Epilepsy is one of the most common and severe neurologic diseases in children, affecting 0.9–2% of the pediatric population.(1,2) Children and adolescents with epilepsy and their parents indicate that quality of life is driven as much or more by cognitive comorbidities as by seizure control.(3–5) Surveys of these families found that cognitive problems were second only to medication side effects in terms of decreasing quality of life.(6) The new International League Against Epilepsy (ILAE) classification considers the cognitive comorbidities seen in epilepsy to be part of the condition.(7) Of the cognitive problems seen in epilepsy, language disorders (Table) are particularly important to identify and address as language dysfunction can contribute to academic underachievement and long-term social, professional and psychological problems.(8)
Table 1.
Speech and Language Disorders (101)
| Auditory agnosia | Inability to recognize the symbolic meaning behind a sound, including an inability to understand speech or meaningful noises (such as a telephone ring) |
|
| |
| Aphasia | Disorders affecting the production or comprehension of spoken and written language due to acquired damage to the language regions of the dominant (typically left) hemisphere. Different components of language are affected depending on the area of brain damage. Though the disorders described below are the canonical aphasias, patients typically have mixed symptoms. |
| Receptive/Fluent/Wernicke’s Aphasia: Inability to understand spoken or written language, classically attributed to damage of the superior temporal gyrus of the dominant temporal lobe. Speech is fluent but nonsensical. | |
| Expressive/Non-fluent/Broca’s Aphasia: Inability to produce speech or writing, classically attributed to damage of the inferior frontal gyrus of the dominant frontal lobe. Speech is halting and grammar is significantly affected, but comprehension is typically spared. | |
| Conduction Aphasia: Inability to repeat secondary to damage to the arcuatefasciculus which connects Wernicke’s and Broca’s areas. | |
|
| |
| Dysarthria | Impairment of speech due to difficulty with strength or coordination of the muscles of speech. This can be a primary muscle problem or secondary to damage to the nerves or brain structures that control the muscles. Mistakes in speech are usually consistent and there can be difficulty in other functions like chewing or swallowing. Dysarthria can be a congenital or acquired condition. |
|
| |
| Prosody | The varying rhythm, intensity, or frequency of speech that, when interpreted as stress or intonation, aid in transmission of meaning. |
| Aprosody: Absence of rhythm or normal pitch variations; “robotic” voicing. | |
| Dysprosody: Impairment in normal speech intonation patterns. | |
|
| |
| Speech Dyspraxia/Apraxia | Difficulty in articulation of syllables or words due to impaired motor planning; mistakes are inconsistent, with intermixed fragments of intact speech. There is often impaired pitch and prosody.(76) Unlike in dysarthria, muscle strength and coordination are otherwise intact. Dyspraxia can be a congenital or acquired condition. |
The impact of epilepsy on language is relevant not only from a clinical perspective but also because it sheds light on the underlying neurobiology of both processes. Advances in imaging and neurophysiology techniques have demonstrated that normal language development is a complex process, subserved by bilateral but usually left-predominant networks (Figure 1).(9) We are increasingly understanding that epilepsy is a network disorder in which even focal seizures have widespread impact on many parts of the brain. Given this, childhood epilepsy likely affects the normal development of language on several levels. First, common underlying pathophysiology, such as a genetic mutations or a structural lesion, may lead to both language disorders and seizures. This is supported by the fact that language problems are noted in children with new onset seizures and do not always resolve completely even with excellent seizure control.(10–12) Second, interictal epileptiform discharges (IEDs), spike waves that occur outside of seizures, are known to cause transient disruptions in local cortical function and likely also affect the function of more widespread networks. Finally, childhood is a critical window for the development of language pathways, and abnormal electrical activity during this time may interfere with typical development. Multiple neurocognitive and imaging studies have shown atypical language lateralization and network connectivity in pediatric subjects with epilepsy, which does not always resolve even after the seizures and IEDs have ceased.
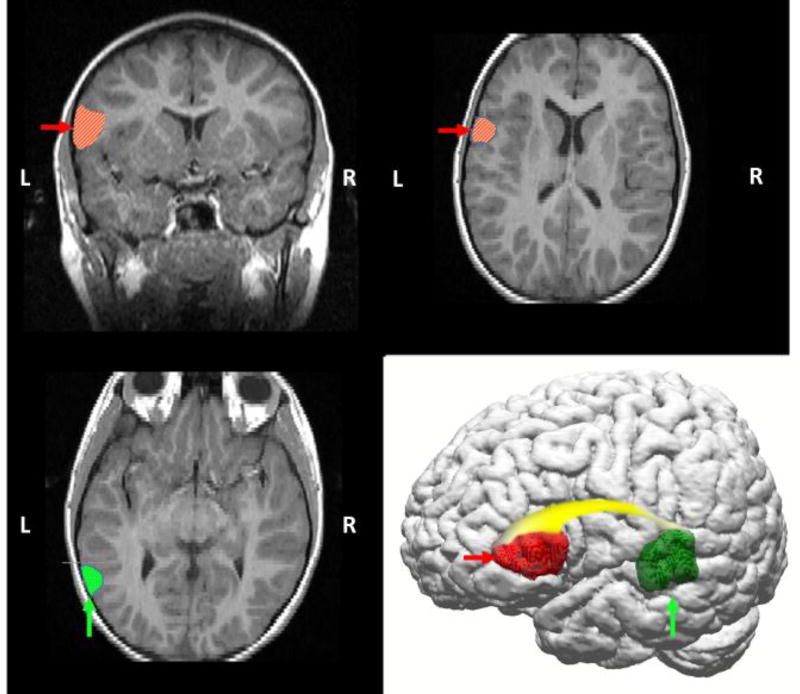
Figure 1.
Diagrammatic representation of primary language areas on anatomical T2-weighted FLAIR MRI and 3D reconstruction images (110), highlighting left temporal lobe (Wernicke’s area in posterior temporal region, vertical arrows/dotted hatching), frontal lobe (Broca’s area in middle frontal region, horizontal arrows/diagonal hatching), and the white matter (arcuate fasciculus, gradient shading) that connects these regions. Historically, language has been conceptualized as a lateralized function, with dominance typically in the left hemisphere. Functional magnetic resonance imaging (fMRI) studies confirm that language is a left-hemispheric dominant process in the vast majority of healthy adults (111), but also that language requires input from distributed networks, including homologous right hemispheric regions.
Many children with epilepsy have underlying conditions that lead to significant intellectual disability (ID), making specific assessment of language extremely difficult; this review will therefore focus on studies of children with normal or near-normal intelligence. This paper will first review studies characterizing language deficits in pediatric epilepsy from the most severe forms with total loss of language to the more common forms of language impairment found in the inappropriately termed “benign” epilepsies of childhood. Next, we will describe what is known about the structural and electrophysiologic changes associated with language dysfunction, reviewing the neuroimaging, electroencephalogram (EEG), and genetic studies related to language dysfunction. Epilepsy surgery planning and resection of epileptic foci provide additional tools to understand the impact of focal epilepsy on language network development, and interaction with overall cognition in children with epilepsy. We will review studies on language mapping in children highlighting the unique challenges and emerging promising techniques to ensure preservation of language.
CLINICAL OVERVIEW
Epilepsy-Aphasia Spectrum
Much of what we know regarding language and epilepsy in children is derived from disorders on the epilepsy-aphasia spectrum. Landau and Kleffner in 1957 first described the relationship between epilepsy and language dysfunction in an article detailing six children with previously normal language development who became aphasic after the onset of focal seizures. These patients all had EEGs with a significant burden of spike waves, especially in sleep. In general, the severity of the patients’ language disorder fluctuated with the degree of EEG epileptiform activity.(13) Since then, a spectrum of disorders, often referred to collectively as the epilepsy-aphasia spectrum, have been described that share features of sleep-potentiated EEG abnormalities, cognitive problems, and rare or even absent clinical seizures.(14) The 2 best-characterized disorders are Landau-Kleffner Syndrome and Benign Epilepsy with Centrotemporal Spikes.
