Abstract
Background
Type 2 diabetes mellitus (T2DM) resolves in over 80% of patients after Roux-en-Y gastric bypass (RYGB). It has been hypothesized that foregut exclusion is mechanistically important to this observation. This study aims (1) to determine if GG fistula, with a loss of foregut exclusion, is associated with T2DM relapse, and (2) to assess if closure of GG fistula is associated with T2DM resolution.
Study Design
A matched cohort study of patients who experienced T2DM remission after RYGB. Cases (patients with GG fistula) were matched to controls (patients without GG fistula) based on age, BMI, weight regain and duration from RYGB. Primary outcome was T2DM relapse. Time-to-event analysis was performed to identify an association between GG fistula and time to T2DM resolution.
Results
126 patients (42 cases/84 controls) were included. Cases experienced a higher rate of T2DM relapse than controls (48%vs13%, OR=18, p<0.0001). On multivariable analysis, GG fistula remained a significant predictor of T2DM relapse after controlling for sex and insulin use (OR=6.3, p=0.02).
Of the 42 cases, 20 experienced T2DM relapse, with one spontaneous resolution. Out of 19, 13 underwent fistula revision and experienced a higher rate of T2DM resolution than the non-revision group (69%vs0%, OR=27, p=0.036). Time to T2DM resolution was shorter in the revision group compared to the non-revision group (p=0.006).
Conclusions
RYGB patients with GG fistula have a higher rate of T2DM relapse, compared to those without GG fistula with similar BMI and weight regain. Successful fistula revision is associated with resolution of T2DM.
Keywords: Foregut hypothesis, duodenal exclusion, glucose, diabetes, metabolic, fistula, RYGB, duodenojejunal bypass liner
INTRODUCTION
Obesity is a pandemic with rising prevalence worldwide. In 2014, more than 1.9 billion adults were overweight (body mass index (BMI) ≥25) with over 600 million adults being obese (body mass index (BMI) ≥30)1. Similarly, in the U.S., more than a third of the U.S. adults had obesity and more than two thirds were overweight2. Consequently, there has been a rapid increase in the prevalence of obesity-related comorbidities including type 2 diabetes mellitus (T2DM). In 2012, 29.1 million Americans, or 9.3% of the U.S. population, had T2DM resulting in a 245 billion U.S. dollar cost to the healthcare system3.
Roux-en-Y gastric bypass (RYGB) is an effective therapy for morbid obesity. In addition, approximately up to 84% of patients who undergo RYGB experience remission of T2DM, defined by the American Diabetes Association (ADA) as the ability to stop all diabetes-related medications and maintain blood glucose within the normal range4–7. A randomized controlled study, the STAMPEDE trial, evaluated the glycemic effect of intensive anti-diabetic medical therapy alone versus intensive medical therapy plus RYGB or sleeve gastrectomy. At 5 years, more patients in the RYGB group achieved the primary outcome (hemoglobin A1c (HbA1c) of 6% or less) than those in the control group (29% vs. 5%, respectively). Furthermore, the RYGB group had a greater reduction in HbA1c than those who received medical therapy alone (2.1% vs. 0.3%, respectively)8. More recently, a joint statement by International Diabetes Organizations has suggested that metabolic surgery be recommended to treat T2DM in patients with class III obesity (BMI ≥40) regardless of glycemic control and class II obesity (BMI 35-39.9) when hyperglycemia is inadequately controlled with lifestyle and optimal medical therapy. Surgery may also be considered for patients with T2DM and class I obesity (BMI 30-34.9) if hyperglycemia remains inadequately controlled despite optimal treatment with either oral or injectable medications9, 10.
While the effect of RYGB on T2DM is well known, the mechanisms behind this metabolic effect are less well understood. It has been observed that glycemic control improves acutely after RYGB, which suggests that the mechanisms may be independent of weight loss. In the foregut hypothesis, exclusion of the proximal small intestine from contact with ingested nutrients is thought to produce direct antidiabetic effects possibly by preventing secretion of unidentified duodenal factors that promote insulin resistance and T2DM. This concept has been supported by studies in rats that underwent duodenal-jejunal bypass (DJB; stomach-sparing bypass of the duodenum and proximal jejunum) who experienced marked improvement in glycemic control. When duodenal passage in DJB was restored, impaired glucose intolerance recurred11. The foregut hypothesis however remains less well proven in human subjects.
