Abstract
Breast fibromatosis, also referred to as desmoid tumor or aggressive fibromatosis, is a very rare, locally aggressive disease that does not metastasize. Bilateral lesions are extremely rare and are found in only 4% of patients with breast fibromatosis. Tumor recurrence following surgery occurs in 18%-29% of patients, most often within the first 2 years after surgery. In this report, we discuss a case of breast fibromatosis, mimicking a breast carcinoma both clinically and radiologically, that presented clinically with dimpling of the skin of the left breast in a 31-year-old woman. The patient relapsed a few months after surgery, with a multicentric and bilateral disease.
Keywords: Breast fibromatosis, Desmoid tumor, Extra-abdominal fibromatosis, Spindle cell tumors
Introduction
Fibromatosis of the breast, also known as aggressive fibromatosis, desmoid tumor, or low-grade fibrosarcoma, may occur in women, typically between the ages of 25 and 45. Breast fibromatosis is a nonmetastasizing benign, but locally invasive, stromal tumor commonly observed in the abdominal wall and in extra-abdominal sites. It rarely occurs in the breast (<0.2% of all breast tumors) where it usually presents as a unilateral solitary lesion, which shares the same clinical and radiological features of breast carcinoma [1], [2], [3]. We report a case of a 31-year-old woman with recurrent breast fibromatosis with multicentric bilateral lesions, which has been initially misinterpreted as a multicentric breast carcinoma.
Case report
A 31-year-old woman presented to the European Institute of Oncology with skin dimpling of the left breast, which she had noticed a few days earlier.
Clinical breast examination showed skin dimpling in the inferior outer quadrant of the left breast; underneath the skin dimpling, there was a palpable breast lump of 2 cm, which was firm, painless, and easily movable under the skin.
The patient had no familiar history of breast cancer, a personal history of prior trauma of the right hand (2013), and splenectomy secondary to a motor vehicle accident (2003). The patient had never been pregnant.
Sonographic evaluation of both breasts demonstrated fibroglandular breasts and revealed in the inferior outer quadrant of the left breast a 20-mm irregular, hypoechoic, solid mass with spiculated margins vascularized on color Doppler, with posterior acoustic shadowing; there was no involvement of the pectoralis muscle (Fig. 1). The ultrasound finding was suspicious for malignancy and was characterized, according to the Breast Imaging Reporting and Data System (BI-RADS), as BI-RADS 4c. A biopsy was requested. No other suspicious findings were present on ultrasound examination. In the left axilla, a 9-mm oval lymph node with a central hyperechoic hylum and minimal thickening of the cortex was observed, compatible with a reactive lymph node, and worthy of cytologic examination.
Fig. 1.
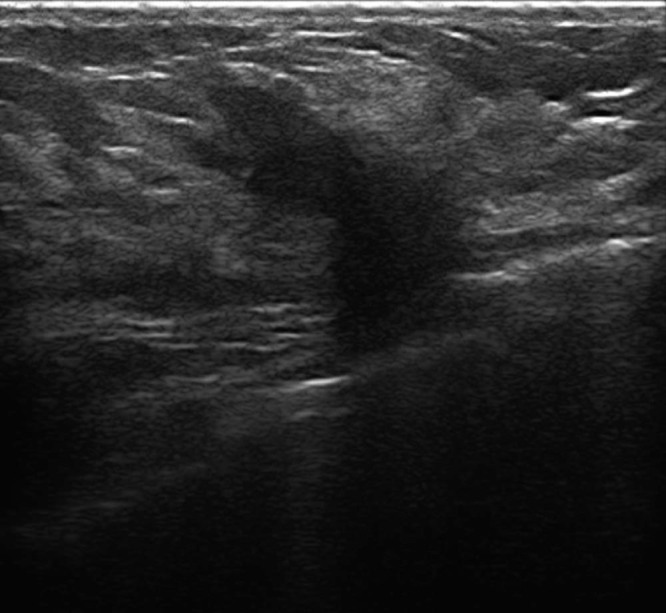
Ultrasound shows a 20-mm irregular, hypoechoic, solid mass with spiculated margins vascularized on color Doppler, with posterior acoustic shadowing in the inferior outer quadrant of the left breast.
