Abstract
While fast plant movements are spectacular but rare, almost all plants exhibit relatively slow, growth-mediated tropic movements that are key to their survival in the natural world. In this brief review, we discuss recent insights into the molecular mechanisms underlying phototropism, gravitropism, hydrotropism, and autostraightening. Careful molecular genetic and physiological studies have helped confirm the importance of lateral auxin gradients in gravitropic and phototropic responses. However, auxin signaling doesn't explain all tropisms: recent work has shown that abscisic acid signaling mediates root hydrotropism and has implicated mechanosensing in autostraightening, the organ straightening process recently modeled as a proprioceptive response. The interactions between distinct tropic signaling pathways and other internal and external sensory processes are also now being untangled.
Keywords: Tropism, auxin, gravitropism, phototropism, hydrotropism, autostraightening, proprioception
Introduction
The power of movement is so firmly associated with animals that the casual observer might be forgiven for thinking that plants that move are the exception rather than the rule. For example, the fast and spectacular action of a Venus flytrap closing on its prey [1] captures the imagination but leaves the impression that plants that move are rare. In fact, plant movements are ubiquitous and have been noted by close observers at least from the time of Alexander the Great, becoming a topic of consuming interest for great botanists of the 19th century such as Sachs, Pfeffer, and Darwin.
The intensive study of plant movements continues to this day. We now appreciate that all plant movements are ultimately controlled by interactions between intracellular turgor pressure and the plant cell wall. Fast movements such as by carnivorous plants are driven by rapid changes in water transport aided by mechanical instabilities in plant structures [2]. In this review, we will examine the basis of the relatively slow plant movements generated by the differential growth of live tissues. These directional growth responses are controlled by anisotropic cell expansion, with the rate and direction of turgor-driven cell growth varying across the cell in a manner determined by local differences in cell wall extensibility and/or elasticity [3]. We will discuss a few recent highlights in the areas of phototropism, gravitropism, hydrotropism, proprioception, focusing on the diverse roles for the growth hormone auxin in these processes (Figure 1).
Figure 1.
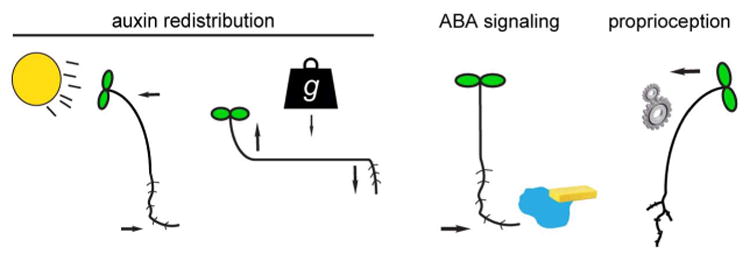
Diverse molecular mechanisms produce tropic movements. Shoot and root gravi-and photo-tropism rely upon the generation of auxin gradients across these organs. However, the rapid gravitropism of roots requires additional signaling pathways as well. Moreover, root hydrotropism is reliant on ABA signaling while proprioceptive (also called autostraightening) movements may depend upon mechanosensing.
Phototropism: Bending towards or away from the light
Most plant shoots grow towards a light source while most roots grow away, allowing plants optimal access to light, water, and nutrients. The primary photoreceptors involved in phototropism are the aptly named phototropins. In Arabidopsis, there are two members of this family, phot1 and phot2. As described more fully in recent reviews [4,5], phot1 and phot2 are plasma membrane-associated proteins with a photosensory region, consisting of two Light, Oxygen, or Voltage (LOV) domains, and a protein kinase domain that becomes activated upon blue light exposure. Exposure to blue light causes phototropins to physically interact with NPH3, another plasma membrane-associated protein essential for phototropism [4], which then undergoes dephosphorylation. Despite the ability of the isolated kinase region of phot2 to confer constitutive phototropin signaling [6], full-length phot1 proteins with a mutated, constitutively activate, kinase domain still require a light stimulus to trigger NPH3 dephosphorylation and downstream responses [7]. Subsequent steps in the signaling pathway are incompletely understood, but result in the polar relocalization of auxin transport carriers and formation of a lateral auxin gradient, leading to differential growth of the shaded and lit sides of the organ. Mechanisms used by auxin to control directional organ growth will be discussed in the following section.
