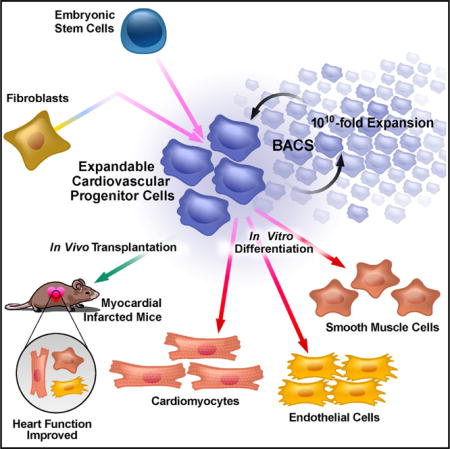
SUMMARY
Stem cell-based approaches to cardiac regeneration are increasingly viable strategies for treating heart failure. Generating abundant and functional autologous cells for transplantation in such a setting, however, remains a significant challenge. Here, we isolated a cell population with extensive proliferation capacity and restricted cardiovascular differentiation potentials during cardiac transdifferentiation of mouse fibroblasts. These induced expandable cardiovascular progenitor cells (ieCPCs) proliferated extensively for more than 18 passages in chemically defined conditions, with 105 starting fibroblasts robustly producing 1016 ieCPCs. ieCPCs expressed cardiac signature genes and readily differentiated into functional cardiomyocytes (CMs), endothelial cells (ECs), and smooth muscle cells (SMCs) in vitro, even after long-term expansion. When transplanted into mouse hearts following myocardial infarction, ieCPCs spontaneously differentiated into CMs, ECs, and SMCs and improved cardiac function for up to 12 weeks after transplantation. Thus, ieCPCs are a powerful system to study cardiovascular specification and provide strategies for regenerative medicine in the heart.
Graphical abstract
INTRODUCTION
Heart failure (HF) is a devastating disease and a major cause of morbidity and mortality worldwide. HF often follows myocardial infarction (MI) that is usually accompanied by a massive loss of cardiomyocytes (CMs). These CMs cannot be regenerated by the adult mammalian heart and cannot yet be replaced and/or regenerated via cell-based therapies. Unfortunately, transplanting CMs into an infarcted heart yields only transient and marginal benefits (Burridge et al., 2012). Shortly after transplantation, most CMs are soon lost. These effects are likely caused by the limited proliferative capacity of fully differentiated CMs and a lack of blood-vessel formation to supply oxygen and nutrients (Lam et al., 2009). Thus, to create more effective regenerative therapies, we need to find a cell type that can be extensively expanded in vitro and robustly differentiated into cardiovascular cells in a diseased heart.
Cardiovascular progenitor cells (CPCs) may offer a promising avenue for cardiac-regenerative therapy. These cells evolve from the mesoderm during cardiogenesis, a well-orchestrated process in developing embryos that is recapitulated in differentiating pluripotent stem cells (PSCs). Patterned mesoderm gives rise to a hierarchy of downstream cellular intermediates that represent lineage-restricted CPCs for fully differentiated heart cells, including CMs, endothelial cells (ECs), and smooth muscle cells (SMCs) (Burridge et al., 2012). Each step in this hierarchy is tightly controlled by multiple stage-specific signals (e.g., Wnt, Activin/Nodal, bone morphogenetic protein [BMP], fibroblast growth factor [FGF], and Notch) (Burridge et al., 2012; Bruneau, 2013). Additionally, the gradual loss of multipotency, or commitment of cell fate, is usually accompanied by a decreased capacity of cellular proliferation. Thus, by isolating CPCs that can extensively self-renew and possess multiple, but restricted, potentials to directly differentiate into these three cardiovascular cell types, we may encourage the development of more effective and potentially safer therapies for cardiac regeneration.
A previous study identified one type of primitive CPCs that express two key marker genes, MESP1 and SSEA1 (Cao et al., 2013); however, these cells more closely represent a mesodermal precursor and are not fully committed to a cardiac fate. To differentiate into CMs in vitro, these primitive CPCs require multiple and sequential developmental signals. This notion is supported by studies in which Mesp1+ cells not only contributed to heart development but also gave rise to non-cardiovascular mesodermal lineages, such as hematopoietic and skeletal muscle cells (Chan et al., 2013; Devine et al., 2014). Consequently, such properties of primitive CPCs may comprise their own ability to efficiently differentiate and restore lost CMs within the damaged heart, which lacks the complex paracrine environment and tight temporal and spatial control seen in developing embryos. Several reports have also described more committed CPCs that are fully specified to a cardiovascular fate. Such line-age-restricted CPCs could be identified by several late-stage marker genes, including insulin gene enhancer protein 1 (Isl1), Nkx2-5, fetal liver kinase 1 (Flk-1 ; also known as vascular endothelial growth factor [VEGF] receptor 2), and platelet-derived growth factor receptor (PdgfR)-α (Moretti et al., 2006; Kattman et al., 2011). These cells directly differentiated into three cardiac lineages without stepwise developmental signals. For example, Isl1+ cells have been observed in postnatal and adult heart and enter fully differentiated cardiovascular lineages without the embryonic heart niche (Laugwitz et al., 2005; Moretti et al., 2006). Unfortunately, although these committed CPCs might be more suitable for cardiac cell therapy in vivo, they have yet to be extensively expanded, thus significantly limiting their applications.
To overcome these limitations, we systematically examined combinations of multiple signaling pathways involved in cardiogenesis and developed chemically defined conditions to identify a specific type of CPCs–reprogrammed from fibroblasts–that extensively self-renews and is restricted to a cardiovascular fate (i.e., directly giving rise to CMs, ECs, and SMCs without stepwise differentiation). These induced expandable CPCs (ieCPCs) can robustly self-renew for more than 18 passages (translated into >1016 ieCPCs from 105 starting fibroblasts) and still retain their original morphology, gene expression pattern, and multiple, but lineage-restricted, potentials to differentiate into three cardiovascular lineages within the heart. Implanted ieCPCs spontaneously differentiate into CMs, ECs, and SMCs in infarcted mouse hearts and benefit heart function following cardiac injury. With this system, similar expandable CPCs could also be differentiated and captured from PSCs. This study provides a proof-of-principle demonstration that cardiovascular-restricted CPCs that can self-renew long term in vitro can also be directly induced from fibroblasts or differentiated from PSCs. These cells offer a useful source, uncompromised by growth arrest, for individualized cardiac cell therapies.
RESULTS
Hypothesis-Driven Screening to Capture ieCPCs
To develop effective and personalized cell therapies, we need to obtain CPCs from an easily accessible source. To that end, we used cell activation and signaling-directed (CASD) lineage conversion, which transiently exposes cells to reprogramming factors and small molecules in conjunction with cardiac-inductive signals (Efe et al., 2011; Wang et al., 2014). Remarkably, we observed cell populations expressing markers corresponding to different stages of cardiogenesis during reprogramming, potentially helping identify and capture the desirable ieCPCs.
To capture and expand putative ieCPCs arising during CASD-lineage conversion, we screened combinations of cardiogenic signals with a functional assay under the hypothesis that ieCPCs would respond and propagate with the right combination of cardiogenic signaling at optimal strength. We performed the conversion from secondary mouse embryonic fibroblasts (2nd MEFs) (Efe et al., 2011). First, we activated the cells by transiently expressing reprogramming factors for 6 days and allowed cardiac specification for another 2 days. From day 8, we treated the activated cells with various combinations of modulators of Wnt, Activin/Nodal, BMP, FGF, VEGF, platelet-derived growth factor (PDGF), and Notch pathways at different concentrations for another 3 days and then passaged these cells three times under the same conditions, each 4 days apart. We then assessed spontaneous cardiac differentiation in cells that extensively propagated without showing morphological changes over the serial passages (Figure 1A). These cells were cultured in defined basal differentiation conditions without serum, knockout serum replacement, or any other exogenous differentiation cues, which is a key criterion in evaluating the committed cardiovascular fate. Cardiac differentiation of these cells was monitored by the presence of contractile patches and the expression of cardiac troponin T (cTnT), a CM-specific marker. After repetitive testing and optimizing, we converged on one promising condition that retained the cardiac potential of induced cells after three passages, indicating that we captured putative ieCPCs. This condition contained BMP4 (5 ng/ml), Activin A (10 ng/ml), CHIR99021 (3 μM; a glycogen synthase kinase 3 inhibitor), and SU5402 (2 μM; an inhibitor of FGF, VEGF, and PDGF signaling), hereafter referred to as BACS.
Figure 1. Generation and Characterization of ieCPCs.