The patient underwent a magnetic resonance imaging (MRI), which showed a marked background enhancement of both breasts; this finding limited diagnostic sensitivity.
On MRI, the lesion appeared as a coarse architectural distortion measuring 23 × 10 mm, isointense to the muscle and to the surrounding gland on T1-weighted image, and hyperintense on T2-weighted image, with a moderate gradual contrast enhancement, without chest wall and pectoralis muscle involvement (Fig. 2). There were 2 other distortions of similar appearance: one in the left outer quadrant near the equator measuring 15 mm and the other in the right inferior outer quadrant; the latter was difficult to measure (Fig 3).
Fig. 2.
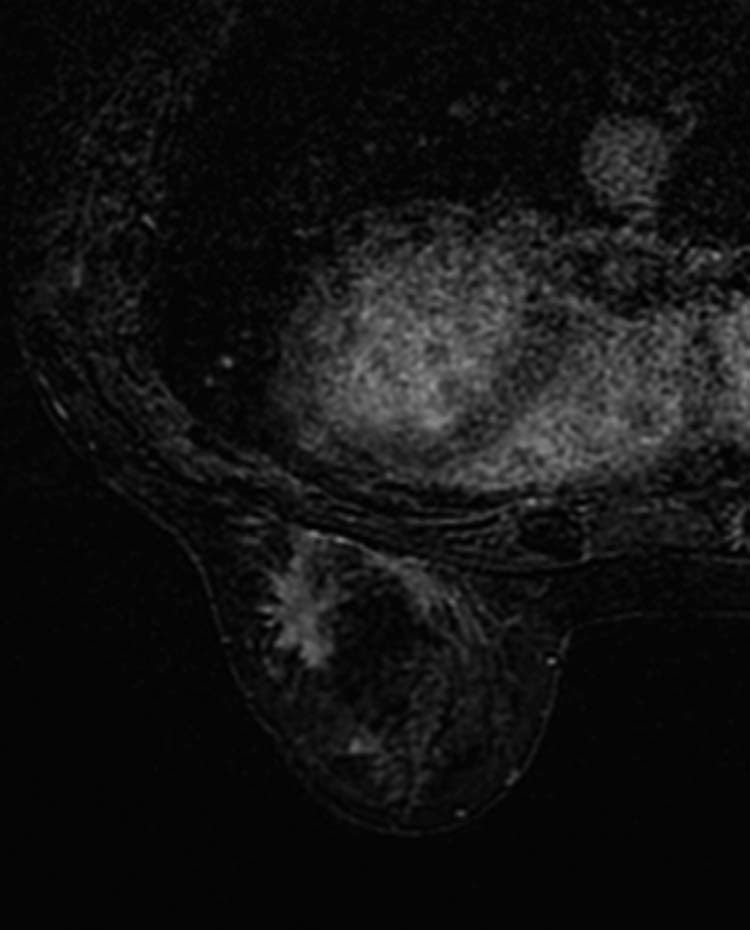
Magnetic resonance imaging shows a coarse architectural distortion measuring 23 × 10 mm, isointense to the muscle and to the surrounding gland on T1-weighted image and hyperintense on T2-weighted image, with a moderate gradual enhancement.
Fig. 3.
