Abstract
Three new species of coccidians (Apicomplexa: Eimeriidae) are described from eastern moles, Scalopus aquaticus (Linnaeus) from Arkansas. Oőcysts of Cyclospora duszynskii n. sp. are subspheroidal with a smooth bi-layered wall, measure 11.4 × 10.0 μm, and have a length/width (L/W) ratio of 1.1; both micropyle and oöcyst residuum are absent, but a single polar granule is present. Sporocysts are ellipsoidal and measure 7.2 × 5.4 μm, L/W 1.3; an indistinct Stieda body is present, but the sub-Stieda and para-Stieda bodies are absent and the sporocyst residuum is composed of medium to large granules of different sizes along the edge of the sporocyst. Oőcysts of Cyclospora yatesi n. sp. are subspheroidal to ovoidal with an ornate outer wall, measure 17.0 × 15.2 μm, L/W 1.1; both micropyle and oöcyst residuum are absent, but a single polar granule is present. Sporocysts are ellipsoidal and measure 9.7 × 7.3 μm, L/W 1.3; an indistinct Stieda body is present, but sub-Stieda and para-Stieda bodies are absent and the sporocyst residuum is composed of medium to large granules of different sizes along the edge of the sporocyst. Oőcysts of Eimeria paulettefordae n. sp. are ovoidal to ellipsoidal with an ornate outer wall, measure 30.0 × 25.4 μm, L/W 1.2; both micropyle and oöcyst residuum are absent, but a single polar granule is present. Sporocysts are ellipsoidal and measure 12.6 × 9.2 μm, L/W 1.4; a button-like Stieda body is present, but sub-Stieda and para-Stieda bodies absent and the sporocyst residuum is composed of medium to large granules of different sizes along the edge of the sporocyst. These are the first coccidians described from Arkansas populations of S. aquaticus. In addition, a summary is provided on the cyclosporans and eimerians from North American talpids.
Introduction
The eastern mole, Scalopus aquaticus (L.) is a robust insectivore that ranges from the northeastern United States west to the Midwest as far as southeastern Wyoming and south through Texas to northern Tamaulipas, México; it is also found in eastern Canada (Yates & Schmidly, 1978; Schmidly, 1994; Reid, 2006). In Arkansas, S. aquaticus is found statewide where it inhabits burrows in suitable soils (Sealander & Heidt 1988).
Much is known about the natural history and ecology of S. aquaticus (Yates & Schmidly, 1978). There are six species of coccidians reported from this host, one Cyclospora Schneider, 1881, four Eimeria Schneider, 1875, and a single Isospora Schneider, 1881 (Duszynski & Upton, 2000). To date, however, nothing has been published on coccidia from S. aquaticus from Arkansas, USA. The present study provides descriptions of three new coccidians infecting S. aquaticus in the state.
Materials and methods
Between April 2013 and January 2017, seven adult S. aquaticus were collected from burrows using Victor® mole traps from Benton (n = 3) and Union (n = 3) counties, Arkansas, USA, and Latimer County (n = 1), Oklahoma. USA. Fresh fecal samples and rectal contents were placed in individual vials containing 2.5% (w/v) aqueous potassium dichromate (K2Cr2O7). Samples were examined for coccidia using an Olympus BX53 light microscope after flotation in Sheather’s sugar solution (specific gravity = 1.30). Measurements were taken on sporulated oőcysts from all infected hosts using Olympus© cellSens 1.14 digital software and reported in micrometers (μm) with the ranges followed by the means in parentheses; photographs were taken using Nomarski interference-contrast optics. Oőcysts were between c. 215–1,125 days old when measured and photographed. A voucher (alcoholic specimen) of S. aquaticus was accessioned into the Henderson State University (HSU) Mammal Collection, Arkadelphia, Arkansas, USA as HSU 706. Photosyntypes of sporulated oöcysts were accessioned into the Harold W. Manter Laboratory of Parasitology (HWML), Lincoln, Nebraska, USA. We follow Wilson and Reeder (2005) in the use of the ordinal name Soricomorpha Gregory for moles in North America.
