Abstract
Background
Radiation therapy is a mainstay in the treatment of many malignancies, but collateral damage to surrounding tissue, with resultant hypovascularity, fibrosis, and atrophy, can be difficult to reconstruct. Fat grafting has been shown to improve the quality of irradiated skin, but volume retention of the graft is significantly decreased. Deferoxamine (DFO) is a FDA-approved iron-chelating medication for acute iron intoxication and chronic iron overload that has also been shown to increase angiogenesis. The present study evaluates the effects of DFO treatment on irradiated skin and subsequent fat graft volume retention.
Methods
Mice underwent irradiation to the scalp followed by treatment with deferoxamine or saline and perfusion and were analyzed using laser Doppler analysis (LDA). Human fat grafts were then placed beneath the scalp and retention was also followed up to eight weeks radiographically. Finally, histologic evaluation of overlying skin was performed to evaluate effects of deferoxamine preconditioning.
Results
Treatment with DFO resulted in significantly increased perfusion, as demonstrated by LDA and CD31 immunofluorescent staining (*p < 0.05). Increased dermal thickness and collagen content secondary to irradiation, however, was not affected by DFO (p > 0.05). Importantly, fat graft volume retention was significantly increased when the irradiated recipient site was preconditioned with DFO (*p < 0.05).
Conclusions
Our results demonstrated increased perfusion with DFO treatment, which was also associated with improved fat graft volume retention. Preconditioning with DFO may thus enhance fat graft outcomes for soft tissue reconstruction following radiation therapy.
Keywords: deferoxamine, fat grafting, irradiation, recipient site, preconditioning
Introduction
Over 5.6 million soft tissue reconstructive procedures are performed annually in the United States, with the majority related to tumor extirpation and sequelae of adjuvant radiation therapy (1). Even with intact overlying epithelium, insufficient underlying soft tissue results in visible asymmetry and contour abnormalities, and may also contribute to unstable wounds and inadequate protection of critical organs and structures including bone, implanted hardware, and large vessels (2–4). While radiation therapy has been shown to be incredibly effective at reducing local recurrence risk for various tumors, collateral damage to adjacent soft tissue resulting in obliteration of microvasculature and fibrosis may significantly complicate reconstructive strategies (5–7).
Autologous fat grafting has emerged as an increasingly popular strategy to manage soft tissue deficiencies throughout the body, and this technique has been applied clinically to reconstruct defects secondary to cancer resection (8, 9). However, transfer of avascular fat to irradiated sites remains challenging, as radiation induced changes to the recipient bed (i.e. hypovascularity) result in poorer fat graft survival (10, 11). Cell-based strategies to enhance volume retention, through enrichment of fat grafts with additional adipose-derived stromal cells, have been shown to improve outcomes (11), but translation of this approach remains difficult due to known heterogeneity of the stromal vascular fraction and regulatory hurdles.
As an alternative, deferoxamine (DFO) has been studied as an angiogenic and antioxidant agent with the potential to improve fat graft survival (12). DFO, an FDA approved iron chelator for treating acute iron poisoning and chronic iron overload associated with frequent blood transfusions, has also been demonstrated to increase hypoxia-inducible factor -1 alpha (HIF-1α) activity and enhance expression of angiogenic growth factors (13, 14). Studies have also shown local injection of DFO to improve ischemic flap survival in both mouse and pig models (15, 16), with increased skin flap blood perfusion and capillary density noted in DFO-treated animals (15). And in the setting of irradiated bone, multiple reports have found DFO to promote bone regeneration following distraction osteogenesis through enhanced vascularity (17–19). Given this ability for local DFO application to promote revascularization, we investigated the potential for DFO preconditioning of irradiated tissue to enhance subsequent fat graft retention.
Materials and Methods
Animal Irradiation Model and Post-Radiation Treatment
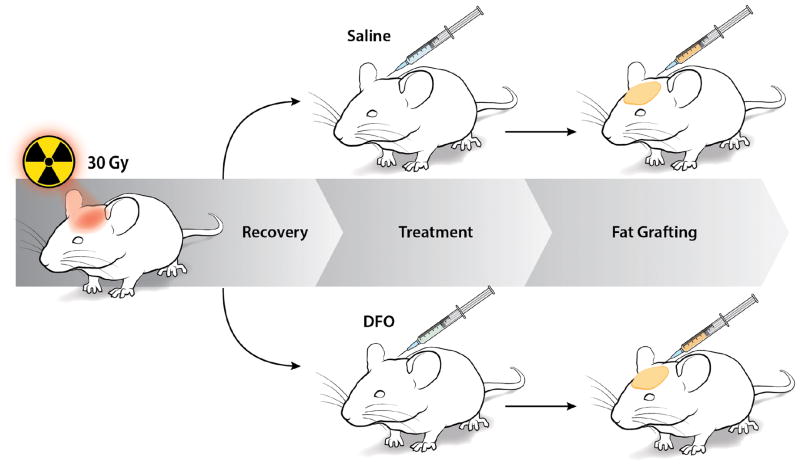
All studies were performed in accordance with Stanford University animal guidelines. Adult 60-day-old male Crl:NU-Fox1NU immunocompromised mice were used for experiments in this study. Twelve mice were treated with a total of 30 Gy external beam radiation, delivered as six fractionated doses of 5 Gy each over 12 days, followed by 5 weeks of recovery. An additional six non-irradiated mice were used as healthy controls for laser Doppler analysis (LDA) and skin analysis. Irradiated mice were divided into two treatment groups: a DFO experimental group and saline control group. Following recovery, mice underwent injection of either DFO (1 mg in 100 μl saline) or 100 μl of saline alone beneath the dermis every other day for a total of seven treatments (Figure 1).
Figure 1.
