Abstract
Movement execution generally occurs in an environment with numerous distractors, and requires the selection of a motor plan from multiple possible alternatives. However, the impact of such distractors on cortical motor function during movement remains largely unknown. Previous studies have identified two movement-related oscillatory responses that are critical to motor planning and execution, and these responses include the peri-movement beta event-related desynchronization (ERD) and the movement-related gamma synchronization (MRGS). In the current study, we investigate how visual distractors cuing alternative movements modulate the beta ERD and MRGS responses. To this end, we recorded magnetoencephalography (MEG) during an arrow-based version of the Eriksen flanker task in 42 healthy adults. All MEG data were transformed in the time-frequency domain and the beta ERD and MRGS responses were imaged using a beamformer. Virtual sensors (voxel time series) were then extracted from the peak voxels of each response for the congruent and incongruent flanker conditions separately, and these data were examined for conditional differences during the movement. Our results indicated that participants exhibited the classic “flanker effect,” as they responded significantly slower during incongruent relative to congruent trials. Our most important MEG finding was a significant increase in the peak frequency of the MRGS in the incongruent compared to the congruent condition, with no conditional effect on response amplitude. In addition, we found significantly stronger peri-movement beta ERD responses in the ipsilateral motor cortex during incongruent compared to congruent trials, but no conditional effect on frequency. These data are the first to show that the peak frequency of the MRGS response is linked to the task parameters, and varies from trial to trial in individual participants. More globally, these data suggest that beta and gamma oscillations are modulated by visual distractors causing response competition.
Keywords: flanker, magnetoencephalography, MEG, oscillations, beta ERD, movement
1. Introduction
In daily life, movement selection is rarely a straightforward process. Multiple movement options are almost always available, and competing environmental cues and distractions are ubiquitously present. One classic experiment that captures this phenomenon is the Eriksen flanker task (Eriksen and Eriksen, 1974). Briefly, during the Eriksen flanker task, participants attend to a central stimulus that is “flanked” by non-target stimuli. In the “congruent” or non-distractor condition, the flanking stimuli indicate the same response as the target stimulus. However, in the “incongruent” or distractor condition, the flanking stimuli indicate a conflicting, often opposite response to the target stimulus. The classic finding in this task is an increase in reaction time in the incongruent relative to the congruent condition. Many studies have identified the brain regions serving congruent and incongruent stimuli processing in the context of selective attention and/or conflict monitoring (e.g., (Botvinick et al., 1999; Bunge et al., 2002; Cavanagh et al., 2009; Clayson and Larson, 2011; Cohen and van Gaal, 2014; Danielmeier et al., 2009; Hazeltine et al., 2000; Hochman et al., 2014; Larson et al., 2012; Nigbur et al., 2012; Nigbur et al., 2011; Padrao et al., 2015; Tillman and Wiens, 2011); others). However, very few studies have focused on the motor response aspect of the task, which is unfortunate as it could provide critical insights on how distracting stimuli and response conflict modulate movement selection during ongoing cognitive processing.
Recent neurophysiological studies of motor control in humans have largely focused on two movement-related oscillatory responses. First, prior to and during movement, there is an event-related desynchronization (ERD) in the beta band (~14–30 Hz), termed the peri-movement beta ERD (Cheyne et al., 2006; Engel and Fries, 2010; Gaetz et al., 2010; Heinrichs-Graham and Wilson, 2015, 2016; Heinrichs-Graham et al., 2014; Jurkiewicz et al., 2006; Pfurtscheller and Lopes da Silva, 1999; Wilson et al., 2014; 2010; 2011). This response typically persists from about 1.0 s prior to movement onset until about 0.5 s after movement and peaks in the bilateral precentral gyri, with stronger responses found contralateral to movement. Other regions that generate a peri-movement beta ERD, especially during complex movements, include the supplementary motor area (SMA), premotor cortices, parietal cortices, and cerebellum (Cheyne et al., 2006; Heinrichs-Graham et al., 2016; Heinrichs-Graham and Wilson, 2015; Kurz et al., 2014; Wilson et al., 2010; 2011). Multiple studies have recently probed the functional roles of the peri-movement beta ERD, and this work overwhelmingly supports the notion that this response is crucial to motor planning and movement selection. For example, the amplitude of the beta ERD is known to be significantly modulated by complexity of the movement to be executed (Heinrichs-Graham and Wilson, 2015), the certainty of the pending movement direction (Doyle et al., 2005; Kaiser et al., 2001; Tzagarakis et al., 2010), the similarity between potential movement plans (Grent-'t-Jong et al., 2014; Praamstra et al., 2009), and other cue-related factors (Alegre et al., 2003; Heinrichs-Graham et al., 2016). Of note, there is a resynchronization in the beta band that follows movement termination, termed the post-movement beta rebound (PMBR), but given the time course of this response, it is not thought to be directly involved in motor planning or execution (Alegre et al., 2008; Alegre et al., 2004; Fry et al., 2016; Heinrichs-Graham et al., 2017; Houdayer et al., 2006; Jurkiewicz et al., 2006; Parkes et al., 2006; Pfurtscheller and Lopes da Silva, 1999; Reyns et al., 2008; Solis-Escalante et al., 2012; Wilson et al., 2010).
In addition to the beta responses, there is also increased gamma activity during movement called the movement-related gamma synchronization (MRGS). The MRGS occurs in the 60–90 Hz range and typically starts about 0.05 s prior to the movement and extends until about 0.1 s after movement onset. This response is primarily isolated to the precentral gyrus contralateral to movement, though it has also been found in the SMA (Cheyne et al., 2008; Gaetz et al., 2011; Gaetz et al., 2010; Muthukumaraswamy, 2010; Wilson et al., 2010). Very few studies have focused on the functional role of the MRGS, but given the temporal characteristics of the response, and its relative isolation in the primary motor cortex, the MRGS has long been thought to be the oscillatory signature of the motor execution signal. For example, Muthukumaraswamy (2010) used magnetoencephalography (MEG) and several simple motor tasks to identify the basic parameter space of the MRGS response. He found bursts of MRGS activity at the onset of both simple transient movements and isometric forces, and that this burst did not persist for the duration of the isometric force. However, during repetitive transient movements, there was a MRGS at the onset of each movement. These data suggest that the MRGS functions specifically as a movement onset response, as it is not sustained throughout motor execution.