Results
Three of seven (43%) S. aquaticus were found to be passing coccidian oöcysts of two cyclosporans and an eimerian. They are described here as new (Fig. 1–3 and Fig. 4–11)
Figs. 1–3.
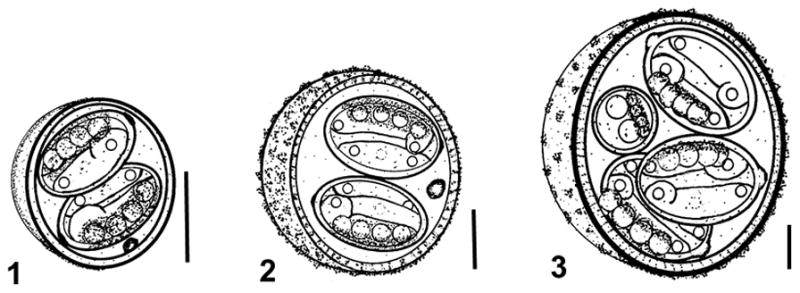
Schematic line drawings of new coccidians from Scalopus aquaticus. 1. Cyclospora duszynskii n. sp. 2. Cyclospora yatesi n. sp. Bar: 5 μm. 3. Eimeria paulettefordae n. sp. Bars: 5 μm.
Figs. 4–11.
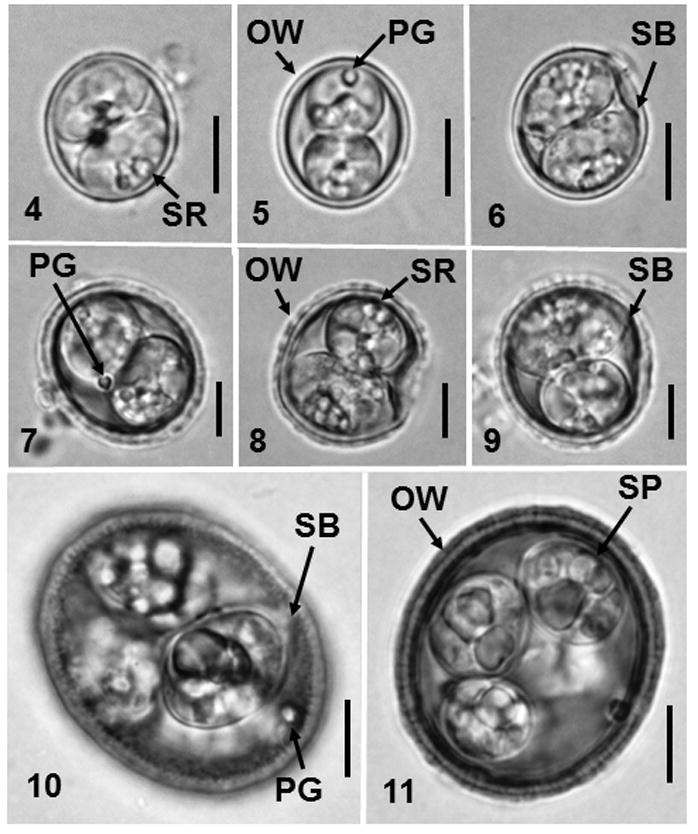
Nomarski-interference contrast microscopy of sporulated oöcysts of new coccidians from Scalopus aquaticus. 4–6. Cyclospora duszynskii. 7–9. Cyclospora yatesi n. sp. 10–11. Eimeria paulettefordae n. sp. (OW) oocyst wall; (PG) polar granule; (SR) sporocyst residuum; (SB) Stieda body; (SP) sporocyst. Bars: 5 μm.
