Abstract
Relapse remains the major cause of mortality post hematopoietic cell transplantation (HCT) for pediatric acute leukemia. Previous research suggests that reducing the intensity of calcineurin inhibitor based graft versus host disease (GVHD) prophylaxis may be an effective strategy in abrogating the risk of relapse in pediatric patients undergoing matched sibling donor (MSD) HCT. We reasoned that benefits of this strategy could be maximized by selectively applying it to those patients least likely to develop GVHD. We conducted a study of risk for GVHD, to risk stratify patients based on age. Patients <18 years with leukemia who received myeloablative, T cell-replete MSD bone marrow transplantation and calcineurin inhibitor based GVHD prophylaxis between 2000–2013 entered into the Center for International Blood and Marrow Transplant Research registry were included. Cumulative incidence of grade 2–4 acute GVHD was 19%, grade 3–4 acute GVHD 7%, and chronic GVHD 16%. Compared to age 13–18 years, age 2–12 years was associated with a lower risk for grade 2–4 acute GVHD (hazard ratio [HR] 0.42, confidence interval [CI] 0.26–0.70, p=0.0008), grade 3–4 acute GVHD (HR 0.24, CI 0.1–0.56, p=0.001) and chronic GVHD (HR 0.32, CI 0.19–0.54, p<0.001). The risk of grade 2–4 acute GVHD was lower for children undergoing transplantation in 2005–2008 (HR 0.36, CI 0.2–0.65, p=0.0007), and 2009–2013 (HR 0.24, CI 0.11–0.53, p=0.0004) compared to 2000–2004. Similarly, the risk of grade 3–4 acute GVHD was lower for children undergoing transplantation in 2005–2008 (HR 0.23, CI 0.08–0.65, p=0.0056) and 2009–2013 (HR 0.16, CI 0.04–0.67, p=0.0126) compared to 2000–2004. We conclude that acute GVHD rates have decreased significantly over time, and children 2–12 years are at very low risk for acute and chronic GVHD. These results should be validated in an independent analysis, as these patients with high-risk malignancies may be good candidates for trials of reduced GVHD prophylaxis.
Keywords: GVHD, matched sibling donor transplantation, children, recipient age, leukemia
Introduction
Relapse is the primary source of failure of allogeneic hematopoietic cell transplantation (HCT) for pediatric acute leukemia.(1–3) Results of randomized controlled trials conducted in the 1980s and 1990s in children and adults receiving myeloablative conditioning, HLA matched sibling donor (MSD) marrow transplantation and cyclosporine with short course methotrexate for graft versus host disease (GVHD) prophylaxis, indicate that the risk for relapse can be mitigated by attenuating the intensity of prophylaxis - by dropping the methotrexate or lowering the cyclosporine dose. (4–7) This research, however, also suggests that the benefits of such reductions could be offset, at least in part, by increases in transplant related mortality (TRM) driven by increased acute GVHD (aGVHD).(6, 7)
Further investigation of attenuated GVHD prophylaxis regimens is needed in pediatric MSD HCT, where the risk for GVHD is low.(8, 9) Studies of risk for GVHD in pediatric patients suggest that even within the pediatric age group, patients can be risk stratified using recipient and donor age.(10, 11) We, therefore, hypothesized that it would be feasible to apply statistical methods to identify an age group within pediatric MSD recipients who are at very low risk of aGVHD, one that would be ideal for trials of attenuated GVHD prophylaxis. As a first step to test this hypothesis, using data drawn from the Center for International Blood and Marrow Transplant Research (CIBMTR) registry, we conducted the largest study to date of risk for GVHD in pediatric HLA MSD HCT. To maximize the relevance of our findings to HCT for pediatric acute leukemia, we limited our sample to patients with acute lymphoblastic leukemia (ALL) and acute myeloid leukemia (AML) and attempted to mirror the approach most commonly employed in this setting by limiting inclusion to marrow grafts and myeloablative conditioning.
Patients and Methods
Patients
This is a retrospective analysis of 476 patients entered into the CIBMTR database. The CIBMTR is a voluntary working group of more than 450 transplantation centers that contribute detailed data on consecutive hematopoietic cell transplantation to a Statistical Center at the Medical College of Wisconsin in Milwaukee, Wisconsin. Participating centers are required to report all transplants consecutively, and compliance is monitored by on-site audits. All patients provided written informed consent in accordance with the Declaration of Helsinki for data submission and research participation. This study was approved by the Institutional Review Board of the Medical College of Wisconsin.
