Abstract
Structural and functional changes in platelets during storage can lead to the loss of platelet reactivity and response. Our aim was to evaluate leukocyte-depleted platelet concentrates on storage days 0, 3 and 5, obtained by in-line filtration. In non-filtered platelet concentrates (NF-PC) group, 180 whole blood units were collected with quadruple blood bags and then compared to another group of 180 whole blood units (leukocyte-depleted platelet concentrates [LD-PC]), collected in Imuflex Whole Blood Filter Saving Platelets (WB-SP) bags with an integrated leukoreduction filter, with regard to the platelet quality and characteristics. The efficacy of the two techniques for platelet concentrate preparation was evaluated by white blood cell (WBC) and platelet count on day 0. The partial pressure of oxygen (pO2), pH, platelets positive for P-selectin (CD62P), CD63, cluster of differentiation 42b (CD42b), phosphatidylserine (PS), and mitochondrial membrane potential (MMP) were analyzed during the storage in both groups. A significantly lower WBC count and higher platelet count was observed in LD-PC compared to NF-PC group, indicating the overall efficacy of the first technique. During the 5-day storage, pH and pO2 decreased in both groups. In LD-PC group, higher pH, increased pO2 and decreased platelet surface expression of CD62P, CD63 and PS were observed compared to NF-PC group. In both groups, the percentage of CD42b positive platelets and MMP did not change significantly during the 5-day period. The assessment of different markers of platelet activation may be an effective tool in evaluating the quality of platelets during storage. A better understanding of platelet activation may provide new insights for developing a novel therapeutic approach in the manipulation of platelet aggregation.
Keywords: Platelet storage, platelet activation markers, leukocyte depletion, in-line filtration
INTRODUCTION
Platelet transfusion is an important step in the treatment of thrombocytopenic patients who suffer from severe bleeding. Although platelet transfusions are extensively used across the world, improving their safety and efficacy remains the primary goal in patients with high risk of bleeding. For optimal results, platelet activation is minimized prior to transfusion, to prevent deleterious changes in platelets [1,2]. During the storage (usually 4-7 days), different biochemical, morphological and functional changes of platelets, known as platelet storage lesion (PSL), may occur, as well as platelet activation and secretion of proinflammatory factors [3]. These changes lead to decreased efficacy of platelet transfusion and may contribute to the development of adverse reactions in the recipients [4].
Platelet concentrates (PCs) can be obtained by apheresis, or derived from the platelet-rich-plasma (PRP) or buffy-coat from the whole blood, with the third method being more common in Europe [5]. The quality of PCs may be impaired during the preparation and storage, as well as by the contamination of white blood cells (WBCs). In addition, due to the risk of bacterial contamination, platelet storage is often limited to a maximum of 5 days [6]. Previous studies also indicated that leukocytes in PCs could mediate adverse effects such as human leukocyte antigen (HLA) alloimmunization, graft-versus-host disease, and febrile reactions [7]. Moreover, functional changes of platelets have been associated with elevated basal levels of platelet activation [8].
Different methods for pathogen reduction and leukocyte depletion in PCs produce platelets that vary greatly in their quality. Thus, a proper and early detection of PSL in platelets remains one of the most important aspects of platelet transfusion [9]. In this study, we evaluated the efficacy of an in-line filtration method for obtaining PCs compared to the standard method. The platelet quality and characteristics were analyzed during 5-day storage.
MATERIALS AND METHODS
Preparation and storage of PCs
Whole blood (450 ± 45 ml), obtained from donors who had not taken any antiplatelet drugs for two weeks prior to donation, was stored 2-3 hours at room temperature (day 0) prior to further processing. All samples showed no reactivity for infectious disease markers (i.e. acquired immune deficiency syndrome [AIDS], syphilis, and hepatitis B and C infection), using serological screening tests (Biokit S.A, Llissá de Munt, Spain and Ortho Clinical Diagnostics, Inc., Raritan, NJ, USA). The collected blood units were randomly assigned to two groups: non-filtered platelet concentrates (NF-PC group) and leukocyte-depleted platelet concentrates (LD-PC group). In NF-PC group, 180 whole blood units were collected with quadruple blood bags (MacoPharma, Tourcoing, France), while in LD-PC group, another 180 whole blood units were collected in Imuflex Whole Blood Filter Saving Platelets (WB-SP) bags (Terumo Medical, Tokyo, Japan) with an integrated leukoreduction filter [10]. Both types of bags contained 63 ml of citrate phosphate dextrose (CPD) and 100 ml of saline-adenine-glucose-mannitol (SAGM) solutions.
