Abstract
Aims
Hospitalizations for heart failure (HF) are common and associated with significant morbidity, mortality and costs. However, precipitating factors leading to HF hospitalization and their importance with respect to subsequent outcomes are not well understood.
Methods and Results
We prospectively collected the symptoms and signs present on admission and investigator-identified factors thought to have contributed to the first adjudicated HF hospitalization in the Candesartan in Heart Failure Assessment of Reduction in Mortality and morbidity program (CHARM), stratified by ejection fraction (EF). Potential precipitants were collected using a specifically designed case report form and then categorized as cardiovascular (CV), non-cardiovascular (non-CV) and unknown. We examined the associations between these factors and subsequent re-hospitalization and mortality rates. Of 1,668 patients who experienced HF hospitalization, 1,152 had reduced EF (HFrEF; EF ≤40%) and 516 had preserved EF (HFpEF). Overall, 54% had CV, 32% non-CV, and 14% unknown factors thought to have precipitated HF, with similar proportions in HFrEF and HFpEF. The most common precipitants were arrhythmia (15%), other non-CV reasons (11%), and respiratory infection (10%). Subsequent CV re-admission rates were highest in those whose initial HF hospitalization was precipitated by CV factors. However, mortality rates were similar among patients with any of the three categories of precipitating factors. Results were similar in HFrEF and HFpEF.
Conclusions
Among chronic HF patients hospitalized for decompensation, the investigator-reported precipitating factor was not associated with the subsequent mortality rate but was associated with the type of re-admission: re-admissions for CV reasons were more likely when the index precipitant was CV.
Keywords: Heart failure, hospitalization, precipitating factors, ejection fraction, prognosis
Introduction
Heart failure (HF) is a leading reason for hospitalization in Western populations over the age of 65 and is associated with significant cost, morbidity, and subsequent mortality.1 Precipitating factors leading to HF hospitalization have been identified in prior, mainly retrospective, studies and include arrhythmias, myocardial ischemia, infections, worsening renal function, uncontrolled hypertension and non-compliance with medications or diet.2–5 However, the relationship between these factors and long-term morbidity and mortality, including recurrent hospitalizations, is not well understood. Similarly, the clinical signs and symptoms on admission in patients hospitalized for HF according to these precipitants are poorly described.
A better understanding of the effect of precipitating factors of HF hospitalizations is important for several reasons. First, the identification of modifiable factors leading to HF hospitalizations may help inform strategies to mitigate recurrent admission. Second, the association of these factors with recurrent hospitalizations may inform the design of future clinical trials, for instance in the selection of high-risk patient populations. Traditionally, only the first hospitalization for HF has been analyzed as an endpoint in clinical trial reports and observational studies. However, this approach does not consider the burden of recurrent events to patients, the healthcare system and payers. Analyses of recurrent hospitalizations among patients with HF are, thus, gaining increasing interest and have the potential to improve the efficiency and reduce cost of future clinical trials.6, 7
In this study we examined prospectively collected, investigator-identified, reasons thought to have contributed to the first hospitalization for HF in the Candesartan in Heart Failure Assessment of Reduction in Mortality and morbidity program (CHARM) and the association between these contributors to HF hospitalization and subsequent recurrent admissions, as well as the rate and cause of subsequent death. Since literature on the precipitants of HF hospitalization in individuals with HFrEF and HFpEFis sparse, we also examined these variables stratified by ejection fraction.
Methods
Patient population
The Candesartan in Heart Failure: Assessment of Reduction in Mortality and morbidity (CHARM) program randomized 7,599 patients with New York Heart Association (NYHA) class II–IV HF to candesartan or placebo in addition to standard HF therapy. The design and main results of this trial have been previously reported.8 Briefly, the program consisted of three concurrent trials (March 1999 – March 2003): CHARM-Alternative included HF patients with an EF≤40% who were intolerant to ACE-inhibitors, CHARM-Added included HF patients with an EF≤40% who were being treated with an ACE-inhibitor, and CHARM-Preserved included HF patients with an EF>40% most of whom were not treated with an ACE-inhibitor. The CHARM trials were approved by institutional review boards for each study site and all enrolled patients provided informed consent for study participation. Patients were excluded from this analysis if there was no primary precipitating factor reported for the first adjudicated HF hospitalization (n=7) or if the ejection fraction was not documented (n=1). For the purpose of this analysis, we focused on patients with a first adjudicated HF hospitalization (n=1,668).
Outcomes
The primary outcome of the overall program was all-cause mortality and for the component trials it was the composite of cardiovascular (CV) death or hospital admission for HF. First hospitalizations for HF were adjudicated, while subsequent HF hospitalizations were investigator reported, as were non-HF hospitalizations. The median follow up for the overall program was 37.7 months. Due to uncertain discharge dates, 2 patients were excluded from the annual incidence rates for HF, CV, non-CV and all-cause readmissions. For patients with missing discharge dates for the first HF hospitalization (n=141), discharge dates were imputed assuming a 5 day length of stay based on the median hospital length of stay in this trial. Patients who ended study while hospitalized (n=7) did not contribute to the calculation of annual incidence rates for HF, CV, non-CV and all-cause readmissions.
