Abstract
Two distinct types of thermogenic fat cells, brown adipocytes and beige adipocytes, play a key role in the regulation of systemic energy homeostasis in mammals. Both brown fat and beige fat possess thermogenic properties in addition to common morphological and biochemical characteristics, including multilocular lipid droplets and cristae-dense mitochondria. Recent studies also identify distinct features between the two types of thermogenic fat cells, such as their developmental regulation and function. Of particular interest is the role of beige fat in the regulation of glucose homeostasis via UCP1-independent mechanisms. A better understanding of the underlying causes of these characteristics of brown and beige fat will allow us to specifically manipulate these cells to improve systemic energy metabolism and glucose homeostasis.
Keywords: Obesity, diabetes, brown adipocytes, beige adipocytes, UCP1
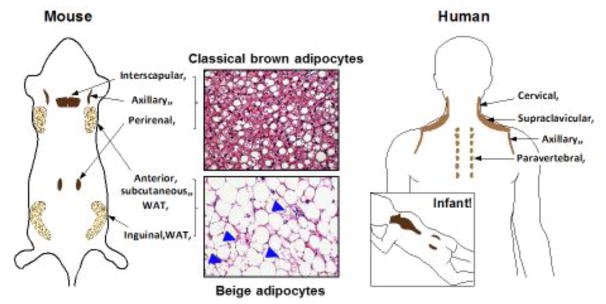
Adipose tissue is a dynamic organ, which can range from 4% to over 40% of total body composition in adult humans. Fat is often noted for its energy-storing function as in white adipose tissue (WAT); however, mammals also possess brown adipose tissue (BAT) that plays a critical role in whole-body energy homeostasis through non-shivering thermogenesis. Emerging evidence suggests that mammals possess, at least, two types of thermogenic adipocytes: classical brown adipocytes and beige adipocytes. In rodents and infants, classical brown adipocytes (or brown fat) are located in the dedicated BAT depots, such as the interscapular regions and around the kidney (perirenal BAT) (Fig. 1). On the other hand, beige adipocytes (also called brite adipocytes) are an inducible form of thermogenic adipocytes that sporadically reside within WAT depots. Similar to brown adipocytes, beige adipocytes also possess abundant cristae-dense mitochondria that express UCP1 (uncoupling protein 1) and multi-locular lipid droplets [1]. However, beige adipocyte biogenesis in WAT is strongly induced in response to certain environmental conditions and external cues, including chronic cold acclimation, exercise, long-term treatment with PPARγ agonists or β3-adrenergic receptor (AR) agonist, cancer cachexia, and tissue injury. This phenomenon is often referred to as the “browning” or “beigeing” of white fat (Fig. 1). While the browning of white fat following cold acclimation was described over 30 years ago [2, 3], recent studies have dissected the molecular regulators and developmental lineages of this browning process [4, 5].
Figure 1. Anatomical locations of thermogenic fat in mice and humans.
Classical brown adipocytes reside in dedicated BAT depots, including interscapular, axillary, and perirenal BAT depots in mice and infants. Beige adipocytes sporadically reside in subcutaneous WAT depots, such as the inguinal and anterior subcutaneous WAT in mice (arrowheads indicate the multilocular beige adipocytes). In adult humans, BAT exist in multiple locations, such as cervical, supraclaviscular, axillary, paravertebral, and abdominal subcutaneous regions. UCP1-positive adipocytes from the supraclavicular region show a molecular signature resembling mouse beige adipocytes, whereas the deep neck regions contain thermogenic fat that resembles classical brown adipocytes in mice.
Beige fat has recently caught mainstream attention for two reasons: the first being that its developmental processes serves as a unique model to better understand how environmental factors control cell fate specification and maintenance. The second reason lies in its relevance to adult humans and its promise as a new therapeutic target in the treatment of obesity and other metabolic disorders. Since the discovery of the brown adipose organ in adult humans [6–10], the prevalence of beige adipocytes in adult humans has been a major debate. The transcriptional analyses in adult human BAT depots demonstrated that adult human BAT expresses molecular markers selective to beige adipocytes in mice [11–13], while the deep neck regions contain thermogenic fat that resembles classical brown fat in mice [14]. Notably, isolation and characterization of clonally-derived beige-like adipocytes from adult humans provide the definitive evidence that beige adipocytes exist in humans [15]. Moreover, chronic cold acclimation promotes the recruitment of new thermogenic fat even in adults who do not possess detectable levels of pre-existing brown fat before cold acclimation, based on glucose uptake assessed by 18F-labeled fluorodeoxyglucose positron emission tomography/computerized tomography (18F-FDG-PET/CT). In parallel with the cold-induced recruitment of new thermogenic adipose tissue, these studies observed a significant increase in non-shivering thermogenesis and/or an improvement in insulin sensitivity [16–19]. A recent study with a refined 18F-FDG-PET/CT imaging further identified a much wider distribution of 18F-FDG PET-positive adipocytes than previously estimated (six locations, including abdominal subcutaneous regions) in adult humans [20]. Together, these results suggest that adult humans also possess the inducible beige-like thermogenic adipocytes that significantly contribute to the regulation of systemic energy homeostasis.
While brown adipocytes and beige adipocytes share many morphological and biochemical characteristics, recent studies suggest discrete characteristics between the two cell types. This review aims to discuss the unique features of each subset of thermogenic adipocytes and their biological significance in the regulation of whole-body energy homeostasis.