The preparation of NF-PCs started within 6 hours following the collection of whole blood units. Thirty PCs were prepared from the 180 whole blood units. Each blood unit was centrifuged in a Cryofuge 8500i centrifuge (Heraeus, Langenselbold, Germany) at 3,890 × g for 10 minutes, at 20 ± 2°C and further processed using a T-ACE II+ Automatic Component Extractor (Terumo Medical, Tokyo, Japan). The separated buffy coat was left at 20 ± 2°C for 2 hours and then centrifuged again at 310 × g for 7 minutes, at 20 ± 2°C. Using the sterile connection system Terumo Sterile Tubing Welder SC-201A [TSCD] (Terumo Medical, Tokyo, Japan), six NF-PCs were pooled together and stored in a horizontal shaker (60 cycles/minute, Teknolabo A.S.S.I. S.r.l., Italy) at 20 ± 2°C for 5 days.
The whole blood from additional 180 donors was collected in Imuflex WB-SP bags for the preparation of 30 LD-PCs. The centrifugation, pooling and storage procedures were performed as described for NF-PCs.
Parameter analysis
The cell count in PCs was performed using a Beckman Coulter AcT Diff Hematology Analyzer (Beckman-Coulter, Fullerton, CA, USA). The contamination of WBCs in NF-PCs and LD-PCs was determined after the samples were diluted in Turk solution (1:10), by manual cell counting in a Nageotte hemocytometer (Hausser Scientific, Horsham, PA, USA), as described previously [11].
The partial pressure of oxygen (pO2) was determined using Cobas b 221 blood gas system (Roche Diagnostics, Rotkreuz, Switzerland), immediately after the preparation of PCs (day 0), as well as on storage day 3 and 5. Extracellular pH was evaluated on day 0, 3 and 5 using a FP20 pH meter (Mettler-Toledo GmbH, Schwerzenbach, Switzerland).
The percentages of platelets positive for P-selectin (CD62P; BD Biosciences, USA), surface glycoprotein gp53 (CD63; BD Biosciences, USA), platelet glycoprotein Ib alpha chain [GP Ib] (CD42b; BD Biosciences, USA), and phosphatidylserine [PS] (annexin V binding; BD Biosciences, USA) on day 0, 3 and 5 were evaluated by flow cytometry analysis, as described previously [12]. In brief, freshly collected platelets were diluted in phosphate-buffered saline (PBS) and adjusted to the final concentration of 1 × 106 cells/ml. The cells (1 × 106 per tube) were incubated with fluorescein isothiocyanate (FITC)-conjugated monoclonal antibodies (anti-CD62P, anti-CD63, and anti-CD42b) and annexin V, according to the manufacturer’s instructions. Control cells were incubated with FITC-conjugated IgG isotype control and run in parallel as negative controls. The labeled cells were fixed in 1% paraformaldehyde for 1 hour and 10000 cells/sample were analyzed on a Coulter Epics XL M CL flow cytometer (Beckman Coulter, Krefeld, Germany). For the flow cytometry analysis, a gate was set around the platelets, as described previously [13].
Changes in the mitochondrial membrane potential (MMP) in platelets were evaluated using the lipophilic cation Rhodamine 123, as previously described [14]. The fluorescence of Rhodamine 123 in the cells was determined by flow cytometry. For each sample, basal intensity values were subtracted from those obtained after different treatments and the results were presented as the ratio of mean fluorescence intensity.
