Abstract
Objectives
The mechanistic target of rapamycin (mTOR) has become a therapeutic target in systemic lupus erythematosus (SLE). mTOR regulates normal T-cell lineage specification, including development of regulatory cells (Tregs). Therefore, we investigated whether mTOR is activated within Tregs and causes their depletion and dysfunction in patients with SLE.
Methods
Activities of mTOR complexes 1 (mTORC1) and 2 (mTORC2) were examined by phosphorylation of 4E-BP1, S6K, and Akt, respectively, in SLE patients relative to healthy control females age-matched for each experiment. Polarization of Tregs from naïve CD4+ T cells was assessed in the presence of IL-6, IL-17, and IL-21. Suppressor function of sorted CD4+CD25+ Tregs was measured by their impact on proliferation of autologous CD4+CD25− responder T cells. Expression of FOXP3, GATA-3, and CTLA-4 was monitored by flow cytometry. Autophagy was assessed by LC3 lipidation using immunoblotting. Impact of mTOR blockade was evaluated by rapamycin treatment.
Results
SLE Tregs exhibited increased mTORC1 and mTORC2 activities but diminished autophagy, GATA-3 and CTLA-4 expression, and suppressor function. IL-21, but not IL-6 or IL-17, blocked Treg development. IL-21 stimulated mTORC1 and mTORC2, and it abrogated autophagy, differentiation, and function of Tregs. Moreover, IL-21 constrained GATA-3 and CTLA-4 expression selectively in Tregs. In turn, mTORC1 blockade by three-day rapamycin treatment enhanced TGF-β production, while dual blockade of mTORC1 and mTORC2 by four-week rapamycin treatment induced autophagy, restored GATA-3 and CTLA-4 expression and corrected Treg function.
Conclusions
IL-21-driven mTOR activation is a pharmacologically targetable checkpoint of deficient autophagy that underlies Treg dysfunction in SLE.
INTRODUCTION
Systemic lupus erythematosus (SLE) is an autoimmune disease of unknown etiology which has been characterized by dysregulated T- and B-cell activation and antinuclear antibody production (1). SLE primarily affects females of childbearing age with mortality still approaching 10% over 5 years (2). There is an unmet medical need, as current treatments are only partially effective and have significant side effects. However, the development of new and efficacious treatments requires a better understanding of pathogenesis. In this regard, a growing body of evidence points to the depletion of Tregs as an important mediator of disease (3–6). Although Treg suppressive function is diminished in patients with active SLE (6, 7), the underlying mechanisms remain unclear.
mTOR is an evolutionally conserved serine-threonine kinase, which translates a variety of environmental cues to signals that dictate cell growth, proliferation, and differentiation (8), and it has emerged as a central regulator of T-cell lineage specification (9). In particular, constitutively active mTOR and Akt abrogate Treg differentiation (10). Genetic ablations of rheb and rictor in mice indicate that dual blockade of mTORC1 and mTORC2 are required to allow Treg differentiation (9). mTOR activation also plays a central role in T-cell dysfunction in SLE (11) that is based on independent lines of evidence: 1) mTOR activity is increased in T cells of patients (12) and mice with SLE (13); 2) mTOR controls T cell specification during development (9, 14) and its skewing in SLE (15, 16); 3) administration of rapamycin improves the clinical outcome in mice (13, 17) and patients with SLE (15, 18) ; 4) rapamycin blocks the production of antiphospholipid antibodies in lupus-prone mice (19) and enhances renal allograft survival in patients with antiphospholipid antibodies (20), which represent a diagnostic criterion and a source of co-morbidity in SLE. However, several important questions remain to be answered: i) is mTOR itself activated within Tregs; ii) which pro-inflammatory cues stimulate mTOR within Tregs; and iii) how mTOR controls Treg dysfunction in patients with SLE?
Among pro-inflammatory cytokines, IL-6 (21), IL-17 (22, 23), and IL-21 have attracted growing interest in lupus pathogenesis (24). These cytokines shift T-cell fate away from Treg to inflammatory T-cell differentiation in a STAT3-dependent manner (25, 26). However, it is not known whether they directly restrict human Treg development via analogous molecular mechanisms in the setting of SLE.
Besides the upstream regulators, much remains to be clarified about the signal transduction machineries that control cellular function downstream of mTOR. In this regard, mTOR has been recognized as a suppressor of autophagy that appears to regulate Treg lineage stability and function (27). However, autophagy is enhanced in lupus T cells (28, 29) and B cells (30). Paradoxically, further enhancement of autophagy via mTOR blockade has therapeutic efficacy both in mice (13, 17) and patients with SLE (15, 18).
Tregs exploit multiple distinct mechanisms to suppress effector cell function. They constitutively express Foxp3 which directly controls the expression of CTLA-4 (31). GATA-3 is a transcription factor, which mediates the expression of FoxP3 (32), and it is also essential for Treg development and function (33). Moreover, TGF-β production is indispensable for peripheral Treg differentiation (34), and it is sensitive to suppression of autophagy in other cell types (35)(1;2)(1;2). However, the relationship of TGF-β production to mTOR activation and autophagy in Tregs in general or in SLE is presently unknown.
Therefore, we examined the state of mTOR activation and its role in dysfunction of Tregs, given the clinical efficacy of mTOR blockade in SLE patients (15, 18, 36). In the present study, we show that SLE Tregs exhibit increased mTORC1 and mTORC2 activities and diminished autophagy and suppressor function which can be effectively restored by treatment with rapamycin.
MATERIALS AND METHODS
Human subjects
39 SLE patients fulfilling the American College of Rheumatology diagnostic criteria were studied (37). In each experiment, peripheral blood was obtained from SLE patients and healthy control (HC) subjects that have been matched at each blood donation for age within 10 years, ethnic background, and gender and processed in parallel. All patients were female. Age of 39 SLE patients was 42.4 ± 1.7 years, while those of 37 HC subjects was 40.6 ± 2.1 years. The demographics and clinical characteristics of study subjects are summarized in Table S1.
Surface and intracellular staining and culture of CD3+ T cells
Immunophenotyping methods are described in the Supplementary Methods Section.
Treg polarization
Naïve CD4+ T cells were isolated from SLE and matched healthy control subjects and cultured for 24 or 72 hours in the presence of plate-bound anti-CD3, soluble anti-CD28, TGF-β, and IL-2 with or without IL-6, IL-17, IL-21, or rapamycin. Frequency of CD4+CD25+FOXP3+ cells and GATA-3 expression in CD4+CD25+FOXP3+ cells were determined by flow cytometry. Additional experimental details have been provided in the Supplementary Methods Section.
Treg suppression assay
CD4+CD25− responder T cells (Tresp cells) and autologous CD4+CD25+ Tregs were magnetically isolated from the peripheral blood of HC and SLE donors. Tresp cells were stained with CFSE and stimulated for 5 days with plate-bound anti-CD3 and irradiated autologous peripheral blood mononuclear cells (PBMC) as antigen-presenting cells (APC) in the presence or absence of equal number of CD4+CD25+ Treg cells. Treg suppressive function was determined by calculating % suppression of division index. Additional experimental details have been provided in the Supplementary Methods Section.
Treg expansion
CD4+CD25+ Tregs from SLE patients were cultured for 4 weeks in the presence of plate-bound anti-CD3, soluble anti-CD28, and IL-2 with or without rapamycin. After 4 weeks of expansion, Tregs were used for suppression assay as described above.
