Abstract
Despite experimental evidence elucidating the anti-tumor activities of tocopherols, clinical trials with α-tocopherol (α-T) have failed to demonstrate its beneficial effects in cancer prevention. This study compared the chemopreventive efficacy of individual tocopherols (α-, δ-, γ-T) and a γ-T rich tocopherol mixture (γ-TmT) in the August Copenhagen Irish (ACI) rat model of estrogen-mediated mammary cancer. Female ACI rats receiving 17β-estradiol (E2) implants were administered with 0.2% α-T, δ-T, γ-T or γ-TmT for 30 weeks. While α-T had no significant effects on mammary tumor growth in ACI rats, δ-T, γ-T and γ-TmT reduced mammary tumor volume by 51% (p < 0.05), 60% (p < 0.01) and 59% (p < 0.01), respectively. Immunohistochemical analysis revealed that δ-T, γ-T and γ-TmT reduced levels of the cell proliferation marker, PCNA, in the rat mammary tumors. To gain further insight into the biological functions of different forms of tocopherols, RNA-seq analysis of the tumors was performed. Treatment with γ-T induced robust gene expression changes in the mammary tumors of ACI rats. IPA analysis identified ‘Cancer’ as a top disease pathway and ‘Tumor growth’ and ‘Metastasis’ as the top signaling pathways modulated by γ-T. Although the results need further functional validation, this study presents an unbiased attempt to understand the differences between biological activities of individual forms of tocopherols at the whole transcriptome level. δ-T, γ-T and γ-TmT could be promising agents for the prevention of estrogen-mediated mammary carcinogenesis. γ-T suppressed growth of mammary tumors most effectively in ACI rats.
Keywords: Breast cancer, estrogen, tocopherols, gene expression, RNA-seq
INTRODUCTION
Breast cancer is one of the most frequently diagnosed malignancies in women worldwide and the second leading cause of cancer related mortality among women in the US (1). Estrogens are known to be critical factors in the etiology of breast cancer (2). Both estrogen receptor (ER)-dependent and ER-independent mechanisms have been implicated in the initiation and progression of mammary cancer (2-4). While activation of ERα enhances cell proliferation and accumulation of mutations resulting from replicative errors (2,4), genotoxic estrogen metabolites can also induce neoplastic transformation (3,4). Understanding the mechanisms underlying estrogen-mediated carcinogenesis could lead to the development of effective strategies for the prevention and treatment of breast cancer.
Recent studies shed light on the role of natural products in the inhibition of estrogen dependent breast cancer (5,6). Dietary components and bioactive natural compounds have been reported to inhibit mammary carcinogenesis by reduction of estrogen-induced oxidative stress as well as downregulation of ER-mediated signaling (7). Tocopherols, members of the vitamin E family present in the diet, have been demonstrated to exert chemopreventive effects in preclinical models of ER positive breast cancer (8-12) as well as lung, colon and prostate cancers (13-16).
Tocopherols are a group of fat soluble phenolic compounds consisting of a chromanol ring and a saturated phytyl side chain (17). Depending on the number and position of methyl groups on the chromanol ring, tocopherols are designated as α, β, δ and γ (17). α-Tocopherol (T) is trimethylated at the 5-, 7- and 8-positions of the chromanol ring, β-T is dimethylated at the 5- and 8-positions, γ-T is dimethylated at the 7- and 8- positions, whereas δ-T is monomethylated at the 8-position (17). Structural differences in the chromanol ring are thought to be responsible for the variation in biological activity of each individual form of tocopherol. α-T has superior antioxidant activity, whereas the unmethylated carbon atoms at 5-position of the chromanol ring make δ- and γ-T more effective in trapping reactive nitrogen species (18). Although tocopherols have been proposed to reduce the risk of cancer due to their antioxidant properties (19,20), large scale chemoprevention clinical trials with α-T have provided inconsistent conclusions (21-23). Thus, detailed investigation of the biological activities and anti-cancer properties of the different forms of tocopherols are fundamental to future intervention studies with tocopherols.
γ-T is the most abundant form of tocopherol in the US diet, being three to five times more abundant than α- or δ-T whereas β-T is present in minute amounts (24). Tocopherols are mostly found in vegetable oils such as soybean, corn and cottonseed oil (24). γ-TmT is a naturally occurring tocopherol mixture rich in γ-T obtained as a byproduct in the distillation of vegetable oil (25). γ-TmT has been shown to inhibit estrogen-induced mammary tumor growth in ACI rats as well as MCF-7 xenografted immunodeficient mice (12). We have previously reported that δ-T, γ-T and γ-TmT suppress N-methyl-N-nitrosourea induced mammary tumor growth in Sprague Dawley rats (9,10). Recently, the tumor inhibitory effects of δ-T, γ-T and γ-TmT in MCF-7 xenografts supplemented with estrogen have been demonstrated (26). August-Copenhagen Irish (ACI) rats exhibit 80-100% tumor incidence upon prolonged exposure to estrogen (27), providing a physiologically relevant model for long-term cancer prevention studies. This study utilized ACI rats to evaluate the comparative chemopreventive efficacy of α-, δ-, γ-T and γ-TmT in estrogen-mediated mammary carcinogenesis.
High-throughput RNA sequencing (RNA-seq) has revolutionized transcriptome profiling, enabling relatively precise quantification of transcript levels in biological samples (28). Currently, RNA-seq is extensively used to analyze differential gene expression in tissues subjected to different treatment conditions. For an unbiased understanding of the mechanisms underlying the anti-cancer activity of the different forms of tocopherols, RNA-seq analysis was performed on mammary tumors from ACI rats treated with α-, δ-, γ-T and γ-TmT. Our analysis identified the top genes and biological networks modulated by individual tocopherols at the whole transcriptome level, providing new insights into their differential chemopreventive activities.
MATERIALS AND METHODS
Diets
Natural γ-TmT was obtained from BASF Corporation (Kankakee, Illinois; Covi-ox T-90, Batch number 0008778732). It contained 56.1% γ-T, 22.3% δ-T, 11.5% α-T and 1.2% β-T. γ-T was purified to ≥ 97% from γ-TmT with no detectable α- and δ-T. α- and δ-T were purified from commercial grade α-T (T3634) and δ-T (T2028) (Sigma-Aldrich, St. Louis, MO) to ≥ 97% purity with no other detectable forms of tocopherol. A CombiFlash Companion XL automated flash chromatographic system (Teledyne ISCO, Lincoln, NE) with a RediSep Rf Gold high performance flash silica gel column (20-40 μm in particle size) was used for the purification. Semipurified AIN-93M diet obtained from Research Diets, Inc. (New Brunswick, NJ) was used as the control diet. Experimental diets were prepared by adding 0.2% each of α-, δ-, γ-T and γ-TmT to the AIN-93M diet.