Inclusion criteria
Patients younger than 18 years with AML or ALL in 1st or 2nd complete remission (CR), who received myeloablative conditioning, T cell-replete HLA MSD bone marrow grafts and calcineurin inhibitor based GVHD prophylaxis between 2000–2013 were included. Patients who received a lymphocyte depleting antibody (ATG/Campath) within the conditioning regimen were excluded. Preparative regimens and GVHD prophylaxis with small cohort sizes (<5) were excluded in an effort to study a homogeneous population.
Statistical analysis
Acute GVHD grading was based on the 1994 Consensus criteria.(12) Chronic GVHD (cGVHD) was described according to the Seattle Criteria,(13) and CIBMTR severity grading was applied.(14) The ‘optimal’ cut points for recipient age were determined using a likelihood ratio test, based on the univariate Cox model for grade III-IV aGVHD. These cut points were used to define the age groups included in all final models.
Patient, disease, and transplant-related factors were compared among the three age groups using χ2 for categorical variables and Kruskal-wallis test for continuous variables. Probabilities for overall survival (OS) were calculated using the Kaplan-Meier method. Comparison of survival curves was done using the log-rank test. Estimates of aGVHD, cGVHD, TRM and relapse were calculated according to the cumulative incidence, with death as a competing risk for GVHD and relapse.
The Cox proportional hazards models were used to examine the effects of recipient age on aGVHD, cGVHD, relapse, TRM, disease-free survival, and OS, while adjusting for other clinical variables. Variables considered included recipient-related variables (age, sex, performance score, diagnosis, and disease stage at transplantation), donor-related variables (donor-recipient birth order, donor–recipient sex match, donor–recipient cytomegalovirus serology), and transplant-related variables (total body irradiation–TBI - containing vs non-TBI containing conditioning, total nucleated cell dose, GVHD prophylaxis, time from diagnosis to transplant and transplant time period). All clinical variables were tested for the affirmation of the proportional hazards assumption. A stepwise forward model selection procedure was used to select adjusted clinical variables for each outcome with a threshold of 0.05 for both entry and stay. The center was adjusted as a random effect. Interactions between the main variable and adjusted covariates were tested and none were detected at 0.01 significance level. To adjust for multiple testing, a 2-sided p-value of <0.01 was considered statistically significant. When the overall p-value of our main testing variable (i.e., patient age) was <0.01 for an endpoint, a threshold of p-value< 0.05/3=0.016 was used for significance of a particular comparison. Analysis was performed using SAS version 9.3 (SAS Institute, Cary, IN).
Results
A total of 476 patients, from 101 centers, met inclusion criteria. Median age of the recipients at time of HCT was 10.1 years. Forty seven percent had AML in 1st CR, 8% AML in 2nd CR, 21% ALL in 1st CR, and 24% ALL in 2nd CR. Using grade III-IV aGVHD as the primary outcome, we identified two cut points, 2 years and 13 years, and used them to define 3 groups for recipient age: < 2 years (n=60), 2 to 12 (n=255) years and 13–17 years (n=162). The majority (73%) of patients received cyclosporine and Methotrexate for GVHD prophylaxis, with similar frequencies across the three age groups. In the youngest age group (<2 years), 76% of patients received busulfan based conditioning, whereas roughly half of the patients in the other age groups received TBI-based conditioning. Total nucleated cell (TNC) dose was higher for the youngest age group. Among the <2 year age group, 41% received a TNC dose >5 × 108/kg, whereas 14% of patients age 2–12 years and 6% of patients 13–17 years received that dose. As expected, donor and recipient age were highly correlated. Baseline patient, donor and transplant characteristics are shown in table 1.
Table 1.
