Abstract
BACKGROUND
Randomized trials support the use of transcatheter aortic valve replacement (TAVR) for treatment of aortic stenosis in high- and intermediate-risk patients, but the generalizability of those results in clinical practice has been challenged.
OBJECTIVES
To determine the safety and effectiveness of TAVR versus surgical aortic valve replacement (SAVR), particularly in intermediate- and high-risk patients, in a nationally representative real-world cohort.
METHODS
Using data from the Transcatheter Valve Therapy Registry and Society of Thoracic Surgeons (STS) National Database linked to Medicare administrative claims for follow-up, we examined 9,464 propensity-matched intermediate- and high-risk (STS Predicted Risk of Mortality [PROM] ≥3%) United States patients who underwent commercial TAVR or SAVR. We compared death, stroke, and days alive and out of hospital (DAOH) to 1 year, as well as discharge to home with subgroup analyses by surgical risk, demographics, and comorbidities.
RESULTS
In a propensity-matched cohort (median age, 82 years; 48% female; median STS PROM 5.6%), TAVR and SAVR patients experienced no difference in 1-year rates of death (17.3% vs. 17.9%; hazard ratio [HR] 0.93, 95% confidence interval [CI] 0.83–1.04) and stroke (4.2% vs. 3.3%; HR 1.18, CI 0.95–1.47), and no difference was observed in the proportion of DAOH to 1 year (rate ratio 1.00, CI 0.98–1.02). However, TAVR patients were more likely to be discharged home after treatment (69.9% vs. 41.2%; odds ratio 3.19, CI 2.84–3.58). Results were consistent across most subgroups, including among intermediate- and high-risk patients.
CONCLUSIONS
Among unselected intermediate- and high-risk patients, TAVR and SAVR resulted in similar rates of death, stroke, and DAOH to 1 year, but TAVR patients were more likely to be discharged to home.
Keywords: transcatheter aortic valve replacement, surgical aortic valve replacement, outcomes, safety and effectiveness
Aortic valve disease is the third most common cause of cardiovascular disease in the United States (U.S.), affecting an estimated 2.5 million adults (5% of those affected are 65 years or older) (1, 2). Severe untreated aortic valve stenosis substantially impacts life expectancy and quality (3); however, patients with aortic valve disease are often older with multiple comorbidities, making recovery from open surgical aortic valve replacement (SAVR) challenging (4). Over the past decade, transcatheter aortic valve replacement (TAVR) has emerged as a less invasive alternative to SAVR, thereby offering potential advantages for this older patient cohort (5). TAVR was approved by the U.S. Food and Drug Administration in 2011; since then, more than 80,000 commercial TAVR procedures have been performed in the U.S. in patients at intermediate, high, and prohibitive surgical risk (J. Matthew Brennan, MD, MPH email communication, February 4, 2017).
To date, three high-quality randomized-controlled trials have supported the use of TAVR in intermediate- and high-risk patients (6–8), but these clinical trials excluded important groups of patients with higher risk comorbidities and were conducted at a select group of high-volume valve centers. Consequently, whether these results are applicable to clinical practice has been questioned (9), and concerns regarding the safety and effectiveness of TAVR have been raised (10, 11). These concerns are of increasing relevance since TAVR is applied to low- and intermediate-risk patients, where the risk of SAVR is less and its long-term outcomes are well-documented (12).
To address these lingering questions, we used observational data from two large U.S. procedural registries to examine the real-world comparative effectiveness of TAVR versus SAVR in a nationally representative real-world cohort of older individuals who may have been considered eligible for either TAVR or SAVR.
METHODS
STUDY DESIGN AND DATA SOURCES
This was a multicenter, non-randomized analysis of older patients with severe, symptomatic aortic valve stenosis at intermediate or high surgical risk who underwent treatment with TAVR or SAVR in the U.S. and may have been considered eligible for either treatment (based on available data). Data for this analysis were drawn from two U.S. procedural registries: 1) SAVR data was drawn from the Society of Thoracic Surgeons (STS) National Database; and 2) TAVR data was drawn from the STS/American College of Cardiology (ACC) Transcatheter Valve Therapy (TVT) Registry. The development and application of these registries have been described previously (13, 14). More than 90% of cardiac surgery programs in the U.S. participate in the STS National Database, and participation in the TVT Registry is necessary for Medicare reimbursement. Notably, the involvement of a heart team is also necessary for Medicare reimbursement in the U.S. For each registry, participants are required to submit 100% of their case records to the registry for quality assessment purposes. Missing data fields trigger critical warnings, and each registry has an independent data auditing program to ensure data accuracy. Records were linked to the Centers for Medicare & Medicaid Services (CMS) fee-for-service administrative insurance claims files to create a longitudinal record including vital status and rehospitalization events, using validated techniques (15).
The most updated Medicare-linked files available were used from the TVT Registry and STS National Database. TVT Registry files are linked with Medicare claims by CMS twice each year, using updated files from the CMS chronic conditions warehouse. STS National Database files are linked with Medicare claims by the Duke Clinical Research Institute annually, using research-identifiable files from ResDAC (Minneapolis, MN) (16). The availability of ResDAC files generally lags 12 to 18 months behind the date of service provision. Detailed clinical information and Medicare claims-based follow-up were available for 25,786 TAVR cases performed between January 1, 2014 and September 30, 2015, and 198,077 SAVR (or SAVR plus coronary artery bypass grafting) cases performed between July 1, 2011 and December 31, 2013.
Patients with characteristics that were thought to strongly favor one treatment or another were excluded (Figure S1). These characteristics included age <65 or >90 years, other major cardiac operations, history of endocarditis, emergency or salvage status, primary aortic insufficiency, hostile chest or porcelain aorta, moderate to severe mitral stenosis, and STS Predicted Risk of Mortality (PROM) <3%. Subsequent aortic valve replacement procedures during the initial aortic valve replacement admission were excluded, and hospitals submitting <10 total SAVR or TAVR records during the study interval were also excluded. Following these exclusions, the population of interest included 17,910 TAVR and 22,618 SAVR patients who were available for propensity matching.
The Patient-Centered Outcomes Research Institute (PCORI) funded this study (Grant #: CER-1306-04350), which was approved by the Institutional Review Board at the Duke University School of Medicine. The Duke Clinical Research Institute (Durham, NC) was responsible for the data management and statistical analysis, with oversight by a multidisciplinary research team that included patient and caregiver representatives, as well as statistical analysts and representatives from both the STS and ACC.
DATA DEFINITIONS AND OUTCOMES
By design, data definitions are identical for most patient characteristics and outcomes across the STS National Database and the TVT Registry, and are available for review in Table S1 and online (17, 18). Outcomes from the index hospitalization were drawn from registry records. A list of potential outcomes available through Medicare claims were reviewed by a broad stakeholder panel that included patients, caregivers, clinicians, health science researchers, and statisticians. Primary outcomes of interest were chosen by consensus, and included death, stroke, and days alive and out of an acute care hospital facility (i.e., days alive and out of hospital [DAOH]) to 1 year, and discharge to home. Stroke and mortality were evaluated to 30 days and 1 year over a median follow-up of 169.5 days for TAVR and 328 days for SAVR. Stroke was identified during the index procedural hospitalization using registry data. Following hospital discharge, stroke was identified using Medicare rehospitalization claims with a primary position International Classification of Diseases, Ninth Revision, Clinical Modification code of 434.x1, 436, 433.x1, 997.02, 437.1, 437.9, 430, 431, or 432.x.
