Abstract
Infections with Candida spp. have different manifestations in humans, ranging from mucosal to bloodstream and deep-seated disseminated infections. Immunocompromised patients have increased susceptibility to these types of infections, due to reduced capacity to elicit effective innate or adaptive immunity. In addition, rare and common genetic variants in the human genome have been identified that influence susceptibility to Candida infections. Genetic determinants of primary immunodeficiencies leading to chronic mucocutaneous candidiasis have been reported, and polymorphisms in genes that are known to be involved in anti-Candida host defense are associated with increased susceptibility to systemic infection. These findings have greatly increased our understanding of pathways important for anti-Candida defense in humans, and patterns of prevalence of Candida infections. In addition, these pathways may offer novel therapeutic targets for treatment. This review provides an overview of the current insights in genetic susceptibility to Candida infections and their consequences for the immune response against Candida.
Keywords: Candida, genetic susceptibility, chronic mucocutaneous candidiasis, candidemia, cytokines, immunity
Introduction
Candida is a dimorphic fungal pathogen that colonizes the mucosal surfaces of approximately 30% of healthy individuals at any given moment [1]. In immunocompetent hosts, the colonization by Candida does not cause disease, but during a breach of the anatomical barriers or in immunocompromised hosts, important clinical consequences may ensue. The spectrum of clinical diseases caused by Candida spp. comprises clinical syndromes ranging from mucocutaneous infections of the oral and vaginal mucosae, to candidemia and deep-seated infections that are often associated with a septic syndrome [2 – 4]. Although C. albicans is still the most prevalent species causing infection, the recent years have witnessed a trend towards an increased prevalence of non-C. albicans Candida species such as C. glabrata and C. krusei [5,6]. Furthermore, increasing reports of resistance to antifungal azoles have occurred, which may be in part promoted by the frequent use of antifungal prophylaxis, preemptive therapeutic strategies in high-risk patients, or alteration of host status [7,8]. Clinical observations have taught us that mucocutaneous candidiasis develops when either T cell immunity is defective (as probably best exemplified in HIV infection), whereas invasive infections tend to occur when phagocytic function falls short or innate immunity is compromised (such as in neutropenia and neutrophil disorders). In recent years the insight in host defense against candidiasis has been greatly augmented and this gain in knowledge has started to be applied to clinical medicine.
The first step in mounting a protective immune response is the recognition of the fungal pathogens by pattern recognition receptors (PRRs), followed by activation of a protective inflammatory reaction. In this early response, neutrophil influx and macrophage activation come into play, followed by the initiation of adaptive immunity through Th1 or Th17 responses. The host-Candida interaction results either in the elimination of the pathogen in the immunocompetent host, or in persistence of infection such as observed in chronic mucocutaneous candidiasis (CMC), candidemia and/or persistent disseminated candidiasis in immunocompromised individuals. Here, we describe the current understanding of the molecular mechanisms involved in the recognition of Candida spp. and activation of host defense by cells of the innate immune system, and we present the recent insights in the genetic defects of innate and adaptive immunity that can lead to an increased susceptibility to Candida infections.
Pattern recognition and host defense mechanisms against Candida infections
Host defense against pathogenic microorganisms, fungi in particular, relies on rapid (hours to days) activation of an acute inflammatory response after the encounter with the fungal pathogen. This is followed by an incremental stimulation of specific immune responses mediated by T-lymphocytes (cellular immunity) or B-lymphocytes (humoral immunity), including production of T cell-dependent cytokines such as IFN-γ and IL-17 [9,10]. The first step in the initiation of an immune response is the recognition of conserved structures of the pathogen named pathogen-associated molecular patterns (PAMPs) by PRRs [11,12]. This recognition is followed by induction of intracellular signals leading to phagocytosis and killing of the fungus, production of proinflammatory cytokines, and the induction of specific adaptive immune responses.
Pattern recognition of Candida
The cell wall of Candida albicans consists of an internal skeleton of chitin and β-glucans that confers the necessary rigidity; this layer is covered by a heavily mannosylated layer of mannoproteins at the surface. Mannans and mannoproteins are recognized by specific PRRs [13–15], as are β-glucans and chitin structures [16,17]. These PAMPs are recognized by receptors grouped in three major families: the Toll-like receptors (TLRs), the C-type lectin receptors (CLRs), and the nucleotide binding domain leucine-rich repeat-containing receptors (NLRs). The fourth major family of PRRs, the retinoic acid-inducible gene-I (RIG-I) receptors (RLRs), is believed to mediate especially the immune responses to viruses [13].
Toll-like receptors
The first suggestion of a fundamental role of TLRs in anti-fungal host defense was made by Lemaitre and colleagues, who observed that Drosophila deficient in the Toll receptor succumbed rapidly to Aspergillus fumigatus infection [18]. Subsequently, mammalian orthologs have been identified that have a leucine-rich repeats extracellular domain that recognizes PAMPs, and a cytoplasmic Toll/IL-1 receptor (TIR) domain responsible for transducing intracellular signals [19]. Ligand recognition by TLRs and subsequent transduction of intracellular signals by several adaptor proteins such as myeloid differentiation factor 88 (MyD88)/Mal and the TRIF/TRAM pathway activates a kinase cascade, followed by nuclear translocation of transcription factors such as NF-κB, AP-1 and IRF3, and production of chemokines and cytokines [13].
Several TLRs, including TLR2, TLR4 and TLR9, have been reported to mediate recognition of components of Candida [20–23]. TLR2 on myeloid cells recognizes the phospholipomannan component of the Candida cell wall [21], while a limited role for TLR1 and especially TLR6, two receptors known for forming heterodimers with TLR2, has been recently reported to play a role in C. albicans recognition in a mouse model of invasive candidiasis [24]. TLR4 recognizes mannans from Saccharomyces cerevisiae and C. albicans [25], and another study found that short linear O-bound mannans of C. albicans are recognized by TLR4 and induce proinflammatory cytokines such as TNFα [26]. Both TLR2 and TLR4 influence susceptibility to murine disseminated candidiasis [20,22,27,28]. TLR7 has been shown to recognize fungal RNA in the autophagosome, which is required for IFN-β release and is associated with prolonged infection by C. glabrata [29]. Unmethylated CpG sequences of DNA are the natural ligands for TLR9. Indeed, TLR9 is able to recognize fungal DNA from C. albicans, and upon recognition induces stimulation of cytokines in dendritic cells [30]. Bellochio et al. have reported that TLR9 knockout mice produced less IL-12 and more IL-4 and IL-10, but this had little effect on the overall mortality of the animals [20,31]. In conclusion, most of the data available at this time suggest a role for TLR9 for the recognition of fungal DNA, but the magnitude of this effect for the overall antifungal defense seems to be overshadowed by redundant signals induced by other PRRs.
C-type lectin receptors
Although TLRs are clearly involved in the recognition of Candida spp., the potent residual production of cytokines in mice with genetic defects in TLRs or MyD88 clearly demonstrates that a second major route of pattern recognition must be involved. CLRs are a large family of PRRs including the mannose receptor (MR), dectin-1, dectin-2, DC-SIGN, Mincle, and circulating mannose-binding lectin (MBL). These receptors share one or more carbohydrate recognition domains that were originally found in MBL [32], and are involved in the recognition of polysaccharide structures from both microorganisms and endogenous ligands [33]. Importantly, over the recent years these receptors have been shown to be central for fungal recognition and induction of the innate immune response.