Characteristics of patients younger than 18 who underwent myeloablative allogeneic transplant for AML, ALL with calcineurin-inhibitor based GVHD prophylaxis and an HLA-identical sibling donor between 2000–2013, as reported to the CIBMTR.
| Characteristic | < 2 | 2 – 12 | 13 – 17 | P-Value |
|---|---|---|---|---|
| Number of patients | 59 | 255 | 162 | |
| Number of centers | 36 | 71 | 67 | |
| Patient-related | ||||
| Recipient age at transplant, years, median (range) | 1 (<1–2) | 8 (2–13) | 16 (13–17) | <0.001 |
| Gender | 0.06 | |||
| Male | 36 (61) | 128 (50) | 99 (61) | |
| Female | 23 (39) | 127 (50) | 63 (39) | |
| Recipient race | 0.04 | |||
| Caucasian | 47 (78) | 211 (83) | 139 (86) | |
| African-American | 5 (8) | 5 (2) | 4 (2) | |
| Asian/Pacific Islander | 2 (3) | 13 (5) | 9 (6) | |
| Others | 4 (8) | 26 (10) | 10 (6) | |
| Missing | 1 (2) | 0 | 0 | |
| Recipient ethnicity | 0.006 | |||
| Hispanic | 11 (19) | 34 (13) | 14 (9) | |
| Non-Hispanic | 27 (46) | 132 (52) | 62 (38) | |
| Not applicable; non-resident of US | 16 (27) | 77 (30) | 76 (47) | |
| Missing | 5 (9) | 12 (5) | 10 (6) | |
| Karnofsky score prior to transplant | 0.001 | |||
| < 90 | 3 (5) | 17 (7) | 19 (12) | |
| ≥ 90 | 51 (86) | 234 (92) | 142 (88) | |
| Missing | 5 (8) | 4 (2) | 1 (<1) | |
| Disease/CR status at transplant | <0.001 | |||
| AML–CR1 | 41 (69) | 114 (45) | 68 (42) | |
| AML–CR2 | 0 | 19 (7) | 19 (12) | |
| ALL–CR1 | 14 (24) | 42 (16) | 43 (27) | |
| ALL–CR2 | 4 (7) | 80 (31) | 32 (20) | |
| Donor-related | ||||
| HLA-id sib donor age, years, median (range) | 5 (<1–23) | 9 (<1–26) | 15 (3–29) | <0.001 |
| HLA-id sib donor age at transplant, years | <0.001 | |||
| < 5 | 27 (46) | 48 (19) | 4 (2) | |
| 5–9 | 24 (41) | 91 (36) | 19 (12) | |
| 10–14 | 3 (5) | 65 (25) | 55 (34) | |
| 15–17 | 0 | 26 (10) | 19 (12) | |
| 18+ | 0 | 21 (8) | 62 (38) | |
| Missing | 5 (8) | 4 (2) | 3 (2) | |
| Donor-recipient age difference, years, median (range) * | 4 (<1–22) | 2 (−9–18) | −1 (−14–13) | <0.001 |
| Donor older than recipient? | <0.001 | |||
| Yes | 55 (93) | 159 (62) | 76 (47) | |
| No | 3 (5) | 95 (37) | 83 (51) | |
| Missing | 1 (2) | 1 (<1) | 3 (2) | |
| Donor-recipient CMV status | 0.02 | |||
| −/− | 14 (24) | 94 (37) | 81 (50) | |
| −/+ | 5 (8) | 20 (8) | 7 (4) | |
| +/− | 18 (31) | 57 (22) | 27 (17) | |
| +/+ | 22 (37) | 76 (30) | 42 (26) | |
| Missing | 0 | 8 (3) | 5 (3) | |
| Donor-recipient gender match | 0.19 | |||
| M/M | 22 (37) | 70 (27) | 46 (28) | |
| M/F | 12 (20) | 65 (25) | 31 (19) | |
| F/M | 14 (24) | 58 (23) | 53 (33) | |
| F/F | 11 (19) | 62 (24) | 32 (20) | |
| Donor-recipient ABO mismatch | 0.97 | |||
| Matched | 41 (69) | 171 (67) | 109 (67) | |
| Minor mismatch | 6 (10) | 32 (13) | 23 (14) | |
| Major mismatch | 9 (15) | 34 (13) | 22 (14) | |