Immunoblotting
Immunoblotting methods and reagents are described in the Supplementary Methods Section.
LUMINEX assay
Measurement of cytokines by the LUMINEX assay is described in the Supplementary Methods Section.
Statistical analysis
Data represent mean ± SEM, unless stated otherwise. Statistical analyses were performed with the Student’s t-test using GraphPad software (San Diego, CA). Differences were considered significant at two-tailed p values < 0.05.
RESULTS
IL-21 inhibits human Treg differentiation and function
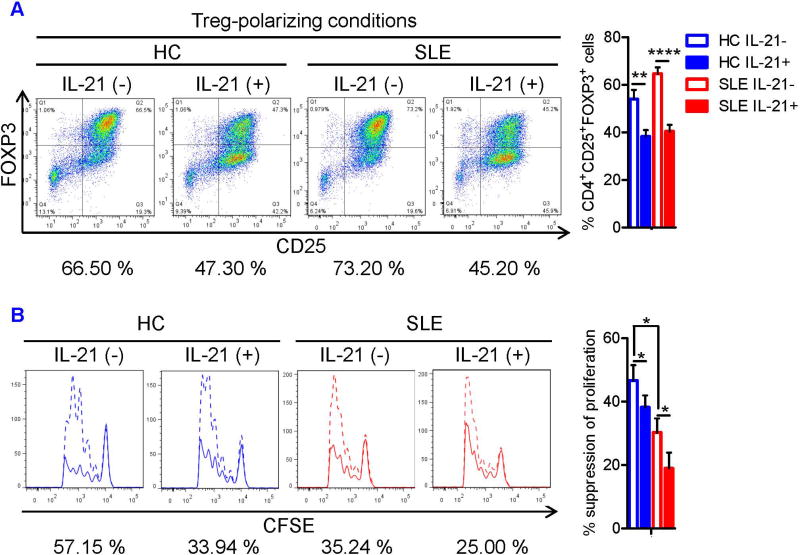
To identify pro-inflammatory cytokines that may antagonize Treg differentiation in SLE, we examined the impact of IL-6, IL-17, and IL-21 that suppress Treg development in mice (25, 26, 38). However, the effect of these cytokines on Treg differentiation from uncommitted CD4+ T cells has not been clarified in human subjects. Therefore, naïve CD4+ T cells were isolated from peripheral blood of SLE and matched HC donors and cultured under Treg-polarizing conditions with or without IL-6, IL-17, or IL-21. The demographics and clinical characteristics of study subjects are shown in the Table S1. Neither IL-6 nor IL-17 inhibited human Treg differentiation (Figures S1A and S1B). In contrast, IL-21 inhibited Treg differentiation both in HC and SLE donors (Figure 1A). Naïve CD4+ T cells were cultured under Treg-polarizing conditions for 3 days in the absence or presence of IL-21 at concentrations of 1 ng/ml, 10 ng/ml, or 100 ng/ml. The concentration of 10 ng/ml was used for subsequent studies because 10 ng/ml IL-21 suppressed Treg differentiation more efficiently than 1 ng/ml whereas there was no difference between 10 ng/ml and 100 ng/ml with regard to their impact on Treg differentiation. Next, we examined the effects of IL-21 on suppressor function. CFSE-stained CD4+ CD25− responder T cells (Tresp cells) from HC and SLE subjects were stimulated for 5 days with plate-bound anti-CD3 and irradiated PBMCs in the presence or absence of equal number of autologous CD4+CD25+ Tregs and IL-21. Suppressive function of SLE Tregs was diminished, which was further inhibited by IL-21 (Figure 1B). IL-21 did not enhance the proliferation of Tresp cells (Figure 1B), suggesting that these effects were mediated by blockade of Treg function.
FIGURE 1. IL-21 inhibits human Treg differentiation and suppressor activity.
(A) Naïve CD4+ T cells were isolated from peripheral blood of SLE and matched healthy control (HC) subjects and cultured for 3 days in the presence of anti-CD3/CD28, TGF-β (5 ng/ml), and IL-2 (50 IU/ml) with or without IL-21 (10 ng/ml). Representative flow cytometry dot plots showing CD4+CD25+FOXP3+ Tregs (left). Numbers below the dot plots represent the frequency of CD4+CD25+FOXP3+ Tregs. Cumulative data from 7 matched HC and SLE subjects (right). Data were analyzed by a paired two-tailed t-test (**, p<0.01; ****, p<0.0001). (B) Magnetically sorted CD4+CD25− responder T cells (Tresp cells) from HC and SLE donors were stained with CFSE and cultured for 5 days in the presence of plate-bound anti-CD3 and irradiated PBMCs with or without IL-21 (100 ng/ml) in the presence or absence of equal number of autologous CD4+CD25+ Tregs. Proliferation of Tresp cells was determined by calculating the division index of CFSE+ cells using FlowJo software. Representative flow cytometry histograms showing CFSE dilution by Tresp cells in the presence (solid line) or absence (dashed line) of Tregs (left). Numbers below the histograms represent % suppression of division index in the presence of Tregs. Cumulative data represent mean ± SE of Treg suppressive function from 8 pairs of matched HC and SLE subjects (right). Statistical analysis was performed by a paired two-tailed t-test (*, p<0.05).
IL-21 activates mTORC1 and mTORC2 and suppresses autophagy during Treg differentiation
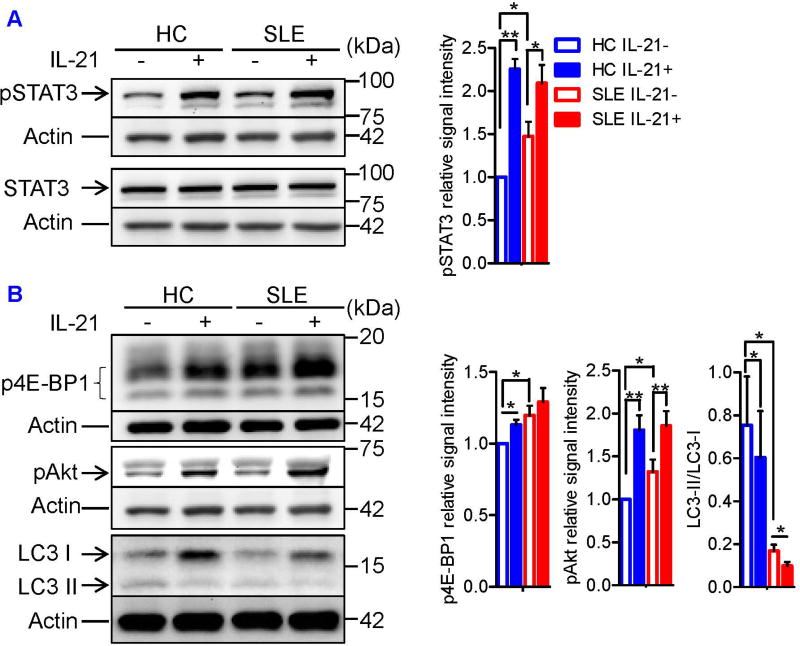
In mice, both IL-6 and IL-21 inhibit Treg differentiation in a STAT3-dependent manner (25, 26), whereas in humans, we found that IL-21, but not IL-6, blocked Treg differentiation (Figures 1 and S1A). These observations prompted us to ask whether IL-21 exploits STAT3-independent mechanism to dampen human Treg differentiation. To delineate the mechanisms by which IL-21 inhibits Treg differentiation, we first quantified the phosphorylation of STAT3 at tyrosine 705 (pSTAT3Y705) in lysates of naïve CD4+ T cells cultured under Treg-polarizing conditions by immunoblotting. pSTAT3 was upregulated in SLE T cells, which was further enhanced by IL-21 (Figure 2A). As measured by phosphorylation of 4E-BP1 and Akt, activities of mTORC1 and mTORC2 were increased in SLE Tregs, respectively, which were further enhanced by IL-21 (Figure 2B). IL-21 did not induce the phosphorylation of S6K1 (data not shown). Unlike IL-21, IL-6 did not activate mTORC1 or mTORC2, although it phosphorylated STAT3 to a level comparable to what was observed in IL-21-treated cells (Figure S1C).
