Abstract
Gangliosides shed by tumors into their microenvironment (TME) are immunoinhibitory. Interferon-γ (IFN-γ) may boost antitumor immune responses. Thus we wondered whether IFN-γ would counteract tumor ganglioside-mediated immune suppression. To test this hypothesis, we exposed human monocyte-derived LPS-activated dendritic cells (DC) to IFN-γ and to a highly purified ganglioside, GD1a. DC ganglioside exposure decreased TLR-dependent p38 signaling, explaining the previously observed ganglioside-induced down-modulation of proinflammatory surface markers and cytokines. Strikingly, while increasing LPS-dependent DC responses, IFN-γ unexpectedly did not counteract the inhibitory effects of GD1a. Rather, induction of indoleamine 2,3-dioxygenase (IDO1), and expression of STAT1/IRF-1 and programmed cell death ligand (PD-L1), indicated that the immunoinhibitory, not an immune stimulatory, IFN-γ-signaling axis, was active. The combination, IFN-γ and DC ganglioside enrichment, markedly impaired DC stimulatory potential of CD8+ T-cells. We suggest that gangliosides and IFN-γ may act in concert as immunosuppressive mediators in the TME, possibly promoting tumor progression.
Keywords: Ganglioside, IFN-γ, tumor immunosuppression, tumor microenvironment
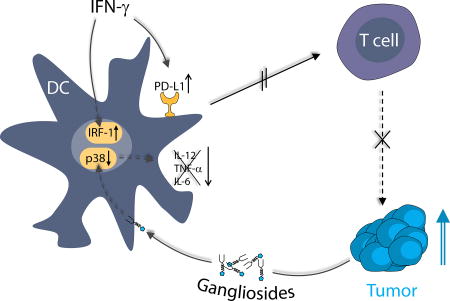
Graphical abstract
1. Introduction
Current concepts of tumor development hold that tumor cell growth is subject to surveillance by the immune system [1], which recognizes and eliminates aberrant tumor cells [2]. However, human tumors are known to protect themselves from immune attack and overcome anti-tumor immune responses. A major factor supporting tumor immune evasion is an immunosuppressive tumor microenvironment (TME) [3], in which infiltration by immune cells may not necessarily translate into an effective anti-tumor immune response.
Gangliosides, amphipathic sialic acid-containing glycosphingolipids, are immunosuppressive molecules influencing the TME and promoting tumor growth [4]. The shedding or release of tumor cell gangliosides is characteristic of many tumors, especially neuroblastoma, lymphoma, glioblastoma, medulloblastoma, and retinoblastoma [5–9], and may have a major impact on tumor evasion and survival, even though tumors may be highly infiltrated with myeloid immune cells, including macrophages and dendritic cells (DCs) [10]. Since ganglioside enrichment has been shown to trigger immunosuppressive mechanisms in DCs, such as inhibition of MyD88 dependent toll-like receptor (TLR) and IL-1R signaling [11], shedding of immunosuppressive gangliosides into the TME [8, 12] may represent a mechanism for tumor immune escape.
Interferon-gamma (IFN-γ) has been proposed as an immunostimulatory adjuvant to cancer treatment and is also highly expressed in the TME [13]. IFN-γ is widely recognized for its pro-inflammatory capability, priming DCs by enhancing TLR expression and promoting NF-kB signaling [14, 15]. Released upon activation of CD4+ T-helper (Th) 1 cells, CD8+ cytotoxic lymphocytes, and natural killer (NK) cells [16], IFN-γ can mediate anti-tumor immunity, reinforcing Th1 and cytotoxic effector responses and enhancing macrophage killing. In the context of cancer, IFN-γ plays an essential role in immune surveillance and in the early tumor elimination phase, as evidenced by the increased incidence of spontaneous tumors in IFN-γ knock-out mice [17].
However, IFN-γ also possesses anti-inflammatory and immunosuppressive properties [18, 19]. This could be especially important in the context of an already developing tumor, because of substantial release of IFN-γ by immune cells [19]. Among its immunosuppressive functions is the induction of the enzymatic activity of Indoleamine-2,3-dioxygenase (IDO1) in DCs [20] and the immunosuppressive receptor programmed death ligand-1 (PD-L1) [21, 22]. PD-L1 upregulation is associated with various cancer types [23]. Its binding partner, programmed death-1 (PD-1), is highly expressed on tumor infiltrating lymphocytes [24], inducing cellular dysfunction such as immunological exhaustion which is associated with decreased inflammatory cytokine secretion and cellular proliferation [25].
Therefore, we wondered, and tested in vitro, whether tumor ganglioside enrichment in the TME, affecting DC function negatively, could be counteracted by the presence of or administration of IFN-γ. Restated, would immune modulatory IFN-γ synergize with or counteract the immunoregulatory effects of a ganglioside-rich microenvironment? We used a representative ganglioside, highly purified di-sialoganglioside GD1a, for these studies. Our findings demonstrate that gangliosides and IFN-γ do not counteract each other but rather cooperate to suppress DC immunostimulatory activity in an inflammatory environment. Such conditions might favor immune suppression in the TME and therefore aid tumor progression.
2. Materials and Methods
2.1. Samples
Human peripheral blood samples from healthy volunteers obtained with informed consent were used for all experiments in accordance with the Declaration of Helsinki.
2.2. DC differentiation, activation and T-cell isolation
Monocytes were isolated and differentiated to immature dendritic cells (iDCs) as previously described, using GM-CSF and IL-4 [26]. Then, iDCs were incubated for 24 hours with highly purified GD1a ganglioside (Matreya). As in our previous studies [11, 27], the rationale for beginning the incubation with gangliosides (rather than with LPS and IFN-γ) was that the initial contact of DC migrating into the TME would likely be with shed tumor gangliosides. Ganglioside incubation was followed by activation for 48 hours with 100 ng/mL Escherichia coli O111:B4 LPS (Merck) and/or 100 U/mL IFN-γ (Peprotec) to promote further maturation. In exploratory studies we had determined that the optimal concentration of IFN-γ for affecting DC activation was 100 U/ml and we used this concentration throughout our experiments. All experiments were conducted with a 48 hour LPS/IFN-γ stimulation. This approach, based on our previous findings [28], was used to assure that a robust control inflammatory DC phenotype would be observed. We selected GD1a for our studies because it is both highly purified and easily obtainable; its immunosuppressive activity is similar to that of many other gangliosides (such as GD3 and GD2) prominent in tumors [29].
To obtain highly enriched (>98%) T-cells, peripheral blood mononuclear cells (PBMCs) were separated by density gradient centrifugation of venous blood. CD3+ T-cells were isolated from the PBMC by magnetic cell sorting (Miltenyi Biotec) according to the manufacturer's instructions.