Developmental timing and lineages of two thermogenic adipocytes
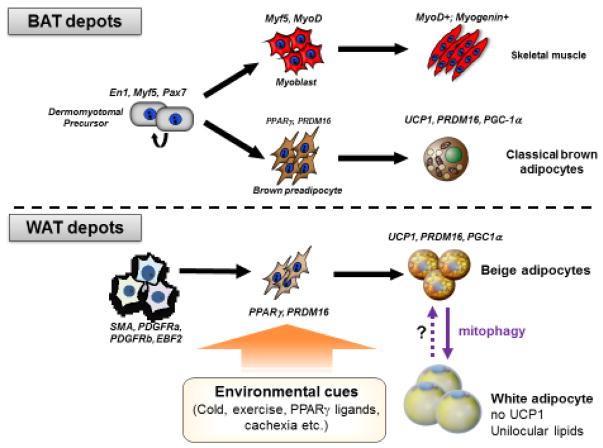
Embryonic BAT development precedes WAT formation during embryogenesis. From a viewpoint of thermogenesis, this is compatible with our understanding of a primary function of BAT in non-shivering thermogenesis following birth, given that newborns need to maintain their body temperature by non-shivering thermogenesis [21]. Lineage tracing studies demonstrated that classical brown adipocytes in the dedicated BAT depots originate from a subpopulation of dermomyotomes marked by the expression of certain transcription factors, including Pax7, Engrailed-1 (En1), and Myf5 [22–25] and that the somite vs. brown preadipocyte divergence occur during the embryonic day (E) 9.5–11.5 in mouse embryos [23] (Fig. 2, upper panel). Early B cell factor 2 (EBF2) is expressed in brown adipocyte precursors that are distinct from the somite population expressing MyoD, downstream of Myf5, within the mouse embryonic somites at E12.5 [26], while MyoD-expressing somites do not give rise to brown adipocytes [27]. Hence, the somite vs. brown adipocyte fate determination likely occurs before the expression of MyoD. Readers can find detailed information regarding the regulatory circuits of brown adipose cell fate determination in recent reviews [5, 28]. Of note, genetic loss of PRDM16 (PR domain containing protein 16) or Myf5-positive precursor-specific deletion of EHMT1 (histone N-methyltransferase), a critical enzymatic co-regulator of PRDM16, halts brown adipocyte differentiation and promotes ectopic activation of the muscle-selective gene program [25, 29], whereas mature adipocyte-specific deletion of PRDM16 (by Adiponectin-Cre) does not affect BAT development [30]. These results indicate that the fate commitment to brown adipocytes vs. somites completes during the precursor stage and that this process seems irreversible after the commitment phase in vivo.
Figure 2. Developmental lineages of thermogenic adipocytes.
Classical brown adipocytes differentiate during embryonic development from a subset of dermomytomal precursors that express En1, Myf5, and Pax7. Development of beige adipocytes in subcutaneous WAT depots is stimulated by enviornmental, such as chronic cold acclimation, exercise, PPARg ligands, tissue injury, and cancer cachexia. When the external stimuli are withdrawn, the recruited beige adipocytes directly acquire a white fat phenotype with unilocular lipid droplets and no UCP1 expression.
On the other hand, beige adipocytes emerge in WAT depots of adults although its developmental origin and cellular heterogeneity appear more multivariable than that of brown adipocytes (Fig. 2, bottom panel). Earlier studies demonstrated that UCP1 expression in the BAT (i.e., brown adipocytes) and the retroperitoneal WAT (i.e., beige adipocytes) were differentially regulated and thus, the developmental mechanisms for beige adipocyte biogenesis are distinct from that of brown adipocytes [31]. To support this notion, lineage tracing studies demonstrated that beige adipocytes are derived from progenitors expressing Sma, Myh11, Pdgfra, or Pdgfrb, in mice [32–35]. Intriguingly, some beige adipocytes, such as ones residing in the anterior-subcutaneous, retroperitoneal, and posterior-subcutaneous WAT, are derived from progenitors expressing Pax3 and/or Myf5 [27]. Furthermore, roscovitine, a pharmacological inhibitor of CDK5, promotes the development of UCP1-positive adipocytes that display a distinct molecular signature from conventional beige adipocytes [36]. Hence, it is conceivable that beige adipocytes are quite heterogeneous in some WAT depots and that multiple subtypes of beige adipocytes emerge depending on the nature of external stimuli. Notably, clonal analyses of adipogenic precursor cells demonstrate that beige fat precursors and white fat precursors are already discrete with distinctive molecular profiles in mice [12] and adult humans [15, 37], although only a few molecular markers are commonly enriched in mouse and human precursors. Future studies will determine the molecular basis of adipose cell heterogeneity that may shed light on the functionally distinct subtypes of adipocytes in mice and humans.
Cellular maintenance of beige adipocytes
A notable feature of beige adipocytes is their inducible and reversible thermogenic capacity in response to environmental stimuli. In response to chronic cold exposure, beige adipocyte differentiation is stimulated primarily from de novo differentiation of precursor cells [34, 38]. It is worth noting, however, that several studies proposed the possibility of trans-differentiation from differentiated white adipocytes into beige adipocytes: besides careful morphological studies [39, 40], a lineage tracing study using adiponectin-CreERT2 demonstrated that most of the newly recruited beige adipocytes in the inguinal WAT were derived from unilocular adipocytes [41]. This view still requires a cautious assessment because of the current technical limitations, including the facts that no specific marker for differentiated white adipocytes is available (note that adiponectin is expressed differentiated brown, beige, and white adipocytes) and that tamoxifen remains persistent within the adipose tissue even following a prolonged washout period [42].
Regardless of the developmental origin of newly formed beige adipocytes, the cold-induced beige adipocytes are gradually replaced by UCP1-negative adipocytes with unilocular lipid droplets (i.e., white adipocytes) when cold-acclimated mice are returned to room temperature or thermoneutrality [43]. Recent studies demonstrated that cold-induced UCP1-positive beige adipocytes directly revert to unilocular white adipocytes within approximately two weeks following re-warming or withdrawal of the β3-AR agonist [44, 45]. Furthermore, isolated beige adipocytes under culture directly acquire a white fat phenotype (i.e., unilocular lipid droplets and loss of UCP1 expression) within ten days following withdrawal of β3-AR agonist bypassing an intermediate precursor stage [45]. On the other hand, classical brown adipocytes retain their multilocular lipid morphology for up to 10 days under the same culture condition [45]. Intriguingly, beige adipocytes in the inguinal WAT of obese mice acquired a white fat state much faster than lean mice, while brown adipocytes retained high UCP1 expression both in obese and lean mice. These results indicate that the beige adipocyte state is distinctly transient and that there is a cell-intrinsic difference between beige adipocytes and brown adipocytes in maintaining their multilocular morphology in the absence of external stimuli. The distinct maintenance mechanism between brown and beige adipocytes may be attributed, in part, to the fact that mitochondrial biogenesis is constitutively active in brown adipocytes, whereas mitochondrial clearance plays a dominant role in beige fat maintenance because mitochondrial biogenesis in beige fat is quickly inactivated to a basal level following the withdrawal of external stimuli.