Animals and experimental procedures
Female ACI rats were purchased from Harlan Laboratories (Indianapolis, IN) at 6-7 weeks of age. After two weeks of acclimatization, the rats were subcutaneously implanted with silastic tubing filled with 9 mg of 17β-estradiol (E2) (Sigma-Aldrich, St. Louis, MO) or sham implants, following a previously described method (29). Rats receiving sham implants were fed with control diet. E2 implanted rats were fed with control diet or diets containing 0.2% α-, δ-, γ-T or γ-TmT. Diets were administered from the day of E2 implantation. Rats were sacrificed at 30 weeks post treatment. Each treatment group included 27 animals. Body weight of the rats was measured weekly and they were palpated for mammary tumors weekly starting from 18 weeks after E2 implantation. Blood was collected at necropsy and serum stored at −80°C. Mammary tumors were snap frozen in liquid nitrogen or fixed in 10% formalin for further analysis. All animal studies were approved by the Institutional Review Board for the Animal Care and Facilities Committee at Rutgers, the State University of New Jersey (Protocol Number: 03-024).
Analysis of tocopherol levels in the rat serum
The levels of tocopherols (α, δ, γ) and their metabolites in rat serum were analyzed by high performance liquid chromatography using previously described methods (16).
Immunohistochemical analysis
Mammary tumors were fixed in 10% formalin, embedded in paraffin and sectioned at 4 μm thickness. Sections were incubated overnight at 4°C with antibody to proliferating cell nuclear antigen (PCNA) (1:4000; M 0879, Dako, Denmark), followed by incubation with biotinylated secondary antibody and avidin/biotin peroxidase complex. The sections were then stained with 3′-diaminobenzamine substrate and counterstained with Modified Harris Haematoxylin. Tumor sections from three different animals per treatment group were stained. Representative images were taken randomly and nuclear staining was quantified using an Aperio ScanScope (Vista, CA) by counting at least 15,000 cells per slide.
RNA-seq analysis
Total RNA was extracted from rat mammary tumors (n=4/group) and the quality was assessed. The samples were subjected to cDNA library construction and Illumina sequencing (NextGen 75bp pair-end) yielding 75 million reads per sample. Quality control on raw reads was performed using FastQC (30). Good quality reads were aligned to the rat reference genome (rn6) using Bowtie2 and differential expression of transcripts was detected using Cuffdiff (31). Analysis of pathways and gene networks of the expression data was performed with QIAGEN’s Ingenuity Pathway Analysis (IPA, QIAGEN Redwood City, www.qiagen.com/ingenuity) for genes with P-value ≤ 0.001 (FDR < 0.05). IPA is a widely used bioinformatics tool to analyze biological molecule interactions, including miRNA, mRNA, and proteins. Pathways with P < 0.001 were found to be significantly differentially expressed. The RNA-Seq datasets described in this study have been deposited in the NCBI Gene Expression Omnibus (GEO) with accession number GSE 103646.
mRNA expression analysis using quantitative polymerase chain reaction (qPCR)
RNA was extracted from frozen mammary tumors. Reverse transcription and qPCR was performed as previously reported (32). Labelled primers were used for chemokine (C-X-C motif) receptor 2 (CXCR2), insulin-like growth factor binding protein 3 (IGFBP3), serpin peptidase inhibitor, clade A, member 1 (SERPINA), Cbp/P300-interacting transactivator with glu/asp-rich carboxy-terminal domain, 1 (CITED1), mesothelin (MSLN), fermitin family member 1 (FERMT1), extracellular matrix protein 1 (ECM1), insulin-like growth factor 1 (IGF1), matrix metalloproteinase 13 (MMP13) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH).
Statistical analysis
Tumor-free survival (TFS), or time to appearance of the first tumor, was estimated by the Kaplan-Meier method. Log-rank test was used to assess the homogeneity of TFS between different treatment groups. Tumor multiplicity was analyzed using log-linear model (Poisson regression). Statistical significance was evaluated using one way analysis of variance model (ANOVA) followed by Dunnett’s multiple comparison post hoc test, preserving the overall type-1 error at the 5% level. The data are represented as ± S.E. Differences were considered statistically significant when P < 0.05.
RESULTS
Administration of α-, δ-, γ-T and γ-TmT increases the serum levels of tocopherols and their metabolites in ACI rats
To determine the bioavailability of tocopherols in the experimental animals, the levels of α-, δ-, γ-T and their respective short chain carboxyethyl hydroxychroman (CEHC) metabolite was measured in the serum of the rats (Table 1). Administration of diets enriched with α-, δ- and γ-T significantly increased the serum concentrations of the corresponding tocopherols by 2-, 81- and 78-fold, respectively. In the γ-TmT treated group, δ- and γ-T levels increased by 30- and 16-fold, respectively. Treatment with α-, δ- and γ-T also led to significant increase in the serum levels of respective CEHC metabolites, by 82-, 204- and 102-fold, respectively. γ-TmT supplementation increased the α-, δ- and γ-CEHC concentrations by 3-, 61- and 36-fold, respectively. Tocopherols are transferred to the blood by α-Tocopherol transfer protein (α-TTP). The affinity of α-TTP is highest for α-T, followed by γ-T and δ-T. Since hepatic α-TTP selectively facilitates the transfer of α-T from liver to blood, α-T is the most abundant tocopherol in blood. Since α-T is already abundant in the blood, supplementation with α-T did not cause a dramatic fold increase in the serum levels of α-T. As most of α-T is transported to blood, only a small percentage of α-T is metabolized. On the other hand, since relatively lower amounts of γ-T and δ-T are transferred into the blood, γ-T and δ-T are more extensively degraded in the liver and their side-chain degradation metabolites are more abundant than those of α-T. Hence, the levels of γ- and δ-CEHC metabolites in the rat serum is higher than that of α-CEHC.
Table 1.
Analysis of tocopherols and their metabolites in the serum of ACI rats
| Treatment | α-T (μM) | δ-T (μM) | γ-T (μM) | α-CEHC (μM) | δ-CEHC (μM) | γ-CEHC (μM) |
|---|---|---|---|---|---|---|
| Negative control | 28.4 ± 3.7 | 0.08 ± 0.03 | 0.30 ± 0.10 | 0.2 ± 0.1 | 0.7 ± 0.1 | 0.5 ± 0.1 |
| E2 control | 34.8 ± 8.0 | 0.05 ± 0.02 | 0.30 ± 0.10 | 0.3 ± 0.0 | 0.9 ± 0.2 | 1.3 ± 0.4 |
| E2 + 0.2% α-T | 74.5 ± 8.7** | 0.06 ± 0.01 | 0.02 ± 0.00 | 24.6 ± 13.8** | 1.2 ± 0.5 | 2.6 ± 1.4 |
| E2 + 0.2% δ-T | 27.9 ± 0.6 | 4.06 ± 0.50 *** | 0.30 ± 0.01 | 0.3 ± 0.1 | 184.1 ± 20.7*** | 2.7 ± 0.7 |
| E2 + 0.2% γ-T | 27.0 ± 1.3 | 0.10 ± 0.01 *** | 23.60 ± 2.80*** | 0.3 ± 0.1 | 1.5 ± 0.2 | 133.3 ± 13.9*** |
| E2 + 0.2% γ-TmT | 34.3 ± 1.7 | 1.50 ± 0.40 ** | 4.80 ± 1.00** | 0.9 ± 0.2* | 55.4 ± 7.9*** | 47.3 ± 11.9** |
The effects of α-, δ-, γ-T and γ-TmT supplementation on the levels of α-, δ- and γ-T and their short chain CEHC metabolite in the serum (μMol/l) of ACI rats were analyzed at 30 weeks. P-values are compared to the E2 control. Statistical significance
P < 0.05,
P < 0.01,
P < 0.001.
