Abstract
Cross-sectional studies indicate consistent associations between low 25(OH)D concentration and increased risk of cardiovascular disease (CVD), but results of randomized control trials (RCTs) are mixed. However, the majority of the RCTs do not focus on type 2 diabetics, potentially obscuring the effects of vitamin D in this population. In vitro 1,25(OH)2D3 downregulates macrophage cholesterol deposition, but the in vivo effects are unknown. To explore potential mechanisms of the effects of vitamin D on CVD risk in patients with type 2 diabetes, we isolated monocytes in a subset of 26 patients from our RCT of diabetics with baseline serum 25(OH)D <25 ng/mL randomized to vitamin D3 4000 IU/day or placebo for 4 months. Upon enrollment, the mean 25(OH)D level was 17 ng/mL, which increased to 36 ng/mL after vitamin D and remained unchanged in the placebo group. Before randomization, groups demonstrated similar mean hemoglobin A1c and plasma lipids levels, none of which was significantly altered by vitamin D supplementation. Moreover, assessment of oxidized LDL uptake in monocytes cultured in the patient’s own serum before vs. after treatment resulted in >50% reduction in the vitamin D group with no change in the placebo group. This was mediated through suppression of endoplasmic reticulum stress and scavenger receptor CD36 protein expression. The reduction in monocyte cholesterol uptake was reflected in a 19% decrease in total monocyte cholesterol content. Interestingly, cross-sectional analysis of circulating monocytes from vitamin D-deficient vs. sufficient diabetic patients revealed 8-fold higher cholesteryl ester content, confirming the capacity of these monocytes to uptake and carry cholesterol in the circulation. This study identifies a unique circulating cholesterol pool within monocytes that is modulated by vitamin D and has the potential to contribute to CVD in type 2 diabetes.
Keywords: Vitamin D, monocytes, CD36, cholesterol metabolism, atherosclerosis
1. Introduction
Type 2 diabetes (T2DM) remains a widely prevalent condition; in the U.S. alone, it affects more than 29 million adults. T2DM often coexists with dyslipidemia and hypertension, and despite potent medications to treat all three risk factors, the risk of myocardial infarction or cardiovascular disease (CVD) death remains nearly twice that of nondiabetics [1], prompting continued interest in the discovery of other modifiable CVD risk factors for this population. A potential target lies in modulating mononuclear cells prior to the development of atherosclerosis. Monocytes and macrophages are critical to cholesterol deposition in the atherosclerotic plaque. Macrophages recruited to the subendothelial space increase expression of scavenger receptors, most notably scavenger receptor A1 (SR-A1) and cluster of differentiation 36 (CD36), enabling them to uptake modified low density lipoprotein (LDL) cholesterol in an unregulated manner. The resulting accumulation of cholesteryl esters transforms the macrophages into foam cells, which are the major contributor to the lipid core of atherosclerotic plaques [2]. The importance of cholesterol metabolism of circulating monocytes may be underrecognized in this process; monocytes from patients with familial hypercholesterolemia (FH) become cholesterol-laden in circulation, suggesting a possible capacity to carry cholesterol into the vessel wall [3]. Our mouse model of atherosclerosis and diabetes has confirmed this capacity for monocyte cholesterol transport into atherosclerotic plaques [4]. Interestingly, monocytes from type 2 diabetics are known to have higher baseline expression of CD36, which is unchanged by acute hyperglycemia [5,6], suggesting that other environmental conditions may regulate monocyte cholesterol metabolism and represent potential treatments for CVD.