Cyclospora duszynskii n. sp
Type-host: Eastern mole, Scalopus aquaticus (Linnaeus, 1758) (Mammalia, Soricomorpha, Talpidae), adult (not measured) collected 26.x.2016).
Type-locality: Bentonville, Benton County, Arkansas, USA (36° 21′ 58.2186″W, 94° 14′ 38.634″W).
Type-material: Photosyntypes of sporulated oocysts are deposited as HWML 139341.
Other hosts and localities: Two adult S. aquaticus (118 and 157 mm total length [TL]) collected on 7–8 × 2013 from El Dorado, Union County, Arkansas, USA (33° 13′ 0.5298″N, 92° 35′ 9.7548″W).
Prevalence: In 3 of 7 (43%) overall; in 1 of 3 (33%) Benton County, Arkansas, USA; in 2 of 3 (50%) Union County, Arkansas, USA.
Sporulation time: Unknown; oöcysts were sporulated when examined.
Site of infection: Unknown; oöcysts recovered from faeces.
ZooBank registration: To comply with the regulations set out in article 8.5 of the amended 2012 version of the International Code of Zoological Nomenclature (ICZN, 2012), details of the new species have been submitted to ZooBank. The Life Science Identifier (LSID) for Cyclospora duszynskii n. sp. is urn:lsid:- zoobank.org:pub:BEA07E8A-93C6-4730-819D-CB1306BC9DF6.
Etymology: The specific epithet is given in honor of Dr. Donald W. Duszynski (Emeritus Professor of Biology, University of New Mexico, Albuquerque, New Mexico, USA), Dean of American coccidiologists, for his many works on the coccidia of vertebrates, including moles.
Description
Sporulated oőcyst
Oőcyst (n = 90) subspheroidal; 10–12 × 9–11 (11.4 × 10), length/width (L/W) ratio 1.0–1.2 (1.1). Wall smooth, thin, bilayered, c.0.7, inner layer c.0.2, light brown outer layer c.0.5. Micropyle and oöcyst residuum absent, single polar granule present.
Sporocyst
Sporocyst (n = 175) two, ellipsoidal 6–10 × 4–7 (7.2 × 5.4); L/W ratio 1.0–1.6 (1.3); wall smooth, thin and uni-layered, light brown, c.0.2 thick. Indistinct Stieda body present, sub-Stieda and para-Stieda bodies absent; sporocyst residuum medium to large granules of different sizes along edge of sporocyst.
Sporozoites
Sporozoites (two, not measured), banana-shaped; with a spheroidal anterior and posterior refractile bodies, nucleus difficult to discern.
Remarks
We limit the comparison of our new taxon only to those cyclosporan species from North American moles of the family Talpidae Fischer (Table 1, also see Duszynski & Upton, 2000). None of the five Cyclospora spp. from moles possess oöcysts as minute as the new species. There is a single previously described species from S. aquaticus (hosts from Texas, USA), Cyclospora megacephali Ford & Duszynski, 1988 which is similar but the subspheroidal oöcysts are larger and measure 18.9 × 15.7 (L/W ratio = 1.2), the sporocysts are larger, 15.0 × 7.2 (L/W of 2.1), and there is a large cap-like SB; oöcysts of Cyclospora ashtabulensis Ford & Duszynski, 1989 from hairy-tailed mole, Parascalops breweri (Bachman) from Ohio, USA, are also larger and measure 18.0 × 14.3 and also do not possess a PG which is present in the new species; Cyclospora parascalopi Ford & Duszynski, 1989 from P. breweri from Massachusetts and Ohio has larger oocysts measuring 16.5 × 13.6 (L/W = 1.2), without a PG; both of these latter species from P. breweri also have a prominent cap-like SB which is not present in the new species (Ford & Duszynski, 1988, 1989). There are also two species of Cyclospora from European moles, Talpa europaea Linnaeus, namely, Cyclospora caryolytica Schaudinn, 1902 from Bulgaria, Germany, and Italy, and Cyclospora talpae Pellérdy & Tanyi, 1968 from Austria, Bulgaria, England, and Hungary (see Duszynski & Upton, 2000). However, neither of these taxa is similar to the new species and, more importantly, they are also widely separated geographically from hosts in the Western Hemisphere.