While these motor-related oscillations have been widely studied, the potential impact of interference and response conflict in modulating these neural responses remains largely unknown. One recent study used a modified Eriksen flanker task and MEG to investigate how these parameters (congruent vs. incongruent) altered the peri-movement beta ERD and MRGS (Grent-'t-Jong et al., 2013). They found that both left sensorimotor beta ERD (contralateral to movement) and mid-frontal MRGS began significantly earlier during incongruent compared to congruent trials, which resulted in a significantly larger beta ERD and MRGS during very early components of each response. However, since these findings were only observed with respect to movement onset (and not stimulus onset), one interpretation of these data is that the findings largely reflect the differential amount of time between stimulus onset and the motor response in congruent relative to incongruent trials. In other words, when the motor response is defined as time zero, the stimulus occurred longer ago in the incongruent relative to the congruent condition, and this alone could underlie such oscillatory differences early in the time course. This scenario seems especially plausible, as one of the main findings was that the conditional difference in the mid-frontal MRGS was significantly correlated with a stronger flanker effect (i.e., larger reaction time differences between congruent and incongruent trials; Grent-‘t-Jong et al., 2013. Another MEG study also probed the impact of interference on motor oscillations, but instead of the flanker task they utilized a conceptually similar multi-source response interference task (Gaetz et al., 2013). As with the flanker task, a key finding was that the MRGS in the primary motor cortex contralateral to movement began earlier during interference trials compared to trials without response interference (Gaetz et al., 2013). Again, these results could reflect differences in reaction time, as participants responded slower in the interference trials and thus stimulus onset occurred longer ago relative to trials without interference. In sum, neither of these studies found differences in motor-related oscillations during the movement (when the responses peak), which raises the question of whether the findings reflect specific motor control differences or just prolonged processing in the interference/response conflict conditions.
The goal of the current study was to clarify the role of motor-related oscillatory activity in the sensorimotor cortices in the context of visual interference causing response competition. To this end, healthy adult participants performed a unimanual, arrow-based version of the Erickson flanker task during MEG. The resulting data were imaged in the time-frequency domain and virtual sensors (voxel time series) were extracted to evaluate the temporal dynamics of both the beta ERD and the MRGS. We hypothesized that the beta ERD and MRGS would be uniquely altered during the actual response (i.e., movement) during the incongruent relative to congruent trials, indicative of each response’s unique role in movement selection and execution.
2. Methods
2.1 Participants
Forty-seven healthy adults (22 females) completed the study. Five participants (3 females) were excluded from analysis due to poor performance, MEG artifacts, and/or no significant movement-related oscillatory response. The mean age of the remaining 42 participants was 27.17 years (SD: 4.53, range: 20–44 years). A total of 7 participants in the final sample were left-handed. Exclusionary criteria included any medical diagnosis affecting CNS function (e.g., psychiatric and/or neurological disease), known brain neoplasm or lesion, history of significant head trauma, current substance dependence, and ferromagnetic implants. Written informed consent was obtained from each participant following the guidelines of the University of Nebraska Medical Center’s Institutional Review Board, who approved the study protocol.
2.2 Stimuli and task
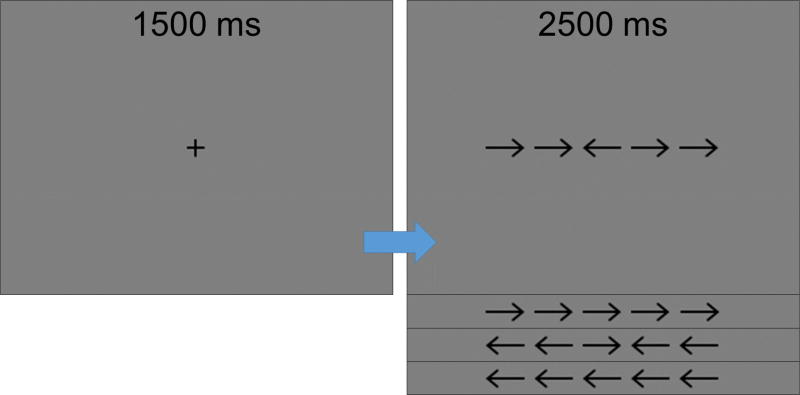
Participants performed an arrow-based version of the Eriksen flanker task (Eriksen and Eriksen, 1974) while seated in a nonmagnetic chair within the magnetically-shielded room. Each trial began with a fixation that was presented for an interval of 1.5 ± .05 s. A row of 5 arrows was then presented for 2.5 s, and participants were instructed to respond with their right hand as to the direction of the middle arrow by pressing their index finger (left arrow) or middle finger (right arrow). In congruent trials, the middle arrow was pointing the same direction as the flanker arrows, while in the incongruent trials, the middle arrow was pointing in the opposite direction of the flanker arrows (Figure 1). Trials were pseudo-randomized and equally split between congruent and incongruent conditions, with left and right pointing arrows being equally represented in each condition. A total of 200 trials were presented, making the overall MEG recording time about 14 minutes for the task.
Figure 1. Flanker task paradigm.
In each trial, participants fixated on a crosshair for 1500 ± 50 ms, then a display with a series of five arrows appeared for 2500 ms, during which participants responded with their right hand as to whether the center arrow was pointing to the left (index finger) or right (middle finger). During “congruent” trials, the flanking arrows matched the target, whereas in “incongruent” trials the flanking arrows pointed in the direction opposite of the center arrow.
2.3 MEG data acquisition
All recordings were conducted in a one-layer magnetically-shielded room with active shielding engaged. Neuromagnetic responses were sampled continuously at 1 kHz with an acquisition bandwidth of 0.1–330 Hz using an Elekta MEG system with 306 magnetic sensors (Elekta, Helsinki, Finland). Using MaxFilter (v2.2; Elekta), MEG data from each participant were individually corrected for head motion and subjected to noise reduction using the signal space separation method with a temporal extension (Taulu and Simola, 2006; Taulu et al., 2005).
2.4 MEG coregistration and structural MRI processing
Prior to MEG measurement, four coils were attached to the subject’s head and localized, together with the three fiducial points and scalp surface, with a 3-D digitizer (Fastrak 3SF0002, Polhemus Navigator Sciences, Colchester, VT, USA). Once the subject was positioned for MEG recording, an electric current with a unique frequency label (e.g., 322 Hz) was fed to each of the coils. This induced a measurable magnetic field and allowed each coil to be localized in reference to the sensors throughout the recording session. Since coil locations were also known in head coordinates, all MEG measurements could be transformed into a common coordinate system. Each participant’s MEG data were coregistered with high-resolution structural T1-weighted MRI data, prior to the application of source space analyses (i.e., beamforming), using BESA MRI (Version 2.0). These structural volumes were acquired with a Philips Achieva 3T X-series scanner using an eight-channel head coil (TR: 8.09 ms; TE: 3.7 ms; field of view: 240 mm; slice thickness: 1 mm with no gap; in-plane resolution: 1.0 × 1.0 mm). The structural volumes were aligned parallel to the anterior and posterior commissures and transformed into standardized space, along with the functional images, after beamforming (see below). All images were then spatially resampled.