Observational studies demonstrate a consistent association between low 25-hydroxy vitamin D [25(OH)D] levels and both T2DM and CVD. 25(OH)D levels <30 ng/mL have been found in 81% of diabetics from National Health and Nutrition Examination Survey (NHANES) data [7]. Within patients with T2DM, low 25(OH)D levels nearly double the relative risk of developing CVD compared to individuals with normal vitamin D levels and T2DM [8]. Several systematic reviews and meta-analyses of randomized clinical trials of the effects of vitamin D on cardiovascular outcomes have been negative [9–12], but these trials have not targeted patients with uncomplicated diabetes. Our group has previously demonstrated that vitamin D and its metabolites have significant effects on the atherogenic functional properties of both macrophages and peripheral blood monocytes from patients with diabetes without known CVD. Ex vivo analysis of human monocyte-derived macrophages from subjects with type 2 diabetes show that vitamin D deficiency in culture increases cholesterol uptake, leading to foam cell formation via an endoplasmic reticulum (ER)-stress-dependent mechanism [13]. Furthermore, the increase in cholesterol uptake is reversible in culture with 1,25-dyhydroxy vitamin D3 [1,25(OH)2D3] supplementation [14]. Next, to mimic in vivo conditions more closely, we looked at freshly isolated human peripheral blood monocytes from type 2 diabetics, finding that systemic 25(OH)D levels correlate inversely with cellular adhesion and migration capabilities and expression of the surface molecules that facilitate these processes, also in an ER-stress-dependent manner [15]. The increased adhesion and migration is suppressed with 25(OH)D supplementation in culture [16]. To investigate these atherogenic properties in vivo, we generated a mouse model with selective knockout of the vitamin D receptor in myeloid cells and found that these mice have cholesterol-laden monocytes with increased adhesion and migration capabilities enabling them to carry cholesterol into atherosclerotic plaques [4]. The effects of vitamin D on monocyte cholesterol metabolism in mice prompted us to investigate whether vitamin D supplementation could alter this mechanism of atherosclerosis in patients with type 2 diabetes.
2. Material and Methods
2.1. Subjects
Subjects were obtained from two settings for longitudinal and cross-sectional assessments. Studies were approved by the Washington University Human Research Protection Office, and all subjects gave informed consent. All procedures were carried out at Washington University School of Medicine in St. Louis, MO.
For longitudinal studies, monocyte assessment was performed in a subset of patients from our randomized clinical trial of the effects of vitamin D3 supplementation on blood pressure in subjects with type 2 diabetes, hypertension, and 25(OH)D level <25 ng/mL. Patients were recruited and enrolled between September 2006 and November 2015. Subjects were screened and included if they were between age 25 and 80 with a diagnosis of type 2 diabetes and hypertension, as well as serum 25(OH)D level <25 ng/ml. Diabetes diagnosis was confirmed by hemoglobin A1c (A1c) >6.5% or ongoing hypoglycemic medications for at least 3 months, and subjects had to have A1c 5.5–9.5% and not be on insulin therapy. Due to the American Diabetes Association recommendations for lower blood pressure (BP) targets in diabetic patients [17], hypertension meeting inclusion criteria was defined as a mean systolic blood pressure (SBP) of ≥120 mm Hg and/or mean diastolic blood pressure (DBP) of ≥80 mm Hg as assessed by 24-hour ambulatory blood pressure monitoring after stopping antihypertensive medications for at least 2 weeks. Antihypertensives were held for the duration of the study. Subjects were excluded from participation for mean SBP >160 mm Hg or mean DBP >100 mm Hg, cardiovascular disease, arrhythmia, congestive heart failure, stage 4 or worse chronic kidney disease, >2+ proteinuria on urine dipstick, untreated thyroid disorders, calcium disorders, recurrent nephrolithiasis, osteoporosis, oral or intravenous immunomodulatory medications, heavy alcohol consumption (males >2 drinks per day and females >1 drink per day), drug use, weight change >5% in the 3 months prior to screening, extreme diets, unwillingness to stop supplements containing calcium or vitamin D for the study duration, and pregnancy. Subjects were then randomized in a 1:1 ratio to one of two groups: vitamin D3 4000 international units (IU) or matching placebo daily (supplied by Tishcon Corp.) for 4 months, with treatment allocation blinded to both investigators and participants. Both groups received calcium carbonate 500 mg twice daily. For safety, patients were seen at 2 weeks, 1 month, 2 months, 3 months, and 4 months for assessment of blood pressure, hypercalciuria, and hypercalcemia, as well as any other adverse events. Monocytes were isolated from venous blood draw at baseline and 4 months.
For cross-sectional studies, patients were screened and included if they were between age 25 and 80 with a self-identified diagnosis of type 2 diabetes and varying levels of 25(OH)D. They were excluded if known to have stage 4 or worse chronic kidney disease, cardiovascular disease, arrhythmia, congestive heart failure, or calcium disorders, if on oral or intravenous immunomodulatory medications, or if pregnant. Subjects underwent a single venous blood draw for serum 25(OH)D level and monocyte isolation.
2.2. Clinical Laboratory Assessment
Serum 25(OH) vitamin D was quantified by liquid chromatography-tandem mass spectrometry (LC-MS/MS) at Mayo Medical Laboratories (Rochester, MN). Lipid levels were quantified by enzymatic colorimetry (with Friedewald calculation for LDL if triglycerides <400 mg/dL), A1c levels were quantified by turbidimetric inhibition immunoassay, and monocyte counts were quantified by electrical impedance at the Core Lab for Clinical Studies at Washington University School of Medicine (St. Louis, MO).