The MR described by Stahl and Ezekowitz [34] has been implicated in the recognition of C. albicans by both mouse and human monocyte-derived macrophages [15], and recently the role of the MR in the recognition of C. albicans has been strengthened by a study showing that it recognizes branched N-bound mannans from C. albicans [26]. In line with this, a recent study has demonstrated an important role of MR for the induction of protective Th17 responses by C. albicans [35]. MR was found to be recruited to the phagosome relatively late after ingestion of C. albicans; there it mediates intracellular signals leading to cytokine production [36]. Additional CLRs involved in the recognition of C. albicans mannans are dectin-2, detected on myeloid cells and maturing inflammatory monocytes recognizing high-mannose structures [37,38], and DC-SIGN and Mincle, primarily expressed on mature DCs, which also recognize mannans [39–41]. Finally, galectin-3 is a receptor mainly expressed by macrophages, and it has been shown to be involved in the recognition of the β–mannosides of C. albicans, in close collaboration with TLR2, especially at the level of the intestinal mucosa [42,43].
In contrast to CLRs that recognize mannan structures, dectin-1 is the main receptor on myeloid cells for β-1,3-glucans [44,45]. Dectin-1 signals through the kinase Syk and the adaptor molecule CARD9, and this pathway has been shown to induce IL-2 and IL-10 in DCs. Moreover, it was demonstrated that infection with C. albicans induces protective dectin-1/CARD9-dependent Th17 responses that have a role in the antifungal host defense [46]. Although dectin-1 signaling alone may be sufficient to induce responses upon fungal recognition, several studies have emphasized that dectin-1 induces stronger proinflammatory responses in collaboration with other PRRs. Two independent studies have shown that dectin-1 in collaboration with TLR2 triggers proinflammatory responses upon stimulation with C. albicans and zymosan [45,47]. Recently, dectin-1 has been found to also amplify TLR4-dependent pathways in both murine and human myeloid cells [48,49].
NLRs and inflammasome activation
In addition to the mainly cell-membrane bound TLRs and CLRs, mammalian host defense has developed a second line of recognition receptors located in the cytoplasm; for the recognition of C. albicans these are the receptors of the NLR family. Some NLR family members, such as NLRP3, are part of the inflammasome complex. The inflammasomes are protein platforms composed of a NLR, the linking molecule ASC and caspase-1. Upon recognition of a microbial PAMP or an endogenous danger signal (e.g., ATP or uric acid), the conformational change in the NLR/ASC complex induces activation of the cysteine protease caspase-1, which in turn processes pro-IL-1β and pro-IL-18 into the bioactive cytokines [50]. IL-1β proved to be important for neutrophil granulocyte recruitment and generation of superoxide [51]. Both IL-1α and IL-1β deficient mice show increased mortality during disseminated candidiasis [51], and NLRP3 and ASC knockout mice have also been reported to be more susceptible to both systemic [52,53] and mucosal [54] Candida infections, although conflicting data exist regarding NLRP3 (van de Veerdonk, personal communication). These data underscore the role of this pathway for host defense against Candida infections. However, the role of the inflammasome components ASC and NLRP3 for antifungal defense in humans is unclear yet, although genetic variation in NLRP3 has been associated with increased risk for developing recurrent vulvovaginal candidiasis (RVVC) [55].
Primary immunodeficiencies with an increased susceptibility to fungal infections
The in vitro and the experimental studies described above provide evidence that PRRs and the mechanisms induced by these receptors are crucial components of the host defense against fungal pathogens. The knowledge regarding the specific roles of PRRs for human antifungal defense has increased during the last few years by the discovery of a number of defects within the innate immune system, and their specific profiles in terms of increased susceptibility to fungal infections.
Recently, Casanova and colleagues have identified patients with defects in the TLR-adaptor molecules IRAK-4 and MyD88 [56,57]. Patients with IRAK-4 or MyD88 deficiency, and thus broad defects in TLR and IL-1 signaling, have a phenotype characterized by increased susceptibility to pus-forming Gram-positive bacteria such as Streptococcus pneumoniae and staphylococci, as well as Gram-negative bacteria such as Pseudomonas spp. [58]. Interestingly, these patients do not seem to have an increased susceptibility to fungal infections (either invasive or mucocutaneous), suggesting that the MyD88/IRAK-4 pathway may be redundant for human antifungal defense.
The role of CLR-dependent pathways for antifungal host defense is supported by the identification of a family bearing mutations in CARD9, an adaptor molecule in the intracellular signaling pathway of dectin-1 and dectin-2, and possibly other CLRs. This family exhibited increased susceptibility to both mucocutaneous and systemic Candida infections [59]. The CARD9-deficient patients also displayed almost complete absence of Th17 responses. In addition to CARD9 deficiency, an early stop codon polymorphism Y238X in dectin-1 (CLEC7A) has been identified in a family with several individuals suffering from recurrent mucocutaneous fungal infections, including RVVC [60]. Myeloid cells of affected patients showed defective β-glucan recognition and impaired cytokine responses (IL-6, TNFα and IL-17). Neutrophils of patients exhibited normal phagocytosis and killing of opsonized C. albicans. This underlines the redundant nature of dectin-1 for the phagocytosis and killing of yeast pathogens by human myeloid cells, explaining the absence of invasive candidiasis in these patients. It is likely that the defective cytokine release of myeloid cells in the patients bearing the Y238X dectin-1 polymorphism, and especially the diminished IL-17 responses, is responsible for the clinical phenotype. However, this genetic variant is not rare: in the Western world the prevalence of heterozygous individuals ranges from 10–15%, suggesting that it behaves as a susceptibility factor, rather than a true immunodeficiency. Indeed, the role of dectin-1 Y238X as a susceptibility polymorphism for mucosal anti-Candida defense has been confirmed in a study showing that individuals heterozygous for the dectin-1 stop polymorphism and undergoing stem cell transplantation are more likely to be colonized with C. albicans and need more often antifungal therapy [61]. However, in a study that assessed the role of common genetic variants of dectin-1 (Y238X) and CARD9 (the S12N polymorphism, not the rare mutation leading to immunodeficiency) in systemic Candida infection, no association was observed with either susceptibility for or clinical outcome of the infection, suggesting that the β-glucan recognition pathway is redundant in systemic immunity to C. albicans [62].
The crucial role of Th17 responses for the host defense against mucosal Candida infections is further supported by the discovery of severe IL-17 defects in patients with two major primary immunodeficiencies syndromes, i.e., hyper IgE syndrome (HIES) and chronic mucocutaneous candidiasis (CMC). Patients with these conditions suffer from chronic mucocutaneous fungal infections [63–65]. In the case of HIES, this is most frequently due to mutations in Signal Transducer and Activator of Transcription (STAT) 3, one of the main signaling molecules of the IL-23 receptor. More rarely, HIES is caused by mutations in DOCK8 (dedicator of cytokinesis 8) or TYK2 (Tyrosine Kinase 2), which also predispose to CMC [66,67]. Furthermore, one study has proposed a polymorphism in TLR3 to be associated with infectious manifestations caused by C. albicans in CMC patients [68]. However, it is likely that this TLR3 polymorphism represents a susceptibility risk factor rather than a genetic cause of CMC, as this polymorphism is a common variant in the healthy population.