2.3. Flow cytometry
The following monoclonal antibodies were used: IgG1-FITC, IgG2a-PE, IgG1-PE, IgG2a-perCP, IgG1-APC, anti–CD3-APC (SK7), anti–CD4-PerCP (SK3), anti–CD25-PE (2A3), anti–CD86-APC (2331), anti–CD14-PerCP-Cy5.5 (M5E2; all from BD Biosciences); anti–CD80-PE (MAB104; Beckman Coulter), anti MHC-II FITC (CR/43, Dako Cytomation). Flow cytometry was performed using a LSR II (BD Biosciences) flow cytometer. Acquired data were analyzed with FlowJo (Tree Star, Inc.).
2.4. Mixed leukocyte reaction
Enriched CD3+ lymphocytes were stained with carboxyfluorescein succinimidyl ester (CFSE, Sigma). 105 T-cells were co-cultured for 7 days in 96 well round bottom plates (NUNC) with 104 allogeneic DCs that had been exposed to GD1a and stimulated with LPS and/or IFN-γ, as above. Proliferation was quantified as the % CFSE-diluted cells at the end of culture, as analyzed by flow cytometry gated on CD3+CD8+ T-cells [20].
2.5. Immunoblotting and enzymatic activity of IDO1
IDO, phospho-Stat1 (BD Biosciences), Stat1, IRF-1, phospho-p38, p38, PD-L1 (all Cell Signaling Technologies) and GAPDH (Ambion) protein expression was determined by immunoblotting and analyzed by ImageJ software. Anti-IDO mouse monoclonal anti-human IDO antibody, a kind gift of Osamu Takikawa, was used as described [20, 30]. IDO1 enzymatic activity was determined by measuring tryptophan and kynurenine levels in cell culture supernatants by high-pressure liquid chromatography [20].
2.6. Cytokine release
Cytokine secretion was analyzed by multiplex-based flow Cytomix array (eBioscience) of culture supernatants according to the manufacturer's instructions.
2.7. Statistical Analysis
The individual effects of LPS, GD1a and IFN-γ on DC functions were analyzed using unpaired two-tailed Student’s t test, with p<0.05 considered statistically significant.
3. Results
3.1. IFN-γ boosted co-stimulatory molecule expression is markedly inhibited by GD1a exposure
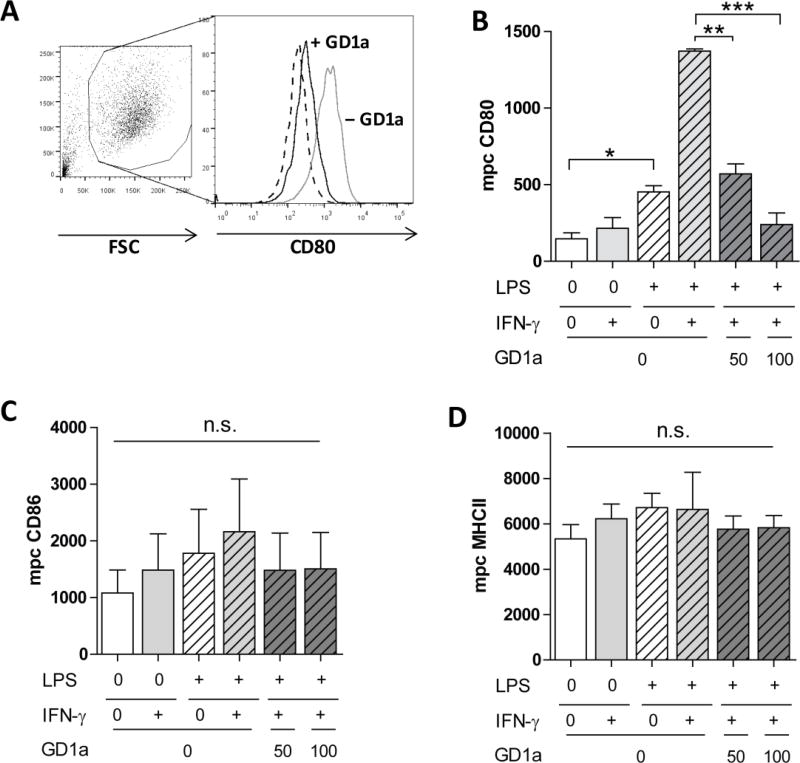
Activation by TLR ligands, either by pathogen-associated molecular patterns, such as LPS, or endogenous damage-associated molecular patterns, such as HMGB-1, both via TLR-4 signaling, increase the expression of DC cell surface molecules responsible for delivering a co-stimulatory signal for T-cell survival and proliferation [31]. Such stimulation can be further amplified by IFN-γ [32]. DC membrane enrichment with gangliosides, such as shed by tumor cells into the TME, has an opposing effect, inhibiting DC cell surface marker expression [27]. We determined the effect of highly purified GD1a ganglioside after combined LPS/IFN-γ activation of DCs on the expression of cell surface molecules essential for antigen-presentation and T-cell co-stimulation. Most affected was CD80; the expression of this co-stimulatory molecule tripled in response to LPS, from a median peak channel of 146 to 452 (Figs. 1A, 1B) and was boosted a further 3-fold by the addition of IFN-γ. Despite this increase, pre-exposure of DCs to GD1a completely overcame the synergistic effect of LPS and IFN-γ. CD86 was mildly increased by the combination of LPS and IFN-γ; once again the increase was suppressed by GD1a exposure (Fig. 1C). The same trend was observed for CD40 and CD83 (not shown). MHC-II expression was unaffected (Fig. 1D). These findings indicate that ganglioside exposure can abrogate the synergistic effect of LPS and IFN-γ on DC maturation, resulting in reduced co-stimulatory molecule expression.
Figure 1. GD1a ganglioside exposure inhibits co-stimulatory CD80 expression of human monocyte-derived DCs.
(A) In a representative FACS histogram analysis, the median peak channel (mpc) of CD80 expression on human monocyte-derived iDCs (dashed line, mpc of 179), DCs activated with 100 ng/mL LPS and 100 U/mL IFN-γ (grey solid line, mpc 1272), and DCs exposed to 100 µM GD1a and then activated with LPS and IFN-γ (black solid line, mpc 308) are shown. (B–D) iDCs were cultured in medium alone (white bar), stimulated with LPS (100 ng/mL, dashed white bar), IFN-γ (100 U/mL, light grey bar) or with both LPS + IFN-γ (dashed grey bars) or in addition treated with GD1a (50 or 100 µM, dashed dark-grey bars). This same shading of bars has been used consistently in figures 1–4. (B), CD80; (C), CD86; (D), MHC-II expression of DCs, analyzed by flow cytometry. Bars indicate mean±SEM mpc of triplicate cultures in one representative of three separate experiments. *, P<0.05; ** P<0.01; ***, P<0.005; no significant differences in (C and D) (P>0.05) analyzed using unpaired two-tailed Student’s t test.