2.3. Monocyte Isolation
Peripheral blood monocytes were isolated as we have previously described from lithium heparin blood collection tubes by standard Ficoll isolation techniques, followed by positive selection with CD14 microbeads (Miltenyi Biotec) [15]. Monocytes were stabilized for 3 hours in the subject’s plasma to mimic in vivo conditions prior to experimentation.
2.4. Monocyte Cholesterol Metabolism
Cholesterol uptake was assessed as we have previously described [13]. Briefly, isolated monocytes were incubated with 10 μg/mL oxidized low density lipoprotein (oxLDL) labeled with 1,1′-dioctadecyl-3,3,3′,3′-tetramethyl indocarbocyanine percholate (DiI; Invitrogen) for 6 hours, then lysed using radioimmunoprecipitation assay (RIPA) buffer. DiI-oxLDL cholesterol uptake was detected using a microplate reader and normalized to cellular protein. Monocyte protein extracts were analyzed by Western blot for CD36 (Santa Cruz) and ER stress protein expression [phospho-pancreatic ER kinase (pPERK, Cell Signaling) and C/EBP homologous protein (CHOP, Santa Cruz)] and normalized to β-actin expression (Cell Signaling). For total cellular cholesterol content and cholesteryl ester quantification, lipids were first extracted from isolated monocytes using the methodology of Bligh and Dyer [18]. Total cellular cholesterol was quantified using enzymatic colorimetry (Wako). For cholesteryl ester quantification, [2,2,4,4,6]2H5-cholesterol and [5,6,22,23]2H4-sitostanol were obtained from Medical Isotopes, and cholesterol was obtained from Sigma. Sitostanol-d4 palmitate (5 μg in toluene) and cholesterol-d5 (2 μg in toluene) were added to unsaponified total lipid extracts as internal standards for cholesteryl ester and free cholesterol, respectively. Samples were applied to silica solid-phase extraction columns (LC-Si SPE Tubes, Sigma) in hexane (0.5 mL per sample). After washing with hexane, sterol esters were eluted with 1% ethyl acetate in hexane, followed by free sterol elution with 25% ethyl acetate in hexane. Both fractions were dried at 60°C. Saponification was performed by adding water (0.5 mL), ethanol (1.16 mL), and 45% aqueous potassium hydroxide (80 μL) to each sample, after which samples were placed at 65°C overnight. Petroleum ether (1 mL) was added and the upper phase was transferred off after 10 minutes, then dried at 60°C. For derivatization, pyridine (50 μL) followed by acetic anhydride (60 μL) were added to each sample, then heated at 75°C prior to drying at 60°C with N2 gas blowing. Samples were redissolved in hexane prior to tandem gas chromatography/mass spectrometry (GC/MS).
2.5. Statistical Analysis
We calculated our sample size for the change in monocyte cholesterol uptake over time between groups, the primary outcome of this study, based upon our previous data of the difference in monocyte cholesterol uptake between mice with and without selective knockout of the vitamin D receptor in myeloid cells, to our knowledge the only assessment of this type in monocytes [19]. Based on the effect size of 1.71 from mice (genetically identical background), we performed the sample size calculation assuming a 25% smaller effect size in human diabetics comparing vitamin D supplementation or placebo, in which case a sample size of 11 per group would give us 80% power to detect a difference between groups at an alpha of 0.05. Subsequently, we assessed cholesteryl ester content in a cross-sectional group of type 2 diabetics with vitamin D insufficiency or sufficiency. Our previous data in macrophages from type 2 diabetics showed a difference in cholesteryl ester content with 8 patients per group; therefore, we used the same number for this study [13]. GraphPad Prism software was used for statistical calculations. Descriptive variables are expressed as the mean ± standard error of the mean (SEM) for continuous data and as a percentage for categorical data. Experiments were carried out with duplicate or triplicate samples. Analytic data are expressed as mean ± SEM for continuous variables. Statistical significance of categorical baseline data between groups was assessed with Fisher’s exact test. Statistical significance of differences for continuous outcomes was calculated using a t-test for parametric data involving two groups. For longitudinal data, statistical significance of differences was calculated using two-way analysis of variance with repeated measures. Results were considered statistically significant if p <0.05.