Schematic of irradiated scalp treatment. Mice received 30 Gy external beam radiation in six fractionated doses. Following recovery, irradiated tissue was then treated with either saline or DFO injections before fat grafting.
Laser Doppler Analysis
LDA was performed to measure perfusion at the irradiated site. A Perimed PIM 3 laser Doppler perfusion imager (Datavagen, Sweden) was used. The signal generated by the LDA, laser Doppler perfusion index (LDPI), was used for comparative purposes. LDPI is a product of the blood cell velocity and concentration, and is represented by a color spectrum, with black/dark blue representing low perfusion and red representing high perfusion. LDA was performed prior to irradiation, following completion of irradiation and recovery, and then twenty-four hours following each treatment with DFO or saline. LDA was also performed every two weeks after fat grafting. Five images were taken of each mouse and the average LDPI of the five images was recorded.
Fat Grafting and Fat Graft Volume Analysis
After informed consent was obtained, lipoaspirate was obtained from three healthy female donors, ages 45, 49, and 51, with no other medical co-morbidities under an approved IRB protocol #2188. Lipoaspirate was allowed to settle for 15 minutes for layers to separate by gravity sedimentation, and then oil and blood layers were removed by vacuum aspiration. The remaining fat layer was centrifuged at 1300 rcf for 3 minutes at 4 °C. Any remaining oil and blood was again removed and the remaining fat was transferred into 1cc syringes for injection through a 14-gauge needle. Fat grafting was performed beneath the scalp by creating a subcutaneous tunnel with the needle and then injecting 200 μl of lipoaspirate in retrograde fashion while pulling the needle out (20).
Mice were imaged using a MicroCAT-II in vivo X-Ray micro-CT scanner (Imtek, Inc.; Knoxville, TN) two days after fat graft injection for baseline volume measurements. Fat graft volume retention was then analyzed every two weeks over a total of 8 weeks using microcomputed tomography, and images were reconstructed as three-dimensional surfaces through cubic-spline interpolation, as previously described (20). All reconstructions were performed by a single investigator (J.F.) to avoid inter-observer variability.
Skin Analysis
Scalp skin biopsy was harvested from both treatment groups following completion of radiation and then 8 weeks following fat grafting. Specimens were fixed in 4% paraformaldehyde, processed, and embedded in paraffin for sectioning. For dermal thickness measurement, sections were stained with hematoxylin and eosin (H&E) and imaged using a Leica DM5000 B Light microscope (Leica Microsystems; Buffalo Grove, IL) at the 20× objective (10). Dermal measurements were made on ten stained sections from each sample. Picrosirius red staining was also performed for collagen content. Vascularity was determined with CD31 immunoflourescent staining (1:100 Ab28364; Abcam; Cambrdige, MA and 1:200 AF547; Thermo Fisher Scientific; Waltham, MA) and DAPI counterstaining to visualize cell nuclei. Fluorescent images were obtained using an X-Cite 120 Fluorescence Illumination System (Lumen Dyanmics Group, Inc.; Ontario, Canada) at the 20× objective. Quantification of CD31 staining was performed using ImageJ (National Institutes of Health; Bethesda, MD), with pixel-positive area per high power field measured to determine vascular density (11). Comparisons for both dermal thickness and CD31 immunofluorescent staining were also made to non-irradiated skin.