STATISTICAL ANALYSIS
An analytic sample was created using propensity score-based matching to correct for differences in characteristics of patients in the two registries. A propensity score, defined as the probability of receiving TAVR given measured covariates, was calculated using logistic regression. Detailed methods, including an extensive list of covariates identified by clinical input regarding factors thought to be related to both procedure selection and outcomes, and common to the two registries, are provided in the Supplementary Appendix (Table S2). Overlap in the covariate distribution and propensity scores between study groups was assessed. Since patients at the tails (<5%, >95%) of the propensity distribution were thought to represent individuals with an overwhelming likelihood of treatment with one or the other of the two treatments, these patients were excluded (Figure S2). Propensity score matching was conducted in a 1:1 ratio, by greedy matching, using a caliper of 0.20 standard deviations in the linear predictor. The adequacy of the propensity model was confirmed by checking covariate balance before and after matching (Figure S3). Furthermore, to assess the potential for unmeasured confounding, the two treatments were compared using two falsification endpoints: lower extremity fracture and urinary tract infection. No statistically significant difference was observed for these outcomes to 1 year in the propensity-matched cohort (Figure S5).
Baseline characteristics of patients receiving SAVR and TAVR were described and compared overall and within pre-specified subgroups based on standardized differences (Figure S4). Cox proportional hazard models were used to compare outcomes of TAVR versus SAVR by hazard ratios (HRs) and 95% confidence intervals (CIs). DOAH was modeled as count data using generalized estimating equations with a log link and a fixed offset (adjusting for differential follow-up time) to obtain rate ratios (RRs) and 95% CI. Models for treatment on outcomes were fit to the matched sample using a robust empirical variance to account for within-hospital clustering. Associations were estimated in pre-specified subgroups, along with 95% CIs and tests of interaction. Statistical significance was defined as p<0.1, and significant values were evaluated for biological plausibility. All analyses were conducted using SAS version 9.4 (SAS Institute, Inc., Cary, NC).
RESULTS
PATIENTS
The propensity-matched cohort included 4,732 SAVR and 4,732 TAVR patients, with a median age of 82 years (interquartile range [IQR]: 77, 85), 47.9% women, and a median STS PROM of 5.6% (4.2%, 8.2%). Baseline characteristics were well-balanced across the two treatment groups (Table 1). Among TAVR patients, transfemoral access was used in 76%, and the valve prosthesis used was CoreValve (Medtronic) in 33% and SAPIEN (Edwards Lifesciences) in 67%.
Table 1.
Baseline Characteristics of the Aortic Valve Replacement Cohort after Propensity Matching*
| SAVR (n=4,732) | TAVR (n=4,732) | Std Diff TAVR vs. SAVR | |
|---|---|---|---|
| Age, median (IQR) | 82 (77,85) | 81 (77,85) | −1.01% |
| Female | 2,278 (48.1) | 2,256 (47.7) | −0.93% |
| Body surface area, median (IQR) | 1.9 (1.7,2.1) | 1.9 (1.7,2.0) | 0.04% |
| Creatinine, median (IQR) | 1.1 (0.9,1.4) | 1.1 (0.9,1.5) | −0.32% |
| Dialysis | 186 (3.9) | 179 (3.8) | −0.77% |
| LVEF, median (IQR) | 55.0 (45.0,55.0) | 55.0 (45.0,55.0) | −1.10% |
| Heart failure symptoms <2 weeks | 4.28% | ||
| None or Class I | 447 (9.4) | 335 (7.1) | |
| Class II | 947 (20.0) | 995 (21.0) | |
| Class III | 2,499 (52.8) | 2,509 (53.0) | |
| Class IV | 839 (17.7) | 893 (18.9) | |
| Chronic lung disease | 1.62% | ||
| None | 2,793 (59.0) | 2,784 (58.8) | |
| Mild | 872 (18.4) | 866 (18.3) | |
| Moderate | 564 (11.9) | 558 (11.8) | |
| Severe | 503 (10.6) | 524 (11.1) | |
| Home oxygen use | 385 (8.1) | 378 (8.0) | −0.54% |
| Prior stroke | 524 (11.1) | 506 (10.7) | −1.22% |
| Peripheral vascular disease | 1,138 (24.0) | 1,113 (23.5) | −1.24% |
| Pre-operative atrial fibrillation/flutter | 1,619 (34.2) | 1,572 (33.2) | −2.10% |
| Prior MI | 2.21% | ||
| Recent | 161 (3.4) | 173 (3.7) | |
| Old | 954 (20.2) | 924 (19.5) | |
| Prior PCI | 1,278 (27.0) | 1,233 (26.1) | −2.15% |
| CAD: diseased vessels | 0.95% | ||
| None | 2,292 (48.4) | 2,326 (49.2) | |
| 1 | 770 (16.3) | 757 (16.0) | |
| 2 | 520 (11.0) | 512 (10.8) | |
| 3 | 1,150 (24.3) | 1,137 (24.0) | |
| Prior CV surgeries | 1,484 (31.4) | 1,406 (29.7) | −3.58% |
| Prior aortic valve replacement | 219 (4.6) | 214 (4.5) | −0.51% |
| Aortic valve mean gradient, median (IQR) | 42.0 (35.0,52.0) | 42.0 (36.0,52.0) | 0.46% |
| Aortic insufficiency (moderate/severe) | 956 (20.2) | 947 (20.0) | −0.47% |
| Mitral insufficiency (moderate/severe) | 1,166 (24.6) | 1,125 (23.8) | −2.02% |
| PA systolic pressure, median (IQR) | 41.0 (37.0,46.0) | 41.0 (37.0,46.0) | 1.09% |
| Pre-operative IABP/inotropes | 128 (2.7) | 123 (2.6) | −0.66% |
| Hematocrit | 36.0 (32.3,39.5) | 36.0 (32.1,39.6) | 0.27% |
| Pre-operative total albumin, median (IQR) | 3.7 (3.5,4.0) | 3.7 (3.5,4.0) | −0.50% |
| Immunosuppression | 363 (7.7) | 344 (7.3) | −1.53% |
| Status (elective, urgent) | 3,871 (81.8) | 3,813 (80.6) | −3.14% |
| STS PROM, median % (IQR) | 5.8 (4.2, 8.6) | 5.5 (4.2, 8.0) | 7.23% |
| PROM 3–5% | 1,850 (39.1) | 1,953 (41.3) | |
| PROM 5–8% | 1,545 (32.7) | 1,596 (33.7) | |
| PROM ≥8% | 1,337 (28.3) | 1,183 (25.0) | |
| Transfemoral access | -- | 3,612 (76.3) | |
| Concomitant CABG | 1565 (33.1) | -- | |
| Medications at hospital discharge | |||
| Aspirin | 3,961 (83.7) | 3,852 (81.4) | −6.07% |
| P2Y12 inhibitor | 646 (13.7) | 2,864 (60.5) | 110.96% |
| Anticoagulants† | 1,871 (39.5) | 1,132 (23.9) | −34.03% |
A more complete listing of patient characteristics and standardized differences before and after propensity matching is included in the Statistical Appendix.
