Abstract
Status epilepticus (SE) is defined as continuous seizure activity lasting more than 5 minutes. It results in neuronal cell death, mediated by endoplasmic reticulum (ER) stress response. Previously, metformin demonstrated neuroprotective effects in primary cortical neurons. In this study, we analyzed the effect of metformin on ER stress via the pro-apoptotic protein kinase RNA-like endoplasmic reticulum kinase (PERK)-eukaryotic initiation factor 2α (eIF2α)-C/EBP homologous protein (CHOP) pathway. SE was induced in rats by pentylenetetrazole. Following SE, the rats were treated with salubrinal, GSK2656157, or metformin. In a control group (normal saline) SE was not induced. CHOP, eIF2α, and PERK expression was determined by Western blot; apoptosis was analyzed by TUNEL assay. CHOP expression was significantly increased at 6 and 24 hours following SE. At both time points, eIF2α and PERK levels were also increased. At 6 hours, CHOP expression was significantly reduced in salubrinal, GSK2656157 and metformin groups versus SE group. eIF2α and PERK levels were decreased in metformin compared to SE group. eIF2α expression was markedly decreased in salubrinal versus SE group, while PERK expression was markedly reduced in GSK2656157 versus SE group. At 6 and 24 hours, the apoptosis rate was significantly increased in SE versus control group, while it was significantly reduced in salubrinal, GSK2656157, and metformin groups compared to SE group. The apoptosis rate also decreased in salubrinal group at 24 hours, although not to the extent observed in metformin group. Overall, CHOP expression and apoptosis induced by SE in rats were reduced with metformin. Further studies are required to evaluate the clinical relevance of metformin for patients with SE.
Keywords: Apoptosis, CHOP expression, metformin, status epilepticus, SE, rat model, C/EBP homologous protein, eukaryotic initiation factor 2α, eIF2α, protein kinase RNA-like endoplasmic reticulum kinase, PERK
INTRODUCTION
Status epilepticus (SE) is a serious neurological disorder resulting from continuous clinical and/or electrographic seizure activity lasting ≥5 minutes, or from recurrent seizure activity without returning to the baseline [1]. Studies in the U.S. have reported the incidence rates of SE in children (<1 year) and older adults (>60 years) of 156/100,000/year and 86/100,000/year, respectively [2,3]. Similarly, the incidence rate of a first episode of SE in Asia is 42/100,000/year [4-6]. The mortality rate of SE is 26% in adults, and it increases to 50% in patients >80 years old [1]. Moreover, neuronal cell death due to continuous seizure activity can result in irreversible neuronal injury [7]. Therefore, rapid diagnosis and management of SE are very important.
Although neuronal loss and microglial activation are assumed to contribute to the pathogenesis of SE [8,9], the underlying cellular and molecular mechanisms regulating neuronal apoptosis and neuronal injury remain largely unknown. Studies have shown that the endoplasmic reticulum (ER) stress response, which is known to trigger apoptosis, plays a role in mediating the pathological reactions in acute brain injuries, including seizures, neuronal damage induced by manganese, and temporal lobe epilepsy [10-13]. Furthermore, in a SE model induced by pentylenetetrazole (PTZ) in rats, the expression of glucose-regulated protein 78 (GRP78) as well as C/EBP homologous protein (CHOP), a proapoptotic transcription factor activated by ER stress, was increased, suggesting the induction of ER stress [14]. Moreover, the CHOP expression was correlated with neuronal apoptosis [14].
Metformin, a medicine commonly used in the treatment of patients with type 2 diabetes mellitus, has demonstrated neuroprotective effects [15-17]. In addition, metformin attenuated the ER stress, insulin receptor substrate 1 phosphorylation, and apoptosis in rat insulinoma cells [18,19]. However, up until know, the metformin action has not been analyzed in SE. Here we hypothesized that metformin reduces brain injury induced by ER stress and inhibits apoptosis through regulating the protein kinase RNA-like endoplasmic reticulum kinase (PERK)-eukaryotic initiation factor 2α (eIF2α)-activating transcription factor 4 (ATF4)-CHOP pathway. We analyzed the effect of metformin, salubrinal (a specific eIF2α phosphatase inhibitor), and GSK2656157 (a PERK inhibitor) on CHOP expression and apoptosis in a SE model induced by PTZ. The aim of this study is to improve the understanding of brain injury in SE and make an important contribution to the treatment and control of SE.