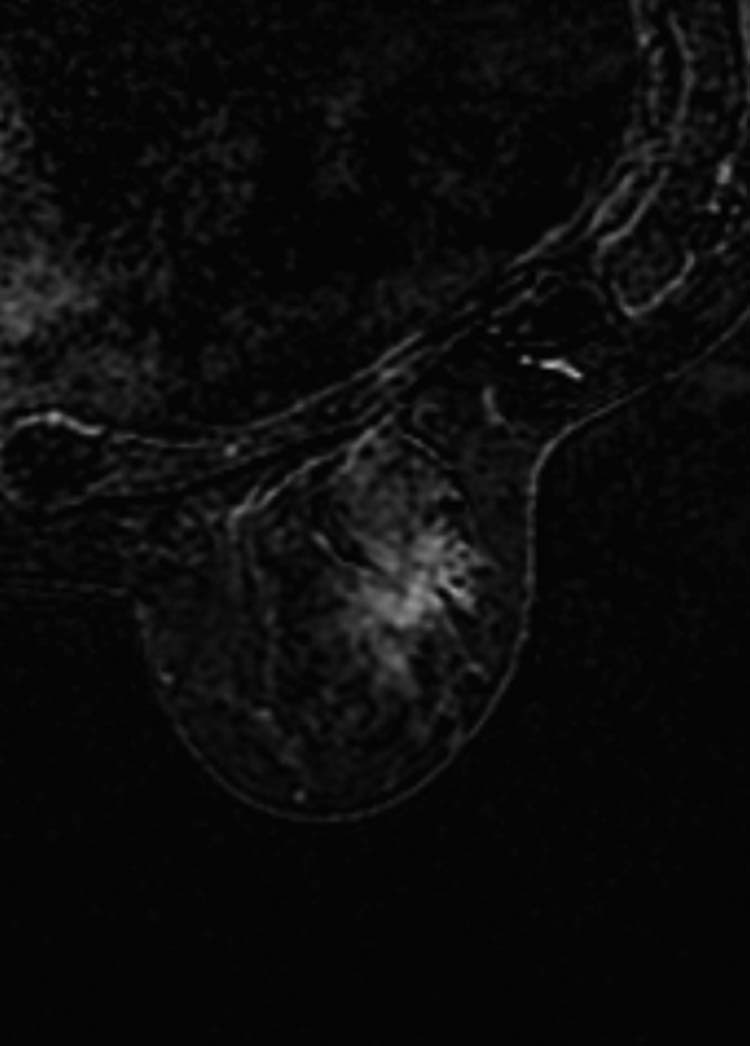
Magnetic resonance imaging shows the area of distortion in the right inferior outer quadrant.
A second-look ultrasound confirmed the presence of 2 additional inhomogeneous hypoechoic areas in the left outer quadrant near the equator (14 mm) and in the right inferior outer quadrant (16 mm) corresponding to the MRI findings.
We performed an ultrasound-guided needle aspiration using a 22-gauge needle, obtaining 4 samples:
-
-
Sample 1: irregular nodule of 20 mm in the inferior outer quadrant of the left breast
-
-
Sample 2: lymph node in the left armpit
-
-
Sample 3: inhomogeneous hypoechoic area of 16 mm to the inferior outer quadrant of the right breast
-
-
Sample 4: inhomogeneous hypoechoic area of 14 mm in the left outer quadrant near the equator.
Samples 1 and 3 were classified as benign lesions: C2 according to the 1997 European guidelines; samples 2 and 4 were insufficient for diagnosis: C1 according to the 1997 European guidelines.
Due to radiological-pathologic discordance, we performed ultrasound-guided core needle biopsy using a 14-gauge needle of the 20-mm nodule located in the inferior outer quadrant of the left breast. This nodule was more suspicious than the others seen on MRI and was associated with skin dimpling. The histologic examination yielded the possibility of a mesenchymal lesion with an uncertain potential of malignancy.
The patient subsequently underwent wide local excision of the lesion, with sentinel lymph node biopsy. The postoperative course was uncomplicated.
The final histopathologic findings were suggestive of cheloid fibromatosis, showing a proliferation of spindle cells, associated with dense connective bundles; the spindle cells were positive for smooth muscle actin and beta-catenin, negative for cytokeratin, desmin, CD34, BCL-2, and CD99 on immunochemistry (Fig. 4). A 3-month follow-up ultrasound was requested.
Fig. 4.