2.5 MEG pre-processing, time-frequency transformation, & statistics
Cardiac artifacts were removed from the data using signal-space projection (SSP), which was accounted for during source reconstruction (Uusitalo and Ilmoniemi, 1997). The continuous magnetic time series was divided into epochs of 4.0 s duration (−2.0 to 2.0 s), with 0.0 s defined as movement onset (i.e., button press) and the baseline defined as the −1.8 to −1.0 s time window. Note that this baseline period was selected to ensure that the onset of the flanker stimulus did not occur during the baseline. Epochs containing artifacts were rejected based on a fixed threshold method, supplemented with visual inspection. After artifact rejection, an average of 84.02 (SD: 5.58) trials in the congruent and 83.76 (SD: 4.41) trials in the incongruent condition remained in each participant; this difference was not significant, t(41) = 0.413, p = .682.
Artifact-free epochs were transformed into the time-frequency domain using complex demodulation with a time-frequency resolution of 2 Hz and 25 ms. The resulting spectral power estimates per sensor were averaged over trials to generate time-frequency plots of mean spectral density. These sensor-level data were normalized by dividing the power value of each time-frequency bin by the respective bin’s baseline power, which was calculated as the mean power during the −1.8 to −1.0 s time period. The specific time-frequency windows used for imaging were determined by statistical analysis of the sensor-level spectrograms across all trials (congruent and incongruent) and the entire array of gradiometers. Each data point in the resulting sensor-specific spectrograms was initially evaluated using a mass univariate approach based on the general linear model. To reduce the risk of false positive results while maintaining reasonable sensitivity, a two-stage procedure was followed to control for Type 1 error. In the first stage, one-sample t-tests were conducted on each data point and the output spectrogram of t-values was thresholded at p < 0.05 to define time-frequency bins containing potentially significant oscillatory deviations across all participants and conditions. In stage two, time-frequency bins that survived the threshold were clustered with temporally and/or spectrally neighboring bins that were also above the (p < 0.05) threshold, and a cluster value was derived by summing all of the t-values of all data points in the cluster. Nonparametric permutation testing was then used to derive a distribution of cluster-values and the significance level of the observed clusters (from stage one) were tested directly using this distribution (Ernst, 2004; Maris and Oostenveld, 2007). For each comparison, at least 10,000 permutations were computed to build a distribution of cluster values. Based on these analyses, the time-frequency windows containing significant oscillatory events around responses of a priori interest (i.e., beta ERD, MRGS) across all participants and conditions were selected for imaging.
2.6 MEG source imaging, voxel time series extraction, & statistics
Cortical networks were imaged through an extension of the linearly constrained minimum variance vector beamformer (Gross et al., 2001), which employs spatial filters in the frequency domain to calculate source power for the entire brain volume. The single images are derived from the cross spectral densities of all combinations of MEG gradiometers averaged over the time-frequency range of interest, and the solution of the forward problem for each location on a grid specified by input voxel space. Following convention, the source power in these images was normalized per participant using a separately averaged pre-stimulus noise period of equal duration and bandwidth (Hillebrand et al., 2005). Such images are typically referred to as pseudo-t maps, with units (pseudo-t) that reflect noise-normalized power differences (i.e. active vs. baseline) per voxel. MEG pre-processing and imaging used the Brain Electrical Source Analysis (BESA version 6.1) software.
Normalized source power was computed for the selected time-frequency bands over the entire brain volume per participant at 4.0 × 4.0 × 4.0 mm resolution. Beamformer images for each time-frequency window of interest were then averaged across both conditions and all participants, and coordinates corresponding to the peak responses (per time-frequency window) were identified. We then extracted virtual sensor data separately per condition from each these peak coordinates, which corresponded to the left and right precentral gyri. To create the virtual sensors, we applied the sensor weighting matrix derived through the forward computation to the preprocessed signal vector, which yielded two time series for each coordinate in source space, and we used the time series with the maximal response for our analyses (Gross et al., 2001). Note that this virtual sensor extraction was done per participant, once the coordinates of interest (i.e., one per cluster) were known. Once these virtual sensors were extracted, characteristics of each response (i.e., peak amplitude, peak frequency) were assessed per condition.
3. Results
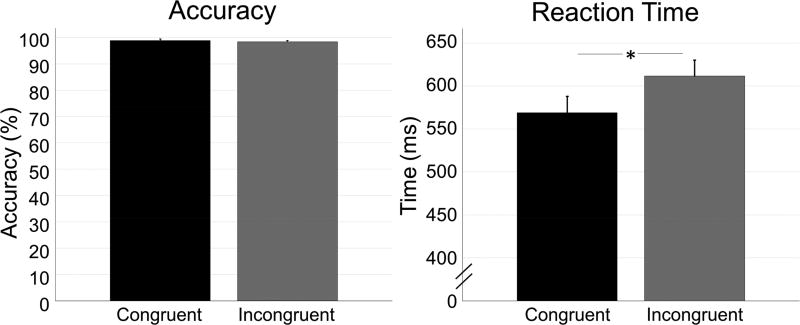
The final 42 participants performed generally well, with an average accuracy of 98.86% (SD: 3.68%) in the congruent condition, and 98.40% (SD: 2.85%) in the incongruent condition. Reaction times for the congruent and incongruent conditions were 569.19 ms (SD: 120.91 ms) and 611.52 ms (SD: 119.98 ms), respectively (Figure 2). While accuracy was not significantly different between conditions, t(41) = 1.740, p = .089, there was a significant difference in reaction time, t(41) = 10.480, p < .001, d = 1.587, 95% CI [35.794, 53.173], such that participants took longer to respond in the incongruent relative to the congruent condition.
Figure 2. Behavioral results.
In each bar graph, the congruent condition is shown in black, while the incongruent condition is shown in gray. Error bars denote the standard error of the mean (SEM). Accuracy (percentage correct) is shown on the left, and reaction time (in ms) is shown on the right. While there was no difference in accuracy between conditions, there was a significant difference in reaction time (p < .001), with participants responding more slowly in the incongruent compared to the congruent condition. * = p < .001.
3.1 Sensor-level results
Sensor level spectrograms across both conditions and all participants were statistically examined using nonparametric permutation testing to derive the precise time-frequency bins for follow up beamforming analyses. The results showed significant beta ERD in a subset of gradiometers near the left and right sensorimotor cortices, which extended from approximately 0.5 s before movement onset until about 0.3 s after onset in the 16–26 Hz range (0.0 s = movement onset; p < 0.001; corrected). A significant resynchronization (i.e., PMBR) in the same 16–26 Hz band was detected during the 0.5 to 1.5 s time window in roughly the same gradiometers (p < 0.001; corrected). These neural response parameters are typical of the peri-movement beta ERD and PMBR responses previously described by our group and many others. Finally, there was a significant MRGS that stretched from 60–80 Hz in a smaller subset of gradiometers near the left sensorimotor cortices. This response began approximately 0.05 s prior to movement onset and extended until about 0.1 s afterward. Given the focus of the study and our hypotheses, we focused the beta ERD and MRGS responses in all remaining analyses.
