Introduction
The number of published papers on PubMed with “arterial stiffness” or “aortic stiffness” in the title over the past 20 years exceeds 4000 papers published, with more than one-half published in the past 5 years (see Figure). The studies published on the topic of arterial stiffness span the full translational continuum from basic mechanistic studies in cell and animal models, to small experimental studies in humans, and large observational cross-sectional and prospective studies relating arterial stiffness to subclinical target organ damage or clinical cardiovascular disease (CVD) events. The reason for this rapidly growing area of investigation is likely attributed to the strong body of evidence indicating that arterial stiffness, specifically aortic stiffness as measured by the “reference standard” carotid-femoral pulse wave velocity (CFPWV), is a robust predictor of clinical CVD events in adults even after adjusting for blood pressure and other conventional CVD risk factors.1, 2 In addition, several prospective cohort studies suggest that elevated CFPWV precedes the development of incident hypertension in middle-aged adults3, 4, consistent with the idea that aortic stiffness may be causal in the genesis of hypertension with aging in humans rather than a subclinical consequence.
Figure.
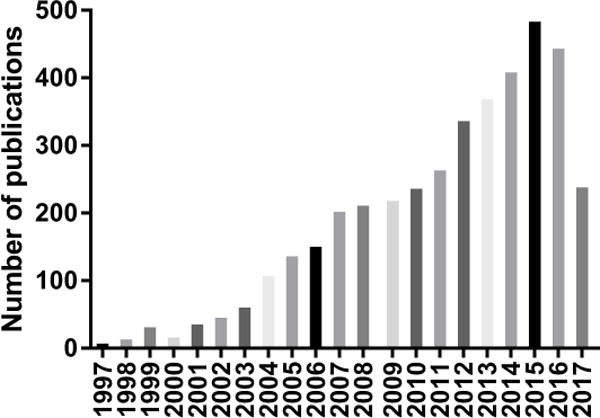
The number of published articles in PubMed with the terms “arterial + stiffness” and “aortic + stiffness” appearing in the title over the past 20 years.
In 2015, the American Heart Association published a comprehensive Scientific Statement addressing “the nomenclature, methodologies, utility, limitations and gaps in knowledge” related to arterial stiffness to inform the research community of the ‘state of field’ and help guide future directions for investigation including identifying targets for intervention.5 Because the putative mechanisms that contribute to the development of aortic stiffness remain elusive, the current review article will focus on the recent advances over the past 2-3 years describing some novel mechanisms that contribute to elevated aortic stiffness and that may be therapeutic targets for intervention. Then, a few of the most exciting recent investigations on the subclinical negative consequences of aortic stiffness on the brain and kidney will be discussed.
Sympathetic and parasympathetic nervous system function
Chronic sympathetic nervous system (SNS) hyperactivation is linked to cardiovascular end organ damage such as vascular hypertrophy6, left ventricular hypertrophy7 and endothelial dysfunction.8 However, the degree to which the SNS activation acutely or chronically modulates mechanical properties of large elastic arteries remains contraversial.9–15 Experimental maneuvers that acutely increase SNS activity and blood pressure (BP) result in reductions in compliance16 and endothelial function of peripheral muscular arteries17, but the influence of transient elevations in SNS activity on the large elastic arteries (e.g., aorta and carotid arteries) show conflicting results. For example, Maki-Petaja et al.9 had a group of young healthy adults perform 4 minutes of static handgrip exercise, a model well established to increase SNS activity and BP. As expected, handgrip exercise resulted in small but significant elevations in BP and CFPWV, however, the rise in CFPWV was completely abrogated after adjustment of increase mean arterial BP. In contrast, acute experimental increases in SNS activity in the absence of increases in BP using the lower body negative pressure (LBNP) technique, resulted in no changes in aortic or carotid elastic compliance in young healthy humans in two studies suggesting a rise in BP is necessary to alter elastic artery stiffness.13, 14 Conversely, extreme levels of LBNP (i.e., −60 to −80 mmHg) that also raise heart rate significantly resulted in a increases in CFPWV (>2 m/sec) in young healthy adults despite no changes in BP, but no change was observed in CFPWV at −50 mmHg indicating that higher levels of SNS may influence CFPWV. In relation to chronic elevations in SNS activity, higher resting tonic SNS activity, as measured by muscle sympathetic nerve activity (MSNA), was positively associated with elevated CFPWV in young/middle-aged healthy men even after accounting for mean arterial BP. 11 Similarly, Tanaka et al. 15 demonstrated an inverse relation between MSNA and carotid artery compliance and adjusting for MSNA abolished the age-related differences in compliance between young and older adults, suggesting that chronically elevated SNS activity mediates the age-related reduction in carotid compliance. Taken together, the data remain unclear whether transient and chronic elevations in SNS activity modulate the mechanical properties of the aorta and carotid arteries. Additional studies are needed but outcomes may differ based on the method of measuring stiffness (e.g., CFPWV vs. local compliance), whether elevated baseline SNS activity is present, and differences in age, sex and other clinical characteristics of the cohort.
