Abstract
The renaissance in the use of encephalography-based research methods to probe the pathophysiology of neuropsychiatric disorders is well afoot and continues to advance. Building on the platform of neuroimaging evidence on brain circuit models, magnetoencephalography, scalp electroencephalography, and even invasive electroencephalography are now being used to characterize brain network dysfunctions that underlie major depressive disorder using brain oscillation measurements and associated treatment responses. Such multiple encephalography modalities provide avenues to study pathologic network dynamics with high temporal resolution and over long time courses, opportunities to complement neuroimaging methods and findings, and new approaches to identify quantitative biomarkers that indicate critical targets for brain therapy. Such goals have been facilitated by the ongoing testing of novel invasive neuromodulation therapies, notably, deep brain stimulation, where clinically relevant treatment effects can be monitored at multiple brain sites in a time-locked causal manner. We review key brain rhythms identified in major depressive disorder as foundation for development of putative biomarkers for objectively evaluating neuromodulation success and for guiding deep brain stimulation or other target-based neuromodulation strategies for treatment-resistant depression patients.
Keywords: Depression, Electrophysiology, Neurocircuitry, Neuroimaging, Neuromodulation, Treatment-resistant
Over 10% of the world population has major depressive disorder (MDD), a disorder associated with the dysregulation of mood, cognition, sensorimotor physiology, and homeostatic processes (1). While treatments are available and generally effective, not all patients respond, leading to continued disability and morbidity. These clinical treatment challenges require new integrative models of disease pathophysiology and treatment effects. Complementary neuroimaging and electrophysiology techniques have emerged as critical contributors to meeting these challenges, providing a versatile platform to characterize brain circuit dysfunction underlying specific symptoms, as well as changes associated with their successful treatment. Discussed here are converging neuroimaging and electrophysiological findings with an eye toward the future application of multimodal network biometrics used in conjunction with targeted neuromodulation interventions for patients with treatment-resistant depression (TRD).
About 10% to 30% of MDD patients develop TRD, defined as MDD unresponsiveness to multiple standard antidepressant interventions (e.g., monotherapies or multiple drugs, psychotherapy) (2). Neuromodulation therapies (3–6) have taken on an increasingly primary role in the treatment of these patients, with the most invasive strategies, such as deep brain stimulation, providing a unique platform to directly modulate and record within specified neural circuits. Brain imaging has been critical to identifying the specific neural circuits to be targeted for neurostimulation.
Neuroimaging studies to date using different MDD cohorts and various interventions have defined a putative depression brain network mediating active symptoms and treatment effects (1,7–15). The most replicable findings involve limbic and cortical regions, notably frontal, ventral, and dorsal anterior cingulate; amygdala; hippocampus; and nucleus accumbens (Figure 1) [reviewed in (1,16)]. Recent studies further identify differential patterns in different patient subgroups using such diverse methods as glucose metabolism positron emission tomography (PET) (17,18), connectivity-based functional magnetic resonance imaging (fMRI) (19,20), diffusion tensor/tractography imaging (21–24), glutamate concentration magnetic resonance spectroscopy (25), and source-localized scalp electroencephalography (EEG) power spectra (26,27). These pattern variations suggest that further TRD subtyping, as well as subtype-specific target selection, may be necessary to optimize the various evolving neuromodulation strategies. Electrophysiology can extend this spatially grounded foundation by characterizing, with high temporal resolution, dynamic activity within putative disease networks determined from neuroimaging (Figure 1). As direct measures of neuron population activity, electrophysiology measures provide valuable windows into region-specific oscillations at millisecond time scales, with distinct oscillations carrying information from different brain processes (28,29). Particularly, for invasive interventions such as deep brain stimulation (DBS), precise anatomical targeting can be combined with simultaneous multimodal EEG and/or intracranial EEG to measure both local and remote network effects before, during, and after acute and chronic stimulation.
Figure 1.
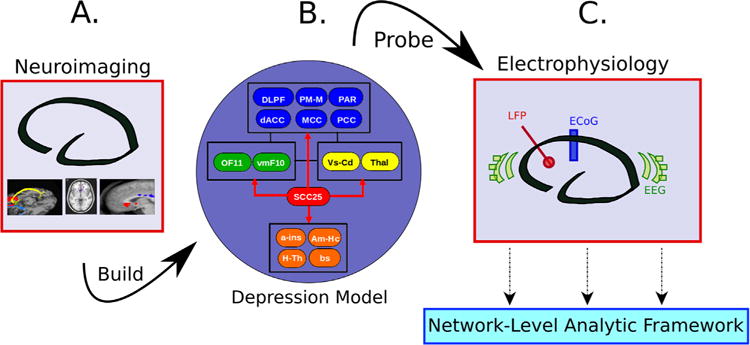
Operational pipeline for imaging-informed electrophysiology studies of depression. A multiregion model of depression (B) is constructed from the synthesis of structural and functional imaging findings (i.e., positron emission tomography, structural magnetic resonance imaging, functional magnetic resonance imaging, diffusion tensor/tractography imaging) (A), providing foundation for hypothesis-driven electrophysiological analyses (C). a-ins, anterior insula; Am-Hc, amygdala-hippocampus; bs, brainstem; dACC, dorsal anterior cingulate cortex; DLPF, dorsolateral prefrontal; ECoG, electrocorticography; EEG, electroencephalography; H-Th, hypothalamus; LFP, local field potential; MCC, mid-cingulate; OF11, orbitofrontal (Brodmann area 11); PAR, parietal; PCC, posterior cingulate; PM-M, premotor-motor; SCC25, subcallosal cingulate (Brodmann area 25); Thal, thalamus; vmF10, ventromedial frontal; Vs-Cd, ventral striatum-caudate.
In this article, we review 1) past studies of brain oscillations in MDD, 2) mechanistic studies of neuromodulation interventions, and 3) strategies for optimizing neuromodulation for TRD using integrated multimodal brain imaging and brain electrophysiology.
RESTING-STATE STUDIES OF BRAIN OSCILLATIONS IN MDD
Historically, noninvasive electrophysiology was the primary probe of brain activity in MDD and thus foundational for later studies using functional imaging methods such as PET, single-photon emission computed tomography, and fMRI. Particularly, alpha and theta rhythms dominated the published literature measured using primarily scalp EEG, findings that continue to guide and influence electrophysiology studies using contemporary tools.
Alpha rhythms, often defined in humans as 8 Hz to 12 Hz oscillations, reflect a potentially important mechanism of depression. The first rhythm seen by EEG discoverer Hans Berger, alpha oscillations have since been ascribed to decreased cortical activity or processing. Early models of depression posited differential activation of positive and negative affect systems (30) located in left and right frontal cortex, respectively (31,32). Demonstration of left frontal alpha increases and right frontal alpha decreases tracked with symptoms of depression (26). Since then, prefrontal rhythm asymmetries have been investigated as biomarkers of depression (33).
Resting alpha asymmetry between the frontal cortices has been shown to predict affective responses to emotive stimuli (34). Studies have also shown an association between increased left frontal alpha and greater depression severity scores (26,35). In a study investigating fluoxetine responder-nonresponder differences, alpha power was found to be similar between the groups, while alpha asymmetry demonstrated decreased alpha in the right hemispheres when compared with the left in the nonresponders (36). These results were seen to support alpha asymmetry as a putative biometric of depressive disease (37). However, enthusiasm for the use of this alpha asymmetry as a clinical biometric was somewhat attenuated by failure to demonstrate a consistent correlation between alpha activity and clinical state over the course of a depressive episode (38). On the other hand, the argument that variability in frontal alpha asymmetries represents a trait marker for depression risk, even outside actual episodes of illness (39), has had more direct support. While reports of alpha abnormalities in depression are among the most robust findings, the temporal instability of alpha asymmetry findings relative to depressive state (39,40) limits current reliance on alpha findings as trait marker of the illness (41,42). Alpha asymmetry over the parietotemporal regions has also been reported (38,43), again paralleling studies with other neuroimaging methods (44,45). Early investigations of whole-brain functional connectivity across the EEG spectrum have further demonstrated increased parietal-temporal alpha coherence (46,47) and distributed cortical synchrony in MDD (47). Such whole-brain level approaches are now the emerging standard, with a focus on identifying diagnostic and prognostic biometrics (48).
Recent investigations of alpha have examined functional connectivity (46), with elevated alpha coherence demonstrated in long-range connections between frontopolar and temporal regions in MDD patients (47). These studies, relying on EEG modalities with higher-order quantitative capabilities, represent a new approach for studying the role of alpha in distributed MDD networks.
