Abstract
In a matched cohort study, we report that the apnea-hypopnea index is significantly higher in neonates with myelomeningocele (34±22) compared with age-matched controls (19±11; P = .021). Assessment of newborns with myelomeningocele for sleep-disordered breathing may facilitate early treatment; the impact on long-term neurodevelopment is unknown.
Keywords: polysomnography, obstructive sleep apnea, central sleep apnea, spina bifida, neonatal
Spina bifida is the most common permanently disabling birth defect in the USA.[1] Myelomeningocele is the most severe form of spina bifida and is usually accompanied by Chiari II malformations. Despite the recent introduction of fetal surgery to close the spinal defect and reduce the need for ventriculo-peritoneal shunts related to Chiari II malformations, long-term neuromotor and intellectual disabilities are expected for most children with myelomeningocele.[2]
Obstructive sleep apnea, central sleep apnea, and sleep-related hypoventilation occur in more than half of children with repaired myelomeningocele and Chiari II malformations.[3–5] The abnormal sleep physiology is multifactorial, related to the level of spinal defect; presence of congenital and acquired brainstem abnormalities; musculoskeletal factors; and pulmonary abnormalities. Yet, in clinical practice, routine assessment for sleep-disordered breathing (SDB) is not commonplace for children with myelomeningocele.
Sleep apnea and hypoventilation may be highly consequential, and are potentially treatable, for this patient population. SDB is associated with sudden death in adults with myelomeningocele (relative risk 4.6, 95% CI 2.9–7.3).[6] Emerging evidence suggests that for otherwise healthy children, even mild symptoms of SDB during infancy, such as parent-reported snoring, lead to long-term risk for adverse neurobehavioral consequences.[7, 8] However, an extensive literature review revealed no published data on the prevalence of SDB in newborns with repaired myelomeningocele.
We hypothesized that for patients with myelomeningocele, SDB could be present as early as the newborn period, despite recent surgical repair. We designed this matched cohort study to assess the frequency and severity of SDB among newborns with myelomeningocele compared with age-matched newborns who required intensive care but did not have congenital anomalies.
Methods
This study was approved by the Institutional Review Board, and a parent of each participant provided written informed consent. Newborns who were admitted to our Level IV neonatal intensive care unit (NICU) after myelomeningocele repair from December 2014 to October 2016 were eligible. Inclusion criteria were: fetal or post-natal repair of myelomeningocele and gestational age at delivery >33 weeks. Exclusion criteria were: additional congenital anomalies that predispose to SDB (eg, severe micrognathia), or prematurity with gestational age <33 weeks. Control infants were newborns >33 weeks gestational age who required NICU care but did not have congenital anomalies and had been recruited for separate research studies. The most common primary admission indication was prematurity. Cases and controls were matched individually by post-menstrual age at the time of the polysomnogram recording.
When medically stable, each newborn underwent a 12-hour attended polysomnogram in the NICU. All infants with myelomeningocele had received their surgical repair prior to polysomnography; none were receiving narcotics or other respiratory depressants at the time of polysomnography. An experienced, registered polysomnographic technologist monitored the study at the bedside and recorded behavioral observations. Polysomnograms included a 9- channel neonatal-montage EEG, bilateral electro-oculogram, chin surface EMG, chest and abdominal excursion belts (inductance plethysmography), nasal pressure, nasal/oral airflow (thermocouples), snoring sensor, oxygen saturation, ECG, bilateral anterior tibialis surface EMG, digital video, and transcutaneous CO2 monitor. Each polysomnogram was scored according to standard neonatal scoring guidelines[9], and reviewed and interpreted by a pediatrician board- certified in sleep medicine.
The primary outcome was the apnea-hypopnea index (AHI; number of scored respiratory events per hour of sleep)[10]. Additional standard, objective measures were extracted from each polysomnogram. A systematic review of each patient’s chart was completed to abstract information regarding the neonatal admission, subsequent subspecialty care, emergency department visits, and hospital admissions. Demographic and clinical data were managed using REDCap.[11]
Parents completed standardized questionnaires to assess basic measures of development (Ages and Stages)[12] and quality of life (Infant Toddler Quality of Life Questionnaire, ITQOL)[13, 14] when the infants reached 6 months.
The data were analyzed using SPSS Statistics 24 (Armonk, New York). Data for each myelomeningocele subject were compared with an individually matched control subject using Mann-Whitney-Wilcoxon tests. In exploratory analyses of categorical variables, Fisher exact and Kruskal-Wallis tests were used. As this was an initial investigation in this area of research, our priority was to maintain sensitivity to potential associations; we did not adjust for multiple comparisons and p≤0.05 was considered significant.