A subgroup of patients with CMC, those with the clinical syndrome named APECED (autoimmune polyendocrinopathy, candidiasis, ectodermal dysplasy) which is due to a defect in the AIRE gene (autoimmune regulator), has a propensity for autoimmune phenomena. In these patients, neutralizing autoantibodies against IL-17 and IL-22 have been found [69]. In addition, approximately 20% of patients with defects in IL12Rβ1, a receptor subunit shared by the IL12 receptor and IL-23 receptor, present with Candida infections [70].
CMC is a relatively heterogeneous immunodeficiency disorder in which several investigators have found strongly decreased IFN-γ and IL-17 production. The availability of next-generation sequencing techniques has allowed for the identification of mutations in the coiled-coil domain of STAT1 as the genetic cause of the disease in families with autosomal dominant CMC (AD-CMC) [71,72]. The discovery of STAT1 mutations as cause of AD-CMC was remarkable, as STAT1 deficiency had been previously reported to be associated with mycobacterial and viral, but not fungal, infections [73,74]. The presence of the AD-CMC mutations in the coiled-coil domain of STAT1, rather than in the Src homology 2 (SH2) or DNA-binding domains of the protein as in patients with mycobacterial/viral infections, is believed to explain the difference [71,72]. Functional studies revealed that these mutations lead to a gain-of-function of STAT1, thereby impairing STAT3 and STAT4 signaling which leads to defects in downstream signaling of the IL-12 receptor and the IL-23 receptor. This results in diminished production of IFN-γ, IL-17 and IL-22, crucial cytokines in mucosal antifungal host defense [72,75,76]. Moreover, in a small number of patients with CMC, loss-of-function mutations were detected in the genes encoding IL-17F and IL-17 receptor A, resulting in defective IL-17 signalling [77]. An overview of primary immunodeficiencies associated with increased susceptibility to Candida infections is listed in Table 1.
Table 1.
Primary immunodeficiencies associated with increased susceptibility to Candida infections.
| Affected gene | Main symptoms | Immune defects | References |
|---|---|---|---|
| STAT1 | CMC, hypothyroidism, esophageal cancer | Diminished production of Candida induced IFN-γ, IL-17 and IL-22 | [71, 72] |
| STAT3 | Serum hyper IgE, Staphylococcus and Candida infections | Diminished production of Candida induced IFN-γ, IL-17 and IL-22 | [109, 110] |
| DOCK8 | Serum hyper IgE, Staphylococcus and Candida infections | Impaired T cell activation, diminished production of Candida induced IFN-γ, IL-17 and IL-22 | [66] |
| TYK2 | Serum hyper IgE, Staphylococcus and Candida infections | Defective cytokine signaling | |
| IL-17RA | CMC | Loss-of-function of IL-17RA | [67] |
| IL-17F | CMC | Loss-of-function of IL-17F | [77] |
| CARD9 | Mucosal and disseminated Candida infections | Diminished production of Candida induced IL-17 | [77] |
| IL-12Rβ1 | Mycobacterial and Salmonella infections and candidiasis | Loss-of-function of IL-12 and IL-23 receptor, diminished production of Candida induced IFN-γ and IL-17 | [59] |
| AIRE | CMC, adrenal insufficiency, hypoparathyroidism | Defective thymic negative selection of autoreactive T cells, autoantibodies against IL-17 and IL-22 | [111, 112] |
Common genetic variation and susceptibility to Candida infections
The prevalence of bloodstream infections with Candida spp. has steadily increased in recent years due to the increased use of invasive procedures, treatment of malignancies and autoimmune diseases with chemotherapy and immunosuppressive drugs, and prolonged ICU stays; the mortality due to this disease remains high at between 30% and 40% [78,79].
Several epidemiological studies have assessed the role of TLR polymorphisms for the susceptibility to disseminated candidiasis. The Asp299Gly TLR4 polymorphism has been proposed to act as a susceptibility trait for systemic candidiasis [80] and the Asp753Gln TLR2 polymorphism resulted in an altered cytokine profile in patients with Candida sepsis [81]. However, these findings could not be confirmed in a much larger cohort including patients and matched controls [82], suggesting that in the previous studies the cohorts were too small, leading to positive associations likely caused by type I errors. Similarly, no role of TLR4 polymorphisms in vaginal colonization with Candida spp. has been observed [83].
Studies dedicated to identify common genetic variants that predispose to bloodstream infections have revealed a significant role for non-synonymous polymorphisms in TLR1, of which three were associated with increased risk of developing candidemia as compared to matched control patients in a hospitalized setting (Plantinga et al., J. Infect Dis in press). These TLR1 polymorphisms result in loss-of-function of the receptor and consequently decreased cytokine responses induced through the TLR1/TLR2 heterodimer. Although no important role for TLR1 was observed in a mouse model of invasive candidiasis [24], several mechanisms could account for the role of human TLR1 in anti-Candida immune responses. The configuration of other TLRs such as TLR2 and TLR6 either as homodimer or heterodimer could be deregulated, that in turn could affect intracellular signaling. Another mechanism through which TLR1 could exert its effect on antifungal host defense is the recent finding that beta-defensin-3 activates immune cells through TLR1/TLR2, with an important lytic activity against C. albicans [84,85]. This is complemented by the observation that polymorphisms in beta-defensin-1 are associated with RVVC [86], emphasizing the important role of beta-defensins in antifungal immunity.
In the same invasive candidiasis cohort, persistence of fungemia was shown to be associated with promoter polymorphisms in the cytokine genes IL-12B and IL-10 [87]. These polymorphisms affect cytokine transcription and thereby influence the IL-10 and IL-12 production capacity of innate immune cells [88–92]. The persistence of infection was demonstrated to correlate with decreased IL-12 and increased IL-10 production induced by Candida, that likely results in inhibition of the T helper 1 response, known to be crucial for anti-Candida systemic immunity [93,94]. In line with this, a decreased production of T helper 2 cytokines such as IL-4 due to genetic variation in the IL4 gene leads to protective effects [95,96].
Multiple studies have been dedicated to investigate the role of mannose binding lectin (MBL) deficiency in infections with Candida spp., since it was demonstrated that MBL could bind and thereby opsonize fungi to facilitate complement activation and phagocytosis [96,97]. Indeed, genetic associations of MBL deficiency with infection risk were observed in cohorts of patients with candidemia, abdominal infections and RVVC [95,98–100].
Another common Candida infection is oropharyngeal candidiasis (OPC), mucosal colonization of the mouth and upper digestive tract that is frequently observed in patients that are infected with human immunodeficiency virus (HIV). About 50–95% of patients encounter this type of candidiasis at least once during their progression to AIDS [101–103]. Hence, occurrence of OPC is associated with decreased numbers of CD4+ T cells. However, also human genetic variation in innate immunity may contribute to the susceptibility for OPC. A recent study addressed the potential role of genetic variants of pattern recognition receptors in susceptibility to OPC in West-African HIV patients, which revealed a potential role for another genetic variant of dectin-1 specific for African populations, I223S [104].