Gastrogastric (GG) fistula is a complication of RYGB where a connection between the gastric pouch and the excluded remnant stomach develops. GG fistulas are seen in 1-2% of RYGB patients with the current laparoscopic procedure, however, they were seen in up to 49% with previous open techniques12–15. Typical symptoms of GG fistula include weight regain (as food does not bypass the remnant stomach), abdominal pain, nausea, vomiting and marginal ulceration. Additionally, since chyme can be reintroduced into the duodenum and proximal jejunum, we hypothesize that patients with GG fistula may experience worsening glycemic control. This study aimed to assess the effect of foregut exclusion on T2DM using patients with GG fistula as a model.
METHODS
Study Design and Data Collection
The study was a retrospective review of a prospectively collected registry. This centralized clinical data registry from two tertiary academic centers was used to identify all patients with obesity and T2DM who underwent RYGB from 2006 to 2016. Only patients who experienced remission of T2DM after RYGB were included in the study. Exclusion criteria included history of other bariatric surgeries (such as sleeve gastrectomy or laparoscopic banding) or endoscopic procedures to treat weight regain (such as endoscopic suturing or argon plasma coagulation to reduce the gastrojejunal anastomotic size). Collected data included age, sex, BMI, amount of weight regain, surgical technique, presence or absence of GG fistula, GG fistula size, fistula revisional surgery, and T2DM status and diabetic medication use prior to RYGB and at time of follow-up.
Part I—Matched cohort study
This section assessed the rate of T2DM recurrence in patients who developed GG fistula compared to those without GG fistula. The case cohort consisted of patients with GG fistula after RYGB, as demonstrated on upper endoscopy or upper GI series (UGIS). The control cohort consisted of those who did not develop GG fistula as confirmed on upper endoscopy or UGIS. Cases were matched 1:2 to controls based on age, body mass index (BMI) at time of follow-up, an amount of weight regain from nadir weight at time of follow-up and time from RYGB. The primary outcome was rate of T2DM recurrence.
Part II—Time-to-event analysis
This part of the study assessed the rate and time to T2DM remission in patients with GG fistula who experienced T2DM relapse. The patient cohort was divided into two groups—those who underwent successful revision of GG fistula and those who did not undergo fistula revision or failed revision. Likelihood of and time to T2DM remission was compared between the two groups. Successful revision of GG fistula was done surgically with confirmed absence of fistula on follow-up upper endoscopy or UGIS at at least six months after the revision.
Definitions
In this study, T2DM was defined as hemoglobin A1c (HbA1c) of greater than or equal to 6.5% or being on at least one anti-diabetic medications. T2DM remission was defined according to the ADA consensus and included both partial and complete remissions—HbA1c of less than 6.5% without being on any anti-diabetic medications7. Recurrence of T2DM was defined as a rise in HbA1c above 6.5% or reintroduction of at least one T2DM medications. Weight regain was calculated using the following formula: (follow-up weight – nadir weight)/(pre-RYGB weight – nadir weight) × 100. Large GG fistula was defined as a fistula diameter of at least 10 mm.
Statistical Analysis
All continuous variables are expressed as mean ± SD, and skewed variables are expressed as median and interquartile range. Categorical variables are expressed as proportions (%). Student’s t-test was used to compare continuous measures, and Pearson’s chi-squared test and McNemar’s test was used for comparison of unmatched and matched categorical variables, respectively. Univariable logistic regression was performed to assess an association between potential risk factors of T2DM recurrence (sex, presence of GG fistula, history of insulin use prior to RYGB, preoperative HbA1c and preoperative T2DM duration) and the return of T2DM. Given collinearity between preoperative HbA1c and insulin use and preoperative T2DM duration and insulin use, only the use of insulin was included in the multivariable logistic regression in addition to sex and presence of GG fistula. Age, BMI, amount of weight regain and time from RYGB were matched characteristics, and therefore were not included in the logistic regression model. A time-to-event analysis with Kaplan Meier analysis was used to evaluate the association between successful fistula revision and remission of T2DM. All tests were performed two-sided and a significance level of P ≤ 0.05 was considered statistical significance. All statistical analyses were performed using SAS Software Version 9.4 (Cary, NC).