Although Darwin established that phototropic cues are sensed at the tip of grass coleoptiles [4], the site of photoreception in dicot shoots has been less clear since PHOT1 and other signaling components are expressed widely in these plants. Investigators have recently examined the ability of PHOT1 expressed under various tissue-specific promoters to rescue phototropism in phot1 phot2 mutants. Light perception in the upper region of the hypocotyl, but not the cotyledons, was found to be necessary and sufficient for phototropic bending [8]. Subsequent studies suggest that normal phototropism depends upon photoreception in the upper hypocotyl, below the shoot apical meristem [9].
Phototropins are also found in green algae, where they have disparate functions such as in regulation of the sexual lifecycle in Chlamydomonas reinhardtii. Intriguingly, expression of the C. reinhardtii phot gene rescues phototropism in Arabidopsis phot1 phot2 double mutants [10]. This conservation of function in phototropic bending is however not seen for all algal phototropins. Expression of the single phototropin found in Ostereococcus tauri rescues some phot1 phot2 phenotypes but not phototropic bending [11]. This may be due to its inability to bind to NPH3. In addition, while a fraction of Arabidopsis phot1 is internalized from the plasma membrane upon blue light stimulation, this response is attenuated for the O. tauri phot [11]. However, it seems unlikely that this is the cause of the inability of the O. tauri phototropin to mediate phototropic bending: it was recently reported that Arabidopsis phot1 constitutively tethered to the plasma membrane mediates phototropic curvature nearly identically to control lines [12].
Auxin and directional growth
Auxin has long been implicated in many growth-mediated plant movements [13]. Intriguingly, depending upon its concentration and the plant tissue involved, auxin can either promote or inhibit cell elongation [14–16]. Auxin is primarily produced at the shoot apex and is moved throughout the plant body by specific auxin carrier proteins that shuttle it into and out of cells (auxin influx and efflux carriers). As first conceptualized in the Cholodny-Went model, tropic stimuli can modify auxin transport, causing the lateral redistribution of auxin across a plant stem and localized organ bending in response to this gradient (reviewed in [5]). Mechanisms controlling the intracellular localization and activity of influx and efflux carriers, and therefore the direction of auxin flow and relative levels of auxin across the plant body, are of intense interest and have recently been reviewed [17].
How does auxin cause rapid changes in growth? A possible role for the plasma membrane-localized AUXIN BINDING PROTEIN (ABP) in the control of growth has long been discussed (reviewed in [18]). However, a number of recent publications have put this model into question [18] and a plausible mechanism for the promotion of cell elongation in stems by a nuclear receptor has been proposed. Nuclear-localized TIR1/AFB (TRANSPORT INHIBITOR RESISTANT1/AUXIN SIGNALING F-BOX) receptor proteins bind auxin in conjunction with Aux/IAA (AUXIN/INDOLE ACETIC ACID) co-receptors to initiate a short signal-transduction pathway that regulates expression of hundreds of genes (see [19] for a recent review). Among these genes are the rapidly auxin-induced SMALL AUXIN UP RNAs (SAURs), which indirectly activate plasma membrane proton pumps, acidifying the apoplast and activating cell wall-loosening enzymes that increase wall extensibility [20,21].
In support of this acid growth model, a recent paper shows that components of the nuclear signaling pathway and induction of gene expression are essential for the acidification of the apoplast and hypocotyl growth in response to auxin [22]. Furthermore, constitutive expression of a normally auxin-induced SAUR is sufficient to confer elongation of aerial organs in Arabidopsis and tomato [21–23]. Together, these findings suggest that the nuclear receptor pathway is sufficient to explain auxin promotion of cell elongation in shoots. The role of auxin in the regulation of root growth is more complex, and will be discussed further below.
Gravitropism: multiple pathways at work in roots
In both shoots and roots, gravity is sensed by statocytes. These cells, found in the columella of the root tip and the endodermis of the shoot, contain starch-storing plastids called amyloplasts that upon plant inclination sediment in the direction of gravity and then trigger differential growth. Remarkably, clever experiments with a centrifugal device show the shoot bending response is independent of gravity intensity; furthermore, when plants are subjected to sustained inclination there is no angular threshold of response [24]. These findings suggest that plants respond directly to organ and statocyte inclination rather than by measuring the force exerted by statoliths upon cellular components. In both roots and shoots, gravistimulation triggers the lateral redistribution of auxin towards the lower side of the organ within minutes to effect directional growth (away from the gravity vector in the case of shoots; towards it in the case of roots; reviewed in [13]). Notably, a recent study has revealed that auxin redistribution is also responsible for the gravitropic responses of woody stems, which cannot undergo elongation growth and instead rely upon asymmetric radial growth to grow away from gravity [25].