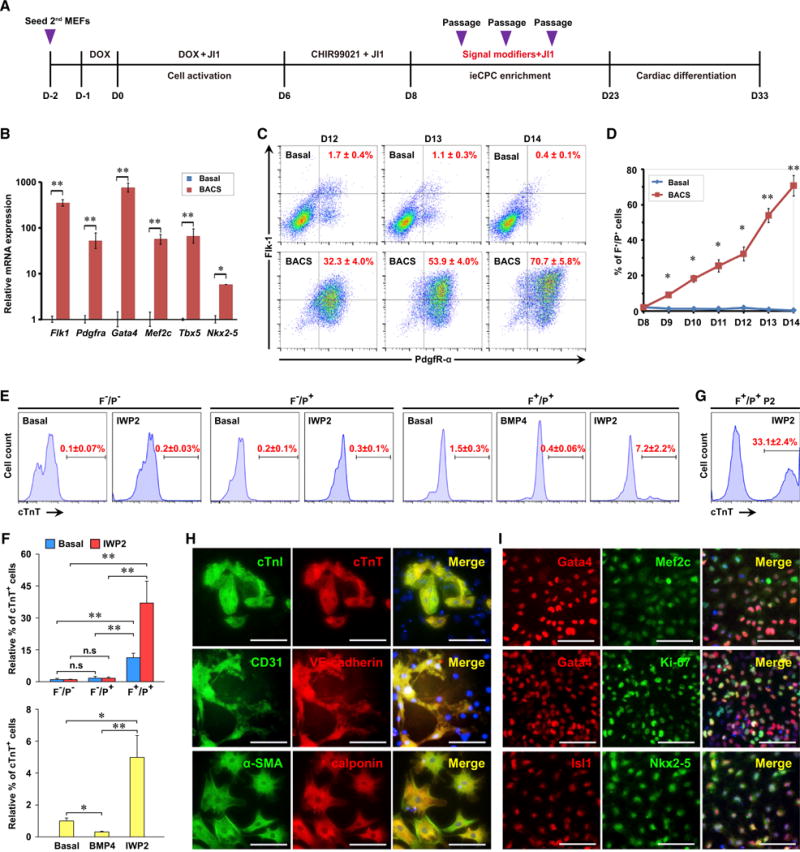
(A) Schematic of hypothesis-driven screening for conditions that expand putative ieCPCs. D, day; DOX, doxycycline; JI1, Jak inhibitor 1.
(B) Expression of CPC markers on day 14 detected by qPCR.
(C and D) Representative (C) and quantitative (D) flow cytometric analysis of the percentage of Flk-1+/PdgfR-α+ (F+/P+) cells treated with BACS. Basal ieCPC medium without BACS served as the control.
(E and F) Representative (E) and quantitative (F) flow cytometric analysis of cTnT in cells differentiated from freshly isolated Flk-1−/PdgfR-α− (F−/P−), Flk-1−/PdgfR-α+ (F−/P+), and F+/P+ cells treated with either basal differentiation medium, BMP4, or IWP2.
(G) Flow cytometric analysis of cTnT in cells differentiated from F+/P+ cells at passage 2 treated with IWP2.
(H) Immunofluorescence analyses of CM markers cTnI and cTnT, EC markersCD31 and VE-cadherin, and SMC markers α-SMA and calponin in ieCPCs cultured in basal differentiation medium for 10 days.
(I) Immunofluorescence analyses of Gata4, Mef2c, Isl1, Nkx2-5, Gata4, and Ki-67 in purified F+/P+ cells.
Scale bars represent 100 μm. Data are mean ± SE, n ≥ 3. *p < 0.05; **p < 0.01; n.s, p > 0.05. See also Figures S1 and S2.
ieCPC Characterization
To characterize the molecular qualities of the putative ieCPCs, we examined the expression of cardiac markers by qPCR. Consistent with our hypothesis, a panel of transcription factors known to be enriched in committed CPCs–Gata4, Mef2c, Tbx5, and Nkx2-5–were highly expressed in the expanded cells (Figure 1B), whereas differentiated CM markers–Tnnt2 and Myh6–were barely detectable (data not shown). Interestingly, Flk1 and Pdgfra, which encode the two CPC surface markers Flk-1 and PdgfR-α, were also highly enriched in expanded ieCPCs (Figure 1B). When expressed simultaneously, these two markers characterize CPCs committed to a cardiovascular lineage (Hirata et al., 2007; Kattman et al., 2011).
We then examined how cells responded to BACS treatment by analyzing Flk-1 and PdgfR-α expression with fluorescence-activated cell sorting (FACS). Flk-1+/PdgfR-α+ cells first appeared at day 8, responded to BACS treatment, and dominated the total population (more than 70%) by 1 week later; however, without BACS, these cells did not grow and eventually attenuated (Figures 1C and 1D). To distinguish whether the initial BACS treatment increased Flk-1+/PdgfR-α+ cells through a mechanism of induction and/or proliferation, we evaluated EdU incorporation in transdifferentiating cells. We added EdU to the culture medium with BACS at day 8 and examined the percentage of EdU+/Flk-1+/PdgfR-α+ cells at day 14. We found that about 33% of Flk-1+/PdgfR-α+ cells did not incorporate EdU (Figure S1A), indicating that they were generated through direct induction and not proliferation. To determine if proliferation is needed to enrich Flk-1+/PdgfR-α+ cells, we treated the cells with the cell-cycle inhibitor NU6140 (4 μM) along with BACS starting on day 8. We found that the ratio of Flk-1+/PdgfR-α+ cells dramatically decreased at day 14 (Figure S1B). These data demonstrate that mechanisms of both cellular induction and proliferation contribute to the generation of Flk-1+/PdgfR-α+ cells during initial BACS treatment.
Next, we isolated the three subpopulations of cells (i.e., Flk-1+/PdgfR-α+, Flk-1−/PdgfR-α+, and Flk-1−/PdgfR-α−) on day 13 by FACS and examined their cardiogenic potentials. We found that only Flk-1+/PdgfR-α+ cells efficiently gave rise to cTnT+ CMs (Figures 1E, 1F, S1C, and S1D) and formed spontaneously beating clusters. Flk-1+/PdgfR-α+ cells that cultured for two passages in BACS to recover from the initial sorting stress showed more robust cardiac differentiation potential (Figure 1G). These data demonstrate that Flk-1+/PdgfR-α+ cells, but not other subpopulations, represent the putative ieCPCs in BACS-treated cells.
ieCPCs Are Committed to a Cardiovascular Fate
We examined whether Flk-1+/PdgfR-α+ ieCPCs are truly committed cardiovascular precursors or more primitive CPCs that require specific signals and steps to be further specified. We found that treating ieCPCs with BMP4 significantly impaired their cardiac differentiation; however, treating them with the Wnt inhibitor IWP2 (5 μM), which promotes CM specification only from late-stage CPCs (Burridge et al., 2012), dramatically enhanced their differentiation (Figures 1E, 1F, and S1E). Even in basal differentiation conditions without any specific induction signaling molecules, Flk-1+/PdgfR-α+ cells directly differentiated into all three cardiovascular lineages, including CMs (cTnT+/cardiac troponin I [cTnI]+), ECs (CD31+/VE-cadherin+), and SMCs (α-smooth muscle actin [α-SMA]+/calponin+) (Figure 1H). These distinct responses suggest that ieCPCs have already committed to a cardiovascular fate. In addition, ieCPCs highly expressed several committed CPC markers, including Gata4, Mef2c, Isl1, and Nkx2-5, and the proliferative marker Ki-67 (Figure 1I); whereas uncommitted mesoderm genes were only transiently expressed at earlier stages during the generation of ieCPCs (Figure S1F).
Next, we determined whether ieCPC differentiation was restricted to a cardiovascular fate. We evaluated gene expression of mesoderm-derived non-cardiovascular lineages for ieCPCs that underwent either CM-, EC-, and SMC-specific differentiation or non-specific differentiation induced by fetal bovine serum (FBS) for 10 days. Under these conditions, we found no induction of non-cardiovascular genes, including markers of hematopoietic precursors, skeletal muscles, adipocytes, and chondrocytes (Figure S1G). To further confirm their restricted cardiovascular potentials, we cultured ieCPCs in the well-established induction conditions specified for each non-cardiovascular mesodermal lineage and compared the differentiation of ieCPCs with mesodermal cells derived from mouse embryonic stem cells (mESCs). In differentiated ieCPCs, we rarely found any c-Kit+/CD45+ hematopoietic progenitors or more differentiated c-Kit−/CD45+ hematopoietic cells, while mESC-derived mesodermal cells well generated those hematopoietic cells (Figure S1H). Consistently, ieCPCs showed limited or no potential to differentiate into myogenin+/MHC+ skeletal muscles, oil red O+ adipocytes, or alizarin red S+ chondrocytes compared with mESC-derived mesodermal cells, even when exposed to each lineage-specific induction cue (Figures S1I–S1K).