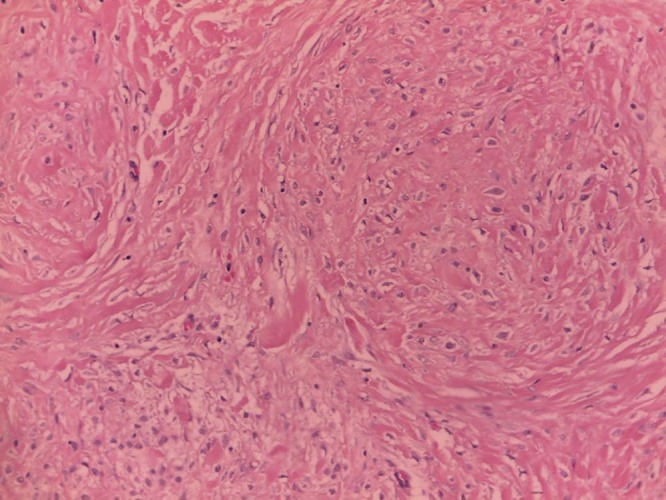
Excision biopsy shows that the final histopathologic findings were suggestive of cheloid fibromatosis, showing a proliferation of spindle cells, associated with dense connective bundles.
At the follow-up ultrasound (about 3 months post surgery), the patient complained about the appearance of a palpable mass in the inferior medial quadrant of the left breast, which was hard, movable, and moderately painful on palpation. Sonographic evaluation showed an inhomogeneously hypoechoic pseudonodular area of 23 mm, with ultrasound features and morphology similar to the lesion that had been surgically removed (Fig. 5). A similar lesion was also observed in the surgical scar site. A postsurgical contrast-enhanced MRI was requested.
Fig. 5.
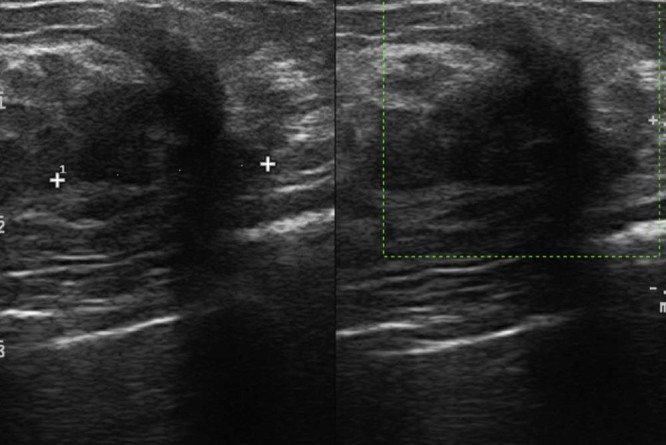
Inhomogeneously hypoechoic pseudonodular area of 23 mm, with ultrasound features and morphology similar to the lesion that had been surgically removed.
On MRI, the area of distortion in the right inferior outer quadrant was unchanged compared with the presurgical MRI earlier. In proximity of the surgical scar site in the left inferior outer quadrant, there were 2 areas of parenchymal distortion with gradual enhancement and features similar to the lesion that had been surgically removed (Fig. 6).
Fig. 6.
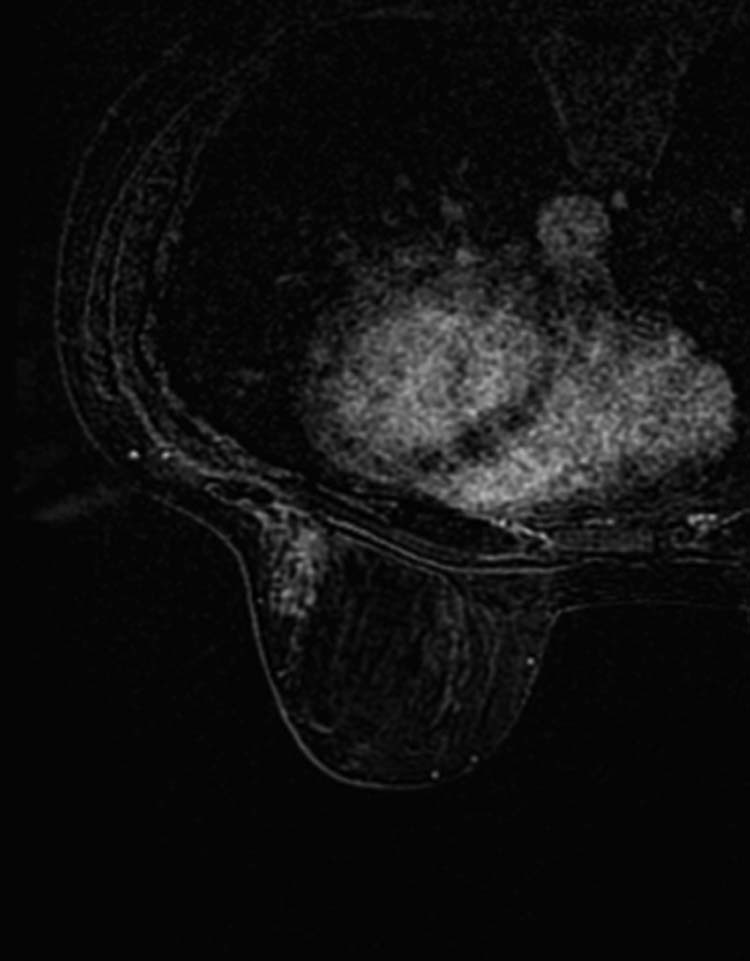
Area of parenchymal distortion with gradual enhancement in proximity of the surgical scar site in the left inferior outer quadrant.