3. Results
3.1. Clinical Characteristics of Study Population
Monocytes were isolated from a subset of 26 total longitudinal study patients. Baseline demographic data (gender, race, ethnicity, and age) and metabolic parameters (body mass index, A1c, lipid levels) were similar between groups (Table 1). Serum 25(OH)D levels increased significantly over 4 months as expected in the vitamin D3 group, from 17 ± 1 to 36 ± 3 ng/mL, compared to no change in the placebo group (18 ± 1 to 16 ± 2 ng/mL), with 73% of supplemented patients achieving a final level of ≥30 ng/mL (Figure 1a). There were no changes over the course of the trial in metabolic parameters including A1c and lipid levels (Figure 1b–f), with similar frequency of statin use between groups (p=0.35).
Table 1.
Baseline Characteristics
| Placebo (n=15) |
Vitamin D (n=11) |
p-value | |
|---|---|---|---|
| Gender (n) | 0.11 | ||
| Male | 67% (10) | 27% (3) | |
| Female | 33% (5) | 73% (8) | |
| Race (n) | 0.23 | ||
| White/Caucasian | 27% (4) | 55% (6) | |
| African- American | 73% (11) | 45% (5) | |
| Ethnicity (n) | 1.00 | ||
| Non-Hispanic | 100% (15) | 100% (11) | |
| Hispanic | 0% (0) | 0% (0) | |
| Age (years) | 57.4 ± 1.8 | 57.6 ± 1.9 | 0.94 |
| BMI (kg/m2) | 31.5 ± 1.6 | 36.8 ± 6.8 | 0.06 |
| Total Cholesterol (mg/dL) | 168 ± 9 | 176 ± 10 | 0.61 |
| LDL (mg/dL) | 101 ± 8 | 106 ± 10 | 0.70 |
| HDL (mg/dL) | 48 ± 4 | 47 ± 3 | 0.79 |
| Triglcyerides (mg/dL) | 96 ± 13 | 112 ± 12 | 0.36 |
| Hemoglobin A1c (%) | 6.6 ± 0.2 | 6.6 ± 0.3 | 0.89 |
Demographic and metabolic baseline characteristics of 26 subjects with type 2 diabetes, hypertension, and serum 25(OH)D level <25 ng/mL. Data presented as mean ± SEM.
Figure 1. Vitamin D supplementation does not change metabolic parameters in patients with type 2 diabetes.
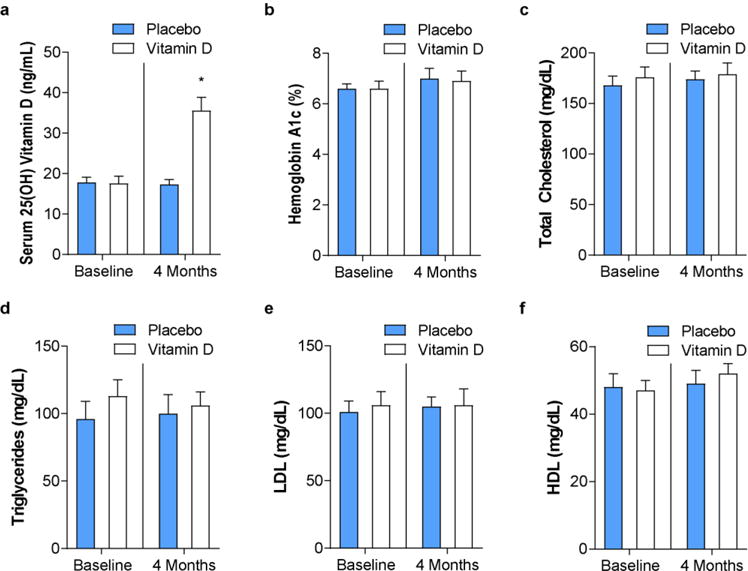
In patients with type 2 diabetes, hypertension, and 25(OH)D <25 ng/mL randomized to vitamin D3 4000 IU daily or placebo for 4 months, (a) serum 25(OH) vitamin D, (b) hemoglobin A1c, and (c–f) plasma lipid levels at baseline and after treatment (n=11 vitamin D, n=15 placebo, *p<0.05 vs. all). Data presented as mean ± SEM.