The sequence of signaling events downstream of gravity perception is not fully resolved, although auxin signaling is clearly key to responses of both shoots and roots. Recent studies have demonstrated that proteins in the land plant-specific LAZY family act downstream of amyloplast sedimentation in both roots and shoots to generate asymmetric distribution of auxin efflux carriers and higher auxin levels on the lower surface side of the gravistimulated organ [26,27]. Phototropism is normal in lazy mutants [26,27], indicating that LAZY proteins act upstream of steps shared between the light- and gravity-sensing pathways in the auxin relocalization process.
In shoots, higher auxin levels promote local acidification of the apoplast and cell elongation, generating upward bending [13]. The role of auxin in the directional growth of roots is more complex. Rapid responses to gravistimulation include increases in cytoplasmic Ca2+ levels and changes in apoplastic pH. Increased cytosolic Ca2+ levels have been reported to lead to changes in the localization of auxin efflux carriers [28]. Auxin can also induce influx of Ca2+ across the plasma membrane in a cyclic nucleotide-gated channel-dependent manner [29]. Mutants in this channel exhibit delays in changes in cell surface pH and in gravitropic bending. Together, these data place calcium signaling both up- and downstream of auxin responses in roots.
Depending upon the species and the hormone concentration, auxin and apoplast acidification have been reported to inhibit or promote root cell elongation [14–16]. Recent work with a fluorescent reporter enabling the assessment of apoplastic pH at cellular resolution suggests a possible explanation for these conflicting reports [15]. Using genetic tools to manipulate endogenous levels of auxin, Barbez and co-workers concluded that low levels of auxin promote apoplast acidification and root cell elongation. However, their results also suggest that high local levels of auxin, such as those produced upon gravistimulation, cause alkalinization of the apoplast and inhibition of cell elongation. Furthermore, they found that auxin-induced apoplast alkalinization and normal gravitropism depend upon the plasma membrane-localized receptor-like kinase FERONIA [15]. Thus the effects of auxin on apoplastic pH and root growth are concentration-dependent and are due to the action of multiple signal transduction pathways [15,29].
Hydrotropism: a tropic response independent of auxin relocalization
In addition to responding to light and gravity, plant roots are hydrotropic: they sense differences in water potential in the local environment and direct their growth accordingly. The molecular details underlying this process are just becoming clear. While pharmacological studies suggest that auxin transport may be involved in hydrotropism in some species, lateral auxin redistribution does not appear to be required for hydrotropism in Arabidopsis or lotus [30,31] [32]. The signaling pathways underlying hydrotropism and gravitropism differ in many other ways as well. While gravitropic signals are sensed by root tip cells and cause differential growth at a distance in the elongation zone, it has recently been shown that the cortical cells of the elongation zone both sense and respond to the water potential signal in hydrotropism [33]. Moreover, signaling components downstream of the abscisic acid (ABA) receptor are required for normal hydrotropism, with low concentrations of exogenous ABA promoting both cell division and expansion in the elongation zone [33–35]. Furthermore, while reactive oxygen species are required for gravitropic bending, they inhibit hydrotropism [36]. Thus although root gravitropism and hydrotropism are superficially similar processes, these directional movements are controlled by very different molecular pathways.
Autostaightening: a proprioceptive response
Plants possess another type of movement response that is currently poorly understood and has variously been called autostraightening, autotropism, or automorphosis (reviewed in [37,38]). There is considerable evidence that plants can sense organ deformation and alter their growth to recover straightness. Thus during phototropism or gravitropism, initial general curving along the entire organ is followed by a period of basipetal straightening, with decurving proceeding from the organ tip so that eventually curvature occurs only at the base of the organ (Figure 2). Mathematical modeling of plant kinematic responses to gravitropic and phototropic signals suggests that the final stable orientation of the plant stem relative to external stimuli depends not only upon the tropic pathways described above, but also upon the concurrent sensing of local curvature via proprioceptive sensing [39,40].
Figure 2.