More recent investigations using magnetoencephalography have thus far failed to confirm the utility of alpha asymmetry as a diagnostic marker, since both depressed and nondepressed subjects exhibit asymmetries at statistically similar rates (49). Notably, frontal alpha asymmetry, when present, was stable following clinically effective repetitive transcranial magnetic stimulation, suggesting an important role for alpha asymmetry variability as a reflection of depression trait but not in differentiating TRD from healthy control subjects or nonresponders from responders (49). Intracranial recordings from implanted DBS electrodes in TRD patients provide new support for a subcortical role for alpha. Recordings from both the subcallosal cingulate (Brodmann area 25) and the nucleus accumbens acquired in different patients in separate studies report increases in resting alpha rhythm in TRD patients when compared with obsessive-compulsive disorder patients (50). While there are no healthy or nondepressed patient control subjects with comparable recordings in these studies, this is the first direct evidence of striatal and limbic alpha rhythm dysfunction in MDD. The presence of alpha asymmetry and its behaviorally specific clinical correlates remains to be determined. Source localization techniques applied to high-density scalp EEG data may provide a needed bridge between these unique invasive recording opportunities and the findings generated using more conventional lower resolution EEG techniques in larger patient and control cohorts.
Theta rhythms, often defined in humans as 4 Hz to 8 Hz oscillations, reflect another potential pathophysiological marker of MDD. Known primarily for its central role in hippocampal circuits, theta has been interpreted as a driving rhythm for downstream regional activity—a possible mechanism for coherent memory and attention processing (51). In addition, theta rhythms are involved in pattern recognition within the place and grid cell systems (52,53). Theta oscillations have also been shown to have properties of a traveling wave within the limbic system, hinting at a richer mechanism of distributed theta-dependent function (54), especially in the context of limbic-cortical interactions and network-level, synchronized activity. Interactions between medial temporal regions and prefrontal cortex appear to be mediated by theta oscillations, revealing a key communication mechanism between regions implicated in memory, executive function (55,56), and depression (44). To date, the focus of studies in MDD have centered on mid-frontal theta and how antidepressant therapy affects its properties.
Mid-frontal theta rhythms have been localized to the anterior cingulate cortex (ACC) in some studies (57), with demonstrations of abnormal theta correlations within corticolimbic networks in MDD using measures of absolute power and low resolution electromagnetic tomography (14,58). Cordance, a composite metric that relates relative power within a band (i.e., the ratio of theta power to total power) to absolute power within that band, has been used to further link theta oscillations to regional perfusion and metabolic activity (59). Cordance studies have demonstrated decreased frontal theta in MDD patients (26,60,61). Thought to reflect ACC activity (60), mid-frontal theta has been proposed as both a potential predictor of antidepressant medication response (61,62) as well as a target of change following treatment (63,64), mirroring findings demonstrated using PET measures of glucose metabolism and blood flow (44,48,65,66).
Expanding on these previous observations, increases in frontal theta cordance seen after 1 month of continuous DBS predicted long-term clinical efficacy (67). Evidence of cingulate asymmetry in resting-state PET and functional connectivity fMRI studies across depression subtypes (44,68,69) suggests that lateralized electrophysiology signals might also be present in theta; however, this has yet to be reported. Further investigation, ideally using bilateral midline cortical sampling using invasive methods, is needed to dissociate lateralized theta changes in ACC from those in adjacent medial prefrontal cortex. This approach might also help to integrate reports of frontal alpha asymmetries with limbic theta patterns. Nonetheless, despite the consistent evidence for theta involvement in MDD, validity of frontal midline theta as a biomarker of depression remains inconsistent (70); further efforts to identify MDD subtypes using localized rhythm abnormalities may be a useful next step.
NEUROMODULATION STUDIES OF BRAIN OSCILLATIONS IN MDD
Neuromodulation is generally designed to impact function of a specific brain target, in turn changing brain network function and corresponding brain rhythms via connections between the target and other brain areas (71). Neuromodulation treatment for TRD is commercially available using various approaches including electroconvulsive therapy (ECT) (72,73), transcranial magnetic stimulation (TMS) (74,75), and vagus nerve stimulation (VNS) (76), with ongoing research evaluating the utility of transcranial direct current stimulation (tDCS) (77,78), magnetic seizure therapy (73,79,80), cortical brain stimulation (81–83), and DBS (69,84–88). Based on the biophysics of each neuromodulation technique, it is likely that each has different effects on neural rhythms. Furthermore, different approaches have different time courses to induce initial and maximal clinical effects. For instance, ECT and TMS can produce acute changes but generally require ongoing multiple sessions over weeks to achieve sustained antidepressant effects (72–75) and maximal VNS effects are reported often after months of ongoing stimulation (89,90). In evaluating rhythm changes with different methods, one must consider target symptoms, time line, and interactions.
While similarities and differences of the various neuromodulation approaches have not been explicitly investigated, differential patterns in oscillatory measures and network function have been identified that correlate with decreased depressive symptoms. ECT decreases ACC-dorsolateral pre-frontal cortex connectivity (91), increases connectivity within frontal cortices (92), and increases subcallosal cingulate cortex (SCC) and frontal theta oscillations (93,94). VNS increases delta oscillations in prefrontal, frontal, and central regions (90,95). TMS reduces connectivity between frontal and deep-brain nuclei (13,96,97); increases prefrontal gamma activity (98); decreases precuneus gamma activity (98); increases alpha rhythms in frontal (99) and dorsal ACC (100) areas; decreases and increases theta oscillations in SCC (101) and prefrontal cortex (102), respectively; decreases prefrontal delta signals (102); and alters activations in other structures (103). Likely due to differences in techniques (cortical targeting, parameters, duration and number of sessions), there is not yet a clear relationship between antidepressant effects and specific brain rhythms for tDCS (104–106). However, tDCS can decrease delta, theta, and alpha rhythms in frontal and cingulate areas in addition to both midline theta increases and alpha decreases (104–106). Such studies might be further extended using longer longitudinal time lines and standardized, multiple signal-acquisition modalities to track the chronology of clinical changes in depressive symptoms.
NETWORK ANALYSES TO STUDY OSCILLATIONS IN MDD
Together, resting-state and neuromodulation studies of MDD oscillations reflect the diseased network’s baseline activity and the network’s response to an induced state change, respectively. The next step is the development of analytic tools and models to integrate these various recording modalities across stimulation techniques within a network perspective.
Toward this goal, higher-order metrics and network-level analyses can extend band-limited power results by explicitly incorporating activity timing information present in rhythm phase. Oscillations have frequencies and magnitudes, often well characterized in basic electrophysiological methods, but oscillation phase may also contribute to region-region interactions and is thought to contain a large portion of the information content of neuronal oscillations (107). Many phase-based analysis techniques have been applied to neural systems, revealing previously unseen dynamics within and between brain regions during physiology and disease (108–110). Here, we will focus on phase locking, coherence, and cross-frequency coupling (CFC) as vital tools for multimodal, multiregional electrophysiological studies in MDD.
Phase locking is an analysis to examine the rapid dynamics in regional brain rhythms that are often lost in power-based analyses; such losses are attributed to large averaging epochs, on the order of minutes. In support of this general observation, longer-range temporal correlation in theta rhythms, occurring at rapid time scales on the order of seconds, has been found to be absent in MDD patients (111). Some phase-based methods have shown transient intervals of phase-locked alpha asymmetry, requiring smaller epoch lengths and a characterization of the phase of ongoing EEG activity (112). Abnormal phase relationships between distinct MDD network nodes may further give rise to mistimed communication between regions, even with unchanged power.
Coherence can quantify spectral synchrony between brain oscillations from distinct regions. Coherence is currently used to determine functional connectivity within distributed electrophysiology channels (47,113). Applied to MDD, long-distance coherence between regions has been shown to increase in alpha and theta rhythms (47,114). Coherence has already demonstrated utility as a functional metric, but extending its use to track neuromodulation effects can more fully probe node-to-node communication at millisecond time scale.
CFC, while not yet applied to MDD, provides a set of methods to quantify the interaction of different brain oscillations. Phase-amplitude coupling (PAC), a type of CFC, determines the statistical relationship between the phase of low-frequency oscillations and the amplitude of high-frequency oscillations (115). PAC is hypothesized to reflect a mechanism by which different subcortical regions exchange control over cortical targets (116). PAC studies have shown rapid changes during learning (117), decision making and reward encoding (118,119), and even disease correction (120). A recent study in Parkinson’s disease demonstrated large clinically relevant changes in coupling between beta and gamma rhythms in the pathway connecting motor cortex and subthalamic nucleus pathway with acute subthalamic nucleus stimulation. This study is particularly noteworthy as it leveraged intraoperative DBS to record multiple structures to link oscillatory changes to clinical effects (120). Furthermore, demonstrations of distinct phase-amplitude couplings across different networks may indicate communication responsible for binding specific regions to elements of a single task (116). Similar methods can be applied in MDD, and animal models have already taken this approach using both multiregion unit activity and field potentials in both genetic and stress models of disease (117,121–126).
