Abstract
Mucopolysaccharidoses (MPS) are rare, inherited, lysosomal storage diseases that cause accumulation of glycosaminoglycans, resulting in anatomic abnormalities and organ dysfunction that can increase the risk of anesthesia complications. We conducted a systematic review of the literature in order to describe the anesthetic management and perioperative outcomes in patients with MPS. We reviewed English-language literature search using an OVID-based search strategy of the following databases: 1) PubMed (1946-present), 2) Medline (1946-present), 3) EMBASE (1946-present), and 4) Web of Science (1946-present), using the following search terms: Mucopolysaccharidosis, Hurler, Scheie, Sanfilippo, Morquio, Maroteaux, anesthesia, perioperative, intubation, respiratory insufficiency, and airway. The review of the literature revealed nine case series and 27 case reports. A substantial number of patients have facial and oral abnormalities posing various challenges for airway management, however, evolving new technologies that include videolaryngoscopy appears to substantially facilitate airway management in these patients. The only type of MPS that appears to have less difficulty with airway management are MPS III patients, as the primary site of glycosaminoglycan deposition is in the central nervous system. All other MPS types have facial and oral characteristics that increase the risk of airway management. To mitigate these risks, anesthesia should be conducted by experienced anesthesiologists with expertise in using of advanced airway intubating devices.
Keywords: General anesthesia, lysosomal storage diseases, tracheal intubation, laryngoscopy
INTRODUCTION
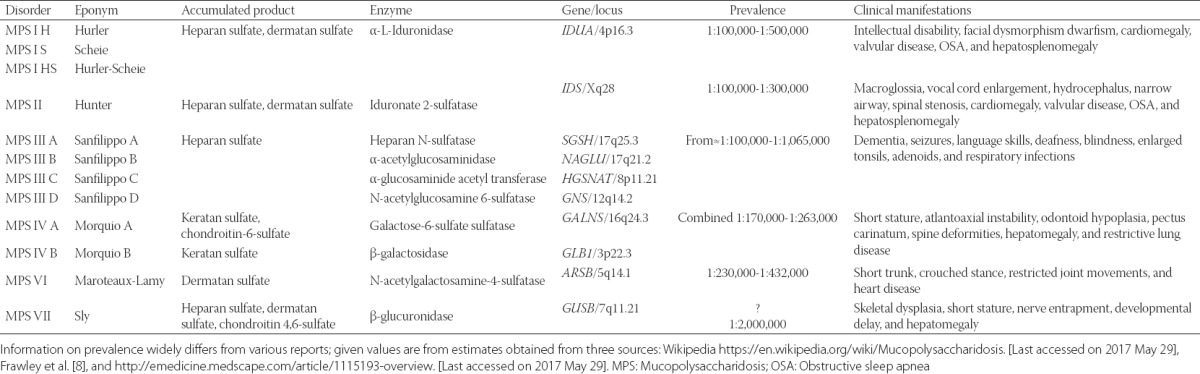
Mucopolysaccharidoses (MPS) are rare, inherited, lysosomal storage diseases characterized by deficiencies in 11 different lysosomal enzymes involved in the metabolism of glycosaminoglycans, previously known as mucopolysaccharides. These enzyme deficiencies result in progressive, widespread accumulation of partially degraded glycosaminoglycans in the lysosomes of various tissues and organs; the characteristic patterns of accumulation form the basis of MPS classification into seven types of progressive MPS diseases (Table 1) [1-5]. With the exception of MPS II which is inherited as an X-linked recessive disorder, all other MPS disorders are inherited in an autosomal recessive pattern; therefore, affecting males and females equally [1]. MPS can be grouped into four broad categories according to their dominant clinical features: 1) MPS I, II, and VII affect soft tissue storage and the skeleton with or without brain disease; 2) MPS VI affects both soft tissues and the skeleton; 3) MPS IVA, IVB are primarily associated with skeletal disorders; and 4) MPS III A-D primarily with central nervous system disorders. Table 1 summarizes enzymatic defects, prevalence, and clinical presentation of various MPS types. The published prevalence estimates vary widely between different studies (http://emedicine.medscape.com/article/1115193-overviewaccessed May 29, 2017). Depending on MPS type, glycosaminoglycan accumulations can occur in various organs resulting in cardiovascular, pulmonary, gastrointestinal, neurologic, and musculoskeletal dysfunction [1]. Glycosaminoglycan accumulation in the upper airway results in hypertrophy of adenoids, tonsils, tongue, and laryngopharynx, which may all pose difficulty for anesthetic airway management. This is especially important because MPS patients frequently require surgical interventions with anesthesia. For example, one MPS I registry showed that 75% of patients underwent at least one procedure requiring anesthesia [6]. The Hunter Outcome Survey that included 527 patients with MPS II reported that 83.7% of patients required a surgical intervention at some point [7].