Identification of precipitating factors
When reporting HF hospitalizations after randomization, investigators were asked to report possible precipitating and aggravating factors and to assign a primary reason for the HF hospitalization to one of several predefined reasons: non-compliance with cardiac medications, inappropriate decrease of anti-failure therapy, excessive salt intake/dietary non-compliance, cardiac arrhythmias, acute myocardial ischemia/myocardial infarction, anemia, febrile illness, other high-output state, excessive alcohol intake, concomitant drug use within previous 48 hours (calcium channel blockers, beta-blockers, antiarrhythmic drugs other than amiodarone, non-steroidal anti-inflammatory drugs), any other non-cardiac precipitating or associated illness or factor, precipitating valvular disease, any other precipitating or aggravating factor(s). Only the primary precipitant identified by investigators was utilized in this analysis. Of those first adjudicated HF hospitalization which were assigned to either “any other non-cardiac precipitating or associated illness or factor” (n=282) or to “any other precipitating or aggravating factor(s)” (n=543) free text descriptions of the primary reason were individually reviewed by a physician and used to reclassify the precipitating factors into specific CV, non-CV and unknown factors.
Statistical analyses
Baseline characteristics for patients according to precipitant factor were compared using chi-squared or Fisher’s exact test, as appropriate, for categorical and ANOVA for continuous variables. In additional analyses, investigator-identified clinical characteristics (HF signs and symptoms) and precipitating factors for HF hospitalizations, stratified by those with reduced (≤40%) and preserved (>40%) EF, were analyzed using chi-squared or Fisher’s exact test, as appropriate.
To assess possible associations between precipitating factors leading to the first HF hospitalization on subsequent hospitalizations and mortality we compared incident all-cause re-admission rates by the 3 precipitating factor groups, stratified by EF group, using chi-squared or Fisher’s exact test, as appropriate. To assess the cumulative incidence rates of subsequent HF hospitalization, the crude number of HF hospitalizations per 100 patient-years of follow up after discharge from the initial hospitalization for HF was calculated by dividing the total number HF hospitalizations by the total follow up time of all patients in each group. The resulting incidence rates, 95% confidence intervals (CI) and p-value were based on the Poisson distribution.9
All tests were two-sided, and a p-value of <0.05 was considered statistically significant. All analyses were conducted using STATA SE, version 12.1 (College Station, TX).
Results
Baseline characteristics, according to type of precipitant
Overall, 1,668 patients enrolled in the CHARM program who were hospitalized for HF based on adjudication criteria, were included in this analysis. Their baseline characteristics, according to type of precipitant, are presented in Table 1. Investigators identified a probable CV precipitant in 54% (n=895) of first HF hospitalizations, a non-CV precipitant in 32% (n=538) and could not identify any precipitant (precipitant unknown) in 14% (n=235). Baseline characteristics were broadly similar across the 3 groups. Of all patients hospitalized for HF, 1,152 (69%) had HFrEF at baseline and 516 (31%) had HFpEF.
Table 1.
Baseline characteristics (n=1,668)
| CV reasons (n=895) | Non-CV reasons (n=538) | Unknown reason (n=235) | P | |
|---|---|---|---|---|
| Demographics | ||||
| Age (years) | 68 (11) | 68 (11) | 67 (11) | 0.199 |
| Men | 614 (69) | 360 (67) | 168 (72) | 0.449 |
| Ethnicity | <0.001 | |||
| European | 786 (88) | 488 (91) | 193 (82) | 0.003 |
| Black | 55 (6.2) | 22 (4.1) | 9 (3.8) | 0.163 |
| Other | 54 (6.0) | 28 (5.2) | 33 (14) | <0.001 |
| Clinical characteristics | ||||
| NYHA class | 0.559 | |||
| II | 290 (32) | 160 (30) | 81 (35) | |
| III | 557 (62) | 348 (65) | 138 (59) | |
| IV | 48 (5.4) | 30 (5.6) | 16 (6.8) | |
| Mean LVEF (%) | 35 (15) | 37 (15) | 35 (14) | 0.281 |
| Heart rate (bpm) | 75 (14) | 74 (12) | 76 (14) | 0.403 |
| Systolic BP (mmHg) | 129 (20) | 130 (20) | 127 (20) | 0.269 |
| Diastolic BP (mmHg) | 75 (11) | 74 (11) | 75 (11) | 0.277 |
| BMI (kg/m2) | 28 (6) | 29 (6) | 27 (6) | 0.021 |
| Clinical evidence of HF | ||||
| Dyspnea when walking on level ground | 634 (71) | 401 (75) | 178 (76) | 0.167 |
| Orthopnea | 246 (28) | 158 (29) | 62 (26) | 0.631 |
| PND | 168 (19) | 101 (19) | 41 (18) | 0.889 |
| JVD | 126 (14) | 69 (13) | 34 (15) | 0.751 |
| Crackles (any) | 202 (23) | 127 (24) | 59 (25) | 0.696 |
| S3 | 143 (16) | 100 (19) | 34 (15) | 0.279 |
| Peripheral edema | 306 (34) | 202 (38) | 77 (33) | 0.316 |
| Medical history | ||||
| Hospital admission for HF | 749 (84) | 449 (84) | 204 (87) | 0.463 |
| Myocardial infarction | 481 (54) | 286 (53) | 129 (55) | 0.906 |
| Stroke | 98 (11) | 59 (11) | 23 (10) | 0.866 |
| Diabetes mellitus | 352 (39) | 239 (44) | 94 (40) | 0.155 |
| Hypertension | 530 (59) | 318 (59) | 124 (53) | 0.181 |
| Atrial fibrillation | 309 (35) | 191 (36) | 81 (35) | 0.924 |
| Pacemaker/ICD | 133 (15) | 82 (15) | 44 (19) | 0.338 |
| ECG: Bundle branch block | 279 (31) | 175 (33) | 74 (32) | 0.698 |
| Current smoker | 108 (12) | 72 (13) | 40 (17) | 0.134 |
| Cancer | 72 (8.0) | 46 (8.6) | 10 (4.3) | 0.099 |
| HF etiology | ||||
| Ischemic | 538 (60) | 329 (61) | 151 (64) | 0.510 |
| Idiopathic dilated cardiomyopathy | 157 (18) | 87 (16) | 43 (18) | 0.715 |
| Hypertensive | 113 (13) | 70 (13) | 23 (9.8) | 0.426 |
| Valvular | 33 (3.7) | 18 (3.4) | 5 (2.1) | 0.549 |
| Diabetes | 3 (0.3) | 0 | 4 (1.7) | 0.007 |
| Alcohol related | 8 (0.9) | 3 (0.6) | 0 | 0.369 |
| Atrial fibrillation | 24 (2.7) | 12 (2.2) | 4 (1.7) | 0.715 |
| Other | 19 (2.1) | 19 (3.5) | 5 (2.1) | 0.268 |
| Medical treatment | ||||
| ACE inhibitor | 414 (46) | 258 (48) | 112 (48) | 0.804 |
| Betablocker | 409 (46) | 236 (44) | 108 (46) | 0.768 |
| Diuretic | 839 (94) | 504 (94) | 226 (96) | 0.337 |
| Spironolactone | 186 (21) | 120 (22) | 54 (23) | 0.679 |
| Digoxin | 491 (55) | 287 (54) | 117 (50) | 0.376 |
| Calcium channel blocker | 169 (19) | 120 (22) | 34 (15) | 0.035 |
| Long acting nitrates | 381 (43) | 204 (38) | 88 (38) | 0.137 |
| Oral anticoagulants | 353 (39) | 204 (38) | 84 (36) | 0.559 |
| Aspirin | 439 (49) | 279 (52) | 120 (51) | 0.567 |
Categorical variables are presented as counts (percentages), continuous variables as means (SD), unless otherwise specified.