INTEGRATED MULTIMODAL FRAMEWORK USING DBS FOR TRD
DBS provides a highly versatile platform to access, test, integrate, and extend these disparate oscillatory findings identified in previous neuromodulation and electrophysiological studies of MDD. Like other neuromodulation techniques, network effects can be electrically triggered, recorded by diverse noninvasive or invasive modalities, and quantified by multiple signal processing techniques within and across modalities over time. Unique to DBS is the anatomical precision of both stimulation and recording within deep network structures. To date, research groups have examined six different DBS targets in TRD patients: the SCC white matter (69), the ventral striatum/ventral anterior internal capsule (84), the nucleus accumbens (85), the inferior thalamic peduncle (87), the lateral habenula (86), and the medial forebrain bundle (88). While there is anatomical evidence that these regions share many structural connections (127–129), it is untested if DBS at these various targets evoke similar quantitative neurophysiology effects. As such, measuring local and remote brain oscillations for each electrically stimulated target will provide important opportunities to test shared and disjoint attributes of different DBS approaches within different theoretical depression models (Figure 2). Further, different targets may optimally impact specific depressive symptoms: inhibiting negative mood may be best achieved with stimulation of the SCC white matter, while motivation and learning may be optimally induced using stimulation of the ventral striatum/ventral anterior internal capsule or medial forebrain bundle. A target comparison strategy addressing the time course of stimulation-induced changes in distinct symptoms or behaviors may also be required to fully delineate DBS effects in MDD toward optimizing the selection of a procedure most likely to result in clinical remission for a given patient (130–132).
Figure 2.
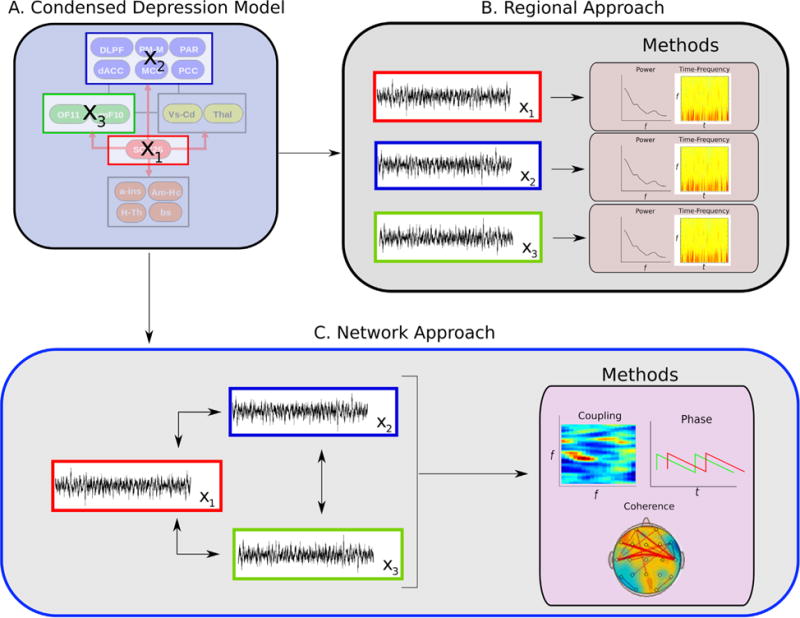
Analytic framework for characterizing regional interactions within a given depression model. Following selection of putative targets (A) derived from a designated multiregion model, two signal processing strategies are implemented: an intraregion approach (B) that uses methods that analyze each node independently and an interregion network approach (C) that considers the interaction between multiple nodes.
There are several examples of DBS studies in MDD patients utilizing scalp or intracranial electrophysiology, but none to date address DBS-specific antidepressant mechanisms. One study found that an increase in frontal theta cordance after 1 month of DBS correlated with the eventual magnitude of long-term antidepressant response to chronic DBS but found no additional differential changes with actual response (67). Other studies have exploited the opportunity to directly measure activity at the DBS target, allowing for direct linking of behavior to the activity of the brain region being modulated, independent of actual stimulation effects (131–134). Such studies have generally been performed in the operating room or in the days thereafter. In separate studies, SCC alpha power was positively correlated with baseline depression severity scores (50); single neurons in the SCC were shown to be preferentially activated by negatively relative to positively valenced emotional images (131); SCC beta coherence changes predicted subsequent decisions in an affective valuation task (134); and the accumbens activity increased in anticipation of an impending reward (118,132). These studies generally confirm previous findings using other imaging modalities. While first observations of the chronology of response-specific changes in negative bias with DBS have been recently reported using scalp EEG (130), longitudinal studies of subcortical electrophysiological changes are not yet available knowledge. Nonetheless, such findings do inform on node and network properties of MDD and perhaps more specifically on TRD dysfunction, but studies thus far provide limited information about mechanisms mediating either acute stimulation-induced rhythmic effects or long-term antidepressant response.
Evolving strategies to address antidepressant mechanisms of DBS are now undergoing testing and development. For example, in studies of SCC DBS for TRD, it has been shown that precise localization of the intended DBS implantation target can be optimized using preoperative diffusion imaging and white matter tractography to define the intersection of the uncinate fasciculus, cingulum, and forceps minor as in and around the SCC (21). Immediately upon implantation of DBS therapy electrodes, detailed measurements of basal activity at the precise target of stimulation can be recorded, confirming localization of the intended contact within the gray-white matter junction. In addition, potential behavioral correlates and changes in local activity with and without therapeutic doses of stimulation can be characterized. Until recently, SCC recordings were restricted to intraoperative recordings or those performed in the immediate postoperative period with externalized leads (50,131,133,134). Devices are now available that not only deliver therapeutic stimulation but also record ongoing oscillations directly off the implanted DBS electrodes (135–138). Consequently, studies can now measure activity changes in targeted regions of interest longitudinally at a high temporal resolution. Integration of signals from multiple regions of interest will require multielectrode data acquisition and multichannel analyses to fully capture mechanistic interactions within the MDD network (Figure 2). Additionally, such studies can be combined with complementary sampling of whole-brain activity changes using scalp EEG, PET, and fMRI (139–141) to define a more comprehensive view of DBS effects throughout the network over time.
The ultimate end product of such multimodal studies will be reliable and sensitive biomarkers of antidepressant response at the cellular and systems levels that guide the optimization of current DBS protocols and facilitate the development of next generation research strategies and medical devices. Toward these goals, current generation devices are now being leveraged using multiple measurements at strategic time points within an individual clinical research trial. Preoperative multi-modal neurophysiology (e.g., PET, fMRI, diffusion tensor/tractography imaging, and EEG) combined with behavioral and psychophysiological measures provides first estimates of the network of interest and eventually of the most appropriate DBS stimulation target based on combined behavioral biometrics and imaging-based inclusion criteria (17,18,142) (Figure 1). Surgical implantation utilizes predefined network maps of individualized white matter pathways to ensure optimal network targeting (21,24) with new methods developing more refined models of likely electrophysiological effects (24,143). Lastly, preoperative, intraoperative, and postoperative multimodal encephalography define, confirm, and help to track acute, subacute, and chronic changes indicative of target engagement (Figures 1 and 2) (136,144,145). Such applications of multimodal encephalography can thus directly inform on mechanisms mediating DBS-induced antidepressant effects at the neuronal level with implications for ongoing, evidence-based modification of algorithms for treatment delivery. Availability of next generation devices for longterm intra-cranial EEG monitoring has already demonstrated feasibility and clinical utility in studies of epilepsy and Parkinson’s disease (120,146,147). Such studies further serve as models for advancing studies of DBS for MDD and for rational development of noninvasive approaches (12). Determining the least invasive and most reliable combination(s) of brain signal modalities, neuromodulation methods, and stimulation targets to achieve the most effective antidepressant response for a given patient is the ultimate goal.
Acknowledgments
Supported in part by Grants from the Dana Foundation, Woodruff Fund, Stanley Medical Research Institute, and Hope for Depression Research Foundation and the National Institute of Neurological Disorders and Stroke (3R01NS079268-02S1) with work performed under Food and Drug Administration physician-sponsored investigational device exemptions G060028 and G130107.
Dr. Mayberg has received consulting and intellectual property licensing fees from St. Jude Medical, Inc.
Footnotes
DISCLOSURES
Other authors report no biomedical financial interests or potential conflicts of interest.
Contributor Information
Otis Lkuwamy Smart, Department of Neurosurgery, Emory University School of Medicine.
Vineet Ravi Tiruvadi, Department of Biomedical Engineering, Georgia Institute of Technology, Emory University School of Medicine.