TABLE 1.
Genotype, phenotype, and clinical manifestations of patients with MPS
Because altered anatomy of the airway and facial structures can complicate airway management [2-5], both mask ventilation and endotracheal intubation, the primary aim of the present study was to perform a comprehensive systematic review of the literature and summarize the published experience of airway management in patients with MPS.
MATERIALS AND Methods
We reviewed the literature for reports of perioperative course and airway-related anesthetic complications in patients with MPS. We conducted an English language literature search using an OVID-based search strategy of the following databases: 1) PubMed (1946-present), 2) Medline (1946-present), 3) EMBASE (1946-present), and 4) Web of Science (1946-present). We used the following search terms: Mucopolysaccharidosis, Hurler, Scheie, Sanfilippo, Morquio, Maroteaux, anesthesia, perioperative, intubation, respiratory insufficiency, and airway. Reference lists of identified reports were searched for additional relevant publications.
RESULTS
The systematic review of the literature identified nine case series, and their airway management is summarized in Table 2 [5,8-15]. In addition, we identified 27 individual case reports and these patients’ characteristics and their airway management is summarized in Tables 3 and 4 [7-10,16-38]. Figure 1 is a pie chart that summarizes airway management in a case series of MPS patients who underwent anesthesia at Mayo Clinic between years 2000 and 2015, which was reported in the Canadian Journal of Anaesthesia [15]. In that report, we described 18 MPS patients who underwent 49 procedures (there were 2, 1, 4, 7, and 4 patients with MPS Type I, II, III, IV, and VI, respectively) [15]. Finally, there is an isolated description of a 5-year-old boy with MPS II who underwent an inguinal hernia repair under a spinal anesthetic out of concern that the airway management would be difficult [39].
TABLE 2.
Outcomes of airway management in various MPS phenotypes from case series
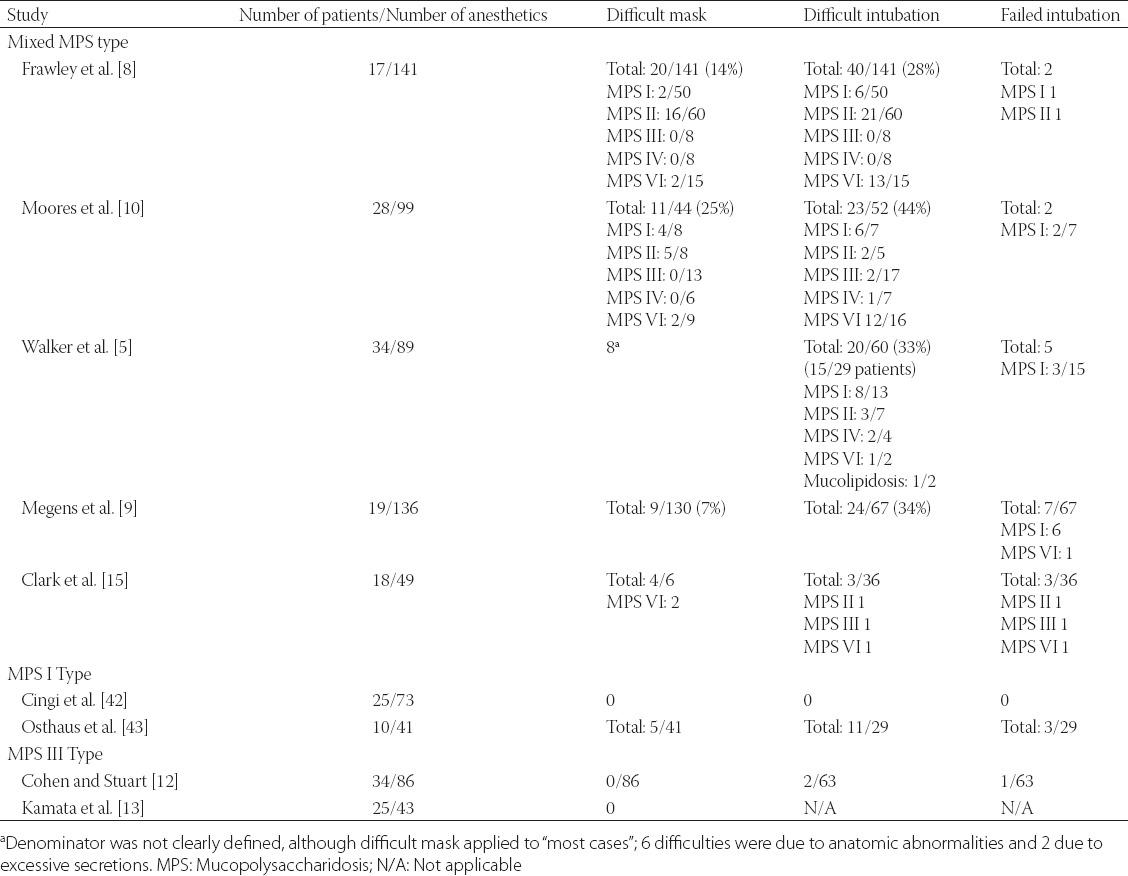
TABLE 3.