3.2. IFN-γ boosted pro-inflammatory cytokine production and stimulatory capacity of DCs is inhibited by GD1a exposure
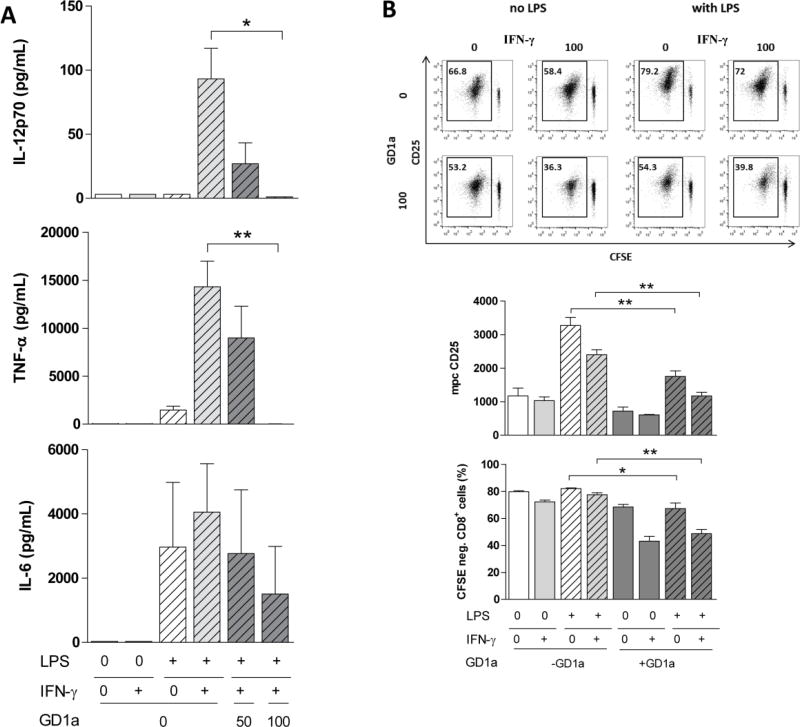
TLR agonists such as LPS actively stimulate DC cytokine production while IFN-γ by itself has limited effects on DC pro-inflammatory cytokine production. However, the pro-inflammatory cytokine IFN-γ is capable of enhancing TLR mediated secretion of inflammatory cytokines [33]. This prompted us to wonder how IFN-γ would affect pro-inflammatory cytokine (IL-12, TNF-α and IL-6) release in DC conditions that downmodulate TLR-4 signaling, i.e., GD1a exposure. As expected, exogenous ganglioside exposure inhibited both LPS-driven and IFN-γ-boosted IL-12p70 and TNF-α cytokine production completely (Fig. 2A). This trend was also seen with respect to IL-6 cytokine production (Fig. 2A). Hence, even in the presence of IFN-γ, ganglioside exposure disables DC cytokine production essential for mounting T-cell immune responses.
Figure 2. DC exposure to GD1a abrogates LPS-stimulated pro-inflammatory cytokine secretion and inhibits allogeneic T-cell responses, not reversed by IFN-γ.
(A) DCs were stimulated as in Figure 1. Cell culture supernatants were analyzed for the cumulative release of IL-12p70, TNF-α, and IL-6. Bars indicate mean±SEM of triplicate cultures in one representative of three similar experiments. (B) DCs cultured and illustrated as in Figure 1, were washed, and then used to stimulate CFSE stained allogeneic T-cells at a DC:T-cell ratio of 1:10. The allogeneic proliferative response of live CFSE-stained CD3+CD8+ lymphocytes was assessed after 7 days of co-culture. The percent CFSE-negative cells and the mpc of CD25 were quantified by flow cytometry as exemplified in a representative experiment shown in Fig. 1B, top panel. Bars indicate mean±SEM combining four cultures of two separate experiments. Proliferation controls: resting T-cells, 0.36% CFSE negative; T-cells stimulated with anti-CD3/anti-CD28 antibodies, 93.6% CFSE negative (Data not shown). *, P<0.05; **, P<0.01, ***, P<0.005 analyzed using a two-tailed Student’s t test.
3.3. Combination of IFN-γ and GD1a exposure of DCs maximally reduces T-cell stimulation
To assess the T-cell stimulatory potential of GD1a-treated DCs, the latter were co-cultured with allogeneic T-cells (Fig. 2B). Pre-incubation of DCs with GD1a slightly inhibited their ability to activate allogeneic T-cells (measured by CD25 expression levels) with resultant reduced proliferative responses, consistent with previous findings [34]. However, there was even greater inhibition upon DC exposure to IFN-γ as well, with CD8+ proliferation significantly decreased, from 80% to 50% (Fig. 2B upper and lower graph), and significantly less CD25 expression (upper and middle graph, Fig. 2B). The results demonstrate that IFN-γ actually further inhibits the stimulatory potential of LPS/GD1a-treated DCs against allogeneic CD8+ T-cells, thereby once again complementing ganglioside-induced inhibition.