MATERIALS AND METHODS
Establishment of an in vivo SE model
Sprague-Dawley rats (N = 60), 21 days old, were obtained from PlusGENE center (Nanjing Medical University). The animals were maintained in a standard 12-hour light/dark cycle and had ad libitum access to food and water at the Nanjing Medical University Medical Laboratory Animal Center. The rats were randomly divided into the following five groups (n = 12/group): control, SE, SE + salubrinal (Sal), SE + GSK2656157 (GSK), and SE + metformin (Met). In the control group, the animals received an intraperitoneal (i.p.) injection of saline. The SE model was established as previously described [20]. Briefly, SE was induced following an i.p. injection of PTZ (Sigma, USA), 70 mg/kg, diluted in normal saline to the concentration of 10 g/L. After 1 hour, the rats in SE + Sal, SE + GSK, and SE + Met groups were treated with 1 mg/kg salubrinal (Selleckchem, Suffolk, UK), 150 mg/kg GSK2656157 (Selleckchem), and 200 mg/kg metformin (Aladdin Chemistry Co., Ltd., Shanghai, China), respectively, administered i.p. Seizure severity was assessed as described by Lado et al. [21]. After 1 hour following a seizure, the rats received an i.p. injection of diazepam (10 mg/kg) to terminate the seizures. All procedures were approved by the Animal Care and Use Committee of Nanjing Medical University and were performed according to the Guide for the Care and Use of Laboratory Animals.
Sample preparation
At 6 and 24 hours after seizures, the rats were anesthetized with 300 mg/kg of 5% chloral hydrate administered i.p. These time points were chosen based on our previous study that showed the onset of ER stress at 6 hours and apoptosis at 24 hours after seizures in rats [14]. Following a thoracotomy, a rapid perfusion with normal saline followed by 4% paraformaldehyde in 0.1M phosphate buffered saline (PBS) was performed through a catheter that was inserted into the ascending aorta through the left ventricle. The brain tissues were collected, fixed in 4% paraformaldehyde in 0.1M PBS for 18-20 hours, and dehydrated in 20% sucrose and 30% sucrose in 0.1M PBS. After 72 hours, they were embedded in optimal cutting temperature (OCT), frozen in liquid nitrogen, and stored at −80°C for future use. The coronal sections (8 µm in thickness) were obtained and adhered to slides that were pretreated with 3-aminopropyltriethoxysilane. The slides were air-dried and stored at −80°C.
Western blot analysis
Western blot analysis of CHOP protein expression was performed as previously described [20]. Briefly, the brain tissue lysates were obtained following homogenization in lysis buffer (50 mM Tris-HCl, pH 7.9; 150 mM NaCl; 1 mM EDTA; 1% NP-40; 0.25% sodium deoxycholate; and 1 mM phenylmethylsulfonyl fluoride) with protease inhibitors (10 µg/mL pepstatin, 10 µg/mL leupeptin, and 5 µg/mL aprotinin) on ice. Protein samples (80 µg) were separated in a sodium dodecyl sulfate-polyacrylamide gel (12%) and transferred to a nitrocellulose membrane (Amersham Biosciences, Little Chalfont, Buckinghamshire, UK). After blocking with 5% nonfat milk in Tris-buffered saline with 0.05% Tween 20, the blots were incubated with a rabbit polyclonal anti-CHOP antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA), anti-eIF2 antibody (Santa Cruz Biotechnology), anti-PERK antibody (Santa Cruz Biotechnology), or a rabbit polyclonal anti-actin antibody (1:2000, Sigma-Aldrich, St. Louis, MO, USA) at 4°C overnight followed by a horseradish peroxidase conjugated anti-rabbit secondary antibody (1:10,000; Santa Cruz) for 1 hour at room temperature; an anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH) antibody (1:1000, 3 µl, Sigma-Aldrich, St. Louis, MO, USA) was used as the loading control. Protein bands were detected with ECL Western blotting detection reagents (Zhongshan Golden Bridge Biotechnology Co., Ltd., Beijing, China). The resulting bands were quantified using ImageJ densitometry software (http://rsbweb.nih.gov/ij/).