Table 1.
Comparison of the sporulated oöcysts of Cyclospora and Eimeria spp. from North American moles (Talpidae).
| Cyclospora or. Eimeria spp. | Type host (current name) Type locality (others) | Oöcyst shape, size, features*† | Sporocyst shape, size, features*† | References |
|---|---|---|---|---|
| C. ashtabulensis |
Parascalops breweri (Bachman) Ohio, USA |
Subpheroidal to ellipsoidal 18.0 × 14.3; L/W 1.3 14–23 × 11–19 M, OR, PG: all − |
Ovoidal 11.6 × 7.2; L/W 1.6 8–14 × 5–9 SB, SR: both + SSB, PSB: both − |
Ford & Duszynski (1989) |
| C. duszynskii |
Scalopus aquaticus (Linnaeus) Arkansas, USA |
Subpheroidal 11.0 × 9.7; L/W 1.1 10–12 × 9–11 M, OR: both − PG: + |
Ellipsoidal 7.2× 5.4; L/W 1.3 6–10 × 4–7 SB, SR: both + SSB, PSB: − |
This report |
| C. megacephali |
S. aquaticus Texas, USA |
Subspheroidal 18.9 × 15.7; L/W 1.2 14–21 × 12–18 M, OR: both − PG: + |
Ellipsoidal 15.0 × 7.2; L/W 2.1 11–17 × 6–9 SB, SR: both + SSB, PSB: both − |
Ford & Duszynski (1988) |
| C. parascalopi |
P. breweri Ohio, USA (Massachusetts, USA) |
Ellipsoidal 16.5 × 13.6; L/W 1.2 13–20 × 11–20 M, OR, PG: all − |
Ovoidal 11.1 × 6.9; L/W 1.6 8–14 × 5–8 SB, SR: both + SSB, PSB: both − |
Ford & Duszynski (1989) |
| C. yatesi |
S. aquaticus Arkansas, USA |
Subspheroidal to ovoidal 17.0 × 15.2; L/W 1.1 12–18 × 10–17 M, OR: both − PG: + |
Ellipsoidal 9.7 × 7.3; L/W 1.3 6–12 × 5–10 SB, SR: both + SSB, PSB: both − |
This report |
| E. aethiospora |
P. breweri Ohio, USA (Massachusetts, USA) |
Subspheroidal to Ellipsoidal 19.3 × 13.0; L/W 1.5 15–24 × 10–16 M, OR: both − PG: + |
Spindle-shaped 10.6 × 5.7; L/W 1.9 8–13 × 4–7 SB, SSB, SR: all + PSB: − |
Ford & Duszynski (1989) |
| E. aquatici |
S. aquaticus Texas, USA |
Ellipsoidal 17.0 × 10.6; L/W 1.6 14–20 × 9–14 M, OR: both − PG: + |
Elongate-ovoidal 9.0 × 5.2; L/W 1.8 SB, SSB, SR: all − PSB: − |
Ford & Duszynski (1988) |
| E. condylurae |
Condylura cristata (Linnaeus) Vermont, USA (Ohio, USA) |
Subspheroidal 17.7 × 15.7; L/W 1.1 17–23 × 14–21 M: − OR, PG: + |
Elongate-ellipsoidal 11.7 × 5.6; L/W 2.1 11–14 × 5–6 SB, SSB, SR: all + PSB: − |
Duszynski (1989) |
| E. heterocapita |
Neurotrichus gibbsii (Baird) Washington, USA |
Subspheroidal to ellipsoidal 25.5 × 21.4; L/W 1.2 23–27 × 18–23 M, OR, PG: all − |
Ovoidal 13.6 × 10.0; L/W 1.3 12–15 × 9–11 SB, SSB, PSB: all + SR: + |
Duszynski (1985) |
| E. motleiensis |
S. aquaticus Texas, USA |
Subspheroidal 17.0 × 15.3; L/W 1.1 15–19 × 13–19 M, PG: both − OR: + |