Power analysis for two paired binomial proportions was conducted using SAS Power and Sample Size application to determine a sufficient sample size for T2DM relapse rate of 20% in the controls and 60% in the cases, with alpha of 0.05 and power of 0.80. We used an estimate of 20% on the basis of a large observational long-term follow-up study indicating 19% incidence of T2DM relapse after initial remission in patients who underwent RYGB6. Although no prior study provided information about an expected increase in T2DM relapse rate in the presence GG fistula, we chose an odds ratio of 3 based on a previous pilot study demonstrating an odds ratio of 3.3 for remission of T2DM in patients without GG fistula compared to those who developed GG fistula16. Based on the aforementioned assumptions, the desired sample size is 37 for the cases and 74 for the controls given 1:2 matching.
RESULTS
Part I: Effect of Gastrogastric Fistula on Recurrence of Type II Diabetes Mellitus
The case cohort consisted of 42 RYGB patients who developed GG fistula. The control cohort consisted of 84 matched RYGB patients without GG fistula (Figure 1). Baseline characteristics of cases and controls are shown in Table 1. All patients had T2DM prior to RYGB and had similar baseline glycemic status. Seventeen percent of both the case and control cohorts were on insulin prior to the gastric bypass. At time of follow-up at approximately 4.3 years, the two arms had similar BMI (35.0 kg/m2 and 35.3 kg/m2 for cases and controls, respectively) and had regained similar amount of weight from their nadir weight (25.6% and 24.5% for cases and controls, respectively) (Table 1).
Figure 1.
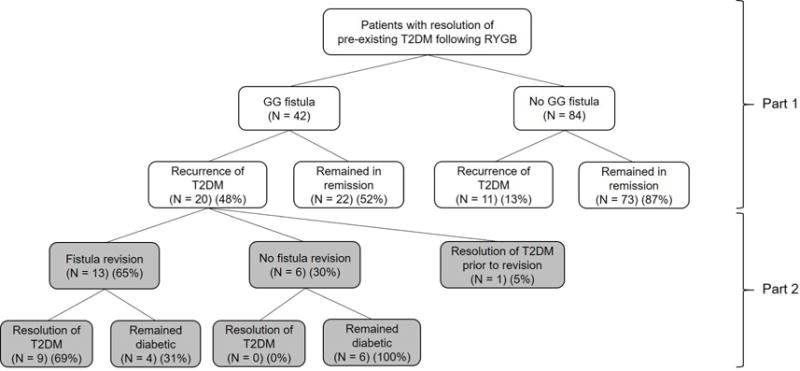
Flow diagram with summary of results. GG, gastrogastric; RYGB, Roux-en-Y gastric bypass; T2DM, type 2 diabetes mellitus.
Table 1.
Baseline Characteristics and Diabetic Medication Usage prior to Roux-en-Y Gastric Bypass in Cases and Controls
| Parameters | Cases (n = 42) | Controls (n = 84) | p Value |
|---|---|---|---|
| Unmatched parameters | |||
| Sex, female, n (%) | 31 (74) | 71 (85) | 0.15 |
| Hemoglobin A1c prior to RYGB, %, mean ± SD | 7.3±1.8 | 7.0±1.3 | 0.54 |
| Duration of DM prior to RYGB, y, mean ± SD | 5.1±5.2 | 5.5±4.4 | 0.79 |
| Diabetic medication usage prior to RYGB, n (%) | |||
| Oral hypoglycemic agents | 30 (71) | 57 (68) | 0.68 |
| Insulin | 7 (17) | 14 (17) | 1.00 |
| Unknown | 11 (26) | 19 (23) | 0.66 |
| Open RYGB (%) | 72.5 | 26.6 | <0.01 |
| Matched parameters | |||
| Age, y, mean ± SD | 51.8±8.3 | 48.7±9.0 | 0.06 |
| BMI at follow-up, kg/m2, mean ± SD | 35.0±8.1 | 35.3±6.9 | 0.84 |
| Amount of weight regain at follow-up, %, | 25.6±22.0 | 24.5±21.1 | 0.79 |
| Duration from RYGB at follow-up, y, mean ± SD | 4.3±3.1 | 4.3±3.0 | 0.90 |
DM, diabetes mellitus; RYGB, Roux-en-Y gastric bypass.
