Abstract
Bone marrow suppression due to exposure to ionizing radiation is a significant clinical problem associated with radiation therapy as well as with nonmedical radiation exposure. Currently, there are no small molecule agents available that can enhance hematopoietic regeneration after radiation exposure. Here, we report on the effective mitigation of acute hematopoietic radiation syndrome in mice by the synthetic triterpenoid, RTA 408. The administration of a brief course of RTA 408 treatment, beginning 24 h after lethal doses of radiation to bone marrow, significantly increased overall survival. Importantly, treatment with RTA 408 led to the full recovery of steady state hematopoiesis with normalization of the frequency of hematopoietic stem and progenitor cells. Moreover, hematopoietic stem cells from RTA 408-mitigated mice showed lineage-balanced, long-term, multilineage potential in serial transplantation assays, indicative of their normal self-renewal activity. The potency of RTA 408 in mitigating radiation-induced bone marrow suppression makes it an attractive candidate for potential clinical use in treating both therapy-related and unanticipated radiation exposure.
INTRODUCTION
Tissue damage due to therapeutic or accidental radiation exposure is a pervasive threat. Dispersal of radioactive materials leading to whole-body exposure may occur as a consequence of nuclear reactor incidents (e.g., Fukushima) or after the detonation of explosive devices laced with radioactive materials (e.g., “dirty bombs”). One of the most highly proliferative tissues in the body, the hematopoietic system, is also the most sensitive to the effects of ionizing radiation. At relatively low doses of exposure, radiation-induced damage to hematopoietic cells can cause bone marrow (BM) failure, leading to anemia, infection and hemorrhage (1, 2). Even exposure to nonlethal doses of radiation causes significant injury to hematopoietic stem cells (HSCs) and can lead to: their depletion, an increase in differentiation, and impaired self-renewal activity (3). To be useful in the setting of unanticipated radiation exposure, therapeutic agents must effectively mitigate radiation-induced damage when administered after the exposure has occurred. To date, no small molecule pharmacological drugs are approved to treat radiation-induced hematopoietic syndrome either in the radioprotection or mitigation setting (4).
Triterpenoids bind to specific cysteine residues on target proteins (5) and elicit both cytoprotective (6) and anti-inflammatory activities (7, 8). While it has not yet been determined which molecular targets of triterpenoids impart cytoprotection, these compounds have been shown to induce antioxidant enzymes in an Nrf2-dependent fashion (9, 10) and inhibit canonical NF-κB signaling (11). Earlier work demonstrated that triterpenoids protect zebrafish embryos against the lethal effects of ionizing radiation (12). More recently, the triterpenoid CDDO-Me administered 24 h after radiation exposure was shown to improve survival in mice exposed to lethal, myelosuppressive doses of total-body irradiation (TBI) (13). Although CDDO-Me advanced to phase III clinical trials to treat diabetes-associated chronic kidney disease, further development of this compound was halted due to adverse events related to fluid overload in a subset of these renal failure patients (14).
In this report, we focus on the mitigation of hematopoietic acute radiation syndrome by the triterpenoid RTA 408, which is currently in clinical development for oncological applications. Recent work demonstrated that RTA 408 protects the skin (15) and gastrointestinal mucosa (Alexeev et al., personal communication) of mice against radiation-induced damage. Based on these findings, we investigated the potential for RTA 408 to increase hematopoietic recovery from radiation-induced damage, and to test its effectiveness in the mitigation setting, i.e., when administered 24 h after radiation exposure. Our study showed that RTA 408 was a highly effective mitigator of hematopoietic syndrome in mice as demonstrated by effective recovery of hematopoiesis after administration of lethal, myeloablative doses of whole-body irradiation. In addition, treatment with RTA 408 restored normal hematopoietic stem and progenitor cell frequency and HSC self-renewal activity.
MATERIALS AND METHODS
Radiation Exposure and Mitigator Treatment
RTA 408 was provided by REATA Pharmaceuticals, Inc. (Irving, TX) and stock solutions for vehicle control (DMSO) were prepared within 1 h before injection. RTA 408 (17.5 mg/kg) or DMSO was administered intraperitoneally at 24, 48 and 72 h after irradiation. Whole-body irradiation (7–8 Gy) was performed using a 250-kVp X-ray machine (Pantak Inc., East Haven, CT) with 50 cm source-to-skin distance and a 2 mm copper filter. The dose rate was approximately 1.4 Gy/min.
Mice
For radiation survival experiments, wild-type C57Bl/6 CD45.2 mice (6–8 weeks old) were used. Congenic wild-type C57Bl/6 CD45.1 and C57Bl/6 CD45.1/CD45.2 hybrid host mice were used as recipients in transplantation experiments. Mice were kept in pathogen-free conditions and handled in accordance with the requirements of the Guideline for Animal Experiments and, after approval of the experimental protocols, by the Institutional Animal Care and Use Committees of Thomas Jefferson University and OHSU.