| Bidirectional mismatch | 1 (2) | 9 (4) | 5 (3) | |
| Missing | 2 (3) | 9 (4) | 3 (2) | |
| Transplant-related | ||||
| TNC pre-cryo dose, 108/kg, median (range) | 5 (<1–67) | 3 (<1–50) | 3 (<1–92) | <0.001 |
| TNC pre-cryo dose, 108/kg | <0.001 | |||
| < 3 | 8 (14) | 98 (38) | 93 (57) | |
| ≥3 | 46 (78) | 137 (54) | 54 (33) | |
| Missing | 5 (8) | 20 (8) | 15 (9) | |
| Conditioning regimen | <0.001 | |||
| TBI-based | 14 (24) | 135 (52) | 84 (52) | |
| BU-based | 45 (76) | 120 (47) | 78 (48) | |
| GVHD prophylaxis | 0.20 | |||
| CSA + MTX ± others | 43 (73) | 183 (72) | 122 (75) | |
| CSA ± others | 12 (20) | 32 (13) | 14 (9) | |
| Tac + MTX ± others | 4 (7) | 31 (12) | 20 (12) | |
| Missing | 0 | 9 (4) | 6 (4) | |
| Year of transplant | 0.47 | |||
| 2000–2004 | 28 (47) | 135 (53) | 84 (52) | |
| 2005–2008 | 17 (29) | 82 (32) | 46 (28) | |
| 2009–2013 | 14 (24) | 38 (15) | 32 (20) | |
| Time from diagnosis to HCT, months, median (range) | 4 (2–14) | 6 (2–103) | 5 (2–130) | <0.001 |
| Time from diagnosis to HCT, months | <0.001 | |||
| < 6 | 52 (88) | 129 (51) | 99 (61) | |
| 6 – <12 | 6 (10) | 32 (13) | 17 (10) | |
| ≥12 | 1 (2) | 94 (37) | 46 (28) | |
| Follow-up of survivors, months, median (range) | 75 (3–168) | 89 (3–172) | 73 (3–170) | |
Abbreviations: CR = Complete Remission, TNC = Total Nucleated Cell, CY = Cyclophosphamide, TBI = Total Body Irradiation, BU = Busulfan, FLU = Fludarabine, CSA = Cyclosporine, MTX = Methotrexate, Tac = Tacrolimus.
: Donor-Recipient age difference is measured as donor age – recipient age (a value of “1.0” indicates the donor is one year older than the recipient, whereas a value of “−1.0” indicates the recipient is 1 year older than the donor).
Acute Graft Versus Host Disease
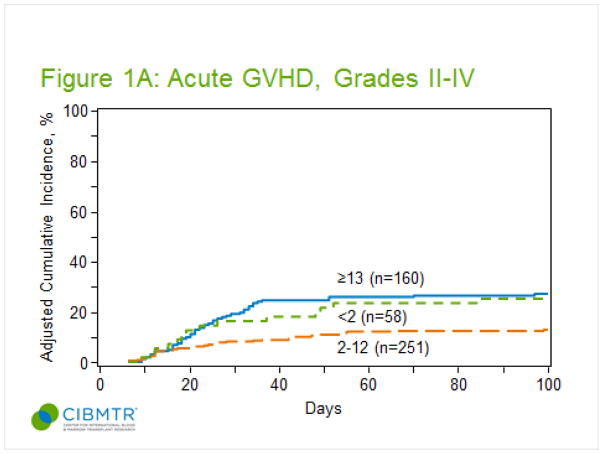
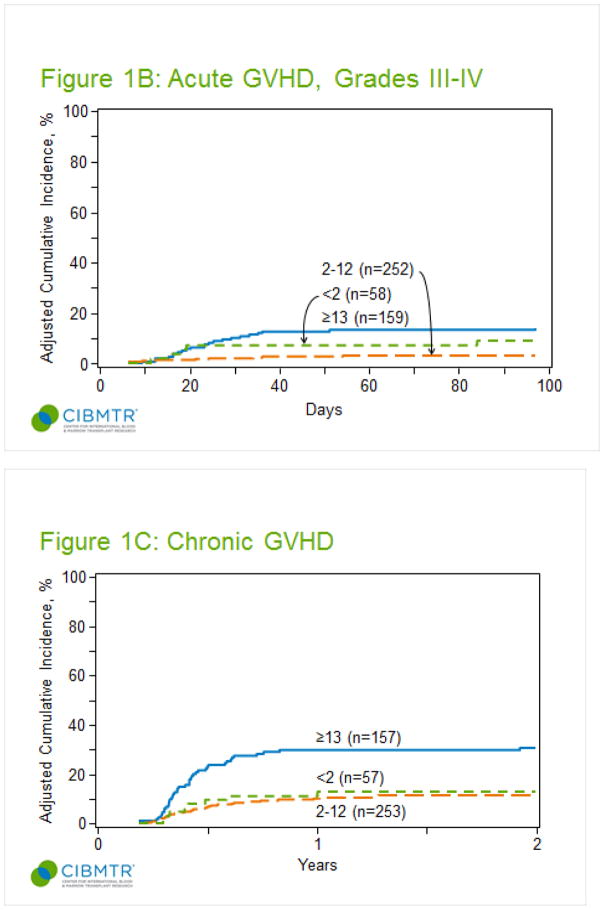
The cumulative incidence of grade II-IV aGVHD for all patients at 100 days post-transplant was 19% (95% confidence interval, CI, 16–23%). As shown in figure 1A, children between 2–12 years at the time of transplant developed significantly less grade II-IV aGVHD (13%) compared to children younger than 2 years (24%) or adolescents older than 13 years (28%, p<0.001). The majority of patients developed grade II-IV aGVHD in the first month post-transplant, and median day of onset did not differ by age (<2, day 21; 2–12, day 24; >=13, day 24). Target organ involvement did not differ by age (supplemental table 1). Out of patients with grade II-IV aGVHD, the proportion of patients with gut GVHD did not differ by age (<2, 8/14; 2–12, 18/35; >=13, 28/45). Multivariate analysis confirmed the protective effect of age 2–12 years (table 2). After adjusting for GVHD prophylaxis regimen, Karnofsky score, and year of transplant, children aged 2–12 were at significantly less risk for grade II-IV