Airway characteristics in patients with MPS I and II with details of airway management from individual case reports

TABLE 4.
Airway characteristics in patients with MPS IV and MPS of unknown type with details of airway management from individual case reports
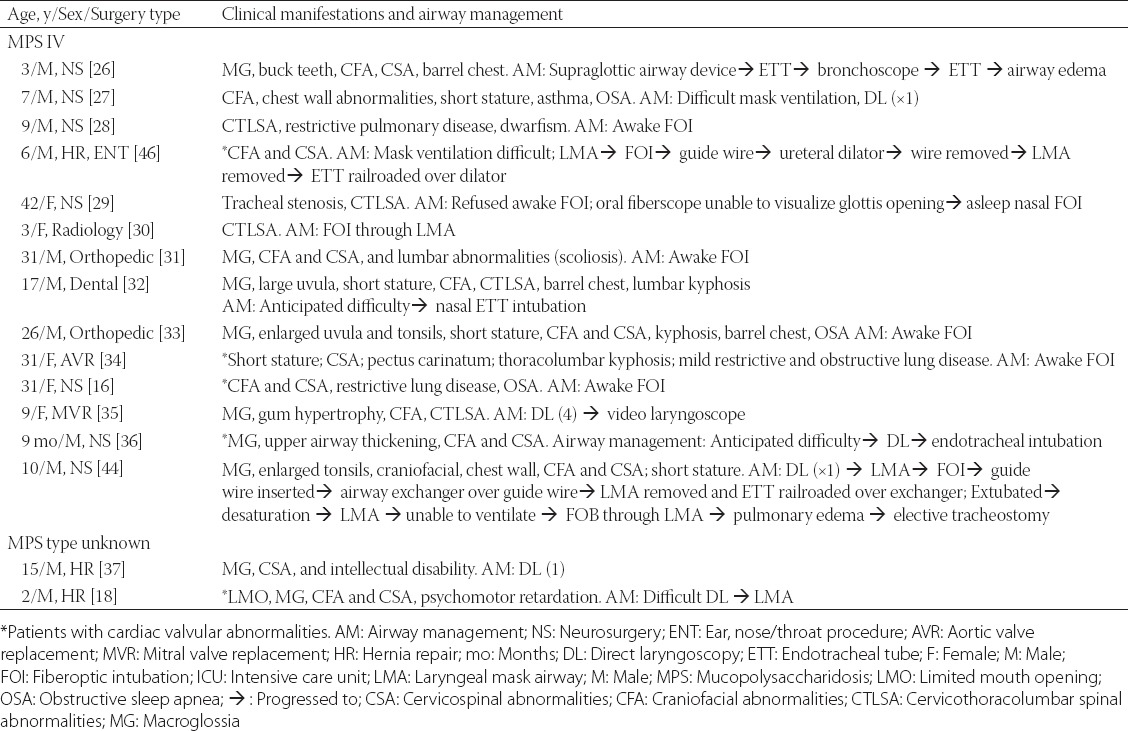
FIGURE 1.
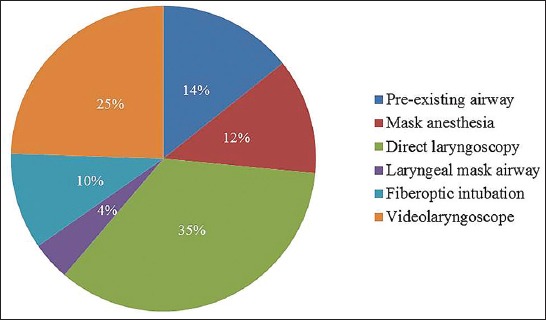
Anesthetic airway management in a series of 49 Mayo Clinic patients with various types of mucopolysaccharoidoses.