Maki-Petaja et al. 9 explored the role of both the parasympathetic nervous system (PNS) and SNS on arterial stiffness. Using heart rate variability obtained from resting ECG in a subset of adults from the Enigma cohort study, participants were first stratified into tertiles of high frequency power, an indirect index of PNS activity. They found that CFPWV was significantly lower with increasing levels of high frequency power (PNS activity), but the differences were abolished after adjustment for mean arterial BP. Consistent with this, in stepwise regression models, only age, sex, BP and heart rate, but not high frequency power, entered the models for CFPWV suggesting that PNS activity does not modulate aortic stiffness. Furthermore, there was no difference in CFPWV or mean BP if the participants were stratified by low frequency power (index of SNS activity), suggesting that SNS activity also does not appreciably modulate CFPWV in young adults. Next, to assess the contribution of the acute changes in SNS and PNS on aortic stiffness, a ganglionic blocker was administered to inhibit SNS and PNS activity, but this resulted in no change in CFPWV despite a paradoxical increase in BP and heart rate. In another study acute sympathoinhibition of SNS with an intravenous ganglionic blocker resulted in reduced CFPWV in postmenopausal but not in younger women10, although the change in CFPWV in the postmenopausal women was abolished after adjusting for the large reduction in BP. Together, these data suggest that neither SNS nor PNS activity appreciable modulates CFPWW in young healthy adults, but more studies are needed in persons at higher CVD risk with elevated SNS activity and arterial stiffness (e.g., aged, hypertension, obesity).
Klotho and Sirtuin-1
Klotho gene is an ‘anti-aging’ gene expressed mostly in the kidney and lesser extent in the brain that when mutated results in myriad premature aging phenotypes and early death.18 In contrast, overexpression of Klotho reverses many of the age-related impairments in physiological function and extends lifespan in mice.19 Klotho gene also results in a secreted protein whereby circulating concentrations are reduced with aging20 and hypertension21, and inversely associated with carotid artery stiffness in humans.22 Therefore, several recent studies investigated whether reduction in Klotho could be a putative mechanism mediating aortic stiffness with aging. Chen et al. (2015)23 demonstrated that mice heterogeneous for Klotho gene exhibited elevated aortic PWV compared with wild-type littermates. Importantly, aortic PWV appeared to be elevated at least 2 weeks before the increase in BP suggesting that aortic stiffness may be causal rather than a result of elevated BP. Klotho haplodeficient mice also exhibited increased collagen I and reduced elastin in media of aortas (but not smaller carotid and femoral arteries), with concomitant elevated aortic expression of matrix-matalloproteinase (MMP)-2 and -9, transforming growth factor-β1 (TGFβ1) and myofibroblast differentiation (indicated by α-smooth muscle cell positive cells). Furthermore, a novel finding was that Klotho-deficient mice also demonstrated elevated circulating aldosterone concentrations and blockade with the mineralocorticoid receptor (MR) antagonist eplerenone for 3 weeks completely abolished the Klotho deficiency-mediated increase in aortic stiffness and aforementioned cellular vascular alterations.
A follow-up study also indicated that heterozygous Klotho-deficient mice fed a high-fat diet (HFD) exhibited increased aortic PWV and glucose within 5 weeks but not in the wild-type mice, suggesting that Klotho deficiency exacerbates HFD-mediated aortic stiffening and impaired glucose metabolism.24 However, it should be noted that systolic BP was also elevated about week 5 making it difficult to determine if the aortic stiffening was a cause or a consequence of the raised BP induced by HFD. In addition to upregulation of collagen I and TGF1β, Klotho-deficient mice on HFD also demonstrated reduced endothelial nitric oxide synthase (eNOS) serine 1177 and adenosine monophosphate-activated protein kinase (AMPK)α phosphorylation. Weekly injection of an AMPK activator abrogated the elevation in aortic PWV, BP and glucose suggesting that AMPK may be a key mechanism in promoting the HFD-mediated stiffening and hypertension in the presence of Klotho-deficiency. In addition, overnight fasting-induced reduction in aortic PWV in high fat/high sucrose (HFS) diet fed mice was prevented in sirtuin-1 (SIRT1) vascular smooth muscle cell (VSMC) knockout mice.25 Similarly overexpression or VSMC SIRT1, an NAD+-dependent deacetylase, or 1 week treatment with SIRT1 activator SRT1720 prevented the HFS diet-related elevation in aortic PWV. However corresponding BPs were not reported so it is unclear whether changes in BP mediated the change in PWV with the fasting, diet- and SIRT1 activation interventions.
Klotho-deficient mice also demonstrated reduced expression of SIRT-1 21 that was associated with antioxidant, antiinflammatory26 and antisenescent27 phenotypes and downregulation of eNOS.28 Because Klotho deficiency downregulated aortic SIRT-1 of the mice and activation of SIRT1 by SRT1720 did not alter circulating Klotho concentrations, this suggests that Klotho signaling is upstream from SIRT1 and acts a hormone given that Klotho gene is expressed in the kidney and brain and not aorta.21 These findings are clinically important because older adults demonstrate lower SIRT1 expression in vascular endothelial cells that is associated with aged-related reduction in endothelial function.29 Importantly, because stiffening precedes the elevation of BP in the Klotho-deficient mice and several large prospective studies suggest that aortic stiffness antecedes development of hypertension3, 4, further investigation is warranted to determine if boosting Klotho is a novel target for treatment of aortic stiffness-related hypertension.