Ovoidal 10.7 × 6.8; L/W 1.6 10–13 × 6–8 SB, SSB, SR: all + PSB: − |
Ford & Duszynski (1988) |
| E. neurotrichi |
N. gibbsi Oregon, USA (Washington, USA) |
Ovoidal 17.6 × 13.6; L/W 1.3 16–20 × 11–16 M, OR: both − PG: + |
Ovoidal 10.7 × 5.5; L/W 2.0 9–12 × 5–6 SB, SR: both + SSB, PSB: both − |
Duszynski (1985) |
| E. parastiedica |
N. gibbsi Washington, USA |
Spheroidal to Subspheroidal 27.4 × 25.5; L/W 1.1 25–30 × 22–28 M, OR, PG: all − |
Ovoidal to football-shaped 18.3 × 10.4; L/W 1.8 16–20 × 9–11 SB, SSB, PSB, SR: all + |
Duszynski (1985) |
| E. paulettefordae |
S. aquaticus Arkansas, USA |
Ovoidal to ellipsoidal 30.0 × 25.4; L/W 1.2 28–32 × 24–27 M, OR: both − PG: + |
Ellipsoidal 12.6 × 9.2; L/W 1.4 9–17 × 8–11 SB, SR: both + SSB, PSB: both − |
This report |
| E. scalopi |
S. aquaticus Texas, USA |
Spheroidal to subspheroidal 13.6 × 12.6; L/W 1.1 11–17 × 11–15 M, PG: both − OR: + |
Lemon-shaped 8.7 × 5.5; L/W 1.6 7–10 × 4–7 SB, SR: both + SSB, PSB: both − |
Ford & Duszynski (1988) |
| E. scapani |
Scapanus latimanus (Bachman) California, USA |
Subspheroidal 19.2 × 16.0; L/W 1.2 16–22 × 14–16 M, OR: both − PG: + (small granules |
Ovoidal L × W (not given)‡ L/W ratio (not given)‡ SB, SR: both + SSB, PSB: both − |
Henry (1932); Duszynski & Upton (2000) |
| E. titthus |
P. breweri Ohio, USA (Massachusetts, USA) |
Subspheroidal 15.8 × 13.5; L/W 1.2 13–19 × 11–17 M, PG: both − OR: + |
Ellipsoidal 11.2 × 5.8; L/W 1.9 9–13 × 4–7 SB, SSB, SR: all + PSB: − |
Ford & Duszynski (1989) |
Measurements in μm.
Descriptions of oöcysts and sporocysts follow guidelines of Wilber et al. (1998) as follows: oöcyst length (L) and width (W), their ranges and ratios (L/W), micropyle (M), oöcyst residuum (OR), polar granule(s) (PG), sporocyst (SP) length (L) and width (W), their ratio (L/W), Stieda body (SB), sub-stieda body (SSB), para-Stieda body (PSB), and sporocyst residuum (SR).
Oöcysts were inadequately described by Henry (1932); see Duszynski & Upton (2000).
Based on the differences in morphological and morphometric characteristics noted above, C. duszynskii is considered a species new to science. Moreover, to date, it is the smallest cyclosporan reported from moles as well as the first species from a mole in Arkansas and the second species known from S. aquaticus.
Cyclospora yatesi n. sp
Type-host: Eastern mole, Scalopus aquaticus (Linnaeus, 1758) (Mammalia, Soricomorpha, Talpidae), adult collected 8.x.2013).
Type-locality: El Dorado, Union County, Arkansas, USA (33° 13′ 0.5298″N, 92° 35′ 9.7548″W).