Dietary δ-T, γ-T and γ-TmT inhibit estradiol induced mammary tumorigenesis in ACI rats
Female ACI rats implanted with E2 were fed with control diet or diets supplemented with 0.2% α-, δ-, γ-T or γ-TmT for 30 weeks. TFS for each treatment group was estimated (Figure 1A). Although no overall difference in tumor free survival time was observed between the E2 control and tocopherol treated groups (Figure 1A), the median time to the appearance of first tumor was 25 weeks in the E2 control group and 26 weeks in the 0.2% α-, δ-, γ-T and γ-TmT treated groups. Administration of 0.2% α-, δ-, γ-T and γ-TmT inhibited mammary tumor growth (Figure 1B). Final tumor measurements revealed that 0.2% α-, δ-, γ-T and γ-TmT reduced tumor volume by 28%, 51% (p < 0.05), 60% (p < 0.01) and 59% (p < 0.01), respectively. δ-T, γ-T and γ-TmT significantly inhibited E2 induced mammary tumor growth in the ACI rats, while α-T had no significant effect on tumor reduction (Figure 1C). Although γ-T inhibited tumor growth more effectively than δ-T, the difference between γ-T and δ-T was not statistically significant. At the end of the 30-week study, the overall tumor multiplicity of the E2 control was not significantly different from the groups treated with 0.2% α-, δ-, γ-T or γ-TmT (Figure 1D). Therefore, tocopherols may act by slowing down tumor growth rather than blocking initiation of tumor development. In comparison to the negative control, the average body weights of rats at 30 weeks were not affected by E2 or any form of tocopherol treatment, indicating that none of the treatments were toxic for the given duration (Figure 1E).
Figure 1.
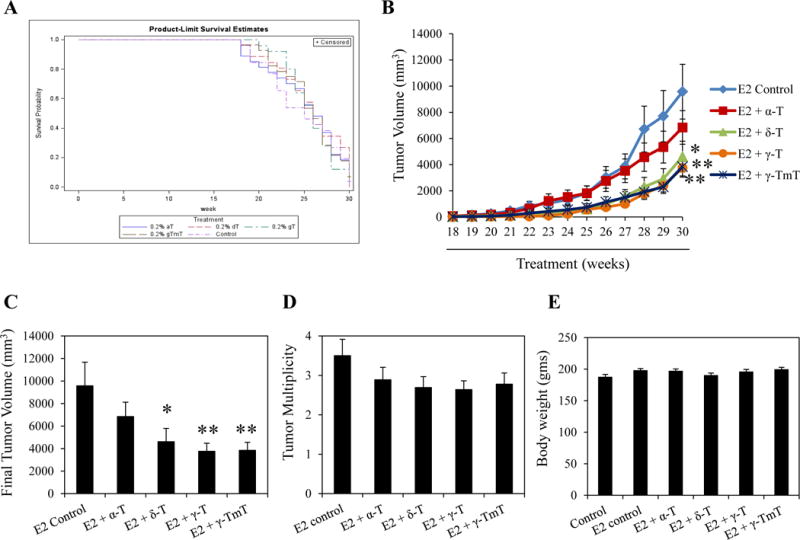
δ-T, γ-T and γ-TmT inhibit estrogen-induced mammary tumorigenesis in ACI rats. ACI rats implanted with E2 were fed with control diet or diet containing 0.2% α-, δ-, γ-T or γ-TmT for 30 weeks (n=27/group). (A) The tumor-free survival curve of each treatment group is shown. (B) Average tumor volume of the different treatment groups at weekly time points starting from 18 weeks is shown. (C) Average final tumor volume of each treatment group at 30 weeks is shown. (D) Average tumor multiplicity of each treatment group at 30 weeks is shown. (E) Average body weight of each treatment group at 30 weeks is shown. Statistical significance, *P < 0.05, **P < 0.01.
Effects of α-, δ-, γ-T and γ-TmT treatment on cell proliferation in ACI rats
Tumor growth data showed that δ-T, γ-T and γ-TmT suppressed estrogen-mediated mammary tumor growth in ACI rats while α-T had no inhibitory effects. To investigate if such differences in tumor inhibition could be attributed to the effects of α-, δ-, γ-T and γ-TmT on cell proliferation, immunohistochemical analysis of the cell proliferation marker, PCNA, was performed in the mammary tumors of ACI rats (Figure 2). α-T treatment had no significant effect on the levels of PCNA in mammary tumors. However, δ-, γ-T and γ-TmT reduced PCNA levels in the mammary tumors of rats by 63% (p < 0.05), 69% (p < 0.05) and 65% (p < 0.05), respectively.
Figure 2.
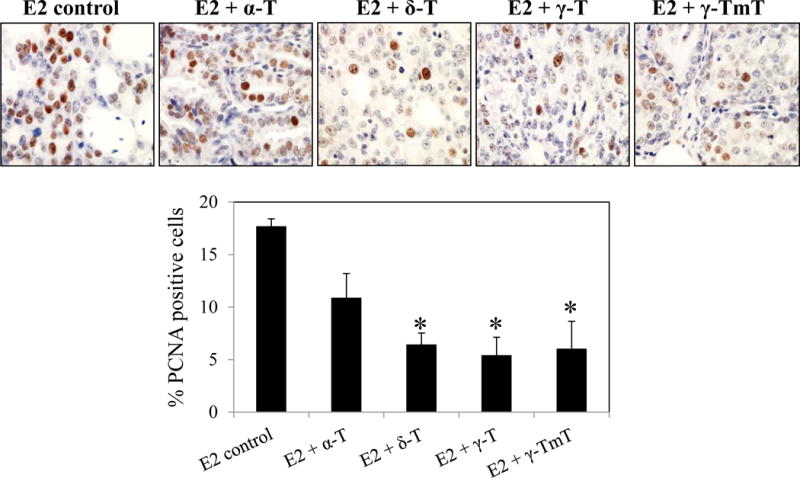
Effect of α-, δ-, γ-T and γ-TmT treatment on cell proliferation of mammary tumors from E2-treated ACI rats. Representative images of PCNA staining in the mammary tumors of ACI rats (40×) and quantification of nuclear PCNA staining is shown (n=3/group). Statistical significance, *P < 0.05.