Statistical analysis
SPSS for Windows, Version 15.0. (SPSS Inc., Chicago, Il, US) was used for statistical analysis. The results are presented as mean ± standard deviation (SD). Differences between groups were determined using Student’s t-test, Mauchly’s sphericity test, or analysis of variance (ANOVA) with repeated measurements, combined with Bonferroni’s post hoc test for multiple comparisons. A p value <0.05 was considered significant.
RESULTS
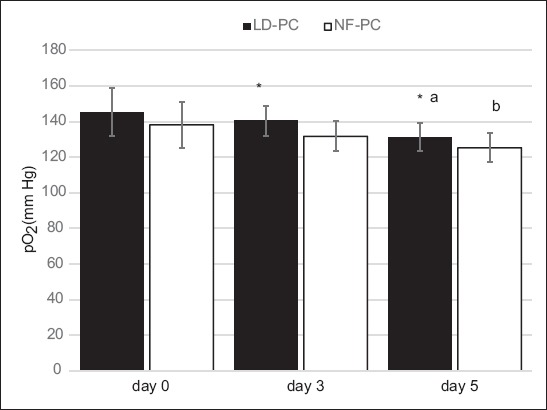
To evaluate the efficacy of the filter-based method for leukocyte depletion, we determined the total WBC and platelet count in NF-PCs and LD-PCs on day 0. A significantly lower WBC count (t = 16.693, p < 0.001) and higher platelet count (t = 7.862, p < 0.001) was observed in LD-PC (WBC count: 0.04 ± 0.02 × 106/unit; platelet count: 88.52 ± 24.31) compared to NF-PC group (WBC count: 5.18 ± 1.5 × 106/unit; platelet count: 49.27 ± 11.46), indicating the overall efficacy of the first technique. Furthermore, no significant changes were observed in the volumes of LD-PCs (64.97 ± 19.22) and NF-PCs [63.21 ± 17.34] (Table 1). During the 5-day storage, we observed decreased overall pO2 (for LD-PC χ2 = 311.006, p < 0.001; for NF-PC χ2 = 223.522, p < 0.001) and pH (for LD-PC χ2 = 306.046, p < 0.001; for NF-PC χ2 = 194.425, p < 0.001) in both groups (Figures 1 and 2, respectively). Nevertheless, on day 3 and 5, the level of pO2 was significantly higher in LD-PC compared to NF-PC group [for day 3 t = 8.544, p < 0.05; for day 5 t = 9.312, p < 0.05] (Figure 1).
TABLE 1.
WBC, platelet count, and volume of platelet concentrates (PCs) obtained with two different methods
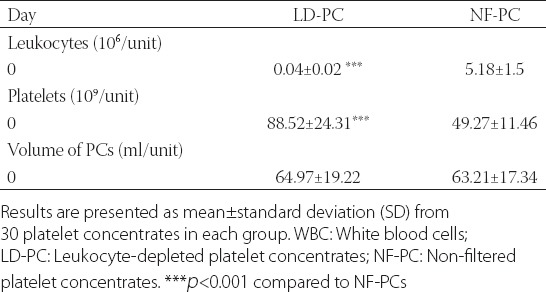
FIGURE 1.
Levels of partial pressure of oxygen (pO2) during 5-day storage in non-filtered (NF-PCs) and leukocyte-depleted platelet concentrates (LD-PCs). A decreased overall pO2 was observed in both groups over the 5-day period (ap<0.001 for LD-PC and bp<0.001 for NF-PC). Nevertheless, on day 3 and 5, the level of pO2 was significantly higher in LD-PC compared to NF-PC group (*p<0.05).
FIGURE 2.
Levels of pH during 5-day storage in non-filtered (NF-PCs) and leukocyte-depleted platelet concentrates (LD-PCs). Over the 5-day storage a decreased overall pH was observed in both groups (ap<0.001 for LD-PC group; bp<0.001 for NF-PC group).