TUNEL assay
The TUNEL assay was performed as previously described using 8-µm-thick coronal sections and an In Situ Cell Death Detection Kit (Roche Applied Science, Germany), according to the manufacturer’s instructions [14]. The negative controls consisted of reaction mixtures in which the terminal deoxynucleotidyl transferase (TdT) was omitted. All tissues were stained with the Hoechst 33528 fluorochrome (Applygen Technologies, Inc., Beijing, China) for 10 minutes, and the images were obtained with a confocal laser scanning microscope (Olympus Fluoview FV1000, Tokyo, Japan).
Statistical analysis
All statistical analyses were carried out with IBM SPSS Statistics for Windows, Version 22.0. (IBM Corp., Armonk, NY, USA). The Western blot and TUNEL assay results are presented as mean ± standard deviation (SD). One-way analysis of variance (ANOVA) with Bonferroni correction for pairwise comparisons was used to compare the Western blot data between the groups. Apoptosis data were compared using the Kruskal–Wallis test and Mann–Whitney U test for pairwise comparisons. All statistical assessments were two-tailed and considered significant at p < 0.05.
RESULTS
Metformin suppresses CHOP levels in SE
As shown in Figure 1, Western blot analysis revealed that CHOP expression was significantly increased at both 6 and 24 hours following SE (both p < 0.01). In addition, eIF2α and PERK levels were increased with SE at both time points.
FIGURE 1.
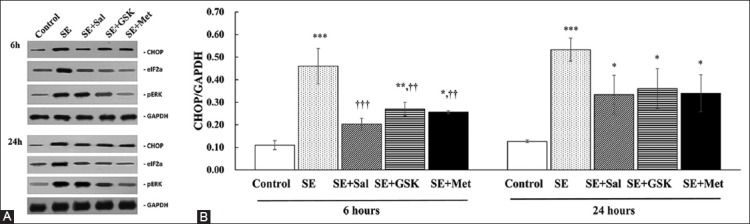
Inhibition of C/EBP homologous protein (CHOP) expression with metformin. (A) The representative CHOP, eukaryotic initiation factor 2α (eIF2α), and protein kinase RNA-like endoplasmic reticulum kinase (PERK) protein expressions were determined by Western blot analysis following the induction of status epilepticus (SE) and treatment with salubrinal (Sal), GSK2656157 (GSK), or metformin (Met) for 6 and 24 hours (n = 3 per group). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) served as the loading control. (B) Quantitative results of CHOP expression analysis were presented as mean ± standard deviation (SD). *p < 0.05, **p < 0.01, and ***p < 0.001, indicated a significance difference as compared with control group; ††p < 0.01 and †††p < 0.001 indicated a significant difference as compared with SE group. No significant differences in the CHOP expression were identified between the 6- and 24-hours values for each group.
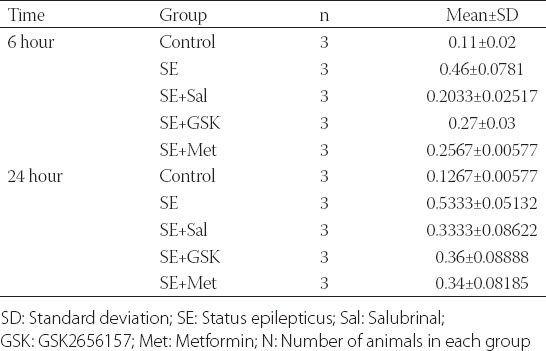
At 6 hours, CHOP expression was significantly reduced in SE + Sal, SE + GSK, and SE + Met groups (all p ≤ 0.002) compared to SE group, however, not to the levels observed in control group. No significant differences in CHOP expression were found between SE + Sal, SE + GSK, and SE + Met groups at 6 hours (all p > 0.05; Figure 1B; Table S1). Although a decreasing trend was also observed at 24 hours following SE and treatments, CHOP levels in the treatment groups were similar to the levels in SE group (all p > 0.05). No significant differences in CHOP expression were observed in each of the treatment groups between 6 and 24-hour time point (all p > 0.05; Figure 1B).