Landau-Kleffner Syndrome (LKS) is the canonical example of the epilepsy-aphasia spectrum. Children with previously normal development undergo a progressive language regression over weeks to months in which they lose the ability to understand speech and sometimes other meaningful sounds, such as a telephone ring. Eventually, speech production diminishes. Neuropsychological testing in LKS suggests that impaired phonologic decoding is the primary deficit that leads to the language regression.(15) Children with LKS may simultaneously develop psychiatric symptoms, including irritability, inattention and autistic traits. Rare and easily controlled seizures, including generalized tonic clonic, focal motor, and atypical absence seizures, emerge. Age of onset is typically between 3–8 years.(16) Corresponding with the acquired auditory agnosia, an EEG pattern of Electrical Status Epilepticus of Sleep (ESES) appears in which extremely frequent spike and slow waves emerge at the onset of sleep and are near continuous through the majority of non-Rapid Eye Movement (non-REM) sleep(14) (Figure 2). Spikes tend to be maximum in the temporal regions.(17)
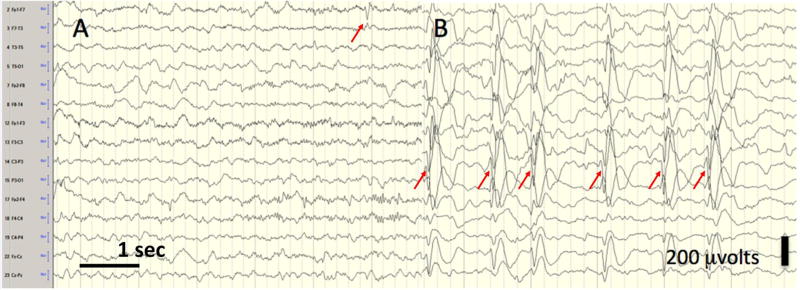
Figure 2.
Electrical Status Epilepticus of Sleep (ESES). The EEG of a young girl with language regression; six seconds each of wakefulness (left panel, A) and slow wave sleep (right panel, B). In sleep, the EEG is similar in appearance to that seen in clinical status epilepticus (bipolor longitudinal montage; 10 uvolts/mm, low filter 0.1 Hz, high filter 70 Hz). In ESES, spikes are typically bitemporal, but may be lateralized, and sleep architecture is interrupted if present. Here, arrows indicate left tempo-parietal spikes with a field to the right hemisphere. When an EEG is evaluated for ESES, the percentage of seconds of non-REM sleep containing spikes is determined, with the cut-off for diagnosis varying between 50–85%.(16)
The ESES pattern is also a defining feature of another epileptic syndrome called continuous spike waves in slow wave sleep (CSWS). The regression in CSWS tends to be more global rather than language-specific and EEG spikes usually have a more frontal predominance.(16,18) One study described language in a small group of children with CSWS and found significant problems with lexical and syntactical skills, but intact comprehension.(19) Overall, LKS and CSWS are quite rare. A clear incidence has not been reported, likely in part due to the lack of agreement among epileptologists regarding the exact definition of ESES, CSWS, and LKS.(14,20) Studies of long-term prognosis of these conditions likewise suffer from imprecise definitions. In general, there is agreement that children with underlying structural or metabolic causes of ESES, EEG abnormalities that persist longer than 18 months, treatment failure, or young age of onset are more likely to have permanent cognitive problems even after resolution of the nocturnal EEG findings.(15,21–23) Approximately 45–70% of affected patients have permanent cognitive impairment, including language dysfunction.(21,22)
Benign Epilepsy with Centrotemporal Spikes (BECTS) or Rolandic epilepsy is the most common focal epilepsy syndrome in childhood, consisting of 15–25% of all cases of pediatric epilepsy.(24) It is an idiopathic epilepsy syndrome characterized by focal sensorimotor seizures that usually involve the face but which often spread to the arm or generalize. The typical EEG findings include centrotemporal spike waves that become markedly more prominent during sleep. Spikes in children with BECTS are not as frequent as in children with ESES. Onset of BECTS is between 3–13 years.(24) Compared with many other causes of epilepsy, seizures are rare, with most patients having only 2 to 10 lifetime seizures.(25) Many children are never treated with anti-seizure medications and, for those who are, seizures are usually easy to control.
BECTS has been considered “benign” because seizures predictably stop after puberty, by age 16, and affected children typically have a normal full-scale IQ.(25) Detailed neuropsychological profiles of children with BECTS, however, show deficits in many subdomains, including language, visual spatial skills, attention, memory,(26,27) fine motor skills,(28–30) and behavior.(31) Smith et al(32) recently performed a meta-analysis of 22 studies that included formal language testing of children with BECTS. Patients consistently demonstrated diminished reading, expressive language and receptive language abilities compared with healthy children; the authors estimated a 0.7 standard deviation between the two groups for these measures. These authors speculate that, as in ESES, phonological processing difficulties might explain the language and reading difficulties, as these were also selectively impaired in children with BECTS. Interestingly, the language differences were more pronounced in older children with BECTS, raising the question as to whether there is a cumulative effect of epilepsy on cognition over time. This question is challenging to answer as many neurocognitive studies of the BECTS population are confounded by the fact that included patients have had epilepsy for varying lengths of time and have pursued differing treatment plans. Interestingly, a recent study of neuropsychological functioning in unmedicated children with newly diagnosed BECTS found only small, non-significant differences in language and cognitive skills compared with healthy controls(30) at the start of their disorder. Prospective studies of children with BECTS that can better account for the effects of anti-seizure medication will be important in disentangling the effects of this disorder on language. Finally, though IQ was an important moderator of language abilities, even children with BECTS and above-average IQs still showed a gap in receptive and expressive language.(33)
These language difficulties translate to meaningful academic problems, even after resolution of the epilepsy. Up to 54% of children with BECTS have educational problems, the majority of which are attributed to language impairment.(34) Follow-up studies have reported mixed outcomes in children with BECTS after remission of the seizures and EEG abnormalities. Interview-based measures (35,36) typically find good psychosocial outcomes in adults with resolved BECTS, but structured neurocognitive testing shows persistent deficits in language processing in almost 50% of subjects.(37,38)
Language Disorders in Other Epilepsy Syndromes
Though the epilepsy aphasia syndromes are the most studied, other types of epilepsy are also associated with language disruption. Several large cohort studies of children with new onset epilepsy have found subtle language abnormalities at the time of seizure onset.(10–12,39) There has been some disagreement as to which types of epilepsy have the biggest impact on language. Several studies(11,40) found that children with absence epilepsy (a generalized seizure disorder) had worse language performance than children with focal epilepsy (seizures originating in one area in the brain). In contrast, Hermann et al (10) found that children with focal but not generalized epilepsy had poorer language than controls; the cognitive differences seen in this cohort remained stable over time .(41) Although patient selection may have contributed to study differences, language dysfunction is common irrespective of epilepsy type.
Older children, particularly adolescents, seem to have worse language performance than younger children. Jones et al (12) found that about 50% of adolescents with epilepsy had reading and language scores greater than one standard deviation below average, compared with only one quarter of children younger than 8 years. Greater duration of illness likely explains some,(39) but not all,(12) of this finding; studies suggest refractory seizures may predict stagnation of language skills during long-term follow-up. Similarly, a recent study from Finland prospectively followed a cohort of children with epilepsy for five decades; 75% of the group were in remission and had been off seizure medications for at least 5 years and 25% of the group still had active epilepsy. The entire cohort had impairments in language and semantic functions in late middle age when compared with healthy controls, but these problems were especially pronounced in those with continued seizures.(42)
ETIOLOGY OF LANGUAGE DYSFUNCTION
Insight from Functional Imaging & Epilepsy Surgery
Functional magnetic resonance imaging (fMRI) studies have demonstrated that epilepsy of various underlying etiologies affects language network consolidation. In children with BECTS, neuropsychological testing (38,43) and fMRI (44–46) indicate that children fail to lateralize cortical functions normally. These children have greater bilateral activation for language tasks that typically involve the left hemisphere – including verb generation(44), sentence generation(45), and semantic decision tasks– as well as right hemisphere tasks, such as prosody discrimination.(46) Similarly, in 58 children with focal epilepsy , fMRI found that patients were significantly more likely to demonstrate bilateral or right hemispheric language dominance compared with left hemispheric language in controls.(47) A more recent series of 23 children with atypical language dominance found that atypical lateralization strongly correlated to a more widespread disruption in network integration.(48) In children with BECTS, there is some evidence that atypical language lateralization persists even after the seizures have abated and the EEG pattern has normalized.(38) In addition to differences in lateralization, children with epilepsy have different connectivity patterns within their language networks. For example, Croft et al (49) showed that children with epilepsy with a left hemispheric focus specifically have decreased activity in the ventral components of the language network which develop earlier in childhood compared with the dorsal components. This decrease in activity correlates with poorer language function. Additionally, the authors found that the deficits persisted in older age groups, suggesting that early-life epilepsy causes chronic deficits rather than just delayed maturation of the network. Taken together, these findings suggest that it is not only acute seizures or epileptiform discharges that cause language dysfunction, but that chronic changes to underlying networks may cause persistent language problems.