Helen S. Mayberg, Departments of Psychiatry, Neurology, and Radiology, Emory University School of Medicine, Atlanta, Georgia
References
- 1.Mayberg HS. Targeted electrode-based modulation of neural circuits for depression. J Clin Invest. 2009;119:717–725. doi: 10.1172/JCI38454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kiening K, Sartorius A. A new translational target for deep brain stimulation to treat depression. EMBO Mol Med. 2013;5:1151–1153. doi: 10.1002/emmm.201302947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Howland RH, Shutt LS, Berman SR, Spotts CR, Denko T. The emerging use of technology for the treatment of depression and other neuropsychiatric disorders. Ann Clin Psychiatry. 2011;23:48–62. [PubMed] [Google Scholar]
- 4.Lipsman N, Sankar T, Downar J, Kennedy SH, Lozano AM, Giacobbe P. Neuromodulation for treatment-refractory major depressive disorder. CMAJ. 2014;186:33–39. doi: 10.1503/cmaj.121317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cook IA, Espinoza R, Leuchter AF. Neuromodulation for depression: Invasive and noninvasive (deep brain stimulation, transcranial magnetic stimulation, trigeminal nerve stimulation) Neurosurg Clin N Am. 2014;25:103–116. doi: 10.1016/j.nec.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 6.Wani A, Trevino K, Marnell P, Husain MM. Advances in brain stimulation for depression. Ann Clin Psychiatry. 2013;25:217–224. [PubMed] [Google Scholar]
- 7.Davidson RJ, Pizzagalli D, Nitschke JB, Putnam K. Depression: Perspectives from affective neuroscience. Annu Rev Psychol. 2002;53:545–574. doi: 10.1146/annurev.psych.53.100901.135148. [DOI] [PubMed] [Google Scholar]
- 8.Carlson PJ, Singh JB, Zarate CA, Jr, Drevets WC, Manji HK. Neural circuitry and neuroplasticity in mood disorders: Insights for novel therapeutic targets. NeuroRx. 2006;3:22–41. doi: 10.1016/j.nurx.2005.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Greicius M. Resting-state functional connectivity in neuropsychiatric disorders. Curr Opin Neurol. 2008;21:424–430. doi: 10.1097/WCO.0b013e328306f2c5. [DOI] [PubMed] [Google Scholar]
- 10.Hamilton JP, Etkin A, Furman DJ, Lemus MG, Johnson RF, Gotlib IH. Functional neuroimaging of major depressive disorder: A meta-analysis and new integration of base line activation and neural response data. Am J Psychiatry. 2012;169:693–703. doi: 10.1176/appi.ajp.2012.11071105. [DOI] [PubMed] [Google Scholar]
- 11.Menon V. Large-scale brain networks and psychopathology: A unifying triple network model. Trends Cogn Sci. 2011;15:483–506. doi: 10.1016/j.tics.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 12.Fox MD, Buckner RL, Liu H, Chakravarty MM, Lozano AM, Pascual-Leone A. Resting-state networks link invasive and noninvasive brain stimulation across diverse psychiatric and neurological diseases. Proc Natl Acad Sci U S A. 2014;111:E4367–E4375. doi: 10.1073/pnas.1405003111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liston C, Chen AC, Zebley BD, Drysdale AT, Gordon R, Leuchter B, et al. Default mode network mechanisms of transcranial magnetic stimulation in depression. Biol Psychiatry. 2014;76:517–526. doi: 10.1016/j.biopsych.2014.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pizzagalli DA, Oakes TR, Davidson RJ. Coupling of theta activity and glucose metabolism in the human rostral anterior cingulate cortex: An EEG/PET study of normal and depressed subjects. Psychophysiology. 2003;40:939–949. doi: 10.1111/1469-8986.00112. [DOI] [PubMed] [Google Scholar]
- 15.Hoflich A, Savli M, Comasco E, Moser U, Novak K, Kasper S, Lanzenberger R. Neuropsychiatric deep brain stimulation for translational neuroimaging. Neuroimage. 2013;79:30–41. doi: 10.1016/j.neuroimage.2013.04.065. [DOI] [PubMed] [Google Scholar]
- 16.Price JL, Drevets WC. Neural circuits underlying the pathophysiology of mood disorders. Trends Cogn Sci. 2012;16:61–71. doi: 10.1016/j.tics.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 17.McGrath CL, Kelley ME, Holtzheimer PE, Dunlop BW, Craighead WE, Franco AR, et al. Toward a neuroimaging treatment selection biomarker for major depressive disorder. JAMA Psychiatry. 2013;70:821–829. doi: 10.1001/jamapsychiatry.2013.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McGrath CL, Kelley ME, Dunlop BW, Holtzheimer PE, 3rd, Craighead WE, Mayberg HS. Pretreatment brain states identify likely nonresponse to standard treatments for depression. Biol Psychiatry. 2014;76:527–535. doi: 10.1016/j.biopsych.2013.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fox MD, Buckner RL, White MP, Greicius MD, Pascual-Leone A. Efficacy of transcranial magnetic stimulation targets for depression is related to intrinsic functional connectivity with the subgenual cingulate. Biol Psychiatry. 2012;72:595–603. doi: 10.1016/j.biopsych.2012.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fox MD, Liu H, Pascual-Leone A. Identification of reproducible individualized targets for treatment of depression with TMS based on intrinsic connectivity. Neuroimage. 2012;66C:151–160. doi: 10.1016/j.neuroimage.2012.10.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Riva-Posse P, Choi KS, Holtzheimer PE, McIntyre CC, Gross RE, Chaturvedi A, et al. Defining critical white matter pathways mediating successful subcallosal cingulate deep brain stimulation for treatment-resistant depression. Biol Psychiatry. 2014;76:963–969. doi: 10.1016/j.biopsych.2014.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lujan JL, Chaturvedi A, Malone DA, Rezai AR, Machado AG, McIntyre CC. Axonal pathways linked to therapeutic and nontherapeutic outcomes during psychiatric deep brain stimulation. Hum Brain Mapp. 2012;33:958–968. doi: 10.1002/hbm.21262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lujan JL, Chaturvedi A, McIntyre CC. Tracking the mechanisms of deep brain stimulation for neuropsychiatric disorders. Front Biosci. 2008;13:5892–5904. doi: 10.2741/3124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lujan JL, Chaturvedi A, Choi KS, Holtzheimer PE, Gross RE, Mayberg HS, McIntyre CC. Tractography-activation models applied to subcallosal cingulate deep brain stimulation. Brain Stimul. 2013;6:737–739. doi: 10.1016/j.brs.2013.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen LP, Dai HY, Dai ZZ, Xu CT, Wu RH. Anterior cingulate cortex and cerebellar hemisphere neurometabolite changes in depression treatment: A 1H magnetic resonance spectroscopy study. Psychiatry Clin Neurosci. 2014;68:357–364. doi: 10.1111/pcn.12138. [DOI] [PubMed] [Google Scholar]
- 26.Saletu B, Anderer P, Saletu-Zyhlarz GM. EEG topography and tomography (LORETA) in diagnosis and pharmacotherapy of depression. Clin EEG Neurosci. 2010;41:203–210. [Google Scholar]
- 27.Korb AS, Hunter AM, Cook IA, Leuchter AF. Rostral anterior cingulate cortex theta current density and response to antidepressants and placebo in major depression. Clin Neurophysiol. 2009;120:1313–1319. doi: 10.1016/j.clinph.2009.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buzsáki G, Draguhn A. Neuronal oscillations in cortical networks. Science. 2004;304:1926–1929. doi: 10.1126/science.1099745. [DOI] [PubMed] [Google Scholar]
- 29.Buzsaki G, Logothetis N, Singer W. Scaling brain size, keeping timing: Evolutionary preservation of brain rhythms. Neuron. 2013;80:751–764. doi: 10.1016/j.neuron.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tucker DM, Stenslie CE, Roth RS, Shearer SL. Right frontal lobe activation and right hemisphere performance. Decrement during a depressed mood. Arch Gen Psychiatry. 1981;38:169–174. doi: 10.1001/archpsyc.1981.01780270055007. [DOI] [PubMed] [Google Scholar]
- 31.Neuper C, Pfurtscheller G. Event-related dynamics of cortical rhythms: Frequency-specific features and functional correlates. Int J Psychophysiol. 2001;43:41–58. doi: 10.1016/s0167-8760(01)00178-7. [DOI] [PubMed] [Google Scholar]
- 32.Davidson RJ. Affective style and affective disorders: Perspectives from affective neuroscience. Cogn Emot. 1998;12:307–330. [Google Scholar]