Statistical Analysis
Data are presented as means ± SE. Two-tailed Student’s t-test was used for comparison between two groups and an analyses of variance with Tukey post-hoc test was used for multiple group comparisons. All analyses were performed using StatPlus software (Analyst-Soft, Inc., Alexandria, Va.). A value of *p < 0.05 was considered significant.
Results
Deferoxamine Treatment Effects on Perfusion
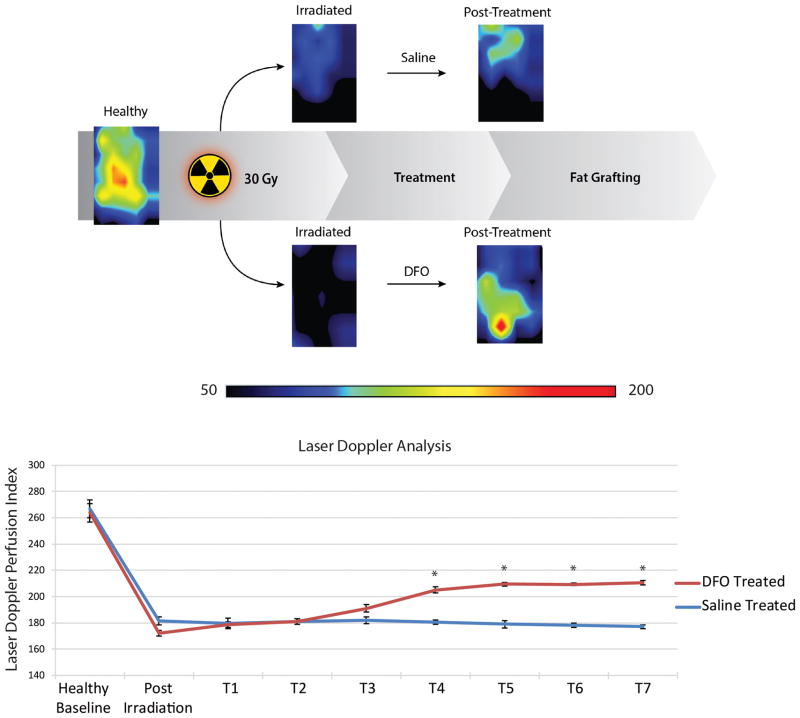
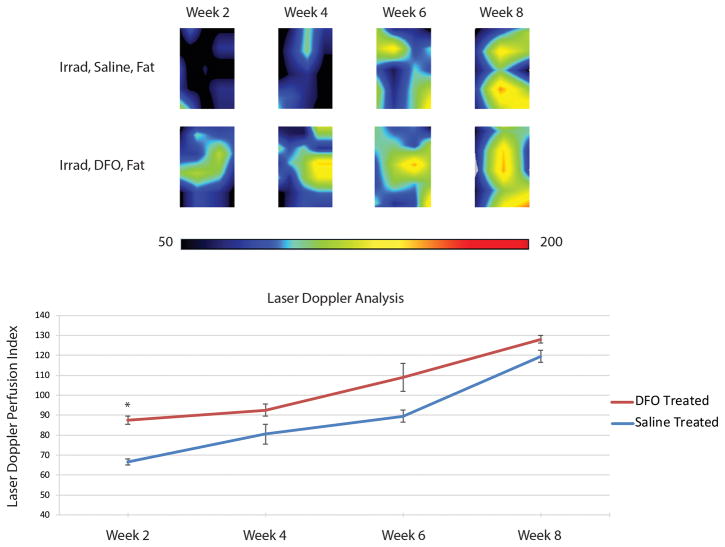
Following completion of irradiation and five-week recovery, perfusion at the scalp was noted to significantly drop from 265.23 ± 7.01 LDPI (pre-radiation baseline) to 176.70 ± 2.59 LDPI (Figure 2). However, treatment of the scalp with 1mg of DFO every other day after radiation recovery resulted in increased LDPI, which became significant after four treatments (205.08 ± 2.30 LDPI) (*p < 0.05). No increase in LDPI measurements was noted after four treatments, though, as three additional treatments of DFO did not result in any significant increase to perfusion. In contrast, injection of saline alone resulted in no change to LDPI measurements over the entire treatment course (Figure 2).
Figure 2.
Laser Doppler analysis of irradiated scalp. A) Heat map representative photos of scalp before irradiation, after irradiation, and after treatment with either saline or DFO. Black/dark blue colors represent lower perfusion and the yellow/red colors represent higher perfusion. B) Quantification of laser Doppler perfusion index demonstrated a significant decrease in perfusion after irradiation. DFO treatments (T) caused a significance rise in perfusion after 4 treatments (T4), compared to saline injection, and plateaued after 5 treatments (T5) (*p < 0.05).
Deferoxamine Preconditioning and Fat Graft Retention
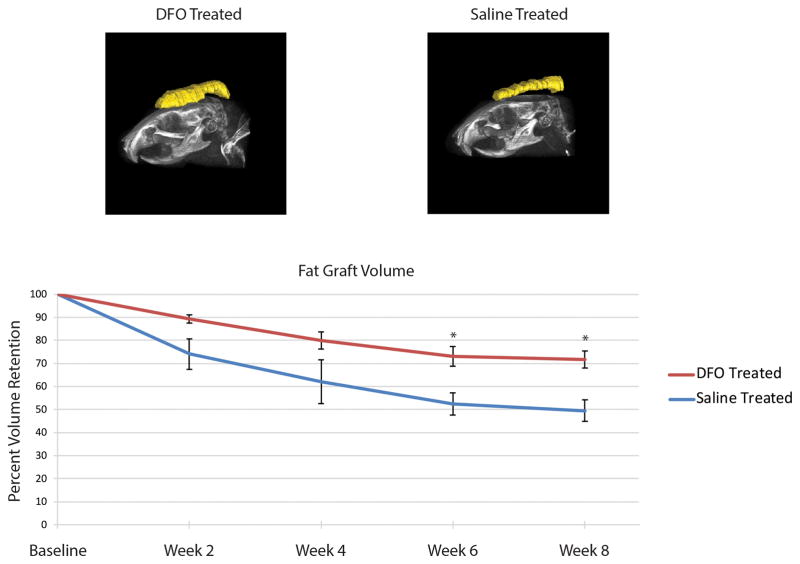
In vivo radiographic analysis of fat grafts showed DFO preconditioned irradiated mice retained more fat volume (89.24% ± 1.69) after two weeks compared to saline injected control mice (74.03 ± 7.91) (Figure 3). Fat graft volume retention was consistently greater among DFO treated mice compared to saline control mice, and at 6 and 8 weeks, these differences were statistically significant (week 6: 73.17% ± 4.26 DFO vs. 52.40% ± 4.83 saline treated, and week 8: 71.75% ± 3.70 DFO vs. 49.47% ± 4.62 saline treated; *p < 0.05) (Figure 3).
Figure 3.
Fat graft volume retention. A) Representative three-dimensional reconstruction of fat grafts after eight weeks in either DFO (left) or saline (right) preconditioned irradiated scalp. B) Quantification of fat graft volumes revealed significantly increased retention in fat grafts placed into DFO treated scalp (red line) when compared to saline treated scalp (blue line) after six and eight weeks (*p < 0.05).