Parental and Early Life Determinants
The heritability of high CFPWV and related hemodynamic variables (e.g., forward and reflected wave amplitude, augmentation index) are estimated to be between 0.21–0.4830, 31, which is in the same range as hypertension heritability.32–34 In this regards, Andersson et al.35 investigated whether normotensive young/middle-aged offspring of parents with hypertension exhibited greater aortic stiffness and hemodynamic variables before the onset of clinical hypertension with the idea that aortic stiffness is causal in the pathogenesis of the development of hypertension. Indeed, offspring with one or two parents with hypertension demonstrated higher CFPWV, forward pressure wave amplitude, augmentation index and mean arterial BP after adjustments for age, sex and height. However, in fully adjusted models only mean arterial BP and forward wave amplitude remained associated with parental hypertension status. Because aortic forward pressure wave amplitude, which is determined from pressure and flow related characteristic impedance in proximal aorta, is a robust predictor of CVD risk in risk factor-adjusted models that include CFPWV, this suggests that parental hypertension could be an important predictor of this understudied hemodynamic biomarker.36
In addition to heritable influences on aortic stiffness, early-life social and psychological factors in childhood may also contribute to higher arterial stiffness and CVD risk in adulthood. Consistent with this, adverse childhood experiences (ACEs) such as physical and sexual abuse, neglect, household dysfunction, appear to have a strong influence on increases in BP in young adulthood after age 30 years, that were not explained by CVD risk factors or socioeconomic status.37 In addition, children and young adults exposed to moderate or severe ACEs demonstrate higher diastolic BP, total peripheral resistance and carotid-radial PWV (i.e., peripheral muscular artery stiffness) after adjustment for demographic factors, education and socioeconomic status.38 However, the comparisons for PWV were not adjusted for BP and CFPWV was not assessed. Thus, future studies investigating whether a BP-independent increase in CFPWV is present in youth exposed to ACEs are still needed. Taken together, these studies underscore that traumatic psychological experiences in childhood may be an underappreciated critical risk factor for CVD that requires additional investigation.
Calcification
Calcification of the arterial media is proposed to be involved with stiffening of the aorta in addition to alterations in load-bearing extracellular matrix (ECM) proteins elastin and collagen, but whether progression of calcification parallels increases in aortic stiffening in humans was unknown until recently. As such, Guo et al. 39 assessed aortic calcification via chest computed tomography from aortic arch to iliac bifurcation and brachial-ankle PWV (baPWV) prospectively in middle-aged men at baseline and 4-7 years later. As predicted, annual change in aortic calcification was significantly associated with increases in baPWV in multivariate adjusted models. In men without calcification at baseline, baPWV was associated with incident aortic calcification at follow-up and baPWV also was associated with progression of aortic calcification in participants with baseline aortic calcification, suggesting that arterial stiffness and calcification are casually linked. However, the direction of the association could not be determined in this study and the relation could be bidirectional. Furthermore, because baPWV includes both peripheral and central arterial stiffness additional studies are needed to confirm these findings with CFPWV.
Cecelja et al. (2016)40 measured expression of genes previously identified to be associated with arterial stiffness in lymphoblastoid cells in the Twins UK cohort in a cross-sectional study and after 4 years follow up. Of the 52 genes analyzed, they found that CFPWV was associated with higher expression of ectonucleotide pyrophosphate/phosphodiesterase (ENPP1), a gene transcript associated with calcification. Importantly, they found that CFPWV and ENPP1 were more strongly correlated among monozygotic than dizygotic twins consistent with a significant genetic effect of ENPP1. In the prospective analysis, baseline ENPP1 expression was also related to progression of CFPWV over the 4 year follow up in addition to collagen type IV α1 (COL4A1), a gene involved in type IV collagen protein expression. Interestingly, progression of decreased carotid distensibility was most closely associated with eNOS (NOS3) and angiotensin converting enzyme (ACE) genes but not ENPP1. Thus, these data suggest that genes involved in regulation of aortic vs. carotid stiffness progression likely differ because of anatomical variances in ECM composition of arterial wall of aorta and carotids or differences in measuring arterial stiffness between arterial sites.
Matrix Gla-protein (MGP) is a strong inhibitor of soft tissue calcification that is fully activated when it undergoes glutamate carboxylation and serine phosphorylation. The caroboxylation of MGP is a vitamin K-dependent process. Because these post-translational steps are not fully accomplished, the inactive form of MGP, dephospho-uncarboxylated (dp-ucMGP) would be predicted to be associated with higher aortic stiffness in part from accelerated vascular calcification. Whereas several studies measuring total or uncarboxylated MGP without phosphorylation status assessed have failed to identify a correlation with aortic stiffness, Piven et al. (2015)41 reported a positive association between circulating dp-ucMGP and CFPWV in a large sample of middle-aged adults after adjustment for BP and CVD risk factors. Given that active MGP resides in the vascular media and inhibits medical calcification of elastin 42, and that inactive dp-ucMGP could be activated by vitamin K administration, vitamin K supplementation may be a novel intervention to attenuate vascular calcification-mediated aortic stiffness but this has not been tested to date.