To more precisely characterize cells after ieCPC differentiation, we analyzed the percentage of CMs (cTnT+), ECs (CD31+), and SMCs (α-SMA+) by FACS in CM, EC, SMC, and FBS differentiation conditions (Figure S2A). Isl1, a well-recognized CPC marker that diminishes as soon as CPCs enter a differentiation program (Moretti et al., 2006), was used to trace undifferentiated ieCPCs (Figure S2B). We found that most (>93%) of the cells in each differentiation condition were CMs, ECs, SMCs, or undifferentiated ieCPCs (Figure S2C), confirming the restricted cardiovascular potentials of ieCPCs. Notably, we did not observe CM generation when ieCPCs were differentiated in FBS-containing conditions at a low seeding density (1 × 104 cells/cm2, suitable for inducing most mesodermal lineages) (Figures S1G and S2A). This deficiency can be prevented by using the cell seeding density optimal for CM differentiation (3 × 105 cells/cm2) and/or using BMP/Wnt inhibitors (Figure S2D).
ieCPCs Can Be Expanded Long Term
We purified Flk-1+/PdgfR-α+ ieCPCs by FACS and tested whether we could expand them in long-term culture. We found that purified ieCPCs showed normal undifferentiated morphology and could stably propagate in BACS conditions for more than 18 passages (>1010-fold expansion) (Figure 2A). To evaluate whether ieCPCs expanded long term retain their original properties, we compared ieCPCs of early (<5), middle (5–10), and late (>10) passages. In these passages, we found that ieCPCs maintained a high degree of similarity in growth rate (Figure 2A), undifferentiated morphology (Figure 2B), and expression of both Flk-1 and PdgfR-α (Figure 2C) over time. By immunostaining, we found that ieCPCs similarly expressed Gata4, Mef2c, Isl1, Nkx2-5, and Ki-67 at early, middle, and late passages (Figures 2D, S3A, and S3B); however, they did not express CM-, EC-, SMC- (Figures S3C–S3E), and pluripotency-related markers (Figures S3F–S3I), even after long-term expansion.
Figure 2. Isolated ieCPCs Expand Long Term in Chemically Defined Conditions.
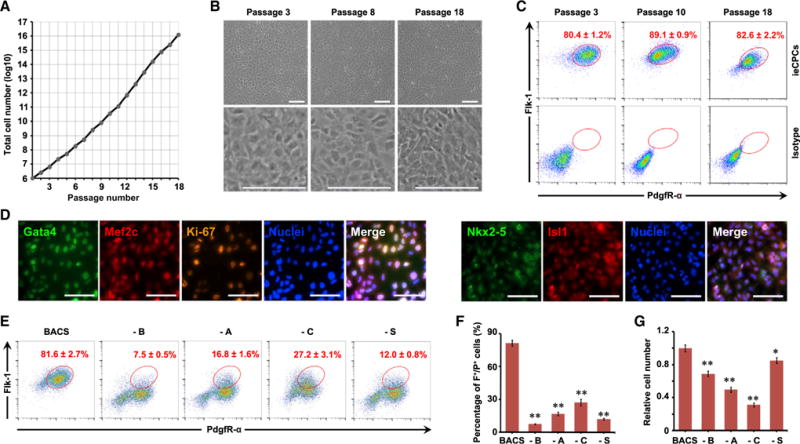
(A) Growth curves of ieCPCs during long-term expansion with BACS.
(B) Representative images showing the typical morphology of ieCPCs at passages 3, 8, and 18.
(C) Percentage of F+/P+ cells detected by flow cytometry at passages 3, 10, and 18.
(D) Immunofluorescence analyses of Gata4, Mef2c, Ki-67, Nkx2-5, and Isl1 in ieCPCs at passage 15.
(E–G) Representative (E) and quantitative (F) results of the percentage of F+/P+ cells detected by flow cytometry and cell number (G) after culturing with BACS or removing individual components. Data were collected after three passages (n = 3). –, omit; A, Activin A; B, BMP4; C, CHIR99021; S, SU5402.
Scale bars represent 100 μm. Data are mean ± SE. *p < 0.05; **p < 0.01. See also Figure S3.
To determine if each component in BACS was required for Flk-1+/PdgfR-α+ cells to self-renew, we omitted each cytokine or chemical individually and evaluated cell expansion. We found that omitting any component of BACS dramatically reduced the percentage of Flk-1+/PdgfR-α+ cells (Figures 2E and 2F) and cell proliferation (Figure 2G) within three passages, suggesting that each component is indispensable. We also found that removing each component of the BACS cocktail caused SMC, EC, or CM marker genes to accumulate (Figure S3J), indicating that some cells spontaneously differentiated. These results suggest that each component of BACS synergistically represses cardiovascular differentiation of ieCPCs to sustain their long-term self-renewal.
To examine the global transcriptional profile of ieCPCs, we compared the transcriptomes of early- and late-passage ieCPCs, their parental MEFs, cells at day 9 of reprogramming (D9), and cardiac derivatives from ieCPCs (ieCPC-CDs) by RNA sequencing (RNA-seq). With hierarchical cluster analyses, we found that early- and late-passage ieCPCs had very similar gene expression profiles that clearly differed from other cell populations (Figure 3A). With Gene Ontology (GO) analysis, we found that genes specifically expressed in ieCPCs were related to cell adhesion and heart-lineage commitment. We pairwise compared ieCPCs with MEFs, D9, and ieCPC-CDs and found that ieCPCs were mainly enriched with GO terms associated with CPC fate and functions, such as heart development and cell proliferation. Conversely, ieCPCs lacked GO terms involved in functions of other cell types, such as the immune response in MEFs, early embryonic development of germ layers in D9, and myocyte contraction in ieCPC-CDs (Figures 3A, 3B, S4A, and S4B). However, comparing early- and late-passage ieCPCs did not yield any GO terms that met the false discovery rate threshold of ≤0.05, suggesting that ieCPCs retained stable transcriptional signatures when expanded long term.
Figure 3. ieCPCs Acquire Transcriptional Signatures of Developing CPCs.
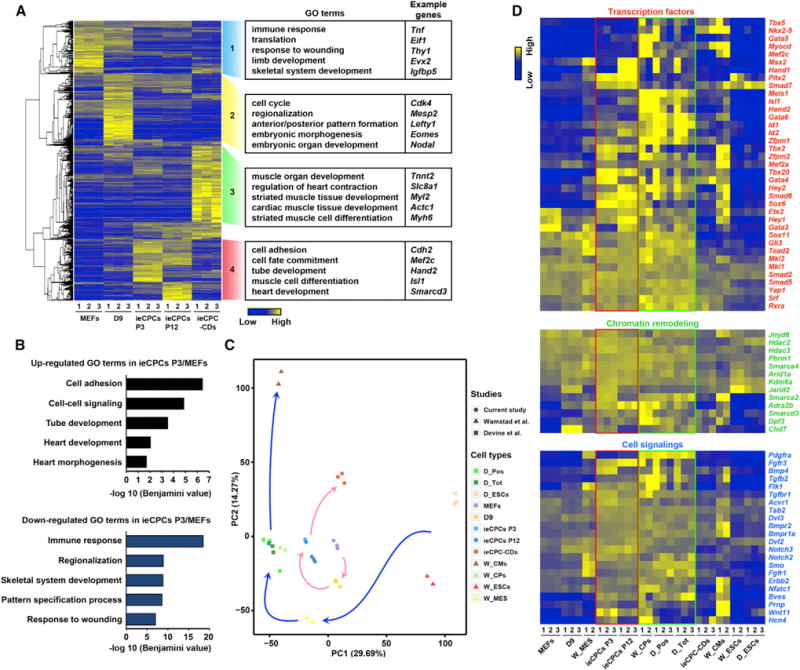
(A) Transcriptome analysis revealing differences in gene expression among passage 3 (P3) and passage 12 (P12) ieCPCs, their parental MEFs, cells at reprogramming D9 (D9), and ieCPC cardiac derivatives (ieCPC-CDs) detected by RNA-seq.
(B) GO analyses of upregulated and downregulated genes in ieCPCs P3/MEFs.
(C) Principal-component (PC) analysis of the global gene expression profile across all tested cell types. CPs, cardiac progenitors; D_, Devine et al. (2014); MES, mesoderm; Pos and Tot, CPCs with or without purification with a Smarcd3-GFP+ reporter, respectively; W_, Wamstad et al. (2012).
(D) Expression of CPC-related marker genes in all tested samples detected by RNA-seq.