aGVHD (HR 0.42, CI 0.26–0.7, p=0.0008) compared to children 13 years or older. Interestingly, children younger than 2 years were also at less risk for grade II-IV aGVHD (HR 0.54, CI 0.26–1.08, p=0.083) after adjustment for these other risk factors, but this result was not statistically significant. The multivariate analysis confirmed the significant effect of other risk factors on the development of grade II-IV aGVHD. Children who received prophylaxis with Cyclosporine +/− other agents (71% CSA only, 17% CSA + mycophenolate, 12% CSA + steroids) had a higher risk compared to those receiving cyclosporine and methotrexate (HR 3.21, CI 1.77–5.83, p =0.0001), but the numbers within each category where too small for further analysis. Risk of grade II-IV GVHD did not differ between CSA/MTX ± other and Tac/MTX ± other prophylaxis. A higher Karnofsky score (>=90) was associated with a reduced risk of developing aGVHD (HR 0.36, CI 0.19–0.65, p =0.0008). Finally, children transplanted after 2004 were at significantly less risk for developing aGVHD (2005–2008, HR 0.36, CI 0.2–0.65, p=0.0007; HCT after 2009, HR 0.24, CI 0.11–0.53, p=0.0004).
Figure 1.
Cumulative incidence of acute GVHD by age group. (A) Cumulative incidence of grade II-IV. (B) Cumulative incidence of grade III-IV. (C) Cumulative incidence of chronic GVHD at 1 year by age group.
Table 2.
Multivariate analysis for grade II–IV acute GVHD and chronic GVHD.
| Factor | n | HR (95% CI) | P value |
|---|---|---|---|
| Grade II-IV acute GVHD | |||
|
| |||
| Recipient age | 0.0032 | ||
| 13–17 | 160 | 1.00* | |
| 2–12 | 251 | 0.42 (0.26 – 0.70) | 0.0008 |
| <2 | 58 | 0.54 (0.26 – 1.08) | 0.083 |
| GVHD prophylaxis# | 0.0018 | ||
| CSA + MTX ± others | 342 | 1.00* | |
| CSA ± others | 57 | 3.21 (1.77 – 5.83) | 0.0001 |
| Tac + MTX ± others | 55 | 1.17 (0.49 – 2.78) | 0.73 |
| missing | 15 | 1.62 (0.57–4.63) | 0.36 |
| Karnofsky score | 0.0006 | ||
| < 90 | 38 | 1.00* | |
| >= 90 | 421 | 0.36 (0.19 – 0.65) | 0.0008 |
| missing | 10 | 1.10 (0.36–3.35) | 0.86 |
| Year of transplant# | <.0001 | ||
| 2000–2004 | 242 | 1.00* | |
| 2005–2008 | 143 | 0.36 (0.20 – 0.65) | 0.0007 |
| 2009–2013 | 84 | 0.24 (0.11 – 0.53) | 0.0004 |
|
| |||
| Chronic GVHD | |||
|
| |||
| Recipient age | 0.0001 | ||
| 13–17 | 154 | 1.00* | |
| 2 – 12 | 253 | 0.32 (0.19 – 0.54) | <.0001 |
| <2 | 56 | 0.36 (0.16–0.82) | 0.0156 |
| D-R birth order | 0.0014 | ||
| Older donor | 282 | 1.00* | |
| Younger donor | 181 | 0.43 (0.26–0.72) | 0.0014 |
| GVHD prophylaxis# | 0.0076 | ||
| CSA + MTX ± others | 340 | 1.00* | |
| CSA ± others | 56 | 2.35 (1.18–4.70) | 0.015 |
| Tac + MTX ± others | 53 | 2.40 (1.22–4.74) | 0.012 |
| missing | 14 | 2.76 (1.12–6.82) | 0.028 |
Reference group
Abbreviations: CSA = Cyclosporine, MTX = Methotrexate, Tac = Tacrolimus.