3.4. GD1a enrichment differentially affects LPS and IFN-γ induced IDO1 expression and function and PD-L1 expression
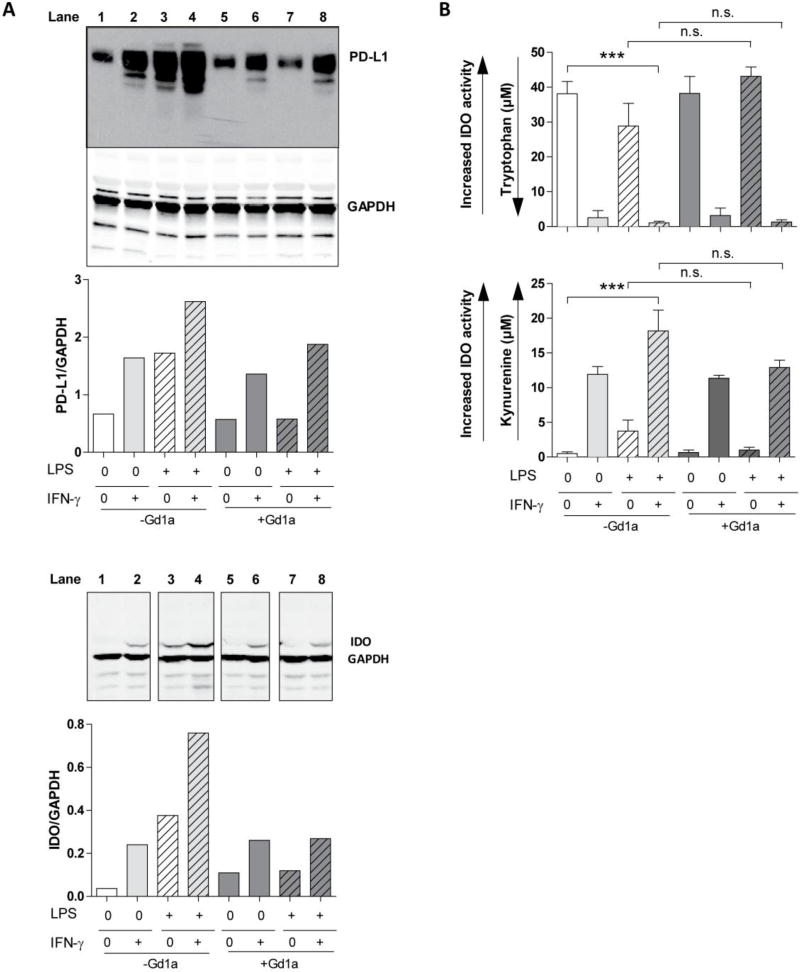
A possible explanation for the apparently opposing effects of IFN-γ is that under strong inflammatory conditions, DCs can acquire immunosuppressive properties by inducing enzymatically active IDO1 [20], also found in many tumor systems [35]. Moreover, a key immunosuppressive mediator in the TME, PD-L1, is upregulated upon IFN-γ or LPS stimulation in mouse macrophages [21] and human monocytes and dendritic cells [22]. Consequently, we tested the effect of GD1a enrichment on IDO1 and PD-L1 protein expression by DC exposed to LPS and/or IFN-γ. Even in the absence of LPS, IFN-γ directly induced protein expression of both key molecules that was not inhibited by DC pre-exposure to GD1a (Fig. 3A). In contrast, GD1a pre-exposure abrogated IDO1 enzyme protein expression driven by LPS alone, explicable by the fact that GD1a exposure impedes DC activation by LPS. It also reduced maximal PD-L1 and IDO1 protein expression (caused by combined activation of DCs with LPS and IFN-γ) back to the levels induced by IFN-γ alone. Thus ganglioside exposure interfered with LPS driven but not IFN-γ driven induction, suggesting two differently affected pathways by ganglioside enrichment of the DC.
Figure 3. PD-L1 expression and IDO1 protein induction and activity by IFN-γ is resistant to GD1a pre-exposure of DCs.
(A) DCs were cultured as in Fig. 1. At the end of culture, PD-L1 (top panel) and IDO1 (lower panel) protein expression was examined by Western blot and quantified by ImageJ; bars indicate relative PD-L1 or IDO1 expression, shown as the PD-L1/GAPDH or IDO/GAPDH ratio. One representative of two separate experiments with similar results is shown. (B) Cell culture supernatants of DCs cultured as in (A) were analyzed for tryptophan and kynurenine concentration to determine IDO1 activity. Increasing IDO1 activity is seen as reduction in tryptophan and increase in kynurenine concentration. Bars indicate the mean ±SEM of triplicate cultures in a representative of three separate experiments. ***, P<0.0005, n.s. not significant analyzed using unpaired two-tailed Student’s t test.
Enzyme protein levels themselves do not necessarily determine enzyme activity. We therefore assessed the functional impact of GD1a exposure, quantifying IDO1 activity under strong inflammatory conditions and analyzing tryptophan depletion and kynurenine accumulation in the cell culture medium. IFN-γ exposure alone, but not LPS exposure alone, caused marked tryptophan depletion (Fig.3B, upper graph) and augmented kynurenine concentrations (Fig. 3B, lower graph). Ganglioside enrichment of DCs did not inhibit this potentially immunosuppressive effect of IFN-γ, and LPS exposure itself did not initiate the process of tryptophan catabolism. These findings highlight IFN-γ as an inducer of IDO1 activity in immature DCs, in LPS-matured DCs, and in GD1a-enriched LPS-matured DCs. They suggest a predominance of anti-inflammatory features of IFN-γ, strongly shifting ganglioside-enriched DCs even further towards immune suppression.
3.5. GD1a inhibits pro-inflammatory p38 signaling but spares immunosuppressive JAK/STAT signaling
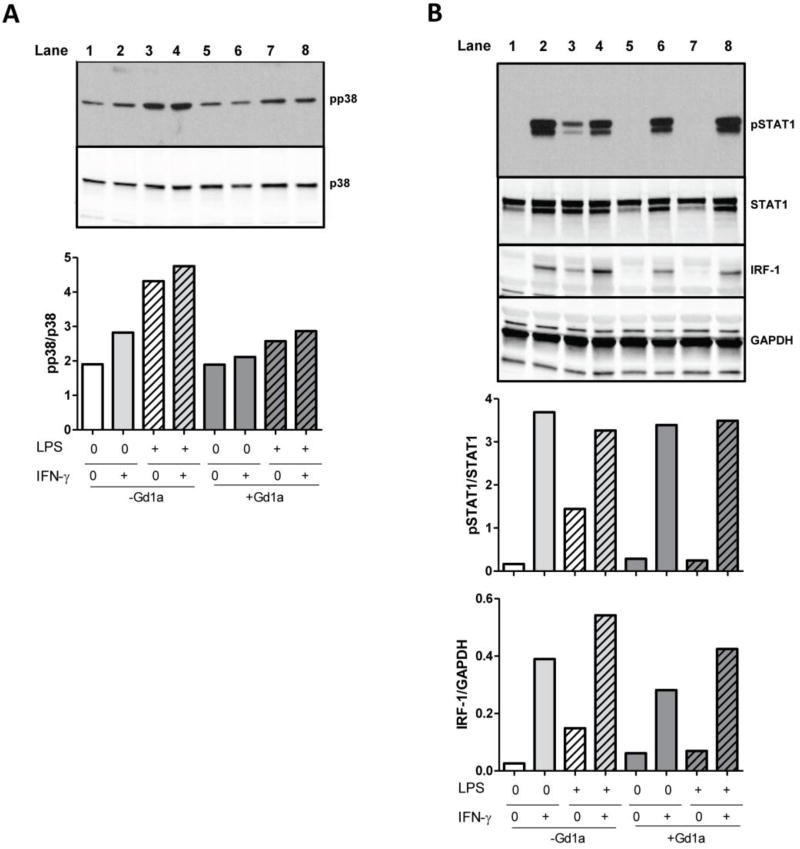
As we observed intact IDO1 expression and activity in ganglioside-enriched DCs stimulated only with IFN-γ, but reduced IDO1 activity in GD1a-treated DCs stimulated with LPS or LPS and IFN-γ (Fig. 3), we reasoned that a complex signaling interaction was taking place. We tested whether intracellular key signaling molecules of two likely pathways — the pro-inflammatory TLR-4 and the immunosuppressive JAK/STAT pathway — had been affected. We confirmed the known inhibiting effect of GD1a on LPS-induced (MyD88 mediated TLR-4) signaling [11, 36] in DCs, and observed reduction of phosphorylation of p38, a key downstream molecule of TLR4 signaling, to baseline levels (Fig. 4A). However, IFN-γ stimulation led to high phosphorylation of signal transducer and activator of transcription 1 (STAT1) and strong expression of its downstream target interferon regulatory factor 1 (IRF-1). Both were unaffected by GD1a treatment (Fig. 4B) in contrast to complete abrogation of the LPS-induced JAK/STAT signaling by GD1a.