3.2 Source imaging
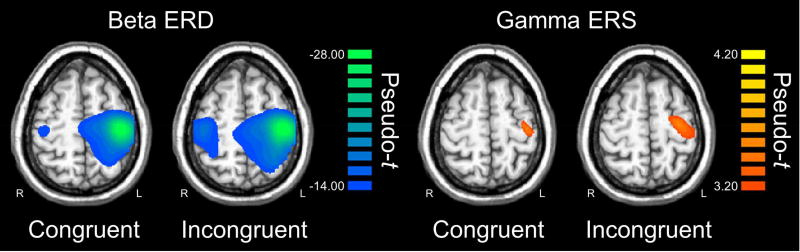
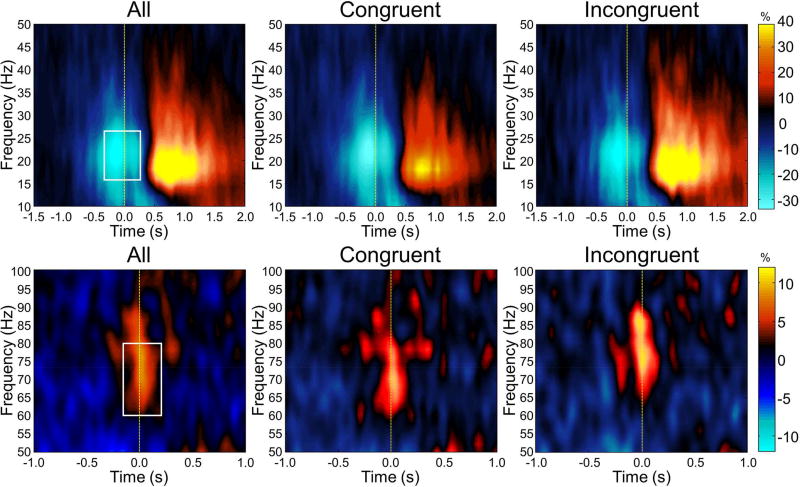
The time-frequency windows corresponding to the maximum beta ERD (−0.2 to 0.3 s, 16–26 Hz) and MRGS (−0.05 to 0.1 s, 60–80 Hz) responses, and a window of equal bandwidth and duration from the baseline period, were individually imaged using a beamformer. These images were then separately averaged across participants to derive the spatial locations of each response for subsequent virtual-sensor analysis (Figure 3). For the beta ERD, this revealed a strong, widespread desynchronization with peaks in the bilateral pre- and postcentral gyri, premotor cortices, SMA, paracentral lobule, parietal cortices, and cerebellum. In contrast, the MRGS was produced by a weaker response that was constrained to the left precentral gyrus. In line with our hypotheses, we extracted separate virtual sensors (i.e., voxel time series) for each condition from the peak voxel of each response in the precentral gyri (see Methods). Thus, the same peak voxel coordinates were used for the congruent and incongruent conditions for each time-frequency response. Of note, four virtual sensor time series were extracted for the beta ERD per participant, as the response was found bilaterally in the left and right precentral gyri. In contrast, the MRGS was only found in the left precentral gyrus; thus, only two virtual sensors (congruent and incongruent) were extracted for each participant. Time-frequency spectra corresponding to the left precentral gyrus peaks, per condition and across conditions, are shown for the beta ERD and MRGS in Figure 4.
Figure 3. Peri-movement beta ERD and MRGS responses.
Group-averaged beamformer images (pseudo-t) for the peri-movement beta ERD (left panel) and MRGS (right panel) are shown for the congruent and incongruent conditions individually. Images are shown in radiologic convention (R = L). In both conditions, the task induced a strong peri-movement beta ERD in the bilateral primary motor cortices (stronger in the contralateral), and a MRGS response that was restricted to the contralateral primary motor cortex. Virtual sensors were extracted from the peak voxel(s) in the grand average image (congruent and incongruent) of each response, and these time series were used to assess the effects of condition on amplitude and peak frequency.
Figure 4. Virtual sensor time-frequency spectra for the peri-movement beta ERD and MRGS response.
Voxel time series were extracted from each participant’s data using the peak voxels corresponding to the peri-movement beta ERD and MRGS in the grand average images. For the beta ERD, virtual sensors were extracted for the left (top row) and right primary motor cortices (not shown), while for the MRGS virtual sensors were extracted solely from the left primary cortex (bottom row). For each voxel time series, frequency (in Hz) is shown on the y-axis, with time (in seconds) on the x-axis. In each spectrogram, color depicts percentage increase (warmer colors) or decrease (cooler colors) from the baseline, with the scale bar shown to the far right of each row. Time-frequency spectrograms combined across both conditions are shown in the left column, while the congruent condition is shown in the middle column and the incongruent condition is shown in the right column. White boxes denote the time-frequency bins that were imaged prior to virtual sensor extraction.
3.3 Virtual-sensor analysis: Beta ERD
We first probed the effects of condition (congruent vs. incongruent) on the relative power and peak frequency of the beta ERD response. To this end, we used two repeated-measures ANOVAs to probe response power and peak frequency separately, with each ANOVA having hemisphere (left, right) and condition (congruent, incongruent) as within-subjects factors. The analysis of beta ERD power revealed a significant effect of condition, F(1,41) = 4.168, p = .048, indicating that participants had greater beta ERD power (i.e., more negative relative from baseline) across hemispheres in the incongruent relative to the congruent condition. There was also a significant main effect of hemisphere, F(1, 41) = 27.910, p < .001, such that there was a stronger beta ERD in the left (contralateral to movement) relative to the right precentral gyrus. Finally, there was a significant hemisphere-by-condition interaction, F(1,41) = 5.754, p = .021. Follow-on analysis showed that beta ERD power was significantly stronger in the right precentral gyrus during incongruent relative to congruent trials, t(41) = 2.871, p = .006, d = .451, 95% CI [1.846, 10.599], and that beta ERD power in the left precentral gyrus did not statistically differ between conditions, t(41) = 0.020, p = .984. Thus, beta ERD power was more bilateral in the incongruent than in the congruent condition, which can be discerned from the beamformer images in Figure 3. In contrast to the beta ERD power analyses, the analysis of peak frequency showed no significant effect of condition, F(1,41) = 0.620, p = .436, or hemisphere, F(1,41) = 1.670, p = .204, nor was there a hemisphere-by-condition interaction, F(1,41) = 0.257, p = .615.