TABLE S1.
Summary of the Western blot data from each group at 6 and 24 hours following SE induction and corresponding treatments
In addition to CHOP, eIF2α and PERK levels were also decreased in SE + Met compared to SE group. As expected, eIF2α expression was markedly decreased in SE + Sal as compared to SE group (at both 6 and 24 hours; Figure 1A). Similarly, PERK expression was markedly decreased in SE + GSK group as compared to SE group (at both 6 and 24 hours; Figure 1A).
Metformin inhibits apoptosis following SE
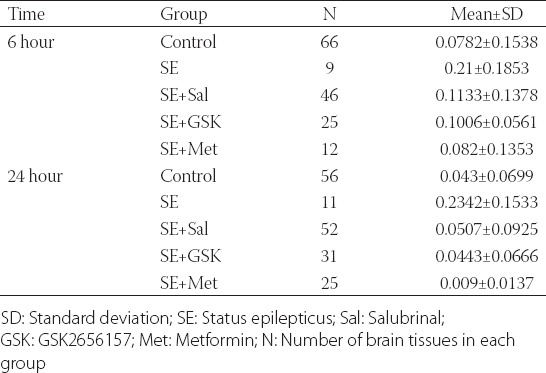
At 6 and 24 hours following SE, the apoptosis rate was significantly higher as compared with control group (p ≤ 0.003; Figure 2; Table S2). At 6 hours, the apoptosis rate was significantly reduced in SE + Sal, SE + GSK and SE + Met groups compared to SE group (p = 0.044, p = 0.037, and p = 0.020, respectively). No significant differences in the apoptosis rates were found between SE + Sal, SE + GSK, and SE + Met groups at 6 hours (all p > 0.05). Similarly, the apoptosis rate was decreased in SE + Sal, SE + GSK, and SE + Met groups compared to SE group, at 24 hours (all p < 0.001; Figure 2; Table S2). In addition, the apoptosis rate was significantly lower in SE + Met group as compared to SE + Sal group (p = 0.007). Finally, the apoptosis rates in all treatment groups following SE were lower at 24 hours compared to the 6-hour time point (all p ≤ 0.014; Figure 2).
FIGURE 2.
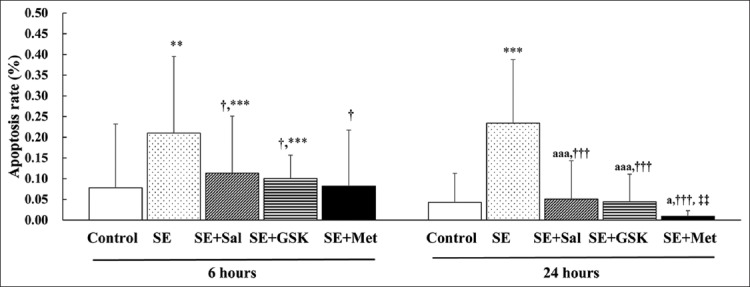
Metformin inhibits apoptosis following status epilepticus (SE). The apoptosis rate for each group was determined by TUNEL analysis following the induction of SE and treatment with salubrinal (Sal), GSK2656157 (GSK), or metformin (Met) for 6 and 24 hours (n = 3 per group). Data were presented as mean ± standard deviation (SD). **p < 0.01 and ***p < 0.001 indicated a significant difference as compared with control group; †p < 0.05 and †††p < 0.01 indicated a significant difference as compared with SE group; ‡‡p < 0.01 indicated a significant difference as compared with SE + Sal group. ap < 0.05 and aaap < 0.001 indicated a significant difference as compared with the corresponding 6-hour value for each group.
TABLE S2.