Figure 4. GD1a interferes with LPS– but not IFN-γ–initiated signaling.
DCs were cultured as in Fig. 1. At the end of culture, (A) pp38, p38 (B) pSTAT1, STAT1, IRF-1 and GAPDH protein expression was determined by Western blot and quantified by ImageJ; bars indicate pp38/p38, pSTAT1/STAT1 and IRF-1/GAPDH relative protein expression.
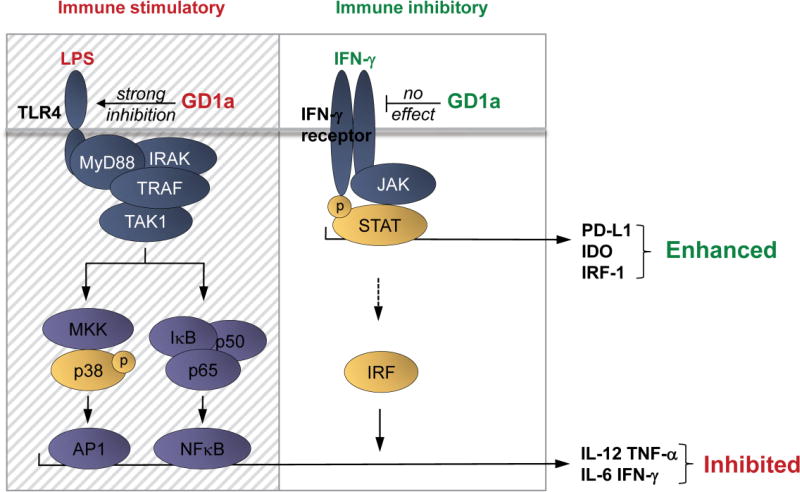
3.6. Model of differential ganglioside effects on immune inhibitory and immune stimulatory pathways
The combined findings suggest a schema (Fig. 5) of DC programming and the impact of membrane ganglioside enrichment on DC function leading to a ganglioside-dependent immunosuppressive phenotype: LPS and IFN-γ exposures of DCs involve p38, MAPK and JAK/STAT signal transduction, respectively. Without membrane ganglioside enrichment, LPS activated DCs release pro-inflammatory factors via AP1 and NFκB transcriptional activity. Production or release of these pro-inflammatory factors is greatly enhanced by the transcriptional activator IRF-1, also induced by IFN-γ. IFN-γ is able to boost this response, but when GD1a exposure blocks the initial LPS activation of DCs [11], the normally pro-inflammatory IFN-γ boost is rendered ineffective because of absence of the primary activation, and inhibition persists. At the same time that GD1a enrichment is able to block TLR4-mediated LPS signaling, it does not affect IFN-γ signaling (as indicated by STAT1 phosphorylation and IRF1 expression). As a result, a selective blockade of TLR signaling by GD1a abrogates the secretion of pro-inflammatory factors, favoring the strong immune suppressive phenotype due to IFN-γ stimulated IDO1 and PD-L1 expression.
Figure 5. Complex interaction of TLR-ligation, ganglioside enrichment, and IFN-γ on dendritic cell signaling.
LPS/TLR-4 mediated activation of p38 drives the secretion of pro-inflammatory factors such as IL-12, TNF-α, IL-6 but also IFN-γ, through active AP1. This also involves the translocation of NFκB transcription factors into the nucleus promoting cytokine production. IFN-γ, by inducing JAK/STAT mediated signal transduction triggers the expression of IRFs, which boost cytokine production in combination with AP1 and NFκB transcription factors. IRF-1 itself is not capable of inducing cytokine production as we have also shown here. IFN-γ as well triggers transcription of IDO1 and PD-L1, inducing an immunosuppressive DC phenotype. In a ganglioside-rich environment, GD1a accumulation in DCs specifically inhibits TLR-4 signaling, strongly reducing p38-mediated AP1 activity and NFκB activity, leading to down modulation of pro-inflammatory cytokine secretion. Of note, IFN-γ signaling is not affected by GD1a enrichment, leaving JAK/STAT-mediated PD-L1 and IDO1 induction by IFN-γ intact. GD1a enriched environments therefore strongly favor immune suppressive properties of DCs in the presence of IFN-γ, which predominantly triggers the expression of enzymatically active IDO1 and PD-L1. In the figure, the hatched area indicates selective inhibitory effects of GD1a on TLR-4 signaling; the clear area depicts the unaffected IFN-γ signaling. TRAF:TNF receptor associated factor, TAK1: Transforming growth factor beta-activated kinase 1, AP1: Activator protein 1, NFκB: Nuclear factor kappa-light-chain-enhancer of activated B cells, IRF: Interferon regulatory factors.
4. Discussion
DCs are critical in generating effective anti-tumor immune responses [37]. To develop strategies optimizing their function, frequently inhibited in the TME by tumor cell gangliosides, we need to understand the causes of such immunosuppression. Ganglioside induced DC dysfunction decreases antigen processing and presentation [34], TLR-4 signaling [11], and cytokine production [36], resulting in a shift of Th1 T-cell polarization towards a Th2 phenotype [38]. Consequently, neutralizing suppressive effects of gangliosides in the TME could favor a more immunologically stimulatory TME. Indirect evidence that supports this concept comes from the effects of knockout of ganglioside synthesis in a tumor cell. In this model, in which robust proliferation of the cell in vitro was unaffected, the elimination of gangliosides synthesis markedly impeded tumor growth in vivo [4].
IFN-γ is generally known as an important factor in stimulating antigen-driven immune responses, thereby affecting the overall immunological state of the TME. Thus, it was unexpected that known IFN-γ–induced pro-inflammatory effects (e.g., CD80 upregulation, and IL-12 and TNF-α secretion) did not counteract ganglioside-induced inhibition. Even more surprisingly, DC immunosuppressive mechanisms induced by IFN-γ, such as the enzymatic activity of IDO1 [20, 30, 39] and upregulation of PD-L1 [21, 22], were not decreased but even enhanced by ganglioside enrichment. Our findings suggest that ganglioside-induced IFN-γ–resistant DC inhibition and resultant impairment of T-cell responses, together with IFN-γ-induced ganglioside-resistant immunosuppressive IDO1 activity and PD-L1 expression, may act in concert with gangliosides to render the TME even more immunosuppressive.