DISCUSSION
Patients with MPS have multiple comorbidities, many of which require surgical interventions. Because MPS is associated with specific phenotypic facial and airway characteristics, substantial challenges for perioperative airway management may be expected. Such challenges were confirmed in our recently reported MPS case series [15], as well as in earlier reports [3,5,8,9]. In addition, failed tracheal intubations requiring emergency tracheostomy have also been reported [3,5,8].
In two case series difficulty with mask ventilation has ranged to 7% and 14% of MPS patients [8,9]. Furthermore, our literature review suggests that mask ventilation is less likely encountered in MPS III patients. Kamata et al. [13] reported no difficulties in mask ventilation in a cohort of MPS III patients, albeit mild upper airway obstruction was noted during 14 procedures (33%), which was resolved with simple head/jaw maneuvering/positioning. Another review of a large MPS III series reported no problems with mask ventilation in 86 anesthetics [12]. All nine MPS III patients in our recent series from Mayo Clinic had uneventful mask ventilation during anesthetic induction [15].
The overall incidence of difficult tracheal intubation, in case series of various types of MPS, ranges between 28% and 44% [5,8,9]. Management of endotracheal intubations in MPS III patients appears to be less difficult. Specifically, Cingi et al. [14] reported 25 children with MPS III who underwent 73 anesthetic with no case of difficult intubation, and all intubations views were graded as Cormack-Lehane 1-2. This may not be surprising because MPS III is associated mostly with central nervous system disorders and less with glycosaminoglycan accumulation in oral soft tissues. Mayo Clinic experience with MPS patients since year 2000 includes 18 MPS patients who underwent 49 procedures (Figure 1) [15]. In seven instances, the patients presented for surgical procedures were already tracheally intubated or presented with tracheostomy (all were MPS IV and VI). Six anesthetics were conducted with mask ventilation as a primary airway management, and ventilation was difficult in two patients on two occasions (both were Type IV MPS [Morquio syndrome]). In 15 procedures, tracheal intubation was electively secured with either fiberoptic intubation or videolaryngoscope (VLG), and VLG was used as a rescue technique in two additional patients who failed the initial planned approach. In 19/36 (53%) procedures, airway management was successful with primary planned approach: direct laryngoscopy or laryngeal mask supraglottic airway. In our series of patients, there were nine patients with MPS III, and while all were “easy masks” one was describe as “difficult direct laryngoscopy”, suggesting that even i
n this MPS group caution should be exercised when managing the airway. Facial characteristics of one our patient with MPS VI (known as Maroteaux-Lamy syndrome) was deceiving, as it did not predict difficult mask ventilation (Figure 2). This 15-year-old female had obstructive sleep apnea, maxillary hypoplasia, high-arched palate, macroglossia, narrow hypopharynx, and compromise of the cervical spinal cord at the foramen magnum. Mask ventilation required two hands and a jaw thrust. Three attempts to place a laryngeal mask airway failed. Endotracheal intubation was successful with a VLG, albeit aided by a fiberoptic bronchoscope. The fiberoptic bronchoscope was used by a second anesthesia provider to locate the glottic opening, as VLG provided only a view of the epiglottis. After inserting the fiberoptic bronchoscope through the glottis opening, our endotracheal tube was guided over the scope into the trachea. In our report, 16.7% (3/18) patients had a true difficult airway (failed primary technique) [15]. This percentage likely represents an underestimate of difficult airway in MPS patients because anesthesiologists electively used a fiberoptic bronchoscope or VLG in 15 out of 36 procedures, suggesting a concern for potential difficult intubation. VLG-assisted intubations have been introduced only recently as an alternative intubation method, and in our series of MPS patients we found that since its introduction in 2009, the majority of cases were intubated using VLG, and all attempts were successful [15]. Theroux et al. [40] retrospectively examined intubations of 28 MPS patients undergoing 108 anesthetics and similarly observed that VLG became a preferred method for tracheal intubation for MPS patients. Megens et al. [9] reviewed the success rate of tracheal intubation using different tools: direct laryngoscopy was difficult in 16 out of 55 anesthetics, VLG was successful in 8 out of 9 anesthetics, and fiberoptic intubations were performed without difficulty in only 2 out of 10 cases. With the more widespread availability of VLG, this technique may become a preferred technique for endotracheal intubation in patients with MPS.
FIGURE 2.