FIGURE 2. IL-21 activates mTORC1 and mTORC2 and suppresses autophagy during Treg differentiation.
Naïve CD4+ T cells from SLE and matched healthy control subjects were cultured for 72 hours (or 24 hours to assess LC3 expression) under Treg-polarizing conditions with or without IL-21. (A) Total STAT3 and its phosphorylation at tyrosine 705 (pSTAT3Y705) were examined by immunoblotting. Representative immunoblot staining of STAT3 and pSTAT3 (left). + and − indicate the presence or absence of IL-21 in the culture media. The signal intensity of pSTAT3 was normalized to that of actin, after which relative pSTAT3 expression to that of IL-21-untreated HC sample was determined. Cumulative data from 5 pairs of matched HC and SLE subjects (right). Data were analyzed by a paired t-test (*p<.0.05, **p<0.01). (B) Phosphorylation of Akt at serine 473 (pAkt) and 4E-BP1 at threonine 37 and 46 (p4E-BP1) as well as LC3 expression was determined by immunoblotting. Representative immunoblot staining of pAkt, p4E-BP1, and LC3 (left). The signal intensity of pAkt and p4E-BP1 was normalized to that of actin, after which relative pAkt and p4E-BP1 expression to that of IL-21-untreated HC sample was determined. The signal intensity of LC3-II relative to that of LC3-I was determined. Cumulative data of pAkt and p4E-BP1 expression and LC3-II/LC3-I ratio from 7, 5, and 9 pairs of matched HC and SLE subjects (right). Results were analyzed by a paired two-tailed t-test (*, p<0.05; **, p<0.01).
In accordance with a controlling role for mTORC1 in autophagy, a key regulatory checkpoint of autophagosome formation, lipidation of LC3, i.e., transition of LC3-I to LC3-II, was examined by western blot. Autophagy was prominently increased in Tregs as compared to effector (Teff) and memory CD4+T cells of HC subjects (Tmem; Figure S2A). Such a distinction was blunted in SLE patients, i.e., autophagy was markedly diminished in Tregs of SLE patients relative to HC subjects (Figures 2B and S2A). While autophagy was enhanced in Teff cells, it was also suppressed in Tmem cells of SLE patients (Figure S2A). IL-21 suppressed autophagy in Tregs of HC subjects (Figure 2B) but it enhanced autophagy in Teff cells of HC subjects. However, IL-21 failed to further increase autophagy of Teff cells of SLE patients that exhibited enhanced autophagy at baseline (Figures S2A and S2B), as reported earlier (13). Moreover, IL-21 inhibited autophagy in Tmem cells of HC subjects (Figure S2C), which may account for diminished autophagy of these cells in SLE patients (Figure S2A).Importantly, IL-21 further suppressed autophagy in lupus Tregs which exhibited such defect already at baseline (Figure 2B). These findings identify IL-21-induced mTOR activation and suppression of autophagy as mechanisms that compromise Treg development in SLE.
Lupus Tregs are GATA-3 and CTLA-4 deficient
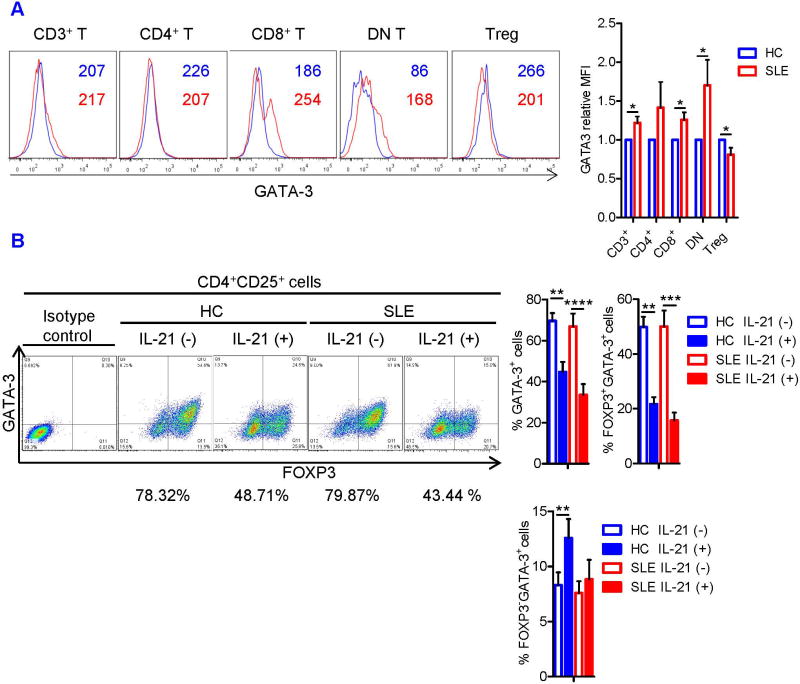
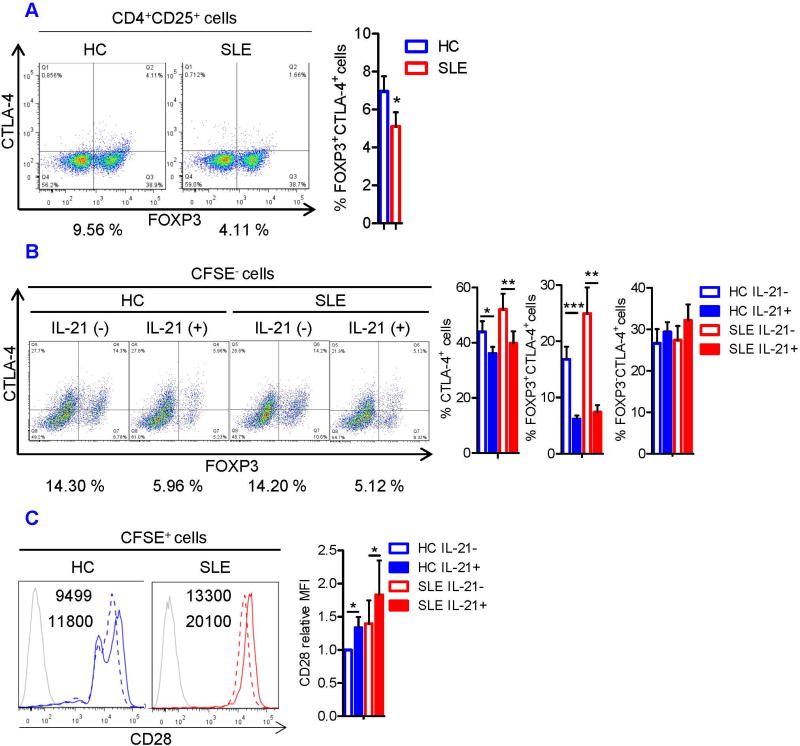
Next, we sought to evaluate intrinsic suppressive machineries of Tregs in SLE patients, which are operational in mouse Tregs (39). To this end, we quantified intracellular expression of GATA-3 and surface CTLA-4 in freshly isolated Tregs from SLE and HC subjects. Although GATA-3 was overexpressed in the majority of T-cell subsets, it was downregulated rather selectively in Tregs of SLE patients (Figure 3A). Likewise, CTLA-4 expression was diminished in freshly isolated CD4+CD25+FOXP3+ cells of SLE patients (Figure 4A). These data suggest that deficiency of GATA-3 and CTLA-4 may contribute the functional defects of SLE Tregs.