The second-look ultrasound showed bilateral hypoechoic pseudonodular areas, with an irregular shape and ultrasound features similar to the lesion that had been previously surgically removed, therefore probably of the same nature (BI-RADS 3). A 6-month follow-up contrast-enhancement MRI was requested.
Discussion
Fibromatosis is a rare clonal proliferation of fibroblasts and myofibroblasts, which can occur at various locations in the body, typically arising from the muscle, the fascia, and aponeurosis. The breast is an unusual location for the development of this tumor (it accounts for only about 0.2% of all breast tumors), with relatively few cases reported in the literature [1]. Breast fibromatosis may arise from the pectoralis muscle or fascia or the mammary tissue.
Although it does not metastasize, breast fibromatosis is frequently locally aggressive and is prone to recur (up to 35%), even after complete surgical excision with clear margins [4].
The patient's age range is between 13 and 83 years, but breast fibromatosis predominantly affects middle-aged women. Few cases have also been reported in men [5]. All racial and ethnic groups are affected and no specific predilection is seen.
The etiology of this disease is unknown, but associations with Gardner syndrome, incidental and surgical trauma, familial multicentric fibromatosis, familial adenomatous polyposis, and silicone and saline implants are reported. Some cases have been associated with sex steroid hormones, mainly estrogens (during childbearing age, the disease tends to be more “cellular,” more mitotically active, with a larger amount of mild cellular atypia), suggesting a hormonal correlation [2], [6].
Breast fibromatosis appears as a solitary, hard, painless nodule, which sometimes can be attached to the skin or to the pectoral muscle fascia. The signs and symptoms of breast fibromatosis may include breast lump, skin retraction or dimpling, and retraction of the nipple. Skin retraction is caused by fibrous tissue contraction vs desmoplastic reaction, which is similar to tethering associated with malignancy [7].
The tumor size may range from a few millimeters to 10 cm (the average size being 2.5 cm). Small-sized tumors may be asymptomatic and show no signs and symptoms.
On mammography, breast fibromatosis often appears as an irregularly shaped, noncalcified, high-density mass with spiculated margins [8].
On ultrasound, breast fibromatosis frequently appears as a poorly defined, hypoechoic mass with a thick echogenic rim and a posterior attenuation. It is not associated with adenopathies [9].
On MRI, breast fibromatosis is ill-defined, hypointense to isointense on T1-weighted images, and heterogeneously hyperintense on T2-weighted images. The lesions usually show slow enhancement after contrast administration. Magnetic resonance is useful to show chest wall involvement, which is important for surgical planning [10], [11], [12].
The clinical presentation and the radiological appearance of breast fibromatosis are highly suspicious for breast carcinoma. There are 2 cases reported in the literature: both patients underwent radical mastectomy because of an erroneous clinical diagnosis of breast carcinoma [3], [13], [14].