The cumulative incidence of grade III-IV aGVHD at 100 days for all patients was 7% (95% CI 5–10%). As shown in figure 1B, children between the ages of 2–12 years developed significantly less grade III-IV aGVHD (3%), compared to children younger than 2 years (9%) and older than 13 years (14%, p <0.001). Multivariate analysis confirmed the protective effect of age 2–12 years. After adjusting for year of transplant, children aged 2–12 years had significantly less risk for grade III-IV aGVHD (HR 0.23, CI 0.1–0.54, p =0.001) compared to children 13 years or older. Children younger than 2 years had a lower risk, but that result was not statistically significant (HR 0.62, CI 0.23–1.71, p =0.35). Similar to the findings for grade II-IV aGVHD, children transplanted after 2004 were at significantly less risk for developing grade III-IV aGVHD (2005–2008, HR 0.23, CI 0.08–0.65, p=0.0056; HCT after 2009, HR 0.16, CI 0.04–0.67, p=0.0126).
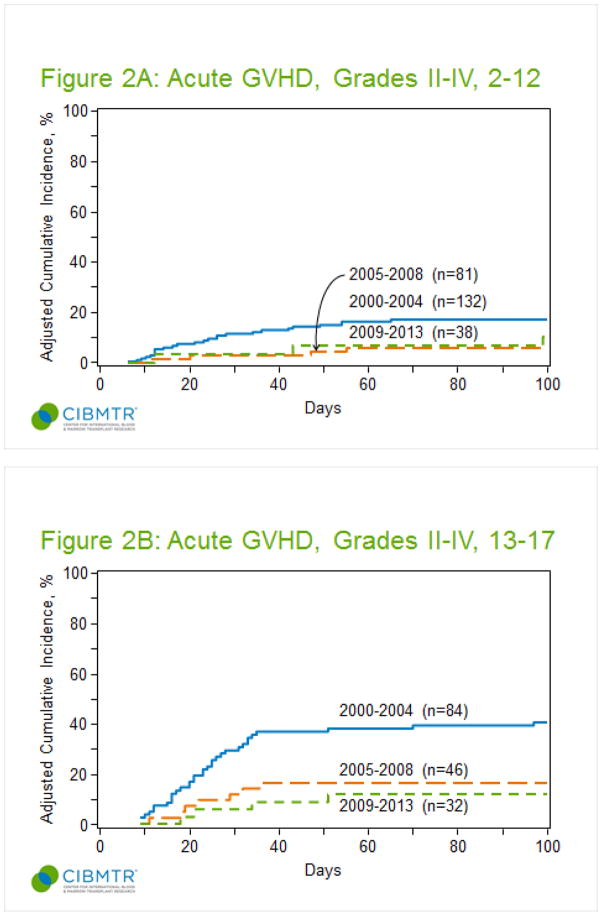
We performed additional analyses in an attempt to identify changes that could have mediated the influence of time period on aGVHD risk. The number of transplants captured decreased over time (247, 2000–2004; 145, 2005–2008; 84, 2009–2013–supplement table 2). The proportion of AML transplants increased (46%, 56% and 81% in the time periods 2000–2004, 2005–2008, and 2009–2013 respectively, p= <0.001) and the proportion of patients receiving TBI decreased over time (57%, 46% and 27%, p=<0.001). GVHD prophylaxis differed across the three time periods (p=<0.001), with increased use of tacrolimus (3%, 16% and 29% respectively). Only 2 out of 84 transplanted patients between 2009–2013 developed grade III-IV aGVHD, and after 2004, there were no reported cases of severe aGVHD among patients 2–12 years (figure 2). Excluding time period from the analysis did not alter the associations between other variables and grade II-IV aGVHD risk (supplement table 3). A subset analysis limited to patients receiving CSA/MTX ± other showed a persistent influence of time period on risk of grade II-IV and III-IV aGVHD (supplement tables 4). Constructing the model with a less stringent p-value of 0.2 showed consistent results with regards to effect of recipient age (data not shown).