Patients who have undergone epilepsy surgery provide insight into language development in the context of epilepsy as well as the potential for recovery after control of seizures. A recent comprehensive review of the behavioral effects of epilepsy surgery supports associations of better outcomes, including language outcomes, with early surgical intervention and post-operative seizure freedom.(50) As described above, children with early left-hemispheric epilepsy are much more likely to have language lateralized to the right hemisphere than the general population.(51) In the most extreme cases, children with hemimegalencephaly, Rasmussen’s encephalitis, or other large unilateral epileptic lesions typically have significant pre-operative deficits in verbal function across multiple behavioral measures.(52–54) Such deficits may be more frequent and severe when the lesions involve the left hemisphere but are common regardless of side.(52) These children often require extensive resections up to hemispherotomy to control their seizures, but many demonstrate improvements in language function after such surgery. When language was formally assessed in a group of 28 children with seizure freedom achieved by hemispherotomy, language skills improved by 50% after surgery, with those patients whose EEG sleep patterns normalized after surgery showing the greatest gains.(55) Similarly, Loddenkemper et al(56) reported that eight patients with ESES due to focal lesions visible on standard MRI had a significant improvement in developmental quotient – with an average increase of 9.8 points – after resective surgery improved the nocturnal EEG.
Remapping of language is likely associated with underlying structural changes. In a group of 10 children after left hemispherotomy, the right hemispheric arcuate fasciculus showed compensatory reorganization on post-surgical diffusion tensor imaging(57) correlating with improved or stable language function. Imaging studies have shown that children with large left hemispheric lesions who undergo early hemispherotomy will remap language to the intact right hemisphere in a thorough enough manner to have complete acquisition of syntax and grammatical construction.(53) Language recovery has been reported to be more rapid and complete in younger children after hemispherotomy(58), suggesting a critical window for remodeling after surgery that would support an “earlier is better” approach to large resections in terms of language recovery. A recent analysis of language reorganization after acquired unilateral insults during childhood supports this notion, suggesting more complete and functional reorganization to the non-dominant hemisphere after acute lesions occurring before, as opposed to after, age 5 years.(59) Not all investigators agree. Curtiss and de Bode (52,60) reported that children with large developmental lesions (present since birth) had worse language if the lesion was in the right hemisphere and children with acquired lesions had worse language outcomes if the lesion was in the left hemisphere. They proposed the ‘critical impact point’ hypothesis in explanation – that there are genetically determined substrates in both hemispheres that are critical for language development at different stages.(52,54) Though more work is still needed to fully elucidate this interaction, it seems most would agree that age at epilepsy surgery may be an important predictor of language outcome.
Interictal Discharges
The impact of IEDs on cognition has been an area of significant research. Substantial evidence suggests that both generalized and focal IEDs cause transient changes in perception, processing, and reactivity, and that the nature of these disruptions is related to the location of the activity.(61) Evidence from rodent studies indicates that single spikes can impair memory formation.(61) Similarly, video-EEG studies in humans have demonstrated transient disruptions in visual, visual-spatial, and language performance based on the location of the spike wave.(61) In children, there is evidence suggesting that spike burden during a task affects performance. Nicolai et al(62) performed a 2-hour neuropsychological battery on 188 children who were simultaneously undergoing EEG recordings. They found a graded difference across various neurocognitive measures, including language – controls did better than those with IEDs on >1% of the recording, who did better than those with subclinical seizures.
It has been hypothesized that in addition to transient cognitive impairment, IEDs can also cause persistent problems with cognition. For example, language skills in patients with LKS fluctuate depending on the severity of nocturnal discharges.(63,64) Several studies of BECTS have found a correlation between diurnal(65) and nocturnal(33,66) spike burden with the degree and type of cognitive dysfunction; for example, left IEDs seem to affect language and right IEDs affect spatial skills.(38,43,67) However, not all studies in BECTS have found this correlation.(68,69) Fewer studies have assessed the effects of IEDs on language in other types of pediatric epilepsy. Ebus et al (70) found that spikes in greater than 10% of the diurnal recording were associated with poorer cognitive function, though they did not look at language-specific measures. In children with lesional epilepsy, IEDs had a negative association with IQ, with additive effects seen for frequent, bilateral, and sleep-potentiated IEDs.(71) Left-lateralized IEDs had a prominent effect on verbal intelligence above that expected from the lesion alone and sleep-potentiated IEDs were associated with poorer expressive and receptive language, reading, spelling, and numerical skills. In summary, although there seems to be a correlation between frequent IEDs, particularly nocturnal ones, and poor language function, the evidence for a causative relationship is relatively weak.
Several interesting studies combining spike detection with fMRI have found that IEDs affect distributed neural networks, influencing both language and resting networks. An EEG-fMRI study of children with BECTS found increased connectivity between the rolandic areas and expressive and receptive language areas during centrotemporal spiking,(72) suggesting that the spikes themselves have a brief, disruptive impact on these remote regions. Ibrahim et al (73) used magnetoencephalography paired with fMRI to test the effect of individual IEDs on several neural networks; they found that children with less perturbation of baseline network activity during IEDs scored better on neurocognitive tests. IEDs can also inappropriately deactivate the default mode network – the “resting network” of the brain that is active during quiet, self-referential thought and deactivated during tasks – in children with BECTS47 and CSWS.(74) Children with BECTS also demonstrate difficulty activating and deactivating the default mode network in response to specific language tasks.(75) Appropriate modulation of the default mode network is necessary for higher order cognitive tasks like language.
Genetics
GRIN2A
In the last several years, there have been major advancements in our knowledge of the genetics of epilepsy-aphasia syndromes. Perhaps the most important discovery has been the identification of mutations in GRIN2A, a gene on chromosome 16p13 that encodes a subunit of the glutamate N-methyl-D-aspartate (NMDA) receptor. The NMDA receptor is a cell surface receptor important in brain development, synaptic plasticity, memory, and sleep and is distributed throughout cortical and subcortical regions.(76,77) Since 2013, a variety of mutations in GRIN2A have been identified in families and individuals with ESES, CSWS and other disorders on the epilepsy aphasia spectrum. GRIN2A mutations have been identified for 9–20% of cohorts with LKS and CSWS but only 3.6% of patients with typical BECTS.(78–80) Turner et al (76) described a language phenotype seen with these mutations which includes speech dyspraxia as well as dysarthria, with poor articulation and disturbances in prosody.