Anticoagulants include warfarin and novel oral anticoagulants.
CABG = coronary artery bypass grafting; CAD = coronary artery disease; CV = cardiovascular; IABP = intra-aortic balloon pump; IQR = interquartile range; LVEF = left ventricular ejection fraction; MI = myocardial infarction; PCI = percutaneous coronary intervention; SAVR = surgical aortic valve replacement; Std Diff = standardized difference; TAVR = transcatheter aortic valve replacement; STS PROM = Society of Thoracic Surgeons Predicted Risk of Mortality
OUTCOMES: PROCEDURE OUTCOMES
On average, TAVR patients spent 31 (IQR: 24, 57) hours in the intensive care unit (ICU) and a total of 4 (IQR: 3, 6) days in the hospital during the index admission, while SAVR patients spent an average of 68 (IQR: 37, 119) hours in the ICU and 8 (IQR: 6, 11) days in the hospital during the index admission. Inhospital mortality was lower among TAVR patients than SAVR (3.0% vs. 5.0%, p<0.001), while the incidence of stroke was no different (2.5% vs. 2.7%, p=0.4). Compared with SAVR, TAVR patients experienced a higher incidence of new pacemaker/implantable cardioverter defibrillator placement (12.8% vs. 6.3%, p<0.001) and major vascular complications (4.2% vs. 0.4%, p<0.001), but a lower incidence of blood transfusions (packed red blood cell units: TAVR 0 [0, 0], SAVR 2 [0, 4], p<0.001), and new requirement for hemodialysis (1.7% vs. 3.2%, p<0.001) during the initial hospitalization.
Discharge to home was more common among TAVR patients than SAVR (69.9% vs. 41.2%), overall and within each subgroup that was studied (Figure S6). Discharge to an extended care facility, transitional care unit, or rehabilitation unit was more common among SAVR patients (41.2% vs. 20.5%, p<0.01).
OUTCOMES: DEATH AND STROKE
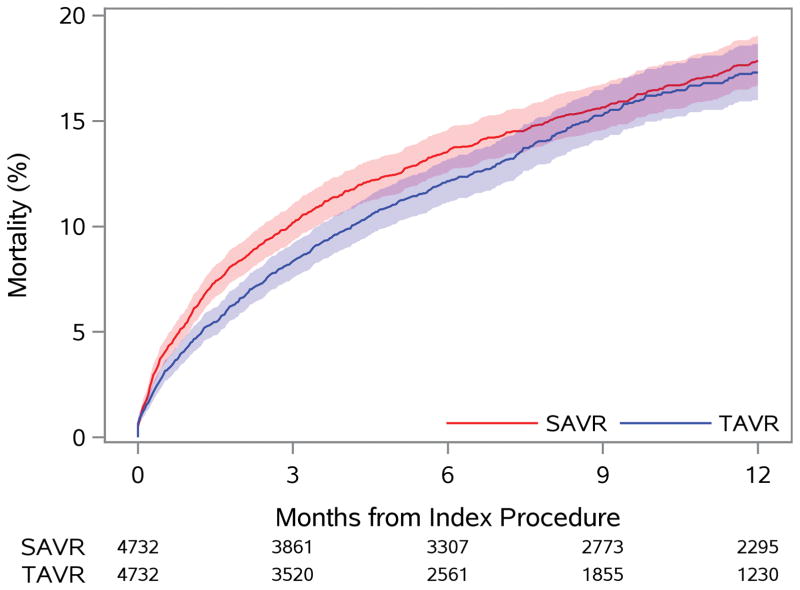
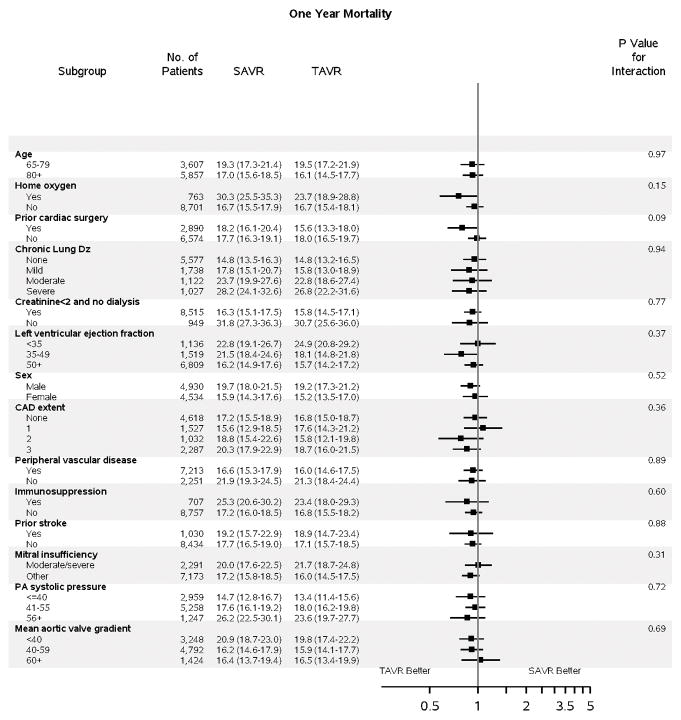
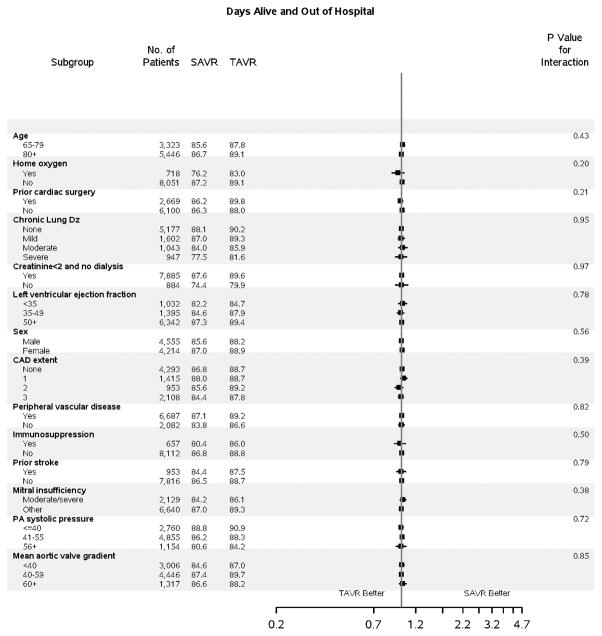
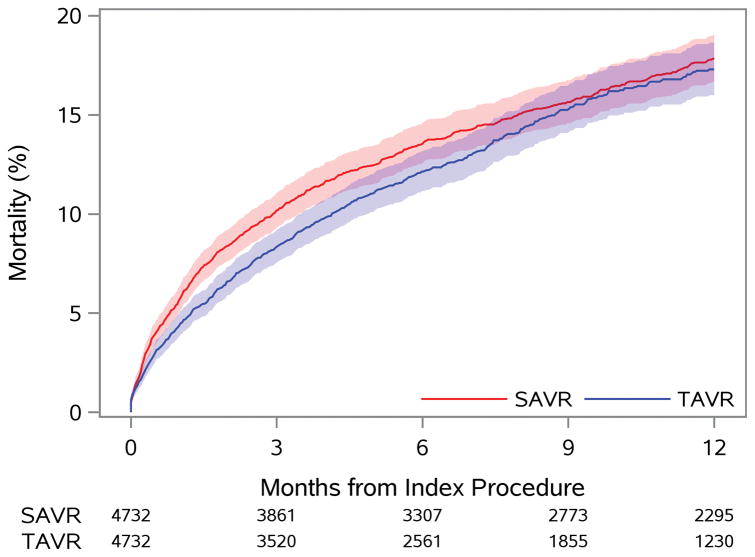
No difference in death at 1 year was observed with TAVR versus SAVR (17.3% vs. 17.9%, p=0.40), although a lower early risk of mortality was observed with TAVR (Figure 1A). A similar 1-year risk of death was observed across most subgroups of interest (Figure 2B); however, those with a prior cardiac surgery experienced a lower 1-year risk of mortality when treated with TAVR versus SAVR (p-interaction=0.09).