Histologic Effects of Deferoxamine and Fat Grafting on Irradiated Skin
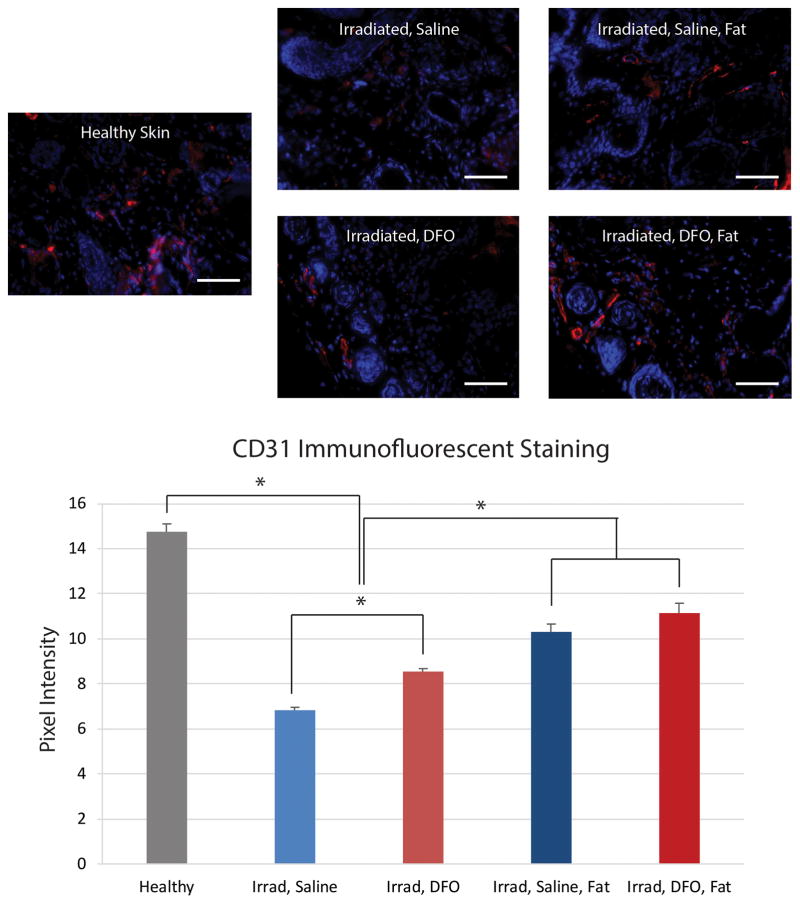
Following irradiation and saline control treatment, vascularity in skin biopsies, as demonstrated by CD31 staining, was found to be significantly lower than non-radiated healthy skin (*p < 0.05) (Figure 4). However, treatment of irradiated skin with DFO resulted in increased CD31 staining, though this did not reach healthy skin levels (Figure 4). As expected following fat grafting, skin biopsies obtained after 8 weeks also demonstrated increased CD31 staining compared to control, saline injected irradiated skin. Interestingly, slightly more CD31 staining following fat grafting was also noted with DFO preconditioned mice relative to saline control fat grafted mice, though this difference was not significant (Figure 4).
Figure 4.
Histologic evaluation of irradiated scalp vascularity. A) Representative images taken at 20× magnification of scalp skin with immunofluorescent staining for CD31 (red) showing increased vascularity with DFO preconditioning. Scale bar represents 100 μm. B) Quantification of CD31 immunofluorescent staining revealed significant drop following irradiation. Significant improvement was noted with DFO treatment, and vascularity was further enhanced with fat grafting (*p < 0.05).
Perfusion of the skin following fat grafting was also measured by LDA, and LDPI values were found to be lower than immediately following completion of DFO or saline preconditioning due to changes in three-dimensional architecture of the region of interest following placement of fat. However, two weeks following injection of fat grafts, significantly more perfusion was still noted in DFO preconditioned mice (86.33 ± 2.00 vs. 65.72 ± 2.02 LDPI for saline control; *p < 0.05) (Figure 5). Perfusion also continued to increase in the DFO preconditioned mice following fat grafting, but perfusion similarly increased in saline injected control mice after fat grafting, and after week 2, no significant difference on LDA was appreciated between the two groups (127.78 ± 2.29 vs. 119.18 ± 4.09 LDPI for DFO and saline treated mice eight weeks following grafting, respectively; p > 0.05) (Figure 5).
Figure 5.
Laser Doppler analysis following fat grafting. A) Representative LDA images of saline (top) and DFO (bottom) treated tissue scalp following fat grafting. Black/dark blue colors represent lower perfusion and yellow/red colors represent higher perfusion. B) Quantification of laser Doppler perfusion index demonstrated DFO treated scalp (red line) had significantly higher perfusion than saline treated scalp (blue line) two weeks after fat grafting (*p < 0.05). Both groups demonstrated increased perfusion after fat grafting with no significant difference appreciated after week 2.