Descriptive studies of affected families have suggested that the GRIN2A mutation may cause language dysfunction independent of causing epilepsy. In families affected by epilepsy-aphasia spectrum disorders, there are some GRIN2A mutation carriers without epilepsy. Additionally, up to 70% of children in these pedigrees have developed speech disorders before onset of epileptiform abnormalities on EEG.(76) GRIN2A mutations seem to be specific for epilepsy-aphasia disorders, as they were not found in screens of patients with other types of epileptic encephalopathies.(79)
RFBOX
Rare mutations in RBFOX1 & RBFOX3 – genes responsible for the splicing of neuronal transcripts important in control of membrane excitability – have also been reported in patients with epilepsy-aphasia disorder. One study of 289 unrelated patients with BECTS found three RFBOX variants compared with no variants in 6503 subjects without BECTS.(81) Ten family members of proband cases were also carriers; five had BECTS, one had ESES and one had the EEG features of centrotemporal spikes. Mutations in RFBOX have also been reported in a variety of other neurologic disorders, including generalized epilepsy, attention disorders, and ID.(81,82) At least one group has argued, however, that RFBOX variants may actually be seen in similar proportions in control populations.(83)
FOXP2/SRPX2/CNTNAP2
FOXP2 encodes an important neuronal transcription factor. It was the first gene to be associated with a severe speech disorder – developmental verbal dyspraxia without epilepsy – in which patients are often unintelligible.(77,84) Two downstream targets of FOXP2 have been associated with speech disturbances and epilepsy. SRPX2 mutations were identified in a family with autosomal dominant rolandic seizures, speech dyspraxia, and ID as well as in patients with bilateral perisylvian polymicrogyria; both conditions are associated with language dysfunction and epilepsy.(85) The role of SRPX2 in language, however, was called into question when the family cohort with autosomal dominant rolandic seizures and speech dyspraxia were later found to also carry a GRIN2A mutation.(77) FOXP2 is also upstream of CNTNAP2, a gene associated with cortical dysplasia, focal epilepsy, and language regression after seizure onset.(86)
BECTS
Many researchers have searched for a genetic cause of BECTS. Family members of children with BECTS have a higher rate of epilepsy, particularly febrile seizures, but the common form of this epilepsy syndrome itself does not seem to have clear Mendelian inheritance (87); several twin studies in fact showed zero concordance among twin pairs.(88) Susceptibility genes identified in families with multiple affected members (i.e. SRPX2) are not causal in the majority of affected patients. Sequencing of ɣ-aminobutyric acid type A receptor (GABAA-R) genes in a large BECTS cohort has shown enrichment of rare variants compared with controls, but a causative role of these variants has not been proven.(89) Interestingly, components of the syndrome may be linked more consistently to specific genes. For example, family association studies have shown that the centrotemporal spike wave trait is often inherited in an autosomal dominant fashion,(90,91) and may be associated with mutations of the Elongator Protein Complex 4 (ELP4) gene on chromosome 11 (92), but this association has not been consistent.(93) Additional analyses have suggested linkage of speech sound disorder in BECTS to 11p13(92) and reading disability to chromosome 7q21 and 1q42.(94) Though BECTS is a common disorder, the genetics underlying it are complex and still being investigated.
Other CNV
Various studies have identified increased frequency of copy number variants (CNVs) – rare microduplications or deletions – in children with BECTS,(87) ESES, and CSWS.(95–97) The importance of each of these has not been clearly verified and a detailed review is beyond the scope of this article.
Seizure Medications and Language
We have focused this review on the impact that epilepsy itself has on language, but pharmacologic treatment for epilepsy can also have significant, complex effects on language and cognition. It can be difficult to disentangle the cognitive benefits of improved seizure control from the negative side effects of these medications. For example, phenobarbital is one of the oldest available seizure medications and is still widely used in pediatric epilepsy, especially for the treatment of neonatal seizures. Hyperactivity and trouble with memory and attention has been reported in exposed children.(98) Farwell et al (99) performed serial developmental assessments on children with febrile seizures randomly assigned to prophylaxis with phenobarbital or placebo. Children in the phenobarbital group had lower IQs than those in the placebo group and this difference persisted even 6 months after phenobarbital was withdrawn. Follow-up of this cohort into the school years showed that the difference in IQs of the groups diminished but that children who had taken phenobarbital had worse performance on reading scores.(100) Of all the seizure medications, topiramate and a similar but newer medication, zonisamide, have most specifically been associated with language problems and speech difficulties in adults (101,102,103) and children.(104,105,106) Several fascinating fMRI studies (107,108,109) have shown that patients receiving topiramate and zonisamide have altered activation of the language networks compared with patient receiving other seizure medications and healthy controls. Although these medications are particularly associated with language difficulties, many patients tolerate them well. Conversely, some patients complain of cognitive problems on other medications that are typically well-tolerated. Clinicians should recognize that some cognitive problems may improve with a change in treatment. For a thorough discussion of the cognitive impact of seizure medications, we refer you to the recent review by Aldenkamp et al. (98)
Concluding Remarks
Epilepsy imparts significant morbidity through its effects on cognition and language. Some of this burden, arising independently from the underlying structural or metabolic causes, may not be directly ameliorated by treatments directed toward seizure. However, a growing body of data, particularly from patients with BECTS and its more severe counterparts in the epilepsy-aphasia spectrum, indicates that IEDs and spike burden affect cognitive and language development in a dynamic manner yet to be fully described. In this context, physicians of children with epilepsy should maintain a high index of suspicion for language disorders and should consider early referral to therapies if problems are suspected, even in children with diagnoses of “benign” syndromes like BECTS. Though modern neuroimaging and genetic testing may provide definitive etiologic diagnoses for many of the children with more severe forms of epilepsy aphasia syndromes, much remains to be learned about their genetic causes. Further research to understand how interictal discharges may disrupt normal network function holds promise to suggest novel therapeutic targets or modalities for the cognitive and language comorbidities of epilepsy.
Acknowledgments
Supported by KL2 Mentored Career Development Award of the Stanford Clinical and Translational Science Award to Spectrum (NIH KL2 TR 001083) and (UL1 TR 001085) (F.B.).
Abbreviations
- BECTS