NYHA: New York Heart Association class, LVEF: Left ventricular ejection fraction, BP: Blood pressure, BMI: Body mass index, HF: Heart failure, PND: Postural nocturnal dyspnea, JVD: Jugular venous distension, ICD: Implanted cardiac defibrillator, ECG: Electrocardiogram, ACE: Angiotensin converting enzyme.
Investigator-identified precipitating factors leading to HF hospitalization
Among the CV precipitants, the five most commonly reported were: 1) an arrhythmia (HFrEF 15% vs. HFpEF 16% of all precipitants; 27% vs. 31% of CV precipitants), 2) non-compliance/inappropriate decrease in HF therapy (11% vs. 7.6%), 3) dietary indiscretion/excessive oral or IV fluids (8.3% vs. 8.5%), 4) myocardial ischemia/angina (7.4 vs. 8.7%) and 5) worsening HF/disease progression (9.0% vs. 3.3%; p<0.001) (Table 2). Although uncommon, uncontrolled hypertension was more often identified as a precipitant in patients with HFpEF (in 3.1% of admissions) compared with HFrEF (1.2%; p=0.007).
Table 2.
Physician-identified primary reason for worsening heart failure leading to first heart failure hospitalization
| n (%) | All patients (n=1,668) | EF≤40% (n=1,152) | EF>40% (n=516) | P* |
|---|---|---|---|---|
| Cardiovascular reasons | 895 (54) | 628 (55) | 267 (52) | 0.294 |
| Arrhythmia | 252 (15) | 169 (15) | 83 (16) | 0.456 |
| Non-compliance/inappropriate decrease in HF therapy | 160 (9.6) | 121 (11) | 39 (7.6) | 0.059 |
| Dietary indiscretion/excessive oral fluid intake/IV fluids | 139 (8.3) | 95 (8.3) | 44 (8.5) | 0.848 |
| Myocardial ischemia/angina | 130 (7.8) | 85 (7.4) | 45 (8.7) | 0.344 |
| Worsening HF/disease progression | 121 (7.3) | 104 (9.0) | 17 (3.3) | <0.001 |
| Valvular disease | 42 (2.5) | 25 (2) | 17 (3.3) | 0.175 |
| Uncontrolled hypertension | 30 (1.8) | 14 (1.2) | 16 (3.1) | 0.007 |
| Other CV reasons | 21 (1.3) | 15 (1.3) | 6 (1.2) | 0.814 |
| Non-cardiovascular reasons | 538 (32) | 358 (31) | 180 (35) | 0.124 |
| Respiratory infection | 174 (10) | 115 (10) | 59 (11) | 0.370 |
| Worsening renal function/renal failure | 61 (3.7) | 36 (3.1) | 25 (4.8) | 0.084 |
| Other infection | 35 (2.1) | 22 (1.9) | 13 (2.5) | 0.422 |
| Anemia | 32 (1.9) | 16 (1.4) | 16 (3.1) | 0.018 |
| COPD/asthma | 16 (1.0) | 13 (1.1) | 3 (0.6) | 0.417 |
| Exertion/increased exercise | 13 (0.8) | 13 (1.1) | 0 | 0.013 |
| Depression/anxiety/emotional stress | 10 (0.6) | 7 (0.6) | 3 (0.6) | 0.949 |
| Diabetes/diabetes medication related reasons | 9 (0.5) | 2 (0.2) | 7 (1.4) | 0.005 |
| NSAID use | 9 (0.5) | 7 (0.6) | 2 (0.4) | 0.729 |
| Other non-CV reasons | 179 (11) | 127 (11) | 52 (10) | 0.564 |
| Unknown reason | 235 (14) | 166 (14) | 69 (13) | 0.573 |
Chi squared or Fisher’s exact test comparing two EF groups, as appropriate.