Out of 42 case patients, 20 (48%) experienced recurrence of their T2DM after initial remission. Baseline characteristics of those who experienced T2DM recurrence and those who did not were similar except that there were more patients with large GGF in the recurrent group (78.6% vs 30.8%, p=0.01) (eTable 1). In comparison, 11 of 84 (13%) of control patients experienced T2DM recurrence. Compared to control patients who had similar BMI and weight regain, case patients had a significantly higher rate of recurrence of T2DM (OR=18.0, p<0.0001).
Univariable logistic regression analysis showed that presence of GG fistula and the use of insulin prior to RYGB were associated with recurrence of T2DM (p<0.0001 and p=0.01, respectively), while sex, preoperative HbA1c and preoperative duration of T2DM were not (p>0.05 for all). Multivariable regression analysis showed that the presence of GG fistula remained a significant predictor of T2DM recurrence after controlling for sex and history of insulin use prior to RYGB (OR=6.3 [1.30, 30.01], β=1.83, p=0.02). Additionally, insulin use prior to RYGB was associated with recurrence of T2DM after initial remission (OR=7.5 [1.35, 41.74], β=2.0, p=0.02) when controlled for sex and presence of GG fistula.
Part II: Effect of Successful Revision of Gastrogastric Fistula on Remission of Type II Diabetes Mellitus
Of the 20 case patients with T2DM recurrence, one experienced spontaneous resolution of diabetes prior to fistula revision. Clinical characteristics of the remainder 19 patients with persistent recurrent T2DM are shown in Table 2. Out of the 19 patients, 13 underwent successful revision of GG fistula. Six did not undergo revision or failed attempted revision as demonstrated on follow-up endoscopy or UGIS.
Table 2.
Baseline Characteristics of Roux-en-Y Gastric Bypass Patients with Gastrogastric Fistula who Experienced Recurrence of Type 2 Diabetes Mellitus
| Successful fistula revision (n = 13) | Unsuccessful or no fistula revision (n = 6) | p Value | |
|---|---|---|---|
| Age, y, mean ± SD | 54±7 | 61±7 | 0.06 |
| Sex, female, n (%) | 10 (77) | 4 (67) | 0.64 |
| At time of fistula diagnosis | |||
| BMI, kg/m2, mean ± SD | 39.9±9.0 | 34.4±8.3 | 0.23 |
| Amount of weight regain from nadir weight, %, mean ± SD | 35.6±35.3 | 24.2±24.0 | 0.49 |
| Insulin use, n (%) | 1 (7) | 1 (17) | 0.50 |
| At time of T2DM follow-up | |||
| BMI, kg/m2, mean ± SD | 38.5±7.8 | 33.7±7.5 | 0.24 |
| %TBWL from fistula weight, %, mean ± SD | 4.4±13.7 | 4.4±14.0 | 1.00 |
Nine of 13 patients (69%) experienced remission of T2DM after fistula revision. Three of 13 patients (23%) remained diabetic at time of last follow-up, which was at 32.0 ± 16.1 months after fistula revision. One patient was lost to follow-up after fistula revision. Median time from fistula revision to remission of T2DM was 3.6 months [range 0 to 47.3]. In comparison, all six patients with persistent fistula were diabetic at time of last follow-up (44.9 ± 27.9 months from time of fistula diagnosis) (p<0.0001). On time-to-event analysis, time to T2DM remission was significantly shorter in the fistula revision group compared to the non-revision group (p=0.006) (Figure 2).
Figure 2.
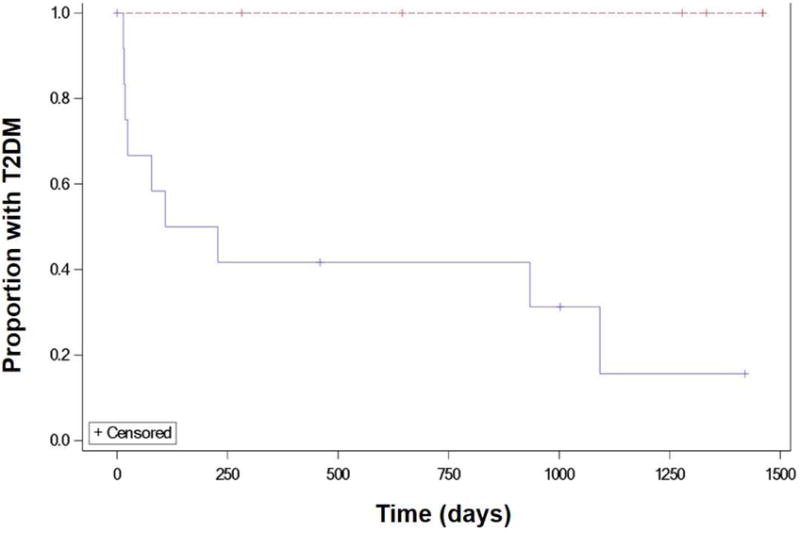
Kaplan-Meier curve showing time to type 2 diabetes mellitus resolution in patients with gastrogastric fistula who underwent fistula surgical revision vs those who did not undergo revision.