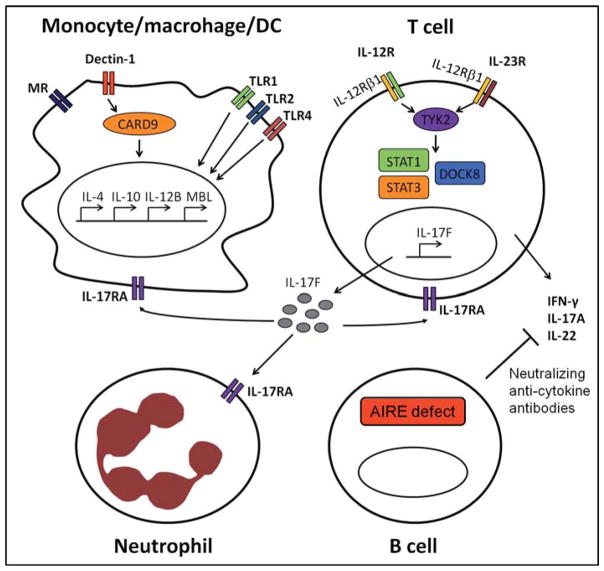
The genetic studies on Candida infections described above are variable in terms of size of patient cohorts and statistical power. While some of the studies do have relatively large cohorts, with appropriate statistical analysis done, others are hampered by small cohorts of patients that preclude the drawing of definitive conclusions. On the other hand, some of the reported genetic associations are strengthened by functional studies that provide mechanistic explanations for the increased susceptibility to infection. However, no validation of these genetic associations in replication studies with independent cohorts have been performed so far, which is needed to accumulate the required evidence of true genetic associations. In Table 2, a complete overview of common genetic variants associated with fungal infection is depicted. Fig. 1 illustrates the role and location in human antifungal defense of proteins encoded by susceptibility genes for both rare congenital disorders and for Candida infections with population-wide occurrence.
Table 2.
Common genetic variants associated with increased susceptibility to Candida infections.
| Affected gene | Polymorphism | Type of infection | Immune defects | References |
|---|---|---|---|---|
| Dectin-1 | Y238X | Recurrent vulvovaginal infections and oral/gastrointestinal colonization | Lack of β-glucan recognition, lower production of Candida induced TNFα, IL-6 and IL-17 | [60,61] |
| I223S | Oropharyngeal candidiasis | Reduced zymosan-binding capacity and IFN-γ production | [104] | |
| TLR1 | R80T N248S S602I |
Candidemia | Impaired production of pro-inflammatory cytokines induced through TLR1-2 heterodimers | [82] |
| TLR3 | L412F | CMC | Defective TLR3 signaling | [68] |
| IL-12B | –2724INS/DEL | Persistent candidemia | Lower production of IFN-γ induced by Candida | [87] |
| IL-10 | –1082A/G | Persistent candidemia | Higher production of IL-10 induced by Candida | [87] |
| MBL2 | Codon 54 and 57 | Candidemia, abdominal Candida infection, recurrent vulvovaginal candidiasis | Lower MBL serum levels | [95, 98–100] |
| IL-4 | –589T/C | Recurrent vulvovaginal candidiasis | Increased levels of vaginal IL-4 and reduced levels of nitric oxide and MBL | [113] |
| –1098T/G –589C/T –33C/T |
Chronic disseminated candidiasis | Unknown | [114] | |
| NLRP3 | Length polymorphism | Recurrent vulvovaginal candidiasis | Impaired production of IL-1β | [55] |
| DEFB1 | –44C/G | C. albicans carriage | Unknown | [86] |
Fig. 1.
Schematic overview of proteins involved in anti-Candida host defense of which genetic variants have been identified to increase susceptibility to either mucosal or systemic Candida infection. Only those proteins, and the context of these proteins within antifungal immunity, are depicted of which genetic variants in the encoding genes are associated with an increased susceptibility to infection. These proteins have diverse functions, ranging from recognition of Candida, cytokine signaling and cellular immunity. TLR, Toll-like receptor; MR, mannose receptor.
Summary and future directions
Genetic association studies, either on rare monogenic disorders or common fungal infections among immunocompromised patients, have provided fundamental insights into the mechanisms involved in conferring resistance to the fungal pathogen C. albicans. Especially cytokines that are crucial in antifungal host defense have been identified, including IFN-γ, IL-17 and IL-22 for mucosal infections, and IL-12 and IFN-γ for systemic infections. These findings have generated the rationale for proposing the treatment with adjuvant immunotherapy in the form of recombinant cytokines for the treatment of Candida infections. Indeed, experimental studies [105,106] and anecdotal case reports [107,108] have provided the proof-of-concept for the use of IFN-γ or colony-stimulating factors for the treatment of systemic fungal infections. Future research should extend the studies recently started to deepen the knowledge of the genetic profile that would predispose to candidemia: from assessing an increasing number of candidate genes, to genome wide arrays when large enough cohorts will be available. In addition, it has been suggested that only 10 – 15% of genetic susceptibility to diseases is to be found in the main effects of the individual common polymorphisms and the rest is most likely hidden in rare genetic variants and complex interactions between genes and the environment. This warrants more detailed assessment of genetic variation by deep-sequencing of candidate genes, pathways and in the future (as assays become more cost-effective) the entire exomes or even genomes of affected patients.
Acknowledgments
This work was supported by a Vici grant from the Nether-lands Organization for Scientific Research to MGN, and National Institutes of Health grants (AI-51537 to MDJ) and (AI-73896 to JRP).
Footnotes
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and the writing of the paper.
References
References for this review were identified through searches of PubMed for articles published from January 1971, to December 2011, by use of the terms ‘Candida’ and ‘infection’. Relevant articles resulting from these searches and relevant references cited in those articles were reviewed.