Type-material: Photosyntypes of sporulated oocysts are deposited as HWML 139342.
Prevalence: In 3 of 7 (43%) overall; in 2 of 3 (67%) Union County, Arkansas, USA.
Sporulation time: Unknown; oöcysts were sporulated when examined.
Site of infection: Unknown; oöcysts recovered from faeces.
ZooBank registration: To comply with the regulations set out in article 8.5 of the amended 2012 version of the International Code of Zoological Nomenclature (ICZN, 2012), details of the new species have been submitted to ZooBank. The Life Science Identifier (LSID) for Cyclospora yatesi n. sp. is urn:lsid: - zoobank.org:pub:29DEFBA7-4ECA-4402-9E23-A2CAAF53E38A.
Etymology: The specific epithet is given in honor of the late Dr. Terry L. Yates (1950–2007) (University of New Mexico, Albuquerque, New Mexico, USA), renowned mammalogist and mole expert who is credited with discovering the source of the hantavirus in the American Southwest in 1993.
Description
Sporulated oőcyst
Oőcyst (n = 53) subspheroidal to ovoidal; 12–18 × 10–17 (17.0 × 15.2), length/width (L/W) ratio 1.0–1.2 (1.1). Wall ornate, bilayered, c.1.3, thick, inner layer c.0.7, yellowish-brown outer layer c.0.6. Micropyle and oöcyst residuum absent, single polar granule present.
Sporocyst
Sporocyst (n =106) two, ellipsoidal 6–12 × 5–10 (9.7 × 7.3); L/W ratio 0.7–1.9 (1.3); wall smooth, uni-layered, colourless, c.0.2 thick. Indistinct Stieda body present, sub-Stieda and para-Stieda bodies absent; sporocyst residuum medium to large granules of different sizes along edge of sporocyst.
Sporozoites
Sporozoites (two, not measured), banana-shaped; with a spheroidal anterior and posterior refractile bodies, nucleus difficult to discern.
Remarks
As noted above, we limit our comparison of our new taxon only to those cyclosporan species from North American moles of the family Talpidae (Table 1). The new taxon possesses oöcysts with sculptured outer walls that are similar in size and wall morphology to two previously described cyclosporans from P. breweri. Cyclospora ashtabulensis and C. parascalopi both have sculptured outer walls on their oöcysts but they also have very different sporocysts with crescent-shaped caps or thick cap-like coverings on their SBs that the new species doesn’t possess. The only cyclosporan from S. aquaticus, C. megacephali has a smooth outer wall.
Based on the differences in morphological and morphometric characteristics noted above, C. yatesi is considered a species new to science. It also represents the third cyclosporan from S. aquaticus as well as another species from an Arkansas mole.
Eimeria paulettefordae n. sp
Type-host: Eastern mole, Scalopus aquaticus (Linnaeus, 1758) (Mammalia, Soricomorpha, Talpidae), adult collected 8.x.2013).
Type-locality: El Dorado, Union County, Arkansas, USA (33° 13′ 0.5298″N, 92° 35′ 9.7548″W).
Type-material: Photosyntypes of sporulated oocysts are deposited as HWML 139343.
Prevalence: In 1 of 7 (14%) overall; in 1 of 3 (33%) Union County, Arkansas, USA.
Sporulation time: Unknown; oöcysts were sporulated when examined.
Site of infection: Unknown; oöcysts recovered from faeces.
ZooBank registration: To comply with the regulations set out in article 8.5 of the amended 2012 version of the International Code of Zoological Nomenclature (ICZN, 2012), details of the new species have been submitted to ZooBank. The Life Science Identifier (LSID) for Eimeria paulettefordae n. sp. is urn:lsid: - zoobank.org:pub:B7D97D61-66EB-47FC-A128-6982816EEB05.