Vascular smooth muscle and endothelial cell intrinsic stiffness
Alterations in the mechanical properties of the large elastic arteries with aging and or hypertension are typically attributed to reductions in elastin and increases in collagen ECM content as well as collagen cross-linking in the vascular media, with additional contributions from impaired NO-mediated vascular tone. However, a series of studies have recently identified increased intrinsic stiffness of aortic VSMCs in animal models of hypertension and aging using novel in vitro biomechanical/chemical techniques. 43–45 Using a reconstituted aortic tissue model and atomic force microscopy in isolated primary aortic VSMCs from monkeys, Qiu et al.45 demonstrated that VSMCs exhibited higher stiffness in the aortic tissue and primary isolated VSMCs higher exhibited greater intrinsic elastic modulus (stiffness) in old compared with young monkeys. Similarly, Sehgel et al. found that cellular stiffness from reconstituted aortic tissue was increased 4-fold, and the intrinsic stiffness of individual VSMCs was increased by 2-fold, in spontaneously hypertensive rats (SHRs) compared with Wistar-Kyoto (WKY) normotensive control rats. 43, 44 A novel finding was that hypertension-mediated increases in individual VSMC stiffness, adhesion to fibronectin, and medial layer thickening were all exacerbated with aging. In contrast, the ECM composition of elastin and collagen in the vascular media was not altered in the aorta of young SHR, but collagen was increased and elastin reduced in the aged SHRs.44 These findings suggest that mechanical properties of VSMCs and perhaps other medial layer remodeling processes (e.g., glycation mediated cross-linking collagen) play a more significant role in hypertension than the ECM proteins elastin and collagen. Moreover, VSMCs and ECM appear to contribute synergistically when aging is superimposed with hypertension, however these results do not provide evidence that age-related changes in VSMC stiffness, adhesion or ECM remodeling contribute to hypertension because BP was not elevated in the aged WKY rats.44
Endothelial function may also contribute, at least in part, to the development of aortic stiffness through chronic decreases in VSMC tone from reduced NO bioavailability. However, much less is known about the potential contribution of intrinsic cortical stiffness of individual endothelial cells. In this regard, DeMarco et al.46 demonstrated that mice fed a HFS diet for 4 months, exhibited a 5-fold increase in endothelial cell and VSMC surface stiffness using atomic force microscopy from aortic explants. This was paralleled by elevation in aortic PWV, endothelial dysfunction, oxidative stress and a pro-inflammatory vascular phenotype despite no increase in BP. Interestingly, treatment with spironolactone, a MR antagonist, completely abolished the increase in aortic PWV and cortical stiffness of endothelial cells and VSMCs, as well as the pro-oxidative stress and -inflammatory phenotype.46 MR antagonism also reversed HFS diet-induced elevation in femoral artery stiffness in the absence of any reduction in BP. This is important because de-stiffening effects of MR antagonism occurred in a BP-independent manner suggesting a direct effect of MR signaling on the vascular remodeling of aorta and femoral arteries making the MR a potential novel target for HFS-induced aortic stiffness.
A follow-up study in this model demonstrated that mice fed the HFS diet and given running wheels for 16 weeks, exhibited reversal of endothelial cell cortical stiffness in aortic tissue in the absence of changes in body weight. Surprisingly, exercise did not reduce aortic PWV despite reductions in endothelial and medial nitrotyrosine, and decreased endothelial TGFβ and adventitial fibronectin. In contrast, wheel running reversed femoral artery stiffness and this was associated with increases in elastin and number of fenestrae in the internal elastic laminae.47 These data suggest that the improvements in the endothelial cell surface stiffness and fenestrae with exercise may precede changes in aortic stiffness, or alternatively, that exercise training was not long enough or stiffness adaptations are specific to the arteries perfusing the exercising muscle in this model.
Subclinical consequences of aortic stiffness
There is a growing body of evidence that the high blood flow, low resistance organs such as the brain and kidney suffer from the hemodynamic consequences (i.e., elevated pulsatile flow and pulse pressure) of increased large elastic artery stiffness with aging and hypertension. Indeed, higher CFPWW was associated with lower memory scores and subcortical white matter hyperintensities, but not processing speed or executive function, in older adults age 70-80 yrs.48, 49 The association between aortic stiffness and memory was mediated in part from greater cerebral vascular resistance and subcortical white matter damage. In another study, higher CFPWV was associated with slower processing speed and executive function and white matter hyperintensities among middle-aged adults (age 45–65 yrs) 50, and the change in CFPWV over 4 years was related to the longitudinal decline in executive function, memory scores and working memory even after adjustment for BP and/or the presence of hypertension.51 Importantly, persons with hypertension and high CFPWV (> 7 m/sec) had the largest decline in executive function over 4 years suggesting an additive effect of hypertension and aortic stiffness on selective domains of cognition. Furthermore, in older adults without dementia at baseline, CFPWV predicted the 10-year incidence of diagnosed mild cognitive impairment and with dementia in non-diabetic aged persons.52 In addition to the brain, previous studies have also linked aortic stiffness and excess flow pulsatility with reduced renal glomerular filtration rate in patients with hypertension and/or chronic kidney disease. 53, 54 An elegant study by Hashimoto and Ito 55 extended these findings, where they revealed that aortic flow reversal, quantified by increased retrograde thoracic aortic flow during diastole, as a result of aortic stiffness explained the link between aortic stiffness and decline in renal function by impairing antegrade aortic and renal flow in patients at risk for or with kidney disease. Taken together, these studies support the idea that interventions targeting aortic stiffness may be a novel approach to starve off the adverse effects of stiffness-associated subclinical damage to these highly susceptible organs.