- 1.Brown GD, Netea MG. Immunology of Fungal Infections. Dordrecht: Springer; 2007. [Google Scholar]
- 2.Marodi L, Johnston RB., Jr Invasive Candida species disease in infants and children: occurrence, risk factors, management, and innate host defense mechanisms. Curr Opin Pediatr. 2007;19:693–697. doi: 10.1097/MOP.0b013e3282f1dde9. [DOI] [PubMed] [Google Scholar]
- 3.Neofytos D, Fishman JA, Horn D, et al. Epidemiology and outcome of invasive fungal infections in solid organ transplant recipients. Transpl Infect Dis. 2010;12:220–229. doi: 10.1111/j.1399-3062.2010.00492.x. [DOI] [PubMed] [Google Scholar]
- 4.Pfaller MA, Diekema DJ. Epidemiology of invasive mycoses in North America. Crit Rev Microbiol. 2010;36:1–53. doi: 10.3109/10408410903241444. [DOI] [PubMed] [Google Scholar]
- 5.Trick WE, Fridkin SK, Edwards JR, Hajjeh RA, Gaynes RP. Secular trend of hospital-acquired candidemia among intensive care unit patients in the United States during 1989–1999. Clin Infect Dis. 2002;35:627–630. doi: 10.1086/342300. [DOI] [PubMed] [Google Scholar]
- 6.Wingard JR, Merz WG, Rinaldi MG, et al. Increase in Candida krusei infection among patients with bone marrow transplantation and neutropenia treated prophylactically with fluconazole. N Engl J Med. 1991;325:1274–1277. doi: 10.1056/NEJM199110313251803. [DOI] [PubMed] [Google Scholar]
- 7.Marr KA, Seidel K, White TC, Bowden RA. Candidemia in allogeneic blood and marrow transplant recipients: evolution of risk factors after the adoption of prophylactic fluconazole. J Infect Dis. 2000;181:309–316. doi: 10.1086/315193. [DOI] [PubMed] [Google Scholar]
- 8.Pfaller MA, Diekema DJ, Gibbs DL, et al. Results from the ARTEMIS DISK Global Antifungal Surveillance study, 1997 to 2005: an 8. 5-year analysis of susceptibilities of Candida species and other yeast species to fluconazole and voriconazole determined by CLSI standardized disk diffusion testing. J Clin Microbiol. 2007;45:1735–1745. doi: 10.1128/JCM.00409-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Netea MG, Brown GD, Kullberg BJ, Gow NA. An integrated model of the recognition of Candida albicans by the innate immune system. Nat Rev Microbiol. 2008;6:67–78. doi: 10.1038/nrmicro1815. [DOI] [PubMed] [Google Scholar]
- 10.Romani L. Immunity to fungal infections. Nat Rev Immunol. 2004;4:1–23. doi: 10.1038/nri1255. [DOI] [PubMed] [Google Scholar]
- 11.Janeway CA, Jr, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 12.Medzhitov R. Recognition of microorganisms and activation of the immune response. Nature. 2007;449:819–826. doi: 10.1038/nature06246. [DOI] [PubMed] [Google Scholar]
- 13.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 14.Gazi U, Martinez-Pomares L. Influence of the mannose receptor in host immune responses. Immunobiology. 2009;214:554–561. doi: 10.1016/j.imbio.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 15.Marodi L, Korchak HM, Johnston RB., Jr Mechanisms of host defense against Candida species. I. Phagocytosis by monocytes and monocyte-derived macrophages. J Immunol. 1991;146:2783–2789. [PubMed] [Google Scholar]
- 16.Gantner BN, Simmons RM, Underhill DM. Dectin-1 mediates macrophage recognition of Candida albicans yeast but not filaments. EMBO J. 2005;24:1277–1286. doi: 10.1038/sj.emboj.7600594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gow NA, Netea MG, Munro CA, et al. Immune recognition of Candida albicans beta-glucan by dectin-1. J Infect Dis. 2007;196:1565–1571. doi: 10.1086/523110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lemaitre B, Nicolas E, Michaut L, Reichhart JM, Hoffmann JA. The dorsoventral regulatory gene cassette spatzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell. 1996;86:973–983. doi: 10.1016/s0092-8674(00)80172-5. [DOI] [PubMed] [Google Scholar]
- 19.Rock FL, Hardiman G, Timans JC, Kastelein RA, Bazan JF. A family of human receptors structurally related to Drosophila Toll. Proc Natl Acad Sci USA. 1998;95:588–593. doi: 10.1073/pnas.95.2.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bellocchio S, Montagnoli C, Bozza S, et al. The contribution of the Toll-like/IL-1 receptor superfamily to innate and adaptive immunity to fungal pathogens in vivo. J Immunol. 2004;172:3059–3069. doi: 10.4049/jimmunol.172.5.3059. [DOI] [PubMed] [Google Scholar]
- 21.Jouault T, Ibata-Ombetta S, Takeuchi O, et al. Candida albicans phospholipomannan is sensed through toll-like receptors. J Infect Dis. 2003;188:165–172. doi: 10.1086/375784. [DOI] [PubMed] [Google Scholar]
- 22.Netea MG, Van der Graaf CA, Vonk AG, et al. The role of toll-like receptor (TLR) 2 and TLR4 in the host defense against disseminated candidiasis. J Infect Dis. 2002;185:1483–1489. doi: 10.1086/340511. [DOI] [PubMed] [Google Scholar]
- 23.Villamon E, Gozalbo D, Roig P, et al. Toll-like receptor-2 is essential in murine defenses against Candida albicans infections. Microbes Infect. 2004;6:1–7. doi: 10.1016/j.micinf.2003.09.020. [DOI] [PubMed] [Google Scholar]
- 24.Netea MG, van de Veerdonk FL, Verschueren I, Van der Meer JW, Kullberg BJ. Role of TLR1 and TLR6 in the host defense against disseminated candidiasis. FEMS Immunol Med Microbiol. 2008;52:118–123. doi: 10.1111/j.1574-695X.2007.00353.x. [DOI] [PubMed] [Google Scholar]
- 25.Tada H, Nemoto E, Shimauchi H, et al. Saccharomyces cerevisiae- and Candida albicans-derived mannan induced production of tumor necrosis factor alpha by human monocytes in a CD14- and Toll-like receptor 4-dependent manner. Microbiol Immunol. 2002;46:503–512. doi: 10.1111/j.1348-0421.2002.tb02727.x. [DOI] [PubMed] [Google Scholar]
- 26.Netea MG, Gow NA, Munro CA, et al. Immune sensing of Candida albicans requires cooperative recognition of mannans and glucans by lectin and Toll-like receptors. J Clin Invest. 2006;116:1642–1650. doi: 10.1172/JCI27114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Netea MG, Sutmuller R, Hermann C, et al. Toll-like receptor 2 suppresses immunity against Candida albicans through induction of IL-10 and regulatory T cells. J Immunol. 2004;172:3712–3718. doi: 10.4049/jimmunol.172.6.3712. [DOI] [PubMed] [Google Scholar]
- 28.Netea MG, Gow NA, Joosten LA, et al. Variable recognition of Candida albicans strains by TLR4 and lectin recognition receptors. Med Mycol. 2010;48:897–903. doi: 10.3109/13693781003621575. [DOI] [PubMed] [Google Scholar]
- 29.Bourgeois C, Majer O, Frohner IE, et al. Conventional dendritic cells mount a type I IFN response against Candida spp. requiring novel phagosomal TLR7-mediated IFN-beta signaling. J Immunol. 2011;186:3104–3112. doi: 10.4049/jimmunol.1002599. [DOI] [PubMed] [Google Scholar]
- 30.Miyazato A, Nakamura K, Yamamoto N, et al. Toll-like receptor 9-dependent activation of myeloid dendritic cells by deoxynucleic acids from Candida albicans. Infect Immun. 2009;77:3056–3064. doi: 10.1128/IAI.00840-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van de Veerdonk FL, Netea MG, Jansen TJ, et al. Redundant role of TLR9 for anti-Candida host defense. Immunobiology. 2008;213:613–620. doi: 10.1016/j.imbio.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 32.Zelensky AN, Gready JE. The C-type lectin-like domain superfamily. FEBS J. 2005;272:6179–6217. doi: 10.1111/j.1742-4658.2005.05031.x. [DOI] [PubMed] [Google Scholar]