Complete Blood Counts and Bone Marrow Analysis
Peripheral blood was collected into tubes containing EDTA tripotassium salt and assayed using a Hemavet 950 FS hematology analyzer. Dissected femurs were flushed with Hanks’ balanced salt solution containing 10 mM HEPES and 3% fetal bovine serum, and then passed through a 70 micron cell strainer. Nucleated cell counts were obtained using Turk’s solution and a hemocytometer.
Colony-Forming Unit Assays
Bone marrow cells (2 × 104) were plated in duplicate or triplicate in 35 mm dishes in mouse methylcellulose complete media (HSC007, R&D Systems™, Minneapolis, MN). Colonies were scored 7–10 days after plating according to the manufacturer’s instructions.
Transplantation Studies
Prior to transplantation, all recipient mice were maintained for at least one week on acidified water. Recipient mice received 7.5 Gy in a single fraction using an RS2000 X-ray irradiator (Rad Source, Alpharetta, GA) with a dose rate of ~1.36 Gy/min. Primary cell recipient mice (CD45.1 or CD45.1/CD45.2 hybrid) received 2 × 106 CD45.2 donor cells together with 1 × 105 carrier bone marrow (CD45.1 or CD45.1/CD45.2 hybrid). Specifically, CD45.1 donor cells were used as carrier cells for transplants into hybrid recipients and hybrid donor cells were used as carrier cells for transplant into CD45.1 recipient mice. For serial transplantation experiments, secondary recipient mice (CD45.1 or CD45.1/CD45.2 hybrid) received 2 × 106 unfractionated bone marrow isolated from primary recipients. Immediately after irradiation, cells were transplanted into anesthetized animals via retro-orbital injection. Recipient mice were maintained on water containing antibiotics for 4 weeks after transplantation, as previously described (16).
Flow Cytometry
Red blood cell depleted peripheral blood was prepared as previously described (17). Live cells were stained with antibodies, washed and then analyzed using a FACSCanto™ or BD™ LSR II (both from BD Biosciences, San Jose, CA). Dead cells were excluded with propidium iodine and doublets were excluded using FSC-A, FSC-H and trigger pulse width parameters. Data were analyzed with FlowJo software (Tree Star, Inc., Philomath, OR). Antibodies (and clones) used in this study included: Mac1 (M1/70), Gr1 (RB6-8C5), B220 (RA3-6B2), CD3 (145-2C11) and c-kit (2B8) from eBioscience (San Diego, CA); CD4 (H29.19), CD5 (53–7.3), CD8 (53.6.7) from BD Pharmingen™ (San Diego, CA); and TER119, Sca1 (D7) and CD150 (TC15-12F12.2), from BioLegend®, Inc. (San Diego, CA). For Lin−Sca1+c-kit+ (LSK) cell analysis, the lineage panel included B220, CD3, CD4, CD5, CD8, Mac1, Gr1 and Ter119.
RESULTS
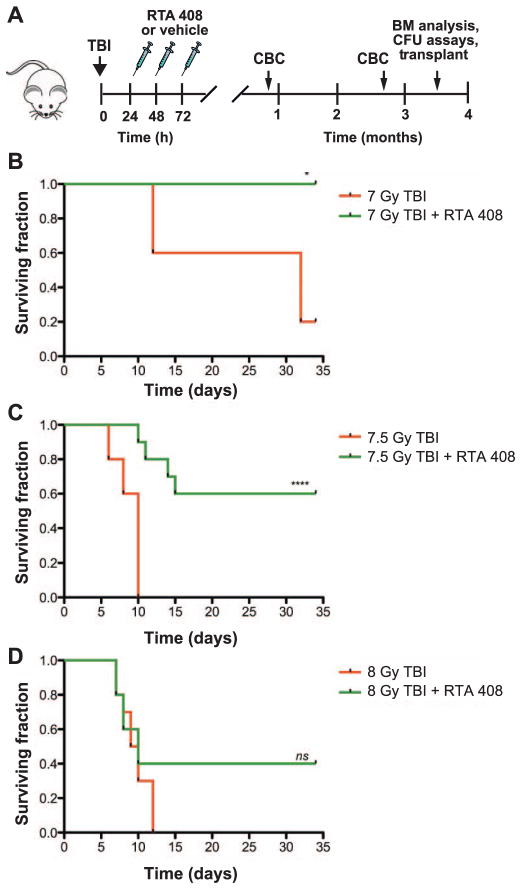
RTA 408 Enhances the Survival of Lethally Irradiated Mice
To determine whether RTA 408 is an effective mitigator of hematopoietic acute radiation syndrome after bone marrow-lethal doses of TBI, mice were administered 3 daily injections of 17.5 mg/kg RTA 408 beginning 24 h after TBI (Fig. 1A). We found that treatment with RTA 408 resulted in the 35 day survival of 100% of 7 Gy (LD40/35) TBI mice (Fig. 1B, P < 0.05) and 60% of 7.5 Gy (LD100/13) TBI mice (Fig. 1C, P < 0.0001). Although 40% of 8 Gy (LD100/10) TBI mice survived after RTA 408 treatment, these results did not reach statistical significance (Fig. 1D).
