Abstract
The Hedgehog (Hh) signaling pathway was first identified in the common fruit fly. It is a highly conserved evolutionary pathway of signal transmission from the cell membrane to the nucleus. The Hh signaling pathway plays an important role in the embryonic development. It exerts its biological effects through a signaling cascade that culminates in a change of balance between activator and repressor forms of glioma-associated oncogene (Gli) transcription factors. The components of the Hh signaling pathway involved in the signaling transfer to the Gli transcription factors include Hedgehog ligands (Sonic Hh [SHh], Indian Hh [IHh], and Desert Hh [DHh]), Patched receptor (Ptch1, Ptch2), Smoothened receptor (Smo), Suppressor of fused homolog (Sufu), kinesin protein Kif7, protein kinase A (PKA), and cyclic adenosine monophosphate (cAMP). The activator form of Gli travels to the nucleus and stimulates the transcription of the target genes by binding to their promoters. The main target genes of the Hh signaling pathway are PTCH1, PTCH2, and GLI1. Deregulation of the Hh signaling pathway is associated with developmental anomalies and cancer, including Gorlin syndrome, and sporadic cancers, such as basal cell carcinoma, medulloblastoma, pancreatic, breast, colon, ovarian, and small-cell lung carcinomas. The aberrant activation of the Hh signaling pathway is caused by mutations in the related genes (ligand-independent signaling) or by the excessive expression of the Hh signaling molecules (ligand-dependent signaling – autocrine or paracrine). Several Hh signaling pathway inhibitors, such as vismodegib and sonidegib, have been developed for cancer treatment. These drugs are regarded as promising cancer therapies, especially for patients with refractory/advanced cancers.
Keywords: Hedgehog signaling pathway, Hh, tumorigenesis, signal transduction
INTRODUCTION
The Hedgehog (Hh) signaling pathway, also known as Hedgehog-Patched (Hh-Ptch), Hedgehog-Gli (Hh-Gli) or Hedgehog-Patched-Smoothened (Hh-Ptch-Smo), is an evolutionarily conserved pathway of signal transmission from the cell membrane to the nucleus. The Hh signaling pathway plays a significant role in the normal embryonic development of invertebrates and vertebrates [1]. The Hh gene is also relevant for proper segregation, i.e. the polarity of the organism and the development of many tissues and organs.
The Hh pathway is mostly inactive or poorly active in the adult organism. If necessary, it can be activated, for example, in wound healing [2]. Furthermore, the pathway is involved in the maintenance of somatic stem cells and pluripotent cells important for tissue repair [3], such as mammary [4], skin [5], neural [6], erythropoietic [7], and lung stem cells [8], as well as some epithelial cells of internal organs [9]. Accordingly, Hh signaling is critical for regeneration of the lung epithelium [8], prostate epithelium [10], and exocrine pancreas cells [11].
In other tissues, the Hh signaling pathway is present only in primary cilia (PC), organelles that consist of microtubules and emanate from the cell surface, receiving mechanical, chemical, and thermal signals [12]. All components of the Hh signal transduction pathway are found in the PC [13].
Some studies indicate that Hh signaling can be involved in various stages of carcinogenesis in different tumors. For example, in pancreatic and esophageal cancer, the activation of this signaling pathway is found in the early stages of tumor as well as in metastatic tumors [14,15]. In other tumors, such as gastric cancer and prostate cancer, the activation of the Hh signaling pathway is associated with tissue invasion and increased metastatic potential. In accordance with those findings, the inhibition of the Hh signaling pathway reduces tumor cell proliferation in prostate and gastric cancer [10,16].
HH SIGNALING PATHWAY
Three proteins are involved in Hh signaling activation: Hedgehog (Hh) ligand, Patched (Ptch) and Smoothened (Smo) [17].
In the absence of Hh ligand, Ptch localizes to the base of the PC and represses the activity of Smo by inhibiting its translocation into the PC [18]. This leads to proteolytic cleavage of full-length glioma-associated oncogene (GliFL) to Gli repressor (GliR) after phosphorylation by protein kinase A (PKA), glycogen synthase kinase-3 (GSK3), and casein kinase 1 (CK1) [19]. GliR binds to Hh target gene promoters and keeps Hh target genes switched off (Figure 1A) [20].
FIGURE 1.
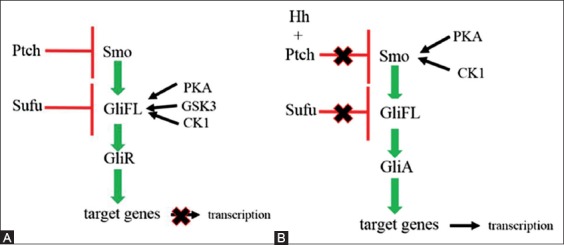
A simplified display of the Hedgehog signaling pathway. (A) In the absence of Hedgehog ligand, full-length Gli is phosphorylated by protein kinase A, glycogen synthase kinase-3, and casein kinase 1. This leads to proteolytic cleavage of the full-length Gli into Gli repressor. Gli repressor represses the expression of target genes, (B) after binding of Hedgehog ligand, Smoothened protein is phosphorylated by protein kinase A and casein kinase 1. The inhibitory effect of Sufu is removed and Gli activator is formed. Gli activator induces the transcription of target genes. Red symbols represent the inhibitory effect, and green arrows show the activating effect. Hh - Hedgehog; Ptch - Patched; Smo - Smoothened; Gli - glioma-associated oncogene; GliFL - full-length Gli; GliA - Gli activator; GliR - Gli repressor; CK1 - casein kinase 1; PKA - protein kinase A; GSK3 - glycogen synthase kinase-3.
The Hh signaling cascade is activated by Hh binding to Ptch1 protein. The Hh-Ptch complex is internalized, and both proteins are degraded in lysosomes [21]. The binding relieves the Smo inhibition, and the Hh signal is now able to be transmitted downstream of Smo via cytoplasmic protein complex composed of kinesin protein (Kif7), Suppressor of fused (Sufu), and GliFL. Smo travels to the tip of the PC and signals Sufu to release Gli activator (GliA). GliA then migrates to the nucleus and activates the expression of the target genes (Figure 1B) [22]. This pathway of signal transduction, where Hh regulates the Gli family of transcription factors, is called the canonical Hh signaling pathway. In the non-canonical Hh signaling pathway, Hh proteins signal through Gli-independent mechanisms.
ELEMENTS OF HH SIGNAL TRANSDUCTION
Hh gene/protein
The Hh gene is highly conserved from fruit flies to humans, and is a key regulator in embryonic development [1]. Unlike Drosophila melanogaster (D. melanogaster), where one Hh gene has been identified, in vertebrates, three Hh gene family members have been detected: the Sonic Hedgehog (SHh), Indian Hedgehog (IHh), and Desert Hedgehog (DHh) gene [23-25]. The products of any of these three genes can bind to Ptch1 receptor and activate the Hh signaling pathway [26], but they perform this activity in various organ systems [23,25]. Hh proteins can act as mitogens, morphogens, and differentiation factors at longer or shorter distances, during different stages of development and in different tissues [17].
The most studied Hh ligand is SHh. It has the highest activity and is involved in the development of various organs during embryogenesis. SHh is expressed in the central nervous system, lungs, teeth, intestines, and hair follicles during development [27-30]. SHh signaling can be autocrine (binds to the same cell from which it is excreted) or paracrine (binds to the nearby cells or induces changes in cells at longer distances).
IHh is involved in endochondral bone formation, as a negative regulator of chondrocyte differentiation [31], and participates in the development of gastrointestinal tract [32] and mammary glands [4].
Of all discovered Hh proteins, DHh is the closest homologue of D. melanogaster Hh ligand [33]. Its expression is largely restricted to gonads, including Sertoli cells in the testis, where it plays a key role in the differentiation of germ cells [34].
All Hh proteins undergo maturation before the active ligand is generated and released from the cell and before the activation of Hh signaling pathway [35]. After the translation, N-terminal signal sequence is removed from a ~45 kDa long polypeptide, which is then autocatalytically cleaved between glycine and cysteine to form an N-terminal fragment. The C-terminal domain (autoprocessing domain) of the Hh polypeptide promotes the binding of cholesterol to glycine at the C-terminus of N-terminal fragment, after which a ~19 kDa N-terminal Hh signaling domain (HhN), associated with a number of known signaling activities, is formed [36]. Subsequently, a palmitic acid moiety is added to the cysteine on the N-terminus of Hh by the acyltransferase Skinny Hedgehog (Ski), resulting in the formation of dually modified Hh signaling domain (HhNp), i.e., active Hh protein [37]. The secretion of mature, functionally active Hh proteins is regulated by Dispatched (Disp) protein, which allows their release from the cells.
Ptch gene/protein
Ptch is the receptor for Hh protein. Two Ptch homologs have been isolated in vertebrates: Ptch1 and Ptch2 [38]. SHh, IHh, and DHh ligands bind with similar affinity to both of them, and both proteins may repress the activity of Smo protein [39]. The human Ptch1 gene is located on chromosome 9q22.3; it contains 23 exons and encodes a glycoprotein of 1447 amino acids [40,41]. The Ptch2 gene is located on 1p34.1 and encodes a 1203-amino acid protein. Ptch1 and Ptch2 genes have different functions based on their different expression during epidermal development [42]. The Ptch1 is primarily expressed in mesenchymal cells that produce SHh proteins, while the Ptch2 is expressed in skin and testicular epithelial cells [43].
Ptch1 protein is a 12-pass transmembrane protein with two large extracellular loops and two large intracellular loops [25]. Within the extracellular loop structure, there is a sterol-sensing domain (SSD) that is thought to interact with cholesterol bound to Hh protein [44]. In the absence of Hh, Ptch blocks the pathway activity [45]. However, when Hh ligand binds to Ptch, Hh relieves the inhibition of Ptch to activate the signal transduction [46]. Subsequently, there is no Smo blockade anymore, which results in the modulation of Gli transcription factors. Ptch also sequesters Hh ligand and restricts the range of signaling of the free ligand [47]. In cells where Ptch is absent, Hh protein is further dispersed [48] and it induces the targeted gene expression at longer distances [49]. The mechanism by which Ptch regulates Smo is still not understood, but it has been shown that Hh binding causes the internalization of Ptch from the cell surface and promotes the accumulation of Smo at the cell surface [18].
Ptch and Hh proteins regulate the cell cycle in two ways [50]. First, Ptch without Hh ligand binds maturation promoting factor (MPF), which is composed of cyclin B1 and cyclin-dependent kinase 1 (CDK1). Binding or retaining MPF in the cytoplasm, prevents its activity. When Hh binds to Ptch, cyclin B1 is released and the cell cycle continues [17]. Second, the Hh signal transmission pathway leads to the transcription of cyclin D and cyclin E, which also promotes the cell cycle progression [51].
Several observations suggest that, besides Ptch1, other receptors bind Hh ligands and participate in the Hh signaling pathway activation [52,53]. Negative receptor of Hh signaling is Hedgehog-interacting protein (Hhip) [54], while Cdo and Boc bind vertebrate Hh proteins and positively regulate Hh signaling. It is not yet fully known if they synergize or compete with Ptch1 for binding to Hh [55]. Some evidence has shown that the membrane protein growth arrest-specific 1 (GAS1), known as a negative regulator of Hh signaling, also modulates Hh signaling positively [56]. These negative and positive receptors most likely play a key role in monitoring the magnitude and range of Hh signaling pathway [57].