Based on our findings, we propose a schema (Fig. 5) to illuminate the consequences on cell signaling of the complex DC interaction with gangliosides and IFN-γ in the TME. As tumor cells proliferate, gangliosides and IFN-γ are released and accumulate in the TME. The gangliosides shed by tumor cells result in their enrichment on tumor-infiltrating DCs in the TME, depriving them of their capacity to stimulate cellular immune responses, blocking p38-driven transcriptional activation of AP-1 and activation and nuclear translocation of NFκB [40] (shaded, LPS-induced area of Fig.5). These ganglioside inhibitory effects (as well as those of IFN-γ, see below) are particularly important in influencing the TME, as decreased NFκB activation reduces inflammatory gene expression and causes M2 polarization of tumor-infiltrating macrophages [41].
While IFN-γ can prime DCs for an enhanced response to antigens by reinforcing LPS-triggered transcription through IRF-1 (Fig. 5, clear IFN-γ-induced area), upregulating CD80, IL-12 and TNF-α, this enhanced DC response remains susceptible to inhibition upon DC ganglioside enrichment. At the same time the direct immunosuppressive effects of IFN-γ — autonomous induction of IDO1 activity and PD-L1 upregulation — remain impervious to inhibition by gangliosides in the DC membrane (Fig. 5). This negates IFN-γ as an effective immunostimulant. To unravel the mechanism of the unexpected synergism between IFN-γ and gangliosides in causing immunosuppression, future detailed studies using inhibitory/blocking/promoting agents may be helpful.
This complex process is particularly relevant to tumors and their microenvironment, favoring tumor immune escape and progression. Summarizing, there are multiple pieces of relevant evidence including our findings here, that address the issue from several perspectives: (i) Gangliosides are expressed by tumors [42], shed in vitro into the culture medium [9] and into the circulation [43] the cerebrospinal fluid [8] in humans. (ii) Ganglioside enrichment of myeloid cells inhibits TLR- or IL-1R-mediated pro-inflammation leading to impaired cellular immunity [11, 44]. (iii) Some tumor gangliosides, such as GD2 in neuroblastoma [6] and GD1b and GD3 in glioma, are prognostic disease markers [45]. (iv) IFN-γ induces IDO1 and PD-L1 while IFN-γ pro-inflammatory responses are inhibited by ganglioside enrichment of DCs and IDO1 activity may be further amplified by substantial secretion of IFN-γ by immune cells [19] and accumulation in the TME. Thus, the role of immunosuppression in tumor progression and the implication of tumor cell ganglioside expression and IFN-γ secretion in these processes, warrants further study.
There may be a problem with the use of IFN-γ as a cytokine to boost host cellular immune responses in cancer. For example, human glioblastoma, with a median patient survival of ~14 months, is a very aggressive form of malignant brain cancer, in part due to its immunosuppressive effects. In a murine glioma model, prolonged survival was associated with IFN-γ administration [46], but in several human studies, IFN-γ injections were not associated with improved outcomes [47, 48]. Since our data in vitro showed that the combination of ganglioside enrichment and IFN-γ stimulation of DCs intensified immune suppression, as an initial approach to determine potential relevance in vivo we queried the degree of expression of the genes controlling ganglioside synthesis enzymes and IFN-γ signaling components in human glioblastoma. In a preliminary analysis of available online expression data of glioma biopsies (Supplementary Fig. 1), we separately analyzed two groups of genes for upregulation: (a) ST3GAL5, ST8SIA1, and B4GALNT1, genes for enzymes that synthesize the gangliosides that are immunosuppressive and highly abundant in brain tumors [6, 9, 11], and (b) genes for IFN-γ and IRF-1 (Supplementary Fig. 1). We correlated highest and lowest levels of gene set expression of the quartiles of patients (n=41 each) retrieved from the University of North Carolina dataset (Supplementary Fig. 1, left panel) and 41 patients per group in the Broad Institute-MIT/Harvard dataset (Supplementary Fig.1, right panel). Although upregulation of neither set of genes alone, i.e., (a) or (b), had a significant effect on survival in either dataset, upregulation of the combined gene signature – ST3GAL5, ST8SIA1, B4GALNT1, IFNG and IRF1 – was associated with a significantly shorter overall survival of these glioblastoma-bearing patients, in both datasets.
In conclusion, this work highlights IFN-γ mediated immunosuppressive mechanisms in a ganglioside-rich immunosuppressive TME, as potentially contributing to tumor escape and progression. Our findings suggest that the combination of immunoregulation by gangliosides and by IFN-γ increases inhibition of immune responses, shifting the balance from possibly immunostimulatory or neutral to immunoregulatory in the TME. Such combined potent ganglioside inhibition of IFN-γ-boosted LPS immune stimulation of DCs and retained integrity of IFN-γ-induced immunosuppressive pathways, may contribute more generally to creating a tolerogenic (and pro-tumorigenic) microenvironment in vivo. Clearly, these in vitro findings require validation in vivo. This is especially important in view of the preliminary data that demonstrated a relationship between the upregulation of the respective genes and patient outcome. The potential impact on the survival of cancer patients provides a compelling rationale for fully exploring a possible prognostic value in cancer. These combined immunosuppressive effects may also be of particular interest in the context of IFN-γ mediated cancer immunotherapy.
Supplementary Material
Gangliosides decrease DC activation, co-stimulation and inflammatory cytokines
IFN-γ does not reverse ganglioside-induced immunosuppression
IFN-γ triggers inhibitory pathways in activated DC
IFN-γ and GD1a combine to maximally promote a regulatory DC phenotype
Acknowledgments
Funding St. Anna Kinderkrebsforschung e.V. (A.M.D) and the National Cancer Institute, NIH (RO1CA61010, S.L.)
We thank David Leitenberg for critically reviewing the manuscript. This work is in memory of our deceased colleague and good friend Andreas Heitger, whose vision initiated this project and whose support throughout this study was invaluable.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors declare no competing financial interests.