EF: Ejection fraction, HF: Heart failure, CV: Cardiovascular, COPD: Chronic obstructive pulmonary disease, NSAID: Non-steroidal anti-inflammatory drugs
Among the non-CV reasons for admission, respiratory infection was by far the most common individual precipitant (10% vs. 11% of all precipitants; 32% vs. 33% of non-CV precipitants), with worsening renal function (3.1% vs. 4.8%), other infection (1.9% vs. 2.5%) and anemia (1.4% vs. 3.1%; p=0.018) the other most commonly identified precipitants. Although less common, a diabetes-related reason was more commonly reported as a precipitating factor in HFpEF (0.2% vs. 1.4%; p=0.005) and physical exertion more frequently in HFrEF (1.1% vs. 0%; p=0.013). There was also a large category of “other” non-CV precipitants (11% vs.10%).
The proportion of patients with an unknown reason for HF hospitalization was similar in the two HF groups (14% vs 13%).
Investigator–identified clinical evidence of HF, according to type of precipitant
Investigator-identified symptoms and signs at the time of the first hospitalization for HF were similar among the precipitating factor groups, both in HFrEF and HFpEF, with the exception of pulmonary edema which was more commonly reported in patients with HFpEF when the precipitating factor was thought to be CV (39.7% CV, 29.4% non-CV, 24.6% unknown precipitant; p=0.016) (Figure 1). The proportions of patients receiving intravenous diuretics (92% HFrEF, 91% HFpEF, p=0.496) and intravenous vasodilators (16% both groups, p=0.935) were similar, but patients with HFrEF were more likely to have received intravenous inotropic agents than patients with HFpEF (21% vs. 13%, p<0.001).
Figure 1. Investigator-identified clinical evidence of worsening heart failure at time of first HF hospitalization.
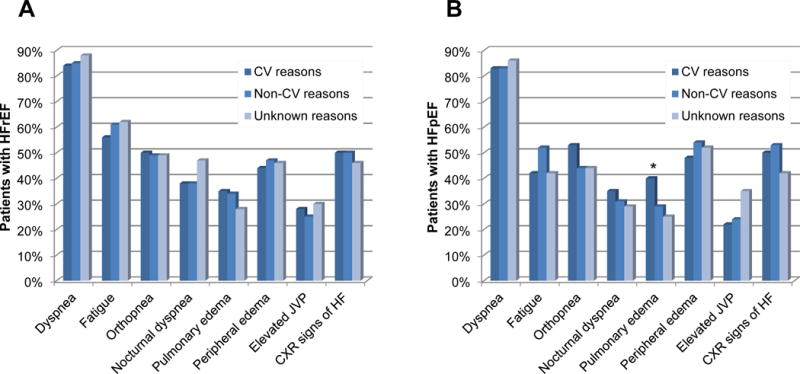
Panel A: Patients with HFrEF (EF≤40%) (n=1,152)
Panel B: Patients with HFpEF (EF>40%) (n=516)
*P<0.05 for comparison between precipitating factor groups
HFrEF: Heart failure with reduced ejection fraction, HFpEF: Heart failure with preserved ejection fraction, CV: Cardiovascular, JVP: Jugular venous pressure, CXR: Chest x-ray
Precipitating causes and subsequent mortality
CV precipitants of hospital admission did not selectively identify patients more likely to die from a CV cause (annual incidence rate: 39 (95% CI: 35, 44) per 100 patient-years for HFrEF, 29 (95% CI: 24, 35) for HFpEF) and a non-CV precipitant of hospitalization (annual incidence rate: 39 (95% CI: 34, 45) per 100 patient-years for HFrEF, 32 (95% CI: 26, 40) for HFpEF) didn’t make a subsequent non-CV death more likely than a CV death (Table 3). Patients with an unknown precipitant were at slightly lower risk of death (both all-cause and CV) and this was also true for both types of HF (HFrEF and HFpEF).
Table 3.
Events following first heart failure hospitalization
| First hospitalization for HF EF≤40% | First hospitalization for HF EF>40% | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| All HFrEF patients (n=1,152) |
Group A CV reasons for HF (n=628) |
Group B: Non-CV reasons for HF (n=358) |
Group C: Unknown reasons (n=166) |
P | All HFpEF patients (n=516) |
Group A: CV reasons for HF (n=267) |
Group B: Non-CV reasons for HF (n=180) |
Group C: Unknown reasons (n=69) |
P | |
| Readmissions following 1st HF hospitalization | ||||||||||
| Annual incidence rate of all-cause readmissions (per 100 pt-yrs; 95% CI)* | 179 (172, 186) | 187 (177, 197) | 174 (162, 187) | 162 (146, 180) | 0.032 | 173 (164, 184) | 181 (168, 196) | 165 (149, 182) | 166 (143, 193) | 0.273 |
| Annual incidence rate of HF readmissions (per 100 pt-yrs; 95% CI)* | 79 (75, 84) | 86 (80, 93) | 70 (62, 78) | 74 (64, 87) | 0.005 | 59 (54, 65) | 61 (53, 69) | 52 (44, 62) | 71 (57, 90) | 0.103 |
| Annual incidence rate of CV readmissions (per 100 pt-yrs; 95% CI)* | 127 (121, 133) | 138 (130, 147) | 111 (102, 121) | 120 (107, 136) | <0.001 | 108 (101, 116) | 118 (108, 130) | 94 (82, 107) | 106 (88, 128) | 0.020 |
| Annual incidence rate of non-CV readmissions (per 100 pt-yrs; 95% CI)* | 52 (48, 56) | 49 (44, 54) | 63 (56, 71) | 42 (34, 51) | <0.001 | 65 (59, 71) | 63 (55, 72) | 71 (61, 82) | 60 (47, 78) | 0.433 |
| Mortality following 1st HF hospitalization | ||||||||||
| Annual incidence of all-cause death (per 100 pt-yrs; 95% CI)** | 39 (36, 42) | 39 (35, 44) | 39 (34, 45) | 36 (29, 45) | 0.913 | 28 (25, 33) | 29 (24, 35) | 32 (26, 40) | 19 (13, 30) | 0.136 |
| Annual incidence of CV death (per 100 pt-yrs; 95% CI)** | 35 (32, 38) | 35 (31, 40) | 34 (29, 40) | 33 (27, 42) | 0.963 | 22 (19, 26) | 22 (17, 27) | 25 (20, 33) | 17 (11, 28) | 0.360 |
P value: Global chi squared or Fisher’s exact test, as appropriate, comparing groups A, B and C stratified by EF group.