Mechanistically, the beige-to-white adipocyte conversion is tightly coupled to a striking decrease in mitochondrial contents and increased mitophagy – a selective form of autophagy for mitochondrial degradation. We reported that blockade of the autophagy pathway by UCP1+ adipocyte-specific deletion of Atg5 or Atg12 prevents the beige-to-white adipocyte conversion even after the withdrawal of external stimuli. Importantly, prolonged retention of thermogenic beige adipocytes by the blockade of autophagy maintains high whole-body energy expenditure and protects mice from diet-induced obesity [45]. These observations are intriguing because not only is autophagy dysregulated in the adipose tissues of obese and type 2 diabetes patients [46–50], beige-to-white adipocyte conversion is also accelerated under an obese state [45]. Accordingly, prolonged retention of beige adipocytes could be an effective strategy, particularly when combined with the browning agents to maintain energy expenditure in obese and diabetic populations.
How is mitochondrial clearance by mitophagy initiated during the beige-to-white adipocyte conversion? It has been well known that “damaged” mitochondria, i.e., a reduction in the membrane potential, triggers mitophagy [51, 52]. However, it remains poorly understood as to how mitochondrial homeostasis in thermogenic adipocytes is regulated, given the fact that mitochondria in thermogenic adipocytes is uncoupled by UCP1 when activated. We recently found that mitophagy in beige adipocytes is regulated by β3-adrenoceptor (β3-AR) signaling at the level of Parkin protein recruitment to the mitochondria, while UCP1-mediated proton uncoupling is dispensable for mitophagy activation and beige fat maintenance (Xu et al. under review). Specifically, Parkin protein recruitment to the mitochondria is highly repressed by β3-AR-induced PKA signaling pathway independent of UCP1, while withdrawal of β3-AR stimuli triggers Parkin recruitment to the mitochondria and mitophagy. In turn, genetic deletion of Parkin potently prevents mitochondrial degradation in beige fat, thereby leading to a prolonged retention of thermogenic beige adipocytes even after the withdrawal of external stimuli. The molecular basis of cell-type specific mitochondrial homeostasis is an intriguing question yet remains unexplored; novel methodologies to accurately monitor mitochondrial biogenesis and clearance in vivo would provide new insights into the cell-type regulation in physiology and disease.
Non-canonical (UCP1-independent) mechanisms in thermogenic fat
UCP1 dissipates energy in the form of heat by uncoupling the mitochondrial proton gradient from mitochondrial ATP synthesis. UCP1 has been considered to be the only thermogenic protein (often referred to as the “thermogenin”) responsible for non-shivering adipose thermogenesis [53, 54]. Thus, the prevailing consensus has been that the action of UCP1 primarily mediates the “anti-obesity” and “anti-diabetic” actions of brown and beige fat. However, genetic studies in rodent models suggest an unexpected incongruity in the metabolic phenotypes between brown/beige fat-deficient mice and UCP1-deficient mice. For example, BAT-deficient mice, caused by the transgenic expression diphtheria toxin (DTA) by the Ucp1 gene enhancer/promoter, exhibit obese and diabetic phenotypes under ambient temperature [55]. Similarly, beige fat-deficient mice, caused by the adipocyte-specific deletion of PRDM16 or EHMT1, develop obesity and systemic insulin resistance even under ambient temperature [29, 30]. In contrast, Ucp1KO mice are not diabetic and display an obese phenotype only when mice are kept under thermoneutrality [56, 57]. This apparent contradiction in the metabolic phenotypes between these mouse models implies the existence of UCP1-independent mechanisms by which brown and/or beige fat contributes to the regulation of whole-body energy homeostasis.
In fact, it has been reported that the inguinal WAT of cold-acclimated Ucp1−/− mice exhibited higher respiration than wild-type mice and Ucp1−/− mice under thermoneutrality [58]. In addition, chronic treatment with β3-AR agonist increases respiration of the epididymal WAT of Ucp1−/− mice [59]. Furthermore, a creatine-driven substrate cycle controls brown fat and beige fat thermogenesis [60–62]. Our recent study identified a distinct UCP1-independent thermogenic mechanism in beige adipocytes that involves ATP-dependent Ca2+ cycling by Sarco/endoplasmic reticulum Ca2+-ATPase 2b (SERCA2b) and ryanodine receptor 2 (RyR2) [63]. Of note, this Ca2+ cycling mechanism is required for beige adipocyte thermogenesis, whereas it is dispensable in brown adipocyte thermogenesis - why is this ATP-dependent Ca2+ cycling mechanism selective to beige fat? It is likely because beige adipocytes have a high capacity to generate ATP via enhanced glycolysis and tricarboxylic acid (TCA) metabolism, and thus, ATP-dependent thermogenesis by SERCA2b remains active in the absence of UCP1. In contrast, brown adipocytes possess a low capacity of ATP synthesis due to the very low expression of ATP synthase [64], thereby the ATP-dependent Ca2+ cycling thermogenesis cannot compensate for the UCP1 loss in vivo.
Our study also demonstrates that the UCP1-independent mechanism in beige fat greatly contributes to the regulation of whole-body energy metabolism and glucose homeostasis (summarized in Fig. 3). Mice with increased beige fat mass by the fat-specific expression of PRDM16 gain significantly less body-weight and adiposity than the littermate control mice under a high-fat diet [65]. Surprisingly even when Prdm16 transgenic (Prdm16 Tg) mice were crossed in Ucp1−/− background (Prdm16 Tg x Ucp1−/− mice), these mice still possessed abundant adipocytes with multi-locular lipid droplets and displayed higher whole-body energy expenditure relative to the littermate Ucp1−/− mice. Importantly, Prdm16 Tg x Ucp1−/− mice were protected from diet-induced body weight gain [63]. Furthermore, both Prdm16 Tg mice and Prdm16 Tg x Ucp1−/− mice displayed a marked increase in glucose tolerance and insulin sensitivity relative to their respective littermate control mice under a high-fat diet. These studies suggest that the anti-obesity and anti-diabetic actions of beige fat are independent of UCP1.