Cytologic examination by fine needle aspiration is usually not diagnostic; differentials include scar or keloid, nodular fasciitis, schwannoma, leiomyoma, solitary fibrous tumor, spindle cell lipoma, myofibroblastoma, myoepithelioma, low-grade fibromyxoid sarcoma, and low-grade fibrosarcoma. The presence of spindle cells admixed with epithelial cells should raise the possibility of fibroadenoma, phyllodes tumor, or metaplastic spindle cell carcinoma [6], [15].
Definitive diagnosis is made by diagnostic surgical biopsy. Histologically, the lesion is composed of bundles of long sweeping and intersecting spindle cells with collagen deposition. Mitotic figures are rare [6].
Multicentric and bilateral disease and recurrence at sites other than the primary have been reported [6], [7], [15], [16], as in this case.
Multicentricity (defined as the presence of 2 or more lesions within different quadrants of the same breast) in fibromatosis has been reported in 10% of cases [16].
Bilateral lesions are extremely rare, found in only 4% of patients [15].
A wide local excision with clear margins remains the treatment of choice. Recurrence is less likely if a wide excision is performed and resection margins are disease-free. Positive excision margins and intralesional excisions are associated with a greater rate of recurrence. Younger age and larger tumor size are also associated with an increased risk of recurrence [17]. Local recurrences usually occur within 3 years since the initial diagnosis. In this time frame, close follow-up is important [6], [18], [19], [20].
Although the first treatment of choice is surgical resection, there are several other options, including radiation therapy, noncytotoxic systemic therapy, and cytotoxic systemic therapy, in patients who are not surgical candidates. The use of radiation has been proposed both as a means to prevent local recurrence as well as to treat local disease. There is not enough evidence in the literature to substantiate or discredit the use of radiotherapy and systemic therapy to treat this disease. Because fibromatosis is not cancer, it has a 100% survival rate [18], [21], [22].
Conclusions
Fibromatosis is a rare, locally aggressive, benign breast tumor that mimics breast cancer on physical examination, mammography, and breast ultrasound. The tumor is best differentiated histologically. It is usually a unilateral, solitary lesion, but it can be multicentric and bilateral, and it may recur after surgery. Complete surgical excision is currently the best treatment option.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
References
- 1.Lee S.M., Lee J.Y., Lee S.Y., Joo M., Kim J.I. Fibromatosis of the breast mimicking an abscess: case report of unusual sonographic features. Clin Imaging. 2015;39:685–688. doi: 10.1016/j.clinimag.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 2.Ha K.Y., DeLeon P., Hamilton R. Breast fibromatosis mimicking breast carcinoma. Proc (Bayl Univ Med Cent) 2013;26(1):22–24. doi: 10.1080/08998280.2013.11928903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosen Y., Papasozomenos S.C., Gardner B. Fibromatosis of the breast. Cancer. 1978;41:1409–1413. doi: 10.1002/1097-0142(197804)41:4<1409::aid-cncr2820410428>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 4.Bhat D., Wear V., Weisenberg E., Alvarado R. Desmoid-type fibromatosis of the breast: a case report. Breast Dis. 2016;36(4):149–152. doi: 10.3233/BD-160227. [DOI] [PubMed] [Google Scholar]
- 5.Li A., Lui C.Y., Ying M., Mak K.L., Lam H.S. Case of fibromatosis of male breast. Australas Radiol. 2007;51(s1):B34–B36. doi: 10.1111/j.1440-1673.2007.01803.x. [DOI] [PubMed] [Google Scholar]