Benign Epilepsy with Centrotemporal Spikes
- CSWS
Continuous Spike-Waves in Slow-Wave Sleep
- EEG
electroencephalogram
- ESES
Electrical Status Epilepticus of Sleep
- fMRI
functional magnetic resonance imaging
- IEDs
interictal epileptiform discharges
- ID
intellectual disability
- LKS
Landau-Kleffner Syndrome
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflicts of interest.
References
- 1.Berg AT, Jallon P, Preux PM. The epidemiology of seizure disorders in infancy and childhood: definitions and classifications. Handb Clin Neurol. 2013;111:391–8. doi: 10.1016/B978-0-444-52891-9.00043-9. [DOI] [PubMed] [Google Scholar]
- 2.Oka E, Ohtsuka Y, Yoshinaga H, Murakami N, Kobayashi K, Ogino T. Prevalence of childhood epilepsy and distribution of epileptic syndromes: a population-based survey in Okayama, Japan. Epilepsia. 2006;47:626–30. doi: 10.1111/j.1528-1167.2006.00477.x. [DOI] [PubMed] [Google Scholar]
- 3.Nickels KC, Zaccariello MJ, Hamiwka LD, Wirrell EC. Cognitive and neurodevelopmental comorbidities in paediatric epilepsy. Nat Rev Neurol. 2016;12:465–76. doi: 10.1038/nrneurol.2016.98. [DOI] [PubMed] [Google Scholar]
- 4.Speechley KN, Ferro MA, Camfield CS, Huang W, Levin SD, Smith ML, et al. Quality of life in children with new-onset epilepsy: a 2-year prospective cohort study. Neurology. 2012;79:1548–55. doi: 10.1212/WNL.0b013e31826e25aa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferro MA, Camfield CS, Levin SD, Smith ML, Wiebe S, Zou G, et al. Trajectories of health-related quality of life in children with epilepsy: a cohort study. Epilepsia. 2013;54:1889–97. doi: 10.1111/epi.12388. [DOI] [PubMed] [Google Scholar]
- 6.Arunkumar G, Wyllie E, Kotagal P, Ong HT, Gilliam F. Parent- and patient-validated content for pediatric epilepsy quality-of-life assessment. Epilepsia. 2000;41:1474–84. doi: 10.1111/j.1528-1157.2000.tb00125.x. [DOI] [PubMed] [Google Scholar]
- 7.Fisher RS, Cross JH, French JA, Higurashi N, Hirsch E, Jansen FE, et al. Operational classification of seizure types by the International League Against Epilepsy: Position Paper of the ILAE Commission for Classification and Terminology. Epilepsia. 2017;58:522–30. doi: 10.1111/epi.13670. [DOI] [PubMed] [Google Scholar]
- 8.Overvliet GM, Besseling RM, Vles JS, Hofman PA, Backes WH, van Hall MH, et al. Nocturnal epileptiform EEG discharges, nocturnal epileptic seizures, and language impairments in children: review of the literature. Epilepsy Behav. 2010;19:550–8. doi: 10.1016/j.yebeh.2010.09.015. [DOI] [PubMed] [Google Scholar]
- 9.Szaflarski JP, Schmithorst VJ, Altaye M, Byars AW, Ret J, Plante E, et al. A longitudinal functional magnetic resonance imaging study of language development in children 5 to 11 years old. Ann Neurol. 2006;59:796–807. doi: 10.1002/ana.20817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hermann B, Jones J, Sheth R, Dow C, Koehn M, Seidenberg M. Children with new-onset epilepsy: neuropsychological status and brain structure. Brain. 2006;129:2609–19. doi: 10.1093/brain/awl196. [DOI] [PubMed] [Google Scholar]
- 11.Fastenau PS, Johnson CS, Perkins SM, Byars AW, deGrauw TJ, Austin JK, et al. Neuropsychological status at seizure onset in children: risk factors for early cognitive deficits. Neurology. 2009;73:526–34. doi: 10.1212/WNL.0b013e3181b23551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jones JE, Siddarth P, Gurbani S, Shields WD, Caplan R. Cognition, academic achievement, language, and psychopathology in pediatric chronic epilepsy: Short-term outcomes. Epilepsy Behav. 2010;18:211–7. doi: 10.1016/j.yebeh.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Landau WM, Kleffner FR. Syndrome of acquired aphasia with convulsive disorder in children. Neurology. 1957;7:523–30. doi: 10.1212/wnl.7.8.523. [DOI] [PubMed] [Google Scholar]
- 14.Sánchez Fernández I, Loddenkemper T, Galanopoulou AS, Moshé SL. Should epileptiform discharges be treated. Epilepsia. 2015;56:1492–504. doi: 10.1111/epi.13108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Metz-Lutz M-N, Filippini M. Neuropsychological findings in Rolandic epilepsy and Landau-Kleffner syndrome. Epilepsia. 2006;47(Suppl 2):71–5. doi: 10.1111/j.1528-1167.2006.00695.x. [DOI] [PubMed] [Google Scholar]
- 16.Nickels K, Wirrell E. Electrical status epilepticus in sleep. Semin Pediatr Neurol. 2008;15:50–60. doi: 10.1016/j.spen.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 17.Nieuwenhuis L, Nicolai J. The pathophysiological mechanisms of cognitive and behavioral disturbances in children with Landau-Kleffner syndrome or epilepsy with continuous spike-and-waves during slow-wave sleep. Seizure. 2006;15:249–58. doi: 10.1016/j.seizure.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 18.Sánchez Fernández I, Chapman KE, Peters JM, Harini C, Rotenberg A, Loddenkemper T. Continuous Spikes and Waves during Sleep: Electroclinical Presentation and Suggestions for Management. Epilepsy Res Treat. 2013;2013:583531. doi: 10.1155/2013/583531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Debiais S, Tuller L, Barthez MA, Monjauze C, Khomsi A, Praline J, et al. Epilepsy and language development: the continuous spike-waves during slow sleep syndrome. Epilepsia. 2007;48:1104–10. doi: 10.1111/j.1528-1167.2007.01015.x. [DOI] [PubMed] [Google Scholar]
- 20.Fernández IS, Chapman KE, Peters JM, Kothare SV, Nordli DR, Jensen FE, et al. The tower of Babel: survey on concepts and terminology in electrical status epilepticus in sleep and continuous spikes and waves during sleep in North America. Epilepsia. 2013;54:741–50. doi: 10.1111/epi.12039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liukkonen E, Kantola-Sorsa E, Paetau R, Gaily E, Peltola M, Granström ML. Long-term outcome of 32 children with encephalopathy with status epilepticus during sleep, or ESES syndrome. Epilepsia. 2010;51:2023–32. doi: 10.1111/j.1528-1167.2010.02578.x. [DOI] [PubMed] [Google Scholar]
- 22.Pera MC, Brazzo D, Altieri N, Balottin U, Veggiotti P. Long-term evolution of neuropsychological competences in encephalopathy with status epilepticus during sleep: a variable prognosis. Epilepsia. 2013;54(Suppl 7):77–85. doi: 10.1111/epi.12313. [DOI] [PubMed] [Google Scholar]
- 23.Caraballo RH, Veggiotti P, Kaltenmeier MC, Piazza E, Gamboni B, Lopez Avaria MF, et al. Encephalopathy with status epilepticus during sleep or continuous spikes and waves during slow sleep syndrome: a multicenter, long-term follow-up study of 117 patients. Epilepsy Res. 2013;105:164–73. doi: 10.1016/j.eplepsyres.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 24.Wirrell EC. Benign epilepsy of childhood with centrotemporal spikes. Epilepsia. 1998;39(Suppl 4):S32–41. doi: 10.1111/j.1528-1157.1998.tb05123.x. [DOI] [PubMed] [Google Scholar]
- 25.Vannest J, Tenney JR, Gelineau-Morel R, Maloney T, Glauser TA. Cognitive and behavioral outcomes in benign childhood epilepsy with centrotemporal spikes. Epilepsy Behav. 2015;45:85–91. doi: 10.1016/j.yebeh.2015.01.041. [DOI] [PubMed] [Google Scholar]
- 26.Filippini M, Boni A, Giannotta M, Gobbi G. Neuropsychological development in children belonging to BECTS spectrum: long-term effect of epileptiform activity. Epilepsy Behav EB. 2013;28:504–11. doi: 10.1016/j.yebeh.2013.06.016. [DOI] [PubMed] [Google Scholar]
- 27.Filippini M, Ardu E, Stefanelli S, Boni A, Gobbi G, Benso F. Neuropsychological profile in new-onset benign epilepsy with centrotemporal spikes (BECTS): Focusing on executive functions. Epilepsy Behav EB. 2016;54:71–9. doi: 10.1016/j.yebeh.2015.11.010. [DOI] [PubMed] [Google Scholar]