FIGURE 3. IL-21 induces GATA-3 deficiency in Tregs.
(A) CD3+ T cells from HC and SLE donors were examined immediately after isolation. Representative flow cytometry histograms of GATA-3 staining (left). Numbers in the histograms denote the mean fluorescence intensity (MFI) of GATA-3 expression in each T-cell subset. Blue and red histograms and numbers represent data from HC and SLE subjects, respectively. DN T cell and Treg denote CD4−CD8− double-negative T cell and CD4+CD25+FOXP3+ Treg, respectively. MFI of GATA-3 was normalized to that of HC cells in each T-cell subset. Cumulative data from 9 pairs of matched HC and SLE subjects (right). The results were analyzed by a paired t-test (*, p<0.05). (B) Naïve CD4+ T cells from SLE and matched healthy control subjects were cultured under Treg-polarizing conditions with or without IL-21. GATA-3 expression in CD4+CD25+FOXP3+ Treg was determined by flow cytometry. Representative flow cytometry dot plots showing FOXP3 versus GATA-3 expression in CD4+CD25+ cells (left panel). Numbers below the dot plots represent the frequency of GATA-3+ cells among the CD4+CD25+FOXP3+ Tregs. Cumulative data showing the frequency of GATA-3+ cells among CD4+CD25+FOXP3+ Tregs, FOXP3+GATA-3+ cells among CD4+CD25+ cells, and FOXP3−GATA-3+ cells among CD4+CD25+ cells from 7 pairs of matched HC and SLE subjects (right panel). The results were analyzed by a paired two-tailed t-test (**, p<0.01; ***, p<0.001; ****, p<0.0001).
FIGURE 4. IL-21 induces CTLA-4 deficiency in Tregs.
(A) Magnetically isolated HC and SLE CD4+CD25+ cells were examined for FOXP3 and CTLA-4 expression by flow cytometry. Representative flow cytometry dot plots showing FOXP3 versus CTLA-4 expression (left). Numbers below the dot plots represent the frequency of CTLA-4+ cells among CD4+CD25+ FOXP3+cells. Cumulative data showing CTLA-4 expression in CD4+CD25+FOXP3+ Tregs from 8 pairs of matched HC and SLE subjects (right). Data were analyzed by a paired two-tailed t-test (*p<0.05). (B) CFSE-stained CD4+CD25− responder T cells from HC and SLE donors were cultured for 5 days with irradiated PBMCs and CD4+CD25+ Tregs as described in Fig 1. Representative flow cytometry dot plots showing FOXP3 versus CTLA-4 expression in CFSE− cells (left). Numbers below the dot plots represent the frequency of FOXP3+CTLA-4+ cells. Cumulative data showing the frequency of CTLA-4+cells, FOXP3+CTLA-4+ cells, and FOXP3−CTLA-4+ cells among the CFSE− cells from 8 pairs of matched HC and SLE subjects (right). The results were analyzed by a paired two-tailed t-test (*, p<0.05; **, p<0.01; ***, p<0.001). (C) Representative histograms of CD28 expression in IL-21-treated (solid line) and –untreated (dashed line) CFSE+ cells (left). Grey histograms represent unstained samples. Numbers in the histograms denote the MFI of CD28. MFI of CD28 was normalized to that of IL-21-untreated HC cells. Cumulative data from 8 pairs of matched HC and SLE subjects (right). Data were analyzed by a paired t-test (*, p<0.05).
IL-21-driven downregulation of GATA-3 and CTLA-4 underlies developmental and functional defects of SLE Tregs
Since IL-21 inhibited the development and function of Tregs, its effects on the expression of GATA-3 and CTLA-4 were examined in naive CD4+ T cells cultured under Treg-polarizing conditions. IL-21 effectively blocked GATA-3 expression in CD4+CD25+FOXP3+ Tregs of both HC (p=0.0075) and SLE subjects (p=8.15×10−5; Figure 3B). During co-culture of Tregs and Tresp cells, IL-21 suppressed GATA-3 expression and expansion of FOXP3+GATA-3+cells in CFSE− cells (Figure S3). Likewise, IL-21 reduced the expression of CTLA-4 (Figure 4B) and the frequency of FOXP3+CTLA-4+ cells in the CFSE− population in both HC and SLE subjects (Figure 4B). Similar to what was seen in GATA-3 expression (Figure 3B), IL-21 suppressed CTLA-4 expression selectively and most markedy in FOXP3+ cells (Figure 4B). Accordingly, among CFSE− cells, the FOXP3+CTLA-4+ population was reduced from 16.8 ± 2.2% to 6.2 ± 0.6% in HC subjects (p=0.00058) and from 24.9 ± 4.6% to 7.4 ± 1.2% in SLE patients (p=0.0014, Figure 4B). In contrast, IL-21 upregulated CD28 expression in the CFSE+ Tresp cells (Figure 4C). As the opposing effects of CTLA-4 and CD28 on T-cell activation state are documented by fatal autoimmunity and excessive CD28 signaling in CTLA-4-deficent mice (40), this finding supports the notion that IL-21-driven downregulation of CTLA-4 contributes to the loss of Treg suppressor activity in SLE.
In contrast to IL-21, IL-2 promotes FoxP3 expression and Treg development (41). In this regard, we noted that IL-2 upregulated FOXP3, GATA-3, and CTLA-4 in Tregs (Figure S4), and adeno-associated virus-mediated GATA-3 overexpression restored FOXP3 expression in SLE Tregs (data not shown). These findings suggest that IL-21 and IL-2 regulate human Treg development and function in a reciprocal fashion by modulating GATA-3 and CTLA-4.