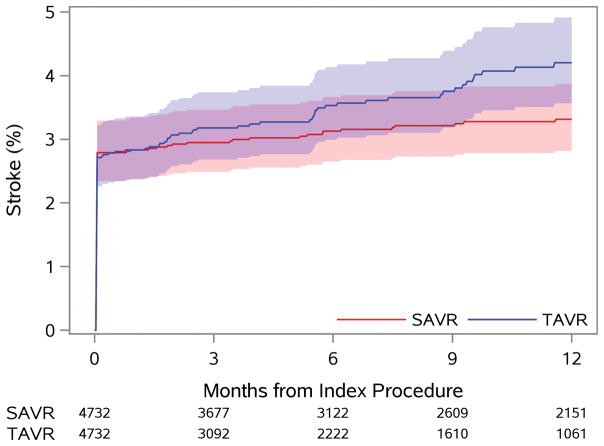
Figure 1. Time-to-Event Curves for Death and Stroke.
The rates of: A) death from any cause; and B) rehospitalization for stroke are similar to 1 year among patients treated with TAVR versus SAVR.
HR = hazard ratio (TAVR vs. SAVR); SAVR = surgical aortic valve replacement; TAVR = transcatheter aortic valve replacement
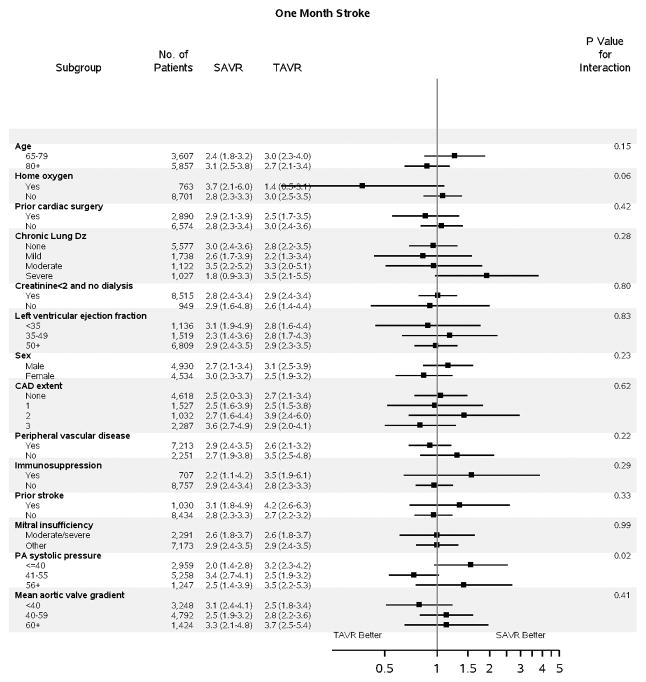
Figure 2. Subgroup Analyses.
Subgroup analyses are shown comparing TAVR vs SAVR for: A) death to 1 year (HR, 95% CI); B) stroke to 30 days (HR, 95% CI); and C) days alive and out of an acute care hospital to 1 year (RR, 95% CI). For DAOH, the proportion (%) of days alive and out of an acute care hospital (% DAOH) in the first year following initial hospital discharge has been calculated for each patient (Figure S8). Subgroup results for stroke to 1 year are presented separately due to non-proportional hazards (Figure S7). A balance of covariates within each subgroup has been forced with inclusion of interaction terms in the propensity score. The p-value for interaction represents the likelihood of interaction between the variable and the relative treatment effect. Comparative treatment effects were similar across most subgroups, with few significant interactions noted.
CAD = coronary artery disease; Dz = disease; PA = pulmonary artery; SAVR = surgical aortic valve replacement; TAVR = transcatheter aortic valve replacement
The risk of stroke was highest in the first 30 days following treatment and was identical between TAVR and SAVR (2.8 vs. 2.8%, p=0.13) patients. An increase in the incidence of stroke was observed among TAVR (vs. SAVR) patients between 30 days and 1 year, with a progressive divergence of the stroke event curves. Nevertheless, the overall risk of stroke remained low during this interval (0.5% vs. 1.4%, Figure 1B), and the overall difference in risk of stroke was not significant to 1 year (TAVR vs. SAVR HR 1.18, 95% CI 0.95–1.47). Patients with home oxygen experienced a lower risk of stroke to 1 month with TAVR versus SAVR (p-interaction=0.06, Figure 2B), but by 1 year neither treatment was favored in these patients (Figure S7).
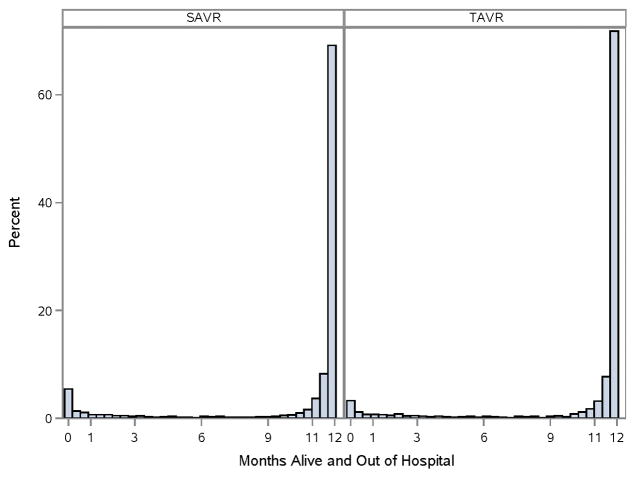
OUTCOMES: DAYS ALIVE AND OUT OF HOSPITAL
In the first year following hospital discharge, ≥80% of patients were alive and out of an acute care hospital for at least 11 of 12 months (Figure 3). The proportion of DAOH was similar between TAVR versus SAVR patients (RR 1.0, 95% CI 0.98–1.02), a result that was consistent across all subgroups (Figure 2C).