DISCUSSION
Our study shows that there is an association between foregut exclusion and improvement in glycemic control in patients with RYGB. Specifically, RYGB patients who develop GG fistula, where the proximal small intestine is no longer excluded, experience a higher rate of T2DM relapse compared to those where foregut exclusion remains preserved. This is independent of BMI and the amount of weight regain. Additionally, restoration of foregut exclusion via surgical revision results in rapid resolution of T2DM compared to patients with patent fistula.
As far as we know, this is the first long-term human study that demonstrates the specific impact of foregut exclusion on glucose homeostasis by analyzing 1) recurrence of T2DM following breakdown of foregut exclusion and 2) remission of T2DM following restoration of foregut exclusion. Also known as the foregut hypothesis, the exclusion of the proximal small bowel from ingested nutrients has been thought to play an important role in improvement of glycemic control after RYGB. In 2006, Rubino et al. performed a stomach-sparing duodenal jejunal bypass (DJB) on rats, where the duodenum and 10 cm of the proximal jejunum were bypassed through creation of a gastrojejunal anastomosis. This was compared to a sham operation and gastrojejunostomy alone without small bowel bypass to allow rapid passage of some nutrients into the distal small bowel (to evaluate the hindgut hypothesis). At follow-up, DJB rats had significantly better oral glucose tolerance compared to sham-operated and GJ rats despite similar weight loss. Subsequently, restoration of duodenal passage was performed while the GJ was left intact in DJB rats. This reversal resulted in a return of impaired glucose tolerance11.
It has been proposed the gut hormones may play an important role in the foregut hypothesis. Specifically, O’Brien et al demonstrated that RYGB patients with GG fistula had a significant lower level of incretin hormones glucagon-like peptide-1 (GLP-1) and peptide YY (PYY) compared to those without GG fistula14. Four months after fistula repair, PYY significantly increased to the same level as those RYGB patients without GG fistula. While GLP-1 similarly increased after fistula repair, the change was not statistically significant. It is important to note that in this study total GLP-1, and not active GLP-1, levels were measured. This study suggests that presence of GG fistula, where the foregut is no longer excluded, may have reversed the gut hormonal effect of RYGB, i.e. GLP-1 and PYY are no longer elevated. Once the fistula is repaired, the hormonal effect of RYGB is restored.
In addition to foregut exclusion, bile rerouting has also been hypothesized to be another potential mechanism of how RYGB cures T2DM. Specifically, previous studies showed that rats who underwent a bile diversion surgery from the bile duct to the mid-jejunum or the mid-ileum experienced an increase in plasma bile acid and a marked improvement in glucose control17–20. This effect was blunted by a bile acid sequestrant. Although the pattern of bile rerouting persists in patients with GG fistula, bile is mixed with chyme earlier, which potentially impacts these bile-related mechanisms.
In humans, data supporting the foregut hypothesis remain less well-established. A few small, short-term human studies demonstrated that performing a mixed meal test orally in patients with RYGB anatomy (thereby excluding the proximal small bowel from glucose passage) resulted in higher incretin and insulin response compared with glucose loading by way of the gastrostomy tube into the remnant stomach (thereby enabling administration of nutrients to the bypassed foregut)21–23. Evidence supporting the foregut hypothesis may also be derived from studies involving an endoscopic device that excludes the proximal small bowel. A duodenaljejunal bypass liner (DJBL) is a fluoropolymer sleeve that is endoscopically implanted in the duodenum and proximal jejunum, causing ingested nutrients to pass from the stomach into the sleeve and directly into the jejunum without contacting the duodenum. A U.S. pivotal study showed that more DJBL patients were able to achieve HbA1c level of 7% or less compared to sham patients (34.8% vs. 9.8%, respectively)24. A recent meta-analysis of 14 studies with 412 patients demonstrated that HbA1c decreased by 1.3% at time of DJBL explant, which was 0.9% greater than that of patients in the control arm25. However, patients in the DJBL arm also lost more weight making it more challenging to separate the effect of weight loss and duodenal exclusion alone on glycemic status. Additionally, glucose homeostasis after the removal of DJBL remains unclear.