Smo gene/protein
As previously mentioned, Smo protein is a co-receptor in the Hh signaling pathway, i.e. it represents a signaling component of the receptor complex. It is a seven-pass transmembrane protein, structurally similar to G-protein-coupled receptors [58], and has an extracellular cysteine-rich domain (CRD) that is indispensable for its function [59].
Smo is considered to be a positive Hh signaling pathway regulator because it is constitutively active in the absence of inhibitory Ptch1, and it promotes the activation of downstream components of this signaling pathway [60].
The mechanism by which Ptch1 inhibits Smo is still not clear. The suggested mechanism [61] based on the physical interaction between Smo and Ptch1, in which they form a membrane-associated receptor complex, has not been confirmed in vivo [47]. Ptch1 possibly functions through the changes in the distribution or concentration of a small molecule that affects Smo [62]. Ciliary localization of Ptch1, enabled by the ciliary localization sequence (CLS) within its carboxyl-terminal tail, has one of the main roles in the inhibition of Smo. CLS also unlocks Ptch1 transport into the PC [63]. Oxysterols [64] and Vitamin D3 derivatives [65] may function as endogenous regulators of Smo activity. Despite these insights on the regulators of Smo activity, the mechanisms by which Ptch1 represses Smo and how Hh ligand counters this effect remain unknown.
After the binding of Hh ligand and degradation of Ptch1 protein, Smo is phosphorylated by PKA and CK1, and its endocytosis and degradation are blocked [66]. Smo transmits a signal to the cytoplasm in a phosphorylation cascade, where Gli protein is the final target (Figure 1B).
Gli gene/protein
Gli family consists of zinc finger proteins, and is named after glioblastoma from which they were initially isolated [67].
In vertebrates, there are three members of Gli gene family: Gli1, Gli2, and Gli3 [68]. GLI1 protein acts as a transcriptional activator [69]. Hh ligands induce the expression of Gli1, which also provides a positive feedback for Hh signaling [70]. The activator domain of GLI1 consists of 18 amino acids, and it probably forms a negatively charged helix that is similar to viral protein 16 [71]. GLI2 primarily functions as a transcriptional activator, while GLI3 mainly functions as a repressor in Hh signaling [72].
Gli proteins regulate the expression of target genes by directly binding to their promoters [73]. GLI1 and GLI3 proteins recognize the 5’-GACCACCCA-3’ sequence in target gene promoters [74], and GLI2 recognizes almost the identical 5’-GAACCACCCA-3’ motive [75].
In the absence of Hh ligand, GliFL is phosphorylated by PKA, GSK3 and CK1, and recognized by β-transducin-repeat containing protein (β-TrCP). This leads to proteolytic cleavage of GliFL into C-terminally truncated repressor form, GliR [76]. GliR translocates to the nucleus where it binds to Hh target gene promoters and represses their expression (Figure 1A). The binding of Hh ligand leads to the release of Gli from Sufu and the formation of GliA [77]. GliA then translocates to the nucleus, binds to target gene promoters, and activates the transcription of Hh target genes (Figure 1B).
The activation of canonical Hh signaling results in the suppression of proteolytic degradation of Gli proteins, thereby increasing their cytoplasmic and nuclear levels, and hence, the transcription of target genes in the Hh signaling pathway. Studies have shown that the expression of Gli transcription factors and their activation is also regulated by other signaling pathways. For example, in esophageal carcinoma, the mammalian target of rapamycin (mTOR)/ribosomal protein S6 kinase beta-1(S6K1) signaling pathway activates GLI1 by phosphorylation, leading to the release of GLI1 from the Sufu protein complex and its translocation into the nucleus [78]. In addition, it has been reported that the transforming growth factor beta (TGF-β), epidermal growth factor receptor (EGFR), mitogen-activated protein kinases (MAPK), and fibroblast growth factor (FGF) signaling pathways can also induce the expression of Gli transcription factors [79].
Suppressed fusion protein (Sufu)
Sufu is a crucial negative regulator of the Hh pathway, and it functions between Smo and Gli transcription factors. It binds directly to Gli proteins [80]. In a stimulated Hh signaling pathway, active Smo leads to the recruitment of Sufu-Gli to cilia, followed by a rapid dissociation of the complex and initiation of target gene transcription [81]. Sufu inhibits Gli proteins by preventing their translocation into the nucleus [82]. It plays a key role in the stabilization and processing of Gli and thus maintains accurate Hh signaling [77]. Sufu also localizes to the nucleus where it can bind to Gli-binding sequences in DNA molecule and prevent gene transcription [83].
Kif7
The kinesin protein Kif7 is a component of Hh signaling that can act as both a positive and negative regulator [84]. In response to Hh activation, Kif7 localizes to the tip of the PC, and it can control the cilium structure and organize a specialized compartment necessary for Hh signaling [85]. When Hh signaling is induced, Gli proteins also translocate to the PC tip [86]. The mechanisms of Kif7 positive and negative roles are not completely understood. One possible mechanism is the post-translational control of Kif7 phosphorylation by protein phosphatase 2A (PP2A) [87].
Cyclic AMP (cAMP)-dependent PKA
cAMP-dependent PKA is also an important negative regulator of the Hh pathway (Figure 1) [88]. It is localized at the base of cilia and regulates the formation of GliR/GliA complex [89]. PKA activity is crucial for Hh signaling. In the absence of Hh ligand, PKA phosphorylates Gli proteins, which are then cleaved to the repressor form of GliR and repress Hh target gene expression (Figure 1A) [90]. PKA may also inhibit Gli proteins by modulating their interaction with Sufu. One explanation as to how PKA inhibits Hh signaling is by blocking the localization of Sufu-Gli complex to cilia and inhibiting the Sufu-Gli dissociation [81]. The basal level of PKA activity maintains the suppression of Hh signaling pathway in the absence of Hh ligand. If the PKA activity is reduced, it causes ectopic expression of Hh target genes [91].
Target genes
The main target genes of Hh signaling pathway (Table 1) include the Ptch1, Ptch2, and Gli1 genes; their activation results in elevated levels of the respective mRNAs and proteins [92]. Increased expression of the Ptch1, Ptch2, and Gli1 genes is a highly reliable indicator of activated signaling pathway and provides negative (Ptch1) and positive (Gli1) regulation of Hh signaling with negative and positive feedback loop mechanisms [93].
TABLE 1.
Hh signaling pathway target genes
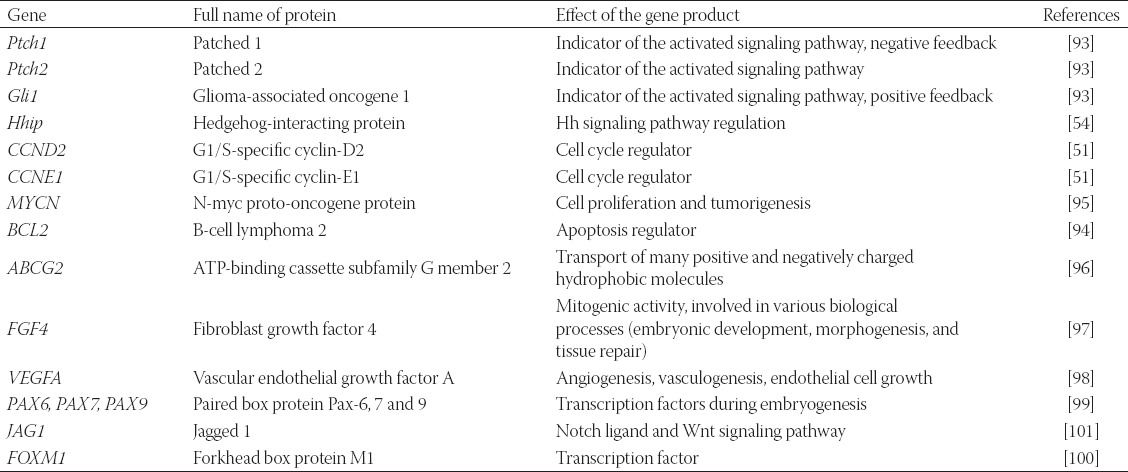
Other target genes include Hedgehog-interacting protein (Hhip) [54], cell cycle regulators (CCND2 and CCNE1) [51], apoptosis regulator (BCL2) [94], MYCN [95], ABCG2 [96], FGF4 [97], VEGFA [98], PAX6, PAX7, PAX9 [99], FOXM1 [100], JAG1, and members of the Wnt signaling pathway [101]. Recent studies show the existence of an interaction between the Wnt and Hh signaling pathways [32,102].
Activation and deactivation of these Hh genes may contribute not only to the development of normal tissues and organs, but also to tumorigenesis.
THE ROLE OF THE HH SIGNALING PATHWAY IN CANCER
Dysfunction or aberrant activation of the Hh signaling pathway is associated with developmental deformities and cancers [103], such as basal cell nevus syndrome (BCNS), also known as Gorlin syndrome, sporadic basal cell carcinoma (BCC), medulloblastomas (MBs), rhabdomyosarcomas, meningiomas [104], and others. According to the latest estimates, the Hh signaling pathway contributes to the development of one-third of all malignant tumors [60]. Deregulation of any component within the Hh pathway leads to its aberrant activation, resulting in malignant transformation. There are three proposed mechanisms of aberrant Hh signaling activation in different cancer types [22]. These are as follows:
Type I - autonomous and ligand-independent type of Hh signaling (Figure 2A);
Type II - ligand-dependent oncogenic Hh signaling in autocrine/juxtacrine manner (Figure 2B);
Type IIIa/b - ligand-dependent Hh signaling in paracrine or reverse paracrine manner (Figure 2C).
FIGURE 2.
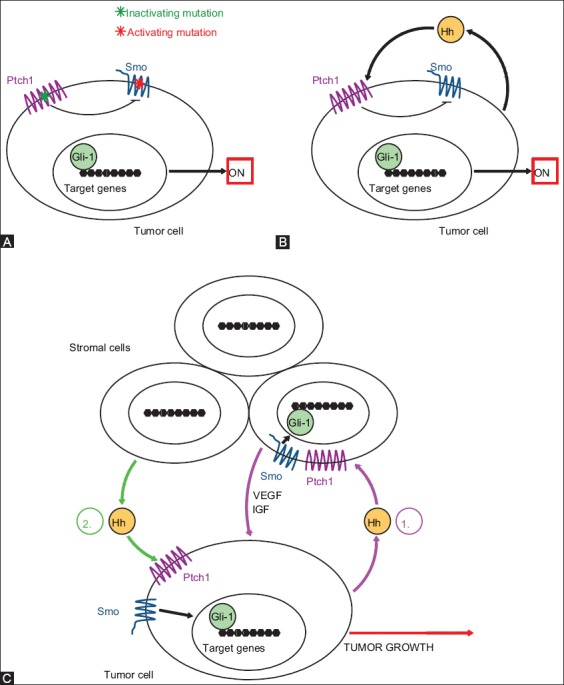
Three basic mechanisms of aberrant activation of Hedgehog signaling. (A) Type I - Ligand-independent Hedgehog signaling. This type includes: Ptch1 inactivating mutation (green asterisk) or Smo activating mutation (red asterisk), thereby Smoothened receptor can no longer be inhibited by Patched 1. The result is a constitutive activation of Hedgehog pathway in the absence of the ligand. (B) Type II - Ligand-dependent autocrine/juxtacrine Hedgehog signaling. The Hedgehog ligand is secreted by the tumor cell and taken up into the same tumor cell (autocrine manner) or into the nearby tumor cells (juxtacrine manner), thus activating the signal cascade downstream of the Hedgehog signaling pathway. (C-1) Type IIIa - Ligand-dependent paracrine signaling. The Hedgehog ligand is secreted by tumor cells and taken up by the stromal cells. Activated stromal cells synthesize and secrete signals, such as vascular endothelial growth factor and insulin-like growth factor, which are then taken back into the tumor cells to support their survival and growth. (C-2) Type IIIb - Ligand-dependent reverse paracrine signaling. The Hedgehog ligand is directly secreted by stromal cells and taken up by the tumor cells. Thus, the ligand helps the tumor cell proliferation and growth. Hh - Hedgehog; Ptch1 - Patched 1; Smo - Smoothened; Gli1 - glioma-associated oncogene 1; VEGF - vascular endothelial growth factor; IGF - insulin-like growth factor.