Figure 3. Days Alive and Out of Hospital to 1 Year.
For DAOH, the proportion (%) of days alive and out of an acute care hospital (% DAOH) in the first year following initial hospital discharge has been calculated for each patient and displayed by treatment group. For ease of interpretation, % DAOH has been displayed across a 12-month interval. Patients with 0 days alive and out of the hospital died prior to discharge from the index hospitalization. Not all patients had a full year of follow-up post-TAVR. For those without a full year of follow-up, statistical methods adjusted for differential patient follow-up.
SAVR = surgical aortic valve replacement; TAVR = transcatheter aortic valve replacement
INFLUENCE OF PRE-OPERATIVE SURGICAL RISK
After verifying covariate balance across 3 risk-levels of STS PROM (3–5%, 5–8%, ≥8%), a stratified analysis was performed. Increasing pre-operative surgical risk was associated with a lower likelihood of discharge to home, fewer DAOH, a higher risk of stroke, and a higher risk of death to 1 year; however, the relative treatment effect (TAVR vs. SAVR) was consistent for each outcome of interest across the spectrum of intermediate to high baseline surgical risk (STS PROM, Table 2).
Table 2.
Clinical Outcomes to 1 Year, Stratified by Surgical Risk (STS PROM)
| Outcome | Overall n=9,464 | STS PROM (≥3%, 5%) n=3,803 |
STS PROM (≥5%, <8%) n=3,141 |
STS PROM (≥8%) n=2,520 |
p-interaction | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SAVR n=4,732 |
TAVR n=4,732 |
HR (95% CI) |
SAVR n=1,850 |
TAVR n=1,953 |
HR (95% CI) |
SAVR n=1,545 |
TAVR n=1,596 |
HR (95% CI) |
SAVR n=1,337 |
TAVR n=1,183 |
HR (95% CI) |
||
| Death | 17.9 | 17.3 | 0.93 (0.83, 1.04) |
11.2 | 12.6 | 1.06 (0.86, 1.31) |
16.2 | 15.3 | 0.92 (0.75, 1.13) |
28.7 | 27.4 | 0.91 (0.78, 1.08) |
0.50 |
| Stroke | 3.3 | 4.2 | 1.18 (0.95, 1.47) |
3.3 | 3.8 | 1.06 (0.73, 1.54) |
3.5 | 4.5 | 1.22 (0.83, 1.79) |
3.1 | 4.4 | 1.33 (0.87, 2.03) |
0.73 |
| Discharge to home | 41.2 | 69.9 | 3.19 (2.84, 3.58)† |
49.5 | 77.5 | 3.33 (2.83, 3.92)† |
41.9 | 70.4 | 2.37 (2.00, 2.80)† |
29.0 | 56.8 | 1.32 (1.13, 1.55)† |
0.89 |
| % DAOH, median | 100 | 100 | 1.00 (0.98,1.02)* |
100 | 100 | 0.99 (0.97,1.01)* |
98.9 | 99.3 | 0.99 (0.95,1.04)* |
95.6 | 96.9 | 0.93 (0.83,1.05)* |
0.598 |
A rate ratio was calculated to compare treatment effects for the proportion (%) of days alive and out of an acute care hospital to 1 year.
An odds ratio was calculated to compare treatment effects for the probability of discharge to home.
CI = confidence interval; DAOH = days alive and out of hospital; HR = hazard ratio (TAVR vs. SAVR); SAVR = surgical aortic valve replacement; STS PROM = Society of Thoracic Surgeons Predicted Risk of Mortality; TAVR = transcatheter aortic valve replacement
DISCUSSION
In a broad cohort of older U.S. patients with severe aortic stenosis who were eligible for treatment with either TAVR or SAVR, no significant difference was observed in death, stroke, or DAOH to 1 year. TAVR patients were more often discharged directly to home, reflecting a less demanding post-operative recovery. Results were consistent across most patient subgroups and across the spectrum of intermediate to high pre-operative surgical risk. These findings are largely consistent with those observed in pivotal randomized clinical trials and support the safety and effectiveness of TAVR in real-world intermediate- and high-risk patients.
In three previous randomized clinical trials among patients with severe aortic stenosis and intermediate or high surgical risk, TAVR has demonstrated similar (or superior) outcomes to 1 year when compared with SAVR. In high-risk patients, Cohort A of the randomized Placement of Aortic Transcatheter Valve trial (PARTNER; n=699, 25 U.S. centers) demonstrated similar rates of death to 1 year with a first-generation balloon-expandable TAVR prosthesis versus SAVR (24.2% vs. 26.8, p=0.44), but with an increased risk of stroke or transient ischemic attack (TIA; 8.3% vs. 4.3%, p=0.04) (6). In a lower risk cohort, the U.S. CoreValve trial (n=795, 45 U.S. centers) demonstrated lower rates of death to 1 year with a self-expanding TAVR prosthesis (14.2% vs. 19.1%, p=0.04 for superiority), without an increased risk of stroke (8.8% vs. 12.6%, p=0.10) (8). These results were consistent across most subgroups of patients. Among intermediate-risk patients in the PARTNER 2A trial (n=2032, 57 centers), patients randomized to a balloon-expandable second generation TAVR prosthesis (vs. SAVR) experienced similar rates of death (12.3% vs. 12.9%, p=0.69) or stroke (8.0% vs. 8.1%, p=0.88) at 1 year (7). Again, no significant subgroup interactions were observed.
Despite favorable results in carefully-controlled randomized trials, the generalizability of trial results to real-world patients has been questioned by some, due to systematic exclusion from clinical trials of patients with certain high-risk comorbidities (e.g., hemodialysis, recent stroke [<6 months prior], very low left ventricular ejection fraction [<20%]) (9). Responding to these concerns, non-randomized evaluations of TAVR versus SAVR have been performed (19, 20) with mixed results—particularly among intermediate-risk patients. A propensity-adjusted comparison of intermediate-risk TAVR patients from the SAPIEN 3 registry cohort (n=963) versus SAVR patients from the PARTNER 2A trial (n=747) demonstrated lower 1-year risks of mortality (7.4% vs. 13.0%) and stroke (4.6% vs. 8.2%) with TAVR using the third-generation SAPIEN 3 balloon-expandable prosthesis versus SAVR (21). By contrast, in a propensity-matched analysis of 5,997 intermediate-risk patients undergoing TAVR versus SAVR as part of the German Aortic Valve Registry (GARY), patients treated with TAVR had a substantially higher 1-year risk of mortality versus SAVR (15.5% vs. 10.9%, p=0.002) (11).
The results of our analyses are largely consistent with those of the pivotal randomized clinical trials. In a broad cohort of both intermediate- and high-risk older patients, the 1-year incidences of mortality and stroke are similar to those previously published for both intermediate- and high-risk patients. Consistent with the PARTNER and PARTNER 2A trial results, we observed a similar comparative risk of mortality for TAVR and SAVR among both intermediate- and high-risk patients. In contrast to results from GARY, we did not observe an increased risk of mortality among intermediate-risk patients. In our study, we used detailed phenotypic information from both the STS National Database and TVT Registry to both exclude patients who would not have been considered for both procedures and closely match the remaining eligible patients; many of these variables were not available in other observational datasets. The availability of these additional data elements may account for differences between our study outcomes and both the GARY and SAPIEN 3 results, allowing for a more accurate approximation of the existing randomized trial results.