Figure 3. Anti-obesity and anti-diabetic actions of beige fat are UCP1-independent.
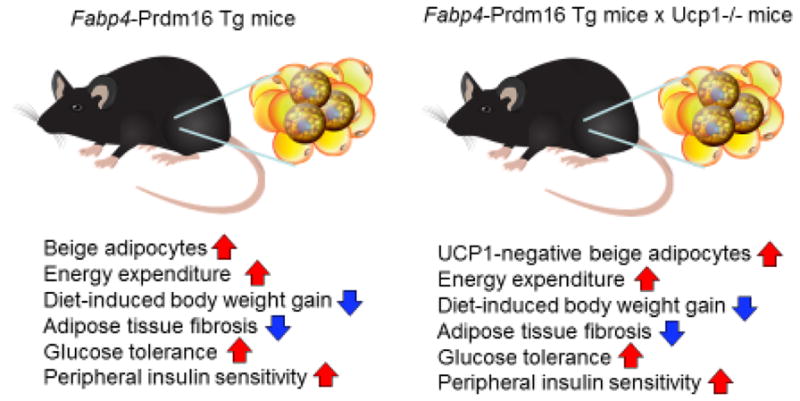
Adipose tissue-selective PRDM16 transgenic (Prdm16 Tg) mice are protected from diet-induced obesity and glucose intolerance (see phenotypes on the right). When crossed in Ucp1−/− background, many of the metabolic phenotypes in wild-type background are preserved in Prdm16 Tg x Ucp1−/− mice [63].
The discovery of UCP1-independent thermogenic mechanisms may offer new opportunities for improving metabolic health, particularly in groups such as elderly populations who do not possess UCP1-positive adipocytes. Through SERCA2-RyR2 signaling, one could potentially pharmacologically activate this non-canonical thermogenesis by enhancing Ca2+ cycling. Indeed, S107, a pharmacological stabilizer of RyR2, which prevents Ca2+ leak and enhances Ca2+ loading in the SR/ER [66], activates UCP1-independent thermogenesis and protects Ucp1−/− mice from cold-induced hypothermia [63]. Increased Ca2+ flux, by inhibiting KCNK3-mediated K+ current in BAT, is also known to enhance intracellular cAMP signaling and subsequent thermogenesis, thereby protecting mice from diet-induced obesity [67]. In turn, dysregulation of Ca2+ homeostasis has been reported in the liver and the pancreas under pathological conditions such as obesity and diabetes [68–73]. Hence, enhanced Ca2+ cycling by S107 treatment potently restores glucose-stimulated Ca2+ flux and insulin secretion in the pancreas [74]. A concern regarding the pharmacological activation of Ca2+ cycling would be potentially harmful effects on the cardiovascular system caused by dysregulated Ca2+ cycling. In fact, mutations in the RYR2 gene mutations cause catecholaminergic polymorphic ventricular tachycardia (CPVT) and arrhythmogenic right ventricular cardiomyopathy type 2 (ARVD2) [75, 76]. Developing new technologies to manipulate Ca2+ cycling in an adipose tissue-selective fashion may circumvent the potential detrimental effects on the cardiovascular system.
Conclusion Remarks and Future Perspectives
Despite the morphological and biochemical similarities between brown fat and beige fat, recent studies identified distinct characteristics in the developmental regulation and function between the two cell types (summarized in Table 1). It is important to note that UCP1 still is a central regulator of BAT thermogenesis, as demonstrated by a number of studies in the past. Meanwhile, emerging evidence suggests alternative thermogenic pathways exist in beige fat and contributes to the regulation of systemic energy expenditure and glucose homeostasis. It remains unknown, however, how the multiple thermogenic mechanisms are coordinately regulated to regulate systemic energy homeostasis in vivo. It is now critical to determine the relative contribution of canonical (UCP1) and non-canonical (UCP1-independent) adipose thermogenesis to whole-body energy homeostasis. Also, the extent to which the two independent ATP-dependent thermogenic mechanisms, creatine cycling and Ca2+ cycling, converge to control non-canonical thermogenesis should be critically determined.
Table 1.
Biochemical and functional characteristics of thermogenic fat.

| ||
|---|---|---|
| Location | • Dedicated depots (infants) • Relatively homogeneous |
• Adults (mice and humans) • Highly heterogeneous |
| Lineage | • Myogenic lineage (Eng, Pax7, Myf5) | • Multiple lineages (SMA, PDGFRα, or PDGFRβ) |
| Enriched markers | Ucp1, Cidea, Pgc1a, Cox7a Prdm16, Zic1 | Ucp1, Cidea, Pgc1a, Cox7a, Prdm16, Cited1, Cd137, Tmem26, Tbx1 |
| Maintenance | • Constitutive | • Transient (direct transition) |
| Thermogenic mechanisms | • UCP1-dependent • Creatin cycling |
UCP1-independent (Ca2+ cycling, creatin cycling) |
It is worth noting that the Ca2+ cycling thermogenesis is an evolutionally conserved mechanism found in adult humans, mice, and even pigs that are a rare mammalian species with mutations in the UCP1 gene and lacks a functional UCP1 protein [63]. Curiously, genome analyses of whales, including minke, fin, bowhead, and sperm whales, found that whales also lack functional UCP1 proteins [77], although the role of Ca2+ cycling thermogenesis in whales is unknown. Tangentially, Ca2+ cycling by SERCA1 is known to control thermogenesis in the “heat-organ” of endothermic fish species, such as swordfish and blue marlin [78]. Because thermogenesis is a fundamental homeostatic system to all the endothermic animals, a better understanding of the divergent evolution in non-model organisms would provide new insights into UCP1-independent thermogenic mechanisms.