Dual blockade of mTORC1 and mTORC2 restores SLE Treg function by inducing autophagy and correcting expression of GATA-3 and CTLA-4
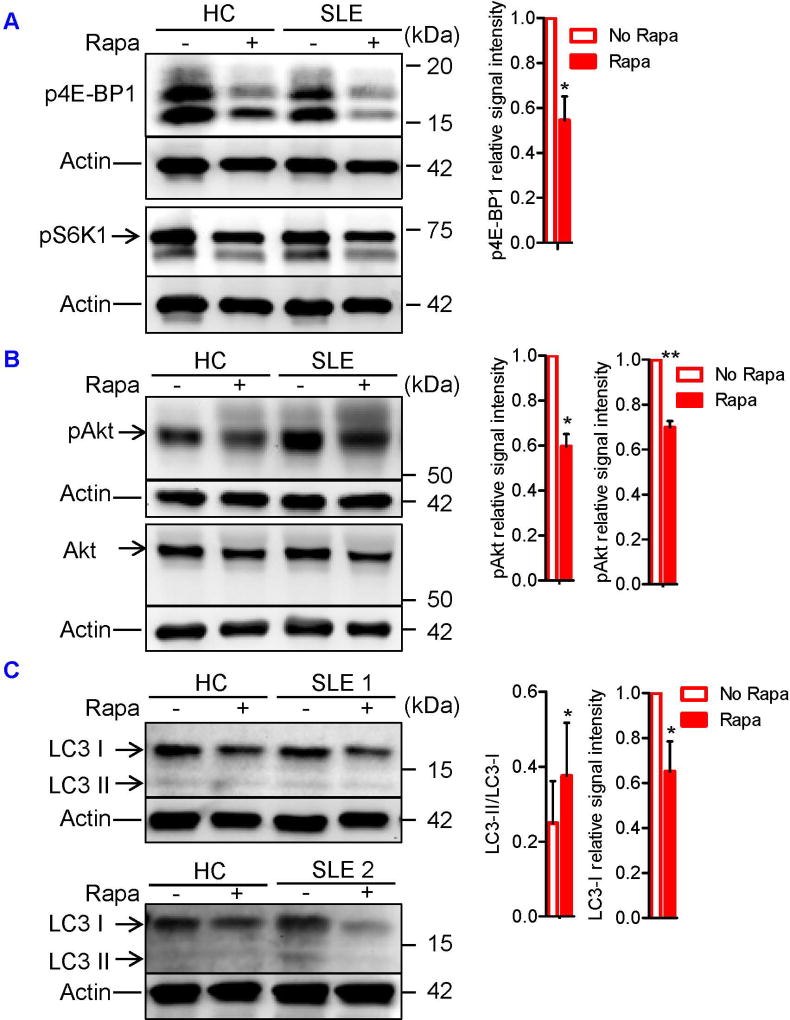
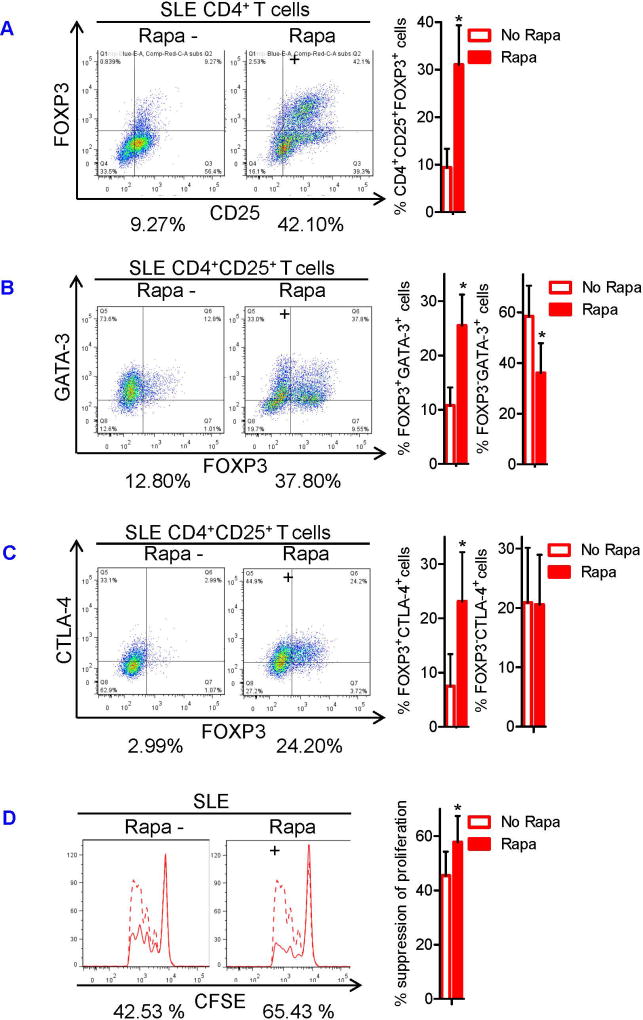
To determine whether mTOR activation caused SLE Treg depletion and dysfunction, we examined mTOR activity and autophagy of SLE Tregs that had been expanded in vitro for 4 weeks in the presence or absence of rapamycin. Rapamycin blocked the phosphorylation of mTORC1 substrate, 4E-BP1 (Figure 5A), and mTORC2 substrate, Akt, in the Tregs (Figure 5B). Blockade of mTOR also induced autophagy in the Tregs, as evidenced by the increased ratio of LC3-II relative to LC3-I as well as reduced LC3-I expression (Figure 5C). We next assessed the effects of mTOR blockade on GATA-3 and CTLA-4 expression and Treg function. Importantly, 4-week rapamycin treatment expanded CD4+CD25+FOXP3+ Tregs in SLE patients by 3.3-fold (p=0.03; Figure 6A) and restored their expression of GATA-3 (Fig. 6B) and CTLA-4 (Figure 6C) as well as suppressor function (Figure 6D). When naïve CD4+ T cells were cultured under Treg-polarizing conditions only for 3 days, addition of rapamycin did not expand Tregs (Figure S5A). This was attributed to the blockade of mTORC1, but not mTORC2 (Figure S5B). Tregs expanded in vitro for 4 weeks did not secrete meaningful amount of IL-21 (data not shown). However, 3-day rapamycin treatment also suppressed the production of pro-inflammatory cytokines, including IL-21, and it restored diminished TGF-β secretion by CD3+ T cells of SLE patients (Figure S6A). This result indicates that mTORC1 suppresses TGF-β production by T cells and explains why mTOR-deficient T cells develop into Tregs even in the absence of exogenous TGF-β (Figure S6B).
FIGURE 5. Dual blockade of mTORC1 and mTORC2 restores autophagy in SLE Tregs.
CD4+CD25+ Tregs were cultured for 4 weeks in the presence of plate-bound anti-CD3, soluble anti-CD28, and IL-2 (100 IU/ml; omitted in the first week of culture) with or without rapamycin (100 nM). Culture media were changed every week. Expression of Akt, pAktSer473, pS6K1Thr389, p4E-BP1Thr37/46, and LC3 in SLE Tregs were determined by immunoblotting. (A) Representative immunoblot staining of p4E-BP1 and pS6K1 from HC and SLE subjects (left). + and − indicate the presence or absence of rapamycin. The signal intensity of p4E-BP1 was normalized to that of actin, after which relative p4E-BP1 expression to that of rapamycin-untreated sample was determined. Cumulative data from 4 SLE subjects were presented (right). Data were analyzed by a paired two-tailed t-test (*p<0.05). (B) Representative immunoblot staining of Akt and pAkt from HC and SLE subjects (left). + and − indicate the presence or absence of rapamycin. The signal intensity of pAkt was normalized to that of actin, after which relative pAkt expression to that of rapamycin-untreated sample was determined (left in the right panel). The signal intensity of pAkt and Akt were first normalized to that of actin. Relative pAkt expression was normalized to that of Akt (pAkt/Akt expression), after which pAkt/Akt expression to that of rapamycin-untreated sample was determined (right in the right panel). Cumulative data from 3 SLE subjects were presented. Data were analyzed by a paired two-tailed t-test (*, p<0.05; **p<0.01). (C) Representative immunoblot staining of LC3 from HC and two SLE subjects (left). + and − indicate the presence or absence of rapamycin. The signal intensity of LC3-II relative to that of LC3-I was determined. Cumulative data from 4 SLE subjects were presented (left in the right panel). The signal intensity of LC3-I was normalized to that of actin, after which relative LC3-I expression to that of rapamycin-untreated sample was determined. Cumulative data from 1 HC and 4 SLE subjects were presented (right in the right panel). Data were analyzed by a paired t-test (*, p<0.05).