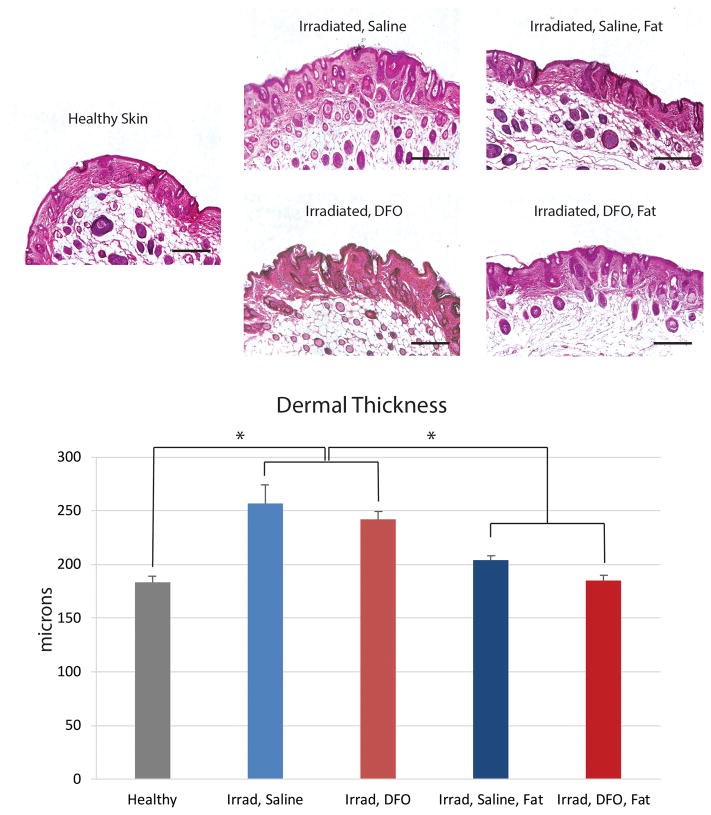
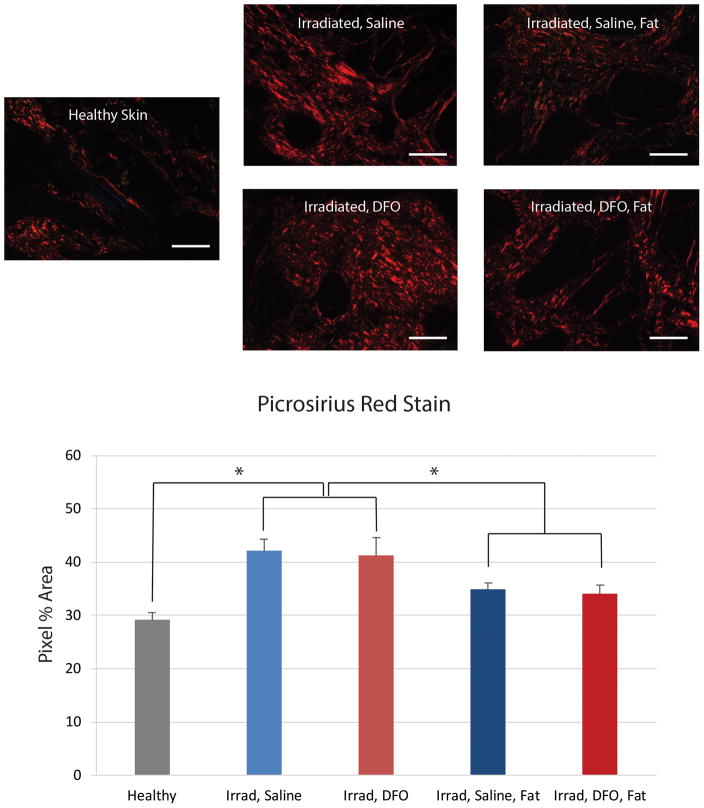
Finally, dermal thickness of irradiated skin following saline treatment was significantly greater than healthy, non-irradiated skin (*p < 0.05) (Figure 6A and B). Compared to saline injected mice (256.71 ± 16.76 μm), DFO treatment on irradiated skin resulted in a slight decrease in dermal thickness, (242.09 ± 7.22 μm) but this was not significantly less. However, fat grafting, whether into saline or DFO preconditioned sites, was found to significantly decrease dermal thickness, though there was no significant difference when comparing these two groups (p > 0.05) (Figure 6A and B). Paralleling these findings, picrosirius red staining revealed significantly increased collagen content following irradiation and saline treatment (*p < 0.05) (Figure 6C and D). DFO treatment on irradiated skin resulted in a slight decrease in collagen content which was not statistically significant. And similar to our observations with dermal thickness, fat grafting, whether into saline or DFO preconditioned sites, was found to significantly reduce collagen content (*p < 0.05) (Figure 6C and D).
Figure 6.
Evaluation of irradiated scalp histology following fat grafting. A) Representative H&E stained sections at 10× magnification of non-irradiated, healthy skin, irradiated skin after saline or DFO treatment, and irradiated skin after saline or DFO treatment and fat grafting. Scale bar represents 200 μm. B) Quantification of dermal thickness demonstrated significant increase following radiation, with no difference between saline or DFO treated skin. Both treatment groups demonstrated significant reduction following fat grafting (*p < 0.05). C) Representative picrosirius red stained sections at 20× magnification demonstrating density of positive-stained collagen (red) after irradiation and saline or DFO preconditioning, followed by fat grafting. Scale bar represents 100 μm. D) Quantification of collagen content revealed significant increase in collagen following radiation, irrespective of saline or DFO treatment. Both groups demonstrated significant reduction following fat grafting (*p < 0.05).
Discussion
After heart disease, cancer remains the leading cause of death in the United States, with an estimated 1.6 million new cancer cases diagnosed and 600,000 cancer-related deaths projected for 2017 (21). However, in recent years, substantial progress in medical care has been made, with surgery, chemotherapy, and radiation therapy increasing both the number of cancer survivors and the length of their survival (22). With this improvement, long-term issues related to treatment of cancer, such as with radiation therapy, have become increasingly apparent, and have been shown to profoundly impact quality of life. Radiation-induced soft tissue injury is one of the most common side effects of radiotherapy, affecting over 90% of patients, and the resulting soft tissue atrophy and fibrosis can lead to both severe cosmetic and long-term functional impairment (23, 24).
Chronic radiation injury is characterized by epidermal thinning, eosinophilic homogenized sclerosis of dermal collagen, scattered large and atypical fibroblasts, and fibrous thickening with luminal obliteration of deep vessels (24–26). Vascular damage and development of fibrosis is thought to result from radiation-induced cytokine expression, generation of reactive oxygen species, and cellular apoptosis (27), and soft tissue reconstruction of such hostile sites remains extremely challenging. While autologous fat grafting has become increasingly popular to address post-radiation soft tissue deficits, fibroinflammatory changes and hypovascularity have been associated with poorer fat graft outcomes (10, 11). Improved retention has been noted with cell-assisted lipotransfer, but the functional heterogeneity among stromal cells used to enrich lipoaspirate, in concert with concerns regarding post-oncologic locoregional recurrence, has limited the wide-spread adoption of this strategy.