- 33.Allen JJB, Urry HL, Hitt SK, Coan JA. The stability of resting frontal electroencephalographic asymmetry in depression. Psychophysiology. 2004;41:269–280. doi: 10.1111/j.1469-8986.2003.00149.x. [DOI] [PubMed] [Google Scholar]
- 34.Wheeler RE, Davidson RJ, Tomarken AJ. Frontal brain asymmetry and emotional reactivity: A biological substrate of affective style. Psychophysiology. 1993;30:82–89. doi: 10.1111/j.1469-8986.1993.tb03207.x. [DOI] [PubMed] [Google Scholar]
- 35.Davidson RJ, Slagter HA. Probing emotion in the developing brain: Functional neuroimaging in the assessment of the neural substrates of emotion in normal and disordered children and adolescents. Ment Retard Dev Disabil Res Rev. 2000;6:166–170. doi: 10.1002/1098-2779(2000)6:3<166::AID-MRDD3>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 36.Bruder GE, Stewart JW, Tenke CE, McGrath PJ, Leite P, Bhattacharya N, Quitkin FM. Electroencephalographic and perceptual asymmetry differences between responders and nonresponders to an SSRI antidepressant. Biol Psychiatry. 2001;49:416–425. doi: 10.1016/s0006-3223(00)01016-7. [DOI] [PubMed] [Google Scholar]
- 37.Jaworska N, Blier P, Fusee W, Knott V. α Power, alpha asymmetry and anterior cingulate cortex activity in depressed males and females. J Psychiatr Res. 2012;46:1483–1491. doi: 10.1016/j.jpsychires.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bruder GE, Fong R, Tenke CE, Leite P, Towey JP, Stewart JE, et al. Regional brain asymmetries in major depression with or without an anxiety disorder: A quantitative electroencephalographic study. Biol Psychiatry. 1997;41:939–948. doi: 10.1016/S0006-3223(96)00260-0. [DOI] [PubMed] [Google Scholar]
- 39.Debener S, Beauducel A, Nessler D, Brocke B, Heilemann H, Kayser J. Is resting anterior eeg alpha asymmetry a trait marker for depression? Neuropsychobiology. 2000;41:31–37. doi: 10.1159/000026630. [DOI] [PubMed] [Google Scholar]
- 40.Pizzagalli DA, Nitschke JB, Oakes TR, Hendrick AM, Horras KA, Larson CL, et al. Brain electrical tomography in depression: The importance of symptom severity, anxiety, and melancholic features. Biol Psychiatry. 2002;52:73–85. doi: 10.1016/s0006-3223(02)01313-6. [DOI] [PubMed] [Google Scholar]
- 41.Carvalho A, Moraes H, Silveira H, Ribeiro P, Piedade RA, Deslandes AC, et al. EEG frontal asymmetry in the depressed and remitted elderly: Is it related to the trait or to the state of depression? J Affect Disord. 2011;129:143–148. doi: 10.1016/j.jad.2010.08.023. [DOI] [PubMed] [Google Scholar]
- 42.Segrave RA, Cooper NR, Thomson RH, Croft RJ, Sheppard DM, Fitzgerald PB. Individualized alpha activity and frontal asymmetry in major depression. Clin EEG Neurosci. 2011;42:45–52. doi: 10.1177/155005941104200110. [DOI] [PubMed] [Google Scholar]
- 43.Kentgen LM, Tenke CE, Pine DS, Fong R, Klein RG, Bruder GE. Electroencephalographic asymmetries in adolescents with major depression: Influence of comorbidity with anxiety disorders. J Abnorm Psychol. 2000;109:797–802. doi: 10.1037//0021-843x.109.4.797. [DOI] [PubMed] [Google Scholar]
- 44.Mayberg HS. Limbic-cortical dysregulation: A proposed model of depression. J Neuropsychiatry Clin Neurosci. 1997;9:471–481. doi: 10.1176/jnp.9.3.471. [DOI] [PubMed] [Google Scholar]
- 45.Anand A, Li Y, Wang Y, Wu J, Gao S, Bukhari L, et al. Activity and connectivity of brain mood regulating circuit in depression: A functional magnetic resonance study. Biol Psychiatry. 2005;57:1079–1088. doi: 10.1016/j.biopsych.2005.02.021. [DOI] [PubMed] [Google Scholar]
- 46.Fingelkurts AA, Fingelkurts AA, Rytsälä H, Suominen K, Isometsä E, Kähkönen S. Impaired functional connectivity at EEG alpha and theta frequency bands in major depression. Hum Brain Mapp. 2007;28:247–261. doi: 10.1002/hbm.20275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Leuchter AF, Cook IA, Hunter AM, Cai C, Horvath S. Resting-state quantitative electroencephalography reveals increased neurophysiologic connectivity in depression. PLoS One. 2012;7:e32508. doi: 10.1371/journal.pone.0032508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kennedy SH, Konarski JZ, Segal ZV, Lau MA, Bieling PJ, McIntyre RS, Mayberg HS. Differences in brain glucose metabolism between responders to CBT and venlafaxine in a 16-week randomized controlled trial. Am J Psychiatry. 2007;164:778–788. doi: 10.1176/ajp.2007.164.5.778. [DOI] [PubMed] [Google Scholar]
- 49.Li CT, Chen LF, Tu PC, Wang SJ, Chen MH, Su TP, Hsieh JC. Impaired prefronto-thalamic functional connectivity as a key feature of treatment-resistant depression: A combined MEG, PET and rTMS study. PLoS One. 2013;8:e70089–e70089. doi: 10.1371/journal.pone.0070089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Neumann WJ, Huebl J, Brucke C, Gabriels L, Bajbouj M, Merkl A, et al. Different patterns of local field potentials from limbic DBS targets in patients with major depressive and obsessive compulsive disorder. Mol Psychiatry. 2014;19:1186–1192. doi: 10.1038/mp.2014.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Green JD, Arduini AA. Hippocampal electrical activity in arousal. J Neurophysiol. 1954;17:533–557. doi: 10.1152/jn.1954.17.6.533. [DOI] [PubMed] [Google Scholar]
- 52.Colgin LL. Mechanisms and functions of theta rhythms. Annu Rev Neurosci. 2013;36:295–312. doi: 10.1146/annurev-neuro-062012-170330. [DOI] [PubMed] [Google Scholar]
- 53.Buzsaki G. Theta oscillations in the hippocampus. Neuron. 2002;33:325–340. doi: 10.1016/s0896-6273(02)00586-x. [DOI] [PubMed] [Google Scholar]
- 54.Lubenov EV, Siapas AG. Hippocampal theta oscillations are travelling waves. Nature. 2009;459:534–539. doi: 10.1038/nature08010. [DOI] [PubMed] [Google Scholar]
- 55.Hyman JM, Hasselmo ME, Seamans JK. What is the functional relevance of prefrontal cortex entrainment to hippocampal theta rhythms? Front Neurosci. 2011;5:24–24. doi: 10.3389/fnins.2011.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mizuhara H, Yamaguchi Y. Human cortical circuits for central executive function emerge by theta phase synchronization. Neuroimage. 2007;36:232–244. doi: 10.1016/j.neuroimage.2007.02.026. [DOI] [PubMed] [Google Scholar]
- 57.Ishii R, Shinosaki K, Ukai S, Inouye T, Ishihara T, Yoshimine T, et al. Medial prefrontal cortex generates frontal midline theta rhythm. Neuroreport. 1999;10:675–679. doi: 10.1097/00001756-199903170-00003. [DOI] [PubMed] [Google Scholar]
- 58.Pascual-Marqui RD, Michel CM, Lehmann D. Low resolution electromagnetic tomography: A new method for localizing electrical activity in the brain. Int J Psychophysiol. 1994;18:49–65. doi: 10.1016/0167-8760(84)90014-x. [DOI] [PubMed] [Google Scholar]
- 59.Leuchter AF, Cook IA, Lufkin RB, Dunkin J, Newton TF, Cummings JL, et al. Cordance: A new method for assessment of cerebral perfusion and metabolism using quantitative electroencephalography. Neuroimage. 1994;1:208–219. doi: 10.1006/nimg.1994.1006. [DOI] [PubMed] [Google Scholar]
- 60.Mitchell DJ, McNaughton N, Flanagan D, Kirk IJ. Frontal-midline theta from the perspective of hippocampal “theta”. Prog Neurobiol. 2008;86:156–185. doi: 10.1016/j.pneurobio.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 61.Mulert C, Juckel G, Brunnmeier M, Karch S, Leicht G, Mergl R, et al. Rostral anterior cingulate cortex activity in the theta band predicts response to antidepressive medication. Clin EEG Neurosci. 2007;38:78–81. doi: 10.1177/155005940703800209. [DOI] [PubMed] [Google Scholar]
- 62.Korb AS, Hunter AM, Cook IA, Leuchter AF. Rostral anterior cingulate cortex activity and early symptom improvement during treatment for major depressive disorder. Psychiatry Res. 2011;192:188–194. doi: 10.1016/j.pscychresns.2010.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Knott VJ, Telner JI, Lapierre YD, Browne M, Horn ER. Quantitative EEG in the prediction of antidepressant response to imipramine. J Affect Disord. 1996;39:175–184. doi: 10.1016/0165-0327(96)00003-1. [DOI] [PubMed] [Google Scholar]