- 28.Ayaz M, Kara B, Soylu N, Ayaz AB. Fine motor skills in children with rolandic epilepsy. Epilepsy Behav EB. 2013;29:322–5. doi: 10.1016/j.yebeh.2013.07.033. [DOI] [PubMed] [Google Scholar]
- 29.Kirby A, Williams N, Koelewijn L, Brindley LM, Muthukumaraswamy SD, Te Water Naudé J, et al. Benign childhood epilepsy with centrotemporal spikes (BECTS) and developmental co-ordination disorder. Epilepsy Behav EB. 2017;72:122–6. doi: 10.1016/j.yebeh.2017.04.014. [DOI] [PubMed] [Google Scholar]
- 30.Vannest J, Tenney JR, Altaye M, Byars AW, Spencer C, Maloney TC, et al. Impact of frequency and lateralization of interictal discharges on neuropsychological and fine motor status in children with benign epilepsy with centrotemporal spikes. Epilepsia. 2016;57:e161–167. doi: 10.1111/epi.13445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kavros PM, Clarke T, Strug LJ, Halperin JM, Dorta NJ, Pal DK. Attention impairment in rolandic epilepsy: systematic review. Epilepsia. 2008;49:1570–80. doi: 10.1111/j.1528-1167.2008.01610.x. [DOI] [PubMed] [Google Scholar]
- 32.Smith AB, Bajomo O, Pal DK. A meta-analysis of literacy and language in children with rolandic epilepsy. Dev Med Child Neurol. 2015;57:1019–26. doi: 10.1111/dmcn.12856. [DOI] [PubMed] [Google Scholar]
- 33.Liu X, Zhang X, Han Q, Guo J, Wang C. Cognition in Chinese children with benign childhood epilepsy with centrotemporal spikes (BCECTS) Neurosci Lett. 2012;507:1–4. doi: 10.1016/j.neulet.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 34.Vinayan KP, Biji V, Thomas SV. Educational problems with underlying neuropsychological impairment are common in children with Benign Epilepsy of Childhood with Centrotemporal Spikes (BECTS) Seizure. 2005;14:207–12. doi: 10.1016/j.seizure.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 35.Callenbach PM, Bouma PA, Geerts AT, Arts WF, Stroink H, Peeters EA, et al. Long term outcome of benign childhood epilepsy with centrotemporal spikes: Dutch Study of Epilepsy in Childhood. Seizure. 2010;19:501–6. doi: 10.1016/j.seizure.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 36.Camfield CS, Camfield PR. Rolandic epilepsy has little effect on adult life 30 years later: a population-based study. Neurology. 2014;82:1162–6. doi: 10.1212/WNL.0000000000000267. [DOI] [PubMed] [Google Scholar]
- 37.Monjauze C, Tuller L, Hommet C, Barthez MA, Khomsi A. Language in benign childhood epilepsy with centro-temporal spikes abbreviated form: rolandic epilepsy and language. Brain Lang. 2005;92:300–8. doi: 10.1016/j.bandl.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 38.Monjauze C, Broadbent H, Boyd SG, Neville BGR, Baldeweg T. Language deficits and altered hemispheric lateralization in young people in remission from BECTS. Epilepsia. 2011;52:e79–83. doi: 10.1111/j.1528-1167.2011.03105.x. [DOI] [PubMed] [Google Scholar]
- 39.Byars AW, deGrauw TJ, Johnson CS, Perkins SM, Fastenau PS, Dunn DW, et al. Language and social functioning in children and adolescents with epilepsy. Epilepsy Behav. 2014;31:167–71. doi: 10.1016/j.yebeh.2013.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Caplan R, Siddarth P, Vona P, Stahl L, Bailey C, Gurbani S, et al. Language in pediatric epilepsy. Epilepsia. 2009;50:2397–407. doi: 10.1111/j.1528-1167.2009.02199.x. [DOI] [PubMed] [Google Scholar]
- 41.Rathouz PJ, Zhao Q, Jones JE, Jackson DC, Hsu DA, Stafstrom CE, et al. Cognitive development in children with new onset epilepsy. Dev Med Child Neurol. 2014;56:635–41. doi: 10.1111/dmcn.12432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Karrasch M, Tiitta P, Hermann B, Joutsa J, Shinnar S, Rinne J, et al. Cognitive Outcome in Childhood-Onset Epilepsy: A Five-Decade Prospective Cohort Study. J Int Neuropsychol Soc JINS. 2017;23:332–40. doi: 10.1017/S1355617716001077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bedoin N, Herbillon V, Lamoury I, Arthaud-Garde P, Ostrowsky K, De Bellescize J, et al. Hemispheric lateralization of cognitive functions in children with centrotemporal spikes. Epilepsy Behav EB. 2006;9:268–74. doi: 10.1016/j.yebeh.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 44.Lillywhite LM, Saling MM, Harvey AS, Abbott DF, Archer JS, Vears DF, et al. Neuropsychological and functional MRI studies provide converging evidence of anterior language dysfunction in BECTS. Epilepsia. 2009;50:2276–84. doi: 10.1111/j.1528-1167.2009.02065.x. [DOI] [PubMed] [Google Scholar]
- 45.Datta AN, Oser N, Bauder F, Maier O, Martin F, Ramelli GP, et al. Cognitive impairment and cortical reorganization in children with benign epilepsy with centrotemporal spikes. Epilepsia. 2013;54:487–94. doi: 10.1111/epi.12067. [DOI] [PubMed] [Google Scholar]
- 46.Vannest J, Szaflarski JP, Eaton KP, Henkel DM, Morita D, Glauser TA, et al. Functional magnetic resonance imaging reveals changes in language localization in children with benign childhood epilepsy with centrotemporal spikes. J Child Neurol. 2013;28:435–45. doi: 10.1177/0883073812447682. [DOI] [PubMed] [Google Scholar]
- 47.You X, Adjouadi M, Guillen MR, Ayala M, Barreto A, Rishe N, et al. Sub-patterns of language network reorganization in pediatric localization related epilepsy: A multisite study. Hum Brain Mapp. 2011;32:784–99. doi: 10.1002/hbm.21066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ibrahim GM, Morgan BR, Doesburg SM, Taylor MJ, Pang EW, Donner E, et al. Atypical language laterality is associated with large-scale disruption of network integration in children with intractable focal epilepsy. Cortex J Devoted Study Nerv Syst Behav. 2015;65:83–8. doi: 10.1016/j.cortex.2014.12.016. [DOI] [PubMed] [Google Scholar]
- 49.Croft LJ, Baldeweg T, Sepeta L, Zimmaro L, Berl MM, Gaillard WD. Vulnerability of the ventral language network in children with focal epilepsy. Brain. 2014;137:2245–57. doi: 10.1093/brain/awu154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Besag F, Caplan R, Aldenkamp A, Dunn DW, Gobbi G, Sillanpää M. Psychiatric and Behavioural Disorders in Children with Epilepsy (ILAE Task Force Report): Behavioural effects of epilepsy surgery. Epileptic Disord Int Epilepsy J Videotape. 2016;18:S68–76. doi: 10.1684/epd.2016.0809. [DOI] [PubMed] [Google Scholar]
- 51.Helmstaedter C, Kurthen M, Linke DB, Elger CE. Patterns of Language Dominance in Focal Left and Right Hemisphere Epilepsies: Relation to MRI Findings, EEG, Sex, and Age at Onset of Epilepsy. Brain Cogn. 1997;33:135–50. doi: 10.1006/brcg.1997.0888. [DOI] [PubMed] [Google Scholar]
- 52.Gröppel G, Dorfer C, Mühlebner-Fahrngruber A, Dressler A, Porsche B, Czech T, et al. Improvement of language development after successful hemispherotomy. Seizure. 2015;30:70–5. doi: 10.1016/j.seizure.2015.05.018. [DOI] [PubMed] [Google Scholar]
- 53.de Bode S, Smets L, Mathern GW, Dubinsky S. Complex syntax in the isolated right hemisphere: Receptive grammatical abilities after cerebral hemispherectomy. Epilepsy Behav EB. 2015;51:33–9. doi: 10.1016/j.yebeh.2015.06.024. [DOI] [PubMed] [Google Scholar]
- 54.Save-Pédebos J, Pinabiaux C, Dorfmuller G, Sorbets SF, Delalande O, Jambaqué I, et al. The development of pragmatic skills in children after hemispherotomy: Contribution from left and right hemispheres. Epilepsy Behav EB. 2016;55:139–45. doi: 10.1016/j.yebeh.2015.12.013. [DOI] [PubMed] [Google Scholar]