FIG. 1.
Mice treated with RTA 408 24 h post TBI survive BM-lethal doses of radiation. Panel A: Experimental schema. Mice exposed to 7–8 Gy TBI were injected intraperitoneally with 17.5 mg/kg RTA 408 per day beginning 24 h post TBI. Mice were monitored for survival through day 35. Complete blood counts (CBC) and bone marrow analysis were performed at the indicated time points. Panels B–D: Survival of TBI mice after RTA 408 or vehicle-only treatment. In panel B, n =5 mice per treatment group and for panels C–D, n =10 mice per treatment group. Statistically significant differences between survival distributions were determined by log-rank test. (*P < 0.05; ****P < 0.0001; ns: not significant).
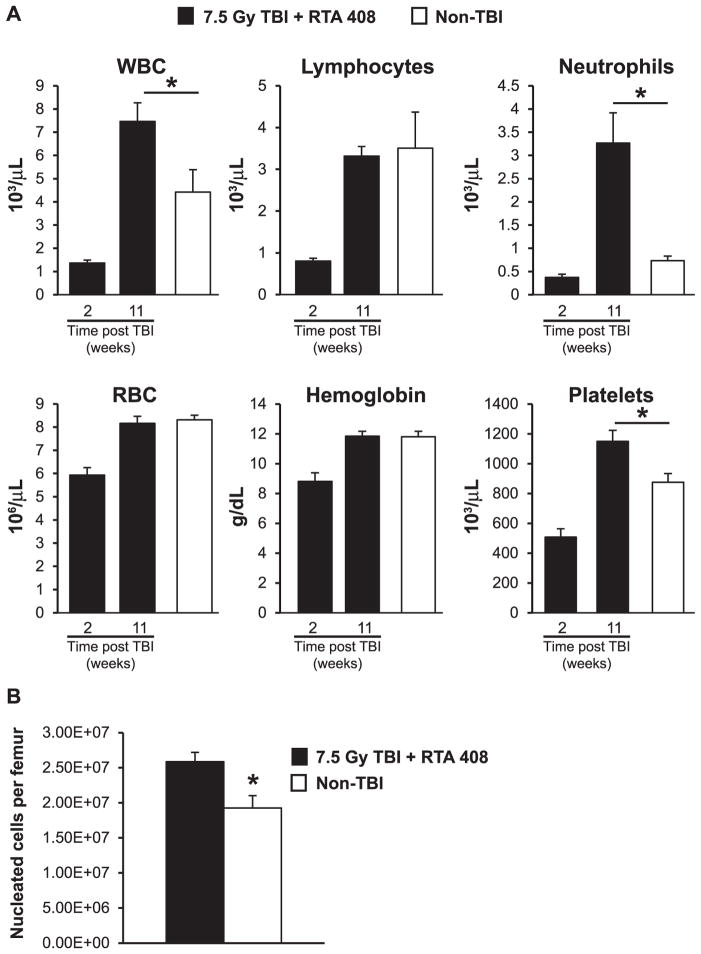
Full Hematologic Recovery in Irradiated Mice Treated with RTA 408
To begin to assess the recovery of hematopoiesis, complete blood counts were obtained at 2 weeks and again at 11 weeks after RTA 408 treatment. As anticipated, both neutrophils and lymphocytes were markedly reduced at 2 weeks postirradiation; however, the hemoglobin remained above 8 g/dL, a level consistent with survival after a bone marrow-lethal dose of radiation. By 11 weeks after radiation exposure, most parameters had returned to normal in the RTA 408-treated mice (Fig. 2A). Circulating neutrophils were increased in RTA 408-treated mice relative to nonirradiated age-matched control mice. This result was not surprising, since the percentage of neutrophils in the blood is typically increased in mice after receiving either bone marrow or hematopoietic stem cell transplant. This myeloid bias may be further amplified since the triterpenoid derivative CDDO-Me is known to promote myelopoiesis in mice (18). In LD(50/30)/LD(70/30) TBI mice that survive radiation-induced injury without any intervention, BM cellularity typically remains significantly decreased throughout life (19). However, when we assessed BM in RTA 408-treated 7.5 Gy TBI mice 14 weeks postirradiation, BM cellularity was not reduced in the TBI + RTA 408-treated mice (Fig. 2B). Together, these findings of the complete restoration of circulating hematopoietic cells and normal BM cellularity in 7.5 Gy TBI mice indicate that RTA 408 is a potent mitigator of hematopoietic syndrome.
FIG. 2.