Type I - ligand-independent signaling
The ligand-independent activation of Hh signaling in Type I is caused either by the activating mutations in the Smo or inactivating mutations in the Ptch1 or Sufu (Hh signaling negative regulators) leading to constitutive activation of Hh signaling in the absence of the ligand (Figure 2A) [105]. This link was first found in patients with a rare autosomal dominant disorder, i.e., BCNS (Gorlin syndrome), where a Ptch1 mutation on chromosome 9 was found [106]. These patients are at high risk for the development of BCCs and have an increased risk of developing other tumors, especially MBs, meningiomas, and rhabdomyosarcomas [104].
Somatic mutations of these genes have also been found in sporadic BCCs and MBs [107]. Sporadic BBC is the most common cancer in humans, and almost all cases are caused by a gene mutation leading to a constitutive activation of Hh signaling pathway.
The confirmation of these findings was found in many preclinical and clinical models. For example, an inactivating mutation in the Ptch1 was found in about 85% of sporadic BCCs [106]. These observations have been supported by the fact that in Ptch1 heterozygous mice the formation of UV-induced BCC was more frequently observed [108]. Activating Smo mutations that reduce its inhibition by Ptch1 have been found in 10% of sporadic BCCs. Rare mutations of the Sufu gene (<10% somatic) have also been found [105]. However, although the Sufu is a tumor suppressor gene, its targeted inactivation in mouse skin does not result in BCC development, suggesting that the Sufu heterozygosity is not sufficient for tumorigenesis [109]. Furthermore, it has been found that the hyperactivation of Hh signaling after Gli overexpression and activation of atypical protein kinase C (aPKC)-ι/λ (GLI1-positive regulator) can promote BCC development regardless of the upstream components [107]. Gli2 gene amplification is seen in 8% of BCCs, suggesting that the increase in the number of Gli2 copies can induce tumorigenesis as well [110]. In 30% cases of BCC, no mutations in Hh signaling pathway genes can be found [39]. Given all the above results, it can be concluded that, in most instances, BCC is a disease related to the aberrant activation of Hh signaling pathway that leads to tumor cell proliferation and survival.
MB is a rare, aggressive tumor, predominantly present in children, and is another malignancy that occurs in 5% of patients with Gorlin syndrome [111]. The Hh signaling pathway plays an important role in the embryonic development of the cerebellum, and consequently, its role in the formation of MB is not surprising. Purkinje cells release SHh, which induces mass proliferation of granule neuron progenitor (GNP) cells and delays neural differentiation. After early postnatal development, Hh signaling is normally downregulated in the brain. The formation of MB occurs when the Hh pathway is constitutively activated in GNP cells and the proliferation continues outside the normal developmental period [112]. More than one-third of sporadic MB cases are associated with aberrant activation of Hh pathway that is either linked to Ptch1 mutation or Ptch1 locus chromosomal loss (45% of cases) [113]. Somatic inactivating Smo or Sufu mutations each occur in about 14% of cases. While Smo mutations are more frequent in adults, mutations of Sufu are mostly found in pediatric patients (0–3 years) [114]. In preclinical animal models, Ptch1+/- heterozygous mice and Sufu+/- heterozygous mice, deficient also in p53 alleles, both develop MBs [112].
In both BCCs and MBs, dysregulation of PKA and guanine nucleotide-binding protein (GNAS), was also found. PKA is the main negative regulator of the Hh pathway, while G-protein alpha subunit, encoded by the GNAS gene, promotes PKA-dependent cAMP activity. Therefore, it is not surprising that reduced PKA activity triggers oncogenic Hh signaling and formation of MBs and BCCs. Furthermore, GNAS gene mutations have been found in human MBs [110] and in lesions similar to BCC [115], confirming their important role in Hh signaling. Ptch2 mutations, although rare, can be found in MBs and in BCCs as well [42].
Ptch1 gene mutations were detected in trichoepitheliomas [116], esophageal carcinomas [117], and bladder carcinomas [118], whereas mutations of Ptch1 and Sufu have been found in a rare muscle tumor, rhabdomyosarcoma [119].
Type II - ligand-dependent autocrine/juxtacrine signaling
Type II is ligand-dependent and responds to Hh in an autocrine/juxtacrine manner leading to tumor formation and growth (Figure 2B). Since the Hh pathway is activated in a cell-autonomous manner, Hh ligand is produced by and taken up by the same or surrounding tumor cells. The overexpression of the ligand-dependent autocrine/juxtacrine Hh signaling pathway has been found in various tumors including stomach, esophageal, pancreatic [120], colorectal [121], ovarian and endometrial [122], breast [123], prostate [16], lung [8], melanomas [124], gliomas [125], and other extracutaneous tumors. Apart from the Hh ligand overexpression, most of these tumors show ectopic expression of Ptch1 and Gli.
Studies of the activation of Hh signaling pathway in colorectal carcinomas (CRCs) are contradictory. A few studies [126,127] found an increased level of the Hh pathway components in CRC. In addition, in CRC cells in vivo, increased SHh expression was detected at both, the mRNA and protein level. These findings suggest that SHh is required for the development of CRC. In contrast, other reports [120,128,129] claim that the Hh signaling pathway is inactivated during the CRC progression.
Type III - ligand-dependent paracrine signaling
Type III is ligand-dependent and uses paracrine signaling. Although paracrine Hh signaling plays an important role during normal embryonic development and is required for the growth and maintenance of many tissues [130], paracrine activation of Hh pathway in stromal cells has also been associated with various cancers, such as those of prostate, pancreas, and colon. Namely, Hh ligands, secreted by tumor cells, bind to Ptch1 receptors on tumor stromal cells, which then undergo Hh pathway activation. In a feedback loop, the stromal cells transmit the growth signals (vascular endothelial growth factor [VEGF], insulin-like growth factor [IGF], Wnt, PDGF, and BMP) to tumor cells, supporting and promoting their proliferation and differentiation (Figure 2C-1) [131]. In addition, Fan et al. [132] suggested that some prostate cancer cells signal to stromal cells in ligand-dependent and paracrine manner, and Theunissen and Sauvage [133] recently supported this finding. This suggests that, while some Hh ligand-expressing epithelial cancer cells do not respond to the ligands themselves, there is an activation of Hh signaling pathway in their surrounding stromal cells.
The “reverse” paracrine signal model has also been recognized, but only in hematological malignancies such as B-cell lymphomas, multiple myelomas, and leukemias. In this model, tumors receive Hh ligand secreted directly from bone marrow or lymph node stromal cells. Thus, in the reverse paracrine ligand-dependent cancers, stromal cells provide a microenvironment that is favorable for tumor growth (Figure 2C-2). For that reason, surrounding stromal cells can also be considered as a therapeutic target [133].
Cancer stem cells (CSCs)
Recently, CSCs have emerged as an important factor in both tumor initiation and progression [134,135]. Within each tumor, there is a set of cells that act as stem cells: they divide slowly but, if necessary, they can very quickly proliferate and create a new population of tumor cells [136]. Activated signaling pathways, including Hh, are involved in their growth, survival, migration, and proliferation [137]. Tumor stem cells have already been detected, for example, in multiple myeloma [137], pancreatic adenocarcinoma [138], breast cancer [139], and chronic myelogenous leukemia (CML) [140]. Due to their slow growth, CSCs are potentially resistant to conventional chemotherapy and radiotherapy, and they are thought to be the major cause of tumor relapse after such therapies. Therefore, new generations of antitumor drugs are being designed in an attempt to target signaling pathways, including Hh, specifically in CSCs [141].
Epigenetic changes
Recent studies show that, apart from mutations, the Hh signaling pathway can be disrupted by epigenetic changes, or more accurately, by methylation of gene promoters. So far, the methylation of the promoter of the Ptch1 gene has been described in dermoid cysts and ovarian fibromas [142] and in breast cancer [143], whereas in MBs, SHh ligand induces a local switch of epigenetic cofactors that cooperate with Gli in controlling the transcription outcomes [144].
CLINICAL AND THERAPEUTIC IMPLICATIONS
Recent findings in the Hh signaling pathway and its role in the tumorigenesis have opened new views toward the development of molecular targeting and tumor prevention associated with the Hh pathway. Special attention has been paid to the targeted Hh signaling pathway inhibition (HPI) as a treatment for locally aggressive BCCs and metastatic BCCs, when radiotherapy and surgery are not effective treatment modalities. Therefore, HPI therapy approach is a new hope for patients with difficult-to-treat BCCs.
More than 50 HPI molecules have been identified, which act at different levels of the Hh signaling pathway. HPIs are categorized as Hh ligand inhibitors, Smo antagonists, Gli inhibitors, inhibitors of bromodomain and extra-terminal domain (BET) family of proteins, atypical protein kinase C (aPKC) inhibitors, and phosphodiesterase inhibitors (Table 2).
TABLE 2.
Targeted tumor therapy associated with Hh signaling pathway
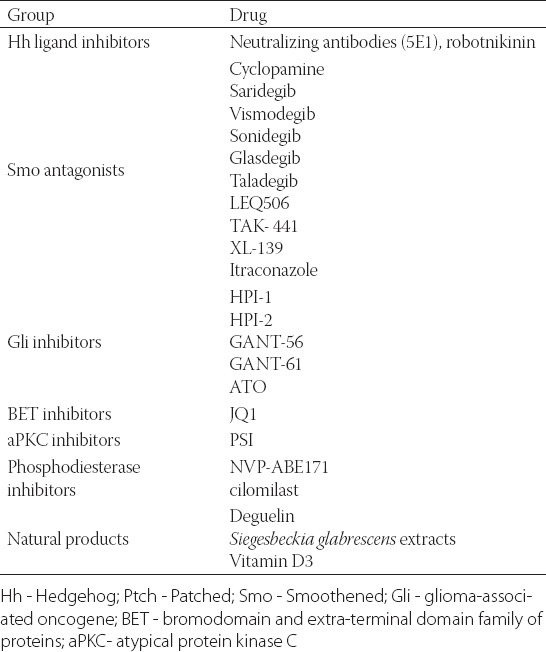
Hh ligand inhibitors act at the highest level of the signaling pathway by inhibiting the binding of Hh protein to Ptch receptor [145]. On the other hand, Smo antagonists bind to the Smo drug-binding pocket, thus preventing the downstream activation of the Hh signaling cascade [146]. Smo inhibitors currently used in clinical trials are IPI-926 (saridegib), BMS833923 (XL-139), PF04449913 (glasdegib), LY2940680 (taladegib), LEQ506, and TAK-441, whereas GDC-0449 (vismodegib) and LDE225 (sonidegib) have been approved by the US Food and Drug Administration, but are not in use yet [22,147,148].