Figure 2.
Cumulative incidence of grade II-IV acute GVHD by time period. (A) Patients 2–12 years. (B) Patients 13–17 years.
Chronic Graft Versus Host Disease
The cumulative incidence of cGVHD at 1 year post-transplant for all age groups was 16% (95% CI 13–20%). As shown in figure 1C, it was lowest for children 2–12 years (10%), with a similar incidence in children younger than 2 years (15%), and the highest incidence observed in children 13 years or older (27%, p <0.001). Fifty percent of patients were diagnosed within the first 6 months and almost all patients were diagnosed within the first year post transplant. Among cGVHD cases, the majority of patients <13 years had mild disease, while 51% of patients 13–17 years had moderate - severe disease (supplement table 3).
Multivariate analysis confirmed the protective effect of age (table 2). Children 2–12 years were at significantly lower risk for cGVHD (HR 0.32, CI 0.19–0.54, P <0.001) compared to children 13 years or older. Children <2 years were also at less risk (HR 0.36, CI 0.16–0.82, p=0.0156), but the result was not statistically significant. GVHD prophylaxis was also important; compared to CSA/MTX ± others, tacrolimus based regimens and the CSA ± others had a HR of 2.35 (CI 1.18–4.7, p=0.015) and 2.40 (CI 1.22–4.7, p=0.013), respectively (table 2).
The use of a donor younger than the recipient significantly decreased the risk of cGVHD (HR 0.43, 95% CI 0.26–0.72, p= 0.0014). Having a younger donor significantly decreased the risk in patients 13–17 years (HR 0.42, 95% CI 0.22–0.80, p=0.0086), but not 2–12 years (HR 0.39, 95% CI 0.13–1.21, p=0.1045). In the 13–17 age group, the protective effect of a younger donor was not explained by recipient age, as the median recipient age was 16 years (range 13–17) for both patients with younger and older donors. In the 2–12 age group, median recipient age was 10 (range 3–12) for patients with younger donors and 7 (3–12) for patients with older donors.
Survival
OS was 81% (95% CI 78–85%) at 1 year post transplant. TRM was 2% (95% CI 1–3%) at 100 days and 4% (95% CI 3–6%) at 1 year. In multivariate analyses (table 3), there was no difference in OS, relapse or leukemia-free survival between the age groups. Analysis of TRM revealed a non-statistically significant decrease in risk for patients 2–12 years (HR 0.34, 95% CI 0.15–0.77, p=0.01). These analyses did not reveal an impact for other patient, donor, or transplant risk factors.
Table 3.
Effect of age on transplant outcomes in multivariate analyses
| Factor | n | HR (95% CI) | P value |
|---|---|---|---|
| Overall Survival | |||
|
| |||
| Recipient age | 0.48 | ||
| 13–17 | 162 | 1.00* | |
| 2–12 | 255 | 0.83 (0.56–1.21) | 0.33 |
| <2 | 59 | 1.06 (0.62–1.80) | 0.83 |
|
| |||
| TRM | |||
|
| |||
| Recipient age | 0.02 | ||
| 13–17 | 159 | 1.00* | |
| 2–12 | 248 | 0.34 (0.15–0.77) | 0.01 |
| <2 | 57 | 1.04 (0.40–2.68) | 0.93 |
|
| |||
| LFS | |||
|
| |||
| Recipient age | 0.21 | ||
| 13–17 | 159 | 1.00* | |
| 2–12 | 248 | 0.89 (0.63–1.26) | 0.51 |
| <2 | 57 | 1.31 (0.82–2.09) | 0.26 |
|
| |||
| Relapse | |||
|
| |||
| Recipient age | 0.43 | ||
| 13–17 | 159 | 1.00* | |
| 2–12 | 248 | 1.09 (0.74–1.61) | 0.67 |
| <2 | 57 | 1.41 (0.83–2.41) | 0.20 |
Reference group
Abbreviations: TRM = Transplant related mortality, LFS = Leukemia free survival.