References
- 1.Zitvogel L, Tesniere A, Kroemer G. Cancer despite immunosurveillance: immunoselection and immunosubversion. Nature reviews. Immunology. 2006;6:715–727. doi: 10.1038/nri1936. [DOI] [PubMed] [Google Scholar]
- 2.Restifo NP, Dudley ME, Rosenberg SA. Adoptive immunotherapy for cancer: harnessing the T cell response. Nature reviews. Immunology. 2012;12:269–281. doi: 10.1038/nri3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nature reviews. 2005;5:263–274. doi: 10.1038/nrc1586. [DOI] [PubMed] [Google Scholar]
- 4.Liu Y, Yan S, Wondimu A, Bob D, Weiss M, Sliwinski K, Villar J, Notario V, Sutherland M, Colberg-Poley AM, Ladisch S. Ganglioside synthase knockout in oncogene-transformed fibroblasts depletes gangliosides and impairs tumor growth. Oncogene. 2010;29:3297–3306. doi: 10.1038/onc.2010.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ladisch S, Gillard B, Wong C, Ulsh L. Shedding and immunoregulatory activity of YAC-1 lymphoma cell gangliosides. Cancer Res. 1983;43:3808–3813. [PubMed] [Google Scholar]
- 6.Valentino L, Moss T, Olson E, Wang HJ, Elashoff R, Ladisch S. Shed tumor gangliosides and progression of human neuroblastoma. Blood. 1990;75:1564–1567. [PubMed] [Google Scholar]
- 7.Portoukalian J, David MJ, Gain P, Richard M. Shedding of GD2 ganglioside in patients with retinoblastoma. Int J Cancer. 1993;53:948–951. doi: 10.1002/ijc.2910530614. [DOI] [PubMed] [Google Scholar]
- 8.Ladisch S, Chang F, Li R, Cogen P, Johnson D. Detection of medulloblastoma and astrocytoma-associated ganglioside GD3 in cerebrospinal fluid. Cancer Lett. 1997;120:71–78. doi: 10.1016/s0304-3835(97)00297-8. [DOI] [PubMed] [Google Scholar]
- 9.Chang F, Li R, Ladisch S. Shedding of gangliosides by human medulloblastoma cells. Exp Cell Res. 1997;234:341–346. doi: 10.1006/excr.1997.3619. [DOI] [PubMed] [Google Scholar]
- 10.Gentles AJ, Newman AM, Liu CL, Bratman SV, Feng W, Kim D, Nair VS, Xu Y, Khuong A, Hoang CD, Diehn M, West RB, Plevritis SK, Alizadeh AA. The prognostic landscape of genes and infiltrating immune cells across human cancers. Nat Med. 2015;21:938–945. doi: 10.1038/nm.3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shen W, Stone K, Jales A, Leitenberg D, Ladisch S. Inhibition of TLR activation and up-regulation of IL-1R-associated kinase-M expression by exogenous gangliosides. J Immunol. 2008;180:4425–4432. doi: 10.4049/jimmunol.180.7.4425. [DOI] [PubMed] [Google Scholar]
- 12.Nakamura O, Iwamori M, Matsutani M, Takakura K. Ganglioside GD3 shedding by human gliomas. Acta Neurochir (Wien) 1991;109:34–36. doi: 10.1007/BF01405694. [DOI] [PubMed] [Google Scholar]
- 13.Alshaker HA, Matalka KZ. IFN-gamma, IL-17 and TGF-beta involvement in shaping the tumor microenvironment: The significance of modulating such cytokines in treating malignant solid tumors. Cancer Cell Int. 2011;11:33. doi: 10.1186/1475-2867-11-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schroder K, Sweet MJ, Hume DA. Signal integration between IFNgamma and TLR signalling pathways in macrophages. Immunobiology. 2006;211:511–524. doi: 10.1016/j.imbio.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 15.Hu X, Ivashkiv LB. Cross-regulation of signaling pathways by interferon-gamma: implications for immune responses and autoimmune diseases. Immunity. 2009;31:539–550. doi: 10.1016/j.immuni.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gajewski TF, Schreiber H, Fu YX. Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol. 2013;14:1014–1022. doi: 10.1038/ni.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaplan DH, Shankaran V, Dighe AS, Stockert E, Aguet M, Old LJ, Schreiber RD. Demonstration of an interferon gamma-dependent tumor surveillance system in immunocompetent mice. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:7556–7561. doi: 10.1073/pnas.95.13.7556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kelchtermans H, Billiau A, Matthys P. How interferon-gamma keeps autoimmune diseases in check. Trends in immunology. 2008;29:479–486. doi: 10.1016/j.it.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 19.Mandai M, Hamanishi J, Abiko K, Matsumura N, Baba T, Konishi I. Dual Faces of IFNgamma in Cancer Progression: A Role of PD-L1 Induction in the Determination of Pro-and Antitumor Immunity. Clin Cancer Res. 2016;22:2329–2334. doi: 10.1158/1078-0432.CCR-16-0224. [DOI] [PubMed] [Google Scholar]
- 20.Jurgens B, Hainz U, Fuchs D, Felzmann T, Heitger A. Interferon-gamma-triggered indoleamine 2,3-dioxygenase competence in human monocyte-derived dendritic cells induces regulatory activity in allogeneic T cells. Blood. 2009;114:3235–3243. doi: 10.1182/blood-2008-12-195073. [DOI] [PubMed] [Google Scholar]
- 21.Yamazaki T, Akiba H, Iwai H, Matsuda H, Aoki M, Tanno Y, Shin T, Tsuchiya H, Pardoll DM, Okumura K, Azuma M, Yagita H. Expression of programmed death 1 ligands by murine T cells and APC. J Immunol. 2002;169:5538–5545. doi: 10.4049/jimmunol.169.10.5538. [DOI] [PubMed] [Google Scholar]
- 22.Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne MC, Horton HF, Fouser L, Carter L, Ling V, Bowman MR, Carreno BM, Collins M, Wood CR, Honjo T. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192:1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang X, Teng F, Kong L, Yu J. PD-L1 expression in human cancers and its association with clinical outcomes. Onco Targets Ther. 2016;9:5023–5039. doi: 10.2147/OTT.S105862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ahmadzadeh M, Johnson LA, Heemskerk B, Wunderlich JR, Dudley ME, White DE, Rosenberg SA. Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood. 2009;114:1537–1544. doi: 10.1182/blood-2008-12-195792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wherry EJ. T cell exhaustion. Nat Immunol. 2011;12:492–499. doi: 10.1038/ni.2035. [DOI] [PubMed] [Google Scholar]
- 26.Lanzinger M, Jurgens B, Hainz U, Dillinger B, Raberger J, Fuchs D, Heitger A. Ambivalent effects of dendritic cells displaying prostaglandin E2-induced indoleamine 2,3-dioxygenase. Eur J Immunol. 2012;42:1117–1128. doi: 10.1002/eji.201141765. [DOI] [PubMed] [Google Scholar]