Due to its pharmacokinetic properties, sonidegib is a highly effective drug [149]. In addition to treating BCC, there is a significant interest in the use of sonidegib in the treatment of MB and renal, lung, pancreatic, and ovarian carcinomas, along with hematologic malignancies, such as myeloid leukemia and lymphoma.
Vismodegib has a similar safety profile as sonidegib, but a Phase II clinical trial with vismodegib revealed its lower therapeutic efficacy and more serious side effects, such as significant fatigue, hyponatremia, hypocalcemia, muscle spasms, and atrial fibrillation [150].
Although Smo inhibitors possess a great potential for the treatment of BCC, mutations in drug-binding pocket may result in the resistance of tumor cells to these drugs. Therefore, the main focus is on the antagonists of Smo receptors that do not bind to the same binding site as sonidegib and vismodegib, such as itraconazole [151] and on the common antagonists of Hh signaling pathway, such as Gli-transcription factor inhibitors HPI-1, HPI-2, GANT-56, GANT-61, and arsenic trioxide.
The viability and proliferation of tumor cells due to aberrant Hh signaling activity in Smo-resistant tumors can also be decreased by BET inhibitors. JQ1 is, among others, a BET inhibitor commonly used in research studies. It was shown that BET inhibitors also inhibit the growth of MB and BCC and increase the survival in mouse models [152].
Phosphodiesterase inhibitors were useful in the treatment of Smo-resistant MB in vivo [153] and aPKC inhibitors could be useful in the treatment of resistant BCCs [154]. Several natural molecules have also shown some benefits in cancer treatment. For example, deguelin is a flavonoid with anticarcinogenic and antiproliferative activities. It induces apoptosis and cell cycle arrest and inhibits blood vessel formation [155]. Many studies refer to deguelin as the regulator of the Hh signaling pathway, and several studies have already shown its excellent potential in the treatment of various malignant tumors such as gastric, lung and breast cancers, and more recently, pancreatic cancer [155].
Furthermore, it has been found that Siegesbeckia glabrescens sesquiterpenes suppress Gli-mediated transcriptional activity and proliferation of human pancreatic cells [156]. Also, recent studies indicate that Smo protein can be repressed by the secretion of Ptch-dependent (pro-)Vitamin D3 [65].
CONCLUSIONS AND FUTURE DIRECTIONS
Novel findings reveal multiple roles of the Hh signaling pathway in the development and progression of various cancers. Although the link between the Hh signaling pathway and tumorigenesis is very heterogeneous, it is known that the aberrant activation of Hh signaling leads to the growth, proliferation, and invasion of tumor cells. Therefore, further research and understanding of the specific role of deregulated activation of Hh signaling in different cancer types will hopefully contribute to the development of novel anti-cancer treatment modalities. Smo inhibitors represent a new and promising treatment option with possible benefits for some cancers. Their entry into clinical use provides a new avenue and hope for many patients with advanced and chemorefractory BCCs. Their use in the treatment of other cancer types, such as pancreatic cancer or MB, has also been proposed. However, due to harmful and potentially toxic side-effects of Smo inhibitors, undetermined safety in children, and the evidence that some patients develop resistance to Smo-inhibitors, efforts are being made to develop new classes of drugs. CSCs seem to be critical for tumorigenesis and HPI resistance in various tumors, so the combination of systemic HPI with other cytotoxic inhibitors is required. Further research studies should elucidate other mechanisms of Hh actions and translate their findings into novel, better, and safer anti-cancer therapies.
ACKNOWLEDGMENTS
This publication was co-financed by the European Union through the Europe Regional Development Fund, Operational Programme Competitiveness and Cohesion, under grant agreement No. KK.01.1.1.01.0008, Reproductive and Regenerative Medicine - Exploring New Platforms and Potentials.
DECLARATION OF INTERESTS
The authors declare no conflict of interests.
REFERENCES
- 1.Varjosalo M, Taipale J. Hedgehog: Functions and mechanisms. Genes Dev. 2008;22(18):2454–72. doi: 10.1101/gad.1693608. https://doi.org/10.1101/gad.1693608. [DOI] [PubMed] [Google Scholar]
- 2.Le H, Kleinerman R, Lerman OZ, Brown D, Galiano R, Gurtner GC, et al. Hedgehog signaling is essential for normal wound healing. Wound Repair Regen. 2008;16(6):768–73. doi: 10.1111/j.1524-475X.2008.00430.x. https://doi.org/10.1111/j.1524-475X.2008.00430.x. [DOI] [PubMed] [Google Scholar]
- 3.Lowry WE, Richter L, Yachechko R, Pyle AD, Tchieu J, Sridharan R, et al. Generation of human induced pluripotent stem cells from dermal fibroblasts. Proc Natl Acad Sci U S A. 2008;105(8):2883–8. doi: 10.1073/pnas.0711983105. https://doi.org/10.1073/pnas.0711983105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lewis MT, Veltmaat JM. Next top, the twilight zone: Hedgehog network regulation of mammary gland development. J Mammary Gland Biol Neoplasia. 2004;9(2):165–81. doi: 10.1023/B:JOMG.0000037160.24731.35. https://doi.org/10.1023/B: JOMG.0000037160.24731.35. [DOI] [PubMed] [Google Scholar]
- 5.Zhou JX, Jia LW, Liu WM, Miao CL, Liu S, Cao YJ, et al. Role of sonic hedgehog in maintaining a pool of proliferating stem cells in the human fetal epidermis. Hum Reprod. 2006;21(7):1698–704. doi: 10.1093/humrep/del086. https://doi.org/10.1093/humrep/del086. [DOI] [PubMed] [Google Scholar]
- 6.Stecca B, Mas C, Clement V, Zbinden M, Correa R, Piguet V, et al. Melanomas require HEDGEHOG-GLI signaling regulated by interactions between GLI1 and the RAS-MEK/AKT pathways. Proc Natl Acad Sci U S A. 2007;104(14):5895–900. doi: 10.1073/pnas.0700776104. https://doi.org/10.1073/pnas.0700776104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Detmer K, Thompson AJ, Garner RE, Walker AN, Gaffield W, Dannawi H, et al. Hedgehog signaling and cell cycle control in differentiating erythroid progenitors. Blood Cells Mol Dis. 2005;34(1):60–70. doi: 10.1016/j.bcmd.2004.08.026. https://doi.org/10.1016/j.bcmd.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 8.Watkins DN, Berman DM, Burkholder SG, Wang B, Beachy PA, Baylin SB. Hedgehog signalling within airway epithelial progenitors and in small-cell lung cancer. Nature. 2003;422(6929):313–7. doi: 10.1038/nature01493. https://doi.org/10.1038/nature01493. [DOI] [PubMed] [Google Scholar]
- 9.Beachy PA, Karhadkar SS, Berman DM. Tissue repair and stem cell renewal in carcinogenesis. Nature. 2004;432(7015):324–31. doi: 10.1038/nature03100. https://doi.org/10.1038/nature03100. [DOI] [PubMed] [Google Scholar]
- 10.Karhadkar SS, Bova GS, Abdallah N, Dhara S, Gardner D, Maitra A, et al. Hedgehog signalling in prostate regeneration, neoplasia and metastasis. Nature. 2004;431(7009):707–12. doi: 10.1038/nature02962. https://doi.org/10.1038/nature02962. [DOI] [PubMed] [Google Scholar]
- 11.Fendrich V, Esni F, Garay MV, Feldmann G, Habbe N, Jensen JN, et al. Hedgehog signaling is required for effective regeneration of exocrine pancreas. Gastroenterology. 2008;135(2):621–31. doi: 10.1053/j.gastro.2008.04.011. https://doi.org/10.1053/j.gastro.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Plotnikova OV, Golemis EA, Pugacheva EN. Cell cycle-dependent ciliogenesis and cancer. Cancer Res. 2008;68(7):2058–61. doi: 10.1158/0008-5472.CAN-07-5838. https://doi.org/10.1158/0008-5472.CAN-07-5838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Michaud EJ, Yoder BK. The primary cilium in cell signaling and cancer. Cancer Res. 2006;66(13):6463–7. doi: 10.1158/0008-5472.CAN-06-0462. https://doi.org/10.1158/0008-5472.CAN-06-0462. [DOI] [PubMed] [Google Scholar]
- 14.Bailey JM, Mohr AM, Hollingsworth MA. Sonic hedgehog paracrine signaling regulates metastasis and lymphangiogenesis in pancreatic cancer. Oncogene. 2009;28(40):3513–25. doi: 10.1038/onc.2009.220. https://doi.org/10.1038/onc.2009.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ma X, Sheng T, Zhang Y, Zhang X, He J, Huang S, et al. Hedgehog signaling is activated in subsets of esophageal cancers. Int J Cancer. 2006;118(1):139–48. doi: 10.1002/ijc.21295. https://doi.org/10.1002/ijc.21295. [DOI] [PubMed] [Google Scholar]
- 16.Sheng T, Li C, Zhang X, Chi S, He N, Chen K, et al. Activation of the hedgehog pathway in advanced prostate cancer. Mol Cancer. 2004;3:29. doi: 10.1186/1476-4598-3-29. https://doi.org/10.1186/1476-4598-3-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cross SS, Bury JP. The Hedgehog signalling pathways in human pathology. Curr Diagnostic Pathol. 2004;10(2):157–68. https://doi.org/10.1016/j.cdip.2003.11.005. [Google Scholar]
- 18.Denef N, Neubüser D, Perez L, Cohen SM. Hedgehog induces opposite changes in turnover and subcellular localization of patched and smoothened. Cell. 2000;102(4):521–31. doi: 10.1016/s0092-8674(00)00056-8. https://doi.org/10.1016/S0092-8674(00)00056-8. [DOI] [PubMed] [Google Scholar]
- 19.Price MA, Kalderon D. Proteolysis of the hedgehog signaling effector cubitus interruptus requires phosphorylation by glycogen synthase kinase 3 and casein kinase 1. Cell. 2002;108(6):823–35. doi: 10.1016/s0092-8674(02)00664-5. https://doi.org/10.1016/S0092-8674(02)00664-5. [DOI] [PubMed] [Google Scholar]
- 20.Goetz SC, Anderson KV. The primary cilium: A signalling centre during vertebrate development. Nat Rev Genet. 2010;11(5):331–44. doi: 10.1038/nrg2774. https://doi.org/10.1038/nrg2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mastronardi FG, Dimitroulakos J, Kamel-Reid S, Manoukian AS. Co-localization of patched and activated sonic hedgehog to lysosomes in neurons. Neuroreport. 2000;11(3):581–5. doi: 10.1097/00001756-200002280-00030. https://doi.org/10.1097/00001756-200002280-00030. [DOI] [PubMed] [Google Scholar]