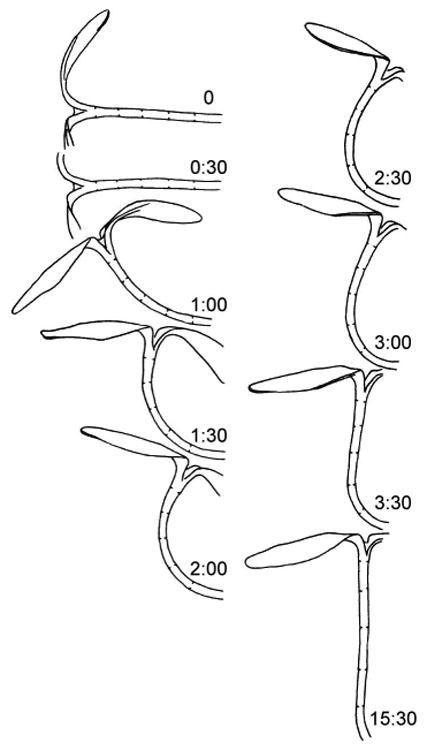
Autostraightening of the hypocotyl of a de-etiolated sunflower seedling subjected to gravistimulation. Hours after the seedling was turned to the horizontal positions are indicated. By one hour after gravistimulation, the hypocotyl exhibits nearly uniform curvature along its length. However, the position of curvature gradually moves basipetally down the hypoctyl until by 3:30 hours the upper portion of the hypocotyl has straightened and curvature is restricted to the base. Re-drawn from Firn and Digby [52].
How are proprioceptive cues sensed and transduced into differential growth? Studies using radiolabeled auxin or examining expression of an auxin-responsive reporter gene suggest that autostraightening does not depend upon lateral redistribution of auxin [41,42]. Instead, local curvature appears to be sensed via an actinomyosin-dependent mechanism that triggers autostraightening via an unknown mechanism. An intriguing recent manuscript revealed that Arabidopsis plants mutant for either two myosin XI family members or an actin isoform hyperbend both aerial organs and roots in response to gravitropic or phototropic stimuli [43]. These myosin XI genes are preferentially expressed in xylem fiber cells, extremely long cells (∼ 1 mm) with actin cables running along their longitudinal axes. It is possible that proprioceptive cues are sensed by the bending of these long actin cables which then activate mechanosensitive channels that produce signals regulating differential growth (reviewed in [44]). These new insights will facilitate studies leading to a better understanding of the molecular mechanisms underlying proprioception and how mechanical cues constrain and inform plant growth pathways.
Future directions
Although plant movements have been intensively studied for decades, many questions remain. Early predictions that phototropic and gravitropic stimuli cause the redistribution of auxin within roots and stems to generate differential growth across these organs are now well-supported. However, although the proximal receptors have been identified, our understanding of the signaling pathways generating these auxin gradients is still far from complete. Additional, currently unknown receptors may also be involved; for example, the extremely rapid movement of ions across root cell plasma membrane after auxin- or gravi-stimulation suggests a role for non-nuclear auxin receptors in these processes [29]. Mutant analysis has also revealed the action of opposing response pathways downstream of the same environmental stimulus. For example, the roots of plants mutant for LAZY proteins demonstrate negative, rather than positive, gravitropism [26,27,45]. This growth away from the gravity vector is accompanied by the accumulation of auxin and auxin transporters on the upper, rather than the lower sides, of gravistimulated roots. Thus these mutants reveal a LAZY-independent mechanism for the establishment of an auxin gradient with an opposite orientation to the one generated in wild-type roots in response to gravity.
Another important area for future research is how tropic response pathways interact with each other. Although in our above discussion we have generally treated growth-mediated tropic response pathways as though they act in isolation from each other, this is far from the truth. Plants are always subject to multiple, often conflicting, environmental cues. For example, young sunflower plants must maintain an upright posture against gravity while still bending to track the sun from east to west each day and bending back again towards the east each night [46]. To allow plants to cope with such conflicting signals, many tropic response pathways are mutually antagonistic, such as hydrotropism and gravitropism [30,36], and phototropism and gravitropism [47]. Highlighting the complicated relationships between tropic response pathways, it was recently reported that in microgravity conditions, Arabidopsis roots exhibit positive, not negative phototropism [48].
Non-directional sensing pathways can also affect tropisms; for example, the red light photoreceptor phytochrome can affect phototropism and gravitropism through control of auxin production and differentiation of plastids, respectively [49,50]. Finally, entirely internal cues can also modulate tropic responses: the circadian clock has been shown to control plant sensitivity to directional light and to gravistimulation [46,51]. Thus a full understanding of growth-mediated plant movements will require not only the unraveling of the complex signaling pathways outlined above, but also how these pathways influence each other.