P value: Log rank test comparing groups A, B and C stratified by EF group.
HF: Heart failure, CV: Cardiovascular, pt: Patient, yrs: Years, CI: Confidence interval
Precipitating causes and subsequent re-hospitalization
The picture regarding re-admission was different than that for mortality. The overall re-admission rate was similar in patients with HFrEF (annual incidence rate: 179 (95% CI: 172, 186) per 100 patient-years) and HFpEF (173 (95% CI: 164, 184) per 100 patient-years) and highest among those with a CV precipitant of their index hospitalization for both HFrEF (187 (95% CI: 177, 197) per 100 patient-years) and HFpEF (181 (95% CI: 168, 196) per 100 patient-years)). Compared to patients with a non-CV precipitant of their index admission, those with a CV precipitant were relatively more likely to have a subsequent HF (and any CV) admission and relatively less likely to have a subsequent non-CV admission. This pattern was apparent in both HFrEF and HFpEF (Table 3).
Discussion
Our main findings were: 1) CV reasons were thought to be the precipitant for HF admissions in more than half of cases and non-CV reasons in one third, with the remainder of admissions having no prospectively identified precipitant. 2) Among the CV and non-CV precipitants, there was no single very common and only a few common causes. 3) The precipitants that were identified were largely similar for HFrEF and HFpEF, although there were a few differences. 4) The type of precipitant (CV or non-CV) was not associated with the subsequent cause of death but was associated with re-admission type.
Investigator-identified precipitating factors leading to HF hospitalization
A number of precipitating factors believed to be associated with HF hospitalizations have previously been reported but these have almost exclusively been collected retrospectively. One exception was the RESOLVD pilot study, in which, among 768 patients with HFrEF, the most common primary causes leading to HF hospitalizations were thought to be non-adherence to salt restriction (15%), other non-cardiac causes (15%), and inappropriate reductions in HF therapy (9%).2 Within the “other” and “other non-cardiac” precipitating factor categories, investigators noted respiratory infections, use of a beta-blocker (the study drug metoprolol) and excessive fluid intake as most common causes. While the proportion of non-cardiac causes and inappropriate reductions in HF therapy were similar in our study, non-adherence in salt restriction was reported less frequently in CHARM. This difference could be due to either patient education efforts regarding salt restriction in the CHARM cohort or alternative primary precipitants (e.g. arrhythmia) which were more commonly identified in CHARM. Although both CHARM and RESOLVD were multi-site international trials, geographic variability in salt intake may contribute to this difference. In the OPTIMIZE-HF registry of 48,612 patients hospitalized for HF (mean EF 39%) in the USA, 61.3% patients had one or more pre-specified precipitating factors identified, with pneumonia/respiratory process (15.3%), myocardial ischemia (14.7%), and an arrhythmia (13.5%) being most frequent.4 The OPTIMIZE-HF report did not differentiate between patients with HFrEF and HFpEF. A more recent international AHF registry (GREAT) of 15,828 patients hospitalized for HF in Europe and Asia identified one precipitating factor in 49% of patients, multiple factors in 6% and no known precipitants in 44%. Of those with a single precipitating factor, the most common reported precipitants were acute coronary syndrome (52%), atrial fibrillation (16%) and infection (14%).10 The higher rates of precipitant infection and myocardial ischemia in the AHF registries as compared with CHARM may be related to the difference between an AHF registry and a chronic HF clinical trial dataset. Since our analysis is based on adjudicated HF hospitalizations, myocardial infarctions complicated by AHF would have been adjudicated as myocardial infarction rather than HF hospitalization based on the pre-defined event definitions and respiratory infections without significant concomitant volume overload may not have qualified as HF admission.
Recently, registry data from the US Get With The Guidelines-HF database, a prospective observational study of patients hospitalized for HF with documented EF, reported that the most common factors thought to precipitate HF hospitalizations included pneumonia/respiratory problem (28.2%), arrhythmia (21.7%), medication non-compliance (15.8%), worsening renal failure (14.7%), and uncontrolled hypertension (14.5%).5 Some of these factors varied by EF group (EF <40%, 40–49%, ≥50%) and were independently associated with hospital length of stay and inpatient mortality. Long term outcomes were not reported. This registry also identified higher rates of respiratory infection, arrhythmias, medication non-compliance, worsening renal function and hypertension than ours. Although the leading precipitating factors were similar to ours, the proportions in these groups were higher than in CHARM. Registry cohorts may differ from clinical trial cohorts due to exclusion criteria which may select a patient population with generally better renal function, blood pressure control and medication compliance, for instance.