3.4 Virtual-sensor analysis: MRGS
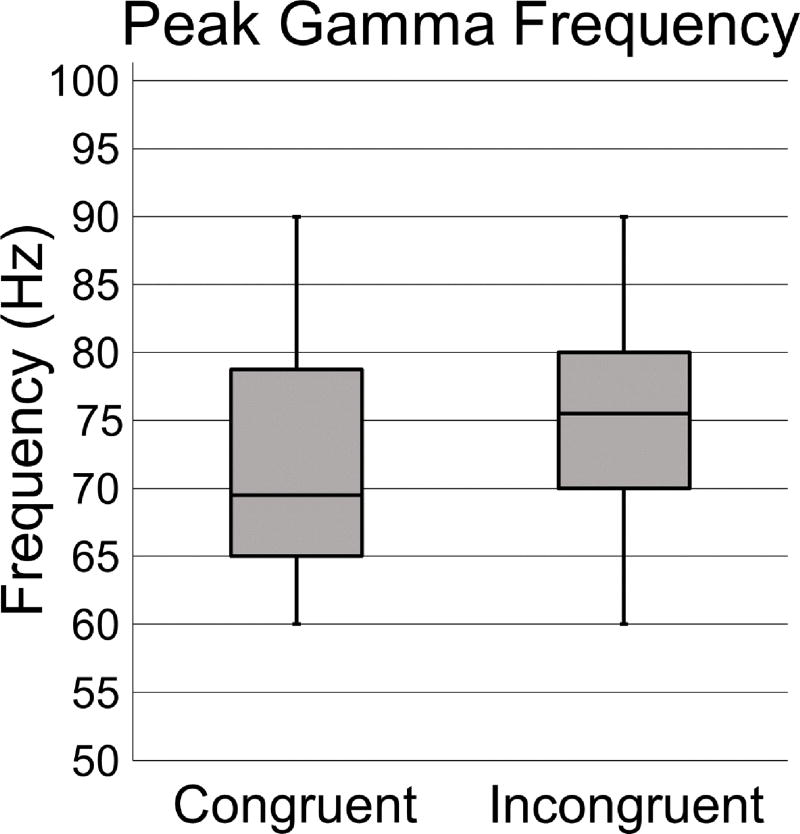
Since there was no hemisphere factor for the MRGS response, we examined the effect of condition on power and peak frequency using paired-samples t-tests. Analysis of response power was not significant, t(41) = 0.840, p = .406. However, analysis of peak frequency was significant, t(41) = 2.159, p = .037, d = 0.335, 95% CI [0.234, 7.004], and showed that the MRGS frequency was significantly higher in the incongruent compared to the congruent condition (Figure 5). Lastly, to determine whether there was a link between the beta ERD and MRGS in the context of response competition, a correlation analysis was run between MRGS and beta ERD characteristics. These correlations were not significant (all p’s > .05).
Figure 5. MRGS peak frequency by condition.
Box plots showing the peak frequency for the congruent (left) and incongruent (right) conditions. Frequency (in Hz) is denoted on the y-axis. The center line within each box denotes the median frequency, and the bottom and top of each box designate the first and third quartile, respectively. Each lower and upper stem reflects the minimum and maximum values. The peak frequency of the MRGS response was higher in the incongruent relative to the congruent condition, t(41) = 2.159, p = .037.
4. Discussion
This study examined how visual interference and response conflict modulate motor-related oscillatory activity during movement execution using an arrow-based version of the Eriksen flanker task. We hypothesized that there would be distinct changes in both peri-movement beta ERD and MRGS activity when distractors were present, indicative of each response’s unique role in movement planning and selection. In line with this hypothesis, we found distinct patterns of beta and gamma activity as a function of condition, which had not been previously described in oscillatory studies of motor control during visual interference (Gaetz et al., 2013; Grent-'t-Jong et al., 2013). Specifically, we observed stronger ipsilateral beta ERD power in the incongruent compared to the congruent condition, whereas no conditional differences in MRGS power were identified. Conversely, we observed an increase in MRGS peak frequency in the incongruent compared to the congruent condition, which was not found in the beta band. Below we discuss the implications of these findings as they relate to understanding the functional role of movement-related oscillations during response selection in the presence of conflict.
Our most important finding was likely the significant effect of condition on MRGS peak frequency, which indicated that the peak gamma frequency was higher in the incongruent compared with the congruent condition. This is the first study to report condition-dependent differences in the MRGS peak frequency within individuals. In fact, recent work suggests that while gamma frequency differs as a function of the type of movement performed (Cheyne et al., 2008; Muthukumaraswamy, 2010), there is remarkable consistency within individuals as to their movement-specific gamma frequency profile (Cheyne and Ferrari, 2013), which is in direct contrast to the current data. Importantly, in this study, participants performed the same movements in each condition (i.e., right-hand movements of either the index finger or middle finger, equally-balanced across conditions), so differences in the movement itself cannot account for the differences in gamma frequency. Thus, our data provides evidence that the MRGS response is not simply a motor execution signal, but is instead modulated by the cognitive demands of the movement to be executed. In other words, some aspect of the movement selection process, in addition to the parameters of the movement itself, is related to MRGS frequency. In the current investigation, the MRGS frequency was elevated in the presence of response conflict. Future research should further investigate whether MRGS frequency increases as a function of increasing response conflict, or whether some other aspect of movement selection, such as difficulty of the movement choice, modulates MRGS frequency. Additionally, given that this is the first report of within-subject modulation of MRGS frequency, replication studies are necessary. Nonetheless, the results from our study suggest that the MRGS frequency is modulated by higher-order cognitive processes accompany movement selection, rather than acting simply as a motor execution signal.
In regard to mechanism, a wealth of literature suggests a tight positive relationship between peak gamma frequency and/or amplitude and γ-aminobutyric acid (GABA) concentration across the visual and sensorimotor domains (Campbell et al., 2014; Edden et al., 2009; Gaetz et al., 2011; Kujala et al., 2015; Magazzini et al., 2016; Muthukumaraswamy et al., 2009), though this is not always the case (Cousijn et al., 2014; Lozano-Soldevilla et al., 2014). Of note, there is evidence that the NMDA receptor antagonist ketamine also increases gamma amplitude (Shaw et al., 2015). GABA is the most abundant inhibitory neurotransmitter in the brain; thus, the increase in MRGS frequency in the incongruent condition observed in the current study may be reflective of an increase in local neural inhibition. Basically, in the incongruent condition, the four flanking arrows pointed in the opposite direction of the target arrow, and thus primed the participant to respond with the incorrect finger. The primed response may have to be actively inhibited within the motor cortex in order for the participant to respond correctly, which could result in a transient increase in GABA concentration in this neural region. On the other hand, response inhibition was not required during congruent trials, as the flanker arrows were the same as the target arrow, and as such primed the participant to respond correctly from the onset. However, this interpretation is only speculative, as we did not measure GABA concentrations in this study, and to date there is no available methods for noninvasively measuring GABA concentration trial-to-trial.