Summary of the apoptosis rate in each group at 6 and 24 hours following SE induction and corresponding treatments
DISCUSSION
In SE, CHOP expression is induced and correlated with neuronal apoptosis [14]. Because metformin has shown neuroprotective effects in primary cortical neurons [15-17], we sought to determine if it could reduce ER-stress-induced CHOP expression, and subsequent apoptosis. The CHOP expression was decreased in our SE + Sal, SE + GSK, and SE + Met groups. All treatments also ameliorated apoptosis induced by SE; however, the highest suppression of apoptosis was observed in SE + Met group. The decreased apoptosis rate induced by metformin in our in vivo SE model is consistent with previously reported anti-apoptotic properties of metformin [18,19]. Nevertheless, the precise mechanism by which metformin inhibits apoptosis in SE remains to be determined.
Previous studies indicated that metformin suppresses the ER stress through the 5’ adenosine monophosphate-activated protein kinase (AMPK)-phosphatidylinositol 3 kinase (PI3K)-c-Jun NH2 pathway [18]. It is also possible that it reduces the level of reactive oxygen species and DNA damage induced by ER stress [22]. Further studies should clarify the anti-apoptotic effects of metformin following SE.
The eIF2α/ATF4 pathway is necessary for the activation of genes essential for the adaptation of cells to environmental and ER stress [23]. In SE, eIF2α is phosphorylated [20], and PERK is induced by the unfolded protein response which is a cellular stress response associated with the ER stress [24,25]. We have previously shown that the ER stress is induced following SE through the PERK/eIF2α/CHOP signaling, in a SE rat model [20]. In the present study, the inhibition of eIF2α and PERK ameliorated the CHOP expression induced by SE, suggesting that the activation of PERK and eIF2α are required for CHOP induction in SE. Given the role of ATF4, a member of the CCAAT-enhancer-binding protein (C/EBP) family of transcription factors, in this apoptotic signaling pathway [26-29], further studies will be undertaken to evaluate its role in activating CHOP expression in SE.
In the present study, both salubrinal and GSK2656257 suppressed apoptosis following SE. This is consistent with previous studies that reported a role of the PERK/eIF2α/CHOP signaling pathway in neuronal apoptosis. Specifically, in an in vitro model of ER stress in cortical neurons, ATF4 induced CHOP expression, which enhanced the expression of p53 upregulated modulator of apoptosis (PUMA), a Bcl-2 family member, and consequently resulted in apoptosis [27]. Previously, we also showed that CHOP expression induced by SE was correlated with neuronal apoptosis in vivo [14]. Lopez-Meraz et al. [30] reported that SE induces necrosis in the CA1-subiculum area, in addition to the dentate gyrus, and that the two processes were mediated by distinct caspase pathways. Further studies are required to identify the downstream molecules associated with apoptosis induced by CHOP, including caspase proteins and the proapoptotic Bcl-2-like protein 11 (Bim) [29].
In our study, metformin reduced apoptosis induced by SE to a greater extent than salubrinal or GSK2656157, despite the similar reductions in CHOP levels in all of the treatment groups. Although both salubrinal and metformin have neuroprotective effects, it has been reported that metformin also has the capability to enhance the proliferation of neural precursors through TAp73 [31], which may result in fewer apoptotic cells after metformin compared to the salubrinal or GSK2656157 treatment. Further studies are necessary to examine the mechanism by which metformin ameliorates apoptosis induced by SE.
The present study is limited in that CHOP was the only apoptosis-related signaling molecule evaluated; therefore, additional studies are required to analyze the up and downstream effector proteins that correspond to the changes in CHOP expression induced by metformin. Furthermore, we did not evaluate in detail the mechanism by which metformin reduced apoptosis following SE. Future studies will analyze whether other apoptotic factors are induced after the administration of metformin in the SE model.
CONCLUSION
The CHOP expression induced by SE was suppressed with metformin, salubrinal, and GSK2656257 to similar extents. However, metformin inhibited apoptosis following SE to a greater extent than salubrinal or GSK2656257. These results form the basis for future research to evaluate the clinical relevance of metformin for patients with SE.