Notably, the rates of stroke reported to 1-year in this cohort are roughly 50% lower than those reported to a year in each of the reported clinical trials, including the intermediate-risk PARTNER 2A clinical trial. The reduced strokes rates observed here are consistent with those reported by others, including the non-randomized SAPIEN 3 intermediate-risk analysis (21); the reason for this finding is unclear. This observation may represent an under-ascertainment or under-reporting of stroke events, since dedicated post-operative neurology evaluations that were available in pivotal trials were likely to reveal a higher incidence of both clinically significant and insignificant strokes. Similar to results from the PARTNER trial, we observed a non-significant, but progressive increase in the 1-year risk of stroke among TAVR patients in our cohort. The cause (and clinical importance) of this observation is unknown. No such increase in stroke risk was observed in either the U.S. CoreValve High Risk trial or the PARTNER 2A trial. However, this finding warrants further investigation. To evaluate alternative strategies to address the excess risk of stroke following TAVR, both the Anti-Thrombotic Strategy After Trans-Aortic Valve Implantation for Aortic Stenosis (ATLANTIS) and the Global Study Comparing a Rivaroxaban-based Antithrombotic Strategy to an Antiplatelet-based Strategy after Transcathether Aortic Valve Replacement to Optimize Clinical Outcomes (GALILEO) study are randomizing patients post-TAVR to various post-TAVR anticoagulation strategies (22). Finally, we observed a significantly lower risk of stroke at 1 month among patients with home oxygen. We would hypothesize that this finding is related to an increase in underlying aortic calcification (from tobacco exposure) among patients with home oxygen use. In these patients, avoiding direct manipulation of the ascending aorta with TAVR (versus SAVR) may lead to a lower stroke incidence.
Importantly, we did not see significant differences in treatment effects across most patient subgroups, including within the intermediate- and high-risk strata. These results are generally consistent with available randomized data (23); however, our analysis does suggest that patients with significant lung disease and prior cardiac surgery may derive additional benefit from TAVR (vs. SAVR) for selected outcomes.
STUDY LIMITATIONS
While this study has important strengths, its limitations must also be clearly acknowledged. First, this is not a randomized treatment comparison, and bias (particularly, through imbalances in patient frailty) may have influenced our results. Second, we found that nearly half of the patients in the U.S. had a very high likelihood of receiving treatment with either SAVR (31.5%) or TAVR (14.8%), making it unlikely that the alternative treatment was considered a reasonable option in nearly half of patients. Consequently, the results reported here are intended to evaluate treatment effects among those generally considered eligible for either procedure, excluding patients with extremely high or low propensities for TAVR. Third, although results of our subgroup analyses have demonstrated general parity of treatment effects across patient subgroups, it is likely that certain comorbidity combinations may favor one treatment over another. The importance of developing decision assistance tools to help optimize individualized patient care cannot be overstated. Fourth, due to differences in the mechanisms of Medicare linkage from the STS National Database and TVT Registry, there was an offset in the interval of inclusion for SAVR (July 1, 2011 to December 31, 2013) and TAVR (January 1, 2014 to September 30, 2015). Consequently, the results reported here may underestimate the safety and effectiveness of SAVR if surgical outcomes significantly improved during the offset interval. Fifth, the outcomes presented here were selected from a list of available outcomes by a broad stakeholder panel that included patients and caregivers; however, there was general agreement that quality of life is an important metric for consideration when choosing between these two procedures. Quality of life data, physical functioning, and New York Heart Association Class at follow-up were not available for surgical aortic valve replacement patients and, therefore, could not be presented in our study. Likewise, several important surrogate outcomes were not available, such as degree of aortic valve insufficiency and left ventricular remodeling. Also, expectations regarding long-term valve durability are key to treatment decisions, especially among younger patients and those at lower preoperative surgical risk. An evaluation of the need for valve re-intervention will be important as this cohort matures over time. Sixth, cause of death was thought to be an important consideration that could not be addressed in our study due to a lack of necessary data. Finally, it is important to recognize that the treatment of aortic valve disease is a rapidly developing field, with frequent modifications in device technology for both minimally-invasive SAVR and TAVR. The data reported here are the most contemporary available in the U.S. and reflect outcomes of patients treated following the interval of early adoption of TAVR technology in the U.S.; however, recent TAVR device modifications have lowered device delivery profiles, improved prosthesis-annular apposition, and improved device repositioning capabilities. These modifications have lowered the incidence of procedural complications, including peri-procedural stroke, acute vascular complications, device malposition, and perivalvular aortic insufficiency. As TAVR and SAVR devices continue to evolve, the relative risks and benefits of these two procedures may change. Diligent monitoring of outcomes will continue to help direct future device innovation in this field.
CONCLUSIONS
In conclusion, we used propensity score methods to compare 1-year outcomes of TAVR versus SAVR in a large, real-world cohort of older U.S. patients with aortic valve stenosis who were at intermediate or high surgical risk. Importantly, our results confirm and extend the observations of existing randomized studies in this field. Compared with SAVR, TAVR patients experienced a lower incidence of inhospital mortality and were more often discharged directly to home. At 1-year follow-up, death, stroke, and DAOH were similar for the two treatments in the overall cohort and across most patient subgroups, including those within the spectrum of intermediate to high surgical risk.
Supplementary Material
Central Illustration. Rate of Mortality for TAVR and SAVR.
Among unselected intermediate- and high-risk patients, TAVR and SAVR resulted in similar rates of death (shown here), stroke, and DAOH to 1 year, but TAVR patients were more likely to be discharged to home. Results were consistent across most subgroups, including among intermediate- and high-risk patients.
DAOH = days alive and out of hospital; SAVR = surgical aortic valve replacement; TAVR = transcatheter aortic valve replacement
PERSPECTIVES.
Competency in Medical Knowledge
The use of transcatheter aortic valve replacement (TAVR) for the treatment of aortic stenosis offers an alternative to surgical aortic valve replacement (SAVR) in intermediate- and high-risk patients.
Translational Outlook
Among unselected intermediate- and high-risk patients, TAVR and SAVR resulted in similar rates of death, stroke, and days alive and out of hospital to 1 year, but TAVR patients were more likely to be discharged to home, reflecting a less demanding post-operative recovery. As TAVR and SAVR devices continue to evolve, the relative risks and benefits of these two procedures may change. Diligent monitoring of outcomes will continue to help direct future device innovation in this field.
Acknowledgments
The authors would like to thank Erin Campbell, MS, for her editorial contributions to this manuscript. Ms. Campbell did not receive compensation for her assistance, apart from her employment at the institution where this study was conducted.
Sources of funding: Research reported in this article was funded through a Patient-Centered Outcomes Research Institute (PCORI) Award (CER-1306-04350).