The current study also found that the amplitude of the peri-movement beta ERD was modulated by condition. Numerous studies have linked the peri-movement beta ERD to motor planning and movement selection (Alegre et al., 2003; Doyle et al., 2005; Grent-'t-Jong et al., 2014; Heinrichs-Graham et al., 2016; Heinrichs-Graham and Wilson, 2015; Kaiser et al., 2001; Praamstra et al., 2009; Tzagarakis et al., 2010). As such, it was not surprising that the presence of conflicting response commands, as in the incongruent condition, modified the beta ERD response. However, it was particularly interesting that the presence of incongruent flanking stimuli caused an increase in beta ERD power in the ipsilateral, but not contralateral primary motor cortex. In other words, the left primary motor cortex, which is known to be primarily responsible for movement selection of the right hand, was not affected despite both response options originating in the right hand; rather the response was more bilateral in the incongruent condition. It is possible that the increased difficulty or ambiguity of the response decision in the incongruent condition induced a more widespread beta ERD response, resulting in greater recruitment of the ipsilateral motor cortex in that condition. Indeed, a classic EEG study by Doyle and colleagues (2005) showed that in the presence of more ambiguous movement cues, the peri-movement beta ERD was more bilateral than when the movement cue was more direct.
The only other MEG studies that probed differences in these responses in the context of response conflict found no differences in beta ERD power or MRGS frequency during and slightly preceding the movement onset (Grent-'t-Jong et al., 2013), which is in direct contrast with the current study. We propose that differences in the characteristics of the movement and/or the cognitive demands of the motor selection process may have contributed to the disparities between studies. Specifically, Gaetz and colleagues (2013) utilized a multi-source interference task, where they presented three numbers to the participant and instructed the participant to identify which number was dissimilar, and to perform an extension-flexion of the finger corresponding to that number. Importantly, in the incongruent condition, the dissimilar number was presented in a location different than the finger being tapped. On the contrary, Grent-‘t-Jong and colleagues (2013) used a vertical, arrow-based Flanker task that required an extension or flexion of the same finger as response options. In the current study, we employed a horizontal, arrow-based Flanker design that required an extension-flexion of either the right index or middle finger. Thus, all three studies utilized flexion-extension movements, but the number of muscle groups involved in the target movements differed, as did the precise visuo-motor response mapping process across each study. Future studies should investigate whether the different movement options modulate MRGS frequency. However, we feel that this possibility unlikely since studies of one (Grent-‘t-Jong et al.) or three (Gaetz et al.) different movement options did not show an effect, while our study of two movement options did. In our view, it is more likely that the disparate findings across these studies and ours reflects a power issue; the current study had almost two- to three-fold more participants than previous studies. Given the notable between-subject variability in MRGS characteristics (Muthukumaraswamy 2010; Cheyne et al. 2008), power was likely a contributor.
To close, the current study sought to identify whether response competition modulated motor-related oscillatory activity, using an arrow-based version of the Eriksen flanker task during MEG. Our results are the first to show a context-dependent modulation of peak gamma frequency, in that the MRGS peak frequency was significantly increased during incongruent relative to congruent processing. We posit that this increase in frequency may be due to a transient increase in GABA concentration, but further work is needed to test this hypothesis. Further, EMG was not recorded in this study, so the possibility that the response characteristics of the congruent vs. incongruent conditions were of different speed, strength, etc. cannot be definitively ruled out and this may have affected MRGS frequency, though there is no evidence to date that MRGS frequency is modulated by these response parameters. In contrast, the presence of response conflict induced an elevation of peri-movement beta ERD power in the motor cortex ipsilateral to movement, which may be due to the increased ambiguity of the response plan in the incongruent compared to congruent conditions. In sum, this pattern of results suggests that the peri-movement beta ERD and MRGS have unique roles in motor planning and movement selection in the presence of response conflict, and that higher-order cognitive functioning may impact critical parameters of the MRGS, which indicates that this response is not simply a motor execution response as previously thought.
Acknowledgments
Grant Support: This work was supported by grant R01-MH103220 from the National Institutes of Health (TWW), grant #1539067 from the National Science Foundation (TWW), and by a Research Support Fund grant from the Nebraska Health System and the University of Nebraska Medical Center. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: None.
References
- Alegre M, Alvarez-Gerriko I, Valencia M, Iriarte J, Artieda J. Oscillatory changes related to the forced termination of a movement. Clin Neurophysiol. 2008;119:290–300. doi: 10.1016/j.clinph.2007.10.017. [DOI] [PubMed] [Google Scholar]
- Alegre M, Gurtubay IG, Labarga A, Iriarte J, Malanda A, Artieda J. Alpha and beta oscillatory changes during stimulus-induced movement paradigms: effect of stimulus predictability. Neuroreport. 2003;14:381–385. doi: 10.1097/00001756-200303030-00017. [DOI] [PubMed] [Google Scholar]
- Alegre M, Gurtubay IG, Labarga A, Iriarte J, Valencia M, Artieda J. Frontal and central oscillatory changes related to different aspects of the motor process: a study in go/no-go paradigms. Exp Brain Res. 2004;159:14–22. doi: 10.1007/s00221-004-1928-8. [DOI] [PubMed] [Google Scholar]
- Botvinick M, Nystrom LE, Fissell K, Carter CS, Cohen JD. Conflict monitoring versus selection-for-action in anterior cingulate cortex. Nature. 1999;402:179–181. doi: 10.1038/46035. [DOI] [PubMed] [Google Scholar]
- Bunge SA, Hazeltine E, Scanlon MD, Rosen AC, Gabrieli JD. Dissociable contributions of prefrontal and parietal cortices to response selection. Neuroimage. 2002;17:1562–1571. doi: 10.1006/nimg.2002.1252. [DOI] [PubMed] [Google Scholar]