Hematological recovery in RTA 408-treated 7.5 Gy TBI mice. Panel A: Circulating blood cell analysis in RTA 408-treated 7.5 Gy TBI mice 2 and 11 weeks postirradiation. Pooled results from n =7–8 mice are shown. Circulating blood cell analysis in control, non-TBI mice (n =6) is also shown. Although platelets are significantly higher in RTA 408-treated mice, they are still within normal range. Panel B: Nucleated bone marrow cell counts in mice treated with RTA 408 (n =8) 14 weeks post 7.5 Gy TBI and in age-matched, non-TBI controls (n =5). A Student’s t test was used for statistical analysis. *P < 0.05. For all graphs, error bars indicate SEM.
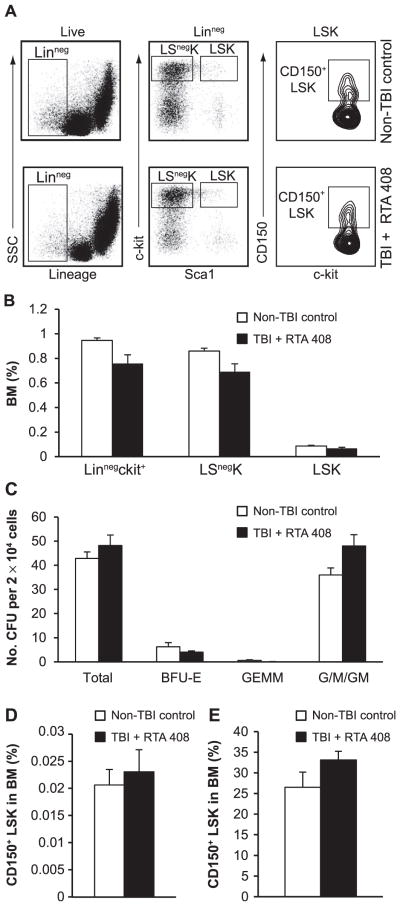
RTA 408 Restores Normal Hematopoietic Stem and Progenitor Cell Frequency in Lethally Irradiated Mice
Acute radiation exposure causes long-term damage to both hematopoietic stem and progenitor cells, resulting in their decreased frequency and a substantive loss of functional activity (3, 19–21). To more fully assess the efficacy of RTA 408 in restoring hematopoietic stem and progenitor cell frequency after lethal irradiation, BM from RTA 408-treated mice was analyzed 14 weeks after 7.5 Gy TBI (Fig. 1A). Bone marrow from age-matched, nonirradiated mice was used for comparison, since none of the vehicle-treated 7.5 Gy TBI mice survived (Fig. 1C). Flow cytometric analysis (Fig. 3A) revealed that the TBI + RTA 408-treated mice had comparable frequencies of phenotypic progenitor cells including Linage−(Lin−) c-kit+ cells and Lin−Sca1−c-kit+ (LSnegK) myeloerythroid committed progenitors. Similarly, a normal frequency of LSK cells, a subpopulation highly enriched for hematopoietic stem cells and multipotent progenitor cells, was observed in RTA 408-mitigated mice (Fig. 3B). Hematopoietic progenitor cell function in RTA 408-mitigated 7.5 Gy TBI mice was further assessed by measuring BM colony-forming unit (CFU) activity on a per cell basis in cytokine supplemented methylcellulose. Both the total frequency and the individual subsets of myeloerythroid colonies formed by RTA 408-mitigated BM were indistinguishable from those formed by non-TBI control BM (Fig. 3C), indicating no loss of progenitor activity in the RTA 408-mitigated BM. Together, these data demonstrate that RTA 408 treatment of 7.5 Gy TBI mice restored both phenotypic and functional hematopoietic progenitors to normal levels.
FIG. 3.
Restoration of hematopoietic stem and progenitor cell frequency in RTA 408-mitigated 7.5 Gy TBI mice to non-TBI levels. Panel A: Representative flow cytometry analysis of 7.5 Gy TBI + RTA 408-treated mice and age-matched control BM, 14 weeks postirradiation. Parental populations are indicated on the top of each plot and gates used for analysis are shown. Panel B: Calculated frequency of hematopoietic progenitor populations based on flow cytometry analysis. No significant differences between control non-TBI mice (n = 5) and 7.5 Gy TBI + RTA 408-treated mice (n = 8) were observed. Panel C: Comparison of in vitro colony-forming unit activity in methylcellulose supplemented with IL3, IL6, TPO and SCF. There were no differences, in either the colonies per input BM or the types of colonies formed, between RTA 408-mitigated BM (n = 6) and age-matched non-TBI BM (n = 5). BFU-E: burst-forming-unit erythroid; GEMM: mixed lineage granulocytic, erythroid, macrophage, megakaryocyte; G/M/GM: myeloid colonies containing granulocytes (G), macrophages (M) or both cell types (GM). Panel D: CD150+LSK cell frequency in RTA 408-mitigated TBI mice (n = 8) is the same as in non-TBI, age-matched mice (n = 5). Panel E: Similar CD150+ cell frequency in the LSK compartments of RTA 408-mitigated BM and non-TBI BM. Unpaired Student’s t tests were used for statistical analysis. Error bars indicate SEM.