See also Figure S4.
To determine whether ieCPCs represent a particular stage of cardiac differentiation of ESCs, we compared them with cells at different stages during cardiac differentiation of mESCs, including undifferentiated ESCs, mesodermal cells, CPCs, and differentiated CMs (Wamstad et al., 2012; Devine et al., 2014). We found that ieCPCs and ESC-derived CPCs had the highest transcriptional similarity compared with other reference cell types (Figures 3C and S4C–S4E) and represented an intermediate cardiogenic population between uncommitted mesoderm and terminally differentiated cardiovascular cells (Figure 3C).
Next, we evaluated a panel of well-studied genes involved in CPC fate commitment. We found that ieCPCs highly expressed CPC-related genes, including transcription factors, chromatin remodelers, and cell-signaling molecules (Figure 3D). However, they weakly expressed markers associated with other cell types, including fibroblasts, early mesoderm, endoderm, ectoderm, non-cardiovascular mesoderm, PSCs, epicardial cells, mesenchymal stem cells, and differentiated CMs, ECs, and SMCs (Figure S4F), with expression levels no higher than the ESC-derived CPCs described by Wamstad et al. (2012) and Devine et al. (2014). Collectively, these results demonstrate that ieCPCs possess a global transcriptional pattern similar to that of normal CPCs derived from ESCs.
Long-Term Expanded ieCPCs Exhibit Multi-lineage Potentials for Cardiovascular Differentiation In Vitro
We tested whether ieCPCs retain their multi-lineage potentials for cardiovascular differentiation when expanded long term. We cultured ieCPCs at late passages in differentiation medium supplemented with IWP2 (5 μM) and monitored their cardiac differentiation daily for spontaneously contracting cells (typically observed first at day 3 of differentiation). The number of beating cells gradually increased until day 10 and remained at a similar level for more than 1 month. Cardiac differentiation was robust, and synchronized beating sheets formed at day 10 (Movie S1). With immunostaining, we observed that CMs derived from ieCPCs (ieCPC-CMs) expressed several CM-specific markers (Figure 4A). We also analyzed gene expression by qPCR and detected many genes important for CM contraction and functional regulation (Figure 4B). FACS analysis of cTnT revealed that the efficiency of CM differentiation from ieCPCs was about 35% at day 10 (Figure 4C). Typically, 10,000 starting ieCPCs produced ~30,000 total cells, from which we estimate a yield of approximately one CM per input ieCPC.
Figure 4. ieCPCs Expanded Long Term Efficiently Differentiate into Functional CMs In Vitro.
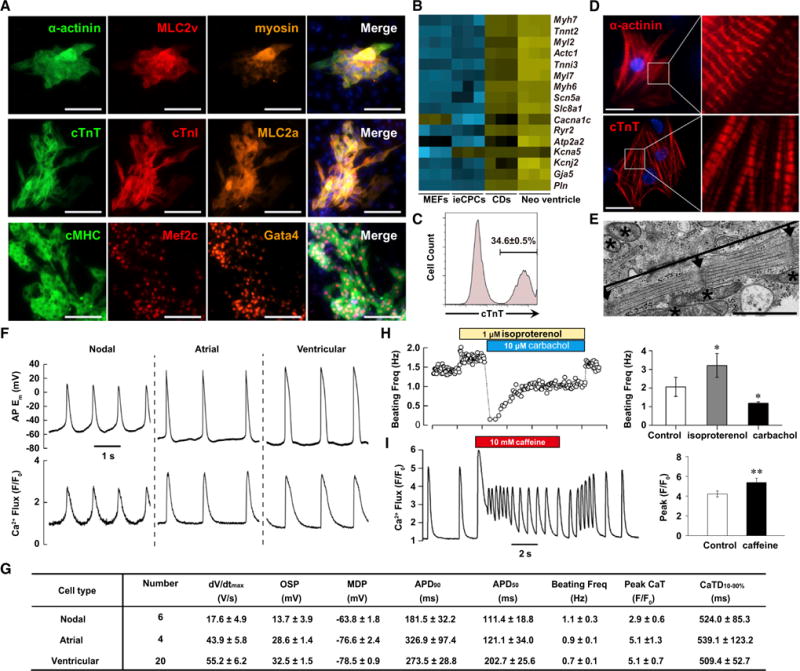
(A) Immunofluorescence analyses of multiple CM markers in ieCPC-CMs. Scale bars represent 100 μm.
(B) Heatmap showing expression of key CM transcripts in MEFs, ieCPCs, ieCPC-CDs (CDs), and primary neonatal ventricle (Neo ventricle).
(C) Flow cytometric analyses of ieCPC-CMs expressing cTnT after 10 days of differentiation from ieCPCs at late passages (P10–P15).
(D) Immunofluorescence analyses of α-actinin and cTnT in ieCPC-CMs. Right panels show boxed areas in left panels at higher magnification. Scale bars represent 20 μm.
(E) Transmission electron microscopy of ieCPC-CMs. Arrows, Z-bands; brackets between two arrows, sarcomeric units; asterisks, mitochondria. The scale bar represents 1 μm.
(F) Representative traces of simultaneous APs (identified as changes in membrane potential [Em]) and Ca2+ transients (Fluo-4 fluorescence expressed relative to baseline [F/F0]) in ieCPC-CMs.
(G) Tabulated parameters describing APs: maximum upstroke velocity (dV/dtmax); overshoot potential (OSP); minimum diastolic potential (MDP); APD50 and APD90; and Ca2+ transients: peak relative fluorescence (Peak CaT) and Ca2+-transient duration from 10% of the rising phase to 90% decay (CaTD10%–90%).
(H) Typical effects of isoproterenol and carbachol on beating frequency in ieCPC-CMs (*p < 0.05, n = 6).
(I) Caffeine-induced release of Ca2+ from sarcoplasmic reticulum in ieCPC-CMs (**p < 0.01, n = 10). Data are mean ± SE.
Next, we further characterized ieCPC-CMs. By immunofluorescence of the cardiac-myofilament proteins, we found that single ieCPC-CMs displayed a well-organized sarcomeric structure with clear cross-striations at day 10 (Figure 4D). We confirmed this finding by transmission electron microscopy, in which the well-organized sarcomeres, myofibrillar bundles, and transverse Z bands were surrounded by ample mitochondria (Figure 4E). In addition, intracellular electrical recordings from single beating ieCPC-CMs at day 10 revealed robust action potentials (APs) that were synchronized 1:1 with rhythmic Ca2+ transients (Figure 4F), which closely resembled CMs derived from murine fetal hearts or PSCs (Kuzmenkin et al., 2009). On the basis of the ratio of AP duration at 90% repolarization (APD90) to AP duration at 50% repolarization (APD50) (Kuzmenkin et al., 2009), we detected nodal-like (20.0%), atrial-like (13.3%), and ventricular-like (66.7%) APs (Figures 4F and 4G). Moreover, 1 μM isoproterenol (a β-adrenergic agonist) significantly increased the frequency of cell contraction and spontaneous Ca2+ transients. These effects were blocked by 10 μM carbachol, a synthetic muscarinic agonist, suggesting functional and coupled cascades of β-adrenergic and muscarinic signaling in ieCPC-CMs (Figure 4H). Moreover, 10 mM caffeine elicited large Ca2+ transients in ieCPC-CMs (Figure 4I), indicating the presence of cardiac ryanodine receptors. Thus, the CMs generated from ieCPCs expanded long-term possess physiological features of bona fide CMs and are functional in vitro.
To test whether ieCPCs expanded long term can give rise to functional ECs in vitro, we examined EC gene expression after 10 days of EC differentiation. ECs generated from ieCPCs (ieCPC-ECs) showed typical morphology and highly expressed the EC-specific markers CD31 and VE-cadherin (Figure 5A). FACS analysis revealed that more than 90% of the total cell population expressed CD31 (Figure 5B). Moreover, in contrast to the parental MEFs, ieCPC-ECs could robustly form vessel-like structures (Figure 5C) and efficiently took up fluorescent-labeled acetylated low-density lipoprotein (ac-LDL) (Figure 5D). These findings confirmed that ieCPC-ECs exhibited a similar phenotype and function to primary ECs (Kaufman et al., 2004).
Figure 5. ieCPCs Expanded Long Term Efficiently Differentiate into Functional ECs and SMCs In Vitro.