Summary and future directions
Aortic stiffness continues to be a robust area of investigation spanning the translational continuum of epidemiology, clinical and basic mechanistic studies. Given that most antihypertensive drugs do not target stiffness of the large elastic arteries, these and other recent discoveries may provide initial guidance in discovering potential new therapeutic targets to treat or prevent the development of aortic stiffness and subsequent subclinical target organ damage. The major hurdle is that promising interventions (e.g., both exercise or pharmacological) in rodent models of aging, hypertension and/or obesity, do not always translate to effective treatments in human clinical studies. Nonetheless, this should not diminish efforts to move basic studies to small, randomized or experimental integrative studies in humans in order to inform additional basic studies and guide larger clinical trials. However, perhaps equally important than treating aortic stiffness once it is established, is maintaining ‘aortic health’ in early life through the middle-age adult years would likely be the most effective strategy for preventing an onslaught of aortic stiffness-associated CVD, kidney disease and cognitive impairment in our aging population.
Acknowledgments
Sources of funding.
Dr. Pierce was supported by grants from the American Heart Association (13SDG143400012 and 15SFRN23760002) and National Institutes of Health (AG043722, AG048170, AG049784 and HL014388).
Footnotes
Disclosures.
None.
References
- 1.Ben-Shlomo Y, Spears M, Boustred C, et al. Aortic pulse wave velocity improves cardiovascular event prediction: an individual participant meta-analysis of prospective observational data from 17,635 subjects. J Am Coll Cardiol. 2014;63:636–646. doi: 10.1016/j.jacc.2013.09.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol. 2010;55:1318–1327. doi: 10.1016/j.jacc.2009.10.061. [DOI] [PubMed] [Google Scholar]
- 3.Kaess BM, Rong J, Larson MG, Hamburg NM, Vita JA, Levy D, Benjamin EJ, Vasan RS, Mitchell GF. Aortic stiffness, blood pressure progression, and incident hypertension. JAMA. 2012;308:875–881. doi: 10.1001/2012.jama.10503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Najjar SS, Scuteri A, Shetty V, Wright JG, Muller DC, Fleg JL, Spurgeon HP, Ferrucci L, Lakatta EG. Pulse wave velocity is an independent predictor of the longitudinal increase in systolic blood pressure and of incident hypertension in the Baltimore Longitudinal Study of Aging. J Am Coll Cardiol. 2008;51:1377–1383. doi: 10.1016/j.jacc.2007.10.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Townsend RR, Wilkinson IB, Schiffrin EL, Avolio AP, Chirinos JA, Cockcroft JR, Heffernan KS, Lakatta EG, McEniery CM, Mitchell GF, Najjar SS, Nichols WW, Urbina EM, Weber T, American Heart Association Council on H Recommendations for improving and standardizing vascular research on arterial stiffness: a scientific statement from the American Heart Association. Hypertension. 2015;66:698–722. doi: 10.1161/HYP.0000000000000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dinenno FA, Jones PP, Seals DR, Tanaka H. Age-associated arterial wall thickening is related to elevations in sympathetic activity in healthy humans. Am J Physiol Heart Circ Physiol. 2000;278:H1205–1210. doi: 10.1152/ajpheart.2000.278.4.H1205. [DOI] [PubMed] [Google Scholar]
- 7.Greenwood JP, Scott EM, Stoker JB, Mary DA. Hypertensive left ventricular hypertrophy: relation to peripheral sympathetic drive. J Am Coll Cardiol. 2001;38:1711–1717. doi: 10.1016/s0735-1097(01)01600-x. [DOI] [PubMed] [Google Scholar]
- 8.Gamboa A, Figueroa R, Paranjape SY, Farley G, Diedrich A, Biaggioni I. Autonomic blockade reverses endothelial dysfunction in obesity-associated hypertension. Hypertension. 2016;68:1004–1010. doi: 10.1161/HYPERTENSIONAHA.116.07681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maki-Petaja KM, Barrett SM, Evans SV, Cheriyan J, McEniery CM, Wilkinson IB. The role of the autonomic nervous system in the regulation of aortic stiffness. Hypertension. 2016;68:1290–1297. doi: 10.1161/HYPERTENSIONAHA.116.08035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harvey RE, Barnes JN, Hart EC, Nicholson WT, Joyner MJ, Casey DP. Influence of sympathetic nerve activity on aortic hemodynamics and pulse wave velocity in women. Am J Physiol Heart Circ Physiol. 2017;312:H340–H346. doi: 10.1152/ajpheart.00447.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Swierblewska E, Hering D, Kara T, Kunicka K, Kruszewski P, Bieniaszewski L, Boutouyrie P, Somers VK, Narkiewicz K. An independent relationship between muscle sympathetic nerve activity and pulse wave velocity in normal humans. J Hypertens. 2010;28:979–984. doi: 10.1097/hjh.0b013e328336ed9a. [DOI] [PubMed] [Google Scholar]