The therapeutic potential of non-canonical thermogenesis by Ca2+ cycling and creatine cycling in treating metabolic diseases is compelling due to the presence of these mechanisms in adult humans. Of note, FGF21 increases intracellular Ca2+ levels in adipocytes [79] and induces the browning in a cell-autonomous fashion [80]; although recent studies reported that pharmacological action of FGF21 on body-weight and glucose metabolism was UCP1-independent [81, 82] and that neither FGF21 nor UCP1are required for cold adaptation in mice [83]. It is conceivable that some of the FGF21 actions to increase whole-body energy metabolism may be mediated through an activation of UCP1-independent thermogenesis in the adipose tissue. Adipose-tissue selective activation of non-canonical thermogenesis (e.g., by enhancing the SERCA2b function), if successfully developed, would open up a new therapeutic potential to improve metabolic health even in populations who lack UCP1-positive adipocytes.
The role of BAT as a thermogenic organ has been well-established. On the other hand, many biological roles of beige fat may go beyond thermogenesis [4]. As an example, we recently demonstrated that beige adipocyte biogenesis is inversely correlated with the development of adipose tissue fibrosis in the subcutaneous WAT. Chronic cold acclimation or PRDM16 expression in the adipose tissue potently prevents the development of adipose tissue fibrosis even in Ucp1 null mice, and the repressive effect is independent of energy expenditure and body-weight [84]. Importantly, repression of adipose tissue fibrosis by the UCP1-independent mechanism leads to a significant improvement in systemic glucose homeostasis and insulin-sensitivity in WAT [84]. As another non-thermogenic role of brown/beige fat, several “batokines” are known to control systemic glucose homeostasis [85–87]. Accordingly, it will be increasingly important to better understand the non-thermogenic roles of brown/beige fat in the regulation of whole-body energy metabolism and glucose/lipid homeostasis.
Trends.
Two types of thermogenic fat (brown adipocytes and beige adipocytes) exist in humans and rodents.
Brown and beige fat possess common and distinct characteristics, including their developmental regulation and function.
Non-canonical (UCP1-independent) thermogenic mechanisms control systemic energy metabolism and glucose homeostasis.
Non-canonical thermogenesis by SEARCA2b mediated Ca2+ cycling is an evolutionally conserved mechanism that is active even in animals that lacks a functional UCP1 protein.
Outstanding Questions.
1. What is the relative contribution of canonical (UCP1) thermogenesis and non-canonical thermogenesis in the regulation of systemic energy homeostasis?
Thermoregulation is a fundamental homeostatic system in endothermic animals, even in pigs and whales that lack a functional UCP1 protein. How are the multiple thermogenic mechanisms coordinately regulated to control core-body and peripheral tissue temperature in physiology and disease?
2. Does non-canonical thermogenesis play any role in systemic and local thermoregulation (e.g., fever, hyperthermia) and age-related disorders?
Thermogenic fat has been known to regulate core-body temperature in response to fever and cancer-related hyperthermia. Also, the prevalence of thermogenic fat is highly age-dependent. Do non-canonical thermogenic mechanisms play any role in fever and other temperature-related diseases?
3. How many types of adipocytes do humans and mice have?
So far, two distinct types of thermogenic adipocytes (brown and beige adipocytes) exist in mammals. Given the heterogeneity in adipose tissues, it is likely that more than two forms of thermogenic fat exist.
Acknowledgments
We apologize for our inability to cite many papers that contributed to the advance of this field due to a space limit. This work was supported by NIH DK97441 and DK108822, and the Edward Mallinckrodt, Jr. Foundation to S.K. K.I. is supported by the Manpei Suzuki Diabetes Foundation.
GLOSSARY
- BAT
Brown adipose tissue
- WAT
White adipose tissue
- UCP1
Uncoupling protein 1
- 18F-FDG-PET/CT
18F-labeled fluorodeoxyglucose positron emission tomography/computerized tomography
- PPARγ
Peroxisome proliferator-activated receptor gamma
- β3-AR
β3-adrenoceptor
- SERCA
Sarco/endoplasmic reticulum Ca2+-ATPase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cinti S. The adipose organ. Editrice Kurtis; Milano, Italy: 1999. [Google Scholar]
- 2.Young P, et al. Brown adipose tissue in the parametrial fat pad of the mouse. FEBS Lett. 1984;167(1):10–4. doi: 10.1016/0014-5793(84)80822-4. [DOI] [PubMed] [Google Scholar]
- 3.Loncar D, et al. Epididymal white adipose tissue after cold stress in rats. I. Nonmitochondrial changes. J Ultrastruct Mol Struct Res. 1988;101(2–3):109–22. doi: 10.1016/0889-1605(88)90001-8. [DOI] [PubMed] [Google Scholar]
- 4.Kajimura S, et al. Brown and Beige Fat: Physiological Roles beyond Heat Generation. Cell Metab. 2015;22(4):546–59. doi: 10.1016/j.cmet.2015.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harms M, Seale P. Brown and beige fat: development, function and therapeutic potential. Nat Med. 2013;19(10):1252–63. doi: 10.1038/nm.3361. [DOI] [PubMed] [Google Scholar]
- 6.van Marken Lichtenbelt WD, et al. Cold-activated brown adipose tissue in healthy men. N Engl J Med. 2009;360(15):1500–8. doi: 10.1056/NEJMoa0808718. [DOI] [PubMed] [Google Scholar]