To improve fat graft outcomes, in this present study we instead focused on preconditioning the irradiated soft tissue site with DFO to enhance vascularity. HIF-1α is typically degraded by prolyl hydroxylase domain-containing protein 2 (PHD2), and DFO, through chelation of the iron co-factor for PHD2 activity, has been shown to stabilize HIF-1α leading to an increase in downstream angiogenic factors and recruitment of endothelial progenitor cells (28, 29). This is the mechanism by which DFO has been thought to promote revascularization of ischemic skin flaps, enhance wound healing in diabetic mice (15, 30), and augment callus size, mineralization, and mechanical strength at irradiated bone injury sites (31, 32). Furthermore, reversal of radiation-induced hypovascularity has also been appreciated with DFO treatment during mandibular distraction osteogenesis (17, 33). All of these findings support the potential for DFO, through stabilization of HIF-1α and increased angiogenic gene expression, to precondition the irradiated recipient site for subsequent fat grafting.
Importantly, DFO was recently suggested to promote fat graft viability in a rat model. Temiz and colleagues described serial injections of 300 mg DFO into inguinal fat pads transplanted to parascapular subcutaneous pockets, which resulted in significantly greater weight measurements after two months (12). However, more inflammation and fibrosis was noted in DFO injected fat grafts, though no change in cellular apoptosis was appreciated. Repeated manipulation of fat grafts with each injection may have contributed to this observation. In addition, adipogenic differentiation of resident stromal cells has been purported to contribute to long-term fat graft retention (34, 35), and direct exposure of DFO to fat grafts may be detrimental to this process. Studies have shown intracellular iron deficiency through DFO administration to severely blunt adipocyte differentiation (36). These findings thus temper enthusiasm for direct injection of DFO into fat grafts.
Alternatively, preconditioning the recipient site to facilitate earlier revascularization of fat grafts remains promising, and this study, to our knowledge, is the first to analyze the effects of DFO local injections to irradiated, hypovascular skin. We noted improved perfusion by laser Doppler analysis of irradiated tissue with DFO treatment. Importantly, laser Doppler analysis allowed for the estimation of in vivo local blood perfusion in the microcirculation through frequency shifts in light that has been scattered by moving red blood cells. This facilitated longitudinal measurements in the same animal following each treatment with DFO. Histologic analysis of treated skin also revealed increased vascularity by CD31 staining following DFO treatment. This translated to enhanced volume retention when fat grafts were placed into DFO preconditioned recipient sites. Interestingly, the addition of fat grafts to DFO treated irradiated tissue led to further improvement in vascularity, even though DFO-related effects were seen to plateau after four treatments. This suggests that alternative mechanisms may also be employed by transferred adipocytes and associated stromal cells to improve vascularity following fat grafting. Finally, the effects of DFO treatment on skin vascularity were not found to be associated with significant changes to dermal thickness and collagen content. Decreased dermal thickness and collagen content were noted, however, following fat grafting, as previously reported (10, 11). Therefore, the architectural changes observed in the dermis with decreased collagen secondary to fat transfer may not necessarily be a result of improved vascularity alone.
While the treatment protocol for DFO preconditioning of irradiated tissue used in our study follows other previously published reports, delivery of DFO on alternate dates through direct injection may limit the clinical translation of this approach. In patients with radiation fibrosis and soft tissue atrophy, preconditioning tissue with serial DFO injections prior to fat grafting may be difficult logistically and not well tolerated by patients. However, recent description of a transdermal drug delivery system for DFO was found to improve wound healing and prevent pressure-induced ulcer formation in diabetic mice (13). Such an approach may also be potentially effective at preconditioning irradiated tissue for fat grafting and would likely be better tolerated by patients. Alternatively, nanoparticle formulations of DFO have also been developed, and their controlled release of DFO may similarly be employed to improve vascularity of irradiated skin. These nanoparticles may also be directly injected with fat grafts to promote earlier revascularization.
As DFO promotes expression of multiple angiogenic factors through stabilization of HIF-1α, concern may also be raised regarding its use in irradiated post-oncologic resection sites. Though no studies, to our knowledge, have demonstrated an increased risk for cancer recurrence following local administration of DFO, several reports have suggested an anti-tumor effect. Iron is necessary for oxygen transport, cell metabolism, and growth, and it is especially important in cells with active growth (37, 38). Not surprisingly, iron chelators such as DFO have been found to reduce liver fibrosis, and its effect on iron metabolism has been shown to clinically reduce progression of hepatocellular carcinoma (39–41). Iron dependency has also been reported in human epidermal growth factor receptor 2 positive breast cancer cells, and multiple breast cancer cell lines have been shown to be vulnerable to iron chelation (42). These reports thus suggest local DFO application may not be associated with increased risk for cancer recurrence.
Collectively, our findings demonstrate that DFO treatment can improve radiation-induced hypovascularity, and this enhanced perfusion may improve the quality of the recipient site for fat grafting. In our study, effects of DFO on vascularity were noted to plateau after four treatments, and it remains unknown whether the improved flow is durable after DFO withdrawal. Another limitation of this study is with DFO dosage and treatment regimen. While these were selected based on previously published studies in other models (15, 17, 18, 31), whether a higher or lower dosage administered more or less frequently would result in increased or decreased vascularity is also unknown. Despite this, following DFO treatment, long-term retention of fat grafts injected into irradiated sites was significantly improved.
Conclusion
Reconstruction of irradiated tissue remains challenging owing to radiation induced alterations to the recipient bed. Fibroinflammatory changes and hypovascularity have been shown to impact fat graft retention, and while cell-based strategies have been shown to improve outcomes, regulatory and safety concerns have, to date, limited their translational potential. As an alternative approach, we have demonstrated that preconditioning irradiated tissue with deferoxamine improves local perfusion, and this was associated with improved radiographic and histologic fat graft outcomes. Preconditioning with deferoxamine prior to fat grafting therefore holds promise for enhancing reconstruction outcomes for irradiated tissue.