The level of platelet activation during the 5-day storage was evaluated by determining the percentage of platelets positive for several platelet activation markers, using flow cytometry analysis. As shown in Table 2, the percentage of platelets expressing CD62P was significantly lower in LD-PC compared to NF-PC group on day 0 (t = 10.642, p < 0.05), 3 (t = 12.754, p < 0.05) and 5 (t = 10.724, p < 0.05). Similarly, a significantly lower percentage of CD63-positive platelets was observed in LD-PC compared to NF-PC group on day 0 (t = 8.226, p < 0.05), 3 (t = 7.852, p < 0.05) and 5 (t = 10.664, p < 0.05). We also observed a decrease in the percentage of CD42b-positive platelets during the 5-day storage in both groups, however, these changes were not statistically significant. In addition, the number of platelets expressing PS on the surface was significantly higher in NF-PC compared to LD-PC group, on day 0 (t = 7.342, p < 0.05), day 3 (t = 8.724, p < 0.05), and day 5 [t = 7.295, p < 0.05] (Table 2). In both groups, MMP did not change significantly during the 5-day period (p > 0.05, Figure 3).
TABLE 2.
Expression of activation markers on platelet surface during 5-day storage in non-filtered and leukocyte-depleted platelet concentrates
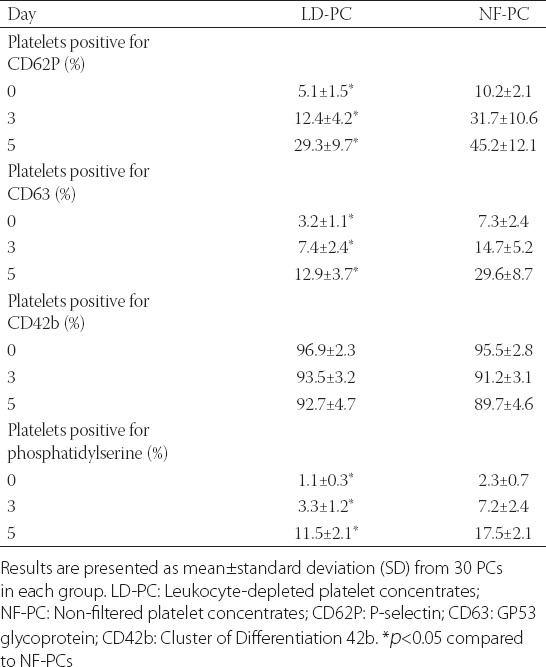
FIGURE 3.
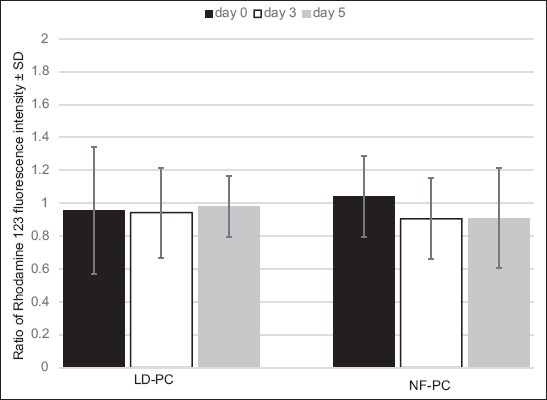
Changes in the mitochondrial membrane potential (MMP) during 5-day storage in non-filtered (NF-PCs) and leukocyte-depleted platelet concentrates (LD-PCs). In both groups, the MMP did not change significantly during the 5-day period. Results are presented as ratio of fluorescence intensity ± standard deviation (SD) of NF-PCs on day 0.
DISCUSSION
In the present study, we evaluated the efficacy of integrated leukoreduction filters for obtaining LD-PCs and analyzed the changes in the percentage of activated platelets during 5-day storage, compared to NF-PCs. We found that the number of WBCs in LD-PCs was significantly decreased compared to NF-PCs. The total number of WBCs was below 5 × 104 leukocytes/unit in the PCs obtained using the Imuflex WB-SP bags with integrated leukoreduction filters, which is below the limit recommended for WBC count in PCs to avoid adverse effects of platelet transfusion [7]. Furthermore, pH and pO2 decreased in both groups during the 5-day storage, with lower values for both parameters in NF-PC compared to LD-PC group, indicating that cells were more metabolically active in NF-PC group. As previously indicated, irreversible damage occurs in platelets below pH 6 [15]. Although we observed pH above 6 in both of our groups, on day 5, pH was 6.8 in NF-PC group which is the value of pH when platelets undergo morphological changes, such as from disk- to spherical-shaped platelets [16].