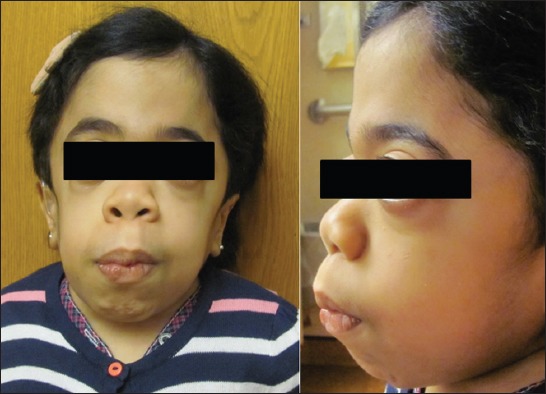
(A and B) A 15-year-old girl with mucopolysaccharidosis Type VI. Despite the fact that her facial characteristics gave impression that her airway is “manageable,” she had difficult mask ventilation, and 3 failed attempts to place a laryngeal mask airway. Placement of endotracheal tube was successful with a video laryngoscope aided by fiberoptic bronchoscope (see discussion for details). Published with the consent of patient’s legal representative.
Besides various airway issues, patients with MPS have other comorbidities that may have an impact on ventilation. Specifically, they frequently have restrictive or obstructive lung disease, recurrent lung infections, and obstructive sleep apnea [5]. Propensities for bronchospasm and oxyhemoglobin desaturation may complicate airway management in MPS patients. Furthermore, skeletal dysplasia such as atlantoaxial instability, spinal cord compression, limited neck mobility, pectus carinatum, and scoliosis is common [16]. Finally, there may be a high degree of tracheal narrowing in patients with MPS IV A (Morquio A). Evaluation of 28 MPS IV A patients with sagittal magnetic resonance imaging (MRI) scans found 68% had at least 25% tracheal narrowing and 29% had >75% narrowing due to a combination of a narrow thoracic inlet, tracheal growth, and tortuous brachiocephalic artery [41].
CONCLUSION
Patients with MPS have multiple comorbidities requiring frequent surgical procedures with anesthesia. With an exception of MPS III, all other MPS types have facial and airway characteristics which may create a challenge for anesthetic airway management. To mitigate the risks of airway mismanagement in patients with MPS, anesthetic planning should include experienced anesthesiologists and expertise in using a full array of advanced airway devices.
ACKNOWLEDGMENTS
This project was supported by the Department of Anesthesiology and Perioperative Medicine, College of Medicine, Mayo Clinic, Rochester, MN 55905, USA.
DECLARATION OF INTERESTS
The authors declare no conflict of interests.
REFERENCES
- 1.Muenzer J. Overview of the mucopolysaccharidoses. Rheumatology (Oxford) 2011;50(Suppl 5):4–12. doi: 10.1093/rheumatology/ker394. https://doi.org/10.1093/rheumatology/ker394. [DOI] [PubMed] [Google Scholar]
- 2.Baines D. Suxamethonium in mucopolysaccharidosis. Anaesth Intensive Care. 1989;17(3):382. [PubMed] [Google Scholar]
- 3.Kempthorne PM, Brown TC. Anaesthesia and the mucopolysaccharidoses: A survey of techniques and problems. Anaesth Intensive Care. 1983;11(3):203–7. doi: 10.1177/0310057X8301100304. [DOI] [PubMed] [Google Scholar]
- 4.King DH, Jones RM, Barnett MB. Anaesthetic considerations in the mucopolysaccharidoses. Anaesthesia. 1984;39(2):126–31. doi: 10.1111/j.1365-2044.1984.tb09499.x. https://doi.org/10.1111/j.1365-2044.1984.tb09499.x. [DOI] [PubMed] [Google Scholar]
- 5.Walker R, Belani KG, Braunlin EA, Bruce IA, Hack H, Harmatz PR, et al. Anaesthesia and airway management in mucopolysaccharidosis. J Inherit Metab Dis. 2013;36(2):211–9. doi: 10.1007/s10545-012-9563-1. https://doi.org/10.1007/s10545-012-9563-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arn P, Wraith JE, Underhill L. Characterization of surgical procedures in patients with mucopolysaccharidosis Type I: Findings from the MPS I Registry. J Pediatr. 2009;154(6):859-64–e3. doi: 10.1016/j.jpeds.2008.12.024. https://doi.org/10.1016/j.jpeds.2008.12.024. [DOI] [PubMed] [Google Scholar]