One newborn with a postnatally repaired myelomeningocele was older than the rest of the cohort at the time of the polysomnogram (44 4/7 weeks postmenstrual age) and an age-matched control was not available. This subject was excluded from the matched analysis; however, the individual’s data are presented with description of the full myelomeningocele cohort.
Results
Twenty newborns with myelomeningocele were included (five fetal repair and 15 post-natal repair). The clinical and demographic details of the cases and age-matched controls are presented in Table I.
Table 1.
Characteristics of 19 newborns with myelomeningocele and their age-matched controls.
| Myelomeningocele Patients (n=19) | Control Patients (n=19) | |
|---|---|---|
|
| ||
| Mean gestational age at birth (weeks) | 36.6±2.1 | 36.9±2.3 |
|
| ||
| Mean postmenstrual age at time of polysomnogram (weeks) | 37.5±2.1 | 37.5±2.2 |
|
| ||
| Mean chronological age at time of polysomnogram (days) | 6.2±3.7 | 4.7±4.6 |
|
| ||
| Female Sex (N) | 8 | 9 |
|
| ||
| Mean duration of initial postnatal admission (days) | 17.3±17.0 | 7.3±3.9 |
|
| ||
| Timing of myelomeningocele repair | ||
| Fetal | 5 | |
| Postnatal | 14 | |
|
| ||
| Myelomeningocele spinal level | ||
| Thoracic | 3 | |
| Lumbar | 10 | |
| Sacral | 6 | |
|
| ||
| Chiari II malformation confirmed at birth | 15* | |
|
| ||
| Ventriculo-peritoneal shunt placed prior to PSG | 4 | |
|
| ||
| Number of ED visits in first 6 months (Median) | 0 (range 0–3) | 0 (range 0–2) |
|
| ||
| Number of post-neonatal hospital admissions in first 6 months (Median) | 1 ( range 0–2) | 0 (range 0–0) |
Two of five newborns with prenatal myelomeningocele repair had a Chiari II malformation.
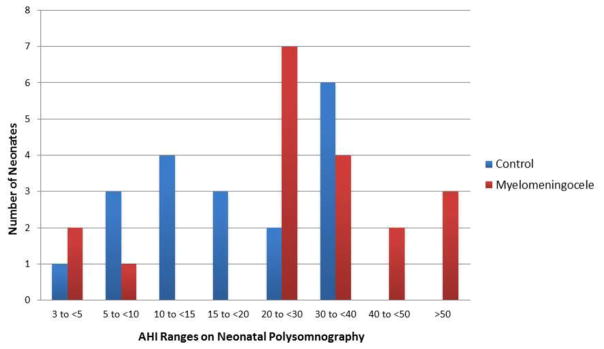
The overall AHI was higher for infants with myelomeningocele than for controls (mean 34.2 ± 21.9 versus 19.3 ± 11.1; p=0.021; Figure; available at www.jpeds.com). AHI was higher for neonates with myelomeningocele during both quiet sleep (p=0.015) and active sleep (p=0.015). Similarly, the hypopnea index was higher in the myelomeningocele cohort than the controls (p=0.044). Most of the respiratory events were hypopneas and most of the apneas were central (Table 2).
Figure.
Apnea Hypopnea Index (AHI) distribution for 19 neonates with myelomeningocele compared with 19 age-matched neonates who required intensive care but did not have congenital malformations.
Table 2.