- 7.Saito M, et al. High incidence of metabolically active brown adipose tissue in healthy adult humans: effects of cold exposure and adiposity. Diabetes. 2009;58(7):1526–31. doi: 10.2337/db09-0530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cypess AM, et al. Identification and importance of brown adipose tissue in adult humans. N Engl J Med. 2009;360(15):1509–17. doi: 10.1056/NEJMoa0810780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Virtanen KA, et al. Functional brown adipose tissue in healthy adults. N Engl J Med. 2009;360(15):1518–25. doi: 10.1056/NEJMoa0808949. [DOI] [PubMed] [Google Scholar]
- 10.Nedergaard J, et al. Unexpected evidence for active brown adipose tissue in adult humans. Am J Physiol Endocrinol Metab. 2007;293(2):E444–52. doi: 10.1152/ajpendo.00691.2006. [DOI] [PubMed] [Google Scholar]
- 11.Lidell ME, et al. Evidence for two types of brown adipose tissue in humans. Nat Med. 2013;19(5):631–4. doi: 10.1038/nm.3017. [DOI] [PubMed] [Google Scholar]
- 12.Wu J, et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell. 2012;150(2):366–76. doi: 10.1016/j.cell.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sharp LZ, et al. Human BAT possesses molecular signatures that resemble beige/brite cells. PLoS One. 2012;7(11):e49452. doi: 10.1371/journal.pone.0049452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cypess AM, et al. Anatomical localization, gene expression profiling and functional characterization of adult human neck brown fat. Nat Med. 2013;19(5):635–9. doi: 10.1038/nm.3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shinoda K, et al. Genetic and functional characterization of clonally derived adult human brown adipocytes. Nat Med. 2015;21(4):389–94. doi: 10.1038/nm.3819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yoneshiro T, et al. Recruited brown adipose tissue as an antiobesity agent in humans. J Clin Invest. 2013;123(8):3404–8. doi: 10.1172/JCI67803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van der Lans AA, et al. Cold acclimation recruits human brown fat and increases nonshivering thermogenesis. J Clin Invest. 2013;123(8):3395–403. doi: 10.1172/JCI68993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee P, et al. Temperature-acclimated brown adipose tissue modulates insulin sensitivity in humans. Diabetes. 2014;63(11):3686–98. doi: 10.2337/db14-0513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hanssen MJ, et al. Short-term cold acclimation improves insulin sensitivity in patients with type 2 diabetes mellitus. Nat Med. 2015;21(8):863–5. doi: 10.1038/nm.3891. [DOI] [PubMed] [Google Scholar]
- 20.Leitner BP, et al. Mapping of human brown adipose tissue in lean and obese young men. Proc Natl Acad Sci U S A. 2017;114(32):8649–8654. doi: 10.1073/pnas.1705287114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sidossis L, Kajimura S. Brown and beige fat in humans: thermogenic adipocytes that control energy and glucose homeostasis. J Clin Invest. 2015;125(2):478–86. doi: 10.1172/JCI78362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Atit R, et al. Beta-catenin activation is necessary and sufficient to specify the dorsal dermal fate in the mouse. Dev Biol. 2006;296(1):164–76. doi: 10.1016/j.ydbio.2006.04.449. [DOI] [PubMed] [Google Scholar]
- 23.Lepper C, Fan CM. Inducible lineage tracing of Pax7-descendant cells reveals embryonic origin of adult satellite cells. Genesis. 2010;48(7):424–36. doi: 10.1002/dvg.20630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sanchez-Gurmaches J, et al. PTEN loss in the Myf5 lineage redistributes body fat and reveals subsets of white adipocytes that arise from Myf5 precursors. Cell Metab. 2012;16(3):348–62. doi: 10.1016/j.cmet.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seale P, et al. PRDM16 controls a brown fat/skeletal muscle switch. Nature. 2008;454(7207):961–7. doi: 10.1038/nature07182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang W, et al. Ebf2 is a selective marker of brown and beige adipogenic precursor cells. Proc Natl Acad Sci U S A. 2014;111(40):14466–71. doi: 10.1073/pnas.1412685111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sanchez-Gurmaches J, Guertin DA. Adipocytes arise from multiple lineages that are heterogeneously and dynamically distributed. Nat Commun. 2014;5:4099. doi: 10.1038/ncomms5099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Inagaki T, et al. Transcriptional and epigenetic control of brown and beige adipose cell fate and function. Nat Rev Mol Cell Biol. 2016;17(8):480–95. doi: 10.1038/nrm.2016.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ohno H, et al. EHMT1 controls brown adipose cell fate and thermogenesis through the PRDM16 complex. Nature. 2013;504(7478):163–7. doi: 10.1038/nature12652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cohen P, et al. Ablation of PRDM16 and Beige Adipose Causes Metabolic Dysfunction and a Subcutaneous to Visceral Fat Switch. Cell. 2014;156(1–2):304–16. doi: 10.1016/j.cell.2013.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xue B, et al. Genetic variability affects the development of brown adipocytes in white fat but not in interscapular brown fat. J Lipid Res. 2007;48(1):41–51. doi: 10.1194/jlr.M600287-JLR200. [DOI] [PubMed] [Google Scholar]