- 33.Ip WK, Takahashi K, Ezekowitz RA, Stuart LM. Mannose-binding lectin and innate immunity. Immunol Rev. 2009;230:9–21. doi: 10.1111/j.1600-065X.2009.00789.x. [DOI] [PubMed] [Google Scholar]
- 34.Stahl PD, Ezekowitz RA. The mannose receptor is a pattern recognition receptor involved in host defense. Curr Opin Immunol. 1998;10:50–55. doi: 10.1016/s0952-7915(98)80031-9. [DOI] [PubMed] [Google Scholar]
- 35.van de Veerdonk FL, Marijnissen RJ, Kullberg BJ, et al. The macrophage mannose receptor induces IL-17 in response to Candida albicans. Cell Host Microbe. 2009;5:329–340. doi: 10.1016/j.chom.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 36.Heinsbroek SE, Taylor PR, Martinez FO, et al. Stage-specific sampling by pattern recognition receptors during Candida albicans phagocytosis. PLoS Pathog. 2008;4:e1000218. doi: 10.1371/journal.ppat.1000218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McGreal EP, Rosas M, Brown GD, et al. The carbohydrate-recognition domain of Dectin-2 is a C-type lectin with specificity for high mannose. Glycobiology. 2006;16:422–430. doi: 10.1093/glycob/cwj077. [DOI] [PubMed] [Google Scholar]
- 38.Saijo S, Ikeda S, Yamabe K, et al. Dectin-2 recognition of alpha-mannans and induction of Th17 cell differentiation is essential for host defense against Candida albicans. Immunity. 2010;32:681–691. doi: 10.1016/j.immuni.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 39.Cambi A, Gijzen K, de Vries JM, et al. The C-type lectin DC-SIGN (CD209) is an antigen-uptake receptor for Candida albicans on dendritic cells. Eur J Immunol. 2003;33:532–538. doi: 10.1002/immu.200310029. [DOI] [PubMed] [Google Scholar]
- 40.Cambi A, Netea MG, Mora-Montes HM, et al. Dendritic cell interaction with Candida albicans critically depends on N-linked mannan. J Biol Chem. 2008;283:20590–20599. doi: 10.1074/jbc.M709334200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wells CA, Salvage-Jones JA, Li X, et al. The macrophage-inducible C-type lectin, mincle, is an essential component of the innate immune response to Candida albicans. J Immunol. 2008;180:7404–7413. doi: 10.4049/jimmunol.180.11.7404. [DOI] [PubMed] [Google Scholar]
- 42.Jawhara S, Thuru X, Standaert-Vitse A, et al. Colonization of mice by Candida albicans is promoted by chemically induced colitis and augments inflammatory responses through galectin-3. J Infect Dis. 2008;197:972–980. doi: 10.1086/528990. [DOI] [PubMed] [Google Scholar]
- 43.Jouault T, El Abed-El BM, Martinez-Esparza M, et al. Specific recognition of Candida albicans by macrophages requires galectin-3 to discriminate Saccharomyces cerevisiae and needs association with TLR2 for signaling. J Immunol. 2006;177:4679–4687. doi: 10.4049/jimmunol.177.7.4679. [DOI] [PubMed] [Google Scholar]
- 44.Brown GD, Gordon S. Immune recognition. A new receptor for beta-glucans. Nature. 2001;413:36–37. doi: 10.1038/35092620. [DOI] [PubMed] [Google Scholar]
- 45.Brown GD, Herre J, Williams DL, et al. Dectin-1 mediates the biological effects of beta-glucans. J Exp Med. 2003;197:1119–1124. doi: 10.1084/jem.20021890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.LeibundGut-Landmann S, Gross O, Robinson MJ, et al. Syk- and CARD9-dependent coupling of innate immunity to the induction of T helper cells that produce interleukin 17. Nat Immunol. 2007;8:630–638. doi: 10.1038/ni1460. [DOI] [PubMed] [Google Scholar]
- 47.Gantner BN, Simmons RM, Canavera SJ, Akira S, Underhill DM. Collaborative induction of inflammatory responses by dectin-1 and toll-like receptor 2. J Exp Med. 2003;197:1107–1117. doi: 10.1084/jem.20021787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dennehy KM, Ferwerda G, Faro-Trindade I, et al. Syk kinase is required for collaborative cytokine production induced through Dectin-1 and Toll-like receptors. Eur J Immunol. 2008;38:500–506. doi: 10.1002/eji.200737741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ferwerda G, Meyer-Wentrup F, Kullberg BJ, Netea MG, Adema GJ. Dectin-1 synergizes with TLR2 and TLR4 for cytokine production in human primary monocytes and macrophages. Cell Microbiol. 2008;10:2058–2066. doi: 10.1111/j.1462-5822.2008.01188.x. [DOI] [PubMed] [Google Scholar]
- 50.Martinon F, Tschopp J. Inflammatory caspases: linking an intracellular innate immune system to autoinflammatory diseases. Cell. 2004;117:561–574. doi: 10.1016/j.cell.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 51.Vonk AG, Netea MG, van Krieken JH, et al. Endogenous interleukin (IL)-1 alpha and IL-1 beta are crucial for host defense against disseminated candidiasis. J Infect Dis. 2006;193:1419–1426. doi: 10.1086/503363. [DOI] [PubMed] [Google Scholar]
- 52.Gross O, Poeck H, Bscheider M, et al. Syk kinase signalling couples to the Nlrp3 inflammasome for anti-fungal host defence. Nature. 2009;459:433–436. doi: 10.1038/nature07965. [DOI] [PubMed] [Google Scholar]
- 53.Kumar H, Kumagai Y, Tsuchida T, et al. Involvement of the NLRP3 inflammasome in innate and humoral adaptive immune responses to fungal beta-glucan. J Immunol. 2009;183:8061–8067. doi: 10.4049/jimmunol.0902477. [DOI] [PubMed] [Google Scholar]
- 54.Hise AG, Tomalka J, Ganesan S, et al. An essential role for the NL-RP3 inflammasome in host defense against the human fungal pathogen Candida albicans. Cell Host Microbe. 2009;5:487–497. doi: 10.1016/j.chom.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lev-Sagie A, Prus D, Linhares IM, et al. Polymorphism in a gene coding for the inflammasome component NALP3 and recurrent vulvovaginal candidiasis in women with vulvar vestibulitis syndrome. Am J Obstet Gynecol. 2009;200:303–306. doi: 10.1016/j.ajog.2008.10.039. [DOI] [PubMed] [Google Scholar]
- 56.Picard C, Puel A, Bonnet M, et al. Pyogenic bacterial infections in humans with IRAK-4 deficiency. Science. 2003;299:2076–2079. doi: 10.1126/science.1081902. [DOI] [PubMed] [Google Scholar]
- 57.von Bernuth H, Picard C, Jin Z, et al. Pyogenic bacterial infections in humans with MyD88 deficiency. Science. 2008;321:691–696. doi: 10.1126/science.1158298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Picard C, von Bernuth H, Ghandil P, et al. Clinical features and outcome of patients with IRAK-4 and MyD88 deficiency. Medicine (Baltimore) 2010;89:403–425. doi: 10.1097/MD.0b013e3181fd8ec3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Glocker EO, Hennigs A, Nabavi M, et al. A homozygous CARD9 mutation in a family with susceptibility to fungal infections. N Engl J Med. 2009;361:1727–1735. doi: 10.1056/NEJMoa0810719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ferwerda B, Ferwerda G, Plantinga TS, et al. Human dectin-1 deficiency and mucocutaneous fungal infections. N Engl J Med. 2009;361:1760–1767. doi: 10.1056/NEJMoa0901053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Plantinga TS, van der Velden WJ, Ferwerda B, et al. Early stop polymorphism in human DECTIN-1 is associated with increased Candida colonization in hematopoietic stem cell transplant recipients. Clin Infect Dis. 2009;49:724–732. doi: 10.1086/604714. [DOI] [PubMed] [Google Scholar]