Etymology: The specific epithet is given in honor of Dr. Paulette L. Ford (Rocky Mountain Station, U.S. Forest Service, Albuquerque, New Mexico, USA), who described (with D.W. Duszynski) several species of coccidians in moles of the world.
Description
Sporulated oőcyst
Oőcyst (n = 6) ovoidal to ellipsoidal; 28–32 × 24–27 (30.0 × 25.4), length/width (L/W) ratio 1.1–1.3 (1.2). Wall ornate, bilayered, c.1.7, thick, dark inner layer c.0.9, yellowish-brown outer layer c.0.8. Micropyle and oöcyst residuum absent, single polar granule present.
Sporocyst
Sporocyst (n =21) two, ellipsoidal 9–17 × 8–11 (12.6 × 9.2); L/W ratio 1.1–1.6 (1.4); wall smooth, uni-layered, colourless, c.0.2 thick. Button-like Stieda body present, sub-Stieda and para-Stieda bodies absent; sporocyst residuum medium to large granules of different sizes along edge of sporocyst.
Sporozoites
Sporozoites (two, not measured) two, banana-shaped; with a spheroidal anterior and posterior refractile bodies, nucleus difficult to discern.
Remarks
We limit comparison of our new taxon only to those eimerian species from North American moles of the family Talpidae (Table 1). Of the three eimerians previously known from S. aquaticus, namely E. aquatici Ford & Duszynski, 1988, E. motleiensis Ford & Duszynski, 1988 and E. scalopi Ford & Duszynski 1988, none possess oöcysts that are as large (17.0 × 10.6, 17.0 × 15.3, and 13.6 × 12.6, respectively) as the new species (30.0 × 25.4). In addition, other eimerians from North American moles, including E. condylurae Duszynski, 1989 from star-nosed mole, Condylura cristata (Linnaeus) from Ohio and Vermont, E. parastiedica Duszynski, 1985 from American shrew mole, Neurotrichus gibbsii (Baird) from Washington, E. aethiospora Ford & Duszynski, 1989 and E. titthus Ford & Duszynski, 1989 from P. breweri from Ohio, and E. scapani Henry, 1932 from broad-footed mole, Scapanus latimanus (Bachman) from California, all have oöcysts that are also considerably smaller (Table 1; Duszynski & Upton, 2000). Only two species possess oöcysts from North American talpids, E. heterocapita Duszynski, 1985 and E. parastiedica from N. gibbsii, that are similar (but still smaller) in size to the new species (25.5 × 21.4 and 27.4 × 25.5). However, oöcysts of E. heterocapita possesses an amicropylar cap at one end and E. parastiedica has a prominent SB, SSB, and PSB as distinctive features of the sporocyst (Duszynski & Upton, 2000), which the new species doesn’t possess.
Based on the differences in morphological and morphometric characteristics noted above, E. paulettefordae is considered a species new to science. It also represents the fourth eimerian from S. aquaticus and, to date, the largest coccidian known from any North American mole.
Discussion
Duszynski & Upton (2000) summarized the coccidians from soricomorphs of the world. Wilson and Reeder (2005) recognized seven species of moles (Talpidae) within five genera in North America. Although five species have been reported to harbor coccidia (see Duszynski & Upton, 2000), the coast mole, Scapanus orarius (True) which ranges from northern California through Oregon and Washington to Central Idaho, and Townsend’s mole, Scapanus townsendii (Bachman) which occupies a limited range along the west coast of Washington, Oregon and into Northern California (Reid, 2006) still remain to be surveyed. Future surveys will likely increase our knowledge of mole coccidians by reporting new host and geographic distributional records, as well as including the possibility of discovering additional species new to science.
Acknowledgments
We thank Drs. Renn Tumlison (HSU) and Scott L. Gardner and Gabor R. Racz (HWML) for expert curatorial assistance, and R. Scott Seville (Univ. Wyoming) for a pre-review of the ms. Scientific Collecting Permits were provided to CTM by the Arkansas Game and Fish Commission (permit number 031120151). Matthew B. Connior (NW Arkansas Community College, Bentonville, Arkansas) provided some of the samples used in this study.