Highlights.
Most plant movements are relatively slow and mediated by differential growth
Lateral gradients in auxin concentration are key for photo- and gravi-tropism
Activity of nuclear signaling pathways can explain auxin promotion of shoot growth
However, hydrotropism and autostraightening don't rely on auxin relocalization
Tropic response pathways don't act in isolation and are often mutually antagonistic
Acknowledgments
This work was supported by the National Institutes of General Medical Sciences of the National Institutes of Health under award number R01GM069418 and the National Science Foundation under award number IOS 1238040.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Guo Q, Dai E, Han X, Xie S, Chao E, Chen Z. Fast nastic motion of plants and bioinspired structures. J R Soc Interface. 2015;12:20150598. doi: 10.1098/rsif.2015.0598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Forterre Y. Slow, fast and furious: understanding the physics of plant movements. J Exp Bot. 2013;64:4745–4760. doi: 10.1093/jxb/ert230. [DOI] [PubMed] [Google Scholar]
- 3.Braidwood L, Breuer C, Sugimoto K. My body is a cage: mechanisms and modulation of plant cell growth. New Phytol. 2014;201:388–402. doi: 10.1111/nph.12473. [DOI] [PubMed] [Google Scholar]
- 4.Liscum E, Askinosie SK, Leuchtman DL, Morrow J, Willenburg KT, Coats DR. Phototropism: growing towards an understanding of plant movement. Plant Cell. 2014;26:38–55. doi: 10.1105/tpc.113.119727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fankhauser C, Christie JM. Plant Phototropic Growth. Curr Biol. 2015;25:R384–R389. doi: 10.1016/j.cub.2015.03.020. [DOI] [PubMed] [Google Scholar]
- 6.Kong SG, Kinoshita T, Shimazaki KI, Mochizuki N, Suzuki T, Nagatani A. The C-terminal kinase fragment of Arabidopsis phototropin 2 triggers constitutive phototropin responses. Plant J. 2007;51:862–873. doi: 10.1111/j.1365-313X.2007.03187.x. [DOI] [PubMed] [Google Scholar]
- 7.Petersen J, Inoue S, Kelly SM, Sullivan S, Kinoshita T, Christie JM. Functional Characterization of a Constitutively Active Kinase Variant of Arabidopsis Phototropin 1. J Biol Chem. 2017 doi: 10.1074/jbc.M117.799643. * A mutant phot1 protein with normal photochemistry but a constitutively active kinase domain was used to rescue Arabidopsis phot1 phot2 mutants. This protein rescues phototropism, but only after exposure to relatively high intensity directional light. Thus initiation of phototropin signaling requires more than just kinase activation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Preuten T, Hohm T, Bergmann S, Fankhauser C. Defining the site of light perception and initiation of phototropism in Arabidopsis. Curr Biol. 2013;23:1934–1938. doi: 10.1016/j.cub.2013.07.079. [DOI] [PubMed] [Google Scholar]
- 9.Sullivan S, Takemiya A, Kharshiing E, Cloix C, Shimazaki K, Christie JM. Functional characterization of Arabidopsis phototropin 1 in the hypocotyl apex. Plant J. 2016;88:907–920. doi: 10.1111/tpj.13313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Onodera A. Phototropin from Chlamydomonas reinhardtii is Functional in Arabidopsis thaliana. Plant Cell Physiol. 2005;46:367–374. doi: 10.1093/pcp/pci037. [DOI] [PubMed] [Google Scholar]
- 11.Sullivan S, Petersen J, Blackwood L, Papanatsiou M, Christie JM. Functional characterization of Ostreococcus tauri phototropin. New Phytol. 2016;209:612–623. doi: 10.1111/nph.13640. [DOI] [PubMed] [Google Scholar]
- 12.Preuten T, Blackwood L, Christie JM, Fankhauser C. Lipid anchoring of Arabidopsis phototropin 1 to assess the functional significance of receptor internalization: should I stay or should I go? New Phytol. 2015;206:1038–1050. doi: 10.1111/nph.13299. [DOI] [PubMed] [Google Scholar]
- 13.Zadnikova P, Smet D, Zhu Q, Straeten DVD, Benkova E. Strategies of seedlings to overcome their sessile nature: auxin in mobility control. Front Plant Sci. 2015;6 doi: 10.3389/fpls.2015.00218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Evans ML, Ishikawa H, Estelle MA. Responses of Arabidopsis roots to auxin studied with high temporal resolution: Comparison of wild type and auxin-response mutants. Planta. 1994;194:215–222. [Google Scholar]