- 62.Rosentul DC, Plantinga TS, Oosting M, et al. Genetic variation in the dectin-1/CARD9 recognition pathway and susceptibility to candidemia. J Infect Dis. 2011;204:1138–1145. doi: 10.1093/infdis/jir458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Milner JD, Brenchley JM, Laurence A, et al. Impaired T(H)17 cell differentiation in subjects with autosomal dominant hyper-IgE syndrome. Nature. 2008;452:773–776. doi: 10.1038/nature06764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ng WF, von Delwig A, Carmichael AJ, et al. Impaired T(H)17 responses in patients with chronic mucocutaneous candidiasis with and without autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy. J Allergy Clin Immunol. 2010;126:1006–1015. doi: 10.1016/j.jaci.2010.08.027. [DOI] [PubMed] [Google Scholar]
- 65.van de Veerdonk FL, Marijnissen RJ, Joosten LA, et al. Milder clinical hyperimmunoglobulin E syndrome phenotype is associated with partial interleukin-17 deficiency. Clin Exp Immunol. 2010;159:57–64. doi: 10.1111/j.1365-2249.2009.04043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Engelhardt KR, McGhee S, Winkler S, et al. Large deletions and point mutations involving the dedicator of cytokinesis 8 (DOCK8) in the autosomal-recessive form of hyper-IgE syndrome. J Allergy Clin Immunol. 2009;124:1289–1302. doi: 10.1016/j.jaci.2009.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Minegishi Y, Saito M, Morio T, et al. Human tyrosine kinase 2 deficiency reveals its requisite roles in multiple cytokine signals involved in innate and acquired immunity. Immunity. 2006;25:745–755. doi: 10.1016/j.immuni.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 68.Nahum A, Dadi H, Bates A, Roifman CM. The L412F variant of Toll-like receptor 3 (TLR3) is associated with cutaneous candidiasis, increased susceptibility to cytomegalovirus, and autoimmunity. J Allergy Clin Immunol. 2011;127:528–531. doi: 10.1016/j.jaci.2010.09.031. [DOI] [PubMed] [Google Scholar]
- 69.Puel A, Doffinger R, Natividad A, et al. Autoantibodies against IL-17A, IL-17F, and IL-22 in patients with chronic mucocutaneous candidiasis and autoimmune polyendocrine syndrome type I. J Exp Med. 2010;207:291–297. doi: 10.1084/jem.20091983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.de Beaucoudrey L, Samarina A, Bustamante J, et al. Revisiting human IL-12Rbeta1 deficiency: a survey of 141 patients from 30 countries. Medicine (Baltimore) 2010;89:381–402. doi: 10.1097/MD.0b013e3181fdd832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liu L, Okada S, Kong XF, et al. Gain-of-function human STAT1 mutations impair IL-17 immunity and underlie chronic mucocutaneous candidiasis. J Exp Med. 2011 doi: 10.1084/jem.20110958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.van de Veerdonk FL, Plantinga TS, Hoischen A, et al. STAT1 mutations in autosomal dominant chronic mucocutaneous candidiasis. N Engl J Med. 2011;365:54–61. doi: 10.1056/NEJMoa1100102. [DOI] [PubMed] [Google Scholar]
- 73.Chapgier A, Wynn RF, Jouanguy E, et al. Human complete Stat-1 deficiency is associated with defective type I and II IFN responses in vitro but immunity to some low virulence viruses in vivo. J Immunol. 2006;176:5078–5083. doi: 10.4049/jimmunol.176.8.5078. [DOI] [PubMed] [Google Scholar]
- 74.Dupuis S, Jouanguy E, Al-Hajjar S, et al. Impaired response to interferon-alpha/beta and lethal viral disease in human STAT1 deficiency. Nat Genet. 2003;33:388–391. doi: 10.1038/ng1097. [DOI] [PubMed] [Google Scholar]
- 75.Conti HR, Shen F, Nayyar N, et al. Th17 cells and IL-17 receptor signaling are essential for mucosal host defense against oral candidiasis. J Exp Med. 2009;206:299–311. doi: 10.1084/jem.20081463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Smeekens SP, Plantinga TS, van de Veerdonk FL, et al. STAT1 hyperphosphorylation and defective IL12R/IL23R signaling underlie defective immunity in autosomal dominant chronic mucocutaneous candidiasis. PLoS One. 2011;6:e29248. doi: 10.1371/journal.pone.0029248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Puel A, Cypowyj S, Bustamante J, et al. Chronic mucocutaneous candidiasis in humans with inborn errors of interleukin-17 immunity. Science. 2011;332:65–68. doi: 10.1126/science.1200439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gudlaugsson O, Gillespie S, Lee K, et al. Attributable mortality of nosocomial candidemia, revisited. Clin Infect Dis. 2003;37:1172–1177. doi: 10.1086/378745. [DOI] [PubMed] [Google Scholar]
- 79.Wisplinghoff H, Bischoff T, Tallent SM, et al. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis. 2004;39:309–317. doi: 10.1086/421946. [DOI] [PubMed] [Google Scholar]
- 80.Van der Graaf CA, Netea MG, Morre SA, et al. Toll-like receptor 4 Asp299Gly/Thr399Ile polymorphisms are a risk factor for Candida bloodstream infection. Eur Cytokine Netw. 2006;17:29–34. [PubMed] [Google Scholar]
- 81.Woehrle T, Du W, Goetz A, et al. Pathogen specific cytokine release reveals an effect of TLR2 Arg753Gln during Candida sepsis in humans. Cytokine. 2008;41:322–329. doi: 10.1016/j.cyto.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 82.Plantinga TS, Johnson MD, Scott WK, et al. Toll-like receptor 1 polymorphisms increase susceptibility to candidemia. J Infect Dis. 2012;205:934–943. doi: 10.1093/infdis/jir867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Morre SA, Murillo LS, Spaargaren J, Fennema HS, Pena AS. Role of the toll-like receptor 4 Asp299Gly polymorphism in susceptibility to Candida albicans infection. J Infect Dis. 2002;186:1377–1379. doi: 10.1086/344328. [DOI] [PubMed] [Google Scholar]
- 84.Funderburg N, Lederman MM, Feng Z, et al. Human-defensin-3 activates professional antigen-presenting cells via Toll-like receptors 1 and 2. Proc Natl Acad Sci USA. 2007;104:18631–18635. doi: 10.1073/PNAS.0702130104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hoover DM, Wu Z, Tucker K, Lu W, Lubkowski J. Antimicrobial characterization of human beta-defensin 3 derivatives. Antimicrob Agents Chemother. 2003;47:2804–2809. doi: 10.1128/AAC.47.9.2804-2809.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jurevic RJ, Bai M, Chadwick RB, White TC, Dale BA. Single-nucleotide polymorphisms (SNPs) in human beta-defensin 1: high-throughput SNP assays and association with Candida carriage in type I diabetics and nondiabetic controls. J Clin Microbiol. 2003;41:90–96. doi: 10.1128/JCM.41.1.90-96.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Johnson MD, Plantinga TS, van de Vosse E, et al. Cytokine gene polymorphisms and the outcome of invasive candidiasis: A Prospective Cohort Study. Clin Infect Dis. 2011 doi: 10.1093/cid/cir827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Morahan G, Huang D, Wu M, et al. Association of IL12B promoter polymorphism with severity of atopic and non-atopic asthma in children. Lancet. 2002;360:455–459. doi: 10.1016/S0140-6736(02)09676-9. [DOI] [PubMed] [Google Scholar]