- 32.Long JZ, et al. A smooth muscle-like origin for beige adipocytes. Cell Metab. 2014;19(5):810–20. doi: 10.1016/j.cmet.2014.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vishvanath L, et al. Pdgfrbeta+ Mural Preadipocytes Contribute to Adipocyte Hyperplasia Induced by High-Fat-Diet Feeding and Prolonged Cold Exposure in Adult Mice. Cell Metab. 2016;23(2):350–9. doi: 10.1016/j.cmet.2015.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Berry DC, et al. Mouse strains to study cold-inducible beige progenitors and beige adipocyte formation and function. Nat Commun. 2016;7:10184. doi: 10.1038/ncomms10184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee YH, et al. In vivo identification of bipotential adipocyte progenitors recruited by beta3-adrenoceptor activation and high-fat feeding. Cell Metab. 2012;15(4):480–91. doi: 10.1016/j.cmet.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang H, et al. Browning of White Adipose Tissue with Roscovitine Induces a Distinct Population of UCP1+ Adipocytes. Cell Metab. 2016;24(6):835–847. doi: 10.1016/j.cmet.2016.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xue R, et al. Clonal analyses and gene profiling identify genetic biomarkers of the thermogenic potential of human brown and white preadipocytes. Nat Med. 2015;21(7):760–8. doi: 10.1038/nm.3881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang QA, et al. Tracking adipogenesis during white adipose tissue development, expansion and regeneration. Nat Med. 2013;19(10):1338–44. doi: 10.1038/nm.3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barbatelli G, et al. The emergence of cold-induced brown adipocytes in mouse white fat depots is determined predominantly by white to brown adipocyte transdifferentiation. Am J Physiol Endocrinol Metab. 2010;298(6):E1244–53. doi: 10.1152/ajpendo.00600.2009. [DOI] [PubMed] [Google Scholar]
- 40.Cinti S. Transdifferentiation properties of adipocytes in the Adipose Organ. Am J Physiol Endocrinol Metab. 2009 doi: 10.1152/ajpendo.00183.2009. [DOI] [PubMed] [Google Scholar]
- 41.Lee YH, et al. Cellular origins of cold-induced brown adipocytes in adult mice. FASEB J. 2015;29(1):286–99. doi: 10.1096/fj.14-263038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ye R, et al. Impact of tamoxifen on adipocyte lineage tracing: Inducer of adipogenesis and prolonged nuclear translocation of Cre recombinase. Mol Metab. 2015;4(11):771–8. doi: 10.1016/j.molmet.2015.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Loncar D. Convertible adipose tissue in mice. Cell Tissue Res. 1991;266(1):149–61. doi: 10.1007/BF00678721. [DOI] [PubMed] [Google Scholar]
- 44.Rosenwald M, et al. Bi-directional interconversion of brite and white adipocytes. Nat Cell Biol. 2013 doi: 10.1038/ncb2740. [DOI] [PubMed] [Google Scholar]
- 45.Altshuler-Keylin S, et al. Beige Adipocyte Maintenance Is Regulated by Autophagy-Induced Mitochondrial Clearance. Cell Metab. 2016;24(3):402–19. doi: 10.1016/j.cmet.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kovsan J, et al. Altered autophagy in human adipose tissues in obesity. J Clin Endocrinol Metab. 2011;96(2):E268–77. doi: 10.1210/jc.2010-1681. [DOI] [PubMed] [Google Scholar]
- 47.Nunez CE, et al. Defective regulation of adipose tissue autophagy in obesity. Int J Obes (Lond) 2013;37(11):1473–80. doi: 10.1038/ijo.2013.27. [DOI] [PubMed] [Google Scholar]
- 48.Jansen HJ, et al. Autophagy activity is up-regulated in adipose tissue of obese individuals and modulates proinflammatory cytokine expression. Endocrinology. 2012;153(12):5866–74. doi: 10.1210/en.2012-1625. [DOI] [PubMed] [Google Scholar]
- 49.Ost A, et al. Attenuated mTOR signaling and enhanced autophagy in adipocytes from obese patients with type 2 diabetes. Mol Med. 2010;16(7–8):235–46. doi: 10.2119/molmed.2010.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kosacka J, et al. Autophagy in adipose tissue of patients with obesity and type 2 diabetes. Mol Cell Endocrinol. 2015;409:21–32. doi: 10.1016/j.mce.2015.03.015. [DOI] [PubMed] [Google Scholar]
- 51.Narendra DP, et al. PINK1 is selectively stabilized on impaired mitochondria to activate Parkin. PLoS Biol. 2010;8(1):e1000298. doi: 10.1371/journal.pbio.1000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Narendra D, et al. Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J Cell Biol. 2008;183(5):795–803. doi: 10.1083/jcb.200809125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Golozoubova V, et al. Only UCP1 can mediate adaptive nonshivering thermogenesis in the cold. FASEB J. 2001;15(11):2048–50. doi: 10.1096/fj.00-0536fje. [DOI] [PubMed] [Google Scholar]
- 54.Nedergaard J, et al. UCP1: the only protein able to mediate adaptive non-shivering thermogenesis and metabolic inefficiency. Biochim Biophys Acta. 2001;1504(1):82–106. doi: 10.1016/s0005-2728(00)00247-4. [DOI] [PubMed] [Google Scholar]
- 55.Lowell BB, et al. Development of obesity in transgenic mice after genetic ablation of brown adipose tissue. Nature. 1993;366(6457):740–2. doi: 10.1038/366740a0. [DOI] [PubMed] [Google Scholar]
- 56.Enerback S, et al. Mice lacking mitochondrial uncoupling protein are cold-sensitive but not obese. Nature. 1997;387(6628):90–4. doi: 10.1038/387090a0. [DOI] [PubMed] [Google Scholar]
- 57.Feldmann HM, et al. UCP1 ablation induces obesity and abolishes diet-induced thermogenesis in mice exempt from thermal stress by living at thermoneutrality. Cell Metab. 2009;9(2):203–9. doi: 10.1016/j.cmet.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 58.Ukropec J, et al. UCP1-independent thermogenesis in white adipose tissue of cold-acclimated Ucp1−/− mice. J Biol Chem. 2006;281(42):31894–908. doi: 10.1074/jbc.M606114200. [DOI] [PubMed] [Google Scholar]
- 59.Granneman JG, et al. White adipose tissue contributes to UCP1-independent thermogenesis. Am J Physiol Endocrinol Metab. 2003;285(6):E1230–6. doi: 10.1152/ajpendo.00197.2003. [DOI] [PubMed] [Google Scholar]
- 60.Kazak L, et al. A Creatine-Driven Substrate Cycle Enhances Energy Expenditure and Thermogenesis in Beige Fat. Cell. 2015;163(3):643–55. doi: 10.1016/j.cell.2015.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kazak L, et al. Genetic Depletion of Adipocyte Creatine Metabolism Inhibits Diet-Induced Thermogenesis and Drives Obesity. Cell Metab. 2017 doi: 10.1016/j.cmet.2017.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bertholet AM, et al. Mitochondrial Patch Clamp of Beige Adipocytes Reveals UCP1-Positive and UCP1-Negative Cells Both Exhibiting Futile Creatine Cycling. Cell Metab. 2017;25(4):811–822. e4. doi: 10.1016/j.cmet.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ikeda K, et al. UCP1-independent signaling involving SERCA2b-mediated calcium cycling regulates beige fat thermogenesis and systemic glucose homeostasis. Nat Med. 2017;23(12):1454–1465. doi: 10.1038/nm.4429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kramarova TV, et al. Mitochondrial ATP synthase levels in brown adipose tissue are governed by the c-Fo subunit P1 isoform. FASEB J. 2008;22(1):55–63. doi: 10.1096/fj.07-8581com. [DOI] [PubMed] [Google Scholar]