- 22.Rubin LL, de Sauvage FJ. Targeting the hedgehog pathway in cancer. Nat Rev Drug Discov. 2006;5(12):1026–33. doi: 10.1038/nrd2086. https://doi.org/10.1038/nrd2086. [DOI] [PubMed] [Google Scholar]
- 23.Echelard Y, Epstein DJ, St-Jacques B, Shen L, Mohler J, McMahon JA, et al. Sonic hedgehog, a member of a family of putative signaling molecules, is implicated in the regulation of CNS polarity. Cell. 1993;75(7):1417–30. doi: 10.1016/0092-8674(93)90627-3. https://doi.org/10.1016/0092-8674(93)90627-3. [DOI] [PubMed] [Google Scholar]
- 24.Krauss S, Concordet JP, Ingham PW. A functionally conserved homolog of the drosophila segment polarity gene hh is expressed in tissues with polarizing activity in zebrafish embryos. Cell. 1993;75(7):1431–44. doi: 10.1016/0092-8674(93)90628-4. https://doi.org/10.1016/0092-8674(93)90628-4. [DOI] [PubMed] [Google Scholar]
- 25.Marigo V, Tabin CJ. Regulation of patched by sonic hedgehog in the developing neural tube. Proc Natl Acad Sci U S A. 1996;93(18):9346–51. doi: 10.1073/pnas.93.18.9346. https://doi.org/10.1073/pnas.93.18.9346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pathi S, Pagan-Westphal S, Baker DP, Garber EA, Rayhorn P, Bumcrot D, et al. Comparative biological responses to human sonic, indian, and desert hedgehog. Mech Dev. 2001;106(1-2):107–17. doi: 10.1016/s0925-4773(01)00427-0. https://doi.org/10.1016/S0925-4773(01)00427-0. [DOI] [PubMed] [Google Scholar]
- 27.Goodrich LV, Johnson RL, Milenkovic L, McMahon JA, Scott MP. Conservation of the hedgehog/patched signaling pathway from flies to mice: Induction of a mouse patched gene by hedgehog. Genes Dev. 1996;10(3):301–12. doi: 10.1101/gad.10.3.301. https://doi.org/10.1101/gad.10.3.301. [DOI] [PubMed] [Google Scholar]
- 28.Bellusci S, Furuta Y, Rush MG, Henderson R, Winnier G, Hogan BL, et al. Involvement of sonic hedgehog (Shh) in mouse embryonic lung growth and morphogenesis. Development. 1997;124(1):53–63. doi: 10.1242/dev.124.1.53. [DOI] [PubMed] [Google Scholar]
- 29.Hardcastle Z, Mo R, Hui CC, Sharpe PT. The shh signalling pathway in tooth development: Defects in gli2 and gli3 mutants. Development. 1998;125(15):2803–11. doi: 10.1242/dev.125.15.2803. [DOI] [PubMed] [Google Scholar]
- 30.Litingtung Y, Lei L, Westphal H, Chiang C. Sonic hedgehog is essential to foregut development. Nat Genet. 1998;20(1):58–61. doi: 10.1038/1717. https://doi.org/10.1038/1717. [DOI] [PubMed] [Google Scholar]
- 31.Vortkamp A, Lee K, Lanske B, Segre GV, Kronenberg HM, Tabin CJ, et al. Regulation of rate of cartilage differentiation by indian hedgehog and PTH-related protein. Science. 1996;273(5275):613–22. doi: 10.1126/science.273.5275.613. https://doi.org/10.1126/science.273.5275.613. [DOI] [PubMed] [Google Scholar]
- 32.van den Brink GR, Bleuming SA, Hardwick JC, Schepman BL, Offerhaus GJ, Keller JJ, et al. Indian hedgehog is an antagonist of wnt signaling in colonic epithelial cell differentiation. Nat Genet. 2004;36(3):277–82. doi: 10.1038/ng1304. https://doi.org/10.1038/ng1304. [DOI] [PubMed] [Google Scholar]
- 33.Robbins DJ, Fei DL, Riobo NA. The hedgehog signal transduction network. Sci Signal. 2012;5:246–re6. doi: 10.1126/scisignal.2002906. https://doi.org/10.1126/scisignal.2002906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bitgood MJ, Shen L, McMahon AP. Sertoli cell signaling by desert hedgehog regulates the male germline. Curr Biol. 1996;6(3):298–304. doi: 10.1016/s0960-9822(02)00480-3. https://doi.org/10.1016/S0960-9822(02)00480-3. [DOI] [PubMed] [Google Scholar]
- 35.Johnson RL, Scott MP. New players and puzzles in the hedgehog signaling pathway. Curr Opin Genet Dev. 1998;8(4):450–6. doi: 10.1016/s0959-437x(98)80117-2. https://doi.org/10.1016/S0959-437X(98)80117-2. [DOI] [PubMed] [Google Scholar]
- 36.Mann RK, Beachy PA. Novel lipid modifications of secreted protein signals. Annu Rev Biochem. 2004;73:891–923. doi: 10.1146/annurev.biochem.73.011303.073933. https://doi.org/10.1146/annurev.biochem.73.011303.073933. [DOI] [PubMed] [Google Scholar]
- 37.Buglino JA, Resh MD. Hhat is a palmitoylacyltransferase with specificity for N-palmitoylation of sonic hedgehog. J Biol Chem. 2008;283(32):22076–88. doi: 10.1074/jbc.M803901200. https://doi.org/10.1074/jbc.M803901200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zaphiropoulos PG, Undén AB, Rahnama F, Hollingsworth RE, Toftgård R. PTCH2, a novel human patched gene, undergoing alternative splicing and up-regulated in basal cell carcinomas. Cancer Res. 1999;59(3):787–92. [PubMed] [Google Scholar]
- 39.Gailani MR, Ståhle-Bäckdahl M, Leffell DJ, Glynn M, Zaphiropoulos PG, Pressman C, et al. The role of the human homologue of Drosophila patched in sporadic basal cell carcinomas. Nat Genet. 1996;14(1):78–81. doi: 10.1038/ng0996-78. https://doi.org/10.1038/ng0996-78. [DOI] [PubMed] [Google Scholar]
- 40.Johnson RL, Rothman AL, Xie J, Goodrich LV, Bare JW, Bonifas JM, et al. Human homolog of patched, a candidate gene for the basal cell nevus syndrome. Science. 1996;272(5268):1668–71. doi: 10.1126/science.272.5268.1668. https://doi.org/10.1126/science.272.5268.1668. [DOI] [PubMed] [Google Scholar]
- 41.Hahn H, Christiansen J, Wicking C, Zaphiropoulos PG, Chidambaram A, Gerrard B, et al. A mammalian patched homolog is expressed in target tissues of sonic hedgehog and maps to a region associated with developmental abnormalities. J Biol Chem. 1996;271(21):12125–8. doi: 10.1074/jbc.271.21.12125. https://doi.org/10.1074/jbc.271.21.12125. [DOI] [PubMed] [Google Scholar]
- 42.Smyth I, Narang MA, Evans T, Heimann C, Nakamura Y, Chenevix-Trench G, et al. Isolation and characterization of human patched 2 (PTCH2), a putative tumour suppressor gene inbasal cell carcinoma and medulloblastoma on chromosome 1p32. Hum Mol Genet. 1999;8(2):291–7. doi: 10.1093/hmg/8.2.291. https://doi.org/10.1093/hmg/8.2.291. [DOI] [PubMed] [Google Scholar]
- 43.Larsson NG, Wang J, Wilhelmsson H, Oldfors A, Rustin P, Lewandoski M, et al. Mitochondrial transcription factor A is necessary for mtDNA maintenance and embryogenesis in mice. Nat Genet. 1998;18(3):231–6. doi: 10.1038/ng0398-231. https://doi.org/10.1038/ng0398-231. [DOI] [PubMed] [Google Scholar]
- 44.Ingham PW. How cholesterol modulates the signal. Curr Biol. 2000;10(5):R180–3. doi: 10.1016/s0960-9822(00)00346-8. https://doi.org/10.1016/S0960-9822(00)00346-8. [DOI] [PubMed] [Google Scholar]
- 45.Ingham PW, Taylor AM, Nakano Y. Role of the Drosophila patched gene in positional signalling. Nature. 1991;353(6340):184–7. doi: 10.1038/353184a0. https://doi.org/10.1038/353184a0. [DOI] [PubMed] [Google Scholar]
- 46.Marigo V, Davey RA, Zuo Y, Cunningham JM, Tabin CJ. Biochemical evidence that patched is the hedgehog receptor. Nature. 1996;384(6605):176–9. doi: 10.1038/384176a0. https://doi.org/10.1038/384176a0. [DOI] [PubMed] [Google Scholar]
- 47.Johnson RL, Milenkovic L, Scott MP. In vivo functions of the patched protein: Requirement of the C terminus for target gene inactivation but not hedgehog sequestration. Mol Cell. 2000;6(2):467–78. doi: 10.1016/s1097-2765(00)00045-9. https://doi.org/10.1016/S1097-2765(00)00045-9. [DOI] [PubMed] [Google Scholar]
- 48.Taylor AM, Nakano Y, Mohler J, Ingham PW. Contrasting distributions of patched and hedgehog proteins in the drosophila embryo. Mech Dev. 1993;42(1-2):89–96. doi: 10.1016/0925-4773(93)90101-3. https://doi.org/10.1016/0925-4773(93)90101-3. [DOI] [PubMed] [Google Scholar]
- 49.Chen Y, Struhl G. Dual roles for patched in sequestering and transducing hedgehog. Cell. 1996;87(3):553–63. doi: 10.1016/s0092-8674(00)81374-4. https://doi.org/10.1016/S0092-8674(00)81374-4. [DOI] [PubMed] [Google Scholar]
- 50.Fan H, Khavari PA. Sonic hedgehog opposes epithelial cell cycle arrest. J Cell Biol. 1999;147(1):71–6. doi: 10.1083/jcb.147.1.71. https://doi.org/10.1083/jcb.147.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Duman-Scheel M, Weng L, Xin S, Du W. Hedgehog regulates cell growth and proliferation by inducing cyclin D and cyclin E. Nature. 2002;417:299–304. doi: 10.1038/417299a. https://doi.org/10.1038/417299a. [DOI] [PubMed] [Google Scholar]
- 52.Bhat KM, Schedl P. Requirement for engrailed and invected genes reveals novel regulatory interactions between engrailed/invected, patched, gooseberry and wingless during Drosophila neurogenesis. Development. 1997;124(9):1675–88. doi: 10.1242/dev.124.9.1675. [DOI] [PubMed] [Google Scholar]
- 53.Ramírez-Weber FA, Casso DJ, Aza-Blanc P, Tabata T, Kornberg TB. Hedgehog signal transduction in the posterior compartment of the Drosophila wing imaginal disc. Mol Cell. 2000;6(2):479–85. doi: 10.1016/s1097-2765(00)00046-0. https://doi.org/10.1016/S1097-2765(00)00046-0. [DOI] [PubMed] [Google Scholar]
- 54.Chuang PT, McMahon AP. Vertebrate hedgehog signalling modulated by induction of a hedgehog-binding protein. Nature. 1999;397(6720):617–21. doi: 10.1038/17611. https://doi.org/10.1038/17611. [DOI] [PubMed] [Google Scholar]
- 55.Tenzen T, Allen BL, Cole F, Kang JS, Krauss RS, McMahon AP, et al. The cell surface membrane proteins cdo and boc are components and targets of the hedgehog signaling pathway and feedback network in mice. Dev Cell. 2006;10(5):647–56. doi: 10.1016/j.devcel.2006.04.004. https://doi.org/10.1016/j.devcel.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 56.Martinelli DC, Fan CM. Gas1 extends the range of hedgehog action by facilitating its signaling. Genes Dev. 2007;21(10):1231–43. doi: 10.1101/gad.1546307. https://doi.org/10.1101/gad.1546307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jeong J, McMahon AP. Growth and pattern of the mammalian neural tube are governed by partially overlapping feedback activities of the hedgehog antagonists patched 1 and hhip1. Development. 2005;132(1):143–54. doi: 10.1242/dev.01566. https://doi.org/10.1242/dev.01566. [DOI] [PubMed] [Google Scholar]