The increased oxygen consumption by the activated platelets, observed in our study, correlates with previous reports [17,18]. Energy production in platelets is based on the two major metabolic pathways, anaerobic glycolysis and oxidative phosphorylation [19]. In stored platelets, increased glycolysis leads to the accumulation of lactic acid and results in lower pH [20]. Furthermore, elevated oxygen consumption by platelets may be associated with mitochondrial dysfunction and increased mitochondrial membrane permeability to cytochrome c [21]. Overall, our results indicate decreased metabolic activity of LD-PCs compared to NF-PCs.
Flow cytometry is considered the gold standard for the evaluation of platelet activation [22]. Using flow cytometry, we showed that the percentage of platelets expressing CD62P and CD63 was significantly higher in NF-PC compared to LD-PC group. These findings are in line with earlier reports, which indicated that increased CD62P and CD63 expression in platelets is associated with PSL [1,23]. P-selectin (CD62P) is located in the membrane of α-granules in platelets, while CD63 is present in the membrane of dense granules [24]. Upon platelet activation, these proteins are translocated to the platelet surface, but the effect of this process on transfusion is still not clarified [23]. Generally, increased expression of platelet activation markers is observed in PCs without leukocyte depletion, due to the presence of WBCs and cytokines [25]. Although the number of CD42b positive platelets slightly decreased over the 5-day period, we did not observe a significant difference between the two groups. CD42b is a receptor for von Willebrand factor (VWF); the binding of CD42b to VWF initiates platelet deposition, and the complex is cleaved upon platelet activation [26]. In some studies, stabile CD42b expression was demonstrated during platelet storage [9,27], while in other studies, decreased CD42b expression was observed [28,29]. These differences in CD42b expression in platelets may be due to the differences in storage time, e.g., in our study the storage time was 5 days, while in other studies it was 12 and 14 days [28,29]. In addition, PS exposure, another marker of platelet activation and apoptosis [19], was significantly increased in NF-PC group, which is in agreement with previous studies [30,31]. However, the significance of this process remains to be clarified [9]. We also evaluated MMP in the two groups, as platelet apoptosis was previously associated with the loss of MMP and intrinsic apoptotic pathway [32]. The MMP remained stable in both of our groups over the 5-day storage. Nevertheless, despite the stability of MMP in our PCs, increased PS exposure alone remains an indicator of apoptosis in platelets, as shown in a previous study [33]. In agreement with our observations, another study indicated that, after stimulation of platelets with thrombin, platelet activation occurred before apoptotic changes [34].
One of the limitations of our study is that we did not analyze soluble mediators in PCs, which may provide an insight into the role of cytokines in platelet activation. Another limitation of our study is the relatively small number of analyzed PCs.
In summary, we showed that WB-SP bags with an integrated leukoreduction filter is an effective method for obtaining LD-PC. The removal of WBCs from PCs decreases platelet activation during storage. Proper detection of PSL is a crucial step in preventing adverse effects of transfusion.
DECLARATION OF INTERESTS
The authors declare no conflict of interests.