Our findings extend those from prior reports. We identified a broad spectrum of CV and non-CV reasons thought to have precipitated the index HF hospitalization, with only small differences between patients with HFrEF and HFpEF. Several of these factors, both CV and non-CV related, are potentially modifiable and could be addressed through close outpatient monitoring, patient education and engagement. Based on our data, these strategies should include improved management of co-morbidities (atrial fibrillation, hypertension, COPD, diabetes), and strategies to improve adherence to evidence based HF therapies. Comprehensive in-hospital and post-discharge programs that focus on these aspects have demonstrated reductions in the rate of subsequent readmissions for HF, although no single intervention alone may be sufficient to address this complex issue.11 In addition, the number of respiratory infections leading to HF exacerbations, which was one of the leading non-CV reasons in our study, could potentially be reduced through vaccination programs for influenza and pneumococcal infections.12, 13 Given the chronicity and trajectory of HF, some hospitalizations for HF will be unavoidable. However, novel strategies for outpatient management through home visits or clinics for IV diuretics have the potential to further reduce hospitalizations for HF even in the setting of worsening HF.14
Investigator–identified clinical evidence of HF
There were no important differences with respect to symptoms and clinical signs between the precipitant factor groups with the exception that pulmonary edema was more commonly reported in HFpEF patients with a CV precipitant. This may be an indicator of a higher degree of volume overload in this subgroup of patients.
Recurrent hospitalizations and mortality
Prior data on the long term outcomes based on precipitants leading to an initial hospitalization for HF are sparse. We found that patients with a CV precipitant of their index HF hospitalization had the highest annual incidence rate of CV readmissions adding information to the previous report about subsequent risk following a hospitalization for HF.15 This insight may be relevant both clinically and for research purposes. If a specific CV cause, such as uncontrolled hypertension, can be addressed, subsequent hospitalization could potentially be prevented. Few other studies have investigated the relationship between potential HF hospitalization precipitating factors and risk of re-admission and mortality. In the OPTIMIZE-HF registry (n=5,791, mean EF 37%), myocardial ischemia and worsening renal function were associated with a higher risk of 60- to 90-day all-cause mortality whereas uncontrolled hypertension as a precipitating factor was associated with lower rates of post-discharge death or readmission. In the GREAT registry (n=15,828, mean EF 38%) 90-day all-cause mortality was highest in patients in whom AHF was thought to have been precipitated by acute coronary syndrome or infection (HR 1.69, 95% CI 1.44–1.97 and HR 1.51, 95% CI 1.18–1.92).10 Analyses were not stratified by EF. In CHARM, rates of CV and all-cause mortality were similar among the three precipitating factor categories in patients with HFrEF and HFpEF but were overall higher in those with HFrEF, in concordance with prior analyses stratified by EF.7
Our findings suggest that precipitating factors leading to the initial HF hospitalization may be associated with the rate of recurrent admissions rather than subsequent mortality. This finding could be due to a number of reasons but it may be that CV precipitants are more persistent or likely to recur (e.g. atrial fibrillation, myocardial ischemia) than non-CV causes, e.g. respiratory infection. It is also possible that based on the precipitant, certain conditions may be more or less likely to be amendable to outpatient management in patients with known HF so that patients re-presenting with CV problems, e.g. arrhythmias, are more likely to be admitted, whereas non-CV problems, e.g. certain infections, may be managed in the outpatient setting.
Limitations
These data should be interpreted in the context of their limitations. First, only the initial HF hospitalization was adjudicated by an independent committee, all subsequent hospitalizations were investigator reported. It is possible that some of these events would not meet the criteria used by an endpoint committee. In addition, subsequent hospitalizations may have been influenced by the type of precipitant (CV vs. non-CV) whereas mortality was adjudicated in all cases. However, the same data collection forms were used for all events and should have led to consistent data collection for subsequent events. Removal of additional events would have led to an underestimation of the number of recurrent hospitalizations in this cohort. Second, this analysis focused on the primary factor leading to the first adjudicated hospitalization for HF, additional secondary factors were not analyzed in this manuscript and precipitating factors leading to subsequent HF hospitalizations were also not analyzed with respect to CV, non-CV and unknown factors. It is possible that both during the initial and subsequent hospitalizations multiple factors contributed to patients’ worsening HF. In addition, patient-identified precipitating factors for HF hospitalizations may differ from investigator-identified reasons for admission but these were not collected in this trial.16, 17 Third, EFs were reported by the study sites and not verified by an independent core imaging laboratory. Fourth, the cut off values for HFrEF (EF≤40%) and HFpEF (EF>40%) in this analysis were based on the study design of CHARM. Due to the small size of the group with an EF ≥40% we were unable to further divide this group into the recently proposed HFmrEF (EF 40–50%) and HFpEF (>50%) classifications.18 Future investigations in larger cohorts should describe precipitating factors based on the new classifications. Finally, although our analyses were stratified by EF, we did not adjust for potential additional confounders in this hypothesis-generating report.
Conclusions
Among chronic HF patients hospitalized for decompensation, the investigator-reported precipitating factor was not associated with the subsequent mortality rate (or cause of death) but was associated with the type of re-admission: re-admissions for CV reasons were more likely when the index precipitant was CV. These findings may have implications for developing strategies to prevent readmissions and inform the design of future trials.
Figure 2. Annual incidence rates of events following first heart failure hospitalization.