FIGURE 6. mTOR blockade induces expansion and restores GATA-3 and CTLA-4 expression and suppressor function of SLE Tregs.
Sorted CD4+CD25+ T cells were expanded in vitro for 4 weeks in the presence or absence of rapamycin as described in Fig 5. (A) Representative flow cytometry dot plots showing CD25 versus FOXP3 expression in the Tregs (left). Numbers below the dot plots represent the frequency CD4+CD25+FOXP3+ cells. Cumulative data from 4 SLE subjects (right). (B) Representative flow cytometry dot plots showing FOXP3 versus GATA-3 expression among CD4+CD25+ cells (left). Numbers below the dot plots represent the frequency FOXP3+GATA-3+cells among CD4+CD25+ Treg cells. Cumulative data of frequency of FOXP3+GATA-3+cells and FOXP3−GATA-3+cells among CD4+CD25+ Treg cells from 4 SLE subjects (right). (C) Representative flow cytometry dot plots showing FOXP3 versus CTLA-4 expression (left). Numbers below the dot plots represent the frequency of FOXP3+CTLA-4+ cells among CD4+CD25+ Treg cells. Cumulative data of frequency of FOXP3+CTLA-4+ cells and FOXP3−CTLA-4+ cells among CD4+CD25+ Treg cells from 4 SLE subjects (right). (D) Using these Tregs, Treg suppression assay was performed as described in Fig 1. Representative flow cytometry histograms showing CFSE dilution by Tresp cells in the presence (solid line) or absence (dashed line) of Tregs (left). Numbers below the histograms represent % suppression of division index in the presence of Tregs. Cumulative data of Treg suppressor function from 4 SLE subjects (right). The results were analyzed by a paired two-tailed t-test (*, p<0.05).
DISCUSSION
IL-21 has emerged as a pro-inflammatory cytokine in SLE (24, 42). The present study reveals a role for IL-21 in blockade of Treg differentiation and function through activating mTORC1 and mTORC2. In mice, IL-6, IL-17 (38), and IL-21 inhibit Treg differentiation in a STAT3-dependent manner (25, 26). As shown in the present study, IL-21, but not IL-6, blocked Treg differentiation, yet both of these cytokines phosphorylated STAT3 to comparable levels. The discrepancy between the two species is intriguing and raises a possibility that IL-21 exploited STAT3-independent mechanisms to abrogate human Treg differentiation. Indeed, we showed that IL-21, but not IL-6, elicited the activation of mTORC1 and mTORC2 and suppressed autophagy in naïve CD4+ T cells cultured under Treg-polarizing conditions, which likely played an important role in curtailing Treg development since mTOR is a robust antagonist of Treg differentiation (9), and Tregs need autophagy to maintain its lineage-commitment and function (27). Our previous work highlighted mTORC1 activation as an important mechanism that drives expansion Th17 cells and IL-4-expressing CD4−CD8− double-negative T cells in SLE (15, 16). The present study unveiled a pro-inflammatory role for mTORC2 in blocking Treg development in SLE, which is consistent with observations that activation of mTORC1 and mTORC2 jointly promote Tfh cell differentiation (43). In this regard, it is noteworthy that IL-21 secretion by T cells was blocked by rapamycin in our study. These findings suggest that the IL-21→mTOR→IL-21 axis serves as a self-amplifying loop for Treg contraction in SLE (Figure S7). Although it has been suspected that IL-21 may activate mTOR via the JAK-STAT and PI3K-AKT signaling cascade (44), concrete evidence has been lacking. Nevertheless, the mechanism is likely to be indirect given that IL-21 binds to a cell surface receptor (44), while mTOR is located in the cytosol where it can traffic to endosomes or lysosomes (11).
Rapamycin is therapeutic in SLE (15, 18), which occurs with a striking reversal of Treg depletion in vivo (15, 16). Expansion of CD4+CD25+FOXP3+ Tregs after 4-week rapamycin treatment enabled dual blockade of mTORC1 and mTORC2, which was in agreement with earlier observations that prolonged rapamycin treatment also suppressed mTORC2 (45). In addition to its critical role in Treg differentiation, mTORC2 blockade is likely to be essential for lineage stabilization and functional maturation of Tregs. Such Treg-lineage stability and protection against autoimmunity depend on the inactivation of mTORC2 (46).
Our understanding of mechanisms by which the mTOR activation curtails Treg development has been evolving. Interestingly, phosphorylation of 4E-BP1, but not S6K1, was sensitive to IL-21 and rapamycin in the current study. In this regard, it is important to note that rapamycin differentially inhibits the phosphorylation of 4E-BP1 and S6K1 depending on the cell types (47). The robust and reciprocal effects of IL-21 and rapamycin on autophagy and phosphorylation of 4E-BP1 indicate that this substrate of mTORC1 controls the transcriptional machinery involved in Treg development and function. Without directly implicating autophagy, mTORC1 activation has been shown to induce proteasomal degradation of FoxP3 (48). In turn, mTORC2 activation phosphorylates Akt, which inhibits FoxP3 expression by inactivating Foxo1 and Foxo3 (49). Similar to earlier studies in mice (27), autophagy was increased in Tregs over Teff cells in HC subjects. However, this difference was abrogated in SLE patients, as autophagy was increased in naïve and pro-inflammatory effector CD4+ T cells but profoundly diminished in Tregs. Such a blockade of autophagy in Tregs is attributed to activation of the IL-21-mTORC1 axis in SLE.
In addition, our data pinpoint specific molecular targets downstream of mTOR pathway activation in lupus Tregs. Meaningful Treg suppression was observed only when irradiated PBMC were used to stimulate Tresp cells, but not when Tresp cells were stimulated with plate-bound anti-CD3 and soluble anti-CD28, suggesting that Treg suppression requires the contribution by APC in primary human cells (Figure S7). Of note, IL-21-driven downregulation of CTLA-4 in Tregs was associated with reciprocal upregulation of CD28 expression in the Tresp cells. These findings are consistent with the notion that CTLA-4-dependent downregulation of CD80/CD86 on APC is critical for suppressor function of Tregs (39). In the current study, IL-21 downregulated while IL-2 upregulated GATA-3 and CTLA-4 expression in Tregs, suggesting that the opposing effects of these cytokines on Treg development are mediated through GATA-3 and CTLA-4. Along these lines, IL-21 inhibited GATA-3 and CTLA-4 expression in CD4+CD25+FOXP3+ cells, but not in CD4+CD25+FOXP3−cells. In a reciprocal fashion, mTOR blockade restored diminished GATA-3 and CTLA-4 expression specifically in FOXP3+ but not in FOXP3− cells of SLE patients. These results indicate that mTOR negatively regulates GATA-3 and CTLA-4 in a Treg-cell specific manner, and that IL-21-driven activation of mTORC1 and mTORC2 abrogates Treg differentiation and function by suppressing GATA-3 and CTLA-4 expression.
Furthermore, we found that mTORC1 blockade restored the diminished TGF-β expression by SLE T cells. Notably, there is evidence that autophagy induces TGF-β expression in other cell types (35). In turn, TGF-β stimulates the expression of Foxp3 (34), which binds to the promoters of the GATA-3 and CTLA4 to control their expression (31). These findings collectively unveil an intriguing paradigm whereby TGF-β connects the IL-21-mTOR-autophagy axis with the deficient, GATA-3/FOXP3-dependent expression of CTLA-4 in lupus Tregs (Figure S6B).