Overview of differentially expressed genes in response to α-, δ-, γ-T treatment in the mammary tumors of ACI rats
In order to determine the effects of different forms of tocopherol treatment on the whole transcriptome, we compared global gene expression profiles of mammary tumors from the E2 control group to those of α-, δ- and γ-T treated groups. RNA-seq analysis was performed on four tumor samples per treatment group. For the γ-T treated group, one outlier was detected after RNA-seq analysis. Hence all further analysis was performed on four samples each from α- and δ-T and three from the γ-T group. Figure 3A shows a heatmap of gene expression changes across four treatment groups, E2, α-T, δ-T and γ-T based on 61 out of 256 differentially expressed genes. Treatment with α-, δ- and γ-T upregulated or downregulated a large number of genes with respect to E2 control. We identified 47 differentially regulated genes (5 upregulated and 42 downregulated) in the α-T treated group, 51 (34 upregulated and 17 downregulated) in the δ-T treated group and 192 (72 upregulated and 120 downregulated) in the γ-T treated group. Overall, the data showed that γ-T induced more significant gene expression changes in the ACI rat mammary tumors compared to α-T and δ-T. Of the 72 genes upregulated by γ-T, 5 were also upregulated by δ-T. The genes upregulated by α-T were unique, sharing no common genes with δ- or γ-T (Figure 3B). Among the 120 genes downregulated by γ-T, 6 were downregulated by δ-T and 18 by α-T. Four genes were downregulated by α-, δ- and γ-T (Figure 3B).
Figure 3.
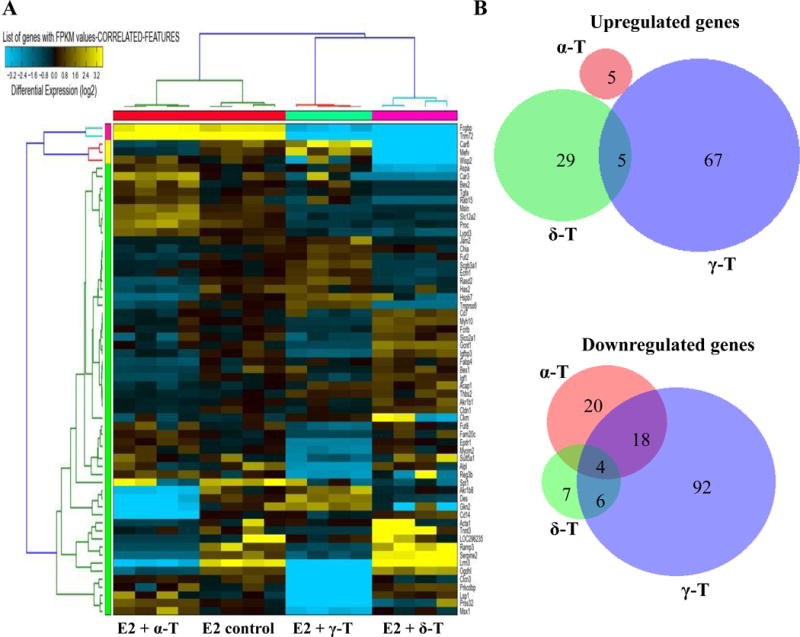
Overview of genes regulated by the different tocopherols. (A) The clustered heatmap of 15 samples based on 61 out of 256 differentially expressed genes across 4 groups (E2 control, α-, δ-, γ-T). (B) Venn diagrams comparing the genes upregulated and downregulated by treatment with α-, δ- and γ-T compared to E2 control.
The main gene networks and canonical pathways modulated by γ-T treatment
Tumor inhibition data showed that γ-T is the most effective form of tocopherol in suppressing E2-mediated mammary tumor growth. Further, RNA-seq analysis demonstrated that γ-T had the most profound influence on tumor transcriptome, when compared to α- or δ-T. Considering the superior chemopreventive activity of γ-T, in-depth analysis was done on RNA-seq data from the γ-T treated group to investigate the possible biological functions and pathways regulated by γ-T. Using the IPA software (www.qiagen.com/ingenuity), we performed core analysis of all the genes significantly upregulated or downregulated by γ-T in comparison to the E2 control group. This analysis identified ‘Cancer’ and ‘Organismal Injury and Abnormalities’ as the top two disease and disorder pathways modulated by γ-T (Figure 4A). Further analysis revealed that the effect of γ-T on the Cancer network involved genes related to different cellular functions such as tumor growth (IGFBP3, TGFA, COL18A1, PLAU, TNFRSF9) and metastasis (ANGTPL4, PDLIM3, SERPINA1, IL2RB) (Figure 4B and Supplementary Figure S1).
Figure 4.
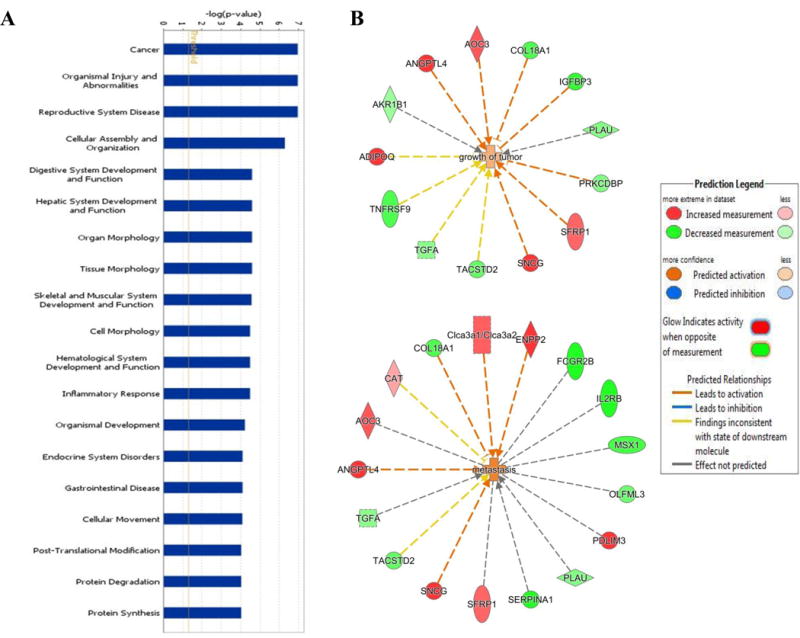
Treatment with γ-T differentially regulates genes involved in various signaling pathways. (A) IPA analysis showing the top disease and disorder gene networks modulated by treatment with γ-T. (B) Network map created by IPA showing the genes differentially regulated by γ-T and their functions.
Effect of γ-T treatment on gene expression in the mammary tumors of ACI rats
IPA analysis revealed that γ-T treatment had a robust effect on genes commonly involved in cancer. To gain a better understanding of the mechanism by which γ-T inhibited mammary tumor growth, the genes differentially regulated by γ-T were further analyzed. The top differentially expressed genes in response to γ-T treatment are reported in Table 2. γ-T modulated the expression of genes involved in different cellular functions which can be broadly classified as follows:
Table 2.