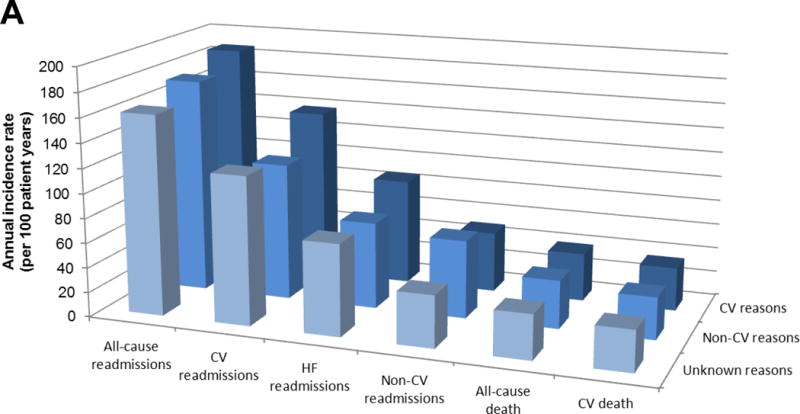
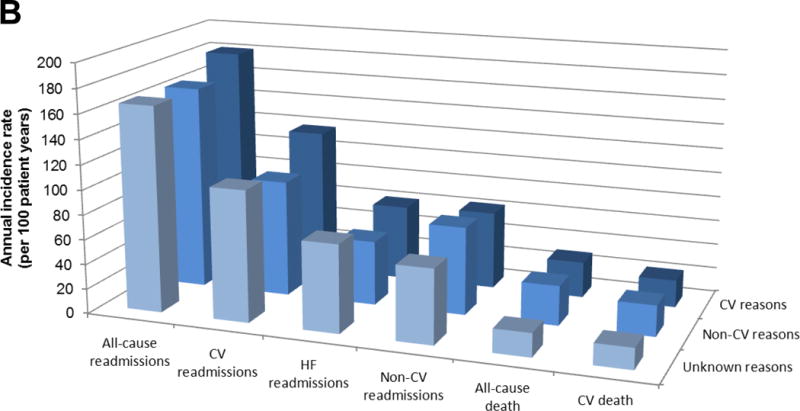
Panel A: HFrEF (EF≤40%) (n=1,152)
Panel B: HFpEF (EF>40%) (n=516)
HFrEF: Heart failure with reduced ejection fraction, HFpEF: Heart failure with preserved ejection fraction, CV: Cardiovascular
Acknowledgments
Sources of Funding
The Candesartan in Heart Failure: Reduction in Mortality and morbidity program was funded by AstraZeneca. The writing of this manuscript was supported by a grant from the National Heart, Lung and Blood Institute (grant number K23HL123533) (EP).
Dr. Platz reports grants from NIH/NHLBI, personal fees from Sanofi and Parexel, outside the submitted work. Dr. Jhund and Dr. Claggett have nothing to disclose. Dr. Pfeffer reports grants from AstraZeneca, during the conduct of the study; personal fees from AstraZeneca, Bayer, Boehringer Ingelheim, DalCor, Gilead, GlaxoSmithKline, Janssen, Lilly USA, The Medicines Company, and Merck, grants and personal fees from Novartis, personal fees from Novo Nordisk, and Relypsa, grants and personal fees from Sanofi, personal fees from Thrasos, Genzyme, and Teva, outside the submitted work. In addition, Dr. Pfeffer has a patent and The Brigham and Women’s Hospital has patents for the use of inhibitors of the RAS in selected survivors of MI with Novartis. Dr. Pfeffer is a co-inventor. His share of the licensing agreement is irrevocably transferred to charity. Dr. Swedberg reports grants from AstraZeneca, during the conduct of the study; personal fees from Novartis, grants and personal fees from Servier, outside the submitted work. Dr. Granger reports grants and personal fees from Astra Zeneca, during the conduct of the study. Dr. Yusuf has nothing to disclose. Dr. Solomon reports grants and personal fees from Astra Zeneca, during the conduct of the study; grants and personal fees from Novartis, grants and personal fees from Amgen, grants and personal fees from Bayer, grants and personal fees from GlaxoSmithKline, grants and personal fees from Alnylam, grants from Gilead, Ionis, and Boston Scientific, personal fees from Merck, and Bristol-Myers Squibb, outside the submitted work.
Footnotes
Conflict of Interest
Dr. McMurray has nothing to disclose.
Contributor Information
Elke Platz, Director, Emergency Ultrasound Research, Department of Emergency Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, USA.
Pardeep S. Jhund, Senior Clinical Lecturer, BHF Glasgow Cardiovascular Research Centre, Institute of Cardiovascular and Medical Sciences, University of Glasgow, Glasgow, UK
Brian L. Claggett, Chief Statistician, CICL, Cardiovascular Division, Brigham and Women’s Hospital, Harvard Medical School, Boston, USA
Marc A. Pfeffer, Dzau Professor of Medicine, Cardiovascular Division, Brigham and Women’s Hospital, Harvard Medical School, Boston, USA
Karl Swedberg, Department of Molecular and Clinical Medicine, University of Gothenburg, Sweden and National Heart and Lung Institute, Imperial College, London, UK.
Christopher B. Granger, Division of Cardiology, Department of Medicine, Duke University Medical Center, Durham, USA
Salim Yusuf, Population Health Research Institute Hamilton Health Sciences and McMaster University, Hamilton, Ontario, Canada.