- 7.Mendelsohn NJ, Harmatz P, Bodamer O, Burton BK, Giugliani R, Jones SA, et al. Importance of surgical history in diagnosing mucopolysaccharidosis Type II (Hunter syndrome): Data from the hunter outcome survey. Genet Med. 2010;12(12):816–22. doi: 10.1097/GIM.0b013e3181f6e74d. https://doi.org/10.1111/j.1460-9592.2012.03825.x. [DOI] [PubMed] [Google Scholar]
- 8.Frawley G, Fuenzalida D, Donath S, Yaplito-Lee J, Peters H. A retrospective audit of anesthetic techniques and complications in children with mucopolysaccharidoses. Paediatr Anaesth. 2012;22(8):737–44. doi: 10.1111/j.1460-9592.2012.03825.x. https://doi.org/10.1111/j.1460-9592.2012.03825.x. [DOI] [PubMed] [Google Scholar]
- 9.Megens JH, de Wit M, van Hasselt PM, Boelens JJ, van der Werff DB, de Graaff JC. Perioperative complications in patients diagnosed with mucopolysaccharidosis and the impact of enzyme replacement therapy followed by hematopoietic stem cell transplantation at early age. Paediatr Anaesth. 2014;24(5):521–7. doi: 10.1111/pan.12370. https://doi.org/10.1111/pan.12370. [DOI] [PubMed] [Google Scholar]
- 10.Moores C, Rogers JG, McKenzie IM, Brown TC. Anaesthesia for children with mucopolysaccharidoses. Anaesth Intensive Care. 1996;24(4):459–63. doi: 10.1177/0310057X9602400408. [DOI] [PubMed] [Google Scholar]
- 11.Cohen-Levy J. Orthodontic treatments of pediatric obstructive sleep apnea syndrome. Med Sommeil. 2011;8(2):61–8. https://doi.org/10.1016/j.msom.2011.03.001. [Google Scholar]
- 12.Cohen MA, Stuart GM. Delivery of anesthesia for children with Mucopolysaccharidosis Type III (Sanfilippo syndrome): A review of 86 anesthetics. Paediatr Anaesth. 2017;27(4):363–9. doi: 10.1111/pan.13075. https://doi.org/10.1111/pan.13075. [DOI] [PubMed] [Google Scholar]
- 13.Kamata M, McKee C, Truxal KV, Flanigan KM, McBride KL, Aylward SC, et al. General anesthesia with a native airway for patients with mucopolysaccharidosis Type III. Paediatr Anaesth. 2017;27(4):370–6. doi: 10.1111/pan.13108. https://doi.org/10.1111/pan.13108. [DOI] [PubMed] [Google Scholar]
- 14.Cingi EC, Beebe DS, Whitley CB, Belani KG. Anesthetic care and perioperative complications in children with Sanfilipo Syndrome Type A. Paediatr Anaesth. 2016;26(5):531–8. doi: 10.1111/pan.12876. https://doi.org/10.1111/pan.12876. [DOI] [PubMed] [Google Scholar]
- 15.Clark BM, Sprung JJ, Weingarten TN, Warner ME. Airway management changes in patients with mucopolysaccharidoses: The role of videolaryngoscopy. Can J Anaesth 2017. doi: 10.1007/s12630-017-0906-0. [Epub ahead of print] https://doi.org/10.1007/s12630-017-0906-0. [DOI] [PubMed] [Google Scholar]
- 16.Cade J, Jansen N. Anesthetic challenges in an adult with mucopolysaccharidosis Type VI. A&A Case Rep. 2014;2(12):152–4. doi: 10.1213/XAA.0000000000000031. https://doi.org/10.1213/XAA.0000000000000031. [DOI] [PubMed] [Google Scholar]
- 17.Gurumurthy T, Shailaja S, Kishan S, Stephen M. Management of an anticipated difficult airway in Hurler’s syndrome. J Anaesthesiol Clin Pharmacol. 2014;30(4):558–61. doi: 10.4103/0970-9185.142862. https://doi.org/10.4103/0970-9185.142862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ziyaeifard M, Azarfarin R, Ferasatkish R, Dashti M. Management of difficult airway with laryngeal mask in a child with mucopolysaccharidosis and mitral regurgitation: A case report. Res Cardiovasc Med. 2014;3:2–e17456. doi: 10.5812/cardiovascmed.17456. https://doi.org/10.5812/cardiovascmed.17456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nadeem A, Siddiqui K. Anaesthesia challenges in a patient with Hurler syndrome: A case report. Anaesth Pain Intensive Care. 2012;16(1):100–1. [Google Scholar]