- 64.Landolt HP, Gillin JC. Different effects of phenelzine treatment on EEG topography in waking and sleep in depressed patients. Neuropsychopharmacology. 2002;27:462–469. doi: 10.1016/S0893-133X(02)00322-6. [DOI] [PubMed] [Google Scholar]
- 65.Mayberg HS. Positron emission tomography imaging in depression: A neural systems perspective. Neuroimaging Clin N Am. 2003;13:805–815. doi: 10.1016/s1052-5149(03)00104-7. [DOI] [PubMed] [Google Scholar]
- 66.Saxena S, Brody AL, Ho ML, Zohrabi N, Maidment KM, Baxter LR., Jr Differential brain metabolic predictors of response to paroxetine in obsessive-compulsive disorder versus major depression. Am J Psychiatry. 2003;160:522–532. doi: 10.1176/appi.ajp.160.3.522. [DOI] [PubMed] [Google Scholar]
- 67.Broadway JM, Holtzheimer PE, Hilimire MR, Parks NA, Devylder JE, Mayberg HS, Corballis PM. Frontal theta cordance predicts 6-month antidepressant response to subcallosal cingulate deep brain stimulation for treatment-resistant depression: A pilot study. Neuropsychopharmacology. 2012;37:1764–1772. doi: 10.1038/npp.2012.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Drevets WC, Price JL, Simpson JR, Jr, Todd RD, Reich T, Vannier M, Raichle ME. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386:824–827. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- 69.Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, et al. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45:651–660. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 70.Gold C, Fachner J, Erkkila J. Validity and reliability of electroencephalographic frontal alpha asymmetry and frontal midline theta as biomarkers for depression. Scand J Psychol. 2013;54:118–126. doi: 10.1111/sjop.12022. [DOI] [PubMed] [Google Scholar]
- 71.Holtzheimer PE, Mayberg HS. Neuromodulation for treatment-resistant depression. F1000 Med Rep. 2012;4:22. doi: 10.3410/M4-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Eschweiler GW, Vonthein R, Bode R, Huell M, Conca A, Peters O, et al. Clinical efficacy and cognitive side effects of bifrontal versus right unilateral electroconvulsive therapy (ECT): A short-term randomised controlled trial in pharmaco-resistant major depression. J Affect Disord. 2007;101:149–157. doi: 10.1016/j.jad.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 73.Kayser S, Bewernick BH, Grubert C, Hadrysiewicz BL, Axmacher N, Schlaepfer TE. Antidepressant effects, of magnetic seizure therapy and electroconvulsive therapy, in treatment-resistant depression. J Psychiatr Res. 2011;45:569–576. doi: 10.1016/j.jpsychires.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 74.Johnson KA, Baig M, Ramsey D, Lisanby SH, Avery D, McDonald WM, et al. Prefrontal rTMS for treating depression: Location and intensity results from the OPT-TMS multi-site clinical trial. Brain Stimul. 2013;6:108–117. doi: 10.1016/j.brs.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gaynes BN, Lloyd SW, Lux L, Gartlehner G, Hansen RA, Brode S, et al. Repetitive transcranial magnetic stimulation for treatment-resistant depression: A systematic review and meta-analysis. J Clin Psychiatry. 2014;75:477–489. doi: 10.4088/JCP.13r08815. quiz 489. [DOI] [PubMed] [Google Scholar]
- 76.Aaronson ST, Carpenter LL, Conway CR, Reimherr FW, Lisanby SH, Schwartz TL, et al. Vagus nerve stimulation therapy randomized to different amounts of electrical charge for treatment-resistant depression: Acute and chronic effects. Brain Stimul. 2013;6:631–640. doi: 10.1016/j.brs.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 77.Murphy DN, Boggio P, Fregni F. Transcranial direct current stimulation as a therapeutic tool for the treatment of major depression: Insights from past and recent clinical studies. Curr Opin Psychiatry. 2009;22:306–311. doi: 10.1097/YCO.0b013e32832a133f. [DOI] [PubMed] [Google Scholar]
- 78.Ferrucci R, Bortolomasi M, Vergari M, Tadini L, Salvoro B, Giacopuzzi M, et al. Transcranial direct current stimulation in severe, drug-resistant major depression. J Affect Disord. 2009;118:215–219. doi: 10.1016/j.jad.2009.02.015. [DOI] [PubMed] [Google Scholar]
- 79.Kosel M, Frick C, Lisanby SH, Fisch HU, Schlaepfer TE. Magnetic seizure therapy improves mood in refractory major depression. Neuropsychopharmacology. 2003;28:2045–2048. doi: 10.1038/sj.npp.1300293. [DOI] [PubMed] [Google Scholar]
- 80.Kayser S, Bewernick BH, Hurlemann R, Soehle M, Schlaepfer TE. Comparable seizure characteristics in magnetic seizure therapy and electroconvulsive therapy for major depression. Eur Neuropsychopharmacol. 2013;23:1541–1550. doi: 10.1016/j.euroneuro.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 81.Kopell BH, Halverson J, Butson CR, Dickinson M, Bobholz J, Harsch H, et al. Epidural cortical stimulation of the left dorsolateral prefrontal cortex for refractory major depressive disorder. Neurosurgery. 2011;69:1015–1029. doi: 10.1227/NEU.0b013e318229cfcd. discussion 1029. [DOI] [PubMed] [Google Scholar]
- 82.Nahas Z, Anderson BS, Borckardt J, Arana AB, George MS, Reeves ST, Takacs I. Bilateral epidural prefrontal cortical stimulation for treatment-resistant depression. Biol Psychiatry. 2010;67:101–109. doi: 10.1016/j.biopsych.2009.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kopell BH, Kondziolka D, Dougherty DD, Howland R, Harsch HH, Halverson JL, et al. Feasibility study of the safety and effectiveness of an implantable cortical stimulation system for subjects with major depression: 863. Neurosurgery. 2007;61:215. [Google Scholar]
- 84.Malone DA, Jr, Dougherty DD, Rezai AR, Carpenter LL, Friehs GM, Eskandar EN, et al. Deep brain stimulation of the ventral capsule/ventral striatum for treatment-resistant depression. Biol Psychiatry. 2009;65:267–275. doi: 10.1016/j.biopsych.2008.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bewernick BH, Hurlemann R, Matusch A, Kayser S, Grubert C, Hadrysiewicz B, et al. Nucleus accumbens deep brain stimulation decreases ratings of depression and anxiety in treatment-resistant depression. Biol Psychiatry. 2010;67:110–116. doi: 10.1016/j.biopsych.2009.09.013. [DOI] [PubMed] [Google Scholar]
- 86.Sartorius A, Henn FA. Deep brain stimulation of the lateral habenula in treatment resistant major depression. Med Hypotheses. 2007;69:1305–1308. doi: 10.1016/j.mehy.2007.03.021. [DOI] [PubMed] [Google Scholar]
- 87.Jimenez F, Velasco F, Salin-Pascual R, Hernandez JA, Velasco M, Criales JL, Nicolini H. A patient with a resistant major depression disorder treated with deep brain stimulation in the inferior thalamic peduncle. Neurosurgery. 2005;57:585–593. doi: 10.1227/01.neu.0000170434.44335.19. [DOI] [PubMed] [Google Scholar]
- 88.Schlaepfer TE, Bewernick BH, Kayser S, Madler B, Coenen VA. Rapid effects of deep brain stimulation for treatment-resistant major depression. Biol Psychiatry. 2013;73:1204–1212. doi: 10.1016/j.biopsych.2013.01.034. [DOI] [PubMed] [Google Scholar]
- 89.Dell’Osso B, Oldani L, Palazzo MC, Balossi I, Ciabatti M, Altamura AC. Vagus nerve stimulation in treatment-resistant depression: Acute and follow-up results of an italian case series. J ECT. 2013;29:41–44. doi: 10.1097/YCT.0b013e3182735ef0. [DOI] [PubMed] [Google Scholar]
- 90.Nahas Z, Teneback C, Chae JH, Mu Q, Molnar C, Kozel FA, et al. Serial vagus nerve stimulation functional MRI in treatment-resistant depression. Neuropsychopharmacology. 2007;32:1649–1660. doi: 10.1038/sj.npp.1301288. [DOI] [PubMed] [Google Scholar]
- 91.Beall EB, Malone DA, Dale RM, Muzina DJ, Koenig KA, Bhattacharrya PK, et al. Effects of electroconvulsive therapy on brain functional activation and connectivity in depression. J ECT. 2012;28:234–241. doi: 10.1097/YCT.0b013e31825ebcc7. [DOI] [PubMed] [Google Scholar]