- 15.Barbez E, Dünser K, Gaidora A, Lendl T, Busch W. Auxin steers root cell expansion via apoplastic pH regulation in Arabidopsis thaliana. Proc Natl Acad Sci. 2017;114:E4884–E4893. doi: 10.1073/pnas.1613499114. ** Using a dye that reports apoplast pH at cellular resolution, the authors demonstrated concentration-dependent effects of auxin on apoplastic pH and root cell elongation. They also used elegant genetic techniques to show a role for the nuclear auxin signaling pathway in promotion of root cell elongation and for the plasma membrane-localized receptor-like protein FERONIA in auxin-mediated inhibition of cell elongation and gravitropism. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pacheco-Villalobos D, Diaz-Moreno SM, van der Schuren A, Tamaki T, Kang YH, Gujas B, Novak O, Jaspert N, Li Z, Wolf S, et al. The Effects of High Steady State Auxin Levels on Root Cell Elongation in Brachypodium. Plant Cell. 2016;28:1009–1024. doi: 10.1105/tpc.15.01057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Armengot L, Marquès-Bueno MM, Jaillais Y. Regulation of polar auxin transport by protein and lipid kinases. J Exp Bot. 2016;67:4015–4037. doi: 10.1093/jxb/erw216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Strader LC, Zhao Y. Auxin perception and downstream events. Curr Opin Plant Biol. 2016;33:8–14. doi: 10.1016/j.pbi.2016.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Salehin M, Bagchi R, Estelle M. SCFTIR1/AFB-Based Auxin Perception: Mechanism and Role in Plant Growth and Development. Plant Cell. 2015;27:9–19. doi: 10.1105/tpc.114.133744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ren H, Gray WM. SAUR Proteins as Effectors of Hormonal and Environmental Signals in Plant Growth. Mol Plant. 2015;8:1153–1164. doi: 10.1016/j.molp.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spartz AK, Ren H, Park MY, Grandt KN, Lee SH, Murphy AS, Sussman MR, Overvoorde PJ, Gray WM. SAUR Inhibition of PP2C-D Phosphatases Activates Plasma Membrane H+-ATPases to Promote Cell Expansion in Arabidopsis. Plant Cell. 2014;26:2129–2142. doi: 10.1105/tpc.114.126037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fendrych M, Leung J, Friml J. TIR1/AFB-Aux/IAA auxin perception mediates rapid cell wall acidification and growth of Arabidopsis hypocotyls. eLife. 2016;5 doi: 10.7554/eLife.19048. ** The importance of the TIR1-family of nuclear-localized auxin receptors in rapid auxin-mediated cell elongation has been long debated. Carrying out careful analysis of the kinetics of growth and changes in apoplastic pH in plants mutant for various auxin signaling components, the authors conclude that the nuclear auxin signaling pathway promotes shoot growth via acidification of the apoplast. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spartz AK, Lor VS, Ren H, Olszewski NE, Miller ND, Wu G, Spalding EP, Gray WM. Constitutive Expression of Arabidopsis SMALL AUXIN UP RNA19 (SAUR19) in Tomato Confers Auxin-Independent Hypocotyl Elongation. Plant Physiol. 2017;173:1453–1462. doi: 10.1104/pp.16.01514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chauvet H, Pouliquen O, Forterre Y, Legue V, Moulia B. Inclination not force is sensed by plants during shoot gravitropism. Sci Rep. 2016;6 doi: 10.1038/srep35431. ** The authors examined shoot gravitropic responses in four angiosperm species under various gravity levels and found the response depended only upon the initial angle of inclination. They therefore conclude that in plants, gravity perception does not depend upon linear acceleration (force) and instead depends only upon the angle of the stem. This may allow plants to ignore transient cues such as those induced by wind shaking. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gerttula S, Zinkgraf M, Muday GK, Lewis DR, Ibatullin FM, Brumer H, Hart F, Mansfield SD, Filkov V, Groover A. Transcriptional and Hormonal Regulation of Gravitropism of Woody Stems in Populus. Plant Cell. 2015 doi: 10.1105/tpc.15.00531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taniguchi M, Furutani M, Nishimura T, Nakamura M, Fushita T, Iijima K, Baba K, Tanaka H, Toyota M, Tasaka M, et al. The Arabidopsis LAZY1 Family Plays a Key Role in Gravity Signaling within Statocytes and in Branch Angle Control of Roots and Shoots. Plant Cell. 2017;29:1984–1999. doi: 10.1105/tpc.16.00575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yoshihara T, Spalding EP. LAZY genes mediate the effects of gravity on auxin gradients and plant architecture. Plant Physiol. 2017 doi: 10.1104/pp.17.00942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang J, Vanneste S, Brewer PB, Michniewicz M, Grones P, Kleine-Vehn J, Löfke C, Teichmann T, Bielach A, Cannoot B, et al. Inositol Trisphosphate-Induced Ca2+ Signaling Modulates Auxin Transport and PIN Polarity. Dev Cell. 2011;20:855–866. doi: 10.1016/j.devcel.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 29.Shih HW, DePew C, Miller N, Monshausen G. The Cyclic Nucleotide-Gated Channel CNGC14 Regulates Root Gravitropism in Arabidopsis thaliana. Curr Biol. 2015;25:3119–3125. doi: 10.1016/j.cub.2015.10.025. [DOI] [PubMed] [Google Scholar]
- 30.Shkolnik D, Krieger G, Nuriel R, Fromm H. Hydrotropism: root bending does not require auxin redistribution. Mol Plant. 2016;9:757–759. doi: 10.1016/j.molp.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 31.Nakajima Y, Nara Y, Kobayashi A, Sugita T, Miyazawa Y, Fujii N, Takahashi H. Auxin transport and response requirements for root hydrotropism differ between plant species. J Exp Bot. 2017 doi: 10.1093/jxb/erx193. [DOI] [PubMed] [Google Scholar]
- 32.Morohashi K, Okamoto M, Yamazaki C, Fujii N, Miyazawa Y, Kamada M, Kasahara H, Osada I, Shimazu T, Fusejima Y, et al. Gravitropism interferes with hydrotropism via counteracting auxin dynamics in cucumber roots: clinorotation and spaceflight experiments. New Phytol. 2017;215:1476–1489. doi: 10.1111/nph.14689. [DOI] [PubMed] [Google Scholar]
- 33.Dietrich D, Pang L, Kobayashi A, Fozard JA, Boudolf V, Bhosale R, Antoni R, Nguyen T, Hiratsuka S, Fujii N, et al. Root hydrotropism is controlled via a cortex-specific growth mechanism. Nat Plants. 2017;3:17057. doi: 10.1038/nplants.2017.57. ** The authors convincingly demonstrate that water potential cues are sensed in the root elongation zone, the same region where differential growth drives hydrotropic bending. They also show that hydrotropic growth depends upon a functional ABA signaling pathway in the cortical cell layer. [DOI] [PubMed] [Google Scholar]
- 34.Takahashi N, Goto N, Okada K, Takahashi H. Hydrotropism in abscisic acid, wavy, and gravitropic mutants of Arabidopsis thaliana. Planta. 2002;216:203–211. doi: 10.1007/s00425-002-0840-3. [DOI] [PubMed] [Google Scholar]
- 35.Xu W, Jia L, Shi W, Liang J, Zhou F, Li Q, Zhang J. Abscisic acid accumulation modulates auxin transport in the root tip to enhance proton secretion for maintaining root growth under moderate water stress. New Phytol. 2013;197:139–150. doi: 10.1111/nph.12004. [DOI] [PubMed] [Google Scholar]
- 36.Krieger G, Shkolnik D, Miller G, Fromm H. Reactive oxygen species tune root tropic responses. Plant Physiol. 2016 doi: 10.1104/pp.16.00660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hamant O, Moulia B. How do plants read their own shapes? New Phytol. 2016;212:333–337. doi: 10.1111/nph.14143. [DOI] [PubMed] [Google Scholar]
- 38.Stankovic B, Volkmann D, Sack FD. Autotropism, automorphogenesis, and gravity. Physiol Plant. 1998;102:328–335. doi: 10.1034/j.1399-3054.1998.1020222.x. [DOI] [PubMed] [Google Scholar]