- 58.Alcedo J, Ayzenzon M, Von Ohlen T, Noll M, Hooper JE. The Drosophila smoothened gene encodes a seven-pass membrane protein, a putative receptor for the hedgehog signal. Cell. 1996;86(2):221–32. doi: 10.1016/s0092-8674(00)80094-x. https://doi.org/10.1016/S0092-8674(00)80094-X. [DOI] [PubMed] [Google Scholar]
- 59.Rana R, Carroll CE, Lee HJ, Bao J, Marada S, Grace CR, et al. Structural insights into the role of the smoothened cysteine-rich domain in hedgehog signalling. Nat Commun. 2013;4:2965. doi: 10.1038/ncomms3965. https://doi.org/10.1038/ncomms3965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Murone M, Rosenthal A, de Sauvage FJ. Hedgehog signal transduction: From flies to vertebrates. Exp Cell Res. 1999;253(1):25–33. doi: 10.1006/excr.1999.4676. https://doi.org/10.1006/excr.1999.4676. [DOI] [PubMed] [Google Scholar]
- 61.Stone DM, Hynes M, Armanini M, Swanson TA, Gu Q, Johnson RL, et al. The tumour-suppressor gene patched encodes a candidate receptor for sonic hedgehog. Nature. 1996;384(6605):129–34. doi: 10.1038/384129a0. https://doi.org/10.1038/384129a0. [DOI] [PubMed] [Google Scholar]
- 62.Taipale J, Cooper MK, Maiti T, Beachy PA. Patched acts catalytically to suppress the activity of smoothened. Nature. 2002;418(6900):892–7. doi: 10.1038/nature00989. https://doi.org/10.1038/nature00989. [DOI] [PubMed] [Google Scholar]
- 63.Kim J, Hsia EY, Brigui A, Plessis A, Beachy PA, Zheng X, et al. The role of ciliary trafficking in hedgehog receptor signaling. Sci Signal. 2015;8:379–ra55. doi: 10.1126/scisignal.aaa5622. https://doi.org/10.1126/scisignal.aaa5622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Corcoran RB, Scott MP. Oxysterols stimulate sonic hedgehog signal transduction and proliferation of medulloblastoma cells. Proc Natl Acad Sci U S A. 2006;103(22):8408–13. doi: 10.1073/pnas.0602852103. https://doi.org/10.1073/pnas.0602852103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bijlsma MF, Spek CA, Zivkovic D, van de Water S, Rezaee F, Peppelenbosch MP, et al. Repression of smoothened by patched-dependent (pro-)vitamin D3 secretion. PLoS Biol. 2006;4:8–e232. doi: 10.1371/journal.pbio.0040232. https://doi.org/10.1371/journal.pbio.0040232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jia J, Tong C, Wang B, Luo L, Jiang J. Hedgehog signalling activity of smoothened requires phosphorylation by protein kinase A and casein kinase I. Nature. 2004;432(7020):1045–50. doi: 10.1038/nature03179. https://doi.org/10.1038/nature03179. [DOI] [PubMed] [Google Scholar]
- 67.Kinzler KW, Bigner SH, Bigner DD, Trent JM, Law ML, O’Brien SJ, et al. Identification of an amplified, highly expressed gene in a human glioma. Science. 1987;236(4797):70–3. doi: 10.1126/science.3563490. https://doi.org/10.1126/science.3563490. [DOI] [PubMed] [Google Scholar]
- 68.Hui CC, Slusarski D, Platt KA, Holmgren R, Joyner AL. Expression of three mouse homologs of the Drosophila segment polarity gene cubitus interruptus, gli, gli-2, and gli-3, in ectoderm-and mesoderm-derived tissues suggests multiple roles during postimplantation development. Dev Biol. 1994;162(2):402–13. doi: 10.1006/dbio.1994.1097. https://doi.org/10.1006/dbio.1994.1097. [DOI] [PubMed] [Google Scholar]
- 69.Hynes M, Stone DM, Dowd M, Pitts-Meek S, Goddard A, Gurney A, et al. Control of cell pattern in the neural tube by the zinc finger transcription factor and oncogene gli-1. Neuron. 1997;19(1):15–26. doi: 10.1016/s0896-6273(00)80344-x. https://doi.org/10.1016/S0896-6273(00)80344-X. [DOI] [PubMed] [Google Scholar]
- 70.Regl G, Neill GW, Eichberger T, Kasper M, Ikram MS, Koller J, et al. Human GLI2 and GLI1 are part of a positive feedback mechanism in basal cell carcinoma. Oncogene. 2002;21(36):5529–39. doi: 10.1038/sj.onc.1205748. https://doi.org/10.1038/sj.onc.1205748. [DOI] [PubMed] [Google Scholar]
- 71.Yoon JW, Liu CZ, Yang JT, Swart R, Iannaccone P, Walterhouse D, et al. GLI activates transcription through a herpes simplex viral protein 16-like activation domain. J Biol Chem. 1998;273(6):3496–501. doi: 10.1074/jbc.273.6.3496. https://doi.org/10.1074/jbc.273.6.3496. [DOI] [PubMed] [Google Scholar]
- 72.Persson M, Stamataki D, te Welscher P, Andersson E, Böse J, Rüther U, et al. Dorsal-ventral patterning of the spinal cord requires gli3 transcriptional repressor activity. Genes Dev. 2002;16(22):2865–78. doi: 10.1101/gad.243402. https://doi.org/10.1101/gad.243402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sasaki H, Hui C, Nakafuku M, Kondoh H. A binding site for gli proteins is essential for HNF-3beta floor plate enhancer activity in transgenics and can respond to shh in vitro. Development. 1997;124(7):1313–22. doi: 10.1242/dev.124.7.1313. [DOI] [PubMed] [Google Scholar]
- 74.Kinzler KW, Vogelstein B. The GLI gene encodes a nuclear protein which binds specific sequences in the human genome. Mol Cell Biol. 1990;10(2):634–42. doi: 10.1128/mcb.10.2.634. https://doi.org/10.1128/MCB.10.2.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tanimura A, Dan S, Yoshida M. Cloning of novel isoforms of the human gli2 oncogene and their activities to enhance tax-dependent transcription of the human T-cell leukemia virus Type 1 genome. J Virol. 1998;72(5):3958–64. doi: 10.1128/jvi.72.5.3958-3964.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pan Y, Bai CB, Joyner AL, Wang B. Sonic hedgehog signaling regulates gli2 transcriptional activity by suppressing its processing and degradation. Mol Cell Biol. 2006;26(9):3365–77. doi: 10.1128/MCB.26.9.3365-3377.2006. https://doi.org/10.1128/MCB.26.9.3365-3377.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Humke EW, Dorn KV, Milenkovic L, Scott MP, Rohatgi R. The output of hedgehog signaling is controlled by the dynamic association between suppressor of fused and the gli proteins. Genes Dev. 2010;24(7):670–82. doi: 10.1101/gad.1902910. https://doi.org/10.1101/gad.1902910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang Y, Ding Q, Yen CJ, Xia W, Izzo JG, Lang JY, et al. The crosstalk of mTOR/S6K1 and hedgehog pathways. Cancer Cell. 2012;21(3):374–87. doi: 10.1016/j.ccr.2011.12.028. https://doi.org/10.1016/j.ccr.2011.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mimeault M, Rachagani S, Muniyan S, Seshacharyulu P, Johansson SL, Datta K, et al. Inhibition of hedgehog signaling improves the anti-carcinogenic effects of docetaxel in prostate cancer. Oncotarget. 2015;6(6):3887–903. doi: 10.18632/oncotarget.2932. https://doi.org/10.18632/oncotarget.2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Stone DM, Murone M, Luoh S, Ye W, Armanini MP, Gurney A, et al. Characterization of the human suppressor of fused, a negative regulator of the zinc-finger transcription factor gli. J Cell Sci. 1999;112:4437–48. doi: 10.1242/jcs.112.23.4437. [DOI] [PubMed] [Google Scholar]
- 81.Tukachinsky H, Lopez LV, Salic A. A mechanism for vertebrate hedgehog signaling: Recruitment to cilia and dissociation of SuFu-Gli protein complexes. J Cell Biol. 2010;191(2):415–28. doi: 10.1083/jcb.201004108. https://doi.org/10.1083/jcb.201004108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Méthot N, Basler K. Suppressor of fused opposes hedgehog signal transduction by impeding nuclear accumulation of the activator form of cubitus interruptus. Development. 2000;127(18):4001–10. doi: 10.1242/dev.127.18.4001. [DOI] [PubMed] [Google Scholar]
- 83.Chen M, Wilson CW, Li Y, Ruel L, Thérond PP, King K, et al. Cilium-independent regulation of gli protein function by sufu in hedgehog signaling is evolutionarily conserved. Genes Dev. 2009;23(16):1910–28. doi: 10.1101/gad.1794109. https://doi.org/10.1101/gad.1794109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liem KF, Jr, He M, Ocbina PJ, Anderson KV. Mouse kif7/Costal2 is a cilia-associated protein that regulates sonic hedgehog signaling. Proc Natl Acad Sci U S A. 2009;106(32):13377–82. doi: 10.1073/pnas.0906944106. https://doi.org/10.1073/pnas.0906944106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.He M, Subramanian R, Bangs F, Omelchenko T, Liem KF, Jr, Kapoor TM, et al. The kinesin-4 protein kif7 regulates mammalian hedgehog signalling by organizing the cilium tip compartment. Nat Cell Biol. 2014;16(7):663–72. doi: 10.1038/ncb2988. https://doi.org/10.1038/ncb2988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Haycraft CJ, Banizs B, Aydin-Son Y, Zhang Q, Michaud EJ, Yoder BK, et al. Gli2 and gli3 localize to cilia and require the intraflagellar transport protein polaris for processing and function. PLoS Genet. 2005;1:4–e53. doi: 10.1371/journal.pgen.0010053. https://doi.org/10.1371/journal.pgen.0010053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Liu YC, Couzens AL, Deshwar AR, McBroom-Cerajewski LD, Zhang X, Puviindran V, et al. The PPFIA1-PP2A protein complex promotes trafficking of kif7 to the ciliary tip and hedgehog signaling. Sci Signal. 2014;7:355–ra117. doi: 10.1126/scisignal.2005608. https://doi.org/10.1126/scisignal.2005608. [DOI] [PubMed] [Google Scholar]
- 88.Jiang J, Struhl G. Protein kinase A and hedgehog signaling in Drosophila limb development. Cell. 1995;80(4):563–72. doi: 10.1016/0092-8674(95)90510-3. https://doi.org/10.1016/0092-8674(95)90510-3. [DOI] [PubMed] [Google Scholar]
- 89.Barzi M, Berenguer J, Menendez A, Alvarez-Rodriguez R, Pons S. Sonic-hedgehog-mediated proliferation requires the localization of PKA to the cilium base. J Cell Sci. 2010;123(1):62–9. doi: 10.1242/jcs.060020. https://doi.org/10.1242/jcs.060020. [DOI] [PubMed] [Google Scholar]
