, Klein, Haire-Joshu.
References
- 1.IOM. The National Academies Collection: Reports funded by National Institutes of Health. In: Rasmussen KM, Yaktine AL, editors. Weight Gain During Pregnancy: Reexamining the Guidelines. National Academy of Sciences; Washington (DC): 2009. [PubMed] [Google Scholar]
- 2.Doherty DA, Magann EF, Francis J, Morrison JC, Newnham JP. Pre-pregnancy body mass index and pregnancy outcomes. International Journal of Gynecology & Obstetrics. 2006;95:242–247. doi: 10.1016/j.ijgo.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 3.Davis EM, Stange KC, Horwitz RI. Childbearing, stress and obesity disparities in women: a public health perspective. Maternal and child health journal. 2012;16:109–118. doi: 10.1007/s10995-010-0712-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kiel DW, Dodson EA, Artal R, Boehmer TK, Leet TL. Gestational weight gain and pregnancy outcomes in obese women: how much is enough? Obstetrics and gynecology. 2007;110:752–758. doi: 10.1097/01.AOG.0000278819.17190.87. [DOI] [PubMed] [Google Scholar]
- 5.Effect of diet and physical activity based interventions in pregnancy on gestational weight gain and pregnancy outcomes: meta-analysis of individual participant data from randomised trials. BMJ. 2017:358. doi: 10.1136/bmj.j3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Herring SJ, Cruice JF, Bennett GG, Rose MZ, Davey A, Foster GD. Preventing excessive gestational weight gain among African American women: A randomized clinical trial. Obesity (Silver Spring, Md) 2016;24:30–36. doi: 10.1002/oby.21240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thomson J, Tussing-Humphreys L, Goodman M, Olender S. Gestational Weight Gain: Results from the Delta Healthy Sprouts Comparative Impact Trial. Journal of Pregnancy. 2016;2016 doi: 10.1155/2016/5703607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anderson CK, Walch TJ, Lindberg SM, Smith AM, Lindheim SR, Whigham LD. Excess Gestational Weight Gain in Low-Income Overweight and Obese Women: A Qualitative Study. Journal of nutrition education and behavior. 2015;47:404–411. e401. doi: 10.1016/j.jneb.2015.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yeo S, Samuel-Hodge CD, Smith R, Leeman J, Ferraro AM, Asafu-Adjei JK. Challenges of Integrating an Evidence-based Intervention in Health Departments to Prevent Excessive Gestational Weight Gain among Low-income Women. Public health nursing (Boston, Mass) 2016;33:224–231. doi: 10.1111/phn.12255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kumanyika SK, Whitt-Glover MC, Haire-Joshu D. What works for obesity prevention and treatment in black Americans? Research directions Obesity reviews: an official journal of the International Association for the Study of Obesity. 2014;15(Suppl 4):204–212. doi: 10.1111/obr.12213. [DOI] [PubMed] [Google Scholar]
- 11.PAT. PAT Quality Assurance Guidelines. 2016. [Google Scholar]
- 12.ACOG Committee opinion. Number 267, January 2002: exercise during pregnancy and the postpartum period. Obstetrics and gynecology. 2002;99:171–173. doi: 10.1016/s0029-7844(01)01749-5. [DOI] [PubMed] [Google Scholar]
- 13.RGC, Evans M, Cahill AG, Franks PW, Gallagher D, Phelan S, et al. Design of lifestyle intervention trials to prevent excessive gestational weight gain in women with overweight or obesity. Obesity (Silver Spring, Md) 2016;24:305–313. doi: 10.1002/oby.21330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.ACOG Practice Bulletin No. 125: Chronic hypertension in pregnancy. Obstetrics and gynecology. 2012;119:396–407. doi: 10.1097/AOG.0b013e318249ff06. [DOI] [PubMed] [Google Scholar]
- 15.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 16.Mari A, Tura A, Gastaldelli A, Ferrannini E. Assessing insulin secretion by modeling in multiple-meal tests: role of potentiation. Diabetes. 2002;51(Suppl 1):S221–226. doi: 10.2337/diabetes.51.2007.s221. [DOI] [PubMed] [Google Scholar]
- 17.Haire-Joshu D, Brownson RC, Nanney MS, Houston C, Steger-May K, Schechtman K, et al. Improving dietary behavior in African Americans: the Parents As Teachers High 5, Low Fat Program. Preventive medicine. 2003;36:684–691. doi: 10.1016/s0091-7435(03)00053-7. [DOI] [PubMed] [Google Scholar]
- 18.Haire-Joshu D, Elliott MB, Caito NM, Hessler K, Nanney MS, Hale N, et al. High 5 for Kids: the impact of a home visiting program on fruit and vegetable intake of parents and their preschool children. Preventive medicine. 2008;47:77–82. doi: 10.1016/j.ypmed.2008.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haire-Joshu DL, Schwarz CD, Peskoe SB, Budd EL, Brownson RC, Joshu CE. A group randomized controlled trial integrating obesity prevention and control for postpartum adolescents in a home visiting program. The international journal of behavioral nutrition and physical activity. 2015;12:88. doi: 10.1186/s12966-015-0247-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.