3.2. Monocyte Cholesterol Metabolism
To evaluate the effects of vitamin D3 on the capacity of patient monocytes to uptake modified cholesterol that could contribute to early atherosclerotic foam cell formation, uptake of DiI-labeled oxLDL was assessed before and after vitamin D3 treatment or placebo. Relative and absolute monocyte counts were no different between groups at baseline and did not change over time (data not shown). All patients had 25(OH)D <25 ng/mL prior to randomization, and uptake was similar between groups at baseline. Vitamin D3 supplementation significantly decreased cholesterol uptake by >50% compared to no effect over time in the placebo group (Figure 2a). In macrophages from T2DM patients, it is known that active vitamin D downregulates ER stress, causing reduction of protein expression of scavenger receptors CD36 and SR-A1 to suppress foam cell formation [13]. In this study, we found that vitamin D3 supplementation similarly downregulated monocyte ER stress activation and CD36 protein expression (Figure 2b). Moreover, this reduction in cholesterol uptake was reflected by a 19% decrease in total monocyte cholesterol content after vitamin D3 supplementation, while total cholesterol in the placebo group did not change (Figure 2c). To clarify the composition of the cholesterol within the monocytes, we performed GC/MS in monocytes isolated from a cross-sectional sample of vitamin D-sufficient [25(OH)D mean 35 ± 2 ng/mL] or insufficient [25(OH)D mean 13 ± 2 ng/mL] T2DM patients. We found that vitamin D-insufficient cells had 8-fold higher cholesteryl ester percentage, suggesting that vitamin D sufficiency decreases cholesterol uptake, cholesterol esterification, and foam cell formation (Figure 2d).
Figure 2. Vitamin D supplementation reduces monocyte cholesterol deposition through suppression of ER stress and CD36.
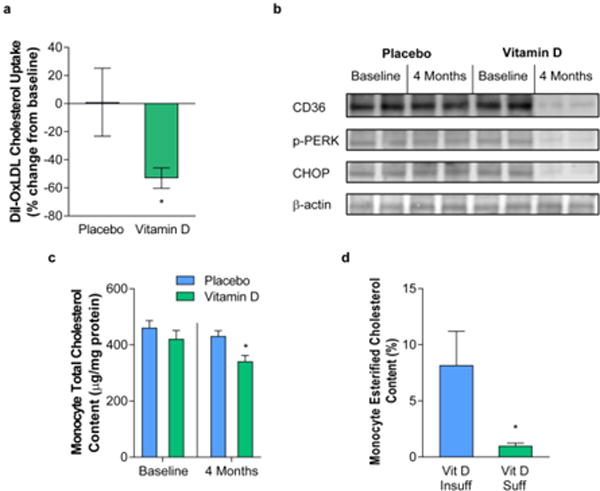
In patients with type 2 diabetes, hypertension, and 25(OH)D <25 ng/mL randomized to vitamin D3 4000 IU daily or placebo for 4 months, (a) change in DiI-oxLDL cholesterol uptake after treatment (n=11 vitamin D, n=15 placebo, *p<0.05 vs. all), (b) Western blot analysis of scavenger receptor CD36 and ER stress proteins phospho-PERK and CHOP at baseline and after treatment (representative of n=6 per group), and (c) total cellular cholesterol content at baseline and after treatment (n=6 per group, *p<0.05 vs. all). In patients with type 2 diabetes and vitamin D insufficiency [25(OH)D <20 ng/mL] or sufficiency [25(OH)D >30 ng/mL], (d) monocyte cholesteryl ester as a percentage of total cholesterol assessed by GC/MS (n=8 per group, *p <0.05). Data presented as mean ± SEM.
4. Discussion
Our murine study demonstrated that vitamin D-deficient monocytes carry cholesterol into the vessel wall, contributing to foam cell formation and atherosclerosis progression [4]. However, the effects of vitamin D on monocyte cholesterol metabolism in humans are unknown. In this study, we demonstrated that in T2DM patients, 4 months of vitamin D3 supplementation reduced total monocyte cholesterol content by suppressing oxLDL cholesterol uptake despite consistent plasma cholesterol, LDL, HDL, and triglyceride levels. These changes were mediated by a reduction of monocyte ER stress and CD36 expression. Furthermore, in cross-sectional analysis of vitamin D-insufficient versus sufficient subjects with type 2 diabetes, quantitative mass spectrometry analysis a revealed lower proportion of monocyte cholesteryl ester in vitamin D sufficient cells. Together, these findings suggest that vitamin D may be an effective treatment to reduce cholesterol deposition in monocytes, an early step in the development of atherosclerosis, in a high-risk type 2 diabetic population.