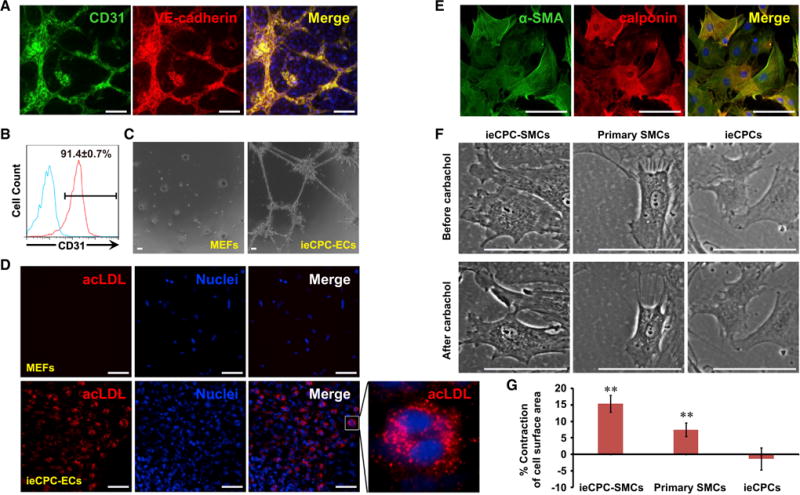
(A) Immunofluorescence analyses of EC markers in ieCPC-ECs.
(B) Flow cytometric analyses of CD31 expression after 10 days of EC differentiation from ieCPCs at late passages. Blue line indicates isotype control.
(C and D) ieCPC-ECs, but not control 2nd MEFs, form a capillary-like network on a thin layer of Matrigel (C) and take up ac-LDL (D).
(E) Immunofluorescence analyses of SMC markers in ieCPC-SMCs.
(F) ieCPC-SMCs, but not control ieCPCs, display similar contractile ability as primary SMCs in response to 100 μM carbachol.
(G) Quantitative results of cell surface area reflecting the contraction of each cell type in (F), summarized from 29 ieCPC-SMCs, 28 primary SMCs, and 28 ieCPCs. Data are means ± SE. **p < 0.01.
Scale bars represent 100 μm. See also Figure S5.
To examine SMC differentiation, we analyzed the expression of SMC-specific markers 10 days after SMC differentiation. With immunofluorescence staining, we found that most cells (>98%) were positive for the SMC-specific markers (Figure 5E). Carbachol (100 μM) induced contraction of SMCs derived from ieCPCs (ieCPC-SMCs), a phenomenon we also observed in primary SMCs but not undifferentiated ieCPCs (Figures 5F and 5G). These findings suggest that ieCPC-SMCs have similar functional properties to primary SMCs.
To determine whether ieCPCs are multipotent at a single-cell level, we performed clonal assays on single ieCPCs and examined their potentials to differentiate into CMs, ECs, and SMCs. After 28 days of differentiation, we found that 31.8% of the single ieCPC-derived clones were tripotent, as demonstrated by co-expression of CM, EC, and SMC genes (Figures S5A–S5C). We also found that 22.7% of the clones were bipotent and 45.5% of them were unipotent (Figures S5B and S5C), in part because of the differentiation conditions and associated efficiency.
ieCPCs Give Rise to CMs, ECs, and SMCs when Transplanted into Mouse Hearts
After demonstrating that ieCPCs expanded long term undergo cardiovascular differentiation in vitro, we extended the approach to the native heart environment in vivo. We labeled ieCPCs at passage 10 with red fluorescent protein (RFP) (Figure S6A) and transplanted them into infarcted hearts of immunodeficient mice. Parental MEFs served as a negative control. For each mouse, 1 million ieCPCs or MEFs were directly injected into the mouse heart immediately after coronary ligation. We sacrificed recipient mice 2 weeks after transplantation and examined the expression of markers for differentiated cardiovascular lineages. As expected, control MEFs did not express CM, EC, and SMC markers (Figures S6B–S6D) and did not convert into cardiovascular cells in the native cardiac niche. In striking contrast, we detected RFP+ ieCPCs co-expressing cTnT and myosin (Figure 6A), CD31 and VE-cadherin (Figures 6B and 6C), or α-SMA and calponin (Figures 6D and 6E) in grafts, indicating that ieCPCs successfully converted into CMs, ECs, and SMCs, respectively, in vivo. We also found RFP+ cells that were CD31+/VE-cadherin+ or α-SMA+/calponin+ in capillaries and arterioles, suggesting that the transplanted cells formed blood vessels, although at a low frequency (Figures 6C and 6E). We found that 96 clusters (30.8%) expressed CM marker cTnT, 184 clusters expressed SMC marker α-SMA (59.2%), and 21 clusters expressed EC marker CD31 (6.8%) in 311 engrafted RFP+ clusters, suggesting that more than 90% of the engrafted ieCPCs efficiently differentiated into three cardiovascular cell types after transplantation (Figure S6E). Similar to our observation in vitro, we did not detect non-cardiovascular-lineage markers in the grafted ieCPCs (Figures S6F–S6H). These results suggest that the in vivo environment of the infarcted mouse ventricle can trigger multi-lineage cardiovascular differentiation of ieCPCs.
Figure 6. ieCPCs Give Rise to CMs, ECs, and SMCs In Vivo and Improve Cardiac Function after MI.
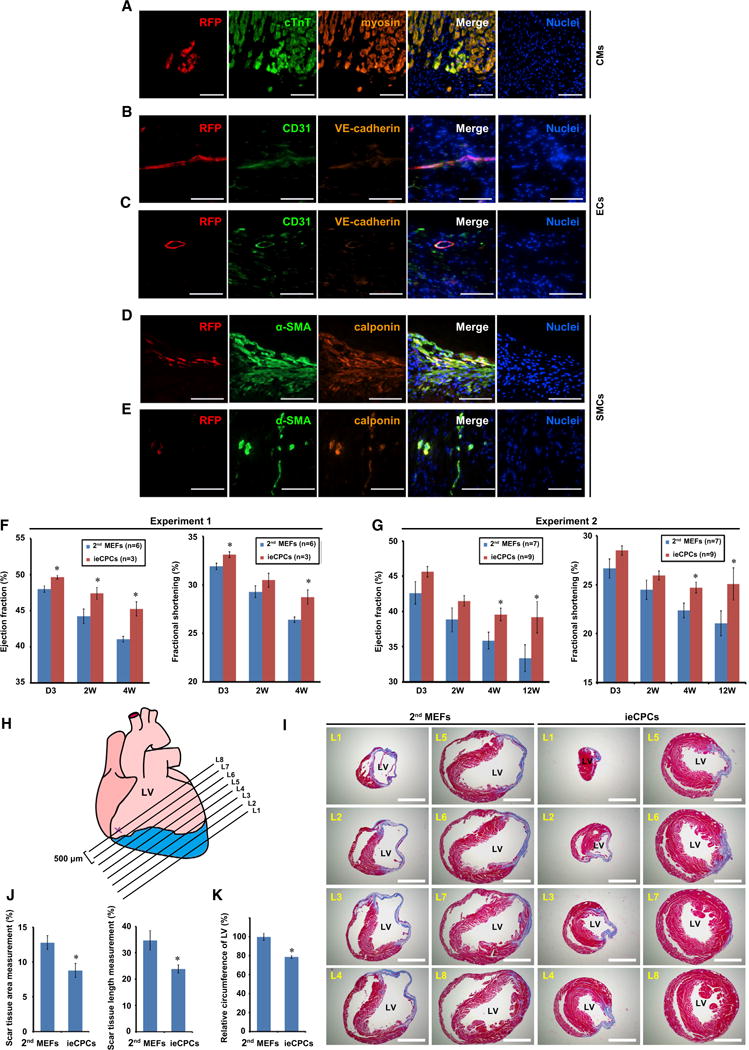
(A–E) Immunofluorescence analyses of RFP and CM (A), EC (B and C), and SMC (D and E) markers in tissue sections collected 2 weeks after transplanting RFP-labeled ieCPCs at passage 10 into infarcted hearts of immunodeficient mice. Scale bars represent 100 μm.
(F and G) Ejection fraction and fractional shortening of the left ventricle (LV) quantified by echocardiography. Results from two independent experiments were shown. D, days; W, weeks.
(H–J) Cardiac fibrosis was evaluated at eight levels (L1–L8) by Masson’s trichrome staining 12 weeks after coronary ligation. The ligation site is marked as X. Sections of representative hearts are shown in (I) with quantification in (J). Scar tissue (%) = (the sum of fibrotic area or length at L1–L8/the sum of LV area or circumference at L1-L8) × 100. Scale bars represent 500 μm.
(K) Quantification of LV circumference of mouse hearts 12 weeks after transplantation of 2nd MEFs or ieCPCs. Data were summarized from 48 sections for each group. Data are mean ± SE. *p < 0.05.
See also Figure S6.