To assess phenotypic HSCs in RTA 408-mitigated 7.5 Gy TBI mice, the frequency of CD150+LSK cells, a population that is highly enriched for functional stem cells (22), was determined by flow cytometry. Both the absolute number and frequency of CD150+LSK cells in TBI + RTA 408-treated mice were the same as in the non-TBI controls (Fig. 3D). It has previously been shown that radiation-induced injury causes a long-term phenotypic skewing of hematopoietic stem cells, reflected by a higher proportion of CD150+ cells within the LSK cell subset (19, 21). However, the proportion of CD150+ cells in the LSK cell population of RTA 408-mitigated TBI mice was the same as that observed in age-matched non-TBI mice (Fig. 3E). Thus, RTA 408 treatment 24 h after TBI effectively mitigates the radiation-induced loss of phenotypic HSCs.
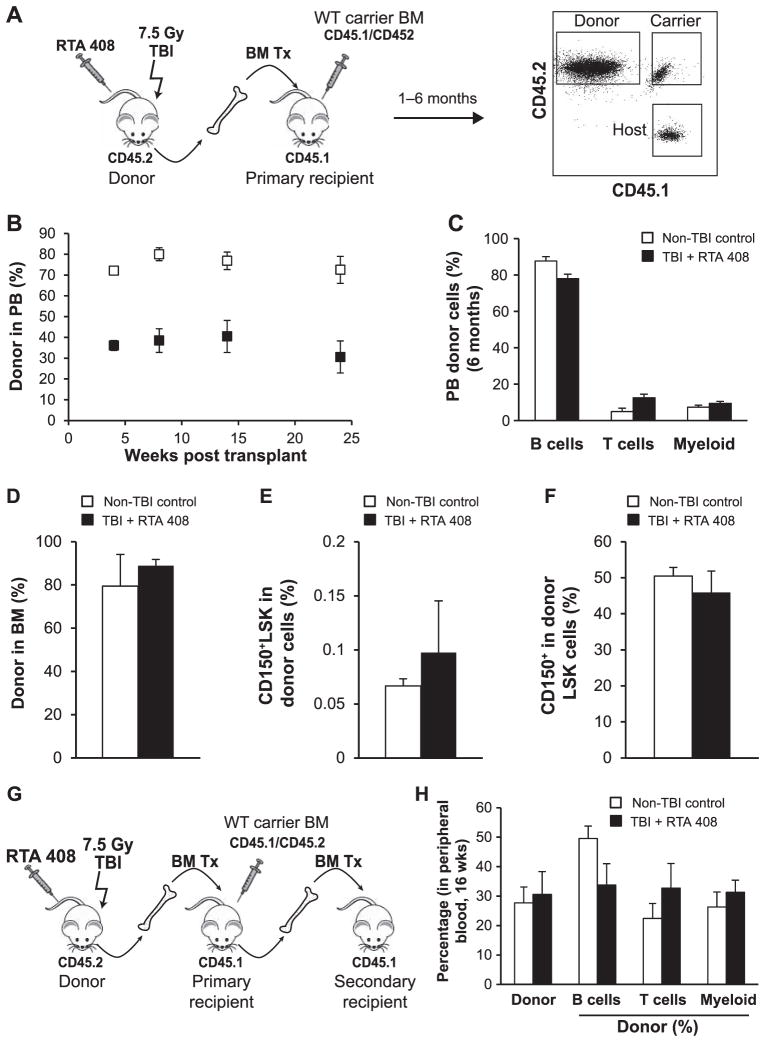
RTA 408 Restores Functional HSCs in Lethally Irradiated Mice
Transplantation assays were performed to assess the long-term functional status of HSCs in RTA 408-rescued mice. For these experiments, BM from individual RTA 408-treated mice harvested 3.5 months after TBI was co-transplanted with a limiting dose of carrier BM into CD45 congenic, lethally irradiated recipients (Fig. 4A). For comparison, non-TBI donor cell contribution to BM HSCs in primary recipients was evaluated. Donor cell engraftment was monitored in the peripheral blood over time. Radiation exposure not only limits HSC self-renewal, it also causes their long-term myeloid lineage skewing (3, 19). As shown in Fig. 4B, BM from RTA 408-rescued donors successfully established robust hematopoietic engraftment in primary hosts. Moreover, RTA 408-treated BM sustained long-term, multilineage hematopoiesis for 6 months after transplantation (Fig. 4C), consistent with the presence of functional HSCs lacking lineage bias. Phenotypic HSCs (CD150+LSK) derived from RTA 408 donor cells were assessed in primary recipient mice BM >6 months after transplantation. Importantly, the frequency of CD150+LSK cells derived from RTA 408-mitigated donor cells was the same as those derived from non-TBI donor cells (Fig. 4D–F). In addition, RTA 408-mitigated BM had the same proportion of CD150+ cells within the LSK subset as BM from non-TBI donors, providing further evidence of sustained HSC lineage balance in RTA 408-mitigated BM.