Recent studies identify differential cholesterol metabolism in monocytes compared to macrophages that suggests they are more resistant to becoming foam cells [20,21]. Human THP-1 monocytes show poor conversion of free cholesterol into cholesteryl ester upon cholesterol loading with labeled micelles, but also decreased hydrolysis of cholesteryl ester into free cholesterol when stimulated with labeled cholesteryl ester, leading to a net lower content of neutral lipids. Free cholesterol, but not cholesteryl ester exposure, stimulates ATP-binding cassette (ABC) transporter proteins in monocytes that drive cholesterol efflux, but decreases enzymes involved in cholesteryl ester synthesis and hydroxylation. In contrast, cholesteryl ester exposure decreases LDL receptor expression, but promotes CD36 expression, promoting differential cholesterol uptake of modified lipoproteins [20]. In this study, we found that achieving higher serum 25(OH)D levels in T2DM has a direct action on monocyte cholesterol metabolism, decreasing total cholesterol content and proportion of cholesteryl ester. These results resemble our previous data in monocyte-derived macrophages from subjects with T2DM in which 1,25(OH)2D3 suppresses cholesterol uptake and cholesterol esterification to reduce total cholesterol content [13]. Therefore, our results suggest similar responses to vitamin D supplementation in monocytes and macrophages, arguing that it could alter atherosclerosis progression by both decreasing monocyte capacity to carry cholesterol into the vessel wall and reducing macrophage cholesterol esterification within the vessel wall.
Monocyte cholesterol metabolism is also influenced by differences in local conditions. In patients with FH and its associated mouse model (knockout of the LDL receptor; LDLR−/−), both of which result in elevated plasma LDL levels, monocytes become cholesterol-laden in the circulation [3,4]. Monocytes from FH patients show a predominance for oxLDL binding capacity and uptake over native LDL through increased expression of CD36, emphasizing the importance of the upregulation of scavenger receptors in monocyte cholesterol deposition [3]. In LDLR−/− mice, we found that monocyte-selective deletion of the vitamin D receptor induced a cholesterol-laden phenotype with increased adhesion to and migration into the vessel wall, facilitating cholesterol transport into the early atherosclerotic plaque [4]. Similarly, we have shown previously that monocytes from subjects with T2DM with lower serum 25(OH)D have increased adhesion and migration [15] and now show that they have elevated total cholesterol content as well. Furthermore, we demonstrated for the first time an intervention that can alter monocyte cholesterol metabolism in vivo. In T2DM patients with baseline 25(OH)D <25 ng/mL, despite no changes in plasma glucose or lipid parameters, vitamin D3 supplementation suppressed monocyte oxLDL cholesterol uptake by downregulation of ER stress and reduction of CD36 expression. Interestingly, patients with T2DM have higher baseline CD36 expression compared to non-diabetics [5], but this does not change during acute hyperglycemia, arguing that the increased CD36 expression is in response to macrophage insulin resistance or chronic hyperglycemia [22]. We have previously shown that 1,25(OH)2D3-supplemented media improves macrophage insulin signaling by increasing insulin-induced Akt phosphorylation, a potential mechanism by which the suppression of CD36 expression and foam cell formation may be more significant in T2DM [13]. Our previous studies in monocyte-derived macrophages from T2DM patients demonstrate that 1,25(OH)2D3 supplementation suppresses foam cell formation by reduction of oxLDL cholesterol uptake. 1,25(OH)2D3 suppresses macrophage ER stress, leading to reduced c-Jun N-terminal kinase (JNK) activation and downregulation of peroxisome proliferator-activated receptor gamma (PPARα) and CD36 expression [13], a response which appears to be similar in monocytes. Mononcyte CD36 can be suppressed by atorvastatin in T2DM patients, though monocyte cholesterol has not been measured in this setting, and the effects are concurrent with lipid-lowering effects, suggesting that vitamin D may have more direct effects on monocyte cholesterol metabolism [23]. These findings reinforce the need for carefully-designed interventional studies of the cardiovascular effects of vitamin D that focus on the high-risk diabetic population.
Highlights.
Vitamin D is first intervention to reduce monocyte cholesterol in type 2 diabetes
Vitamin D replacement decreases monocyte CD36 expression and cholesterol uptake
Vitamin D is a monocyte ER stress reliever in type 2 diabetes
Acknowledgments
This publication was made possible by National Institutes of Health R01 HL094818 and American Diabetes Association 1-12-CT-08. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NIH.