- 89.Muller-Berghaus J, Kern K, Paschen A, et al. Deficient IL-12p70 secretion by dendritic cells based on IL12B promoter genotype. Genes Immun. 2004;5:431–434. doi: 10.1038/sj.gene.6364102. [DOI] [PubMed] [Google Scholar]
- 90.Schaaf BM, Boehmke F, Esnaashari H, et al. Pneumococcal septic shock is associated with the interleukin-10 - 1082 gene promoter polymorphism. Am J Respir Crit Care Med. 2003;168:476–480. doi: 10.1164/rccm.200210-1164OC. [DOI] [PubMed] [Google Scholar]
- 91.Stanilova SA, Miteva LD, Karakolev ZT, Stefanov CS. Interleukin-10 - 1082 promoter polymorphism in association with cytokine production and sepsis susceptibility. Intensive Care Med. 2006;32:260–266. doi: 10.1007/s00134-005-0022-4. [DOI] [PubMed] [Google Scholar]
- 92.Zeng L, Gu W, Chen K, et al. Clinical relevance of the interleukin 10 promoter polymorphisms in Chinese Han patients with major trauma: genetic association studies. Crit Care. 2009;13:R188. doi: 10.1186/cc8182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Romani L, Mencacci A, Grohmann U, et al. Neutralizing antibody to interleukin 4 induces systemic protection and T helper type 1-associated immunity in murine candidiasis. J Exp Med. 1992;176:19–25. doi: 10.1084/jem.176.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Romani L, Puccetti P, Mencacci A, et al. Neutralization of IL-10 up-regulates nitric oxide production and protects susceptible mice from challenge with Candida albicans. J Immunol. 1994;152:3514–3521. [PubMed] [Google Scholar]
- 95.Aydemir C, Onay H, Oguz SS, et al. Mannose-binding lectin codon 54 gene polymorphism in relation to risk of nosocomial invasive fungal infection in preterm neonates in the neonatal intensive care unit. J Matern Fetal Neonatal Med. 2010 doi: 10.3109/14767058.2010.536865. [DOI] [PubMed] [Google Scholar]
- 96.Brouwer N, Dolman KM, van HM, et al. Mannose-binding lectin (MBL) facilitates opsonophagocytosis of yeasts but not of bacteria despite MBL binding. J Immunol. 2008;180:4124–4132. doi: 10.4049/jimmunol.180.6.4124. [DOI] [PubMed] [Google Scholar]
- 97.van Asbeck EC, Hoepelman AI, Scharringa J, Herpers BL, Verhoef J. Mannose binding lectin plays a crucial role in innate immunity against yeast by enhanced complement activation and enhanced uptake of polymorphonuclear cells. BMC Microbiol. 2008;8:229. doi: 10.1186/1471-2180-8-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Babula O, Lazdane G, Kroica J, Ledger WJ, Witkin SS. Relation between recurrent vulvovaginal candidiasis, vaginal concentrations of mannose-binding lectin, and a mannose-binding lectin gene polymorphism in Latvian women. Clin Infect Dis. 2003;37:733–737. doi: 10.1086/377234. [DOI] [PubMed] [Google Scholar]
- 99.Giraldo PC, Babula O, Goncalves AK, et al. Mannose-binding lectin gene polymorphism, vulvovaginal candidiasis, and bacterial vaginosis. Obstet Gynecol. 2007;109:1123–1128. doi: 10.1097/01.AOG.0000260386.17555.a5. [DOI] [PubMed] [Google Scholar]
- 100.van Till JW, Modderman PW, de BM, et al. Mannose-binding lectin deficiency facilitates abdominal Candida infections in patients with secondary peritonitis. Clin Vaccine Immunol. 2008;15:65–70. doi: 10.1128/CVI.00297-07. This paper was first published online on Early Online on 6 June 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rabeneck L, Crane MM, Risser JM, Lacke CE, Wray NP. A simple clinical staging system that predicts progression to AIDS using CD4 count, oral thrush, and night sweats. J Gen Intern Med. 1993;8:5–9. doi: 10.1007/BF02600284. [DOI] [PubMed] [Google Scholar]
- 102.Samaranayake LP, Holmstrup P. Oral candidiasis and human immunodeficiency virus infection. J Oral Pathol Med. 1989;18:554–564. doi: 10.1111/j.1600-0714.1989.tb01552.x. [DOI] [PubMed] [Google Scholar]
- 103.Samaranayake LP. Oral mycoses in HIV infection. Oral Surg Oral Med Oral Pathol. 1992;73:171–180. doi: 10.1016/0030-4220(92)90191-r. [DOI] [PubMed] [Google Scholar]
- 104.Plantinga TS, Hamza OJ, Willment JA, et al. Genetic variation of innate immune genes in HIV-infected african patients with or without oropharyngeal candidiasis. J Acquir Immune Defic Syndr. 2010;55:87–94. doi: 10.1097/QAI.0b013e3181e53c64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Baltch AL, Bopp LH, Smith RP, et al. Effects of voriconazole, granulocyte-macrophage colony-stimulating factor, and interferon gamma on intracellular fluconazole-resistant Candida glabrata and Candida krusei in human monocyte-derived macrophages. Diagn Microbiol Infect Dis. 2005;52:299–304. doi: 10.1016/j.diagmicrobio.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 106.Vonk AG, Netea MG, van Krieken JH, et al. Treatment of intra-abdominal abscesses caused by Candida albicans with antifungal agents and recombinant murine granulocyte colony-stimulating factor. Antimicrob Agents Chemother. 2003;47:3688–3693. doi: 10.1128/AAC.47.12.3688-3693.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Dignani MC, Rex JH, Chan KW, et al. Immunomodulation with interferon-gamma and colony-stimulating factors for refractory fungal infections in patients with leukemia. Cancer. 2005;104:199–204. doi: 10.1002/cncr.21142. [DOI] [PubMed] [Google Scholar]
- 108.Poynton CH, Barnes RA, Rees J. Interferon gamma and granulocyte-macrophage colony-stimulating factor for the treatment of hepatosplenic candidosis in patients with acute leukemia. Clin Infect Dis. 1998;26:239–240. doi: 10.1086/517077. [DOI] [PubMed] [Google Scholar]
- 109.Holland SM, DeLeo FR, Elloumi HZ, et al. STAT3 mutations in the hyper-IgE syndrome. N Engl J Med. 2007;357:1608–1619. doi: 10.1056/NEJMoa073687. [DOI] [PubMed] [Google Scholar]
- 110.Minegishi Y, Saito M, Tsuchiya S, et al. Dominant-negative mutations in the DNA-binding domain of STAT3 cause hyper-IgE syndrome. Nature. 2007;448:1058–1062. doi: 10.1038/nature06096. [DOI] [PubMed] [Google Scholar]
- 111.Nagamine K, Peterson P, Scott HS, et al. Positional cloning of the APECED gene. Nat Genet. 1997;17:393–398. doi: 10.1038/ng1297-393. [DOI] [PubMed] [Google Scholar]
- 112.Pearce SH, Cheetham T, Imrie H, et al. A common and recurrent 13-bp deletion in the autoimmune regulator gene in British kindreds with autoimmune polyendocrinopathy type 1. Am J Hum Genet. 1998;63:1675–1684. doi: 10.1086/302145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Babula O, Lazdane G, Kroica J, et al. Frequency of interleukin-4 (IL-4) -589 gene polymorphism and vaginal concentrations of IL-4, nitric oxide, and mannose-binding lectin in women with recurrent vulvovaginal candidiasis. Clin Infect Dis. 2005;40:1258–1262. doi: 10.1086/429246. [DOI] [PubMed] [Google Scholar]
- 114.Choi EH, Foster CB, Taylor JG, et al. Association between chronic disseminated candidiasis in adult acute leukemia and common IL4 promoter haplotypes. J Infect Dis. 2003;187:1153–1156. doi: 10.1086/368345. [DOI] [PubMed] [Google Scholar]