Genes regulated by γ-T treatment
| Gene symbol | Gene description | log2(fold change) | p-value |
|---|---|---|---|
| Genes downregulated by γ-T | |||
|
| |||
| Cell proliferation, apoptosis, signal transduction | |||
| KLHDC8A | Kelch domain containing 8A | −5.5 | 5.00E-05 |
| FERIL4 | Fer-1 like family member 4, pseudogene | −4.0 | 5.00E-04 |
| CXCR2 | Chemokine (C-X-C motif) receptor 2 | −3.8 | 1.00E-04 |
| IGFBP3 | Insulin-like growth factor binding protein 3 | −2.9 | 5.00E-05 |
|
| |||
| Serine protease inhibitor | |||
| SERPINA1 | Serpin peptidase inhibitor, clade A, member 1 | −6.6 | 5.00E-05 |
| SERPINB9 | Serpin peptidase inhibitor, clade B, member 9 | −1.5 | 8.00E-04 |
|
| |||
| Transcription | |||
| CITED1 | Cbp/P300-interacting transactivator with glu/asp-rich carboxy terminal domain,1 | −4.4 | 5.00E-05 |
|
| |||
| Cell adhesion, extracellular matrix | |||
| MSLN | Mesothelin | −3.3 | 5.00E-05 |
| FERMT1 | Fermitin family member 1 | −3.3 | 5.00E-05 |
| ECM1 | Extracellular matrix protien 1 | −2.3 | 5.00E-04 |
| CLDN1 | Claudin 1 | −2.1 | 5.00E-05 |
|
| |||
| Immune response, growth factors | |||
| SFTPD | Surfactant protein D | −5.6 | 5.00E-05 |
| IL2RB | Interleukin 2 receptor, beta | −5.5 | 5.00E-05 |
| TPSB2 | Tryptase beta 2 | −3.9 | 5.00E-05 |
| IGF1 | Insulin-like growth factor 1 | −3.8 | 1.00E-04 |
| TNFRSF9 | Tumor necrosis factor receptor superfamily, member 9 | −2.4 | 5.00E-05 |
| WISP2 | Wnt-1-inducible signaling pathway protein 2 | −3.1 | 7.50E-04 |
|
| |||
| Angiogenesis, metastasis | |||
| MMP13 | Matrix metalloproteinase 13 | −2.1 | 6.50E-04 |
| ESM1 | Endothelial cell-specific molecule 1 | −2.0 | 7.00E-04 |
|
| |||
| Others | |||
| CMA1 | Chymase 1 | −5.2 | 5.00E-05 |
| CPA3 | Carboxypeptidase A3 | −4.2 | 5.00E-05 |
| HDC | Histidine decarboxylase | −4.2 | 1.00E-04 |
|
| |||
| Genes upregulated by γ-T | |||
|
| |||
| Cell proliferation, apoptosis, signal transduction | |||
| SCGB3A1 | Secretoglobin, family 3A, member 1 | Below detection limit | 5.00E-05 |
| G0S2 | G0/G1 switch 2 | 2.3 | 5.00E-05 |
| SFRP-1 | Secreted frizzled-related protein 1 | 2.3 | 5.00E-05 |
|
| |||
| Cell adhesion, extracellular matrix, cytoskeleton | |||
| PDLIM3 | PDZ and LIM domain 3 | 5.5 | 5.00E-05 |
| JAM2 | Junctional adhesion molecule 2 | 2.2 | 5.00E-05 |
|
| |||
| Lipid metabolism | |||
| ADIPOQ | Adiponectin, C1Q and collagen domain containing | 4.4 | 5.00E-05 |
|
| |||
| Others | |||
| RPLP2 | Ribosomal protein, large, P2 | Below detection limit | 5.00E-05 |
Cell proliferation, apoptosis, signal transduction
γ-T downregulated the expression of KLHDC8A, FERIL4, CXCR2, IGFBP-3 while SCGB3A1, G0S2, SFRP-1 expression was upregulated.
Serine protease inhibitors
SERPINA1 and SERPINB9 expression was downregulated by γ-T.
Transcription
Expression of the transcriptional coactivator CITED1 was downregulated in response to γ-T treatment.
Cell adhesion and extracellular matrix components
γ-T downregulated the expression of MSLN, FERMT1, ECM1, CLDN1 while PDLIM3 and JAM2 were upregulated.
Immune response and growth factors
Expression of genes involved in immune response such as SFTPD, IL2RB, TPSBP2, TNFRSF9 and the growth factors IGF1 and WISP2 were downregulated upon γ-T treatment.
Angiogenesis and metastasis
γ-T downregulated the angiogenic marker ESM1 and the metastatic marker MMP13.
Others
Gene expression of the enzymes CMA1, CPA3, HDC, the regulator of lipid metabolism, ADIPOQ and the ribosomal component RPLP2 were modulated by γ-T treatment.
Nine genes belonging to the different cellular pathways regulated by γ-T were randomly selected and their mRNA expression levels were determined by qPCR (Figure 5). The results further confirmed the downregulation of these genes in response to γ-T treatment in support of the RNA-seq analysis.
Figure 5.
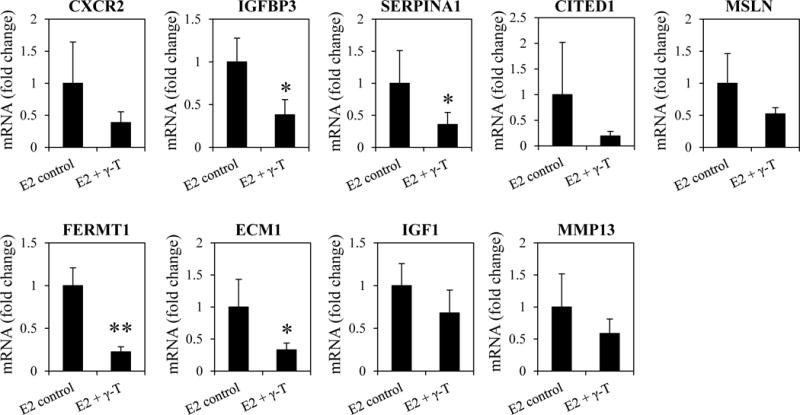
Validation of RNA-seq analysis by qPCR. Nine genes were randomly selected from the different cellular pathways modulated by γ-T and their mRNA levels in the mammary tumors of ACI rats were determined by qPCR (n=6-8/group).
DISCUSSION
Tocopherols have been known to reduce the risk of cancer (33). However, clinical trials with α-T, the most abundant form of tocopherol in human tissues, have failed to demonstrate its cancer preventive properties (21). Previously, we reported that the natural tocopherol mixture, γ-TmT, rich in γ-T, inhibited E2-mediated mammary tumor growth in ACI rats at the doses of 0.3% and 0.5% (12). The current study assessed the chemopreventive potential of individual forms of tocopherols in estrogen-mediated breast cancer. The anti-cancer activities of a single dose (0.2%) of α-, δ-, γ-T and γ-TmT were tested using the ACI rat model. Based on the results of previous studies, the dose of 0.2% was chosen to evaluate the relative activities of the different tocopherols. The 30-week study revealed that δ-T, γ-T and γ-TmT inhibited mammary tumorigenesis in ACI rats, while α-T had not significant inhibitory effect (Figure 1). γ-T reduced E2-induced mammary tumor growth most effectively, followed by γ-TmT and δ-T. This finding is in accordance with previous studies which have reported the superior cancer preventive properties of δ-T and γ-T over α-T in colon cancer (14) and NMU-induced mammary cancer (9). Supporting the tumor inhibition results, δ-T, γ-T and γ-TmT inhibited cell proliferation in E2-treated mammary tumors, as evident from the reduced percentage of PCNA positive cells, while α-T did not (Figure 2).