- 20.Gupta N, Rath GP, Bala R, Reddy BK, Chaturvedi A. Anesthetic management in children with Hurler’s syndrome undergoing emergency ventriculoperitoneal shunt surgery. Saudi J Anaesth. 2012;6(2):178–80. doi: 10.4103/1658-354X.97036. https://doi.org/10.4103/1658-354X.97036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ard JL, Jr, Bekker A, Frempong-Boadu AK. Anesthesia for an adult with mucopolysaccharidosis I. J Clin Anesth. 2005;17(8):624–6. doi: 10.1016/j.jclinane.2005.01.012. https://doi.org/10.1016/j.jclinane.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 22.Walker RW, Dearlove OR. Anaesthesia for children with mucopolysaccharidoses. Anaesth Intensive Care. 1997;25(2):197–8. [PubMed] [Google Scholar]
- 23.Wilder RT, Belani KG. Fiberoptic intubation complicated by pulmonary edema in a 12-year-old child with Hurler syndrome. Anesthesiology. 1990;72(1):205–7. doi: 10.1097/00000542-199001000-00033. https://doi.org/10.1097/00000542-199001000-00033. [DOI] [PubMed] [Google Scholar]
- 24.Busoni P, Fognani G. Failure of the laryngeal mask to secure the airway in a patient with Hunter’s syndrome (mucopolysaccharidosis Type II) Paediatr Anaesth. 1999;9(2):153–5. doi: 10.1046/j.1460-9592.1999.9220289.x. https://doi.org/10.1046/j.1460-9592.1999.9220289.x. [DOI] [PubMed] [Google Scholar]
- 25.Kaur J, Swami AC, Kumar A, Lata S. Anesthetic management of a child with Hunter’s syndrome. J Anaesthesiol Clin Pharmacol. 2012;28(2):255–7. doi: 10.4103/0970-9185.94914. https://doi.org/10.4103/0970-9185.94914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dhanger S, Adinarayanan S, Vinayagam S, Kumar MP. I-gel assisted fiberoptic intubation in a child with Morquio’s syndrome. Saudi J Anaesth. 2015;9(2):217–9. doi: 10.4103/1658-354X.152893. https://doi.org/10.4103/1658-354X.152893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morgan KA, Rehman MA, Schwartz RE. Morquio’s syndrome and its anaesthetic considerations. Paediatr Anaesth. 2002;12(7):641–4. doi: 10.1046/j.1460-9592.2002.00838.x. https://doi.org/10.1046/j.1460-9592.2002.00838.x. [DOI] [PubMed] [Google Scholar]
- 28.Bartz HJ, Wiesner L, Wappler F. Anaesthetic management of patients with mucopolysaccharidosis IV presenting for major orthopaedic surgery. Acta Anaesthesiol Scand. 1999;43(6):679–83. doi: 10.1034/j.1399-6576.1999.430614.x. https://doi.org/10.1034/j.1399-6576.1999.430614.x. [DOI] [PubMed] [Google Scholar]
- 29.Nielsen RM, Pedersen NA, Olsen KS. Airway management in a patient with Morquio-Brailsford syndrome. Eur J Anaesthesiol. 2013;30(3):133–4. doi: 10.1097/EJA.0b013e32835c8dc5. https://doi.org/10.1097/EJA.0b013e32835c8dc5. [DOI] [PubMed] [Google Scholar]
- 30.Chaudhuri S, Duggappa AK, Mathew S, Venkatesh S. Safe intubation in Morquio-Brailsford syndrome: A challenge for the anesthesiologist. J Anaesthesiol Clin Pharmacol. 2013;29(2):258–61. doi: 10.4103/0970-9185.111666. https://doi.org/10.4103/0970-9185.111666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kadic L, Driessen JJ. General anaesthesia in an adult patient with Morquio syndrom with emphasis on airway issues. Bosn J Basic Med Sci. 2012;12(2):130–3. doi: 10.17305/bjbms.2012.2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shivajirao TP, Wasmatkar NP, Gore PG, Lakhe JN, Vinayak SR. Anesthetic considerations in Morquio syndrome: A case report. Anaest Pain Intensive Care. 2013;17(1):75–8. [Google Scholar]