- 92.Perrin JS, Merz S, Bennett DM, Currie J, Steele DJ, Reid IC, Schwarzbauer C. Electroconvulsive therapy reduces frontal cortical connectivity in severe depressive disorder. Proc Natl Acad Sci U S A. 2012;109:5464–5468. doi: 10.1073/pnas.1117206109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.McCormick LM, Yamada T, Yeh M, Brumm MC, Thatcher RW. Antipsychotic effect of electroconvulsive therapy is related to normalization of subgenual cingulate theta activity in psychotic depression. J Psychiatr Res. 2009;43:553–560. doi: 10.1016/j.jpsychires.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 94.Heikman P, Salmelin R, Makela JP, Hari R, Katila H, Kuoppasalmi K. Relation between frontal 3–7 Hz MEG activity and the efficacy of ECT in major depression. J ECT. 2001;17:136–140. doi: 10.1097/00124509-200106000-00009. [DOI] [PubMed] [Google Scholar]
- 95.Neuhaus AH, Luborzewski A, Rentzsch J, Brakemeier EL, Opgen-Rhein C, Gallinat J, Bajbouj M. P300 is enhanced in responders to vagus nerve stimulation for treatment of major depressive disorder. J Affect Disord. 2007;100:123–128. doi: 10.1016/j.jad.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 96.Downar J, Geraci J, Salomons TV, Dunlop K, Wheeler S, McAndrews MP, et al. Anhedonia and reward-circuit connectivity distinguish nonresponders from responders to dorsomedial prefrontal repetitive transcranial magnetic stimulation in major depression. Biol Psychiatry. 2014;76:176–185. doi: 10.1016/j.biopsych.2013.10.026. [DOI] [PubMed] [Google Scholar]
- 97.Harvey PO, Van den Eynde F, Zangen A, Berlim MT. Neural correlates of clinical improvement after deep transcranial magnetic stimulation (DTMS) for treatment-resistant depression: A case report using functional magnetic resonance imaging. Neurocase. 2015;21:16–22. doi: 10.1080/13554794.2013.860173. [DOI] [PubMed] [Google Scholar]
- 98.Kito S, Pascual-Marqui RD, Hasegawa T, Koga Y. High-frequency left prefrontal transcranial magnetic stimulation modulates resting EEG functional connectivity for gamma band between the left dorsolateral prefrontal cortex and precuneus in depression. Brain Stimul. 2014;7:145–146. doi: 10.1016/j.brs.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 99.Noda Y, Nakamura M, Saeki T, Inoue M, Iwanari H, Kasai K. Potentiation of quantitative electroencephalograms following pre-frontal repetitive transcranial magnetic stimulation in patients with major depression. Neurosci Res. 2013;77:70–77. doi: 10.1016/j.neures.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 100.Vanneste S, Ost J, Langguth B, De Ridder D. TMS by double-cone coil prefrontal stimulation for medication resistant chronic depression: A case report. Neurocase. 2014;20:61–68. doi: 10.1080/13554794.2012.732086. [DOI] [PubMed] [Google Scholar]
- 101.Narushima K, McCormick LM, Yamada T, Thatcher RW, Robinson RG. Subgenual cingulate theta activity predicts treatment response of repetitive transcranial magnetic stimulation in participants with vascular depression. J Neuropsychiatry Clin Neurosci. 2010;22:75–84. doi: 10.1176/appi.neuropsych.22.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Maihofner C, Ropohl A, Reulbach U, Hiller M, Elstner S, Kornhuber J, Sperling W. Effects of repetitive transcranial magnetic stimulation in depression: A magnetoencephalographic study. Neuroreport. 2005;16:1839–1842. doi: 10.1097/01.wnr.0000183903.77654.64. [DOI] [PubMed] [Google Scholar]
- 103.Hernandez-Ribas R, Deus J, Pujol J, Segalas C, Vallejo J, Menchon JM, et al. Identifying brain imaging correlates of clinical response to repetitive transcranial magnetic stimulation (rTMS) in major depression. Brain Stimul. 2013;6:54–61. doi: 10.1016/j.brs.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 104.da Silva MC, Conti CL, Klauss J, Alves LG, do Nascimento Cavalcante HM, Fregni F, et al. Behavioral effects of trans-cranial direct current stimulation (tDCS) induced dorsolateral pre-frontal cortex plasticity in alcohol dependence. J Physiol Paris. 2013;107:493–502. doi: 10.1016/j.jphysparis.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 105.Palm U, Keeser D, Schiller C, Fintescu Z, Reisinger E, Baghai TC, et al. Transcranial direct current stimulation in a patient with therapy-resistant major depression. World J Biol Psychiatry. 2009;10:632–635. doi: 10.1080/15622970802480905. [DOI] [PubMed] [Google Scholar]
- 106.Powell TY, Boonstra TW, Martin DM, Loo CK, Breakspear M. Modulation of cortical activity by transcranial direct current stimulation in patients with affective disorder. PLoS One. 2014;9:e98503. doi: 10.1371/journal.pone.0098503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Stam CJ. Nonlinear dynamical analysis of EEG and MEG: Review of an emerging field. Clin Neurophysiol. 2005;116:2266–2301. doi: 10.1016/j.clinph.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 108.Varela F, Lachaux JP, Rodriguez E, Martinerie J. The brainweb: Phase synchronization and large-scale integration. Nat Rev Neurosci. 2001;2:229–239. doi: 10.1038/35067550. [DOI] [PubMed] [Google Scholar]
- 109.Palva JM, Palva S, Kaila K. Phase synchrony among neuronal oscillations in the human cortex. J Neurosci. 2005;25:3962–3972. doi: 10.1523/JNEUROSCI.4250-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Siegel M, Warden MR, Miller EK. Phase-dependent neuronal coding of objects in short-term memory. Proc Natl Acad Sci U S A. 2009;106:21341–21346. doi: 10.1073/pnas.0908193106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Linkenkaer-Hansen K, Monto S, Rytsala H, Suominen K, Isometsa E, Kahkonen S. Breakdown of long-range temporal correlations in theta oscillations in patients with major depressive disorder. J Neurosci. 2005;25:10131–10137. doi: 10.1523/JNEUROSCI.3244-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Allen JJ, Cohen MX. Deconstructing the “resting” state: Exploring the temporal dynamics of frontal alpha asymmetry as an endophenotype for depression. Front Hum Neurosci. 2010;4:232. doi: 10.3389/fnhum.2010.00232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Srinivasan R, Winter WR, Ding J, Nunez PL. EEG and MEG coherence: Measures of functional connectivity at distinct spatial scales of neocortical dynamics. J Neurosci Methods. 2007;166:41–52. doi: 10.1016/j.jneumeth.2007.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Olbrich S, Trankner A, Chittka T, Hegerl U, Schonknecht P. Functional connectivity in major depression: Increased phase synchronization between frontal cortical EEG-source estimates. Psychiatry Res. 2014;222:91–99. doi: 10.1016/j.pscychresns.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 115.Canolty RT, Edwards E, Dalal SS, Soltani M, Nagarajan SS, Kirsch HE, et al. High gamma power is phase-locked to theta oscillations in human neocortex. Science. 2006;313:1626–1628. doi: 10.1126/science.1128115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Voytek B, Canolty RT, Shestyuk A, Crone NE, Parvizi J, Knight RT. Shifts in gamma phase-amplitude coupling frequency from theta to alpha over posterior cortex during visual tasks. Front Hum Neurosci. 2010;4:191. doi: 10.3389/fnhum.2010.00191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Tort AB, Kramer MA, Thorn C, Gibson DJ, Kubota Y, Graybiel AM, Kopell NJ. Dynamic cross-frequency couplings of local field potential oscillations in rat striatum and hippocampus during performance of a T-maze task. Proc Natl Acad Sci U S A. 2008;105:20517–20522. doi: 10.1073/pnas.0810524105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Cohen MX, Axmacher N, Lenartz D, Elger CE, Sturm V, Schlaepfer TE. Good vibrations: Cross-frequency coupling in the human nucleus accumbens during reward processing. J Cogn Neurosci. 2009;21:875–889. doi: 10.1162/jocn.2009.21062. [DOI] [PubMed] [Google Scholar]
- 119.Cohen MX, Elger CE, Fell J. Oscillatory activity and phase-amplitude coupling in the human medial frontal cortex during decision making. J Cogn Neurosci. 2009;21:390–402. doi: 10.1162/jocn.2008.21020. [DOI] [PubMed] [Google Scholar]