Measured sleep variables for 19 neonates with myelomeningocele and their age-matched controls1
| Myelomeningocele patients (n=19) | Control patients (n=19) | p-value | |
|---|---|---|---|
|
| |||
| Apnea Hypopnea Index (AHI) * | 34.2 ± 21.9 | 19.3 ± 11.1 | 0.021 |
| Active sleep AHI * | 52.2 ± 28.0 | 30.5 ± 19.2 | 0.015 |
| Quiet sleep AHI* | 27.0 ± 30.0 | 10.5 ± 8.1 | 0.015 |
|
| |||
| AHI Ranges (# of subjects per group) | |||
| 3 to <5 | 2 | 1 | |
| 5 to <10 | 1 | 3 | |
| 10 to <20 | 0 | 7 | |
| 20 to <30 | 7 | 2 | |
| 30 to <40 | 4 | 6 | |
| 40 to <50 | 2 | 0 | |
| >50 | 3 | 0 | |
|
| |||
| Obstructive apnea index | 3.0 ± 3.5 | 2.5 ± 2.5 | 0.74 |
|
| |||
| Central apnea index | 10.0 ± 17.2 | 4.4 ± 3.4 | 0.26 |
|
| |||
| Hypopnea index * | 21.2 ± 14.3 | 12.4 ± 7.8 | 0.044 |
|
| |||
| Apneic events > 10 seconds (Median, IQR) | 8 (3,26) | 12 (7,20) | |
|
| |||
| Apneic events > 20 seconds (Median, IQR) | 0 (0,0) | 0.5 (0,1) | |
|
| |||
| Mean oxygen saturation (%)* | 94.4 ± 2.6 | 96.3 ± 2.2 | 0.020 |
|
| |||
| Minimum oxygen saturation (%) | 82 ± 5.4 | 80.5 ± 6.4 | 0.22 |
|
| |||
| Percent of time spent with oxygen saturation < 90% | 11.5 ± 12.3 | 6.2 ± 12.5 | 0.13 |
|
| |||
| Percent of time spent in: | |||
| Active sleep* | 41.7 ± 6.7 | 47.1 ± 8.3 | 0.013 |
| Quiet sleep | 41.5 ± 10.3 | 35.2 ± 9.5 | 0.080 |
| Intermediate sleep | 16.8 ± 6.8 | 17.7 ± 8.5 | 0.58 |
Data are presented as mean ± standard deviation unless otherwise indicated
Statistically significant differences via Mann–Whitney–Wilcoxon test.
The duration of admission was significantly longer for neonates with myelomeningocele than for control subjects (Table 1, 17.3±17.0 vs 7.3±3.9, p<0.001). Regression analysis did not reveal an association between AHI and length of stay.
The AHI was not different between the five infants who underwent fetal myelomeningocele repair (median AHI 29.0; IQR 26.8, 33.0) and the fifteen who had postnatal repair (median AHI 29.6; IQR 20.8, 44.6). The AHI did not vary significantly by myelomeningocele level (N=10 lumbar 24.9±5.5, N=6 sacral 43.7±10.9, N=3 thoracic 6.2±9.0, p=0.09), nor by the presence of a Chiari II malformation (N=16 with Chiari II 31.4±23.4, N=4 with no Chiari II 39.3±16.2, p=0.32). Among the 20 neonates with myelomeningocele, 5 underwent ventriculo-peritoneal shunt placement before their neonatal polysomnogram, 7 underwent shunt placement after the polysomnogram, and 8 did not require shunt placement in the first 6 months of life. Of the 5 with fetal repairs, 2 required shunt placement (1 at age 3 months and 1 at 7 months). Both infants received shunts after they presented with brief resolved unresponsive events (BRUE) 4 to 6 weeks after their neonatal discharge. One had neonatal AHI=33, and the other had neonatal AHI=9.9.
Six-month follow-up surveys were returned for 15 myelomeningocele subjects and 11 control subjects. There were no scored delays in any domains for 9 of the 11 control subjects with completed questionnaires. One control infant had delayed problem solving and another had personal-social delay. Four of the 15 infants with myelomeningocele had delays, and in multiple domains including gross motor, fine motor, problem solving, and personal-social. One was delayed in all five domains. There was no difference in neonatal AHI among infants with or without developmental delay at age 6 months (36.0±24.4 versus 29.0±13.1. p=0.78).
On the ITQOL questionnaire, parents of control infants reported their infants’ overall health as “fair” (N=1), or “excellent” (N=11). Parents of neonates with myelomeningocele rated their infants’ health as “good” (N=4), “very good” (N=2), or “excellent” (N=6). There was no association between neonatal AHI and overall health rating on this question. None of the respondents (9/19 controls and 14/19 myelomeningocele) reported any limitations in their 6-month-old infant’s sleep. Similarly, all surveys reported satisfaction with the child’s sleeping habits (one response was neutral). Yet, three of nine surveys from families of control subjects, and three of 13 from families of infants with myelomeningocele identified that their infant had had trouble sleeping in the last four weeks.
Seven of the 20 myelomeningocele patients (35%) vs. 1 (5%) of the 19 control subjects had at least one emergency department visit, and 12 (60%) vs. none (0%) had at least one hospital admission in the first 6 months of life. There were a variety of reasons for emergency department visits and admissions, including upper respiratory infections, urinary tract infections, sepsis, BRUE, and hydrocephalus/need for VP shunt placement. The number of admissions was not associated with neonatal AHI.