REFERENCES
- 1.Ohto H, Nollet KE. Overview on platelet preservation: Better controls over storage lesion. Transfus Apher Sci. 2011;44(3):321–5. doi: 10.1016/j.transci.2011.03.008. https://doi.org/10.1016/j.transci.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 2.Springer DL, Miller JH, Spinelli SL, Pasa-Tolic L, Purvine SO, Daly DS, et al. Platelet proteome changes associated with diabetes and during platelet storage for transfusion. J Proteome Res. 2009;8(5):2261–72. doi: 10.1021/pr800885j. https://doi.org/10.1021/pr800885j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heal JM, Phipps RP, Blumberg N. One big unhappy family: Transfusion alloimmunization, thrombosis, and immune modulation/inflammation. Transfusion. 2009;49(6):1032–6. doi: 10.1111/j.1537-2995.2009.02182.x. https://doi.org/10.1111/j.1537-2995.2009.02182.x. [DOI] [PubMed] [Google Scholar]
- 4.Slichter SJ, Corson J, Jones MK, Christoffel T, Pellham E, Bailey SL, et al. Exploratory studies of extended storage of apheresis platelets in a platelet additive solution (PAS) Blood. 2014;123(2):271–80. doi: 10.1182/blood-2013-05-501247. https://doi.org/10.1182/blood-2013-05-501247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andreu G, Vasse J, Sandid I, Tardivel R, Semana G. Use of random versus apheresis platelet concentrates. Transfus Clin Biol. 2007;14(6):514–21. doi: 10.1016/j.tracli.2008.01.004. https://doi.org/10.1016/j.tracli.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 6.Lannan KL, Refaai MA, Ture SK, Morrell CN, Blumberg N, Phipps RP, et al. Resveratrol preserves the function of human platelets stored for transfusion. Br J Haematol. 2016;172(5):794–806. doi: 10.1111/bjh.13862. https://doi.org/10.1111/bjh.13862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferrer F, Rivera J, Corral J, González-Conejero R, Vicente V. Evaluation of leukocyte-depleted platelet concentrates obtained by in-line filtration. Vox Sang. 2000;78(4):235–41. doi: 10.1159/000031187. https://doi.org/10.1046/j.1423-0410.2000.7840235.x. [DOI] [PubMed] [Google Scholar]
- 8.Apelseth TO, Hervig T. In vitro evaluation of platelet concentrates during storage: Platelet counts and markers of platelet destruction. Transfus Apher Sci. 2007;37(3):261–8. doi: 10.1016/j.transci.2007.02.006. https://doi.org/10.1016/j.transci.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 9.Tynngard N, Wallstedt M, Södergren AL, Faxälv L, Ramström S. Platelet adhesion changes during storage studied with a novel method using flow cytometry and protein-coated beads. Platelets. 2015;26(2):177–85. doi: 10.3109/09537104.2014.891728. https://doi.org/10.3109/09537104.2014.891728. [DOI] [PubMed] [Google Scholar]
- 10.Paunovic D, van der Meer P, Kjeldsen-Kragh J, Kekomaki R, Larsson S, Greppi N, et al. Multicenter evaluation of a whole-blood filter that saves platelets. Transfusion. 2004;44(8):1197–203. doi: 10.1111/j.1537-2995.2004.03350.x. https://doi.org/10.1111/j.1537-2995.2004.03350.x. [DOI] [PubMed] [Google Scholar]
- 11.Mokhtar MB, Hashim HB, Joshi SR. Assessment of quality of platelets preserved in plasma and platelet additive solution: A Malaysian experience. Asian J Transfus Sci. 2016;10(1):84–7. doi: 10.4103/0973-6247.172177. https://doi.org/10.4103/0973-6247.172177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Skripchenko A, Gelderman MP, Awatefe H, Turqeon A, Thompson-Montgomery D, Cheng C, et al. Automated cold temperature cycling improves platelet properties and in vivo recovery in a mouse model compared to continuous cold storage. Transfusion. 2016;56(1):24–32. doi: 10.1111/trf.13273. https://doi.org/10.1111/trf.13273. [DOI] [PubMed] [Google Scholar]