Most recently, checkpoint inhibitors, such as anti-CTLA-4, have revolutionized cancer immunotherapy but often result in unwanted development of autoimmunity (50). Thus, beyond unveiled a direct role of mTOR in Treg depletion during pathogenesis and therapeutic intervention in SLE (11), the present findings have broad implications for normal Treg development as well as autoimmunity induced by treatment with checkpoint inhibitors (51).
Supplementary Material
Acknowledgments
We thank all patients and healthy donors who contributed to this study. We appreciate insightful suggestions of Megan Levings (University of British Columbia) on setting up the human Treg suppression assay. This work was supported by grants R01-AI072648 and R01-AI122176 from the National Institutes of Health and the Department of Medicine at the SUNY Upstate Medical University.
Footnotes
Online Supplemental Materials: Supplementary Methods, Table S1 and Supplementary Figures S1–S7.
The authors declare no competing financial interests.
References
- 1.Tsokos GC. Systemic lupus erythematosus. N Engl J Med. 2011;365(22):2110–21. doi: 10.1056/NEJMra1100359. [DOI] [PubMed] [Google Scholar]
- 2.Trager J, Ward MM. Mortality and causes of death in systemic lupus erythematosus. Curr Opin Rheumatol. 2001;13(5):345–51. doi: 10.1097/00002281-200109000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Yang J, Chu Y, Yang X, Gao D, Zhu L, Wan L, et al. Th17 and natural Treg cell population dynamics in systemic lupus erythematosus. Arthritis Rheum. 2009;60(5):1472–83. doi: 10.1002/art.24499. [DOI] [PubMed] [Google Scholar]
- 4.Miyara M, Amoura Z, Parizot C, Badoual C, Dorgham K, Trad S, et al. Global natural regulatory T cell depletion in active systemic lupus erythematosus. J Immunol. 2005;175(12):8392–400. doi: 10.4049/jimmunol.175.12.8392. [DOI] [PubMed] [Google Scholar]
- 5.Mellor-Pita S, Citores MJ, Castejon R, Tutor-Ureta P, Yebra-Bango M, Andreu JL, et al. Decrease of regulatory T cells in patients with systemic lupus erythematosus. Ann Rheum Dis. 2006;65(4):553–4. doi: 10.1136/ard.2005.044974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonelli M, Savitskaya A, von Dalwigk K, Steiner CW, Aletaha D, Smolen JS, et al. Quantitative and qualitative deficiencies of regulatory T cells in patients with systemic lupus erythematosus (SLE) Int Immunol. 2008;20(7):861–8. doi: 10.1093/intimm/dxn044. [DOI] [PubMed] [Google Scholar]
- 7.Valencia X, Yarboro C, Illei G, Lipsky PE. Deficient CD4+CD25high T regulatory cell function in patients with active systemic lupus erythematosus. J Immunol. 2007;178(4):2579–88. doi: 10.4049/jimmunol.178.4.2579. [DOI] [PubMed] [Google Scholar]
- 8.Bhaskar PT, Hay N. The two TORCs and Akt. Dev Cell. 2007;12(4):487–502. doi: 10.1016/j.devcel.2007.03.020. [DOI] [PubMed] [Google Scholar]
- 9.Delgoffe GM, Pollizzi KN, Waickman AT, Heikamp E, Meyers DJ, Horton MR, et al. The kinase mTOR regulates the differentiation of helper T cells through the selective activation of signaling by mTORC1 and mTORC2. Nat Immunol. 2011;12(4):295–303. doi: 10.1038/ni.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haxhinasto S, Mathis D, Benoist C. The AKT-mTOR axis regulates de novo differentiation of CD4+Foxp3+ cells. J Exp Med. 2008;205(3):565–74. doi: 10.1084/jem.20071477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perl A. Activation of mTOR (mechanistic target of rapamycin) in rheumatic diseases. Nat Rev Rheumatol. 2016;12(3):169–82. doi: 10.1038/nrrheum.2015.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fernandez DR, Telarico T, Bonilla E, Li Q, Banerjee S, Middleton FA, et al. Activation of mammalian target of rapamycin controls the loss of TCRzeta in lupus T cells through HRES-1/Rab4-regulated lysosomal degradation. J Immunol. 2009;182(4):2063–73. doi: 10.4049/jimmunol.0803600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caza TN, Fernandez DR, Talaber G, Oaks Z, Haas M, Madaio MP, et al. HRES-1/Rab4-mediated depletion of Drp1 impairs mitochondrial homeostasis and represents a target for treatment in SLE. Ann Rheum Dis. 2014;73(10):1888–97. doi: 10.1136/annrheumdis-2013-203794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chi H. Regulation and function of mTOR signalling in T cell fate decisions. Nat Rev Immunol. 2012;12(5):325–38. doi: 10.1038/nri3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lai ZW, Borsuk R, Shadakshari A, Yu J, Dawood M, Garcia R, et al. Mechanistic target of rapamycin activation triggers IL-4 production and necrotic death of double-negative T cells in patients with systemic lupus erythematosus. J Immunol. 2013;191(5):2236–46. doi: 10.4049/jimmunol.1301005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kato H, Perl A. Mechanistic target of rapamycin complex 1 expands Th17 and IL-4+ CD4−CD8− double-negative T cells and contracts regulatory T cells in systemic lupus erythematosus. J Immunol. 2014;192(9):4134–44. doi: 10.4049/jimmunol.1301859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Warner LM, Adams LM, Sehgal SN. Rapamycin prolongs survival and arrests pathophysiologic changes in murine systemic lupus erythematosus. Arthritis Rheum. 1994;37(2):289–97. doi: 10.1002/art.1780370219. [DOI] [PubMed] [Google Scholar]
- 18.Fernandez D, Bonilla E, Mirza N, Niland B, Perl A. Rapamycin reduces disease activity and normalizes T cell activation-induced calcium fluxing in patients with systemic lupus erythematosus. Arthritis Rheum. 2006;54(9):2983–8. doi: 10.1002/art.22085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oaks Z, Winans T, Caza T, Fernandez D, Liu Y, Landas SK, et al. Mitochondrial Dysfunction in the Liver and Antiphospholipid Antibody Production Precede Disease Onset and Respond to Rapamycin in Lupus-Prone Mice. Arthritis Rheumatol. 2016;68(11):2728–39. doi: 10.1002/art.39791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Canaud G, Bienaime F, Tabarin F, Bataillon G, Seilhean D, Noel LH, et al. Inhibition of the mTORC pathway in the antiphospholipid syndrome. N Engl J Med. 2014;371(4):303–12. doi: 10.1056/NEJMoa1312890. [DOI] [PubMed] [Google Scholar]
- 21.Maeda K, Kosugi T, Sato W, Kojima H, Sato Y, Kamimura D, et al. CD147/basigin limits lupus nephritis and Th17 cell differentiation in mice by inhibiting the interleukin-6/STAT-3 pathway. Arthritis Rheumatol. 2015;67(8):2185–95. doi: 10.1002/art.39155. [DOI] [PubMed] [Google Scholar]
- 22.Amarilyo G, Lourenco EV, Shi FD, La Cava A. IL-17 promotes murine lupus. J Immunol. 2014;193(2):540–3. doi: 10.4049/jimmunol.1400931. [DOI] [PubMed] [Google Scholar]