- 90.Wang B, Fallon JF, Beachy PA. Hedgehog-regulated processing of gli3 produces an anterior/posterior repressor gradient in the developing vertebrate limb. Cell. 2000;100(4):423–34. doi: 10.1016/s0092-8674(00)80678-9. https://doi.org/10.1016/S0092-8674(00)80678-9. [DOI] [PubMed] [Google Scholar]
- 91.Li W, Ohlmeyer JT, Lane ME, Kalderon D. Function of protein kinase a in hedgehog signal transduction and Drosophila imaginal disc development. Cell. 1995;80(4):553–62. doi: 10.1016/0092-8674(95)90509-x. https://doi.org/10.1016/0092-8674(95)90509-X. [DOI] [PubMed] [Google Scholar]
- 92.Dai P, Akimaru H, Tanaka Y, Maekawa T, Nakafuku M, Ishii S, et al. Sonic hedgehog-induced activation of the gli1 promoter is mediated by GLI3. J Biol Chem. 1999;274(12):8143–52. doi: 10.1074/jbc.274.12.8143. https://doi.org/10.1074/jbc.274.12.8143. [DOI] [PubMed] [Google Scholar]
- 93.Bonifas JM, Pennypacker S, Chuang PT, McMahon AP, Williams M, Rosenthal A, et al. Activation of expression of hedgehog target genes in basal cell carcinomas. J Invest Dermatol. 2001;116(5):739–42. doi: 10.1046/j.1523-1747.2001.01315.x. https://doi.org/10.1046/j.1523-1747.2001.01315.x. [DOI] [PubMed] [Google Scholar]
- 94.Bigelow RL, Chari NS, Unde AB, Spurgers KB, Lee S, Roop DR, et al. Transcriptional regulation of bcl-2 mediated by the sonic hedgehog signaling pathway through gli-1. J Biol Chem. 2004;279:1197–205. doi: 10.1074/jbc.M310589200. https://doi.org/10.1074/jbc.M310589200. [DOI] [PubMed] [Google Scholar]
- 95.Oliver TG, Grasfeder LL, Carroll AL, Kaiser C, Gillingham CL, Lin SM, et al. Transcriptional profiling of the sonic hedgehog response: A critical role for N-myc in proliferation of neuronal precursors. Proc Natl Acad Sci U S A. 2003;100(12):7331–6. doi: 10.1073/pnas.0832317100. https://doi.org/10.1073/pnas.0832317100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Singh RR, Kunkalla K, Qu C, Schlette E, Neelapu SS, Samaniego F, et al. ABCG2 is a direct transcriptional target of hedgehog signaling and involved in stroma-induced drug tolerance in diffuse large B-cell lymphoma. Oncogene. 2011;30(49):4874–86. doi: 10.1038/onc.2011.195. https://doi.org/10.1038/onc.2011.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bouldin CM, Harfe BD. Aberrant FGF signaling, independent of ectopic hedgehog signaling, initiates preaxial polydactyly in dorking chickens. Dev Biol. 2009;334(1):133–41. doi: 10.1016/j.ydbio.2009.07.009. https://doi.org/10.1016/j.ydbio.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 98.Morrow D, Cullen JP, Liu W, Guha S, Sweeney C, Birney YA, et al. Sonic hedgehog induces notch target gene expression in vascular smooth muscle cells via VEGF-A. Arterioscler Thromb Vasc Biol. 2009;29(7):1112–8. doi: 10.1161/ATVBAHA.109.186890. https://doi.org/10.1161/ATVBAHA.109.186890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Macdonald R, Barth K, Xu Q, Holder N, Mikkola I, Wilson SW. Midline signalling is required for pax gene regulation and pattering of the eyes. Development. 1995;121(10):3267–78. doi: 10.1242/dev.121.10.3267. [DOI] [PubMed] [Google Scholar]
- 100.Teh MT, Wong ST, Neill GW, Ghali LR, Philpott MP, Quinn AG, et al. FOXM1 is a downstream target of gli1 in basal cell carcinomas. Cancer Res. 2002;62(16):4773–80. [PubMed] [Google Scholar]
- 101.Katoh M, Katoh M. Notch ligand, JAG1, is evolutionarily conserved target of canonical WNT signaling pathway in progenitor cells. Int J Mol Med. 2006;17(4):681–5. https://doi.org/10.3892/ijmm.17.4.681. [PubMed] [Google Scholar]
- 102.Mullor JL, Dahmane N, Sun T, Ruizi Altaba A. Wnt signals are targets and mediators of gli function. Curr Biol. 2001;11(10):769–73. doi: 10.1016/s0960-9822(01)00229-9. https://doi.org/10.1016/S0960-9822(01)00229-9. [DOI] [PubMed] [Google Scholar]
- 103.Taipale J, Beachy PA. The hedgehog and wnt signalling pathways in cancer. Nature. 2001;411(6835):349–54. doi: 10.1038/35077219. https://doi.org/10.1038/35077219. [DOI] [PubMed] [Google Scholar]
- 104.Hahn H, Wicking C, Zaphiropoulous PG, Gailani MR, Shanley S, Chidambaram A, et al. Mutations of the human homolog of Drosophila patched in the nevoid basal cell carcinoma syndrome. Cell. 1996;85(6):841–51. doi: 10.1016/s0092-8674(00)81268-4. https://doi.org/10.1016/S0092-8674(00)81268-4. [DOI] [PubMed] [Google Scholar]
- 105.Reifenberger J, Wolter M, Knobbe CB, Köhler B, Schönicke A, Scharwächter C, et al. Somatic mutations in the PTCH, SMOH, SUFUH and TP53 genes in sporadic basal cell carcinomas. Br J Dermatol. 2005;152(1):43–51. doi: 10.1111/j.1365-2133.2005.06353.x. https://doi.org/10.1111/j.1365-2133.2005.06353.x. [DOI] [PubMed] [Google Scholar]
- 106.Johnson RL, Rothman AL, Xie J, Goodrich LV, Bare JW, Bonifas JM, et al. Human homolog of patched, a candidate gene for the basal cell nevus syndrome. Science. 1996;272(5268):1668–71. doi: 10.1126/science.272.5268.1668. https://doi.org/10.1126/science.272.5268.1668. [DOI] [PubMed] [Google Scholar]
- 107.Dahmane N, Lee J, Robins P, Heller P, Ruizi Altaba A. Activation of the transcription factor gli1 and the sonic hedgehog signalling pathway in skin tumours. Nature. 1997;389(6653):876–81. doi: 10.1038/39918. https://doi.org/10.1038/39918. [DOI] [PubMed] [Google Scholar]
- 108.Aszterbaum M, Epstein J, Oro A, Douglas V, LeBoit PE, Scott MP, et al. Ultraviolet and ionizing radiation enhance the growth of BCCs and trichoblastomas in patched heterozygous knockout mice. Nat Med. 1999;5(11):1285–91. doi: 10.1038/15242. https://doi.org/10.1038/15242. [DOI] [PubMed] [Google Scholar]
- 109.Lee Y, Kawagoe R, Sasai K, Li Y, Russell HR, Curran T, et al. Loss of suppressor-of-fused function promotes tumorigenesis. Oncogene. 2007;26(44):6442–7. doi: 10.1038/sj.onc.1210467. https://doi.org/10.1038/sj.onc.1210467. [DOI] [PubMed] [Google Scholar]
- 110.Kool M, Jones DT, Jäger N, Northcott PA, Pugh TJ, Hovestadt V, et al. Genome sequencing of SHH medulloblastoma predicts genotype-related response to smoothened inhibition. Cancer Cell. 2014;25(3):393–405. doi: 10.1016/j.ccr.2014.02.004. https://doi.org/10.1016/j.ccr.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lo Muzio L. Nevoid basal cell carcinoma syndrome (Gorlin syndrome) Orphanet J Rare Dis. 2008;3(32):1–16. doi: 10.1186/1750-1172-3-32. DOI: 10.1186/1750-1172-3-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Romer JT, Kimura H, Magdaleno S, Sasai K, Fuller C, Baines H, et al. Suppression of the shh pathway using a small molecule inhibitor eliminates medulloblastoma in ptc1(+/-)p53(-/-) mice. Cancer Cell. 2004;6(3):229–40. doi: 10.1016/j.ccr.2004.08.019. https://doi.org/10.1016/j.ccr.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 113.Raffel C, Jenkins RB, Frederick L, Hebrink D, Alderete B, Fults DW, et al. Sporadic medulloblastomas contain PTCH mutations. Cancer Res. 1997;57(5):842–5. [PubMed] [Google Scholar]
- 114.Taylor MD, Liu L, Raffel C, Hui CC, Mainprize TG, Zhang X, et al. Mutations in SUFU predispose to medulloblastoma. Nat Genet. 2002;31(3):306–10. doi: 10.1038/ng916. https://doi.org/10.1038/ng916. [DOI] [PubMed] [Google Scholar]
- 115.Iglesias-Bartolome R, Torres D, Marone R, Feng X, Martin D, Simaan M, et al. Inactivation of a Gαs–PKA tumour suppressor pathway in skin stem cells initiates basal-cell carcinogenesis. Nat Cell Biol. 2015;17(6):793–803. doi: 10.1038/ncb3164. https://doi.org/10.1038/ncb3164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Vorechovský I, Undén AB, Sandstedt B, Toftgård R, Ståhle-Bäckdahl M. Trichoepitheliomas contain somatic mutations in the overexpressed PTCH gene: Support for a gatekeeper mechanism in skin tumorigenesis. Cancer Res. 1997;57(21):4677–81. [PubMed] [Google Scholar]
- 117.Maesawa C, Tamura G, Iwaya T, Ogasawara S, Ishida K, Sato N, et al. Mutations in the human homologue of the Drosophila patched gene in esophageal squamous cell carcinoma. Genes Chromosomes Cancer. 1998;21(3):276–9. https://doi.org/10.1002/(SICI)1098-2264(199803)21:3<276: AID-GCC15>3.0.CO;2-N. [PubMed] [Google Scholar]
- 118.McGarvey TW, Maruta Y, Tomaszewski JE, Linnenbach AJ, Malkowicz SB. PTCH gene mutations in invasive transitional cell carcinoma of the bladder. Oncogene. 1998;17(9):1167–72. doi: 10.1038/sj.onc.1202045. https://doi.org/10.1038/sj.onc.1202045. [DOI] [PubMed] [Google Scholar]
- 119.Almazán-Moga A, Zarzosa P, Molist C, Velasco P, Pyczek J, Simon-Keller K, et al. Ligand-dependent Hedgehog pathway activation in rhabdomyosarcoma: The oncogenic role of the ligands. Br J Cancer. 2017;117(9):1314–25. doi: 10.1038/bjc.2017.305. https://doi.org/10.1038/bjc.2017.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Berman DM, Karhadkar SS, Maitra A, Montes De Oca R, Gerstenblith MR, Briggs K, et al. Widespread requirement for hedgehog ligand stimulation in growth of digestive tract tumours. Nature. 2003;425(6960):846–51. doi: 10.1038/nature01972. https://doi.org/10.1038/nature01972. [DOI] [PubMed] [Google Scholar]
- 121.Gulino A, Ferretti E, De Smaele E. Hedgehog signalling in colon cancer and stem cells. EMBO Mol Med. 2009;1(6-7):300–2. doi: 10.1002/emmm.200900042. https://doi.org/10.1002/emmm.200900042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Szkandera J, Kiesslich T, Haybaeck J, Gerger A, Pichler M. Hedgehog signaling pathway in ovarian cancer. Int J Mol Sci. 2013;14(1):1179–96. doi: 10.3390/ijms14011179. https://doi.org/10.3390/ijms14011179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kubo M, Nakamura M, Tasaki A, Yamanaka N, Nakashima H, Nomura M, et al. Hedgehog signaling pathway is a new therapeutic target for patients with breast cancer. Cancer Res. 2004;64(17):6071–4. doi: 10.1158/0008-5472.CAN-04-0416. https://doi.org/10.1158/0008-5472.CAN-04-0416. [DOI] [PubMed] [Google Scholar]