- 12.Phillips AA, Bredin SS, Cote AT, Drury CT, Warburton DE. Aortic distensibility is reduced during intense lower body negative pressure and is related to low frequency power of systolic blood pressure. Eur J Appl Physiol. 2013;113:785–792. doi: 10.1007/s00421-012-2489-3. [DOI] [PubMed] [Google Scholar]
- 13.Sonesson B, Vernersson E, Hansen F, Lanne T. Influence of sympathetic stimulation on the mechanical properties of the aorta in humans. Acta Physiol Scand. 1997;159:139–145. doi: 10.1046/j.1365-201X.1997.581343000.x. [DOI] [PubMed] [Google Scholar]
- 14.Pannier B, Slama MA, London GM, Safar ME, Cuche JL. Carotid arterial hemodynamics in response to LBNP in normal subjects: methodological aspects. J Appl Physiol (1985) 1995;79:1546–1555. doi: 10.1152/jappl.1995.79.5.1546. [DOI] [PubMed] [Google Scholar]
- 15.Tanaka H, Dinenno FA, Seals DR. Reductions in central arterial compliance with age are related to sympathetic vasoconstrictor nerve activity in healthy men. Hypertens Res. 2017;40:493–495. doi: 10.1038/hr.2016.182. [DOI] [PubMed] [Google Scholar]
- 16.Boutouyrie P, Lacolley P, Girerd X, Beck L, Safar M, Laurent S. Sympathetic activation decreases medium-sized arterial compliance in humans. Am J Physiol. 1994;267:H1368–1376. doi: 10.1152/ajpheart.1994.267.4.H1368. [DOI] [PubMed] [Google Scholar]
- 17.Hijmering M, Stroes E, Olijhoek J, Hutten B, Blankestijn P, Rabelink T. Sympathetic activation markedly reduces endothelium-dependent, flow-mediated vasodilation. J Amer Coll Cardiol. 2002;39:683–688. doi: 10.1016/s0735-1097(01)01786-7. [DOI] [PubMed] [Google Scholar]
- 18.Kuro-o M, Matsumura Y, Aizawa H, et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390:45–51. doi: 10.1038/36285. [DOI] [PubMed] [Google Scholar]
- 19.Kurosu H, Yamamoto M, Clark JD, Pastor JV, Nandi A, Gurnani P, McGuinness OP, Chikuda H, Yamaguchi M, Kawaguchi H, Shimomura I, Takayama Y, Herz J, Kahn CR, Rosenblatt KP, Kuro-o M. Suppression of aging in mice by the hormone Klotho. Science. 2005;309:1829–1833. doi: 10.1126/science.1112766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xiao NM, Zhang YM, Zheng Q, Gu J. Klotho is a serum factor related to human aging. Chin Med J (Engl) 2004;117:742–747. [PubMed] [Google Scholar]
- 21.Gao D, Zuo Z, Tian J, Ali Q, Lin Y, Lei H, Sun Z. Activation of SIRT1 attenuates klotho deficiency-induced arterial stiffness and hypertension by enhancing AMP-activated protein kinase activity. Hypertension. 2016;68:1191–1199. doi: 10.1161/HYPERTENSIONAHA.116.07709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matsubara T, Miyaki A, Akazawa N, Choi Y, Ra SG, Tanahashi K, Kumagai H, Oikawa S, Maeda S. Aerobic exercise training increases plasma Klotho levels and reduces arterial stiffness in postmenopausal women. Am J Physiol Heart Circ Physiol. 2014;306:H348–355. doi: 10.1152/ajpheart.00429.2013. [DOI] [PubMed] [Google Scholar]
- 23.Chen K, Zhou X, Sun Z. Haplodeficiency of klotho gene causes arterial stiffening via upregulation of scleraxis expression and induction of autophagy. Hypertension. 2015;66:1006–1013. doi: 10.1161/HYPERTENSIONAHA.115.06033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin Y, Chen J, Sun Z. Antiaging gene klotho deficiency promoted high-fat diet-induced arterial stiffening via inactivation of AMP-activated protein kinase. Hypertension. 2016;67:564–573. doi: 10.1161/HYPERTENSIONAHA.115.06825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fry JL, Al Sayah L, Weisbrod RM, Van Roy I, Weng X, Cohen RA, Bachschmid MM, Seta F. Vascular smooth muscle sirtuin-1 protects against diet-induced aortic stiffness. Hypertension. 2016;68:775–784. doi: 10.1161/HYPERTENSIONAHA.116.07622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gano LB, Donato AJ, Pasha HM, Hearon CM, Jr, Sindler AL, Seals DR. The SIRT1 activator SRT1720 reverses vascular endothelial dysfunction, excessive superoxide production, and inflammation with aging in mice. Am J Physiol Heart Circ Physiol. 2014;307:H1754–1763. doi: 10.1152/ajpheart.00377.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zu Y, Liu L, Lee MY, Xu C, Liang Y, Man RY, Vanhoutte PM, Wang Y. SIRT1 promotes proliferation and prevents senescence through targeting LKB1 in primary porcine aortic endothelial cells. Circ Res. 2010;106:1384–1393. doi: 10.1161/CIRCRESAHA.109.215483. [DOI] [PubMed] [Google Scholar]