- Campbell AE, Sumner P, Singh KD, Muthukumaraswamy SD. Acute effects of alcohol on stimulus-induced gamma oscillations in human primary visual and motor cortices. Neuropsychopharmacology. 2014;39:2104–2113. doi: 10.1038/npp.2014.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh JF, Cohen MX, Allen JJ. Prelude to and resolution of an error: EEG phase synchrony reveals cognitive control dynamics during action monitoring. J Neurosci. 2009;29:98–105. doi: 10.1523/JNEUROSCI.4137-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheyne D, Bakhtazad L, Gaetz W. Spatiotemporal mapping of cortical activity accompanying voluntary movements using an event-related beamforming approach. Hum Brain Mapp. 2006;27:213–229. doi: 10.1002/hbm.20178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheyne D, Bells S, Ferrari P, Gaetz W, Bostan AC. Self-paced movements induce high-frequency gamma oscillations in primary motor cortex. Neuroimage. 2008;42:332–342. doi: 10.1016/j.neuroimage.2008.04.178. [DOI] [PubMed] [Google Scholar]
- Cheyne D, Ferrari P. MEG studies of motor cortex gamma oscillations: evidence for a gamma "fingerprint" in the brain? Front Hum Neurosci. 2013;7:575. doi: 10.3389/fnhum.2013.00575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayson PE, Larson MJ. Conflict adaptation and sequential trial effects: support for the conflict monitoring theory. Neuropsychologia. 2011;49:1953–1961. doi: 10.1016/j.neuropsychologia.2011.03.023. [DOI] [PubMed] [Google Scholar]
- Cohen MX, van Gaal S. Subthreshold muscle twitches dissociate oscillatory neural signatures of conflicts from errors. Neuroimage. 2014;86:503–513. doi: 10.1016/j.neuroimage.2013.10.033. [DOI] [PubMed] [Google Scholar]
- Cousijn H, Haegens S, Wallis G, Near J, Stokes MG, Harrison PJ, Nobre AC. Resting GABA and glutamate concentrations do not predict visual gamma frequency or amplitude. Proc Natl Acad Sci U S A. 2014;111:9301–9306. doi: 10.1073/pnas.1321072111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielmeier C, Wessel JR, Steinhauser M, Ullsperger M. Modulation of the error-related negativity by response conflict. Psychophysiology. 2009;46:1288–1298. doi: 10.1111/j.1469-8986.2009.00860.x. [DOI] [PubMed] [Google Scholar]
- Doyle LM, Yarrow K, Brown P. Lateralization of event-related beta desynchronization in the EEG during pre-cued reaction time tasks. Clin Neurophysiol. 2005;116:1879–1888. doi: 10.1016/j.clinph.2005.03.017. [DOI] [PubMed] [Google Scholar]
- Edden RA, Muthukumaraswamy SD, Freeman TC, Singh KD. Orientation discrimination performance is predicted by GABA concentration and gamma oscillation frequency in human primary visual cortex. J Neurosci. 2009;29:15721–15726. doi: 10.1523/JNEUROSCI.4426-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel AK, Fries P. Beta-band oscillations--signalling the status quo? Curr Opin Neurobiol. 2010;20:156–165. doi: 10.1016/j.conb.2010.02.015. [DOI] [PubMed] [Google Scholar]
- Eriksen BA, Eriksen CW. Effects of noise letters upon identification of a target letter in a non-search task. Perception and Psychophysics. 1974;16:143–149. [Google Scholar]
- Ernst MD. Permutation methods: a basis for exact inference. Statistical Science. 2004;19:676–685. [Google Scholar]
- Fry A, Mullinger KJ, O'Neill GC, Barratt EL, Morris PG, Bauer M, Folland JP, Brookes MJ. Modulation of post-movement beta rebound by contraction force and rate of force development. Hum Brain Mapp. 2016;37:2493–2511. doi: 10.1002/hbm.23189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaetz W, Edgar JC, Wang DJ, Roberts TP. Relating MEG measured motor cortical oscillations to resting gamma-aminobutyric acid (GABA) concentration. Neuroimage. 2011;55:616–621. doi: 10.1016/j.neuroimage.2010.12.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaetz W, Liu C, Zhu H, Bloy L, Roberts TP. Evidence for a motor gamma-band network governing response interference. Neuroimage. 2013;74:245–253. doi: 10.1016/j.neuroimage.2013.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaetz W, Macdonald M, Cheyne D, Snead OC. Neuromagnetic imaging of movement-related cortical oscillations in children and adults: age predicts post-movement beta rebound. Neuroimage. 2010;51:792–807. doi: 10.1016/j.neuroimage.2010.01.077. [DOI] [PubMed] [Google Scholar]
- Grent-'t-Jong T, Oostenveld R, Jensen O, Medendorp WP, Praamstra P. Oscillatory dynamics of response competition in human sensorimotor cortex. Neuroimage. 2013;83:27–34. doi: 10.1016/j.neuroimage.2013.06.051. [DOI] [PubMed] [Google Scholar]
- Grent-'t-Jong T, Oostenveld R, Jensen O, Medendorp WP, Praamstra P. Competitive interactions in sensorimotor cortex: oscillations express separation between alternative movement targets. J Neurophysiol. 2014;112:224–232. doi: 10.1152/jn.00127.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross J, Kujala J, Hamalainen M, Timmermann L, Schnitzler A, Salmelin R. Dynamic imaging of coherent sources: Studying neural interactions in the human brain. Proc Natl Acad Sci U S A. 2001;98:694–699. doi: 10.1073/pnas.98.2.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazeltine E, Poldrack R, Gabrieli JD. Neural activation during response competition. J Cogn Neurosci. 2000;12(Suppl 2):118–129. doi: 10.1162/089892900563984. [DOI] [PubMed] [Google Scholar]
- Heinrichs-Graham E, Arpin DJ, Wilson TW. Cue-related Temporal Factors Modulate Movement-related Beta Oscillatory Activity in the Human Motor Circuit. J Cogn Neurosci. 2016;28:1039–1051. doi: 10.1162/jocn_a_00948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs-Graham E, Kurz MJ, Gehringer JL, Wilson TW. The functional role of post-movement beta oscillations in movement termination. Brain Struct Funct. 2017 doi: 10.1007/s00429-017-1387-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs-Graham E, Wilson TW. Coding complexity in the human motor circuit. Hum Brain Mapp. 2015;36:5155–5167. doi: 10.1002/hbm.23000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs-Graham E, Wilson TW. Is an absolute level of cortical beta suppression required for proper movement? Magnetoencephalographic evidence from healthy aging. Neuroimage. 2016;134:514–521. doi: 10.1016/j.neuroimage.2016.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs-Graham E, Wilson TW, Santamaria PM, Heithoff SK, Torres-Russotto D, Hutter-Saunders JA, Estes KA, Meza JL, Mosley RL, Gendelman HE. Neuromagnetic evidence of abnormal movement-related beta desynchronization in Parkinson's disease. Cereb Cortex. 2014;24:2669–2678. doi: 10.1093/cercor/bht121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillebrand A, Singh KD, Holliday IE, Furlong PL, Barnes GR. A new approach to neuroimaging with magnetoencephalography. Hum Brain Mapp. 2005;25:199–211. doi: 10.1002/hbm.20102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochman EY, Vaidya AR, Fellows LK. Evidence for a role for the dorsal anterior cingulate cortex in disengaging from an incorrect action. PLoS One. 2014;9:e101126. doi: 10.1371/journal.pone.0101126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houdayer E, Labyt E, Cassim F, Bourriez JL, Derambure P. Relationship between event-related beta synchronization and afferent inputs: analysis of finger movement and peripheral nerve stimulations. Clin Neurophysiol. 2006;117:628–636. doi: 10.1016/j.clinph.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Jurkiewicz MT, Gaetz WC, Bostan AC, Cheyne D. Post-movement beta rebound is generated in motor cortex: evidence from neuromagnetic recordings. Neuroimage. 2006;32:1281–1289. doi: 10.1016/j.neuroimage.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Kaiser J, Birbaumer N, Lutzenberger W. Event-related beta desynchronization indicates timing of response selection in a delayed-response paradigm in humans. Neurosci Lett. 2001;312:149–152. doi: 10.1016/s0304-3940(01)02217-0. [DOI] [PubMed] [Google Scholar]