Intramyocardial Transplantation of ieCPCs Retards Adverse Remodeling and Improves Heart Outcome after MI
To determine whether the transplantation of ieCPCs could affect cardiac function following cardiac injury, we examined heart function via high-resolution echocardiography over the course of 12 weeks after injecting cells into immunodefcient mice subjected to coronary ligation. All studies were performed in a blinded fashion and were revealed only at the end of the experiments. We found that heart function in the control group, injected with parental 2nd MEFs, continued to decline overtime, reflected by left ventricular fractional shortening and ejection fraction evaluated by echocardiography (Figures 6F and 6G). Strikingly, we found that the natural reduction of heart performance post-MI was significantly attenuated after transplantation of ieCPCs compared with control, and these differences became more pronounced over time (Figures 6F and 6G). Furthermore, we observed significantly smaller scar size in mice transplanted with ieCPCs 12 weeks post-MI (Figures 6H–6J). Adverse remodeling, such as dilation and compensatory hypertrophy that are part of the natural course of events after MI, was also reduced in mouse hearts that received ieCPCs (Figures 6I and 6K). Moreover, we did not observe the formation of teratomas in ieCPC-transplanted mice 8 weeks after transplantation (Figures S6I and S6J). These observations suggest that transplantation of ieCPCs improves cardiac function after acute ischemic myocardial injury.
ieCPCs Can Be Derived from Tail-Tip Fibroblasts
To determine whether ieCPCs could be reprogrammed from other types of fibroblasts that are genetically unmodified, we isolated mouse tail-tip fibroblasts (TTFs) and infected them with lentivirus harboring a doxycycline-inducible transgene encoding the reprogramming factors. Under the same conditions as used for 2nd MEFs, a similar Flk-1+/PdgfR-α+ population was induced from TTFs after BACS treatment. After FACS sorting, these Flk-1+/PdgfR-α+ cells showed normal undifferentiated morphology (Figure S7A), sustained expression of CPC and proliferative markers (Figures S7B and S7C), and steadily expanded in BACS for more than 12 passages. When cultured in the differentiation conditions of 2nd MEF-derived ieCPCs, TTF-derived ieCPCs rapidly differentiated into all three cardiovascular line-ages with comparable efficiencies (Figures S7D and S7E; Movie S2). These results suggest that fibroblasts from different tissues of origin can be stably reprogrammed into multipotent ieCPCs, independent of the transgenic system.
BACS Captures and Expands CPCs Derived from PSCs
Next, we evaluated whether ieCPCs could be captured during cardiac differentiation of PSCs (Kattman et al., 2011; Burridge et al., 2012), which represents embryonic cardiac development. Upon cardiac differentiation of mESCs, we observed Flk-1+/PdgfR-α+ CPCs at day 3 of differentiation. We isolated this population by FACS and cultured it in ieCPC-expansion medium supplemented with BACS. Similar to what were observed in fibroblast-derived ieCPCs, the Flk-1+/PdgfR-α+ CPCs derived from mESCs showed similar morphology (Figure 7A), expressed CPC and proliferative markers (Figures 7B and 7C), robustly expanded in BACS for more than 12 passages, and differentiated into all three cardiovascular lineages with similar efficiencies as fibroblast-derived ieCPCs when stimulated (Figures 7D and 7E; Movie S3). In addition, ieCPCs and mESC-derived CPCs expanded in BACS had very similar gene expression profiles, exhibiting CPC-specific gene signatures (Figure 7F). These results demonstrate that ieCPCs that appear during the cardiac transdifferentiation of fibroblasts also exist during normal cardiac differentiation of mESCs.
Figure 7. BACS Captures and Expands CPCs Derived from mESCs.
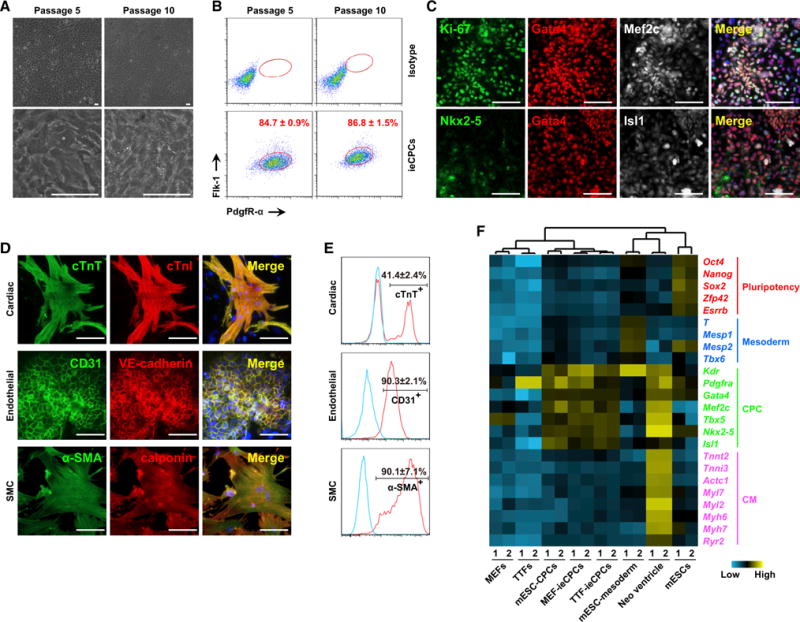
(A) Representative images showing the typical morphology of mESC-CPCs cultured in BACS at passages 5 and 10.
(B) Percentage of F+/P+ cells detected by flow cytometry at passages 5 and 10.
(C) Immunofluorescence analyses of Gata4, Mef2c, Ki-67, Nkx2-5, and Isl1 in mESC-CPCs at passage 10.
(D) Immunofluorescence analyses of CM, EC, and SMC markers in mESC-CPCs cultured in CM-, EC-, and SMC-specific differentiation conditions for 10 days.
(E) Flow cytometric analyses of cTnT, CD31, and α-SMA in mESC-CPCs cultured in the same differentiation conditions as in (D). Blue lines indicate isotype controls.
(F) Hierarchical clustering analysis ofindicated celltypes on the basis ofexpression of pluripotent, mesodermal, CPC-, and CM-specific markers detected by qPCR. Scale bars represent 100 μm. See also Figure S7 and Movie S3.
DISCUSSION
Despite the prospects of cell-based therapies to regenerate damaged heart tissue, a cardiac-restricted cell type is needed that allows autologous transplantation, can be robustly expanded in vitro, and can be rapidly and directly differentiated into cardiovascular cells in vivo. Unfortunately, this discovery is remarkably challenging to accomplish. Here, we identified and captured a cardiovascular-restricted, yet highly expandable, progenitor cell type reprogrammed from fibroblasts. These ieCPCs can self-renew long term and directly differentiate into the three major cardiovascular lineages within the adult heart without being sequentially induced with embryonic cardiogenic signals.
A distinct feature of ieCPCs is their extensive proliferative capacity. Terminally differentiated CMs usually stop proliferating and survive poorly after transplantation, which inhibits their ability to repopulate and replenish a diseased heart (Lam et al., 2009). Although previous studies have identified various types of CPCs that proliferate, they only transiently expanded (e.g., 1 week) and displayed a limited capacity to replicate (<100 population doublings) (Moretti et al., 2006; Qyang et al., 2007). Moreover, many undefined components used in these systems, such as feeder cells and high-level serum, may hamper their application. Of note, a recent publication reported the efficient propagation of PSC-derived CPCs (~107-fold expansion) by combining chemical and genetic manipulation (Birket et al., 2015). This system required exogenous expression of the oncogenic transgene MYC for the self-renewal of CPCs, which limits the translational applicability of these cells. In contrast, the ieCPCs we describe here can be propagated long term (>18 passages) in chemically defined conditions (>1010-fold expansion) without foreign transgenes (Figure 2A).
Moreover, expanded ieCPCs directly differentiate into three cardiovascular lineages required for heart regeneration, which will provide not only a more efficient starting point for regenerative medicine but also a powerful platform to dissect the onset of cardiac development. Currently, we do not know if the cardiovascular derivatives of ieCPCs originated from a homogeneous population or, alternatively, diverse subpopulations within ieCPCs. Because ieCPCs can robustly differentiate into each cardiovascular lineage (~35% for CMs and >90% for SMCs and ECs) in vitro, we speculate that each lineage is less likely to be generated from a distinct subtype of ieCPCs. Clonal assays of single ieCPCs showed that at least a fraction of ieCPCs are multipotent and serve as a common cardiovascular precursor for the three main lineages of the developing heart. Of note, a portion of residual, undifferentiated progenitors was observed when ieCPCs were induced to differentiate in various conditions within the time frame (Figure S2B). This result is consistent with many other systems in which stem cell and precursor differentiation is usually unsynchronized and can hardly achieve 100% differentiation efficiency. In most cases, the differentiated populations are a mixture that contains both undifferentiated stem cells and their derivatives with a ratio that depends on the differentiation contexts (Murry and Keller, 2008). In the SMC and EC differentiation conditions, as well as in the local environment of injured hearts, ieCPCs differentiated in a more synchronized manner, achieving >90% efficiency. Given that the field of stem cell differentiation has experienced rapid growth in knowledge and technical advances, developing more robust and uniform systems for ieCPC differentiation will be of great interest and may be within the reach.