Our findings suggest not only an association between the presence or absence of GG fistula (and therefore duodenal exclusion) and the change in glycemic status, but also a possible causal relationship. Specifically, the study establishes six of Branford Hill’s criteria for causation including 1) strong association (odds ratio of T2DM relapse of 6.3 in the fistula to non-fistula groups after controlling for age, sex, BMI, weight regain, duration from RYGB and insulin use prior to RYGB) 2) consistency (similar findings to endoscopic DJBL placement) 3) temporality or reversibility (revision of fistula led to resolution of T2DM, p<0.0001) 4) plausibility (the presence of GG fistula caused ingested nutrients to be reintroduced into the duodenum and proximal jejunum which may cause secretion of unidentified duodenal factors that promote insulin resistance and T2DM) 5) coherence (the findings make sense with all the current knowledge) and 6) experiment (data from experimental rats in Rubino’s study)26.
In addition to the breakdown of duodenal exclusion via GG fistula, our study also showed that the use of insulin prior to RYGB was associated with a return of T2DM. This finding was consistent with those from previous studies where risk factors for relapse were identified as older age, weight regain, and insulin use prior to RYGB6, 27, 28. In our study, age and weight regain were matched parameters and therefore were not analyzed in the regression model. Additionally, as shown in eTable 1, there appeared to be an association between large-sized GG fistula (≥10 mm) and T2DM recurrence. Further study is warranted to confirm this finding as this may suggest that a large GG fistula should prompt a repair regardless of whether or not the patient is symptomatic.
While this study provides evidence to support the foregut hypothesis, the mechanisms of glycemic control after RYGB are most likely multifactorial. In the hindgut hypothesis, improvement in diabetes is thought to be due to expedited delivery of nutrients to the distal small bowel, which may stimulate L cells in the terminal ileum and proximal colon to secrete more GLP-1 and PYY and other hormones. These hormones then lead to enhanced insulin secretion by pancreatic β cells and decreased insulin resistance29, 30. More recently, it has been hypothesized that gut microbiota may also play an important role in the metabolic changes seen after RYGB. Specifically, it has been observed that the ratio of Firmicutes to Bacteroidaceae bacteria decreases after RYGB, which leads to increased conversion of primary bile acids to secondary bile acids via their 7α-hydroxylase enzyme31, 32. More secondary bile acids then lead to an increase in GLP-1 and GIP levels, which then lead to an improvement in insulin sensitivity.
The limitation of our study includes its retrospective nature. However, the data was analyzed from prospectively collected registry and our sample size was large enough to detect clinically relevant odds ratio of 3 in rates of T2DM relapse. In addition, we were able to collect data on most known potential confounders through extensive medical chart review. Nevertheless, statistical analysis may not be able to fully adjust for differences in study cohorts, and residual bias between cases and controls may account for a portion of our findings. Additionally, we were unable to perform Cox multivariate regression analysis in the time-to-event analysis section to further adjust for potential differences between the revision and non-revision arms. This was due to the fact that none of the patients in the non-revision arm experienced resolution of T2DM. However, as demonstrated in Table 2, baseline characteristics of the two groups were evenly distributed. In fact, there was a trend favoring resolution of T2DM in the non-revision arm as suggested by lower BMI and weight regain in this group at time of fistula diagnosis and follow-up. Lastly, in this study, risk factors for GG fistula development, such as an open surgical technique or postoperative leak, may have an effect on T2DM recurrence, but were not included in the regression model. Since these factors were on the same causal pathway towards the outcome of interest, i.e. T2DM recurrence, as GG fistula, they were not potential confounders and therefore were not included in the regression model.
In conclusion, this study clarifies the potential importance of foregut exclusion on glycemic control. In T2DM patients who respond to RYGB, loss of foregut exclusion is associated with recurrence of T2DM. Following successful fistula revision and restoration of foregut exclusion, T2DM appears to resolve. This observation remains after controlling for BMI and amount of weight regain. Future studies to better understand the mechanisms of foregut exclusion at a molecular level are needed. Additionally, this study provides rationale for minimally invasive endoscopic therapies that focus on foregut exclusion.