- 28.Nisoli E, Tonello C, Cardile A, Cozzi V, Bracale R, Tedesco L, Falcone S, Valerio A, Cantoni O, Clementi E, Moncada S, Carruba MO. Calorie restriction promotes mitochondrial biogenesis by inducing the expression of eNOS. Science. 2005;310:314–317. doi: 10.1126/science.1117728. [DOI] [PubMed] [Google Scholar]
- 29.Donato AJ, Magerko KA, Lawson BR, Durrant JR, Lesniewski LA, Seals DR. SIRT-1 and vascular endothelial dysfunction with ageing in mice and humans. J Physiol. 2011;589:4545–4554. doi: 10.1113/jphysiol.2011.211219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mitchell GF, DeStefano AL, Larson MG, Benjamin EJ, Chen MH, Vasan RS, Vita JA, Levy D. Heritability and a genome-wide linkage scan for arterial stiffness, wave reflection, and mean arterial pressure: the Framingham Heart Study. Circulation. 2005;112:194–199. doi: 10.1161/CIRCULATIONAHA.104.530675. [DOI] [PubMed] [Google Scholar]
- 31.Medda E, Fagnani C, Schillaci G, et al. Heritability of arterial stiffness and carotid intima-media thickness: an Italian twin study. Nutr Metab Cardiovasc Dis. 2014;24:511–517. doi: 10.1016/j.numecd.2013.10.031. [DOI] [PubMed] [Google Scholar]
- 32.An P, Rice T, Gagnon J, Borecki IB, Perusse L, Leon AS, Skinner JS, Wilmore JH, Bouchard C, Rao DC. Familial aggregation of resting blood pressure and heart rate in a sedentary population: the HERITAGE Family Study. Health, Risk Factors, Exercise Training, and Genetics. Am J Hypertens. 1999;12:264–270. doi: 10.1016/s0895-7061(98)00261-1. [DOI] [PubMed] [Google Scholar]
- 33.Bochud M, Bovet P, Elston RC, Paccaud F, Falconnet C, Maillard M, Shamlaye C, Burnier M. High heritability of ambulatory blood pressure in families of East African descent. Hypertension. 2005;45:445–450. doi: 10.1161/01.HYP.0000156538.59873.86. [DOI] [PubMed] [Google Scholar]
- 34.Fava C, Burri P, Almgren P, Groop L, Hulthen UL, Melander O. Heritability of ambulatory and office blood pressure phenotypes in Swedish families. J Hypertens. 2004;22:1717–1721. doi: 10.1097/00004872-200409000-00015. [DOI] [PubMed] [Google Scholar]
- 35.Andersson C, Quiroz R, Enserro D, Larson MG, Hamburg NM, Vita JA, Levy D, Benjamin EJ, Mitchell GF, Vasan RS. Association of parental hypertension with arterial stiffness in nonhypertensive offspring: the framingham heart study. Hypertension. 2016;68:584–589. doi: 10.1161/HYPERTENSIONAHA.116.07426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cooper LL, Rong J, Benjamin EJ, Larson MG, Levy D, Vita JA, Hamburg NM, Vasan RS, Mitchell GF. Components of hemodynamic load and cardiovascular events: the Framingham Heart Study. Circulation. 2015;131:354–361. doi: 10.1161/CIRCULATIONAHA.114.011357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Su S, Wang X, Pollock JS, Treiber FA, Xu X, Snieder H, McCall WV, Stefanek M, Harshfield GA. Adverse childhood experiences and blood pressure trajectories from childhood to young adulthood: the Georgia stress and Heart study. Circulation. 2015;131:1674–1681. doi: 10.1161/CIRCULATIONAHA.114.013104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Su S, Wang X, Kapuku GK, Treiber FA, Pollock DM, Harshfield GA, McCall WV, Pollock JS. Adverse childhood experiences are associated with detrimental hemodynamics and elevated circulating endothelin-1 in adolescents and young adults. Hypertension. 2014;64:201–207. doi: 10.1161/HYPERTENSIONAHA.113.02755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guo J, Fujiyoshi A, Willcox B, et al. Increased aortic calcification is associated with arterial stiffness progression in multiethnic middle-aged men. Hypertension. 2017;69:102–108. doi: 10.1161/HYPERTENSIONAHA.116.08459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cecelja M, Jiang B, Mangino M, Spector TD, Chowienczyk PJ. Association of cross-sectional and longitudinal change in arterial stiffness with gene expression in the Twins UK Cohort. Hypertension. 2016;67:70–76. doi: 10.1161/HYPERTENSIONAHA.115.05802. [DOI] [PubMed] [Google Scholar]
- 41.Pivin E, Ponte B, Pruijm M, et al. Inactive matrix Gla-Protein is associated with arterial stiffness in an adult population-based study. Hypertension. 2015;66:85–92. doi: 10.1161/HYPERTENSIONAHA.115.05177. [DOI] [PubMed] [Google Scholar]
- 42.Schurgers LJ, Teunissen KJ, Knapen MH, Kwaijtaal M, van Diest R, Appels A, Reutelingsperger CP, Cleutjens JP, Vermeer C. Novel conformation-specific antibodies against matrix gamma-carboxyglutamic acid (Gla) protein: undercarboxylated matrix Gla protein as marker for vascular calcification. Arterioscler Thromb Vasc Biol. 2005;25:1629–1633. doi: 10.1161/01.ATV.0000173313.46222.43. [DOI] [PubMed] [Google Scholar]