Significantly, ieCPCs have a more restricted cardiovascular fate than previously reported CPCs, which seem less differentiated and depend on multiple sequential steps or signals to further differentiate into CMs (Blin et al., 2010; Cao et al., 2013). The ieCPCs express genes of committed CPCs, but not of early mesoderm or other lineages. They respond to late- but not early-stage cardiogenic signals and directly give rise to beating CMs in a single step within 3 days in vitro. Additionally, they show very limited potential to differentiate into non-cardiovascular derivatives of the mesoderm, even when induced with specific cues. Furthermore, when exposed to the infarcted heart environment in vivo, they can directly generate CMs, ECs, and SMCs, but not non-cardiovascular mesodermal derivatives. Because the adult heart lacks the complex signals of embryonic cardiogenesis, these features may make ieCPCs a promising cell source for in vivo applications.
Indeed, we found that transplantation of ieCPCs into mouse infarcted hearts provides long-term functional benefits (3 months), in contrast to transplantation of CMs that only transiently improve cardiac performance (<1 month) (van Laake et al., 2007). This improvement may result from ieCPCs generating de novo CMs to facilitate and restore heart contractility and contributing to blood-vessel formation that supplies oxygen and nutrients to increase graft survival and integration into surrounding tissues (Lam et al., 2009), thereby sustaining long-term functional improvement after cardiac injury. In addition, the beneficial effects of ieCPC transplantation may partially result from paracrine mechanisms. Further studies are needed to better understand the therapeutic mechanisms that occur after ieCPC transplantation and to enhance the engraftment of ieCPCs.
With global transcriptome analysis by RNA-seq, we found high similarities between ieCPCs and CPCs derived from mESCs. Moreover, we could capture ESC-derived CPCs and propagate them long term in BACS conditions; ESC-derived CPCs expanded in BACS possess very similar differentiation capacity to ieCPCs, generating three cardiovascular lineages with equal efficiencies. These results suggest that although ieCPCs can be derived via cardiac transdifferentiation of fibroblasts, they can also exist during embryonic cardiogenesis. Interestingly, ieCPCs co-express multiple CPC markers detected at both early (i.e., Flk-1 and PdgfR-α) and late (i.e., Isl1, Gata4, and Mef2c) stages of normal cardiac development and differentiation. This expression pattern is stable when ieCPCs are expanded long term in vitro, regardless of their origin (i.e., 2nd MEFs, TTFs, or PSCs). This phenomenon is also observed in some native CPC populations in developing hearts or differentiated from PSCs (Figure 3D) (Moretti et al., 2006; Yang et al., 2008), suggesting that there may not be a sharp distinction in expression of those selected genes between these stages and some overlap may exist.
In this study, we identified BACS as a robust condition for the efficient generation and long-term renewal of ieCPCs. BMP, Activin A, and Wnt are key signaling molecules that synergistically induce mesoderm and subsequent CPC formation (Yang et al., 2008; Kattman et al., 2011). Particularly, Wnt signaling plays a biphasic role in cardiogenesis (i.e., it stimulates mesoderm induction from early stem cells but inhibits CM differentiation of late-stage CPCs) (Naito et al., 2006; Burridge et al., 2012). In addition, VEGF, PDGF, and FGF signals induce further differentiation of CPCs (Kattman et al., 2011; Cheung et al., 2012). As a chemical inhibitor of these pathways by targeting their receptors, SU5402 may block further differentiation of ieCPCs. Because BACS contains BMP4, Activin A, and CHIR99021 (a Wnt agonist), it is therefore consistent with what have been mechanistically characterized for these signaling that those reprogramming cells treated with BACS are induced or patterned into mesoderm and CPCs, and, initially, one third of the Flk-1+/PdgfR-α+ cells are directly induced by BACS without replication (Figure S1A). In addition, we also confirmed that the generated ieCPCs can self-renew/expand under the BACS condition, and this expansion step contributes to the rapid enrichment of Flk-1+/PdgfR-α+ cells. Thus, we concluded that mechanisms of both cellular induction and proliferation contribute to the initial generation of Flk-1+/PdgfR-α+ cells after BACS treatment. After initial generation of ieCPCs, BACS also support long-term selfrenewal and proliferation of ieCPCs by synergistically repressing further differentiation. Given that early embryonic cardiogenesis is similar between mice and humans, with CPCs in both species expressing the same key markers (i.e., Flk-1 and PdgfR-α) and responding to similar developmental cues (Kattman et al., 2011), our results may have implications in reprogramming and expanding human ieCPCs for various applications.
EXPERIMENTAL PROCEDURES
CASD System-Based Cardiac Reprogramming
Reprogramming of 2nd MEFs was initiated by seeding cells onto Geltrex (GIBCO)-coated plates in MEF medium. Doxycycline (2 μg/ml; Sigma) was added 1 day later. The next day, cells were cultured in reprogramming medium for 6 days with doxycycline and 0.5 μM JI1 (Millipore). On day 6, medium was changed to transdifferentiation medium containing 3 μM CHIR99021 (Stemgent) and 0.5 μM JI1. On day 8, medium was switched to ieCPC basal medium supplemented with BACS (5 ng/ml BMP4, 10 ng/ml Activin A, 3 mM CHIR99021, and 2 μM SU5402 [Tocris]) and 0.5 μM JI1. Medium was renewed every 2 days until cells were sorted on day 13.
Long-Term Expansion of ieCPCs
Flk-1+/PdgfR-α+ cells were purified by FACS and seeded onto Geltrex-coated plates in ieCPC basal medium supplemented with BACS and 250 μM ascorbic acid (Sigma). ieCPCs were routinely passaged every 4 days, and medium was renewed every 2 days.
ieCPC Differentiation
For the generation of CMs, ieCPCs were seeded onto Matrigel-coated plates at a density of 3 × 105 cells/cm2 and kept in serum-free differentiation (SFD) medium for 10 days. In some experiments, 5 μM IWP2 was included during the first 6 days to increase the yield of CMs. For the generation of SMCs and ECs, ieCPCs were seeded onto Matrigel-coated plates at a density of 1 × 104 cells/cm2 and cultured in SFD medium supplemented with 2 ng/ml TGF-β1 and 10 ng/ml PDGF-BB (for SMCs) or in EGM-2 medium (Lonza) (for ECs) for 10 days.
Cell Transplantation
In cell transplantation studies, a mouse MI model was induced by permanent ligation of the left anterior descending artery. One million donor cells were injected along the boundary between the infarct and border zones. All mouse work was conducted in accordance with institutional guidelines.
Statistical Analysis
Statistical significance of differences was estimated by Student’s t test in Microsoft Excel. p < 0.05 was regarded as indicating statistical significance.
Supplementary Material
Highlights.
Fibroblasts can be reprogrammed into cardiac progenitor cells (CPCs)
CPCs expanded long term under defined conditions generate cardiovascular cells
Transplanting expanded CPCs improves heart function after myocardial infarction
CPCs can be captured during PSC differentiation and expanded in the same conditions
Acknowledgments
We thank Reuben Thomas, Alisha Holloway, Alexander Williams, Jinny Wong, Robert Chadwick, Yanxia Hao, Caroline Miller, Fengrong Yan, and Shaohe Wang for technical assistance. We thank Crystal Herron, Itedale Namro Redwan, and Min Xie for editorial assistance. Y.Z. and N.C. are supported by the California Institute for Regenerative Medicine (CIRM). J.-d.F. is supported by the American Heart Association (AHA) (13SDG14580035). S.D. is supported by CIRM, the National Institute of Child Health and Human Development, the National Heart, Lung, and Blood Institute (NHLBI), the National Institutes of Health (NIH), the Roddenberry Foundation (RF), and the William K. Bowes, Jr. Foundation. D.S. and B.G.B are supported by the NHLBI (U01HL098179, U01 HL100406). D.S. is supported by CIRM, the NIH, the RF, the Younger Family Foundation, and the Whittier Foundation. B.G.B. is supported by the NHLBI (R01HL114948). We dedicate this paper to the memory of our wonderful colleague Dr. C. Ian Spencer.