FIG. 4.
Treatment with RTA 408 restores functional HSCs in 7.5 Gy TBI mice. Panel A: Primary transplant schema. Donor cells from 4 individual 7.5 Gy donor TBI + RTA 408-treated mice were transplanted into 4 cohorts of 2–3 recipient mice. Flow cytometry analysis was used to evaluate blood lineages and distinguish donor, host and carrier cells. As a control, donor cells from 2 individual non-TBI mice were each transplanted into a cohort of 3 recipient mice. Panel B: Contribution of 7.5 Gy TBI + RTA 408-treated donor BM cells to peripheral blood leukocytes after transplantation over time. Pooled results from 10 to 11 TBI + RTA 408-treated BM recipient mice and 6 control BM recipient mice are shown. Panel C: Lineage analysis of circulating donor cells derived from TBI + RTA 408 treatment or control BM 6 months after transplantation. Donor cells from TBI + RTA 408-treated BM contributed to myeloid (Mac1+ and/or Gr1+) and lymphoid (B220+ B cells, CD3+ T cells) lineages similarly to donor cells from control BM. Pooled results from 10 to 11 TBI + RTA 408-treated BM recipient mice and 6 control BM recipient mice are shown. Panels D–F: Donor cell analysis in BM >6 months after transplantation. Recipient mice (n =3) transplanted with TBI + RTA 408-treated BM from 3 different donors were analyzed. A separate cohort of mice (n = 3) that received BM from 2 non-TBI donors was used for controls. Panel D: Similar levels of BM donor cell engraftment by non-TBI donor cells and 7.5 Gy TBI + RTA 408-treated donor cells. Panel E: Similar contribution to CD150+LSK cells by non-TBI donor cells and 7.5 Gy TBI + RTA 408-treated donor cells. Panel F: The same proportion of CD150+ cells in the LSK compartment were derived from non-TBI donor cells or TBI + RTA 408-treated donor cells. Panel G: Secondary transplant schema. Bone marrow from two primary recipient mice reconstituted with non-TBI or 7.5 Gy TBI + RTA 408-treated donor cells were serially transplanted into cohorts of 4–5 secondary recipients. Panel H: Peripheral blood analysis of secondary recipient mice 16 weeks after transplantation.
To stringently test functional HSC activity, serial transplantation was performed (Fig. 4G). RTA 408-treated BM gave rise to multilineage, donor cell engraftment in all secondary recipients providing direct evidence for HSC self-renewal activity (Fig. 4H). Consistent with our finding that phenotypic HSCs in RTA 408-mitigated BM lack a myeloid-bias, we found a similar overall contribution of control donor cells and RTA 408 donor cells to the total number of circulating myeloid cells. These data confirm that RTA 408 supports the regeneration of bona fide, functionally competent, lineage-balanced, long-term HSCs after exposure to bone marrow-lethal doses of radiation.
DISCUSSION
The results of our studies are consistent with the previous published article by Kim et al., demonstrating that the CDDO variant CDDO-Me, beginning 24 h after exposure to 7.5 Gy TBI, enhances hematologic recovery and results in 20% survival (13). In the current study, we show that the triterpenoid RTA 408 can prevent death caused by hematopoietic acute radiation syndrome in 60% of mice that received 7.5 Gy TBI. Moreover, we found that the administration of only three doses of RTA 408 beginning 24 h postirradiation was sufficient to restore hematopoietic stem and progenitor cell frequency and function to levels seen in nonirradiated mice. The combined results from these studies strongly suggest that RTA 408 and other related triterpenoids are promising candidates, and should be evaluated through further preclinical studies for the pharmacological treatment of hematopoietic acute radiation syndrome.