ABBREVIATIONS
- ACC
American College of Cardiology
- CI
confidence interval
- CMS
Centers for Medicare & Medicaid Services
- DAOH
days alive and out of hospital
- GARY
German Aortic Valve Registry
- HR
hazard ratio
- IQR
interquartile range
- PARTNER
Placement of Aortic Transcatheter Valve Trial
- RR
rate ratio
- SAVR
surgical aortic valve replacement
- STS
Society of Thoracic Surgeons
- STS PROM
Society of Thoracic Surgeons Predicted Risk of Mortality
- TAVR
transcatheter aortic valve replacement
- TVT
Transcatheter Valve Therapy (Registry)
- U.S
United States
Footnotes
Disclaimer: The statements presented in this article are solely the responsibility of the author(s) and do not necessarily represent the views of the Patient-Centered Outcomes Research Institute (PCORI), its Board of Governors or Methodology Committee.
AUTHOR CONTRIBUTIONS
All authors have been involved in the study design, analysis, and manuscript revision. All authors read and approved the final manuscript. Dr. Brennan is the guarantor who accepts full responsibility for the work and the conduct of the study, had access to the data, and controlled the decision to publish.
JM Brennan: Dr. Brennan had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Dr. Brennan contributed to the conception and design of the study, the data interpretation, the manuscript drafting, and the critical revision of the manuscript.
L Thomas: Dr. Thomas contributed to the conception and design of the study, the data analysis, the data interpretation, the manuscript drafting, and the critical revision of the manuscript.
DJ Cohen: Dr. Cohen contributed to the conception and design of the study, the data interpretation, the manuscript drafting, and the critical revision of the manuscript.
D Shahian: Dr. Shahian contributed to the conception and design of the study, the data interpretation, the manuscript drafting, and the critical revision of the manuscript.
A Wang: Dr. Wang reports contributed to the conception and design of the study, the data interpretation, the manuscript drafting, and the critical revision of the manuscript.
MJ Mack: Dr. Mack reports contributed to the conception and design of the study, the data interpretation, the manuscript drafting, and the critical revision of the manuscript.
DR Holmes: Dr. Holmes contributed to the conception and design of the study, the data interpretation, the manuscript drafting, and the critical revision of the manuscript.
FH Edwards: Dr. Edwards contributed to the conception and design of the study, the data interpretation, the manuscript drafting, and the critical revision of the manuscript.
NZ Frankel: Mr. Frankel contributed to the conception and design of the study, the data interpretation, and the critical revision of the manuscript.
SJ Baron: Dr. Baron contributed to the conception and design of the study, the data interpretation, the manuscript drafting, and the critical revision of the manuscript.
J Carroll: Dr. Carroll contributed to the conception and design of the study, the data interpretation, the manuscript drafting, and the critical revision of the manuscript.
V Thourani: Dr. Thourani reports contributed to the conception and design of the study, the data interpretation, the manuscript drafting, and the critical revision of the manuscript.
EM Tuzcu: Dr. Tuzcu contributed to the conception and design of the study, the data interpretation, the manuscript drafting, and the critical revision of the manuscript.
SV Arnold: Dr. Arnold contributed to the conception and design of the study, the data interpretation, the manuscript drafting, and the critical revision of the manuscript.
R Cohn: Ms. Cohn contributed to the conception and design of the study, the data interpretation, and the critical revision of the manuscript.
T Maser: Mr. Maser contributed to the conception and design of the study, the data interpretation, and the critical revision of the manuscript.
B Schawe: Ms. Schawe contributed to the conception and design of the study, the data interpretation, and the critical revision of the manuscript.
S Strong: Ms. Strong contributed to the conception and design of the study, the data interpretation, and the critical revision of the manuscript.
A Stickfort: Mr. Stickfort contributed to the conception and design of the study, the data interpretation, and the critical revision of the manuscript.
E Patrick-Lake: Ms. Patrick-Lake contributed to the conception and design of the study, the data interpretation, and the critical revision of the manuscript.
FL Graham: Ms. Graham reports contributed to the conception and design of the study, the data interpretation, the manuscript drafting, and the critical revision of the manuscript.
D Dai: Dr. Dai contributed to the conception and design of the study, the data analysis, the data interpretation, the manuscript drafting, and the critical revision of the manuscript.
F Li: Mr. Li contributed to the conception and design of the study, the data analysis, the data interpretation, the manuscript drafting, and the critical revision of the manuscript.
RA Matsouaka: Dr. Matsouaka contributed to the conception and design of the study, the data analysis, the data interpretation, the manuscript drafting, and the critical revision of the manuscript.
S O’Brien: Dr. O’Brien contributed to the conception and design of the study, the data analysis, the data interpretation, the manuscript drafting, and the critical revision of the manuscript.
F Li: Dr. Li reports contributed to the conception and design of the study, the data analysis, the data interpretation, the manuscript drafting, and the critical revision of the manuscript.
MJ Pencina: Dr. Pencina contributed to the conception and design of the study, the data analysis, the data interpretation, the manuscript drafting, and the critical revision of the manuscript.
ED Peterson: Dr. Peterson contributed to the conception and design of the study, the supervision, data acquisition and interpretation, the manuscript drafting, and the critical revision of the manuscript.
Conflict of interest disclosures:
JM Brennan: Dr. Brennan reports no relevant disclosures.
L Thomas: Dr. Thomas reports no relevant disclosures.
DJ Cohen: Dr. Cohen reports research grant support—Edwards Lifesciences, Medtronic, Boston Scientific, Abbott Vascular; consulting income—Edwards Lifesciences, Medtronic
D Shahian: Dr. Shahian reports no relevant disclosures.
A Wang: Dr. Wang reports no relevant disclosures.
MJ Mack: Dr. Mack reports no relevant disclosures.
DR Holmes: Dr. Holmes reports no relevant disclosures.
FH Edwards: Dr. Edwards reports no relevant disclosures.
NZ Frankel: Mr. Frankel reports no relevant disclosures.
SJ Baron: Dr. Baron no relevant disclosures.
J Carroll: Dr. Carroll reports being a site investigator in PARTNER2 and the Medtronic low risk trial.
V Thourani: Dr. Thourani reports no relevant disclosures.
EM Tuzcu: Dr. Tuzcu reports being a member of the executive committee of the PARTNER trial.
SV Arnold: Dr. Arnold no relevant disclosures.
R Cohn: Ms. Cohn no relevant disclosures.
T Maser: Mr. Maser no relevant disclosures.
B Schawe: Ms. Schawe no relevant disclosures.
S Strong: Ms. Strong no relevant disclosures.
A Stickfort: Mr. Stickfort no relevant disclosures.
E Patrick-Lake: Ms. Patrick-Lake reports no relevant disclosures.
FL Graham: Ms. Graham reports no relevant disclosures.
D Dai: Dr. Dai reports no relevant disclosures.
F Li: Mr. Li reports no relevant disclosures.
RA Matsouaka: Dr. Matsouaka reports no relevant disclosures.
S O’Brien: Dr. O’Brien reports no relevant disclosures.
F Li: Dr. Li reports no relevant disclosures.
MJ Pencina: Dr. Pencina reports no relevant disclosures.
ED Peterson: Dr. Peterson reports no relevant disclosures.