- 39.Bastien R, Douady S, Moulia B. A Unified Model of Shoot Tropism in Plants: Photo-, Gravi- and Propio-ception. PLOS Comput Biol. 2015;11:e1004037. doi: 10.1371/journal.pcbi.1004037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bastien R, Bohr T, Moulia B, Douady S. Unifying model of shoot gravitropism reveals proprioception as a central feature of posture control in plants. Proc Natl Acad Sci. 2013;110:755–760. doi: 10.1073/pnas.1214301109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Haga K, Iino M. Asymmetric distribution of auxin correlates with gravitropism and phototropism but not with autostraightening (autotropism) in pea epicotyls. J Exp Bot. 2006;57:837–847. doi: 10.1093/jxb/erj069. [DOI] [PubMed] [Google Scholar]
- 42.Yamamoto KT, Watahiki MK, Matsuzaki J, Satoh S, Shimizu H. Space–time analysis of gravitropism in etiolated Arabidopsis hypocotyls using bioluminescence imaging of the IAA19 promoter fusion with a destabilized luciferase reporter. J Plant Res. 2017;130:765–777. doi: 10.1007/s10265-017-0932-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Okamoto K, Ueda H, Shimada T, Tamura K, Kato T, Tasaka M, Morita MT, Hara-Nishimura I. Regulation of organ straightening and plant posture by an actin-myosin XI cytoskeleton. Nat Plants. 2015;1:15031. doi: 10.1038/nplants.2015.31. * The authors report remarkable phenotypes in Arabidopsis plants mutant for either myosin XI or ACTIN8 genes: these plants show enhanced organ bending in response to gravitropic or phototropic stimuli, leading to stem kinking and coiling under some conditions. Their data suggest that the long cytoskeletal cables in xylem fiber cells sense organ bending and trigger subsequent straightening. [DOI] [PubMed] [Google Scholar]
- 44.Monshausen GB, Haswell ES. A force of nature: molecular mechanisms of mechanoperception in plants. J Exp Bot. 2013;64:4663–4680. doi: 10.1093/jxb/ert204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ge L, Chen R. Negative gravitropism in plant roots. Nat Plants. 2016;2:16155. doi: 10.1038/nplants.2016.155. [DOI] [PubMed] [Google Scholar]
- 46.Atamian HS, Creux NM, Brown EA, Garner AG, Blackman BK, Harmer SL. Circadian regulation of sunflower heliotropism, floral orientation, and pollinator visits. Science. 2016;353:587–590. doi: 10.1126/science.aaf9793. * Using physiological approaches, the authors demonstrate that the circadian clock is essential for the solar tracking of young sunflowers and that it modulates, or ‘gates’, phototropic responsiveness. The latter finding implicates the clock and phototropism in the uniform eastward orientation of mature sunflowers. [DOI] [PubMed] [Google Scholar]
- 47.Vitha S, Zhao L, Sack FD. Interaction of root gravitropism and phototropism in Arabidopsis wild-type and starchless mutants. Plant Physiol. 2000;122:453–462. doi: 10.1104/pp.122.2.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vandenbrink JP, Herranz R, Medina FJ, Edelmann RE, Kiss JZ. A novel blue-light phototropic response is revealed in roots of Arabidopsis thaliana in microgravity. Planta. 2016;244:1201–1215. doi: 10.1007/s00425-016-2581-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Goyal A, Karayekov E, Galvão VC, Ren H, Casal JJ, Fankhauser C. Shade Promotes Phototropism through Phytochrome B-Controlled Auxin Production. Curr Biol CB. 2016;26:3280–3287. doi: 10.1016/j.cub.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 50.Kim K, Shin J, Lee SH, Kweon HS, Maloof JN, Choi G. Phytochromes inhibit hypocotyl negative gravitropism by regulating the development of endodermal amyloplasts through phytochrome-interacting factors. Proc Natl Acad Sci. 2011;108:1729–1734. doi: 10.1073/pnas.1011066108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vinterhalter D, Vinterhalter B, Miljuš-Djukić J, Jovanović ž, Orbović V. Daily Changes in the Competence for Photo- and Gravitropic Response by Potato Plantlets. J Plant Growth Regul. 2014;33:539–550. [Google Scholar]
- 52.Firn RD, Digby J. A study of the autotropic straightening reaction of a shoot previously curved during geotropism. Plant Cell Environ. 1979;2:149–154. [Google Scholar]