- 13.Hosseini E, Ghasemzadeh M, Nassaji F, Jamaat ZP. GPVI modulation during platelet activation and storage: Its expression levels and ectodomain shedding compared to markers of platelet storage lesion. Platelets. 2017;28(5):498–508. doi: 10.1080/09537104.2016.1235692. https://doi.org/10.1080/09537104.2016.1235692. [DOI] [PubMed] [Google Scholar]
- 14.Pavlovic V, Cekic S, Ciric M, Krtinic D, Jovanovic J. Curcumin attenuates Mancozeb-induced toxicity in rat thymocytes through mitochondrial pathway. Food Chem Toxicol. 2016;88:105–11. doi: 10.1016/j.fct.2015.12.029. https://doi.org/10.1016/j.fct.2015.12.029. [DOI] [PubMed] [Google Scholar]
- 15.Heddle NM, Barty RL, Sigouin CS, Boye DM, Nelson EJ, Blajchman MA, et al. In vitro evaluation of prestorage pooled leukoreduced whole bloodderived platelets stored for up to 7 days. Transfusion. 2005;45(6):904–10. doi: 10.1111/j.1537-2995.2005.04234.x. https://doi.org/10.1111/j.1537-2995.2005.04234.x. [DOI] [PubMed] [Google Scholar]
- 16.Glas M, Bauer JV, Eichler H, Volk T. Impedance aggregometric analysis of platelet function of apheresis platelet concentrates as a function of storage time. Scand J Clin Lab Invest. 2016;76(8):664–70. doi: 10.1080/00365513.2016.1238505. https://doi.org/10.1080/00365513.2016.1238505. [DOI] [PubMed] [Google Scholar]
- 17.Zhang JG, Carter CJ, Culibrk B, Devine DV, Levin E, Scammell K, et al. Buffy-coat platelet variables and metabolism during storage in additive solutions or plasma. Transfusion. 2008;48(5):847–56. doi: 10.1111/j.1537-2995.2008.01645.x. https://doi.org/10.1111/j.1537-2995.2008.01645.x. [DOI] [PubMed] [Google Scholar]
- 18.Slichter SJ, Bolgiano D, Jones MK, Christoffel T, Corson J, Rose L, et al. Viability and function of 8-day-stored apheresis platelets. Transfusion. 2006;46(10):1763–9. doi: 10.1111/j.1537-2995.2006.00970.x. https://doi.org/10.1111/j.1537-2995.2006.00970.x. [DOI] [PubMed] [Google Scholar]
- 19.Johnson L, Schubert P, Tan S, Devine DV, Marks DC. Extended storage and glucose exhaustion are associated with apoptotic changes in platelets stored in additive solution. Transfusion. 2016;56(2):360–8. doi: 10.1111/trf.13345. https://doi.org/10.1111/trf.13345. [DOI] [PubMed] [Google Scholar]
- 20.Tudisco C, Jett BW, Byrne K, Oblitas J, Leitman SF, Stroncek DF. The value of pH as a quality control indicator for apheresis platelets. Transfusion. 2005;45(5):773–8. doi: 10.1111/j.1537-2995.2005.04344.x. https://doi.org/10.1111/j.1537-2995.2005.04344.x. [DOI] [PubMed] [Google Scholar]
- 21.Ekaney ML, Grable MA, Powers WF, 4th, McKillop IH, Evans SL. Cytochrome c and resveratrol preserve platelet function during cold storage. J Trauma Acute Care Surg. 2017;83(2):271–7. doi: 10.1097/TA.0000000000001547. https://doi.org/10.1097/TA.0000000000001547. [DOI] [PubMed] [Google Scholar]
- 22.Beard MJ, Jeewa Z, Bashir S, Cardigan R, Thomas S. Comparison of platelet activation in platelet concentrates measured by flow cytometry or ADVIA 2120. Vox Sang. 2011;101(2):122–30. doi: 10.1111/j.1423-0410.2011.01469.x. https://doi.org/10.1111/j.1423-0410.2011.01469.x. [DOI] [PubMed] [Google Scholar]