- 27.Shen W, Ladisch S. Ganglioside GD1a impedes lipopolysaccharide-induced maturation of human dendritic cells. Cellular immunology. 2002;220:125–133. doi: 10.1016/s0008-8749(03)00004-2. [DOI] [PubMed] [Google Scholar]
- 28.Soukup K, Halfmann A, Le Bras M, Sahin E, Vittori S, Poyer F, Schuh C, Luger R, Niederreiter B, Haider T, Stoiber D, Bluml S, Schabbauer G, Kotlyarov A, Gaestel M, Felzmann T, Dohnal AM. The MAPK-Activated Kinase MK2 Attenuates Dendritic Cell-Mediated Th1 Differentiation and Autoimmune Encephalomyelitis. J Immunol. 2015;195:541–552. doi: 10.4049/jimmunol.1401663. [DOI] [PubMed] [Google Scholar]
- 29.Ladisch S, Becker H, Ulsh L. Immunosuppression by human gangliosides: I. Relationship of carbohydrate structure to the inhibition of T cell responses. Biochim Biophys Acta. 1992;1125:180–188. doi: 10.1016/0005-2760(92)90043-u. [DOI] [PubMed] [Google Scholar]
- 30.Takikawa O, Kuroiwa T, Yamazaki F, Kido R. Mechanism of interferon-gamma action. Characterization of indoleamine 2,3-dioxygenase in cultured human cells induced by interferon-gamma and evaluation of the enzyme-mediated tryptophan degradation in its anticellular activity. The Journal of biological chemistry. 1988;263:2041–2048. [PubMed] [Google Scholar]
- 31.Van Gool SW, Vandenberghe P, de Boer M, Ceuppens JL. CD80, CD86 and CD40 provide accessory signals in a multiple-step T-cell activation model. Immunological reviews. 1996;153:47–83. doi: 10.1111/j.1600-065x.1996.tb00920.x. [DOI] [PubMed] [Google Scholar]
- 32.Sheng KC, Day S, Wright MD, Stojanovska L, Apostolopoulos V. Enhanced Dendritic Cell-Mediated Antigen-Specific CD4+ T Cell Responses: IFN-Gamma Aids TLR Stimulation. Journal of drug delivery. 2013;2013:516749. doi: 10.1155/2013/516749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hayes MP, Wang J, Norcross MA. Regulation of interleukin-12 expression in human monocytes: selective priming by interferon-gamma of lipopolysaccharide-inducible p35 and p40 genes. Blood. 1995;86:646–650. [PubMed] [Google Scholar]
- 34.Heitger A, Ladisch S. Gangliosides block antigen presentation by human monocytes. Biochim Biophys Acta. 1996;1303:161–168. doi: 10.1016/0005-2760(96)00091-4. [DOI] [PubMed] [Google Scholar]
- 35.Zhai L, Dey M, Lauing KL, Gritsina G, Kaur R, Lukas RV, Nicholas MK, Rademaker AW, Dostal CR, McCusker RH, Raizer JJ, Parsa AT, Bloch O, Wainwright DA. The kynurenine to tryptophan ratio as a prognostic tool for glioblastoma patients enrolling in immunotherapy. J Clin Neurosci. 2015;22:1964–1968. doi: 10.1016/j.jocn.2015.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang Y, Cui Y, Cao F, Qin Y, Li W, Zhang J. Ganglioside GD1a suppresses LPS-induced pro-inflammatory cytokines in RAW264.7 macrophages by reducing MAPKs and NF-kappaB signaling pathways through TLR4. Int Immunopharmacol. 2015;28:136–145. doi: 10.1016/j.intimp.2015.05.044. [DOI] [PubMed] [Google Scholar]
- 37.Apetoh L, Locher C, Ghiringhelli F, Kroemer G, Zitvogel L. Harnessing dendritic cells in cancer. Seminars in immunology. 2011;23:42–49. doi: 10.1016/j.smim.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 38.Shen W, Falahati R, Stark R, Leitenberg D, Ladisch S. Modulation of CD4 Th cell differentiation by ganglioside GD1a in vitro. Journal of immunology. 2005;175:4927–4934. doi: 10.4049/jimmunol.175.8.4927. [DOI] [PubMed] [Google Scholar]
- 39.Von Bubnoff D, Scheler M, Wilms H, Fimmers R, Bieber T. Identification of IDO-positive and IDO-negative human dendritic cells after activation by various proinflammatory stimuli. Journal of immunology. 2011;186:6701–6709. doi: 10.4049/jimmunol.1003151. [DOI] [PubMed] [Google Scholar]
- 40.Caldwell S, Heitger A, Shen W, Liu Y, Taylor B, Ladisch S. Mechanisms of ganglioside inhibition of APC function. Journal of immunology. 2003;171:1676–1683. doi: 10.4049/jimmunol.171.4.1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mieczkowski J, Kocyk M, Nauman P, Gabrusiewicz K, Sielska M, Przanowski P, Maleszewska M, Rajan WD, Pszczolkowska D, Tykocki T, Grajkowska W, Kotulska K, Roszkowski M, Kostkiewicz B, Kaminska B. Down-regulation of IKKbeta expression in glioma-infiltrating microglia/macrophages is associated with defective inflammatory/immune gene responses in glioblastoma. Oncotarget. 2015;6:33077–33090. doi: 10.18632/oncotarget.5310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hakomori S. Glycolipids of tumor cell membrane. Adv Cancer Res. 1973;18:265–315. doi: 10.1016/s0065-230x(08)60755-1. [DOI] [PubMed] [Google Scholar]
- 43.Ladisch S, Wu ZL. Detection of a tumour-associated ganglioside in plasma of patients with neuroblastoma. Lancet. 1985;1:136–138. doi: 10.1016/s0140-6736(85)91906-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wurdinger T, Deumelandt K, van der Vliet HJ, Wesseling P, de Gruijl TD. Mechanisms of intimate and long-distance cross-talk between glioma and myeloid cells: how to break a vicious cycle. Biochim Biophys Acta. 2014;1846:560–575. doi: 10.1016/j.bbcan.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 45.Comas TC, Tai T, Kimmel D, Scheithauer BW, Burger PC, Pearl DK, Jewell SD, Yates AJ. Immunohistochemical staining for ganglioside GD1b as a diagnostic and prognostic marker for primary human brain tumors. Neuro Oncol. 1999;1:261–267. doi: 10.1093/neuonc/1.4.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ehtesham M, Samoto K, Kabos P, Acosta FL, Gutierrez MA, Black KL, Yu JS. Treatment of intracranial glioma with in situ interferon-gamma and tumor necrosis factor-alpha gene transfer. Cancer Gene Ther. 2002;9:925–934. doi: 10.1038/sj.cgt.7700516. [DOI] [PubMed] [Google Scholar]
- 47.Farkkila M, Jaaskelainen J, Kallio M, Blomstedt G, Raininko R, Virkkunen P, Paetau A, Sarelin H, Mantyla M. Randomised, controlled study of intratumoral recombinant gamma-interferon treatment in newly diagnosed glioblastoma. Br J Cancer. 1994;70:138–141. doi: 10.1038/bjc.1994.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wolff JE, Wagner S, Reinert C, Gnekow A, Kortmann RD, Kuhl J, Van Gool SW. Maintenance treatment with interferon-gamma and low-dose cyclophosphamide for pediatric high-grade glioma. J Neurooncol. 2006;79:315–321. doi: 10.1007/s11060-006-9147-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.