Abbreviations
- ABC
ATP binding cassette
- CD36
cluster of differentiation 36
- CHOP
CEBP homologous protein
- CVD
cardiovascular disease
- DBP
diastolic blood pressure
- DiI
1,1′-dioctadecyl-3,3,3′,3′-tetramethyl indocarbocyanine percholate
- ER
endoplasmic reticulum
- FH
familial hypercholesterolemia
- GC/MS
gas chromatography mass spectrometry
- JNK
c-Jun N-terminal kinase
- IU
international units
- oxLDL
oxidized low density lipoprotein
- PPARα
peroxisome proliferator-activated receptor gamma
- p-PERK
phospho-pancreatic ER kinase
- RCT
randomized controlled trial
- SBP
systolic blood pressure
- SEM
standard error of the mean
- SR-A1
scavenger receptor class A, type 1
- T2DM
type 2 diabetes mellitus
- 25(OH)D
25-hydroxy vitamin D
- 1
25(OH)2D, 1,25-dihydroxy vitamin D
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Centers for Disease Control and Prevention. National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States, 2014. U.S. Department of Health and Human Services; Atlanta, GA: 2014. [Google Scholar]
- 2.Moore KJ, Tabas I. Macrophages in the pathogenesis of atherosclerosis. Cell. 2011;145:341–355. doi: 10.1016/j.cell.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mosig S, Rennert K, Büttner P, Krause S, Lütjohann D, Soufi M, Heller R, Funke H. Monocytes of patients with familial hypercholesterolemia show alterations in cholesterol metabolism. BMC Med Genomics. 2008;1:60. doi: 10.1186/1755-8794-1-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oh J, Riek AE, Darwech I, Funai K, Shao J, Chin K, Sierra OL, Carmeliet G, Ostlund RE, Bernal-Mizrachi C. Deletion of macrophage Vitamin D receptor promotes insulin resistance and monocyte cholesterol transport to accelerate atherosclerosis in mice. Cell Rep. 2015;10:1872–1886. doi: 10.1016/j.celrep.2015.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sampson MJ, Davies IR, Braschi S, Ivory K, Hughes DA. Increased expression of a scavenger receptor (CD36) in monocytes from subjects with Type 2 diabetes. Atherosclerosis. 2003;167:129–134. doi: 10.1016/S0021-9150(02)00421-5. [DOI] [PubMed] [Google Scholar]
- 6.Spartano NL, Lamon-Fava S, Matthan NR, Ronxhi J, Greenberg AS, Obin MS, Lichtenstein AH. Regulation of ATP-binding cassette transporters and cholesterol efflux by glucose in primary human monocytes and murine bone marrow-derived macrophages. Exp Clin Endocrinol Diabetes Off J Ger Soc Endocrinol Ger Diabetes Assoc. 2014;122:463–468. doi: 10.1055/s-0034-1374600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Soderstrom LH, Johnson SP, Diaz VA, Mainous AG. Association between vitamin D and diabetic neuropathy in a nationally representative sample: results from 2001–2004 NHANES. Diabet Med J Br Diabet Assoc. 2012;29:50–55. doi: 10.1111/j.14645491.2011.03379.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cigolini M, Iagulli MP, Miconi V, Galiotto M, Lombardi S, Targher G. Serum 25hydroxyvitamin D3 concentrations and prevalence of cardiovascular disease among type 2 diabetic patients. Diabetes Care. 2006;29:722–724. doi: 10.2337/diacare.29.03.06.dc05-2148. [DOI] [PubMed] [Google Scholar]
- 9.Wang L, Manson JE, Song Y, Sesso HD. Systematic review: Vitamin D and calcium supplementation in prevention of cardiovascular events. Ann Intern Med. 2010;152:315–323. doi: 10.7326/0003-4819-152-5-201003020-00010. [DOI] [PubMed] [Google Scholar]
- 10.Elamin MB, Elnour NO, Abu Elamin KB, Fatourechi MM, Alkatib AA, Almandoz JP, Liu H, Lane MA, Mullan RJ, Hazem A, Erwin PJ, Hensrud DD, Murad MH, Montori VM. Vitamin D and cardiovascular outcomes: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2011;96:1931–1942. doi: 10.1210/jc.2011-0398. [DOI] [PubMed] [Google Scholar]