Footnotes
ACCESSION NUMBERS
The accession number for the RNA-seq data reported in this paper is GEO: GSE77375.
SUPPLEMENTAL INFORMATION
Supplemental Information includes Supplemental Experimental Procedures, seven figures, and three movies and can be found with this article online at http://dx.doi.org/10.1016/j.stem.2016.02.001.
AUTHOR CONTRIBUTIONS
Conceptualization, Y.Z., N.C., and S.D.; Investigation, Y.Z., N.C., Y.H., C.I.S, J.-d.F., and T.X.; Formal Analysis, C.Y., K.L., and B.N.; Writing – Original Draft, Y.Z., N.C., and S.D.; Writing – Review and Editing, Y.Z., N.C., and S.D.; Funding Acquisition, B.G.B., D.S., and S.D.; Resources, K.L. and S.X.; Supervision, B.G.B., D.S., and S.D.
References
- Birket MJ, Ribeiro MC, Verkerk AO, Ward D, Leitoguinho AR, den Hartogh SC, Orlova VV, Devalla HD, Schwach V, Bellin M, et al. Expansion and patterning of cardiovascular progenitors derived from human pluripotent stem cells. Nat Biotechnol. 2015;33:970–979. doi: 10.1038/nbt.3271. [DOI] [PubMed] [Google Scholar]
- Blin G, Nury D, Stefanovic S, Neri T, Guillevic O, Brinon B, Bellamy V, Rücker-Martin C, Barbry P, Bel A, et al. A purified population of multipotent cardiovascular progenitors derived from primate pluripotent stem cells engrafts in postmyocardial infarcted nonhuman primates. J Clin Invest. 2010;120:1125–1139. doi: 10.1172/JCI40120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruneau BG. Signaling and transcriptional networks in heart development and regeneration. Cold Spring Harb Perspect Biol. 2013;5:a008292. doi: 10.1101/cshperspect.a008292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burridge PW, Keller G, Gold JD, Wu JC. Production of de novo cardiomyocytes: human pluripotent stem cell differentiation and direct reprogramming. Cell Stem Cell. 2012;10:16–28. doi: 10.1016/j.stem.2011.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao N, Liang H, Huang J, Wang J, Chen Y, Chen Z, Yang HT. Highly efficient induction and long-term maintenance of multipotent cardiovascular progenitors from human pluripotent stem cells under defined conditions. Cell Res. 2013;23:1119–1132. doi: 10.1038/cr.2013.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan SS, Shi X, Toyama A, Arpke RW, Dandapat A, Iacovino M, Kang J, Le G, Hagen HR, Garry DJ, Kyba M. Mesp1 patterns mesoderm into cardiac, hematopoietic, or skeletal myogenic progenitors in a context-dependent manner. Cell Stem Cell. 2013;12:587–601. doi: 10.1016/j.stem.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung C, Bernardo AS, Trotter MW, Pedersen RA, Sinha S. Generation of human vascular smooth muscle subtypes provides insight into embryological origin-dependent disease susceptibility. Nat Biotechnol. 2012;30:165–173. doi: 10.1038/nbt.2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devine WP, Wythe JD, George M, Koshiba-Takeuchi K, Bruneau BG. Early patterning and specification of cardiac progenitors in gastrulating mesoderm. eLife. 2014;3:3. doi: 10.7554/eLife.03848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efe JA, Hilcove S, Kim J, Zhou H, Ouyang K, Wang G, Chen J, Ding S. Conversion of mouse fibroblasts into cardiomyocytes using a direct reprogramming strategy. Nat Cell Biol. 2011;13:215–222. doi: 10.1038/ncb2164. [DOI] [PubMed] [Google Scholar]
- Hirata H, Kawamata S, Murakami Y, Inoue K, Nagahashi A, Tosaka M, Yoshimura N, Miyamoto Y, Iwasaki H, Asahara T, Sawa Y. Coexpression of platelet-derived growth factor receptor alpha and fetal liver kinase 1 enhances cardiogenic potential in embryonic stem cell differentiation in vitro. J Biosci Bioeng. 2007;103:412–419. doi: 10.1263/jbb.103.412. [DOI] [PubMed] [Google Scholar]
- Kattman SJ, Witty AD, Gagliardi M, Dubois NC, Niapour M, Hotta A, Ellis J, Keller G. Stage-specific optimization of activin/nodal and BMP signaling promotes cardiac differentiation of mouse and human pluripotent stem cell lines. Cell Stem Cell. 2011;8:228–240. doi: 10.1016/j.stem.2010.12.008. [DOI] [PubMed] [Google Scholar]
- Kaufman DS, Lewis RL, Hanson ET, Auerbach R, Plendl J, Thomson JA. Functional endothelial cells derived from rhesus monkey embryonic stem cells. Blood. 2004;103:1325–1332. doi: 10.1182/blood-2003-03-0799. [DOI] [PubMed] [Google Scholar]
- Kuzmenkin A, Liang H, Xu G, Pfannkuche K, Eichhorn H, Fatima A, Luo H, Saric T, Wernig M, Jaenisch R, Hescheler J. Functional characterization of cardiomyocytes derived from murine induced pluripotent stem cells in vitro. FASEB J. 2009;23:4168–4180. doi: 10.1096/fj.08-128546. [DOI] [PubMed] [Google Scholar]
- Lam JT, Moretti A, Laugwitz KL. Multipotent progenitor cells in regenerative cardiovascular medicine. Pediatr Cardiol. 2009;30:690–698. doi: 10.1007/s00246-009-9450-1. [DOI] [PubMed] [Google Scholar]
- Laugwitz KL, Moretti A, Lam J, Gruber P, Chen Y, Woodard S, Lin LZ, Cai CL, Lu MM, Reth M, et al. Postnatal isl1+ cardioblasts enter fully differentiated cardiomyocyte lineages. Nature. 2005;433:647–653. doi: 10.1038/nature03215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moretti A, Caron L, Nakano A, Lam JT, Bernshausen A, Chen Y, Qyang Y, Bu L, Sasaki M, Martin-Puig S, et al. Multipotent embryonic isl1+ progenitor cells lead to cardiac, smooth muscle, and endothelial cell diversification. Cell. 2006;127:1151–1165. doi: 10.1016/j.cell.2006.10.029. [DOI] [PubMed] [Google Scholar]
- Murry CE, Keller G. Differentiation of embryonic stem cells to clinically relevant populations: lessons from embryonic development. Cell. 2008;132:661–680. doi: 10.1016/j.cell.2008.02.008. [DOI] [PubMed] [Google Scholar]
- Naito AT, Shiojima I, Akazawa H, Hidaka K, Morisaki T, Kikuchi A, Komuro I. Developmental stage-specific biphasic roles of Wnt/beta-catenin signaling in cardiomyogenesis and hematopoiesis. Proc Natl Acad Sci USA. 2006;103:19812–19817. doi: 10.1073/pnas.0605768103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qyang Y, Martin-Puig S, Chiravuri M, Chen S, Xu H, Bu L, Jiang X, Lin L, Granger A, Moretti A, et al. The renewal and differentiation of Isl1+ cardiovascular progenitors are controlled by a Wnt/beta-catenin pathway. Cell Stem Cell. 2007;1:165–179. doi: 10.1016/j.stem.2007.05.018. [DOI] [PubMed] [Google Scholar]
- van Laake LW, Passier R, Monshouwer-Kloots J, Verkleij AJ, Lips DJ, Freund C, den Ouden K, Ward-van Oostwaard D, Korving J, Tertoolen LG, et al. Human embryonic stem cell-derived cardiomyocytes survive and mature in the mouse heart and transiently improve function after myocardial infarction. Stem Cell Res (Amst) 2007;1:9–24. doi: 10.1016/j.scr.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Wamstad JA, Alexander JM, Truty RM, Shrikumar A, Li F, Eilertson KE, Ding H, Wylie JN, Pico AR, Capra JA, et al. Dynamic and coordinated epigenetic regulation of developmental transitions in the cardiac lineage. Cell. 2012;151:206–220. doi: 10.1016/j.cell.2012.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Cao N, Spencer CI, Nie B, Ma T, Xu T, Zhang Y, Wang X, Srivastava D, Ding S. Small molecules enable cardiac reprogramming of mouse fibroblasts with a single factor, Oct4. Cell Rep. 2014;6:951–960. doi: 10.1016/j.celrep.2014.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Soonpaa MH, Adler ED, Roepke TK, Kattman SJ, Kennedy M, Henckaerts E, Bonham K, Abbott GW, Linden RM, et al. Human cardiovascular progenitor cells develop from a KDR+ embryonicstem-cell-derived population. Nature. 2008;453:524–528. doi: 10.1038/nature06894. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