The mechanism(s) underlying the regenerative effect that RTA 408 has on radiation-damaged hematopoietic cells are obscured by the multiplicity of molecular targets of thiol modifying compounds. Relevant targets include JAK/STAT3 (23) and canonical NF-κB signaling pathways (11), both of which are reportedly inhibited by triterpenoids. In addition, triterpenoids induce Nrf2-dependent transcription of a plethora of antioxidant enzymes by disrupting the interaction of Nrf2 with its inhibitor Keap1, thereby preventing the proteolytic degradation of Nrf2 and facilitating its nuclear translocation (9, 10, 24). Nfr2 has radioprotective activity in the hematopoietic system (13), lending support to the hypothesis that induction of antioxidant enzymes is critical for the bone marrow-protective effects of triterpenoids, including RTA 408. However, it is also likely that triterpenoids are activating additional signaling pathways that also make a significant contribution to the regenerative process. For example, CDDO derivatives have been shown to skew the differentiation of myelomonocytic cells in an Erk1/2 and SMAD-dependent manner (25). Furthermore, CDDO induces granulocytic differentiation of HL-60 cells presumably through induction of CCAAT enhancer-binding protein alpha (CEBPA), a transcription factor critical for granulocytic differentiation (26, 27). The marked increase in circulating neutrophils in RTA 408-mitigated mice (Fig. 2) is consistent with these previous findings. These results suggest that the complex effects of synthetic triterpenoids on multiple signaling pathways regulate hematopoietic cell survival and differentiation.
Acknowledgments
We are grateful to REATA Pharmaceuticals for providing RTA 408 and we also thank Carly Hernandez for technical support. This work was supported by the NIH [U19 AI091175 (CG, UR, WHF)], REATA Pharmaceuticals (UR) and the Department of Defense [W81XWH-12-1-0477 (UR)].
References
- 1.Chao NJ. Accidental or intentional exposure to ionizing radiation: biodosimetry and treatment options. Exp Hematol. 2007;35(4 Suppl 1):24–7. doi: 10.1016/j.exphem.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 2.Mauch P, Constine L, Greenberger J, Knospe W, Sullivan J, Liesveld JL, et al. Hematopoietic stem cell compartment: acute and late effects of radiation therapy and chemotherapy. Int J Radiat Oncol Biol Phys. 1995;31:1319–39. doi: 10.1016/0360-3016(94)00430-S. [DOI] [PubMed] [Google Scholar]
- 3.Shao L, Luo Y, Zhou D. Hematopoietic stem cell injury induced by ionizing radiation. Antioxid Redox Signal. 2014;20:1447–62. doi: 10.1089/ars.2013.5635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xiao M, Whitnall MH. Pharmacological countermeasures for the acute radiation syndrome. Curr Mol Pharmacol. 2009;2:122–33. doi: 10.2174/1874467210902010122. [DOI] [PubMed] [Google Scholar]
- 5.Suh N, Wang Y, Honda T, Gribble GW, Dmitrovsky E, Hickey WF, et al. A novel synthetic oleanane triterpenoid, 2-cyano-3,12-dioxoolean-1,9-dien-28-oic acid, with potent differentiating, anti-proliferative, and anti-inflammatory activity. Cancer Res. 1999;59:336–41. [PubMed] [Google Scholar]
- 6.Yates MS, Tran QT, Dolan PM, Osburn WO, Shin S, McCulloch CC, et al. Genetic versus chemoprotective activation of Nrf2 signaling: overlapping yet distinct gene expression profiles between Keap1 knockout and triterpenoid-treated mice. Carcinogenesis. 2009;30:1024–31. doi: 10.1093/carcin/bgp100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Auletta JJ, Alabran JL, Kim BG, Meyer CJ, Letterio JJ. The synthetic triterpenoid, CDDO-Me, modulates the proinflammatory response to in vivo lipopolysaccharide challenge. J Interferon Cytokine Res. 2010;30:497–508. doi: 10.1089/jir.2009.0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Place AE, Suh N, Williams CR, Risingsong R, Honda T, Honda Y, et al. The novel synthetic triterpenoid, CDDO-imidazolide, inhibits inflammatory response and tumor growth in vivo. Clin Cancer Res. 2003;9:2798–806. [PubMed] [Google Scholar]
- 9.Liby K, Hock T, Yore MM, Suh N, Place AE, Risingsong R, et al. The synthetic triterpenoids, CDDO and CDDO-imidazolide, are potent inducers of heme oxygenase-1 and Nrf2/ARE signaling. Cancer Res. 2005;65:4789–98. doi: 10.1158/0008-5472.CAN-04-4539. [DOI] [PubMed] [Google Scholar]
- 10.Thimmulappa RK, Scollick C, Traore K, Yates M, Trush MA, Liby KT, et al. Nrf2-dependent protection from LPS induced inflammatory response and mortality by CDDO-Imidazolide. Biochem Biophys Res Commun. 2006;351:883–9. doi: 10.1016/j.bbrc.2006.10.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ahmad R, Raina D, Meyer C, Kharbanda S, Kufe D. Triterpenoid CDDO-Me blocks the NF-kappaB pathway by direct inhibition of IKKbeta on Cys-179. J Biol Chem. 2006;281:35764–9. doi: 10.1074/jbc.M607160200. [DOI] [PubMed] [Google Scholar]