- 23.Plaza EM, Lozano ML, Guiu IS, Egea JM, Vicente V, de Terán LC, et al. Evaluation of platelet function during extended storage in additive solution, prepared in a new container that allows manual buffy-coat platelet pooling and leucoreduction in the same system. Blood Transfus. 2012;10(4):480–9. doi: 10.2450/2012.0112-11. DOI: 10.2450/2012.0112-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Velzen JF, Laros-van Gorkom BA, Pop GA, van Heerde WL. Multicolor flow cytometry for evaluation of platelet surface antigens and activation markers. Thromb Res. 2012;130(1):92–8. doi: 10.1016/j.thromres.2012.02.041. https://doi.org/10.1016/j.thromres.2012.02.041. [DOI] [PubMed] [Google Scholar]
- 25.Ahmed AS, Leheta O, Younes S. In vitro assessment of platelet storage lesions in leucoreduced random donor platelet concentrates. Blood Transfus. 2010;8(1):28–35. doi: 10.2450/2009.0077-09. DOI: 10.2450/2009.0077-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morrison A, McMillan L, Radwanski K, Blatchford O, Min K, Petrik J. Storage of apheresis platelet concentrates after manual replacement of >95% of plasma with PAS 5. Vox Sang. 2014;107(3):247–53. doi: 10.1111/vox.12157. https://doi.org/10.1111/vox.12157. [DOI] [PubMed] [Google Scholar]
- 27.Albanyan AM, Harrison P, Murphy MF. Markers of platelet activation and apoptosis during storage of apheresis- and buffy coat-derived platelet concentrates for 7 days. Transfusion. 2009;49(1):108–17. doi: 10.1111/j.1537-2995.2008.01942.x. https://doi.org/10.1111/j.1537-2995.2008.01942.x. [DOI] [PubMed] [Google Scholar]
- 28.Sandgren P, Hansson M, Gulliksson H, Shanwell A. Storage of buffy-coat-derived platelets in additive solutions at 4 degrees C and 22 degrees C: Flow cytometry analysis of platelet glycoprotein expression. Vox Sang. 2007;93(1):27–36. doi: 10.1111/j.1423-0410.2007.00912.x. https://doi.org/10.1111/j.1423-0410.2007.00912.x. [DOI] [PubMed] [Google Scholar]
- 29.Cookson P, Sutherland J, Turner C, Bashir S, Wiltshire M, Hancoc V, et al. Platelet apoptosis and activation in platelet concentrates stored for up to 12 days in plasma or additive solution. Transfus Med. 2010;20(6):392–402. doi: 10.1111/j.1365-3148.2010.01034.x. https://doi.org/10.1111/j.1365-3148.2010.01034.x. [DOI] [PubMed] [Google Scholar]
- 30.Albanyan AM, Murphy MF, Rasmussen JT, Heegaard CW, Harrison P. Measurement of phosphatidylserine exposure during storage of platelet concentrates using the novel probe lactadherin: A comparison study with annexin V. Transfusion. 2009;49(1):99–107. doi: 10.1111/j.1537-2995.2008.01933.x. https://doi.org/10.1111/j.1537-2995.2008.01933.x. [DOI] [PubMed] [Google Scholar]
- 31.Saunders C, Rowe G, Wilkins K, Holme S, Collins P. In vitro storage characteristics of platelet concentrates suspended in 70% SSP+(TM) additive solution versus plasma over a 14-day storage period. Vox Sang. 2011;101(2):112–21. doi: 10.1111/j.1423-0410.2011.01468.x. https://doi.org/10.1111/j.1423-0410.2011.01468.x. [DOI] [PubMed] [Google Scholar]
- 32.Gyulkhandanyan AV, Mutlu A, Freedman J, Leytin V. Markers of platelet apoptosis: Methodology and applications. J Thromb Thrombolysis. 2012;33(4):397–411. doi: 10.1007/s11239-012-0688-8. https://doi.org/10.1007/s11239-012-0688-8. [DOI] [PubMed] [Google Scholar]
- 33.Perrotta PL, Perrotta CL, Snyder EL. Apoptotic activity in stored human platelets. Transfusion. 2003;43(4):526–35. doi: 10.1046/j.1537-2995.2003.00349.x. https://doi.org/10.1046/j.1537-2995.2003.00349.x. [DOI] [PubMed] [Google Scholar]
- 34.Leytin V, Allen DJ, Mutlu A, Mykhaylov S, Lyubimov E, Freedman J. Platelet activation and apoptosis are different phenomena: Evidence from the sequential dynamics and the magnitude of responses during platelet storage. Br J Haematol. 2008;142(3):494–7. doi: 10.1111/j.1365-2141.2008.07209.x. https://doi.org/10.1111/j.1365-2141.2008.07209.x. [DOI] [PubMed] [Google Scholar]