Supplementary Material
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Information: Nothing to disclose.
Presented at Digestive Diseases Week 2017, Chicago, IL, May 2017.
References
- 1.WHO. Obesity and overweight. WHO; http://www.who.int/mediacentre/factsheets/fs311/en/. Accessed February 17, 2017. [Google Scholar]
- 2.Ogden CL, Carroll MD, Kit BK, et al. Prevalence of childhood and adult obesity in the United States, 2011-2012. JAMA. 2014;311:806–814. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Statistics About Diabetes. American Diabetes Association; Alex ADA 1701 NBS, ria, 1-800-Diabetes V 22311. http://www.diabetes.org/diabetes-basics/statistics/. Accessed August 19, 2016. [Google Scholar]
- 4.Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292:1724–1737. doi: 10.1001/jama.292.14.1724. [DOI] [PubMed] [Google Scholar]
- 5.Pournaras DJ, Aasheim ET, Søvik TT, et al. Effect of the definition of type II diabetes remission in the evaluation of bariatric surgery for metabolic disorders. Br J Surg. 2012;99:100–103. doi: 10.1002/bjs.7704. [DOI] [PubMed] [Google Scholar]
- 6.Brethauer SA, Aminian A, Romero-Talamás H, et al. Can diabetes be surgically cured? Long-term metabolic effects of bariatric surgery in obese patients with type 2 diabetes mellitus. Ann Surg. 2013;258:628–636. doi: 10.1097/SLA.0b013e3182a5034b. discussion 636-637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buse JB, Caprio S, Cefalu WT, et al. How do we define cure of diabetes? Diabetes Care. 2009;32:2133–2135. doi: 10.2337/dc09-9036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schauer PR, Bhatt DL, Kirwan JP, et al. Bariatric surgery versus intensive medical therapy for diabetes - 5-year outcomes. N Engl J Med. 2017;376:641–651. doi: 10.1056/NEJMoa1600869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brito JP, Montori VM, Davis AM. Metabolic surgery in the treatment algorithm for type 2 diabetes: a joint statement by International Diabetes Organizations. JAMA. 2017;317:635–636. doi: 10.1001/jama.2016.20563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rubino F, Schauer PR, Kaplan LM, et al. Metabolic surgery to treat type 2 diabetes: clinical outcomes and mechanisms of action. Annu Rev Med. 2010;61:393–411. doi: 10.1146/annurev.med.051308.105148. [DOI] [PubMed] [Google Scholar]
- 11.Rubino F, Forgione A, Cummings DE, et al. The mechanism of diabetes control after gastrointestinal bypass surgery reveals a role of the proximal small intestine in the pathophysiology of type 2 diabetes. Ann Surg. 2006;244:741–749. doi: 10.1097/01.sla.0000224726.61448.1b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cucchi SG, Pories WJ, MacDonald KG, et al. Gastrogastric fistulas. A complication of divided gastric bypass surgery. Ann Surg. 1995;221:387–391. doi: 10.1097/00000658-199504000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carrodeguas L, Szomstein S, Soto F, et al. Management of gastrogastric fistulas after divided Roux-en-Y gastric bypass surgery for morbid obesity: analysis of 1,292 consecutive patients and review of literature. Surg Obes Relat Dis Off J Am Soc Bariatr Surg. 2005;1:467–474. doi: 10.1016/j.soard.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 14.O’Brien CS, Wang G, McGinty J, et al. Effects of gastrogastric fistula repair on weight loss and gut hormone levels. Obes Surg. 2013;23:1294–1301. doi: 10.1007/s11695-013-0917-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Capella JF, Capella RF. Gastro-gastric fistulas and marginal ulcers in gastric bypass procedures for weight reduction. Obes Surg. 1999;9:22–27. doi: 10.1381/096089299765553674. discussion 28. [DOI] [PubMed] [Google Scholar]
- 16.Flicker MS, Dayyeh BKA, Thompson CC. Gastro-gastric fistula formation following roux-en-y bypass surgery leads to persistence and recurrence of diabetes mellitus. Gastroenterol. 2011;140:S304. [Google Scholar]