- 11.Ford JA, MacLennan GS, Avenell A, Bolland M, Grey A, Witham M, RECORD Trial Group Cardiovascular disease and vitamin D supplementation: trial analysis, systematic review, and meta-analysis. Am J Clin Nutr. 2014;100:746–755. doi: 10.3945/ajcn.113.082602. [DOI] [PubMed] [Google Scholar]
- 12.Bjelakovic G, Gluud LL, Nikolova D, Whitfield K, Wetterslev J, Simonetti RG, Bjelakovic M, Gluud C. Vitamin D supplementation for prevention of mortality in adults. Cochrane Database Syst Rev. 2014:CD007470. doi: 10.1002/14651858.CD007470.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oh J, Weng S, Felton SK, Bhandare S, Riek A, Butler B, Proctor BM, Petty M, Chen Z, Schechtman KB, Bernal-Mizrachi L, Bernal-Mizrachi C. 1,25(OH)2 vitamin d inhibits foam cell formation and suppresses macrophage cholesterol uptake in patients with type 2 diabetes mellitus. Circulation. 2009;120:687–698. doi: 10.1161/CIRCULATIONAHA.109.856070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Riek AE, Oh J, Bernal-Mizrachi C. 1,25(OH)2 vitamin D suppresses macrophage migration and reverses atherogenic cholesterol metabolism in type 2 diabetic patients. J Steroid Biochem Mol Biol. 2013;136:309–312. doi: 10.1016/j.jsbmb.2012.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Riek AE, Oh J, Sprague JE, Timpson A, de las Fuentes L, Bernal-Mizrachi L, Schechtman KB, Bernal-Mizrachi C. Vitamin D suppression of endoplasmic reticulum stress promotes an antiatherogenic monocyte/macrophage phenotype in type 2 diabetic patients. J Biol Chem. 2012;287:38482–38494. doi: 10.1074/jbc.M112.386912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Riek AE, Oh J, Darwech I, Moynihan CE, Bruchas RR, Bernal-Mizrachi C. 25(OH) vitamin D suppresses macrophage adhesion and migration by downregulation of ER stress and scavenger receptor A1 in type 2 diabetes. J Steroid Biochem Mol Biol. 2014;144(Pt A):172–179. doi: 10.1016/j.jsbmb.2013.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.American Diabetes Association. Cardiovascular disease and risk management, sec. 8, In standards of medical care in diabetes-2016. Diabetes Care. 2016;39:S60–S71. doi: 10.2337/dc16-S011. [DOI] [PubMed] [Google Scholar]
- 18.Bligh EG, Dyer WJ. A Rapid Method of Total Lipid Extraction and Purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 19.Oh J, Riek AE, Darwech I, Funai K, Shao J, Chin K, Sierra OL, Carmeliet G, Ostlund RE, Bernal-Mizrachi C. Deletion of macrophage Vitamin D receptor promotes insulin resistance and monocyte cholesterol transport to accelerate atherosclerosis in mice. Cell Rep. 2015;10:1872–1886. doi: 10.1016/j.celrep.2015.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fernandez-Ruiz I, Puchalska P, Narasimhulu CA, Sengupta B, Parthasarathy S. Differential lipid metabolism in monocytes and macrophages: influence of cholesterol loading. J Lipid Res. 2016;57:574–586. doi: 10.1194/jlr.M062752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martinez FO, Gordon S, Locati M, Mantovani A. Transcriptional Profiling of the Human Monocyte-to-Macrophage Differentiation and Polarization: New Molecules and Patterns of Gene Expression. J Immunol. 2006;177:7303–7311. doi: 10.4049/jimmunol.177.10.7303. [DOI] [PubMed] [Google Scholar]
- 22.Spartano NL, Lamon-Fava S, Matthan NR, Ronxhi J, Greenberg AS, Obin MS, Lichtenstein AH. Regulation of ATP-binding Cassette Transporters and Cholesterol Efflux by Glucose in Primary Human Monocytes and Murine Bone Marrow-derived Macrophages. Exp Clin Endocrinol Diabetes. 2014;122:463–468. doi: 10.1055/s-0034-1374600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mandosi E, Fallarino M, Gatti A, Carnovale A, Rossetti M, Lococo E, Buchetti B, Filetti S, Lenti L, Morano S. Atorvastatin downregulates monocyte CD36 expression, nuclear NFkappaB and TNFalpha levels in type 2 diabetes. J Atheroscler Thromb. 2010;17:539–545. doi: 10.5551/jat.2956. [DOI] [PubMed] [Google Scholar]