Clinical follow-up for abnormal polysomnogram results was offered to all patients. Of the nine neonates with myelomeningocele who returned for follow-up, five were treated with caffeine. Four received supplemental oxygen for central sleep apnea or sleep-related hypoventilation.
Discussion
Results of this matched cohort study suggest for the first time that SDB is ubiquitous among newborn infants with repaired myelomeningocele. Moreover, the mean AHI of newborns with myelomeningocele was nearly twice that of age-matched controls who also required intensive care. In this small sample, newborns who had fetal myelomeningocele repairs and those with post-natal repairs had equally abnormal sleep physiology. Results of this study raise the possibility that an opportunity exists for early postnatal intervention to control SDB and minimize its long-term consequences for all patients with myelomeningocele.
Older children with myelomeningocele usually have SDB. Among 83 school-aged children with myelomeningocele, 20% had moderate-to-severe sleep apnea (AHI>5; 12 with predominantly central apneas and 5 with predominantly obstructive apnea), and 42% had mild apnea (AHI 1– 4.9/hr).[3] This result was echoed in a retrospective study of 435 patients evaluated in a spina bifida clinic; sleep apnea was diagnosed in 81% of the 51 patients (age 8.3±0.9 years) who had undergone clinically-indicated polysomnography.[5] Across these studies, most of the sleep apnea was central rather than obstructive, perhaps because of compression and dysplasia of the brainstem. Our study uniquely evaluates sleep physiology among newborn infants with myelomeningocele.
Polysomnographic data are lacking regarding SDB in neonates who require intensive care. The most appropriate threshold for SDB diagnosis in neonates is unclear; some amount of apnea or hypopnea is expected even for neonates without obvious risk factors for SDB. Our control subjects’ AHI data suggest that neonates who require intensive care may have a high risk for SDB; this risk is compounded for newborns with myelomeningocele, who are often premature and always require NICU care.
Notably, routine bedside monitoring which assesses for apneic events >20-seconds in duration would have underestimated the degree of SDB in both neonates with myelomeningocele and controls, as even the most severely affected infants had fewer than 3 apneas >20-seconds during the polysomnogram. The clinical implications of the markedly elevated AHI in newborns with myelomeningocele remain uncertain. In our study, higher AHI was not correlated with a longer length of NICU stay or more frequent ED visits or hospitalizations in the first six months of life.
However, SDB has clearly been associated with important effects on general health, behavior, and cognitive functioning in otherwise healthy older children.[15] SDB was associated with sudden death in adults with myelomeningocele[6], but to our knowledge no study has systematically evaluated for subtler neurocognitive or behavioral effects of SDB in this patient population.
Several limitations of this study warrant discussion. As this was a novel, intensive bedside study from a single level IV NICU, the sample size was necessarily restricted. Notably, we might have underestimated SDB in newborns with myelomeningocele, because wound healing from their surgical repairs precluded supine positioning. Conversely, neonates in the control group spent the majority of their sleep lying supine, according to safe sleep protocols. We hypothesize that supine positioning could increase the likelihood of obstructive events. Therefore, the difference in obstructive apneas between the myelomeningocele (potentially underestimated) and control infants (potentially overestimated) may actually be greater than what we measured.
The polysomnography results of the five included newborns with fetal repair were comparable with the 15 who had post-natal repairs. If larger studies demonstrate that fetal myelomeningocele repair does not protect against SDB, this will be very important information for counseling and clinical care of these individuals.
Comprehensive care for children with complex congenital malformations, such as myelomeningocele, requires coordination across multiple medical, surgical, and supportive specialties. Abnormal sleep may have important effects on the physical health, cognitive development, and behavior of infants with myelomeningocele. Our results raise the possibility that infants with myelomeningocele may benefit from systematic assessment for SDB, as early as the first week of life; future studies should evaluate the long-term impact of SDB treatment for this patient population.
Acknowledgments
Supported by the American Sleep Medicine Foundation (≪≫ to R.S.), the National Institutes of Health (NIH) (R21HD083409 and K23HD068402 to R.S.), and the University of Michigan Barwick Scholar Award (to R.S.). R.S. receives research support from the Patient-Centered Outcomes Research Institute, the NIH, the Pediatric Epilepsy Research Foundation, the American Sleep Medicine Foundation, and the University of Michigan. R.S. serves on the Board of the Child Neurology Society and on the Steering Committee of the Pediatric Epilepsy Research Consortium and receives royalties from UpToDate. R.C. serves on the Board of the American Academy of Sleep Medicine and receives royalties from UpToDate.