- 55.Gröppel G, Dorfer C, Mühlebner-Fahrngruber A, Dressler A, Porsche B, Czech T, et al. Improvement of language development after successful hemispherotomy. Seizure. 2015;30:70–5. doi: 10.1016/j.seizure.2015.05.018. [DOI] [PubMed] [Google Scholar]
- 56.Loddenkemper T, Cosmo G, Kotagal P, Haut J, Klaas P, Gupta A, et al. Epilepsy surgery in children with electrical status epilepticus in sleep. Neurosurgery. 2009;64:328–337. doi: 10.1227/01.NEU.0000336767.14252.76. [DOI] [PubMed] [Google Scholar]
- 57.Goradia D, Chugani HT, Govindan RM, Behen M, Juhász C, Sood S. Reorganization of the right arcuate fasciculus following left arcuate fasciculus resection in children with intractable epilepsy. J Child Neurol. 2011;26:1246–51. doi: 10.1177/0883073811402689. [DOI] [PubMed] [Google Scholar]
- 58.Vining EPG, Freeman JM, Pillas DJ, Uematsu S, Carson BS, Brandt J, et al. Why Would You Remove Half a Brain? The Outcome of 58 Children After Hemispherectomy—The Johns Hopkins Experience: 1968 to 1996. Pediatrics. 1997;100:163–71. doi: 10.1542/peds.100.2.163. [DOI] [PubMed] [Google Scholar]
- 59.Lidzba K, Küpper H, Kluger G, Staudt M. The time window for successful right-hemispheric language reorganization in children. Eur J Paediatr Neurol EJPN Off J Eur Paediatr Neurol Soc. 2017;21:715–21. doi: 10.1016/j.ejpn.2017.06.001. [DOI] [PubMed] [Google Scholar]
- 60.Curtiss S, Bode S de. Age and Etiology as Predictors of Language Outcome following Hemispherectomy. Dev Neurosci. 1999;21:174–81. doi: 10.1159/000017396. [DOI] [PubMed] [Google Scholar]
- 61.Holmes GL, Lenck-Santini P-P. Role of interictal epileptiform abnormalities in cognitive impairment. Epilepsy Behav EB. 2006;8:504–15. doi: 10.1016/j.yebeh.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 62.Nicolai J, Ebus S, Biemans DP, Arends J, Hendriksen J, Vles JS, et al. The cognitive effects of interictal epileptiform EEG discharges and short nonconvulsive epileptic seizures. Epilepsia. 2012;53:1051–9. doi: 10.1111/j.1528-1167.2012.03491.x. [DOI] [PubMed] [Google Scholar]
- 63.Caraballo RH, Cejas N, Chamorro N, Kaltenmeier MC, Fortini S, Soprano AM. Landau-Kleffner syndrome: a study of 29 patients. Seizure. 2014;23:98–104. doi: 10.1016/j.seizure.2013.09.016. [DOI] [PubMed] [Google Scholar]
- 64.Soprano AM, Garcia EF, Caraballo R, Fejerman N. Acquired epileptic aphasia: neuropsychologic follow-up of 12 patients. Pediatr Neurol. 1994;11:230–5. doi: 10.1016/0887-8994(94)90108-2. [DOI] [PubMed] [Google Scholar]
- 65.Riva D, Vago C, Franceschetti S, Pantaleoni C, D’Arrigo S, Granata T, et al. Intellectual and language findings and their relationship to EEG characteristics in benign childhood epilepsy with centrotemporal spikes. Epilepsy Behav EB. 2007;10:278–85. doi: 10.1016/j.yebeh.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 66.Ebus SCM, Overvliet GM, Arends JBaM, Aldenkamp AP. Reading performance in children with rolandic epilepsy correlates with nocturnal epileptiform activity, but not with epileptiform activity while awake. Epilepsy Behav EB. 2011;22:518–22. doi: 10.1016/j.yebeh.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 67.Wolff M, Weiskopf N, Serra E, Preissl H, Birbaumer N, Kraegeloh-Mann I. Benign partial epilepsy in childhood: selective cognitive deficits are related to the location of focal spikes determined by combined EEG/MEG. Epilepsia. 2005;46:1661–7. doi: 10.1111/j.1528-1167.2005.00255.x. [DOI] [PubMed] [Google Scholar]
- 68.Miziara CS, de Manreza ML, Mansur L, Reed UC, Guilhoto LM, Serrano VA, et al. Impact of benign childhood epilepsy with centrotemporal spikes (BECTS) on school performance. Seizure. 2012;21:87–91. doi: 10.1016/j.seizure.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 69.Cerminara C, D’Agati E, Lange KW, Kaunzinger I, Tucha O, Parisi P, et al. Benign childhood epilepsy with centrotemporal spikes and the multicomponent model of attention: a matched control study. Epilepsy Behav EB. 2010;19:69–77. doi: 10.1016/j.yebeh.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 70.Ebus S, Arends J, Hendriksen J, van der Horst E, de la Parra N, Hendriksen R, et al. Cognitive effects of interictal epileptiform discharges in children. Eur J Paediatr Neurol. 2012;16:697–706. doi: 10.1016/j.ejpn.2012.05.010. [DOI] [PubMed] [Google Scholar]
- 71.Glennon JM, Weiss-Croft L, Harrison S, Cross JH, Boyd SG, Baldeweg T. Interictal epileptiform discharges have an independent association with cognitive impairment in children with lesional epilepsy. Epilepsia. 2016;57:1436–42. doi: 10.1111/epi.13479. [DOI] [PubMed] [Google Scholar]
- 72.Xiao F, An D, Lei D, Li L, Chen S, Wu X, et al. Real-time effects of centrotemporal spikes on cognition in rolandic epilepsy: An EEG-fMRI study. Neurology. 2016;86:544–51. doi: 10.1212/WNL.0000000000002358. [DOI] [PubMed] [Google Scholar]
- 73.Ibrahim GM, Cassel D, Morgan BR, Smith ML, Otsubo H, Ochi A, et al. Resilience of developing brain networks to interictal epileptiform discharges is associated with cognitive outcome. Brain. 2014;137:2690–702. doi: 10.1093/brain/awu214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Japaridze N, Muthuraman M, Dierck C, von Spiczak S, Boor R, Mideksa KG, et al. Neuronal networks in epileptic encephalopathies with CSWS. Epilepsia. 2016;57:1245–55. doi: 10.1111/epi.13428. [DOI] [PubMed] [Google Scholar]
- 75.Oser N, Hubacher M, Specht K, Datta AN, Weber P, Penner I-K. Default mode network alterations during language task performance in children with benign epilepsy with centrotemporal spikes (BECTS) Epilepsy Behav EB. 2014;33:12–7. doi: 10.1016/j.yebeh.2014.01.008. [DOI] [PubMed] [Google Scholar]
- 76.Turner SJ, Mayes AK, Verhoeven A, Mandelstam SA, Morgan AT, Scheffer IE. GRIN2A: an aptly named gene for speech dysfunction. Neurology. 2015;84:586–93. doi: 10.1212/WNL.0000000000001228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Turner SJ, Morgan AT, Perez ER, Scheffer IE. New genes for focal epilepsies with speech and language disorders. Curr Neurol Neurosci Rep. 2015;15:35. doi: 10.1007/s11910-015-0554-0. [DOI] [PubMed] [Google Scholar]
- 78.Lesca G, Rudolf G, Bruneau N, Lozovaya N, Labalme A, Boutry-Kryza N, et al. GRIN2A mutations in acquired epileptic aphasia and related childhood focal epilepsies and encephalopathies with speech and language dysfunction. Nat Genet. 2013;45:1061–6. doi: 10.1038/ng.2726. [DOI] [PubMed] [Google Scholar]
- 79.Carvill GL, Regan BM, Yendle SC, O’Roak BJ, Lozovaya N, Bruneau N, et al. GRIN2A mutations cause epilepsy-aphasia spectrum disorders. Nat Genet. 2013;45:1073–6. doi: 10.1038/ng.2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lemke JR, Lal D, Reinthaler EM, Steiner I, Nothnagel M, Alber M, et al. Mutations in GRIN2A cause idiopathic focal epilepsy with rolandic spikes. Nat Genet. 2013;45:1067–72. doi: 10.1038/ng.2728. [DOI] [PubMed] [Google Scholar]
- 81.Lal D, Pernhorst K, Klein KM, Reif P, Tozzi R, Toliat MR, et al. Extending the phenotypic spectrum of RBFOX1 deletions: Sporadic focal epilepsy. Epilepsia. 2015;56:e129–33. doi: 10.1111/epi.13076. [DOI] [PubMed] [Google Scholar]
- 82.Zhao WW. Intragenic deletion of RBFOX1 associated with neurodevelopmental/neuropsychiatric disorders and possibly other clinical presentations. Mol Cytogenet. 2013;6:26. doi: 10.1186/1755-8166-6-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kamien B, Lionel AC, Bain N, Scherer SW, Hunter M. Outfoxed by RBFOX1-a caution about ascertainment bias. Am J Med Genet A. 2014;164A:1411–8. doi: 10.1002/ajmg.a.36458. [DOI] [PubMed] [Google Scholar]