Acknowledgments
Michael T. Longaker, MD, MBA was supported by National Institutes of Health grants R01 DE021683, R56 DE025597, and U24 DE026914, the Oak Foundation, and the Hagey Laboratory for Pediatric Regenerative Medicine. Derrick C. Wan, MD was supported by National Institutes of Health grant K08 DE024269, the Hagey Laboratory for Pediatric Regenerative Medicine, the California Institute for Regenerative Medicine, and Stanford University Child Health Research Institute.
Footnotes
Financial Disclosure: None of the authors has a financial interest in any of the products, devices, or drugs mentioned in this manuscript.
Presentations: Data presented in this manuscript was previously presented at the 13th Annual Academic Surgical Congress
References
- 1.American Society Of Plastic Surgeons. Reconstructive Surgery Procedure Trends. Available at: http://www.plasticsurgery.org/Documents/news-resources/statistics/2012-Plastic-Surgery-Statistics/Reconstructive-Surgery-Procedure-Trends-2012.pdf.
- 2.Clark N, Sherman R. Soft-tissue reconstruction of the foot and ankle. Orthop Clin North Am. 1993;24:489–503. [PubMed] [Google Scholar]
- 3.Lim AA, Fan K, Allam KA, et al. Autologous fat transplantation in the craniofacial patient: the UCLA experience. The Journal of craniofacial surgery. 2012;23:1061–1066. doi: 10.1097/SCS.0b013e31824e695b. [DOI] [PubMed] [Google Scholar]
- 4.Siebert JW, Longaker MT, Angrigiani C. The inframammary extended circumflex scapular flap: an aesthetic improvement of the parascapular flap. Plastic and reconstructive surgery. 1997;99:70–77. doi: 10.1097/00006534-199701000-00010. [DOI] [PubMed] [Google Scholar]
- 5.Jacobson AS, Eloy JA, Park E, Roman B, Genden EM. Vessel-depleted neck: techniques for achieving microvascular reconstruction. Head Neck. 2008;30:201–207. doi: 10.1002/hed.20676. [DOI] [PubMed] [Google Scholar]
- 6.Wong KK, Higgins KM, Enepekides DJ. Microvascular reconstruction in the vessel-depleted neck. Curr Opin Otolaryngol Head Neck Surg. 2010;18:223–226. doi: 10.1097/MOO.0b013e32833a2e50. [DOI] [PubMed] [Google Scholar]
- 7.Yarnold J, Brotons MC. Pathogenetic mechanisms in radiation fibrosis. Radiother Oncol. 2010;97:149–161. doi: 10.1016/j.radonc.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 8.Spear SL, Wilson HB, Lockwood MD. Fat injection to correct contour deformities in the reconstructed breast. Plastic and reconstructive surgery. 2005;116:1300–1305. doi: 10.1097/01.prs.0000181509.67319.cf. [DOI] [PubMed] [Google Scholar]
- 9.Delay E, Garson S, Tousson G, Sinna R. Fat injection to the breast: technique, results, and indications based on 880 procedures over 10 years. Aesthetic surgery journal/the American Society for Aesthetic Plastic surgery. 2009;29:360–376. doi: 10.1016/j.asj.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 10.Garza RM, Paik KJ, Chung MT, et al. Studies in fat grafting: Part III. Fat grafting irradiated tissue--improved skin quality and decreased fat graft retention. Plastic and reconstructive surgery. 2014;134:249–257. doi: 10.1097/PRS.0000000000000326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luan A, Duscher D, Whittam AJ, et al. Cell-Assisted Lipotransfer Improves Volume Retention in Irradiated Recipient Sites and Rescues Radiation-Induced Skin Changes. Stem cells. 2016;34:668–673. doi: 10.1002/stem.2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Temiz G, Sirinoglu H, Yesiloglu N, Filinte D, Kacmaz C. Effects of Deferoxamine on Fat Graft Survival. Facial Plast Surg. 2016;32:438–443. doi: 10.1055/s-0036-1584236. [DOI] [PubMed] [Google Scholar]
- 13.Duscher D, Neofytou E, Wong VW, et al. Transdermal deferoxamine prevents pressure-induced diabetic ulcers. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:94–99. doi: 10.1073/pnas.1413445112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thangarajah H, Vial IN, Grogan RH, et al. HIF-1alpha dysfunction in diabetes. Cell Cycle. 2010;9:75–79. doi: 10.4161/cc.9.1.10371. [DOI] [PubMed] [Google Scholar]
- 15.Wang C, Cai Y, Zhang Y, Xiong Z, Li G, Cui L. Local injection of deferoxamine improves neovascularization in ischemic diabetic random flap by increasing HIF-1alpha and VEGF expression. PloS one. 2014;9:e100818. doi: 10.1371/journal.pone.0100818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weinstein GS, Maves MD, McCormack ML. Deferoxamine decreases necrosis in dorsally based pig skin flaps. Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery. 1989;101:559–561. doi: 10.1177/019459988910100508. [DOI] [PubMed] [Google Scholar]
- 17.Donneys A, Deshpande SS, Tchanque-Fossuo CN, et al. Deferoxamine expedites consolidation during mandibular distraction osteogenesis. Bone. 2013;55:384–390. doi: 10.1016/j.bone.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Momeni A, Rapp S, Donneys A, Buchman SR, Wan DC. Clinical Use of Deferoxamine in Distraction Osteogenesis of Irradiated Bone. The Journal of craniofacial surgery. 2016;27:880–882. doi: 10.1097/SCS.0000000000002633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Farberg AS, Sarhaddi D, Donneys A, Deshpande SS, Buchman SR. Deferoxamine enhances bone regeneration in mandibular distraction osteogenesis. Plastic and reconstructive surgery. 2014;133:666–671. doi: 10.1097/01.prs.0000438050.36881.a9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chung MT, Hyun JS, Lo DD, et al. Micro-computed tomography evaluation of human fat grafts in nude mice. Tissue engineering Part C, Methods. 2013;19:227–232. doi: 10.1089/ten.tec.2012.0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.American Cancer Society. Cancer Facts & Figures. 2017 Available at: https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2017.html.