Scott D. Solomon, Director, Non-invasive Cardiology, Cardiovascular Division, Brigham and Women’s Hospital, Harvard Medical School, Boston, USA
John J. McMurray, Professor of Medical Cardiology, BHF Glasgow Cardiovascular Research Centre, Institute of Cardiovascular and Medical Sciences, University of Glasgow, Glasgow, UK
References
- 1.Heidenreich PA, Albert NM, Allen LA, Bluemke DA, Butler J, Fonarow GC, Ikonomidis JS, Khavjou O, Konstam MA, Maddox TM, Nichol G, Pham M, Pina IL, Trogdon JG. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail. 2013;6:606–19. doi: 10.1161/HHF.0b013e318291329a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tsuyuki RT, McKelvie RS, Arnold JM, Avezum A, Jr, Barretto AC, Carvalho AC, Isaac DL, Kitching AD, Piegas LS, Teo KK, Yusuf S. Acute precipitants of congestive heart failure exacerbations. Arch Intern Med. 2001;161:2337–42. doi: 10.1001/archinte.161.19.2337. [DOI] [PubMed] [Google Scholar]
- 3.Opasich C, Rapezzi C, Lucci D, Gorini M, Pozzar F, Zanelli E, Tavazzi L, Maggioni AP, Italian Network on Congestive Heart Failure I Precipitating factors and decision-making processes of short-term worsening heart failure despite “optimal” treatment (from the IN-CHF Registry) Am J Cardiol. 2001;88:382–7. doi: 10.1016/s0002-9149(01)01683-6. [DOI] [PubMed] [Google Scholar]
- 4.Fonarow GC, Abraham WT, Albert NM, Stough WG, Gheorghiade M, Greenberg BH, O’Connor CM, Pieper K, Sun JL, Yancy CW, Young JB, Investigators O-H and Hospitals Factors identified as precipitating hospital admissions for heart failure and clinical outcomes: findings from OPTIMIZE-HF. Arch Intern Med. 2008;168:847–54. doi: 10.1001/archinte.168.8.847. [DOI] [PubMed] [Google Scholar]
- 5.Kapoor JR, Kapoor R, Ju C, Heidenreich PA, Eapen ZJ, Hernandez AF, Butler J, Yancy CW, Fonarow GC. Precipitating Clinical Factors, Heart Failure Characterization, and Outcomes in Patients Hospitalized With Heart Failure With Reduced, Borderline, and Preserved Ejection Fraction. JACC Heart Fail. 2016;4:464–72. doi: 10.1016/j.jchf.2016.02.017. [DOI] [PubMed] [Google Scholar]
- 6.Rogers JK, Pocock SJ, McMurray JJ, Granger CB, Michelson EL, Ostergren J, Pfeffer MA, Solomon SD, Swedberg K, Yusuf S. Analysing recurrent hospitalizations in heart failure: a review of statistical methodology, with application to CHARM-Preserved. Eur J Heart Fail. 2014;16:33–40. doi: 10.1002/ejhf.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chun S, Tu JV, Wijeysundera HC, Austin PC, Wang X, Levy D, Lee DS. Lifetime analysis of hospitalizations and survival of patients newly admitted with heart failure. Circ Heart Fail. 2012;5:414–21. doi: 10.1161/CIRCHEARTFAILURE.111.964791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pfeffer MA, Swedberg K, Granger CB, Held P, McMurray JJ, Michelson EL, Olofsson B, Ostergren J, Yusuf S, Pocock S. Effects of candesartan on mortality and morbidity in patients with chronic heart failure: the CHARM-Overall programme. Lancet. 2003;362:759–66. doi: 10.1016/s0140-6736(03)14282-1. [DOI] [PubMed] [Google Scholar]
- 9.Glynn RJ, Buring JE. Ways of measuring rates of recurrent events. BMJ. 1996;312:364–7. doi: 10.1136/bmj.312.7027.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arrigo M, Gayat E, Parenica J, Ishihara S, Zhang J, Choi DJ, Park JJ, Alhabib KF, Sato N, Miro O, Maggioni AP, Zhang Y, Spinar J, Cohen-Solal A, Iwashyna TJ, Mebazaa A, Network G Precipitating factors and 90-day outcome of acute heart failure: a report from the intercontinental GREAT registry. Eur J Heart Fail. 2017;19:201–208. doi: 10.1002/ejhf.682. [DOI] [PubMed] [Google Scholar]
- 11.Ziaeian B, Fonarow GC. The Prevention of Hospital Readmissions in Heart Failure. Prog Cardiovasc Dis. 2016;58:379–85. doi: 10.1016/j.pcad.2015.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Udell JA, Farkouh ME, Solomon SD, Vardeny O. Does influenza vaccination influence cardiovascular complications? Expert Rev Cardiovasc Ther. 2015;13:593–6. doi: 10.1586/14779072.2015.1044439. [DOI] [PubMed] [Google Scholar]
- 13.Wu WC, Jiang L, Friedmann PD, Trivedi A. Association between process quality measures for heart failure and mortality among US veterans. Am Heart J. 2014;168:713–20. doi: 10.1016/j.ahj.2014.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Desai AS, Stevenson LW. Rehospitalization for heart failure: predict or prevent? Circulation. 2012;126:501–6. doi: 10.1161/CIRCULATIONAHA.112.125435. [DOI] [PubMed] [Google Scholar]
- 15.Abrahamsson P, Swedberg K, Borer JS, Bohm M, Kober L, Komajda M, Lloyd SM, Metra M, Tavazzi L, Ford I. Risk following hospitalization in stable chronic systolic heart failure. Eur J Heart Fail. 2013;15:885–91. doi: 10.1093/eurjhf/hft032. [DOI] [PubMed] [Google Scholar]
- 16.Albert NM, Barnason S, Deswal A, Hernandez A, Kociol R, Lee E, Paul S, Ryan CJ, White-Williams C, American Heart Association Complex Cardiovascular P, Family Care Committee of the Council on C, Stroke Nursing CoCC, Council on Quality of C and Outcomes R Transitions of care in heart failure: a scientific statement from the American Heart Association. Circ Heart Fail. 2015;8:384–409. doi: 10.1161/HHF.0000000000000006. [DOI] [PubMed] [Google Scholar]
- 17.Annema C, Luttik ML, Jaarsma T. Reasons for readmission in heart failure: Perspectives of patients, caregivers, cardiologists, and heart failure nurses. Heart Lung. 2009;38:427–34. doi: 10.1016/j.hrtlng.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 18.Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, Gonzalez-Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P, Authors/Task Force M 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129–200. doi: 10.1093/eurheartj/ehw128. [DOI] [PubMed] [Google Scholar]