ACKNOWLEDGMENTS
This study was funded by “Six talent peaks project” in Jiangsu Province (NO: 2014-WSN-062).
DECLARATION OF INTERESTS
The authors declare no conflict of interests.
REFERENCES
- 1.Sánchez S, Rincon F. Status epilepticus: Epidemiology and public health needs. J Clin Med. 2016;5(8):E71. doi: 10.3390/jcm5080071. https://doi.org/10.3390/jcm5080071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.DeLorenzo RJ, Hauser WA, Towne AR, Boggs JG, Pellock JM, Penberthy L, et al. A prospective, population-based epidemiologic study of status epilepticus in Richmond Virginia. Neurology. 1996;46(4):1029–35. doi: 10.1212/wnl.46.4.1029. https://doi.org/10.1212/WNL.46.4.1029. [DOI] [PubMed] [Google Scholar]
- 3.Hesdorffer DC, Logroscino G, Cascino G, Annegers JF, Hauser WA. Incidence of status epilepticus in Rochester, Minnesota, 1965-1984. Neurology. 1998;50(3):735–41. doi: 10.1212/wnl.50.3.735. https://doi.org/10.1212/WNL.50.3.735. [DOI] [PubMed] [Google Scholar]
- 4.Hui AC, Joynt GM, Li H, Wong KS. Status epilepticus in Hong Kong Chinese: aetiology, outcome and predictors of death and morbidity. Seizure. 2003;12(7):478–82. doi: 10.1016/s1059-1311(03)00024-4. https://doi.org/10.1016/S1059-1311(03)00024-4. [DOI] [PubMed] [Google Scholar]
- 5.Li JM, Chen L, Zhou B, Zhu Y, Zhou D. Convulsive status epilepticus in adults and adolescents of Southwest China: mortality, etiology, and predictors of death. Epilepsy Behav. 2009;14(1):146–9. doi: 10.1016/j.yebeh.2008.09.005. https://doi.org/10.1016/j.yebeh.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 6.Nishiyama I, Ohtsuka Y, Tsuda T, Kobayashi K, Inoue H, Narahara K, et al. An epidemiological study of children with status epilepticus in Okayama, Japan: incidence, etiologies, and outcomes. Epilepsy Res. 2011;96(1-2):89–95. doi: 10.1016/j.eplepsyres.2011.05.004. https://doi.org/10.1016/j.eplepsyres.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 7.Henshall DC, Murphy BM. Modulators of neuronal cell death in epilepsy. Curr Opin Pharmacol. 2008;8(1):75–81. doi: 10.1016/j.coph.2007.07.005. https://doi.org/10.1016/j.coph.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 8.Lopes MW, Soares FM, de Mello N, Nunes JC, Cajado AG, de Brito D, et al. Time-dependent modulation of AMPA receptor phosphorylation and mRNA expression of NMDA receptors and glial glutamate transporters in the rat hippocampus and cerebral cortex in a pilocarpine model of epilepsy. Exp Brain Res. 2013;226(2):153–63. doi: 10.1007/s00221-013-3421-8. https://doi.org/10.1007/s00221-013-3421-8. [DOI] [PubMed] [Google Scholar]
- 9.Laurén HB, Ruohonen S, Kukko-Lukjanov TK, Virta JE, Grönman M, Lopez-Picon FR, et al. Status epilepticus alters neurogenesis and decreases the number of GABAergic neurons in the septal dentate gyrus of 9-day-old rats at the early phase of epileptogenesis. Brain Res. 2013;1516:33–44. doi: 10.1016/j.brainres.2013.04.028. https://doi.org/10.1016/j.brainres.2013.04.028. [DOI] [PubMed] [Google Scholar]
- 10.Pelletier MR, Wadia JS, Mills LR, Carlen PL. Seizure-induced cell death produced by repeated tetanic stimulation in vitro: possible role of endoplasmic reticulum calcium stores. J Neurophysiol. 1999;81(6):3054–64. doi: 10.1152/jn.1999.81.6.3054. [DOI] [PubMed] [Google Scholar]