- 12.Daroczi B, Kari G, Ren Q, Dicker AP, Rodeck U. Nuclear factor kappaB inhibitors alleviate and the proteasome inhibitor PS-341 exacerbates radiation toxicity in zebrafish embryos. Mol Cancer Ther. 2009;8:2625–34. doi: 10.1158/1535-7163.MCT-09-0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim JH, Thimmulappa RK, Kumar V, Cui W, Kumar S, Kombairaju P, et al. NRF2-mediated Notch pathway activation enhances hematopoietic reconstitution following myelosuppressive radiation. J Clin Invest. 2014;124:730–41. doi: 10.1172/JCI70812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang DD. Bardoxolone brings Nrf2-based therapies to light. Antioxid Redox Signal. 2013;19:517–8. doi: 10.1089/ars.2012.5118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reisman SA, Lee CY, Meyer CJ, Proksch JW, Sonis ST, Ward KW. Topical application of the synthetic triterpenoid RTA 408 protects mice from radiation-induced dermatitis. Radiat Res. 2014;181:512–20. doi: 10.1667/RR13578.1. [DOI] [PubMed] [Google Scholar]
- 16.Li B, Bailey AS, Jiang S, Liu B, Goldman DC, Fleming WH. Endothelial cells mediate the regeneration of hematopoietic stem cells. Stem Cell Res. 2010;4:17–24. doi: 10.1016/j.scr.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goldman DC, Bailey AS, Pfaffle DL, Al Masri A, Christian JL, Fleming WH. BMP4 regulates the hematopoietic stem cell niche. Blood. 2009;114:4393–401. doi: 10.1182/blood-2009-02-206433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ames E, Harouna S, Meyer C, Welniak LA, Murphy WJ. The triterpenoid CDDO-Me promotes hematopoietic progenitor expansion and myelopoiesis in mice. Biol Blood Marrow Transplant. 2012;18:396–405. doi: 10.1016/j.bbmt.2011.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chua HL, Plett PA, Sampson CH, Joshi M, Tabbey R, Katz BP, et al. Long-term hematopoietic stem cell damage in a murine model of the hematopoietic syndrome of the acute radiation syndrome. Health Phys. 2012;103:356–66. doi: 10.1097/HP.0b013e3182666d6f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shao L, Feng W, Li H, Gardner D, Luo Y, Wang Y, et al. Total body irradiation causes long-term mouse BM injury via induction of HSC premature senescence in an Ink4a- and Arf-independent manner. Blood. 2014;123:3105–15. doi: 10.1182/blood-2013-07-515619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Simonnet AJ, Nehme J, Vaigot P, Barroca V, Leboulch P, Tronik-Le Roux D. Phenotypic and functional changes induced in hematopoietic stem/progenitor cells after gamma-ray radiation exposure. Stem Cells. 2009;27:1400–9. doi: 10.1002/stem.66. [DOI] [PubMed] [Google Scholar]
- 22.Kiel MJ, Yilmaz OH, Iwashita T, Yilmaz OH, Terhorst C, Morrison SJ. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121:1109–21. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 23.Ahmad R, Raina D, Meyer C, Kufe D. Triterpenoid CDDO-methyl ester inhibits the Janus-activated kinase-1 (JAK1)–>signal transducer and activator of transcription-3 (STAT3) pathway by direct inhibition of JAK1 and STAT3. Cancer Res. 2008;68:2920–6. doi: 10.1158/0008-5472.CAN-07-3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thimmulappa RK, Fuchs RJ, Malhotra D, Scollick C, Traore K, Bream JH, et al. Preclinical evaluation of targeting the Nrf2 pathway by triterpenoids (CDDO-Im and CDDO-Me) for protection from LPS-induced inflammatory response and reactive oxygen species in human peripheral blood mononuclear cells and neutrophils. Antioxid Redox Signal. 2007;9:1963–70. doi: 10.1089/ars.2007.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ji Y, Lee HJ, Goodman C, Uskokovic M, Liby K, Sporn M, et al. The synthetic triterpenoid CDDO-imidazolide induces monocytic differentiation by activating the Smad and ERK signaling pathways in HL60 leukemia cells. Mol Cancer Ther. 2006;5:1452–8. doi: 10.1158/1535-7163.MCT-06-0136. [DOI] [PubMed] [Google Scholar]
- 26.Konopleva M, Tsao T, Ruvolo P, Stiouf I, Estrov Z, Leysath CE, et al. Novel triterpenoid CDDO-Me is a potent inducer of apoptosis and differentiation in acute myelogenous leukemia. Blood. 2002;99:326–35. doi: 10.1182/blood.v99.1.326. [DOI] [PubMed] [Google Scholar]
- 27.Koschmieder S, D’Alo F, Radomska H, Schoneich C, Chang JS, Konopleva M, et al. CDDO induces granulocytic differentiation of myeloid leukemic blasts through translational up-regulation of p42 CCAAT enhancer binding protein alpha. Blood. 2007;110:3695–705. doi: 10.1182/blood-2006-11-058941. [DOI] [PMC free article] [PubMed] [Google Scholar]