We thank the families of the newborns who participated in this study. We thank the research assistants, Stephanie Rau and Shannon Lester, and the sleep technologists, especially Mark Kingen, RPSGT, and Laura Merley, RPSGT, for their tireless work on this project.
Footnotes
The other authors declare no conflicts of interest.
Portions of this study were presented as an abstract at the Pediatric Academic Societies Meeting, May 6–9, 2017, San Francisco, California and the 31st Annual Meeting of the Associated Professional Sleep Societies, LLC, June 3–7, 2017, Boston, Massachusetts.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Boulet SL, Yang Q, Mai C, Kirby RS, Collins JS, Robbins JM, et al. Trends in the postfortification prevalence of spina bifida and anencephaly in the United States. Birth Defects Research A Clin Mol Teratol. 2008;82:527–32. doi: 10.1002/bdra.20468. [DOI] [PubMed] [Google Scholar]
- 2.Adzick NS, Thom EA, Spong CT, Brock JW, III, Burrows PK, Johnson MP, et al. A Randomized trial of prenatal versus postnatal repair of myelomeningocele. New England Journal of Medicine. 2011;364:993–1004. doi: 10.1056/NEJMoa1014379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Waters KA, Forbes P, Morielli A, Hum C, O’Gorman AM, Vernet O, et al. Sleep-disordered breathing in children with myelomeningocele. Journal of Pediatrics. 1998;132:672–81. doi: 10.1016/s0022-3476(98)70359-2. [DOI] [PubMed] [Google Scholar]
- 4.Alsaadi MM, Iqbal SM, Elgamal EA, Gozal D. Sleep-disordered breathing in children with Chiari malformation type II and myelmeningocele. Pediatrics International. 2012;54:623–6. doi: 10.1111/j.1442-200X.2012.03660.x. [DOI] [PubMed] [Google Scholar]
- 5.Patel DM, Rocque BG, Hopson B, Arynchyna A, Bishop ER, Lozano D, et al. Sleep- disordered breathing in patients with myelomeningocele. Journal of neurosurgery Pediatrics. 2015;16:30–5. doi: 10.3171/2014.11.PEDS14314. [DOI] [PubMed] [Google Scholar]
- 6.Jernigan SC, Berry JG, Graham DA, Bauer SB, Karlin LI, Hobbs NM, et al. Risk factors of sudden death in young adult patients with myelomeningocele. J Neurosurg Pediatrics. 2012;9:149–55. doi: 10.3171/2011.11.PEDS11269. [DOI] [PubMed] [Google Scholar]
- 7.Piteo AM, Kennedy JD, Roberts RM, Martin AJ, Nettelbeck T, Kohler MJ, et al. Snoring and cognitive development in infancy. Sleep Medicine. 2011;12:981–7. doi: 10.1016/j.sleep.2011.03.023. [DOI] [PubMed] [Google Scholar]
- 8.Chervin RD, Ruzicka DL, Archbold KH, Dillon JE. Snoring predicts hyperactivity four years later. Sleep. 2005;28:885–90. doi: 10.1093/sleep/28.7.885. [DOI] [PubMed] [Google Scholar]
- 9.Anders T, Emde R, Parmalee A. A Manual of Standardized Terminology, Techniques and Criteria for the Scoring of States of Sleep and Wakefulness in Newborn Infants. Los Angeles, CA: UCLA Brain Information Services; 1971. [Google Scholar]
- 10.Berry RB, Brooks R, Gamaldo CE, Harding SM, Lloyd RM, Marcus CL, et al. AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications Version 2.2.0. Darien, IL: 2015. [Google Scholar]
- 11.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–81. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Squires J, Bricker D. Ages & Stages Questionnaires (ASQ-3): User’s Guide. 3. Brookes Publishing Co; 2009. [Google Scholar]
- 13.Klassen AF, Landgraf JM, Lee SK, Barer M, Raina P, Chan HW, et al. Health related quality of life in 3 and 4 year old children and their parents: preliminary findings about a new questionnaire. Health and quality of life outcomes. 2003;1:81. doi: 10.1186/1477-7525-1-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Landgraf JM. The Infant/Toddler Child Health Questionnaire: Conceptual framework, logic content, and preliminary psychometric results: Boston Health Act. 1994. [Google Scholar]
- 15.Marcus CL, Brooks LJ, Draper KA, Gozal D, Halbower AC, Jones J, et al. Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 2012;130:e714–55. doi: 10.1542/peds.2012-1672. [DOI] [PubMed] [Google Scholar]