- 6.Chummun S., McLean N.R., Abraham S., Youseff M. Desmoid tumour of the breast. J Plast. 2010;63:339–345. doi: 10.1016/j.bjps.2008.09.024. [DOI] [PubMed] [Google Scholar]
- 7.Taylor T.V., Sosa J. Bilateral breast fibromatosis: case report and review of the literature. J Surg Educ. 2011;68(4):320–325. doi: 10.1016/j.jsurg.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 8.Cederlund C.G., Gustavsson S., Linell F., Moquist-Olsson I., Andersson I. Fibromatosis of the breast mimicking carcinoma at mammography. Br J Radiol. 1984;57:98–101. doi: 10.1259/0007-1285-57-673-98. [DOI] [PubMed] [Google Scholar]
- 9.Leibman A.J., Kossoff M.B. Sonographic features of fibromatosis of the breast. J Ultrasound Med. 1991;10:43–45. doi: 10.7863/jum.1991.10.1.43. [DOI] [PubMed] [Google Scholar]
- 10.Ebrahim L., Parry J., Taylor D.B. Fibromatosis of the breast: a pictorial review of the imaging and histopathology findings. Clin Radiol. 2014;69(10):1077–1083. doi: 10.1016/j.crad.2014.05.105. [DOI] [PubMed] [Google Scholar]
- 11.Nakazono T., Satoh T., Hamamoto T., Kudo S. Dynamic MRI of fibromatosis of the breast. Am J Roentgenol. 2003;181(6):1718–1719. doi: 10.2214/ajr.181.6.1811718. [DOI] [PubMed] [Google Scholar]
- 12.Milos R.I., Moritz T., Bernathova M., Amann G., Panotopoulos J., Noebauer-Huhmann I.M. Superficial desmoid tumors: MRI and ultrasound imaging characteristics. Eur J Radiol. 2015;84(11):2194–2201. doi: 10.1016/j.ejrad.2015.08.012. [DOI] [PubMed] [Google Scholar]
- 13.Nichols R.W. Desmoid tumors: a report of 31 cases. Arch Surg. 1923;7:227–236. [Google Scholar]
- 14.Schremmer C.N. Pektoralis desmoid-ein seltener tumor im bereich der mamma. Z Gynakologie. 1971;11:341–347. [PubMed] [Google Scholar]
- 15.Garg P., Chufal S.S., Gupta N., Pant P., Thapliyal N.C. Multicentric aggressive mammary fibromatosis with cytological features and review of literature. J Clin Diagn Res. 2014;8(5):FD1–FD3. doi: 10.7860/JCDR/2014/6056.4328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wongmaneerung P., Somwangprasert A., Watcharachan K., Ditsatham C. Bilateral desmoid tumor of the breast: case series and literature review. Int Med Case Rep J. 2016;9:247–251. doi: 10.2147/IMCRJ.S106325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Neuman H.B., Brogi E., Ebrahim A., Brennan M.F., Van Zee K.J. Desmoid tumors (fibromatoses) of the breast: a 25-year experience. Ann Surg Oncol. 2008;15(1):274–280. doi: 10.1245/s10434-007-9580-8. [DOI] [PubMed] [Google Scholar]
- 18.Matherne T.H., Green A., Jr, Tucker J.A., Dyess D.L. Fibromatosis: the breast cancer imitator. South Med J. 2004;97(11) doi: 10.1097/01.SMJ.0000125109.51375.5C. [DOI] [PubMed] [Google Scholar]
- 19.Nonnis R., Paliogiannis P., Giangrande D., Marras V., Trignano M. Low-grade fibromatosis-like spindle cell metaplastic carcinoma of the breast: a case report and literature review. Clin Breast Cancer. 2012;12(2):147–150. doi: 10.1016/j.clbc.2012.01.011. [DOI] [PubMed] [Google Scholar]
- 20.Dwyer J.B., Clark B.Z. Low-grade fibromatosis-like spindle cell carcinoma of the breast. Arch Pathol Lab Med. 2015;139(4):552–557. doi: 10.5858/arpa.2013-0555-RS. [DOI] [PubMed] [Google Scholar]
- 21.Plaza M.J., Yepes M. Breast fibromatosis response to tamoxifen: dynamic MRI findings and review of the current treatment options. J Radiol Case Rep. 2012;6(3):16–23. doi: 10.3941/jrcr.v6i3.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scheer L., Lodi M., Molière S., Kurtz J.E., Mathelin C. Medical treatment of mammary desmoid-type fibromatosis: which benefit? World J Surg Oncol. 2017;15(1):86. doi: 10.1186/s12957-017-1148-x. [DOI] [PMC free article] [PubMed] [Google Scholar]