This study reports for the first time, the transcriptomic analysis of mammary tumors from ACI rats treated with α-, δ- and γ-T (Figure 3). α-T, which did not significantly inhibit the growth of rat mammary tumors, had minimal effect on the transcriptome (47 differentially expressed genes). Moreover, the fact that the 5 genes upregulated by α-T were unique, sharing no common genes with δ- or γ-T, could account for its reduced tumor inhibitory activity. δ-T, with moderate tumor inhibitory potential, modulated the expression of 51 genes. γ-T, which was most effective in inhibiting mammary tumorigenesis, had the most profound influence on the rat transcriptome, leading to the differential expression of 192 genes. Of these 192 genes, 159 were unique to γ-T. IPA analysis revealed γ-T as the only form of tocopherol which modulated ‘Cancer’ as the top disease pathway (Figure 4). Recent studies indicate a correlation between the chemopreventive/therapeutic potential of compounds and their effects on differential gene expression. Treatment of pancreatic cancer cells with single agents metformin and aspirin as well as their combination revealed that the combination inhibited cell growth more effectively and modulated the expression of a larger number of genes compared to the single agents (34). Thus, the dramatic effect of γ-T treatment on the rat tumor transcriptome could be linked to its superior chemopreventive activity.
γ-T regulated the expression of genes related to different cellular functions (Table 2). Of the top genes downregulated by γ-T treatment, KLHDC8A has been reported to be overexpressed in aggressive gliomas which have lost dependence on mutant EGFR (35). However, its role in breast cancer is still unknown. CXCR2 is a G-protein coupled receptor which binds CXC chemokines, namely CXCL1-3 and 5-8 to trigger their function. Treatment with γ-T downregulated CXCR2 gene expression. CXCR2 has been associated with poor outcomes for different cancers through its effects on migration, invasion and angiogenesis (36). In breast cancer, targeting CXCR2 has been reported to enhance chemotherapeutic response and inhibit tumor growth, angiogenesis and lung metastasis in vivo (37). Thus, inhibition of CXCR2 by γ-T could be critical to its chemopreventive potential. γ-T was also found to downregulate IGFBP3, which can exert pro-survival or pro-apoptotic effects on tumor cells depending on cell type and context (38). γ-T reduced expression of the serine protease inhibitor, SERPINA1 and the transcriptional coregulator, CITED1. Recently, SERPINA1 has been reported to be a direct ER target gene (39). CITED1 is a nuclear protein that binds directly to ERα and activates ER-mediated transcription (40). Therefore, downregulation of SERPINA1 and CITED1 could be indicative of selective inhibition of ER-dependent transcription by γ-T. In future studies, functional validation of these genes modulating ER function could help in improved understanding of the mechanism by which γ-T suppresses E2-mediated mammary tumor growth.
γ-T downregulated gene expression of the cell adhesion molecules MSLN and FERMT1. MSLN is a tumor differentiation antigen that is highly expressed in several human cancers such as pancreatic, lung, ovarian and triple negative breast cancer. MSLN is an important target for cancer immunotherapy (41). Although γ-T reduced MSLN expression, the significance of targeting MSLN in E2-dependent breast cancer is yet unknown. FERMT1 regulates integrin functions and has been implicated in breast cancer growth and lung metastasis (42). ECM1 is a marker for tumorigenesis associated with tumor recurrence in breast cancer. It promotes epithelial to mesenchymal transition and maintenance of breast cancer stem cells (43). Inhibition of FERMT1 and ECM1 by γ-T might open new opportunities for prevention of cancer progression and metastasis by a natural, dietary compound.
SFTPD, a component of innate immune response responsible for maintaining lung homeostasis was downregulated by γ-T. Recently, SFTPD has been reported to inhibit lung cancer progression by binding to epidermal growth factor receptor (EGFR) for suppression of EGF signaling (44). However, the role of SFTPD in breast cancer has not been studied. γ-T reduced the expression of IGF1. IGF1, acting through type 1 insulin-like growth factor receptor (IGF-1R), regulates multiple aspects of breast cancer like cell proliferation, survival and metastasis (45). High levels of circulating IGF1 have been correlated with increased risk of breast cancer (45) and drugs targeting the IGF axis are being tested in clinical trials. Inhibition of IGF1 by γ-T is particularly significant in an E2-dependent model of mammary cancer because of the crosstalk that exists between ER and IGF signaling pathways (46). Blocking of IGF action by γ-T could inhibit the activity of ER. γ-T also downregulated expression of the metastatic biomarker, MMP13. Elevated levels of MMP13 have been associated with decreased overall survival and osteolytic bone metastasis in breast cancer (47). Thus, by suppressing MMP13, γ-T may inhibit breast cancer metastasis.
Among the genes upregulated by γ-T, SCGB3A1, also known as HIN1 was the most dramatically induced. SCGB3A1 is silenced in a substantial fraction of breast cancers due to methylation, suggesting a tumor suppressive function. It inhibits cell growth, invasion and Akt activation (48). γ-T upregulated the tumor suppressor G0S2 which has been reported to inhibit oncogenic transformation through repression of Myc activity (49). Thus, treatment of ACI rats with γ-T regulated the expression of genes that control cell proliferation, ER-dependent signaling, apoptosis, tumor progression and metastasis.
To summarize, this study demonstrated that δ-T, γ-T and γ-TmT are potent inhibitors of E2-mediated mammary tumorigenesis in ACI rats, γ-T exhibiting the maximum anti-cancer activity. Tocopherols, especially γ-T, exerted dramatic effects on the rat transcriptome, regulating the expression of genes involved in cell proliferation, metastasis and tumor progression. These findings provide new insights into the anti-cancer activities of γ-T, mediated via anti-proliferative effects, distinct from its widely reported antioxidant effects. In conclusion, δ-T and γ-T have superior cancer preventive properties compared to α-T in E2-dependent mammary cancer and deserve more attention in future chemoprevention studies.
Supplementary Material
Acknowledgments
The authors would like to thank Dr. Philip Furmanski for his helpful suggestions. Research reported in this publication was supported by the National Center for Complementary and Integrative Health of the National Institutes of Health under Award Number R01 AT007036, the National Institute of Environmental Health Sciences grant ES005022, Charles and Johanna Busch Memorial Fund at Rutgers University, the Trustees Research Fellowship Program at Rutgers, and the New Jersey Commission on Cancer Research Postdoctoral Fellowship to Soumyasri Das Gupta. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Financial Support: This work was supported by the National Institutes of Health Grant R01 AT007036 (N. Suh), the National Institute of Environmental Health Sciences Grant ES005022 (H. Zarbl), Charles and Johanna Busch Memorial Fund at Rutgers University (N. Suh), the Trustees Research Fellowship Program at Rutgers (N. Suh) and the New Jersey Commission on Cancer Research Postdoctoral Fellowship (S. Das Gupta).