- 23.Crispin JC, Oukka M, Bayliss G, Cohen RA, Van Beek CA, Stillman IE, et al. Expanded double negative T cells in patients with systemic lupus erythematosus produce IL-17 and infiltrate the kidneys. J Immunol. 2008;181(12):8761–6. doi: 10.4049/jimmunol.181.12.8761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dolff S, Abdulahad WH, Westra J, Doornbos-van der Meer B, Limburg PC, Kallenberg CG, et al. Increase in IL-21 producing T-cells in patients with systemic lupus erythematosus. Arthritis Res Ther. 2011;13(5):R157. doi: 10.1186/ar3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nurieva R, Yang XO, Martinez G, Zhang Y, Panopoulos AD, Ma L, et al. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature. 2007;448(7152):480–3. doi: 10.1038/nature05969. [DOI] [PubMed] [Google Scholar]
- 26.Yang XO, Nurieva R, Martinez GJ, Kang HS, Chung Y, Pappu BP, et al. Molecular antagonism and plasticity of regulatory and inflammatory T cell programs. Immunity. 2008;29(1):44–56. doi: 10.1016/j.immuni.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wei J, Long L, Yang K, Guy C, Shrestha S, Chen Z, et al. Autophagy enforces functional integrity of regulatory T cells by coupling environmental cues and metabolic homeostasis. Nat Immunol. 2016;17(3):277–85. doi: 10.1038/ni.3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gros F, Arnold J, Page N, Decossas M, Korganow AS, Martin T, et al. Macroautophagy is deregulated in murine and human lupus T lymphocytes. Autophagy. 2012;8(7):1113–23. doi: 10.4161/auto.20275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alessandri C, Barbati C, Vacirca D, Piscopo P, Confaloni A, Sanchez M, et al. T lymphocytes from patients with systemic lupus erythematosus are resistant to induction of autophagy. FASEB J. 2012;26(11):4722–32. doi: 10.1096/fj.12-206060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clarke AJ, Ellinghaus U, Cortini A, Stranks A, Simon AK, Botto M, et al. Autophagy is activated in systemic lupus erythematosus and required for plasmablast development. Ann Rheum Dis. 2015;74(5):912–20. doi: 10.1136/annrheumdis-2013-204343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu Y, Borde M, Heissmeyer V, Feuerer M, Lapan AD, Stroud JC, et al. FOXP3 controls regulatory T cell function through cooperation with NFAT. Cell. 2006;126(2):375–87. doi: 10.1016/j.cell.2006.05.042. [DOI] [PubMed] [Google Scholar]
- 32.Wang Y, Su MA, Wan YY. An essential role of the transcription factor GATA-3 for the function of regulatory T cells. Immunity. 2011;35(3):337–48. doi: 10.1016/j.immuni.2011.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sakaguchi S, Vignali DA, Rudensky AY, Niec RE, Waldmann H. The plasticity and stability of regulatory T cells. Nat Rev Immunol. 2013;13(6):461–7. doi: 10.1038/nri3464. [DOI] [PubMed] [Google Scholar]
- 34.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, et al. Conversion of peripheral CD4+CD25− naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198(12):1875–86. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li J, Yang B, Zhou Q, Wu Y, Shang D, Guo Y, et al. Autophagy promotes hepatocellular carcinoma cell invasion through activation of epithelial-mesenchymal transition. Carcinogenesis. 2013;34(6):1343–51. doi: 10.1093/carcin/bgt063. [DOI] [PubMed] [Google Scholar]
- 36.Lai ZW, Hanczko R, Bonilla E, Caza TN, Clair B, Bartos A, et al. N-acetylcysteine reduces disease activity by blocking mammalian target of rapamycin in T cells from systemic lupus erythematosus patients: a randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2012;64(9):2937–46. doi: 10.1002/art.34502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tan EM, Cohen AS, Fries JF, Masi AT, McShane DJ, Rothfield NF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25(11):1271–7. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- 38.Gu ZW, Wang YX, Cao ZW. Neutralization of interleukin-17 suppresses allergic rhinitis symptoms by downregulating Th2 and Th17 responses and upregulating the Treg response. Oncotarget. 2017;8(14):22361–9. doi: 10.18632/oncotarget.15652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T, Miyara M, Fehervari Z, et al. CTLA-4 control over Foxp3+ regulatory T cell function. Science. 2008;322(5899):271–5. doi: 10.1126/science.1160062. [DOI] [PubMed] [Google Scholar]
- 40.Tai X, Van Laethem F, Sharpe AH, Singer A. Induction of autoimmune disease in CTLA-4−/− mice depends on a specific CD28 motif that is required for in vivo costimulation. Proc Natl Acad Sci U S A. 2007;104(34):13756–61. doi: 10.1073/pnas.0706509104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Burchill MA, Yang J, Vogtenhuber C, Blazar BR, Farrar MA. IL-2 receptor beta-dependent STAT5 activation is required for the development of Foxp3+ regulatory T cells. J Immunol. 2007;178(1):280–90. doi: 10.4049/jimmunol.178.1.280. [DOI] [PubMed] [Google Scholar]
- 42.Choi JY, Ho JH, Pasoto SG, Bunin V, Kim ST, Carrasco S, et al. Circulating follicular helper-like T cells in systemic lupus erythematosus: association with disease activity. Arthritis Rheumatol. 2015;67(4):988–99. doi: 10.1002/art.39020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zeng H, Cohen S, Guy C, Shrestha S, Neale G, Brown SA, et al. mTORC1 and mTORC2 Kinase Signaling and Glucose Metabolism Drive Follicular Helper T Cell Differentiation. Immunity. 2016;45(3):540–54. doi: 10.1016/j.immuni.2016.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tian Y, Zajac AJ. IL-21 and T cell differentiation: consider the context. Trends Immunol. 2016;37(8):557–68. doi: 10.1016/j.it.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lamming DW, Ye L, Katajisto P, Goncalves MD, Saitoh M, Stevens DM, et al. Rapamycin-induced insulin resistance is mediated by mTORC2 loss and uncoupled from longevity. Science. 2012;335(6076):1638–43. doi: 10.1126/science.1215135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shrestha S, Yang K, Guy C, Vogel P, Neale G, Chi H. Treg cells require the phosphatase PTEN to restrain TH1 and TFH cell responses. Nat Immunol. 2015;16(2):178–87. doi: 10.1038/ni.3076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Choo AY, Yoon SO, Kim SG, Roux PP, Blenis J. Rapamycin differentially inhibits S6Ks and 4E-BP1 to mediate cell-type-specific repression of mRNA translation. Proc Natl Acad Sci U S A. 2008;105(45):17414–9. doi: 10.1073/pnas.0809136105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dang EV, Barbi J, Yang HY, Jinasena D, Yu H, Zheng Y, et al. Control of T(H)17/T(reg) balance by hypoxia-inducible factor 1. Cell. 2011;146(5):772–84. doi: 10.1016/j.cell.2011.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Merkenschlager M, von Boehmer H. PI3 kinase signalling blocks Foxp3 expression by sequestering Foxo factors. J Exp Med. 2010;207(7):1347–50. doi: 10.1084/jem.20101156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tanaka A, Sakaguchi S. Regulatory T cells in cancer immunotherapy. Cell Res. 2017;27(1):109–18. doi: 10.1038/cr.2016.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Suarez-Almazor ME, Kim ST, Abdel-Wahab N, Diab A. Review: Immune-Related Adverse Events With Use of Checkpoint Inhibitors for Immunotherapy of Cancer. Arthritis Rheumatol. 2017;69(4):687–99. doi: 10.1002/art.40043. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.