Funding This study was supported, in part, by a grant from the National Institute of General Medical Sciences (2P20GM103432), National Institutes of Health (NIH) to R.S. Seville. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Conflict of interest The authors declare that they have no conflict of interest.
Compliance with ethical standards
Ethical approval All applicable institutional, national and international guidelines for the care and use of animals were followed.
Contributor Information
Chris T. McAllister, Science and Mathematics Division, Eastern Oklahoma State College, Idabel, Oklahoma 74745, USA
Dagmara Motriuk-Smith, Department of Zoology and Physiology, University of Wyoming, Casper, Wyoming 82601, USA.
Catherine M. Kerr, Department of Zoology and Physiology, University of Wyoming, Casper, Wyoming 82601, USA
References
- Duszynski DW. Coccidian parasites (Apicomplexa: Eimeriidae) from insectivores: New species from shrew moles (Talpidae) in the United States. Journal of Protozoology. 1985;32:577–580. doi: 10.1111/j.1550-7408.1985.tb03080.x. [DOI] [PubMed] [Google Scholar]
- Duszynski DW. Coccidian parasites (Apicomplexa: Eimeriidae) from insectivores. VIII. Four new species from the star-nosed mole, Condylura cristata. Journal of Parasitology. 1989;75:514–518. [PubMed] [Google Scholar]
- Duszynski DW, Upton SJ. Coccidia (Apicomplexa: Eimeriidae) of the mammalian order Insectivora. Special Publication of the Museum of Southwestern Biology. 2000;4:1–67. [Google Scholar]
- Ford PL, Duszynski DW. Coccidian parasites (Apicomplexa: Eimeriidae) from insectivores. VI. Six new species from the eastern mole, Scalopus aquaticus. Journal of Protozoology. 1988;35:223–226. doi: 10.1111/j.1550-7408.1988.tb04328.x. [DOI] [PubMed] [Google Scholar]
- Ford PL, Duszynski DW. Coccidian parasites (Apicomplexa: Eimeriidae) from insectivores. VII. Six new species from the hairy-tailed mole, Parascalops breweri. Journal of Parasitology. 1989;75:503–513. [PubMed] [Google Scholar]
- Henry DP. Observations on the coccidia of small mammals in California, with descriptions of seven species. University of California Publications in Zoology. 1932;37:279–290. [Google Scholar]
- ICZN. International Commission on Zoological Nomenclature: Amendment of articles 8, 9, 10, 21 and 78 of the International Code of Zoological Nomenclature to expand and refine methods of publication. Zootaxa. 2012;3450:1–7. doi: 10.3897/zookeys.219.3994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid FA. Mammals of North America. Boston: Houghton Mifflin Company; 2006. p. 579. [Google Scholar]
- Schmidly DJ. The mammals of Texas. Austin: University of Texas Press; 1994. p. 501. [Google Scholar]
- Sealander JA, Heidt GA. Arkansas mammals: Their natural history, classification, and distribution. Fayetteville: University of Arkansas Press; 1988. p. 308. [Google Scholar]
- Wilber PG, Duszynski DW, Upton SJ, Seville RS, Corliss JO. A revision of the taxonomy and nomenclature of the Eimeria spp. (Apicomplexa: Eimeriidae) from rodents in the Tribe Marmotini (Sciuridae) Systematic Parasitology. 1998;39:113–135. [Google Scholar]
- Wilson DE, Reeder DM, editors. Mammal species of the world: A taxonomic and geographic reference. Vol. 2. Baltimore: Johns Hopkins University Press; 2005. p. 142. [Google Scholar]
- Yates TL, Schmidly DJ. Scalopus aquaticus. Mammalian Species. 1978;105:1–4. [Google Scholar]