- 33.McLaughlin AM, Farooq M, Donnelly MB, Foley K. Anaesthetic considerations of adults with Morquio’s syndrome - A case report. BMC Anesthesiol. 2010;6:10–2. doi: 10.1186/1471-2253-10-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pagel PS, Almassi GH. Perioperative implications of Morquio syndrome in a 31-year-old woman undergoing aortic valve replacement. J Cardiothorac Vasc Anesth. 2009;23(6):855–7. doi: 10.1053/j.jvca.2008.12.009. https://doi.org/10.1053/j.jvca.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 35.Sayilgan C, Yuceyar L, Akbas S, Erolcay H. Anesthesia in a child with Maroteaux-Lamy syndrome undergoing mitral valve replacement. Clinics (Sao Paulo) 2012;67(6):693–6. doi: 10.6061/clinics/2012(06)26. https://doi.org/10.6061/clinics/2012(06)26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Suh SH, Okutani R, Nakasuji M, Nakata K. Anesthesia in a patient with mucopolysaccharidosis Type VI (Maroteaux-Lamy syndrome) J Anesth. 2010;24(6):945–8. doi: 10.1007/s00540-010-1029-8. https://doi.org/10.1007/s00540-010-1029-8. [DOI] [PubMed] [Google Scholar]
- 37.Barbosa FT, Borges EL, Brandão RR. General anesthesia after failed spinal block for emergency surgery in a patient with mucopolysaccharidosis: Case report. [Article in Portuguese] Rev Bras Anestesiol. 2007;57(6):658–64. doi: 10.1590/s0034-70942007000600008. [DOI] [PubMed] [Google Scholar]
- 38.Arn P, Whitley C, Wraith JE, Webb HW, Underhill L, Rangachari L, et al. High rate of postoperative mortality in patients with mucopolysaccharidosis I: Findings from the MPS I Registry. J Pediatr Surg. 2012;47(3):477–84. doi: 10.1016/j.jpedsurg.2011.09.042. https://doi.org/10.1016/j.jpedsurg.2011.09.042. [DOI] [PubMed] [Google Scholar]
- 39.Kumar KR, Kumar H, Baidya DK, Arora MK. Successful use of spinal anesthesia for inguinal hernia repair in a child with Hunter syndrome with difficult airway. J Clin Anesth. 2016;30:99–100. doi: 10.1016/j.jclinane.2015.08.025. https://doi.org/10.1016/j.jclinane.2015.08.025. [DOI] [PubMed] [Google Scholar]
- 40.Theroux MC, Nerker T, Ditro C, Mackenzie WG. Anesthetic care and perioperative complications of children with Morquio syndrome. Paediatr Anaesth. 2012;22(9):901–7. doi: 10.1111/j.1460-9592.2012.03904.x. https://doi.org/10.1111/j.1460-9592.2012.03904.x. [DOI] [PubMed] [Google Scholar]
- 41.Tomatsu S, Averill LW, Sawamoto K, Mackenzie WG, Bober MB, Pizarro C, et al. Obstructive airway in Morquio A syndrome, the past, the present and the future. Mol Genet Metab. 2016;117(2):150–6. doi: 10.1016/j.ymgme.2015.09.007. https://doi.org/10.1016/j.ymgme.2015.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cingi E, Belani K, Beebe D, Apostolidou I, Whitley C. Anesthetic care and outcome in children with Sanfilippo syndrome type A. Mol Gen Metab. 2013;108(2):S30. http://dx.doi.org/10.1016/j.ymgme.2012.11.056. [Google Scholar]
- 43.Osthaus WA, Harendza T, Witt LH, Jüttner B, Dieck T, Grigull L, et al. Paediatric airway management in mucopolysaccharidosis 1: A retrospective case review. Eur J Anaesthesiol. 2012;29(4):204–7. doi: 10.1097/EJA.0b013e328350677b. https://doi.org/10.1097/EJA.0b013e328350677b. [DOI] [PubMed] [Google Scholar]
- 44.Walker PP, Rose E, Williams JG. Upper airways abnormalities and tracheal problems in Morquio’s disease. Thorax. 2003;58(5):458–9. doi: 10.1136/thorax.58.5.458. https://doi.org/10.1136/thorax.58.5.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hack HA, Walker R, Gardiner P. Anaesthetic implications of the changing management of patients with mucopolysaccharidosis. Anaesth Intensive Care. 2016;44(6):660–8. doi: 10.1177/0310057X1604400612. [DOI] [PubMed] [Google Scholar]
- 46.Walker RW, Allen DL, Rothera MR. A fibreoptic intubation technique for children with mucopolysaccharidoses using the laryngeal mask airway. Paediatr Anaesth. 1997;7(5):421–6. doi: 10.1046/j.1460-9592.1997.d01-102.x. https://doi.org/10.1046/j.1460-9592.1997.d01-102.x. [DOI] [PubMed] [Google Scholar]