References
- 1.Goldbarg SH, Elmariah S, Miller MA, Fuster V. Insights into degenerative aortic valve disease. J Am Coll Cardiol. 2007;50:1205–13. doi: 10.1016/j.jacc.2007.06.024. [DOI] [PubMed] [Google Scholar]
- 2.Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez-Sarano M. Burden of valvular heart diseases: a population-based study. Lancet. 2006;368:1005–11. doi: 10.1016/S0140-6736(06)69208-8. [DOI] [PubMed] [Google Scholar]
- 3.Bonow RO, Mann DL, Zipes DP, Libby P, editors. Braunwald’s Heart Disease: A Textbook of Cardiovascular Medicine. 8. Philadelphia, PA: W. B. Saunders Company; 2007. [Google Scholar]
- 4.Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:e57–185. doi: 10.1016/j.jacc.2014.02.536. [DOI] [PubMed] [Google Scholar]
- 5.Holmes DR, Jr, Mack MJ, Kaul S, et al. 2012 ACCF/AATS/SCAI/STS expert consensus document on transcatheter aortic valve replacement: developed in collabration with the American Heart Association, American Society of Echocardiography, European Association for Cardio-Thoracic Surgery, Heart Failure Society of America, Mended Hearts, Society of Cardiovascular Anesthesiologists, Society of Cardiovascular Computed Tomography, and Society for Cardiovascular Magnetic Resonance. J Thorac Cardiovasc Surg. 2012;144:e29–84. doi: 10.1016/j.jtcvs.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 6.Smith CR, Leon MB, Mack MJ, et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med. 2011;364:2187–98. doi: 10.1056/NEJMoa1103510. [DOI] [PubMed] [Google Scholar]
- 7.Leon MB, Smith CR, Mack MJ, et al. Transcatheter or surgical aortic-valve replacement in intermediate-risk patients. N Engl J Med. 2016;374:1609–20. doi: 10.1056/NEJMoa1514616. [DOI] [PubMed] [Google Scholar]
- 8.Adams DH, Popma JJ, Reardon MJ. Transcatheter aortic-valve replacement with a self-expanding prosthesis. N Engl J Med. 2014;371:967–8. doi: 10.1056/NEJMc1408396. [DOI] [PubMed] [Google Scholar]
- 9.Jilaihawi H, Chakravarty T, Weiss RE, Fontana GP, Forrester J, Makkar RR. Meta-analysis of complications in aortic valve replacement: comparison of Medtronic-Corevalve, Edwards-Sapien and surgical aortic valve replacement in 8,536 patients. Catheter Cardiovasc Interv. 2012;80:128–38. doi: 10.1002/ccd.23368. [DOI] [PubMed] [Google Scholar]
- 10.Summers MR, Cremer PC, Jaber WA. Three mechanisms of early failure of transcatheter aortic valves: valve thrombosis, cusp rupture, and accelerated calcification. J Thorac Cardiovasc Surg. 2016 doi: 10.1016/j.jtcvs.2016.12.011. pii: S0022-5223(16)31676-2. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 11.Werner N, Zahn R, Beckmann A, et al. Patients at intermediate surgical risk undergoing isolated interventionaly or surgical aortic valve replacement for severe symptomatic aortic valve stenosis. One year results from the German Aortic Valve Registry (GARY). Presented at: American Heart Association Scientific Sessions; November 14, 2016; New Orleans, LA. [Google Scholar]
- 12.Brennan JM, Edwards FH, Zhao Y, O’Brien SM, Douglas PS, Peterson ED. Long-term survival after aortic valve replacement among high-risk elderly patients in the United States: insights from the Society of Thoracic Surgeons Adult Cardiac Surgery Database, 1991 to 2007. Circulation. 2012;126:1621–9. doi: 10.1161/CIRCULATIONAHA.112.091371. [DOI] [PubMed] [Google Scholar]
- 13.Shahian DM, Jacobs JP, Edwards FH, et al. The Society of Thoracic Surgeons National Database. Heart. 2013;99:1494–501. doi: 10.1136/heartjnl-2012-303456. [DOI] [PubMed] [Google Scholar]
- 14.Carroll JD, Edwards FH, Marinac-Dabic D, et al. The STS-ACC transcatheter valve therapy national registry: a new partnership and infrastructure for the introduction and surveillance of medical devices and therapies. J Am Coll Cardiol. 2013;62:1026–34. doi: 10.1016/j.jacc.2013.03.060. [DOI] [PubMed] [Google Scholar]
- 15.Hammill BG, Hernandez AF, Peterson ED, Fonarow GC, Schulman KA, Curtis LH. Linking inpatient clinical registry data to Medicare claims data using indirect identifiers. Am Heart J. 2009;157:995–1000. doi: 10.1016/j.ahj.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jacobs JP, Edwards FH, Shahian DM, et al. Successful linking of the Society of Thoracic Surgeons Adult Cardiac Surgery Database to Centers for Medicare and Medicaid Services Medicare data. Ann Thorac Surg. 2010;90:1150–6. doi: 10.1016/j.athoracsur.2010.05.042. discussion 1156–7. [DOI] [PubMed] [Google Scholar]
- 17.The Society of Thoracic Surgeons. Data collection. [Accessed February 8, 2017];The Society of Thoracic Surgeons web site. http://www.sts.org/sts-national-database/database-managers/adult-cardiac-surgery-database/data-collection.
- 18.The Society of Thoracic Surgeons and American College of Cardiology Transcatheter Valve Therapy Registry. Data collection. [Accessed February 8, 2017];National Cardiovascular Data Registry web site. https://www.ncdr.com/WebNCDR/tvt/publicpage/data-collection.
- 19.Mack MJ, Brennan JM, Brindis R, et al. Outcomes following transcatheter aortic valve replacement in the United States. JAMA. 2013;310:2069–77. doi: 10.1001/jama.2013.282043. [DOI] [PubMed] [Google Scholar]
- 20.Holmes DR, Jr, Brennan JM, Rumsfeld JS, et al. Clinical outcomes at 1 year following transcatheter aortic valve replacement. JAMA. 2015;313:1019–28. doi: 10.1001/jama.2015.1474. [DOI] [PubMed] [Google Scholar]
- 21.Thourani VH, Kodali S, Makkar RR, et al. Transcatheter aortic valve replacement versus surgical valve replacement in intermediate-risk patients: a propensity score analysis. Lancet. 2016;387:2218–25. doi: 10.1016/S0140-6736(16)30073-3. [DOI] [PubMed] [Google Scholar]
- 22.ClinicalTrials.gov. Anti-Thrombotic Strategy After Trans-Aortic Valve Implantation for Aortic Stenosis (ATLANTIS) [Accessed February 20, 2017];ClinicalTrials.gov web site. https://clinicaltrials.gov/ct2/show/NCT02664649. Updated February 2, 2017.
- 23.Siontis GC, Praz F, Pilgrim T, et al. Transcatheter aortic valve implantation vs. surgical aortic valve replacement for treatment of severe aortic stenosis: a meta-analysis of randomized trials. Eur Heart J. 2016;37:3503–12. doi: 10.1093/eurheartj/ehw225. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.