- 17.Goncalves D, Barataud A, De Vadder F, et al. Bile routing modification reproduces key features of gastric bypass in rat. Ann Surg. 2015;262:1006–1015. doi: 10.1097/SLA.0000000000001121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Becker JH, Pournaras DJ, Ghatei MA, et al. The role of bile after Roux-en-Y gastric bypass in promoting weight loss and improving glycaemic control. Eur Surg Res. 2013;50:69. doi: 10.1210/en.2011-2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pournaras DJ, Glicksman C, Vincent RP, et al. The role of bile after Roux-en-Y gastric bypass in promoting weight loss and improving glycaemic control. Endocrinology. 2012;153:3613–3619. doi: 10.1210/en.2011-2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kohli R, Setchell KDR, Kirby M, et al. A surgical model in male obese rats uncovers protective effects of bile acids post-bariatric surgery. Endocrinology. 2013;154:2341–2351. doi: 10.1210/en.2012-2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pournaras DJ, Aasheim ET, Bueter M, et al. Effect of bypassing the proximal gut on gut hormones involved with glycemic control and weight loss. Surg Obes Relat Dis. 2012;8:371–374. doi: 10.1016/j.soard.2012.01.021. [DOI] [PubMed] [Google Scholar]
- 22.Lindqvist A, Spegel P, Ekelund M, et al. Effects of ingestion routes on hormonal and metabolic profiles in gastric-bypassed humans. J Clin Endocrinol Metab. 2013;98:E856–E861. doi: 10.1210/jc.2012-3996. [DOI] [PubMed] [Google Scholar]
- 23.McLaughlin T, Peck M, Holst J, et al. Reversible Hyperinsulinemic hypoglycemia after gastric bypass: a consequence of altered nutrient delivery. J Clin Endocrinol Metab. 2010;95:1851–1855. doi: 10.1210/jc.2009-1628. [DOI] [PubMed] [Google Scholar]
- 24.Kaplan LM, Buse JB, Mullin C, et al. EndoBarrier therapy is associated with glycemic improvement, weight loss and safety issues in patients with obesity and type 2 diabetes on oral antihyperglycemic agents. American Diabetes Association 76th Scientific Sessions; New Orleans, LA. June 12, 2016. [Google Scholar]
- 25.Rohde U, Hedbäck N, Gluud LL, et al. Effect of the EndoBarrier Gastrointestinal Liner on obesity and type 2 diabetes: a systematic review and meta-analysis. Diabetes Obes Metab. 2016;18(3):300–305. doi: 10.1111/dom.12603. [DOI] [PubMed] [Google Scholar]
- 26.Hill AB. The environment and disease: association or causation? Proc R Soc Med. 1965;58:295–300. [PMC free article] [PubMed] [Google Scholar]
- 27.Ganguly S, Tan HC, Lee PC, et al. Metabolic bariatric surgery and type 2 diabetes mellitus: an endocrinologist’s perspective. J Biomed Res. 2015;29:105–111. doi: 10.7555/JBR.29.20140127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boza C, Gamboa C, Salinas J, et al. Laparoscopic Roux-en-Y gastric bypass versus laparoscopic sleeve gastrectomy: a case-control study and 3 years of follow-up. Surg Obes Relat Dis Off J Am Soc Bariatr Surg. 2012;8:243–249. doi: 10.1016/j.soard.2011.08.023. [DOI] [PubMed] [Google Scholar]
- 29.Mason EE. The mechanisms of surgical treatment of type 2 diabetes. Obes Surg. 2005;15:459–461. doi: 10.1381/0960892053723330. [DOI] [PubMed] [Google Scholar]
- 30.Meek CL, Lewis HB, Reimann F, et al. The effect of bariatric surgery on gastrointestinal and pancreatic peptide hormones. Peptides. 2016;77:28–37. doi: 10.1016/j.peptides.2015.08.013. [DOI] [PubMed] [Google Scholar]
- 31.Aron-Wisnewsky J, Doré J, Clement K. The importance of the gut microbiota after bariatric surgery. Nat Rev Gastroenterol Hepatol. 2012;9:590–598. doi: 10.1038/nrgastro.2012.161. [DOI] [PubMed] [Google Scholar]
- 32.Li JV, Ashrafian H, Bueter M, et al. Metabolic surgery profoundly influences gut microbial-host metabolic cross-talk. Gut. 2011;60:1214–1223. doi: 10.1136/gut.2010.234708. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