Footnotes
Disclosure of potential conflicts of interest: No potential conflicts of interest were disclosed.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA: a cancer journal for clinicians. 2016;66(1):7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Yager JD, Davidson NE. Estrogen carcinogenesis in breast cancer. N Engl J Med. 2006;354(3):270–82. doi: 10.1056/NEJMra050776. [DOI] [PubMed] [Google Scholar]
- 3.Cavalieri E, Chakravarti D, Guttenplan J, Hart E, Ingle J, Jankowiak R, et al. Catechol estrogen quinones as initiators of breast and other human cancers: implications for biomarkers of susceptibility and cancer prevention. Biochim Biophys Acta. 2006;1766(1):63–78. doi: 10.1016/j.bbcan.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 4.Yue W, Yager JD, Wang JP, Jupe ER, Santen RJ. Estrogen receptor-dependent and independent mechanisms of breast cancer carcinogenesis. Steroids. 2013;78(2):161–70. doi: 10.1016/j.steroids.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 5.Hemachandra LP, Madhubhani P, Chandrasena R, Esala P, Chen SN, Main M, et al. Hops (Humulus lupulus) inhibits oxidative estrogen metabolism and estrogen-induced malignant transformation in human mammary epithelial cells (MCF-10A) Cancer prevention research (Philadelphia, Pa) 2012;5(1):73–81. doi: 10.1158/1940-6207.capr-11-0348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singh B, Shoulson R, Chatterjee A, Ronghe A, Bhat NK, Dim DC, et al. Resveratrol inhibits estrogen-induced breast carcinogenesis through induction of NRF2-mediated protective pathways. Carcinogenesis. 2014;35(8):1872–80. doi: 10.1093/carcin/bgu120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bak MJ, Das Gupta S, Wahler J, Suh N. Role of dietary bioactive natural products in estrogen receptor-positive breast cancer. Seminars in cancer biology. 2016;40-41:170–91. doi: 10.1016/j.semcancer.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smolarek AK, So JY, Thomas PE, Lee HJ, Paul S, Dombrowski A, et al. Dietary tocopherols inhibit cell proliferation, regulate expression of ERalpha, PPARgamma, and Nrf2, and decrease serum inflammatory markers during the development of mammary hyperplasia. Molecular carcinogenesis. 2013;52(7):514–25. doi: 10.1002/mc.21886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smolarek AK, So JY, Burgess B, Kong AN, Reuhl K, Lin Y, et al. Dietary administration of delta- and gamma-tocopherol inhibits tumorigenesis in the animal model of estrogen receptor-positive, but not HER-2 breast cancer. Cancer prevention research (Philadelphia, Pa) 2012;5(11):1310–20. doi: 10.1158/1940-6207.CAPR-12-0263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee HJ, Ju J, Paul S, So JY, DeCastro A, Smolarek A, et al. Mixed tocopherols prevent mammary tumorigenesis by inhibiting estrogen action and activating PPAR-gamma. Clin Cancer Res. 2009;15(12):4242–9. doi: 10.1158/1078-0432.CCR-08-3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Das Gupta S, So JY, Wall B, Wahler J, Smolarek AK, Sae-Tan S, et al. Tocopherols inhibit oxidative and nitrosative stress in estrogen-induced early mammary hyperplasia in ACI rats. Molecular carcinogenesis. 2014 doi: 10.1002/mc.22164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gupta SD, Sae-tan S, Wahler J, So JY, Bak MJ, Cheng LC, et al. Dietary γ-Tocopherol–Rich Mixture Inhibits Estrogen-Induced Mammary Tumorigenesis by Modulating Estrogen Metabolism, Antioxidant Response, and PPARγ. Cancer Prevention Research. 2015;8(9):807–16. doi: 10.1158/1940-6207.CAPR-15-0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li G-X, Lee M-J, Liu AB, Yang Z, Lin Y, Shih WJ, et al. δ-tocopherol is more active than α-or γ-tocopherol in inhibiting lung tumorigenesis in vivo. Cancer Prevention Research. 2011;4(3):404–13. doi: 10.1158/1940-6207.CAPR-10-0130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guan F, Li G, Liu AB, Lee M-J, Yang Z, Chen Y-K, et al. δ-and γ-tocopherols, but not α-tocopherol, inhibit colon carcinogenesis in azoxymethane-treated F344 rats. Cancer Prevention Research. 2012;5(4):644–54. doi: 10.1158/1940-6207.CAPR-11-0521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen JX, Li G, Wang H, Liu A, Lee M-J, Reuhl K, et al. Dietary tocopherols inhibit PhIP-induced prostate carcinogenesis in CYP1A-humanized mice. Cancer letters. 2016;371(1):71–8. doi: 10.1016/j.canlet.2015.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen JX, Liu A, Lee MJ, Wang H, Yu S, Chi E, et al. delta- and gamma-tocopherols inhibit phIP/DSS-induced colon carcinogenesis by protection against early cellular and DNA damages. Molecular carcinogenesis. 2017;56(1):172–83. doi: 10.1002/mc.22481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Constantinou C, Papas A, Constantinou AI. Vitamin E and cancer: An insight into the anticancer activities of vitamin E isomers and analogs. Int J Cancer. 2008;123(4):739–52. doi: 10.1002/ijc.23689. [DOI] [PubMed] [Google Scholar]
- 18.Christen S, Woodall AA, Shigenaga MK, Southwell-Keely PT, Duncan MW, Ames BN. gamma-tocopherol traps mutagenic electrophiles such as NO(X) and complements alpha-tocopherol: physiological implications. Proc Natl Acad Sci U S A. 1997;94(7):3217–22. doi: 10.1073/pnas.94.7.3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Edefonti V, Hashibe M, Parpinel M, Ferraroni M, Turati F, Serraino D, et al. Vitamin E intake from natural sources and head and neck cancer risk: a pooled analysis in the International Head and Neck Cancer Epidemiology consortium. British journal of cancer. 2015;113(1):182–92. doi: 10.1038/bjc.2015.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhu YJ, Bo YC, Liu XX, Qiu CG. Association of dietary vitamin E intake with risk of lung cancer: a dose-response meta-analysis. Asia Pacific journal of clinical nutrition. 2017;26(2):271–7. doi: 10.6133/apjcn.032016.04. [DOI] [PubMed] [Google Scholar]