- 43.Sehgel NL, Zhu Y, Sun Z, Trzeciakowski JP, Hong Z, Hunter WC, Vatner DE, Meininger GA, Vatner SF. Increased vascular smooth muscle cell stiffness: a novel mechanism for aortic stiffness in hypertension. Am J Physiol Heart Circ Physiol. 2013;305:H1281–1287. doi: 10.1152/ajpheart.00232.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sehgel NL, Sun Z, Hong Z, Hunter WC, Hill MA, Vatner DE, Vatner SF, Meininger GA. Augmented vascular smooth muscle cell stiffness and adhesion when hypertension is superimposed on aging. Hypertension. 2015;65:370–377. doi: 10.1161/HYPERTENSIONAHA.114.04456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Qiu H, Zhu Y, Sun Z, Trzeciakowski JP, Gansner M, Depre C, Resuello RR, Natividad FF, Hunter WC, Genin GM, Elson EL, Vatner DE, Meininger GA, Vatner SF. Short communication: vascular smooth muscle cell stiffness as a mechanism for increased aortic stiffness with aging. Circ Res. 2010;107:615–619. doi: 10.1161/CIRCRESAHA.110.221846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.DeMarco VG, Habibi J, Jia G, Aroor AR, Ramirez-Perez FI, Martinez-Lemus LA, Bender SB, Garro M, Hayden MR, Sun Z, Meininger GA, Manrique C, Whaley-Connell A, Sowers JR. Low-dose mineralocorticoid receptor blockade prevents western diet-induced arterial stiffening in female mice. Hypertension. 2015;66:99–107. doi: 10.1161/HYPERTENSIONAHA.115.05674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Padilla J, Ramirez-Perez FI, Habibi J, Bostick B, Aroor AR, Hayden MR, Jia G, Garro M, DeMarco VG, Manrique C, Booth FW, Martinez-Lemus LA, Sowers JR. Regular exercise reduces endothelial cortical stiffness in western diet-fed female mice. Hypertension. 2016;68:1236–1244. doi: 10.1161/HYPERTENSIONAHA.116.07954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mitchell GF, van Buchem MA, Sigurdsson S, Gotal JD, Jonsdottir MK, Kjartansson O, Garcia M, Aspelund T, Harris TB, Gudnason V, Launer LJ. Arterial stiffness, pressure and flow pulsatility and brain structure and function: the Age, Gene/Environment Susceptibility–Reykjavik study. Brain. 2011;134:3398–3407. doi: 10.1093/brain/awr253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cooper LL, Woodard T, Sigurdsson S, van Buchem MA, Torjesen AA, Inker LA, Aspelund T, Eiriksdottir G, Harris TB, Gudnason V, Launer LJ, Mitchell GF. Cerebrovascular damage mediates relations between aortic stiffness and Memory. Hypertension. 2016;67:176–182. doi: 10.1161/HYPERTENSIONAHA.115.06398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pase MP, Himali JJ, Mitchell GF, Beiser A, Maillard P, Tsao C, Larson MG, DeCarli C, Vasan RS, Seshadri S. Association of aortic stiffness with cognition and brain aging in young and middle-aged adults: The Framingham Third Generation Cohort Study. Hypertension. 2016;67:513–519. doi: 10.1161/HYPERTENSIONAHA.115.06610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hajjar I, Goldstein FC, Martin GS, Quyyumi AA. Roles of arterial stiffness and blood pressure in hypertension-associated cognitive decline in healthy adults. Hypertension. 2016;67:171–175. doi: 10.1161/HYPERTENSIONAHA.115.06277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pase MP, Beiser A, Himali JJ, Tsao C, Satizabal CL, Vasan RS, Seshadri S, Mitchell GF. Aortic stiffness and the risk of incident mild cognitive impairment and dementia. Stroke. 2016;47:2256–2261. doi: 10.1161/STROKEAHA.116.013508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Woodard T, Sigurdsson S, Gotal JD, Torjesen AA, Inker LA, Aspelund T, Eiriksdottir G, Gudnason V, Harris TB, Launer LJ, Levey AS, Mitchell GF. Mediation analysis of aortic stiffness and renal microvascular function. J Am Soc Nephrol. 2015;26:1181–1187. doi: 10.1681/ASN.2014050450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ford ML, Tomlinson LA, Chapman TP, Rajkumar C, Holt SG. Aortic stiffness is independently associated with rate of renal function decline in chronic kidney disease stages 3 and 4. Hypertension. 2010;55:1110–1115. doi: 10.1161/HYPERTENSIONAHA.109.143024. [DOI] [PubMed] [Google Scholar]
- 55.Hashimoto J, Ito S. Aortic blood flow reversal determines renal function: potential explanation for renal dysfunction caused by aortic stiffening in hypertension. Hypertension. 2015;66:61–67. doi: 10.1161/HYPERTENSIONAHA.115.05236. [DOI] [PubMed] [Google Scholar]