- 124.O’Reilly KE, de Miera EV, Segura MF, Friedman E, Poliseno L, Han SW, et al. Hedgehog pathway blockade inhibits melanoma cell growth in vitro and in vivo . Pharmaceuticals (Basel) 2013;6(11):1429–50. doi: 10.3390/ph6111429. https://doi.org/10.3390/ph6111429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Becher OJ, Hambardzumyan D, Fomchenko EI, Momota H, Mainwaring L, Bleau AM, et al. Gli activity correlates with tumor grade in platelet-derived growth factor-induced gliomas. Cancer Res. 2008;68(7):2241–9. doi: 10.1158/0008-5472.CAN-07-6350. https://doi.org/10.1158/0008-5472.CAN-07-6350. [DOI] [PubMed] [Google Scholar]
- 126.Monzo M, Moreno I, Artells R, Ibeas R, Navarro A, Moreno J, et al. Sonic hedgehog mRNA expression by real-time quantitative PCR in normal and tumor tissues from colorectal cancer patients. Cancer Lett. 2006;233(1):117–23. doi: 10.1016/j.canlet.2005.03.001. https://doi.org/10.1016/j.canlet.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 127.Douard R, Moutereau S, Pernet P, Chimingqi M, Allory Y, Manivet P, et al. Sonic hedgehog-dependent proliferation in a series of patients with colorectal cancer. Surgery. 2006;139(5):665–70. doi: 10.1016/j.surg.2005.10.012. https://doi.org/10.1016/j.surg.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 128.van den Brink GR, Bleuming SA, Hardwick JC, Schepman BL, Offerhaus GJ, Keller JJ, et al. Indian hedgehog is an antagonist of wnt signaling in colonic epithelial cell differentiation. Nat Genet. 2004;36(3):277–82. doi: 10.1038/ng1304. https://doi.org/10.1038/ng1304. [DOI] [PubMed] [Google Scholar]
- 129.Akiyoshi T, Nakamura M, Koga K, Nakashima H, Yao T, Tsuneyoshi M, et al. Gli1, downregulated in colorectal cancers, inhibits proliferation of colon cancer cells involving wnt signalling activation. Gut. 2006;55(7):991–9. doi: 10.1136/gut.2005.080333. https://doi.org/10.1136/gut.2005.080333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ingham PW, McMahon AP. Hedgehog signaling in animal development: Paradigms and principles. Genes Dev. 2001;15(23):3059–87. doi: 10.1101/gad.938601. https://doi.org/10.1101/gad.938601. [DOI] [PubMed] [Google Scholar]
- 131.Jiang J, Hui CC. Hedgehog signaling in development and cancer. Dev Cell. 2008;15(6):801–12. doi: 10.1016/j.devcel.2008.11.010. https://doi.org/10.1016/j.devcel.2008.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Fan L, Pepicelli CV, Dibble CC, Catbagan W, Zarycki JL, Laciak R, et al. Hedgehog signaling promotes prostate xenograft tumor growth. Endocrinology. 2004;145(8):3961–70. doi: 10.1210/en.2004-0079. https://doi.org/10.1210/en.2004-0079. [DOI] [PubMed] [Google Scholar]
- 133.Theunissen J, Sauvage FJ De. Paracrine hedgehog signaling in cancer. Cancer Res. 2009;69(15):6007–11. doi: 10.1158/0008-5472.CAN-09-0756. https://doi.org/10.1158/0008-5472.CAN-09-0756. [DOI] [PubMed] [Google Scholar]
- 134.Read TA, Fogarty MP, Markant SL, McLendon RE, Wei Z, Ellison DW, et al. Identification of CD15 as a marker for tumor-propagating cells in a mouse model of medulloblastoma. Cancer Cell. 2009;15(2):135–47. doi: 10.1016/j.ccr.2008.12.016. https://doi.org/10.1016/j.ccr.2008.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zhao C, Chen A, Jamieson CH, Fereshteh M, Abrahamsson A, Blum J, et al. Hedgehog signalling is essential for maintenance of cancer stem cells in myeloid leukaemia. Nature. 2009;458(7239):776–9. doi: 10.1038/nature07737. https://doi.org/10.1038/nature07737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Nicolis SK. Cancer stem cells and “stemness” genes in neuro-oncology. Neurobiol Dis. 2007;25(2):217–29. doi: 10.1016/j.nbd.2006.08.022. https://doi.org/10.1016/j.nbd.2006.08.022. [DOI] [PubMed] [Google Scholar]
- 137.Peacock CD, Wang Q, Gesell GS, Corcoran-Schwartz IM, Jones E, Kim J, et al. Hedgehog signaling maintains a tumor stem cell compartment in multiple myeloma. Proc Natl Acad Sci U S A. 2007;104(10):4048–53. doi: 10.1073/pnas.0611682104. https://doi.org/10.1073/pnas.0611682104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Dembinski JL, Krauss S. Characterization and functional analysis of a slow cycling stem cell-like subpopulation in pancreas adenocarcinoma. Clin Exp Metastasis. 2009;26(7):611–23. doi: 10.1007/s10585-009-9260-0. https://doi.org/10.1007/s10585-009-9260-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Liu S, Dontu G, Mantle ID, Patel S, Ahn NS, Jackson KW, et al. Hedgehog signaling and bmi-1 regulate self-renewal of normal and malignant human mammary stem cells. Cancer Res. 2006;66(12):6063–71. doi: 10.1158/0008-5472.CAN-06-0054. https://doi.org/10.1158/0008-5472.CAN-06-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Long B, Zhu H, Zhu C, Liu T, Meng W. Activation of the hedgehog pathway in chronic myelogeneous leukemia patients. J Exp Clin Cancer Res. 2011;30:1–8. doi: 10.1186/1756-9966-30-8. https://doi.org/10.1186/1756-9966-30-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lou H, Dean M. Targeted therapy for cancer stem cells: The patched pathway and ABC transporters. Oncogene. 2007;26(9):1357–60. doi: 10.1038/sj.onc.1210200. https://doi.org/10.1038/sj.onc.1210200. [DOI] [PubMed] [Google Scholar]
- 142.Cretnik M, Musani V, Oreskovic S, Leovic D, Levanat S. The patched gene is epigenetically regulated in ovarian dermoids and fibromas, but not in basocellular carcinomas. Int J Mol Med. 2007;19(6):875–83. https://doi.org/10.3892/ijmm.19.6.875. [PubMed] [Google Scholar]
- 143.Wolf I, Bose S, Desmond JC, Lin BT, Williamson EA, Karlan BY, et al. Unmasking of epigenetically silenced genes reveals DNA promoter methylation and reduced expression of PTCH in breast cancer. Breast Cancer Res Treat. 2007;105(2):139–55. doi: 10.1007/s10549-006-9440-4. https://doi.org/10.1007/s10549-006-9440-4. [DOI] [PubMed] [Google Scholar]
- 144.Shi X, Zhang Z, Zhan X, Cao M, Satoh T, Akira S, et al. An epigenetic switch induced by Shh signalling regulates gene activation during development and medulloblastoma growth. Nat Commun. 2014;5:5425. doi: 10.1038/ncomms6425. https://doi.org/10.1038/ncomms6425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Huang S, He J, Zhang X, Bian Y, Yang L, Xie G, et al. Activation of the hedgehog pathway in human hepatocellular carcinomas. Carcinogenesis. 2006;27(7):1334–40. doi: 10.1093/carcin/bgi378. https://doi.org/10.1093/carcin/bgi378. [DOI] [PubMed] [Google Scholar]
- 146.Lauressergues E, Heusler P, Lestienne F, Troulier D, Rauly-Lestienne I, Tourette A, et al. Pharmacological evaluation of a series of smoothened antagonists in signaling pathways and after topical application in a depilated mouse model. Pharmacol Res Perspect. 2016;4(2):e00214. doi: 10.1002/prp2.214. DOI: 10.1002/prp2.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Yang L, Xie G, Fan Q, Xie J. Activation of the hedgehog-signaling pathway in human cancer and the clinical implications. Oncogene. 2010;29(4):469–81. doi: 10.1038/onc.2009.392. https://doi.org/10.1038/onc.2009.392. [DOI] [PubMed] [Google Scholar]
- 148.Low JA, de Sauvage FJ. Clinical experience with hedgehog pathway inhibitors. J Clin Oncol. 2010;28(36):5321–6. doi: 10.1200/JCO.2010.27.9943. https://doi.org/10.1200/JCO.2010.27.9943. [DOI] [PubMed] [Google Scholar]
- 149.Goel V, Hurh E, Stein A, Nedelman J, Zhou J, Chiparus O, et al. Population pharmacokinetics of sonidegib (LDE225), an oral inhibitor of hedgehog pathway signaling, in healthy subjects and in patients with advanced solid tumors. Cancer Chemother Pharmacol. 2016;77(4):745–55. doi: 10.1007/s00280-016-2982-1. https://doi.org/10.1007/s00280-016-2982-1. [DOI] [PubMed] [Google Scholar]
- 150.Basset-Seguin N, Sharpe HJ, de Sauvage FJ. Efficacy of hedgehog pathway inhibitors in basal cell carcinoma. Mol Cancer Ther. 2015;14(3):633–41. doi: 10.1158/1535-7163.MCT-14-0703. https://doi.org/10.1158/1535-7163.MCT-14-0703. [DOI] [PubMed] [Google Scholar]
- 151.Kim J, Aftab BT, Tang JY, Kim D, Lee AH, Rezaee M, et al. Itraconazole and arsenic trioxide inhibit hedgehog pathway activation and tumor growth associated with acquired resistance to smoothened antagonists. Cancer Cell. 2013;23(1):23–34. doi: 10.1016/j.ccr.2012.11.017. https://doi.org/10.1016/j.ccr.2012.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Long J, Li B, Rodriguez-Blanco J, Pastori C, Volmar CH, Wahlestedt C, et al. The BET bromodomain inhibitor I-BET151 acts downstream of smoothened protein to abrogate the growth of hedgehog protein-driven cancers. J Biol Chem. 2014;289(51):35494–502. doi: 10.1074/jbc.M114.595348. https://doi.org/10.1074/jbc.M114.595348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Ge X, Milenkovic L, Suyama K, Hartl T, Purzner T, Winans A, et al. Phosphodiesterase 4D acts downstream of neuropilin to control hedgehog signal transduction and the growth of medulloblastoma. Elife. 2015;4:e07068. doi: 10.7554/eLife.07068. https://doi.org/10.7554/eLife.07068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Atwood SX, Li M, Lee A, Tang JY, Oro AE. GLI activation by atypical protein kinase C ι/λ regulates the growth of basal cell carcinomas. Nature. 2013;494(7438):484–8. doi: 10.1038/nature11889. https://doi.org/10.1038/nature11889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Mehta R, Katta H, Alimirah F, Patel R, Murillo G, Peng X, et al. Deguelin action involves c-met and EGFR signaling pathways in triple negative breast cancer cells. PLoS One. 2013;8:6–e65113. doi: 10.1371/journal.pone.0065113. https://doi.org/10.1371/journal.pone.0065113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Cho YR, Choi SW, Seo DW. The in vitro antitumor activity of Siegesbeckia glabrescens against ovarian cancer through suppression of receptor tyrosine kinase expression and the signaling pathways. Oncol Rep. 2013;30(1):221–6. doi: 10.3892/or.2013.2468. https://doi.org/10.3892/or.2013.2468. [DOI] [PubMed] [Google Scholar]