- 11.Yamamoto A, Murphy N, Schindler CK, So NK, Stohr S, Taki W, et al. Endoplasmic reticulum stress and apoptosis signaling in human temporal lobe epilepsy. J Neuropathol Exp Neurol. 2006;65(3):217–25. doi: 10.1097/01.jnen.0000202886.22082.2a. https://doi.org/10.1097/01.jnen.0000202886.22082.2a. [DOI] [PubMed] [Google Scholar]
- 12.Sokka AL, Putkonen N, Mudo G, Pryazhnikov E, Reijonen S, Khiroug L, et al. Endoplasmic reticulum stress inhibition protects against excitotoxic neuronal injury in the rat brain. J Neurosci. 2007;27(4):901–8. doi: 10.1523/JNEUROSCI.4289-06.2007. https://doi.org/10.1523/JNEUROSCI.4289-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu B, Shan M, Wang F, Deng Y, Liu W, Feng S, et al. Endoplasmic reticulum stress signaling involvement in manganese-induced nerve cell damage in organotypic brain slice cultures. Toxicol Lett. 2013;222(3):239–46. doi: 10.1016/j.toxlet.2013.08.001. https://doi.org/10.1016/j.toxlet.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 14.Chen J, Guo H, Zheng G, Shi ZN. Region-specific vulnerability to endoplasmic reticulum stress-induced neuronal death in rat brain after status epilepticus. J Biosci. 2013;38(5):877–86. doi: 10.1007/s12038-013-9391-y. https://doi.org/10.1007/s12038-013-9391-y. [DOI] [PubMed] [Google Scholar]
- 15.Berthier A, Payá M, García-Cabrero AM, Ballester MI, Heredia M, Serratosa JM, et al. Pharmacological interventions to ameliorate neuropathological symptoms in a mouse model of Lafora disease. Mol Neurobiol. 2016;53(2):1296–309. doi: 10.1007/s12035-015-9091-8. https://doi.org/10.1007/s12035-015-9091-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chung MM, Chen YL, Pei D, Cheng YC, Sun B, Nicol CJ, et al. The neuroprotective role of metformin in advanced glycation end product treated human neural stem cells is AMPK-dependent. Biochim Biophys Acta. 2015;1852(5):720–31. doi: 10.1016/j.bbadis.2015.01.006. https://doi.org/10.1016/j.bbadis.2015.01.006. [DOI] [PubMed] [Google Scholar]
- 17.El-Mir MY, Detaille D, R-Villanueva G, Delgado-Esteban M, Guigas B, Attia S, et al. Neuroprotective role of antidiabetic drug metformin against apoptotic cell death in primary cortical neurons. J Mol Neurosci. 2008;34(1):77–87. doi: 10.1007/s12031-007-9002-1. https://doi.org/10.1007/s12031-007-9002-1. [DOI] [PubMed] [Google Scholar]
- 18.Jung TW, Lee MW, Lee YJ, Kim SM. Metformin prevents endoplasmic reticulum stress-induced apoptosis through AMPK-PI3K-c-Jun NH2 pathway. Biochem Biophys Res Commun. 2012;417(1):147–52. doi: 10.1016/j.bbrc.2011.11.073. https://doi.org/10.1016/j.bbrc.2011.11.073. [DOI] [PubMed] [Google Scholar]
- 19.Simon-Szabó L, Kokas M, Mandl J, Kéri G, Csala M. Metformin attenuates palmitate-induced endoplasmic reticulum stress, serine phosphorylation of IRS-1 and apoptosis in rat insulinoma cells. PLoS One. 2014;9(6):e97868. doi: 10.1371/journal.pone.0097868. DOI: 10.1371/journal.pone.0097868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen J, Zheng G, Guo H, Shi ZN. Role of endoplasmic reticulum stress via the PERK signaling pathway in brain injury from status epilepticus. J Mol Neurosci. 2014;53(4):677–83. doi: 10.1007/s12031-014-0236-4. https://doi.org/10.1007/s12031-014-0236-4. [DOI] [PubMed] [Google Scholar]