- 22.National Cancer Institute. A Snapshot of Head and Neck Cancer. 2017 [Google Scholar]
- 23.Hegedus F, Mathew LM, Schwartz RA. Radiation dermatitis: an overview. Int J Dermatol. 2016 doi: 10.1111/ijd.13371. [DOI] [PubMed] [Google Scholar]
- 24.Hymes SR, Strom EA, Fife C. Radiation dermatitis: clinical presentation, pathophysiology, and treatment 2006. J Am Acad Dermatol. 2006;54:28–46. doi: 10.1016/j.jaad.2005.08.054. [DOI] [PubMed] [Google Scholar]
- 25.Ryan JL. Ionizing radiation: the good, the bad, and the ugly. The Journal of investigative dermatology. 2012;132:985–993. doi: 10.1038/jid.2011.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barnett GC, West CM, Dunning AM, et al. Normal tissue reactions to radiotherapy: towards tailoring treatment dose by genotype. Nat Rev Cancer. 2009;9:134–142. doi: 10.1038/nrc2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bentzen SM. Preventing or reducing late side effects of radiation therapy: radiobiology meets molecular pathology. Nat Rev Cancer. 2006;6:702–713. doi: 10.1038/nrc1950. [DOI] [PubMed] [Google Scholar]
- 28.Chang EI, Loh SA, Ceradini DJ, et al. Age decreases endothelial progenitor cell recruitment through decreases in hypoxia-inducible factor 1alpha stabilization during ischemia. Circulation. 2007;116:2818–2829. doi: 10.1161/CIRCULATIONAHA.107.715847. [DOI] [PubMed] [Google Scholar]
- 29.Weng R, Li Q, Li H, Yang M, Sheng L. Mimic hypoxia improves angiogenesis in ischaemic random flaps. Journal of plastic, reconstructive & aesthetic surgery : JPRAS. 2010;63:2152–2159. doi: 10.1016/j.bjps.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 30.Thangarajah H, Yao D, Chang EI, et al. The molecular basis for impaired hypoxia-induced VEGF expression in diabetic tissues. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:13505–13510. doi: 10.1073/pnas.0906670106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Donneys A, Ahsan S, Perosky JE, et al. Deferoxamine restores callus size, mineralization, and mechanical strength in fracture healing after radiotherapy. Plastic and reconstructive surgery. 2013;131:711e–719e. doi: 10.1097/PRS.0b013e3182865c57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Donneys A, Weiss DM, Deshpande SS, et al. Localized deferoxamine injection augments vascularity and improves bony union in pathologic fracture healing after radiotherapy. Bone. 2013;52:318–325. doi: 10.1016/j.bone.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Felice PA, Ahsan S, Donneys A, Deshpande SS, Nelson NS, Buchman SR. Deferoxamine administration delivers translational optimization of distraction osteogenesis in the irradiated mandible. Plastic and reconstructive surgery. 2013;132:542e–548e. doi: 10.1097/PRS.0b013e31829fe548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eto H, Kato H, Suga H, et al. The fate of adipocytes after nonvascularized fat grafting: evidence of early death and replacement of adipocytes. Plastic and reconstructive surgery. 2012;129:1081–1092. doi: 10.1097/PRS.0b013e31824a2b19. [DOI] [PubMed] [Google Scholar]
- 35.Suga H, Eto H, Aoi N, et al. Adipose tissue remodeling under ischemia: death of adipocytes and activation of stem/progenitor cells. Plastic and reconstructive surgery. 2010;126:1911–1923. doi: 10.1097/PRS.0b013e3181f4468b. [DOI] [PubMed] [Google Scholar]
- 36.Moreno-Navarrete JM, Ortega F, Moreno M, Ricart W, Fernandez-Real JM. Fine-tuned iron availability is essential to achieve optimal adipocyte differentiation and mitochondrial biogenesis. Diabetologia. 2014;57:1957–1967. doi: 10.1007/s00125-014-3298-5. [DOI] [PubMed] [Google Scholar]
- 37.Gkouvatsos K, Papanikolaou G, Pantopoulos K. Regulation of iron transport and the role of transferrin. Biochimica et biophysica acta. 2012;1820:188–202. doi: 10.1016/j.bbagen.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 38.Andrews NC. Disorders of iron metabolism. N Engl J Med. 1999;341:1986–1995. doi: 10.1056/NEJM199912233412607. [DOI] [PubMed] [Google Scholar]
- 39.Yu Y, Gutierrez E, Kovacevic Z, et al. Iron chelators for the treatment of cancer. Curr Med Chem. 2012;19:2689–2702. doi: 10.2174/092986712800609706. [DOI] [PubMed] [Google Scholar]
- 40.Torti SV, Torti FM. Iron and cancer: more ore to be mined. Nat Rev Cancer. 2013;13:342–355. doi: 10.1038/nrc3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yamasaki T, Terai S, Sakaida I. Deferoxamine for advanced hepatocellular carcinoma. N Engl J Med. 2011;365:576–578. doi: 10.1056/NEJMc1105726. [DOI] [PubMed] [Google Scholar]
- 42.Ozer U. The role of Iron on breast cancer stem-like cells. Cell Mol Biol (Noisy-le-grand) 2016;62:25–30. [PubMed] [Google Scholar]