- 84.Lai CS, Fisher SE, Hurst JA, Vargha-Khadem F, Monaco AP. A forkhead-domain gene is mutated in a severe speech and language disorder. Nature. 2001;413:519–23. doi: 10.1038/35097076. [DOI] [PubMed] [Google Scholar]
- 85.Roll P, Rudolf G, Pereira S, Royer B, Scheffer IE, Massacrier A, et al. SRPX2 mutations in disorders of language cortex and cognition. Hum Mol Genet. 2006;15:1195–207. doi: 10.1093/hmg/ddl035. [DOI] [PubMed] [Google Scholar]
- 86.Pal DK. Epilepsy and neurodevelopmental disorders of language. Curr Opin Neurol. 2011;24:126–31. doi: 10.1097/WCO.0b013e328344634a. [DOI] [PubMed] [Google Scholar]
- 87.Dimassi S, Labalme A, Lesca G, Rudolf G, Bruneau N, Hirsch E, et al. A subset of genomic alterations detected in rolandic epilepsies contains candidate or known epilepsy genes including GRIN2A and PRRT2. Epilepsia. 2014;55:370–8. doi: 10.1111/epi.12502. [DOI] [PubMed] [Google Scholar]
- 88.Xiong W, Zhou D. Progress in unraveling the genetic etiology of rolandic epilepsy. Seizure. 2017;47:99–104. doi: 10.1016/j.seizure.2017.02.012. [DOI] [PubMed] [Google Scholar]
- 89.Reinthaler EM, Dejanovic B, Lal D, Semtner M, Merkler Y, Reinhold A, et al. Rare variants in γ-aminobutyric acid type A receptor genes in rolandic epilepsy and related syndromes. Ann Neurol. 2015;77:972–86. doi: 10.1002/ana.24395. [DOI] [PubMed] [Google Scholar]
- 90.Bali B, Kull LL, Strug LJ, Clarke T, Murphy PL, Akman CI, et al. Autosomal dominant inheritance of centrotemporal sharp waves in rolandic epilepsy families. Epilepsia. 2007;48:2266–72. doi: 10.1111/j.1528-1167.2007.01221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kugler SL, Bali B, Lieberman P, Strug L, Gagnon B, Murphy PL, et al. An autosomal dominant genetically heterogeneous variant of rolandic epilepsy and speech disorder. Epilepsia. 2008;49:1086–90. doi: 10.1111/j.1528-1167.2007.01517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pal DK, Li W, Clarke T, Lieberman P, Strug LJ. Pleiotropic effects of the 11p13 locus on developmental verbal dyspraxia and EEG centrotemporal sharp waves. Genes Brain Behav. 2010;9:1004–12. doi: 10.1111/j.1601-183X.2010.00648.x. [DOI] [PubMed] [Google Scholar]
- 93.Reinthaler EM, Lal D, Jurkowski W, Feucht M, Steinböck H, Gruber-Sedlmayr U, et al. Analysis of ELP4, SRPX2, and interacting genes in typical and atypical rolandic epilepsy. Epilepsia. 2014;55:e89–93. doi: 10.1111/epi.12712. [DOI] [PubMed] [Google Scholar]
- 94.Strug LJ, Addis L, Chiang T, Baskurt Z, Li W, Clarke T, et al. The genetics of reading disability in an often excluded sample: novel loci suggested for reading disability in Rolandic epilepsy. PLoS One. 2012;7:e40696. doi: 10.1371/journal.pone.0040696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kevelam SH, Jansen FE, Binsbergen E, Braun KP, Verbeek NE, Lindhout D, et al. Copy number variations in patients with electrical status epilepticus in sleep. J Child Neurol. 2012;27:178–82. doi: 10.1177/0883073811416006. [DOI] [PubMed] [Google Scholar]
- 96.Lesca G, Rudolf G, Labalme A, Hirsch E, Arzimanoglou A, Genton P, et al. Epileptic encephalopathies of the Landau-Kleffner and continuous spike and waves during slow-wave sleep types: genomic dissection makes the link with autism. Epilepsia. 2012;53:1526–38. doi: 10.1111/j.1528-1167.2012.03559.x. [DOI] [PubMed] [Google Scholar]
- 97.Mefford HC, Muhle H, Ostertag P, von Spiczak S, Buysse K, Baker C, et al. Genome-wide copy number variation in epilepsy: novel susceptibility loci in idiopathic generalized and focal epilepsies. PLoS Genet. 2010;6:e1000962. doi: 10.1371/journal.pgen.1000962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Aldenkamp A, Besag F, Gobbi G, Caplan R, Dunn DW, Sillanpää M. Psychiatric and Behavioural Disorders in Children with Epilepsy (ILAE Task Force Report): Adverse cognitive and behavioural effects of antiepileptic drugs in children. Epileptic Disord Int Epilepsy J Videotape. 2016;18:S55–67. doi: 10.1684/epd.2016.0809. [DOI] [PubMed] [Google Scholar]
- 99.Farwell JR, Lee YJ, Hirtz DG, Sulzbacher SI, Ellenberg JH, Nelson KB. Phenobarbital for febrile seizures--effects on intelligence and on seizure recurrence. N Engl J Med. 1990;322:364–9. doi: 10.1056/NEJM199002083220604. [DOI] [PubMed] [Google Scholar]
- 100.Sulzbacher S, Farwell JR, Temkin N, Lu AS, Hirtz DG. Late cognitive effects of early treatment with phenobarbital. Clin Pediatr. 1999;38:387–94. doi: 10.1177/000992289903800702. [DOI] [PubMed] [Google Scholar]
- 101.Ojemann LM, Ojemann GA, Dodrill CB, Crawford CA, Holmes MD, Dudley DL. Language Disturbances as Side Effects of Topiramate and Zonisamide Therapy. Epilepsy Behav. 2001;2:579–84. doi: 10.1006/ebeh.2001.0285. [DOI] [PubMed] [Google Scholar]
- 102.Mula M, Trimble MR, Thompson P, Sander JWAS. Topiramate and word-finding difficulties in patients with epilepsy. Neurology. 2003;60:1104–7. doi: 10.1212/01.wnl.0000056637.37509.c6. [DOI] [PubMed] [Google Scholar]
- 103.Donegan S, Dixon P, Hemming K, Tudur-Smith C, Marson A. A systematic review of placebo-controlled trials of topiramate: How useful is a multiple-indications review for evaluating the adverse events of an antiepileptic drug. Epilepsia. 2015;56:1910–20. doi: 10.1111/epi.13209. [DOI] [PubMed] [Google Scholar]
- 104.de Araujo Filho GM, Pascalicchio TF, Lin K, Sousa PS, Yacubian EMT. Neuropsychiatric profiles of patients with juvenile myoclonic epilepsy treated with valproate or topiramate. Epilepsy Behav. 2006;8:606–9. doi: 10.1016/j.yebeh.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 105.Eun S-H, Kim HD, Eun B-L, Lee IK, Chung HJ, Kim JS, et al. Comparative trial of low- and high-dose zonisamide as monotherapy for childhood epilepsy. Seizure. 2011;20:558–63. doi: 10.1016/j.seizure.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 106.Kim SJ, Kim MY, Choi YM, Song MK. Effects of topiramate on language functions in newly diagnosed pediatric epileptic patients. Pediatr Neurol. 2014;51:324–9. doi: 10.1016/j.pediatrneurol.2014.05.033. [DOI] [PubMed] [Google Scholar]
- 107.De Ciantis A, Muti M, Piccolini C, Principi M, Di Renzo A, De Ciantis R, et al. A functional MRI study of language disturbances in subjects with migraine headache during treatment with topiramate. Neurol Sci. 2008;29:S141–143. doi: 10.1007/s10072-008-0906-5. [DOI] [PubMed] [Google Scholar]
- 108.Tang Y, Xia W, Yu X, Zhou B, Wu X, Lui S, et al. Altered cerebral activity associated with topiramate and its withdrawal in patients with epilepsy with language impairment: An fMRI study using the verb generation task. Epilepsy Behav. 2016;59:98–104. doi: 10.1016/j.yebeh.2016.03.013. [DOI] [PubMed] [Google Scholar]
- 109.Wandschneider B, Burdett J, Townsend L, Hill A, Thompson PJ, Duncan JS, et al. Effect of topiramate and zonisamide on fMRI cognitive networks. Neurology. 2017;88:1165–71. doi: 10.1212/WNL.0000000000003736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Fuchs M, Wagner M. CURRY Neuroimaging Suite. 7. Charlotte, NC: Compumedics Neuroscan; 2012. [Google Scholar]
- 111.Pujol J, Deus J, Losilla JM, Capdevila A. Cerebral lateralization of language in normal left-handed people studied by functional MRI. Neurology. 1999;52:1038–1038. doi: 10.1212/wnl.52.5.1038. [DOI] [PubMed] [Google Scholar]
- 112.Stedman . Stedman’s Medical Dictionary Online [Internet] 28. Philadelphia (PA): Lippincott Williams & Wilkins; 2006. [cited 2017 Sep 1]. Available from: http://stedmansonline.com/public/Learnmore.aspx?resourceID=Medical. [Google Scholar]