- 120.de Hemptinne C, Ryapolova-Webb ES, Air EL, Garcia PA, Miller KJ, Ojemann JG, et al. Exaggerated phase-amplitude coupling in the primary motor cortex in Parkinson disease. Proc Natl Acad Sci U S A. 2013;110:4780–4785. doi: 10.1073/pnas.1214546110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Covington HE, 3rd, Lobo MK, Maze I, Vialou V, Hyman JM, Zaman S, et al. Antidepressant effect of optogenetic stimulation of the medial prefrontal cortex. J Neurosci. 2010;30:16082–16090. doi: 10.1523/JNEUROSCI.1731-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Veerakumar A, Challis C, Gupta P, Da J, Upadhyay A, Beck SG, Berton O. Antidepressant-like effects of cortical deep brain stimulation coincide with pro-neuroplastic adaptations of serotonin systems. Biol Psychiatry. 2014;76:203–212. doi: 10.1016/j.biopsych.2013.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Tooker A, Madsen TE, Yorita A, Crowell A, Shah KG, Felix S, et al. Microfabricated polymer-based neural interface for electrical stimulation/recording, drug delivery, and chemical sensing–development. Conf Proc IEEE Eng Med Biol Soc. 2013;2013:5159–5162. doi: 10.1109/EMBC.2013.6610710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ryan SJ, Ehrlich DE, Jasnow AM, Daftary S, Madsen TE, Rainnie DG. Spike-timing precision and neuronal synchrony are enhanced by an interaction between synaptic inhibition and membrane oscillations in the amygdala. PLoS One. 2012;7:e35320. doi: 10.1371/journal.pone.0035320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kumar S, Black SJ, Hultman R, Szabo ST, DeMaio KD, Du J, et al. Cortical control of affective networks. J Neurosci. 2013;33:1116–1129. doi: 10.1523/JNEUROSCI.0092-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Dzirasa K, McGarity DL, Bhattacharya A, Kumar S, Takahashi JS, Dunson D, et al. Impaired limbic gamma oscillatory synchrony during anxiety-related behavior in a genetic mouse model of bipolar mania. J Neurosci. 2011;31:6449–6456. doi: 10.1523/JNEUROSCI.6144-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Holtzheimer PE, Kelley ME, Gross RE, Filkowski MM, Garlow SJ, Barrocas A, et al. Subcallosal cingulate deep brain stimulation for treatment-resistant unipolar and bipolar depression. Arch Gen Psychiatry. 2012;69:150–158. doi: 10.1001/archgenpsychiatry.2011.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Gutman DA, Holtzheimer PE, Behrens TE, Johansen-Berg H, May-berg HS. A tractography analysis of two deep brain stimulation white matter targets for depression. Biol Psychiatry. 2009;65:276–282. doi: 10.1016/j.biopsych.2008.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Johansen-Berg H, Gutman DA, Behrens TE, Matthews PM, Rushworth MF, Katz E, et al. Anatomical connectivity of the subgenual cingulate region targeted with deep brain stimulation for treatment-resistant depression. Cereb Cortex. 2008;18:1374–1383. doi: 10.1093/cercor/bhm167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hilimire MR, Mayberg HS, Holtzheimer PE, Broadway JM, Parks NA, DeVylder JE, Corballis PM. Effects of subcallosal cingulate deep brain stimulation on negative self-bias in patients with treatment-resistant depression [published online ahead of print November 24] Brain Stimul. 2014 doi: 10.1016/j.brs.2014.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Laxton AW, Neimat JS, Davis KD, Womelsdorf T, Hutchison WD, Dostrovsky JO, et al. Neuronal coding of implicit emotion categories in the subcallosal cortex in patients with depression. Biol Psychiatry. 2013;74:714–719. doi: 10.1016/j.biopsych.2013.03.029. [DOI] [PubMed] [Google Scholar]
- 132.Cohen MX, Axmacher N, Lenartz D, Elger CE, Sturm V, Schlaepfer TE. Neuroelectric signatures of reward learning and decision-making in the human nucleus accumbens. Neuropsychopharmacology. 2009;34:1649–1658. doi: 10.1038/npp.2008.222. [DOI] [PubMed] [Google Scholar]
- 133.Cohen MX, Axmacher N, Lenartz D, Elger CE, Sturm V, Schlaepfer TE. Nuclei accumbens phase synchrony predicts decision-making reversals following negative feedback. J Neurosci. 2009;29:7591–7598. doi: 10.1523/JNEUROSCI.5335-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lipsman N, Kaping D, Westendorff S, Sankar T, Lozano AM, Womelsdorf T. Beta coherence within human ventromedial prefrontal cortex precedes affective value choices. Neuroimage. 2014;85:769–778. doi: 10.1016/j.neuroimage.2013.05.104. [DOI] [PubMed] [Google Scholar]
- 135.Stanslaski S, Afshar P, Cong P, Giftakis J, Stypulkowski P, Carlson D, et al. Design and validation of a fully implantable, chronic, closed-loop neuromodulation device with concurrent sensing and stimulation. IEEE Trans Neural Syst Rehabil Eng. 2012;20:410–421. doi: 10.1109/TNSRE.2012.2183617. [DOI] [PubMed] [Google Scholar]
- 136.Rouse AG, Stanslaski SR, Cong P, Jensen RM, Afshar P, Ullestad D, et al. A chronic generalized bi-directional brain-machine interface. J Neural Eng. 2011;8:036018. doi: 10.1088/1741-2560/8/3/036018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Afshar P, Khambhati A, Stanslaski S, Carlson D, Jensen R, Linde D, et al. A translational platform for prototyping closed-loop neuromodulation systems. Front Neural Circuits. 2012;6:117. doi: 10.3389/fncir.2012.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Sun FT, Morrell MJ. Closed-loop neurostimulation: The clinical experience. Neurotherapeutics. 2014;11:553–563. doi: 10.1007/s13311-014-0280-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Figee M, de Koning P, Klaassen S, Vulink N, Mantione M, van den Munckhof P, et al. Deep brain stimulation induces striatal dopamine release in obsessive-compulsive disorder. Biol Psychiatry. 2014;75:647–652. doi: 10.1016/j.biopsych.2013.06.021. [DOI] [PubMed] [Google Scholar]
- 140.Figee M, Luigjes J, Smolders R, Valencia-Alfonso CE, van Wingen G, de Kwaasteniet B, et al. Deep brain stimulation restores frontostriatal network activity in obsessive-compulsive disorder. Nat Neurosci. 2013;16:386–387. doi: 10.1038/nn.3344. [DOI] [PubMed] [Google Scholar]
- 141.Smolders R, Mazaheri A, van Wingen G, Figee M, de Koning PP, Denys D. Deep brain stimulation targeted at the nucleus accumbens decreases the potential for pathologic network communication. Biol Psychiatry. 2013;74:e27–e28. doi: 10.1016/j.biopsych.2013.03.012. [DOI] [PubMed] [Google Scholar]
- 142.Konarski JZ, Kennedy SH, Segal ZV, Lau MA, Bieling PJ, McIntyre RS, Mayberg HS. Predictors of nonresponse to cognitive behavioural therapy or venlafaxine using glucose metabolism in major depressive disorder. J Psychiatry Neurosci. 2009;34:175–180. [PMC free article] [PubMed] [Google Scholar]
- 143.Chaturvedi A, Butson CR, Lempka SF, Cooper SE, McIntyre CC. Patient-specific models of deep brain stimulation: Influence of field model complexity on neural activation predictions. Brain Stimul. 2010;3:65–67. doi: 10.1016/j.brs.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Denison T, Litt B. Advancing neuromodulation through control systems: A general framework and case study in posture-responsive stimulation. Neuromodulation. 2014;17(suppl 1):48–57. doi: 10.1111/ner.12170. [DOI] [PubMed] [Google Scholar]
- 145.Yamazaki M, Terrill M, Fujimoto A, Yamamoto T, Tucker DM. Integrating dense array EEG in the presurgical evaluation of temporal lobe epilepsy. ISRN Neurol. 2012;2012:924081. doi: 10.5402/2012/924081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Whitmer D, de Solages C, Hill B, Yu H, Henderson JM, Bronte-Stewart H. High frequency deep brain stimulation attenuates subthalamic and cortical rhythms in Parkinson’s disease. Front Hum Neurosci. 2012;6:155. doi: 10.3389/fnhum.2012.00155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Heck CN, King-Stephens D, Massey AD, Nair DR, Jobst BC, Barkley GL, et al. Two-year seizure reduction in adults with medically intractable partial onset epilepsy treated with responsive neurostimulation: Final results of the RNS System pivotal trial. Epilepsia. 2014;55:432–441. doi: 10.1111/epi.12534. [DOI] [PMC free article] [PubMed] [Google Scholar]