- Kujala J, Jung J, Bouvard S, Lecaignard F, Lothe A, Bouet R, Ciumas C, Ryvlin P, Jerbi K. Gamma oscillations in V1 are correlated with GABA(A) receptor density: A multi-modal MEG and Flumazenil-PET study. Sci Rep. 2015;5:16347. doi: 10.1038/srep16347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurz MJ, Becker KM, Heinrichs-Graham E, Wilson TW. Neurophysiological abnormalities in the sensorimotor cortices during the motor planning and movement execution stages of children with cerebral palsy. Dev Med Child Neurol. 2014;56:1072–1077. doi: 10.1111/dmcn.12513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson MJ, Clayson PE, Baldwin SA. Performance monitoring following conflict: internal adjustments in cognitive control? Neuropsychologia. 2012;50:426–433. doi: 10.1016/j.neuropsychologia.2011.12.021. [DOI] [PubMed] [Google Scholar]
- Lozano-Soldevilla D, ter Huurne N, Cools R, Jensen O. GABAergic modulation of visual gamma and alpha oscillations and its consequences for working memory performance. Curr Biol. 2014;24:2878–2887. doi: 10.1016/j.cub.2014.10.017. [DOI] [PubMed] [Google Scholar]
- Magazzini L, Muthukumaraswamy SD, Campbell AE, Hamandi K, Lingford-Hughes A, Myers JF, Nutt DJ, Sumner P, Wilson SJ, Singh KD. Significant reductions in human visual gamma frequency by the gaba reuptake inhibitor tiagabine revealed by robust peak frequency estimation. Hum Brain Mapp. 2016;37:3882–3896. doi: 10.1002/hbm.23283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maris E, Oostenveld R. Nonparametric statistical testing of EEG- and MEG-data. J Neurosci Methods. 2007;164:177–190. doi: 10.1016/j.jneumeth.2007.03.024. [DOI] [PubMed] [Google Scholar]
- Muthukumaraswamy SD. Functional properties of human primary motor cortex gamma oscillations. J Neurophysiol. 2010;104:2873–2885. doi: 10.1152/jn.00607.2010. [DOI] [PubMed] [Google Scholar]
- Muthukumaraswamy SD, Edden RA, Jones DK, Swettenham JB, Singh KD. Resting GABA concentration predicts peak gamma frequency and fMRI amplitude in response to visual stimulation in humans. Proc Natl Acad Sci U S A. 2009;106:8356–8361. doi: 10.1073/pnas.0900728106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigbur R, Cohen MX, Ridderinkhof KR, Sturmer B. Theta dynamics reveal domain-specific control over stimulus and response conflict. J Cogn Neurosci. 2012;24:1264–1274. doi: 10.1162/jocn_a_00128. [DOI] [PubMed] [Google Scholar]
- Nigbur R, Ivanova G, Sturmer B. Theta power as a marker for cognitive interference. Clin Neurophysiol. 2011;122:2185–2194. doi: 10.1016/j.clinph.2011.03.030. [DOI] [PubMed] [Google Scholar]
- Padrao G, Rodriguez-Herreros B, Perez Zapata L, Rodriguez-Fornells A. Exogenous capture of medial-frontal oscillatory mechanisms by unattended conflicting information. Neuropsychologia. 2015;75:458–468. doi: 10.1016/j.neuropsychologia.2015.07.004. [DOI] [PubMed] [Google Scholar]
- Parkes LM, Bastiaansen MC, Norris DG. Combining EEG and fMRI to investigate the post-movement beta rebound. Neuroimage. 2006;29:685–696. doi: 10.1016/j.neuroimage.2005.08.018. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Lopes da Silva FH. Event-related EEG/MEG synchronization and desynchronization: basic principles. Clin Neurophysiol. 1999;110:1842–1857. doi: 10.1016/s1388-2457(99)00141-8. [DOI] [PubMed] [Google Scholar]
- Praamstra P, Kourtis D, Nazarpour K. Simultaneous preparation of multiple potential movements: opposing effects of spatial proximity mediated by premotor and parietal cortex. J Neurophysiol. 2009;102:2084–2095. doi: 10.1152/jn.00413.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyns N, Houdayer E, Bourriez JL, Blond S, Derambure P. Post-movement beta synchronization in subjects presenting with sensory deafferentation. Clin Neurophysiol. 2008;119:1335–1345. doi: 10.1016/j.clinph.2008.02.020. [DOI] [PubMed] [Google Scholar]
- Shaw AD, Saxena N, L EJ, Hall JE, Singh KD, Muthukumaraswamy SD. Ketamine amplifies induced gamma frequency oscillations in the human cerebral cortex. Eur Neuropsychopharmacol. 2015;25:1136–1146. doi: 10.1016/j.euroneuro.2015.04.012. [DOI] [PubMed] [Google Scholar]
- Solis-Escalante T, Muller-Putz GR, Pfurtscheller G, Neuper C. Cue-induced beta rebound during withholding of overt and covert foot movement. Clin Neurophysiol. 2012;123:1182–1190. doi: 10.1016/j.clinph.2012.01.013. [DOI] [PubMed] [Google Scholar]
- Taulu S, Simola J. Spatiotemporal signal space separation method for rejecting nearby interference in MEG measurements. Phys Med Biol. 2006;51:1759–1768. doi: 10.1088/0031-9155/51/7/008. [DOI] [PubMed] [Google Scholar]
- Taulu S, Simola J, Kajola M. Applications of the signal space separation method (SSS) IEEE Trans Signal Process. 2005;53:3359–3372. [Google Scholar]
- Tillman CM, Wiens S. Behavioral and ERP indices of response conflict in Stroop and flanker tasks. Psychophysiology. 2011;48:1405–1411. doi: 10.1111/j.1469-8986.2011.01203.x. [DOI] [PubMed] [Google Scholar]
- Tzagarakis C, Ince NF, Leuthold AC, Pellizzer G. Beta-band activity during motor planning reflects response uncertainty. J Neurosci. 2010;30:11270–11277. doi: 10.1523/JNEUROSCI.6026-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uusitalo MA, Ilmoniemi RJ. Signal-space projection method for separating MEG or EEG into components. Med Biol Eng Comput. 1997;35:135–140. doi: 10.1007/BF02534144. [DOI] [PubMed] [Google Scholar]
- Wilson TW, Heinrichs-Graham E, Becker KM. Circadian modulation of motor-related beta oscillatory responses. Neuroimage. 2014;102(Pt 2):531–539. doi: 10.1016/j.neuroimage.2014.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson TW, Slason E, Asherin R, Kronberg E, Reite ML, Teale PD, Rojas DC. An extended motor network generates beta and gamma oscillatory perturbations during development. Brain Cogn. 2010;73:75–84. doi: 10.1016/j.bandc.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson TW, Slason E, Asherin R, Kronberg E, Teale PD, Reite ML, Rojas DC. Abnormal gamma and beta MEG activity during finger movements in early-onset psychosis. Dev Neuropsychol. 2011;36:596–613. doi: 10.1080/87565641.2011.555573. [DOI] [PMC free article] [PubMed] [Google Scholar]