- 21.Lippman SM, Klein EA, Goodman PJ, Lucia MS, Thompson IM, Ford LG, et al. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT) Jama. 2009;301(1):39–51. doi: 10.1001/jama.2008.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Albanes D, Till C, Klein EA, Goodman PJ, Mondul AM, Weinstein SJ, et al. Plasma tocopherols and risk of prostate cancer in the Selenium and Vitamin E Cancer Prevention Trial (SELECT) Cancer prevention research (Philadelphia, Pa) 2014;7(9):886–95. doi: 10.1158/1940-6207.capr-14-0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gaziano JM, Glynn RJ, Christen WG, Kurth T, Belanger C, MacFadyen J, et al. Vitamins E and C in the prevention of prostate and total cancer in men: the Physicians’ Health Study II randomized controlled trial. Jama. 2009;301(1):52–62. doi: 10.1001/jama.2008.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang CS, Suh N, Kong A-NT. Does vitamin E prevent or promote cancer? Cancer prevention research. 2012;5(5):701–5. doi: 10.1158/1940-6207.CAPR-12-0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ju J, Hao X, Lee MJ, Lambert JD, Lu G, Xiao H, et al. A gamma-tocopherol-rich mixture of tocopherols inhibits colon inflammation and carcinogenesis in azoxymethane and dextran sulfate sodium-treated mice. Cancer prevention research (Philadelphia, Pa) 2009;2(2):143–52. doi: 10.1158/1940-6207.CAPR-08-0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bak MJ, Das Gupta S, Wahler J, Lee HJ, Li X, Lee MJ, et al. Inhibitory Effects of gamma- and delta-Tocopherols on Estrogen-Stimulated Breast Cancer In Vitro and In Vivo. Cancer prevention research (Philadelphia, Pa) 2017;10(3):188–97. doi: 10.1158/1940-6207.capr-16-0223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shull JD, Spady TJ, Snyder MC, Johansson SL, Pennington KL. Ovary-intact, but not ovariectomized female ACI rats treated with 17beta-estradiol rapidly develop mammary carcinoma. Carcinogenesis. 1997;18(8):1595–601. doi: 10.1093/carcin/18.8.1595. [DOI] [PubMed] [Google Scholar]
- 28.Wang Z, Gerstein M, Snyder M. RNA-Seq: a revolutionary tool for transcriptomics. Nature reviews Genetics. 2009;10(1):57–63. doi: 10.1038/nrg2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Turan VK, Sanchez RI, Li JJ, Li SA, Reuhl KR, Thomas PE, et al. The effects of steroidal estrogens in ACI rat mammary carcinogenesis: 17beta-estradiol, 2-hydroxyestradiol, 4-hydroxyestradiol, 16alpha-hydroxyestradiol, and 4-hydroxyestrone. J Endocrinol. 2004;183(1):91–9. doi: 10.1677/joe.1.05802. [DOI] [PubMed] [Google Scholar]
- 30.Andrews S. FastQC: a quality control tool for high throughput sequence data. 2010 [Google Scholar]
- 31.Trapnell C, Roberts A, Goff L, Pertea G, Kim D, Kelley DR, et al. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nature protocols. 2012;7(3):562–78. doi: 10.1038/nprot.2012.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee HJ, Liu H, Goodman C, Ji Y, Maehr H, Uskokovic M, et al. Gene expression profiling changes induced by a novel Gemini Vitamin D derivative during the progression of breast cancer. Biochem Pharmacol. 2006;72(3):332–43. doi: 10.1016/j.bcp.2006.04.030. [DOI] [PubMed] [Google Scholar]
- 33.Ju J, Picinich SC, Yang Z, Zhao Y, Suh N, Kong AN, et al. Cancer-preventive activities of tocopherols and tocotrienols. Carcinogenesis. 2010;31(4):533–42. doi: 10.1093/carcin/bgp205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yue W, Wang T, Zachariah E, Lin Y, Yang CS, Xu Q, et al. Transcriptomic analysis of pancreatic cancer cells in response to metformin and aspirin: an implication of synergy. Scientific reports. 2015;5:13390. doi: 10.1038/srep13390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mukasa A, Wykosky J, Ligon KL, Chin L, Cavenee WK, Furnari F. Mutant EGFR is required for maintenance of glioma growth in vivo, and its ablation leads to escape from receptor dependence. Proceedings of the National Academy of Sciences. 2010;107(6):2616–21. doi: 10.1073/pnas.0914356107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jaffer T, Ma D. The emerging role of chemokine receptor CXCR2 in cancer progression. Translational Cancer Research. 2016;5(4):S616–S28. [Google Scholar]
- 37.Sharma B, Nawandar DM, Nannuru KC, Varney ML, Singh RK. Targeting CXCR2 enhances chemotherapeutic response, inhibits mammary tumor growth, angiogenesis, and lung metastasis. Molecular cancer therapeutics. 2013;12(5):799–808. doi: 10.1158/1535-7163.MCT-12-0529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baxter RC. IGF binding proteins in cancer: mechanistic and clinical insights. Nature Reviews Cancer. 2014;14(5):329–41. doi: 10.1038/nrc3720. [DOI] [PubMed] [Google Scholar]
- 39.Chan HJ, Li H, Liu Z, Yuan Y-C, Mortimer J, Chen S. SERPINA1 is a direct estrogen receptor target gene and a predictor of survival in breast cancer patients. Oncotarget. 2015;6(28):25815. doi: 10.18632/oncotarget.4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yahata T, Shao W, Endoh H, Hur J, Coser KR, Sun H, et al. Selective coactivation of estrogen-dependent transcription by CITED1 CBP/p300-binding protein. Genes & development. 2001;15(19):2598–612. doi: 10.1101/gad.906301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pastan I, Hassan R. Discovery of mesothelin and exploiting it as a target for immunotherapy. Cancer research. 2014;74(11):2907–12. doi: 10.1158/0008-5472.CAN-14-0337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sin S, Bonin F, Petit V, Meseure D, Lallemand F, Bièche I, et al. Role of the focal adhesion protein kindlin-1 in breast cancer growth and lung metastasis. Journal of the National Cancer Institute. 2011;103(17):1323–37. doi: 10.1093/jnci/djr290. [DOI] [PubMed] [Google Scholar]
- 43.Lee K, Nam K, Oh S, Lim J, Kim R, Shim D, et al. ECM1 regulates tumor metastasis and CSC-like property through stabilization of β-catenin. Oncogene. 2015;34(50):6055–65. doi: 10.1038/onc.2015.54. [DOI] [PubMed] [Google Scholar]
- 44.Hasegawa Y, Takahashi M, Ariki S, Asakawa D, Tajiri M, Wada Y, et al. Surfactant protein D suppresses lung cancer progression by downregulation of epidermal growth factor signaling. Oncogene. 2015;34(7):838–45. doi: 10.1038/onc.2014.20. [DOI] [PubMed] [Google Scholar]
- 45.Sachdev D. Targeting the type I insulin-like growth factor system for breast cancer therapy. Current drug targets. 2010;11(9):1121–32. doi: 10.2174/138945010792006816. [DOI] [PubMed] [Google Scholar]
- 46.Fagan DH, Yee D. Crosstalk between IGF1R and estrogen receptor signaling in breast cancer. Journal of mammary gland biology and neoplasia. 2008;13(4):423. doi: 10.1007/s10911-008-9098-0. [DOI] [PubMed] [Google Scholar]
- 47.Shah M, Huang D, Blick T, Connor A, Reiter LA, Hardink JR, et al. An MMP13-selective inhibitor delays primary tumor growth and the onset of tumor-associated osteolytic lesions in experimental models of breast cancer. PloS one. 2012;7(1):e29615. doi: 10.1371/journal.pone.0029615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Krop I, Parker MT, Bloushtain-Qimron N, Porter D, Gelman R, Sasaki H, et al. HIN-1, an inhibitor of cell growth, invasion, and AKT activation. Cancer research. 2005;65(21):9659–69. doi: 10.1158/0008-5472.CAN-05-1663. [DOI] [PubMed] [Google Scholar]
- 49.Yim CY, Sekula DJ, Hever-Jardine MP, Liu X, Warzecha JM, Tam J, et al. G0S2 suppresses oncogenic transformation by repressing a MYC-regulated transcriptional program. Cancer research. 2016;76(5):1204–13. doi: 10.1158/0008-5472.CAN-15-2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