- 65.Seale P, et al. Prdm16 determines the thermogenic program of subcutaneous white adipose tissue in mice. J Clin Invest. 2011;121(1):96–105. doi: 10.1172/JCI44271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Andersson DC, Marks AR. Fixing ryanodine receptor Ca leak - a novel therapeutic strategy for contractile failure in heart and skeletal muscle. Drug Discov Today Dis Mech. 2010;7(2):e151–e157. doi: 10.1016/j.ddmec.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chen Y, et al. Crosstalk between KCNK3-Mediated Ion Current and Adrenergic Signaling Regulates Adipose Thermogenesis and Obesity. Cell. 2017;171(4):836–848. e13. doi: 10.1016/j.cell.2017.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ravier MA, et al. Mechanisms of control of the free Ca2+ concentration in the endoplasmic reticulum of mouse pancreatic beta-cells: interplay with cell metabolism and [Ca2+]c and role of SERCA2b and SERCA3. Diabetes. 2011;60(10):2533–45. doi: 10.2337/db10-1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Luciani DS, et al. Roles of IP3R and RyR Ca2+ channels in endoplasmic reticulum stress and beta-cell death. Diabetes. 2009;58(2):422–32. doi: 10.2337/db07-1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Park SW, et al. Sarco(endo)plasmic reticulum Ca2+-ATPase 2b is a major regulator of endoplasmic reticulum stress and glucose homeostasis in obesity. Proc Natl Acad Sci U S A. 2010;107(45):19320–5. doi: 10.1073/pnas.1012044107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tong X, et al. SERCA2 Deficiency Impairs Pancreatic beta-Cell Function in Response to Diet-Induced Obesity. Diabetes. 2016;65(10):3039–52. doi: 10.2337/db16-0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fu S, et al. Aberrant lipid metabolism disrupts calcium homeostasis causing liver endoplasmic reticulum stress in obesity. Nature. 2011;473(7348):528–31. doi: 10.1038/nature09968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kono T, et al. PPAR-gamma activation restores pancreatic islet SERCA2 levels and prevents beta-cell dysfunction under conditions of hyperglycemic and cytokine stress. Mol Endocrinol. 2012;26(2):257–71. doi: 10.1210/me.2011-1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Santulli G, et al. Calcium release channel RyR2 regulates insulin release and glucose homeostasis. J Clin Invest. 2015;125(5):1968–78. doi: 10.1172/JCI79273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tiso N, et al. Identification of mutations in the cardiac ryanodine receptor gene in families affected with arrhythmogenic right ventricular cardiomyopathy type 2 (ARVD2) Hum Mol Genet. 2001;10(3):189–94. doi: 10.1093/hmg/10.3.189. [DOI] [PubMed] [Google Scholar]
- 76.Marks AR, et al. Involvement of the cardiac ryanodine receptor/calcium release channel in catecholaminergic polymorphic ventricular tachycardia. J Cell Physiol. 2002;190(1):1–6. doi: 10.1002/jcp.10031. [DOI] [PubMed] [Google Scholar]
- 77.Keane M, et al. Insights into the evolution of longevity from the bowhead whale genome. Cell Rep. 2015;10(1):112–22. doi: 10.1016/j.celrep.2014.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Block BA. Thermogenesis in muscle. Annu Rev Physiol. 1994;56:535–77. doi: 10.1146/annurev.ph.56.030194.002535. [DOI] [PubMed] [Google Scholar]
- 79.Moyers JS, et al. Molecular determinants of FGF-21 activity-synergy and cross-talk with PPARgamma signaling. J Cell Physiol. 2007;210(1):1–6. doi: 10.1002/jcp.20847. [DOI] [PubMed] [Google Scholar]
- 80.Fisher FM, et al. FGF21 regulates PGC-1alpha and browning of white adipose tissues in adaptive thermogenesis. Genes Dev. 2012;26(3):271–81. doi: 10.1101/gad.177857.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Veniant MM, et al. Pharmacologic Effects of FGF21 Are Independent of the “Browning” of White Adipose Tissue. Cell Metab. 2015;21(5):731–8. doi: 10.1016/j.cmet.2015.04.019. [DOI] [PubMed] [Google Scholar]
- 82.Samms RJ, et al. Discrete Aspects of FGF21 In Vivo Pharmacology Do Not Require UCP1. Cell Rep. 2015;11(7):991–9. doi: 10.1016/j.celrep.2015.04.046. [DOI] [PubMed] [Google Scholar]
- 83.Keipert S, et al. Long-Term Cold Adaptation Does Not Require FGF21 or UCP1. Cell Metab. 2017;26(2):437–446. e5. doi: 10.1016/j.cmet.2017.07.016. [DOI] [PubMed] [Google Scholar]
- 84.Hasegawa Y, et al. Repression of adipose tissue fibrosis through a PRDM16-GTF2IRD1 complex improves systemic glucose homeostasis. Cell Metab. 2018 doi: 10.1016/j.cmet.2017.12.005. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Svensson KJ, et al. A Secreted Slit2 Fragment Regulates Adipose Tissue Thermogenesis and Metabolic Function. Cell Metab. 2016;23(3):454–66. doi: 10.1016/j.cmet.2016.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Long JZ, et al. The Secreted Enzyme PM20D1 Regulates Lipidated Amino Acid Uncouplers of Mitochondria. Cell. 2016;166(2):424–435. doi: 10.1016/j.cell.2016.05.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang GX, et al. The brown fat-enriched secreted factor Nrg4 preserves metabolic homeostasis through attenuation of hepatic lipogenesis. Nat Med. 2014;20(12):1436–43. doi: 10.1038/nm.3713. [DOI] [PMC free article] [PubMed] [Google Scholar]