- 21.Lado FA, Sperber EF, Moshé SL. Anticonvulsant efficacy of gabapentin on kindling in the immature brain. Epilepsia. 2001;42(4):458–63. doi: 10.1046/j.1528-1157.2001.30900.x. https://doi.org/10.1046/j.1528-1157.2001.30900.x. [DOI] [PubMed] [Google Scholar]
- 22.Algire C, Moiseeva O, Deschênes-Simard X, Amrein L, Petruccelli L, Birman E, et al. Metformin reduces endogenous reactive oxygen species and associated DNA damage. Cancer Prev Res (Phila) 2012;5(4):536–43. doi: 10.1158/1940-6207.CAPR-11-0536. https://doi.org/10.1158/1940-6207.CAPR-11-0536. [DOI] [PubMed] [Google Scholar]
- 23.B’chir W, Maurin AC, Carraro V, Averous J, Jousse C, Muranishi Y, et al. The eIF2a/ATF4 pathway is essential for stress-induced autophagy gene expression. Nucleic Acids Res. 2013;41(16):7683–99. doi: 10.1093/nar/gkt563. https://doi.org/10.1093/nar/gkt563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harding HP, Zhang Y, Ron D. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature. 1999;397(6716):271–4. doi: 10.1038/16729. https://doi.org/10.1038/16729. [DOI] [PubMed] [Google Scholar]
- 25.Harding HP, Zhang Y, Bertolotti A, Zeng H, Ron D. Perk is essential for translational regulation and cell survival during the unfolded protein response. Mol Cell. 2000;5(5):897–904. doi: 10.1016/s1097-2765(00)80330-5. https://doi.org/10.1016/S1097-2765(00)80330-5. [DOI] [PubMed] [Google Scholar]
- 26.Chen YJ, Su JH, Tsao CY, Hung CT, Chao HH, Lin JJ, et al. Sinulariolide induced hepatocellular carcinoma apoptosis through activation of mitochondrial-related apoptotic and PERK/eIF2α/ATF4/CHOP pathway. Molecules. 2013;18(9):10146–61. doi: 10.3390/molecules180910146. https://doi.org/10.3390/molecules180910146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Galehdar Z, Swan P, Fuerth B, Callaghan SM, Park DS, Cregan SP. Neuronal apoptosis induced by endoplasmic reticulum stress is regulated by ATF4-CHOP-mediated induction of the Bcl-2 homology 3-only member PUMA. J Neurosci. 2010;30(50):16938–48. doi: 10.1523/JNEUROSCI.1598-10.2010. https://doi.org/10.1523/JNEUROSCI.1598-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Masuda M, Miyazaki-Anzai S, Levi M, Ting TC, Miyazaki M. PERK-eIF2a-ATF4-CHOP signaling contributes to TNFa-induced vascular calcification. J Am Heart Assoc. 2013;2(5):e000238. doi: 10.1161/JAHA.113.000238. https://doi.org/10.1161/JAHA.113.000238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sovolyova N, Healy S, Samali A, Logue SE. Stressed to death-mechanisms of ER stress-induced cell death. Biol Chem. 2014;395(1):1–13. doi: 10.1515/hsz-2013-0174. https://doi.org/10.1515/hsz-2013-0174. [DOI] [PubMed] [Google Scholar]
- 30.Lopez-Meraz ML, Niquet J, Wasterlain CG. Distinct caspase pathways mediate necrosis and apoptosis in subpopulations of hippocampal neurons after status epilepticus. Epilepsia. 2010;51(Suppl 3):56–60. doi: 10.1111/j.1528-1167.2010.02611.x. https://doi.org/10.1111/j.1528-1167.2010.02611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fatt M, Hsu K, He L, Wondisford F, Miller FD, Kaplan DR, et al. Metformin acts on two different molecular pathways to enhance adult neural precursor proliferation/self-renewal and differentiation. Stem Cell Reports. 2015;5(6):988–95. doi: 10.1016/j.stemcr.2015.10.014. https://doi.org/10.1016/j.stemcr.2015.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]