Discussion
Our analysis of GVHD risk in pediatric patients receiving MSD bone marrow transplantation (BMT) for acute leukemia, most of whom received a calcineurin inhibitor with methotrexate for prophylaxis, underscores the important influence of age. Our results indicate that children 2–12 are at very low risk for GVHD. In this age group, the cumulative incidence of grade III-IV aGVHD was only 3% and cGVHD, most of which was mild, was only 8%. Remarkably, in our dataset, there were no cases of grade III-IV in this age group out of 120 HCT performed after 2004. It is important to note that the vast majority of patients in our sample were drawn from North America. Our results, therefore, may not be applicable to European centers, where the use of cyclosporine alone for MSD transplantation for children with acute leukemia is standard.(15) Our data set was not large enough to accommodate a validation set.
Other studies have shown that recipient age is a risk factor for GVHD. (8–11) The goal of this study was to identify a group of children at minimal risk for GVHD in the setting of MSD BMT, using statistical methods. The impetus for doing so comes from research demonstrating an association between the intensity of calcineurin inhibitor immune suppression, whether gauged by the number of agents combined with the calcineurin inhibitor, the dose or the duration of the calcineurin inhibitor, and relapse. Conversely, reducing the intensity of immune suppression can decrease the risk for relapse.(3, 5, 6, 16, 17) We reasoned that strategies to promote a graft versus leukemia (GVL) effect through lessening immunosuppression intensity in children would be optimized by applying them to patients who have the lowest risk of GVHD.
One such strategy that deserves further assessment is using cyclosporine alone. Recently published, non-comparative experience in children suggests that the European strategy of using cyclosporine alone may effectively augment the GVL effect without substantively raising the risk for GVHD and TRM.(18) This observation, however, should be confirmed in a prospective, comparative study, so that the potential of this strategy for increasing the incidence of GVHD and TRM as well as its potential to reduce relapse can be rigorously evaluated. Importantly, the possible risk of increasing TRM should be closely scrutinized. In our analysis, children 13–17 years of age had similar aGVHD incidence to that reported in adults undergoing MSD BMT for leukemia.(19) In a randomized trial of low versus standard dose cyclosporine as a single agent for GVHD prophylaxis, in children and adults, lower cyclosporine dose was associated with increased transplant related complications in patients older than 30 years, but was well tolerated in younger patients, without increase in TRM.(4) This further emphasizes the need to define a candidate age group for a large, multicenter randomized controlled trial, where reduction in GVHD prophylaxis through a calcineurin inhibitor only approach, could safely be applied to capitalize on the reduction in relapse observed in the European studies, while preserving a low TRM rate in MSD BMT.
While it is unclear why the risk for relapse in the 2 to 12 year group was not increased given the very low risk for GVHD, it is important to keep two things in mind. First, the GVL effect of allogeneic transplantation is only partly mediated by GVHD. This is especially true in AML (the majority of our sample), where the contribution of GVHD to the GVL effect is modest.(20) Secondly, our sample was not well suited for comparing relapse across age groups as it was heterogeneous with respect to disease and remission status. Moreover, we did not consider important determinants of relapse, like minimal residual disease, or other prognostic factors delineating age related differences in leukemia biology.
As in a similar CIBMTR study of aGVHD risk in adults, we only assessed the influence of recipient age.(21) Although increased donor age has been shown to be a risk factor for GVHD in two previous pediatric studies, both including patients with non-malignant as well as malignant diseases and one including unrelated as well as related donors (10, 11), in our sample, donor and recipient age were too closely associated to discriminate their effects. We were, however, able to validate previously reported single center and CIBMTR results on the effect of birth order in MSD HCT, where a donor younger than the recipient favorably impacted risk of acute and chronic GVHD. (22, 23) In our analysis, adolescents receiving grafts from younger siblings were at decreased risk for cGVHD.
The incidence of GVHD in patients <2 years was unexpectedly higher than in those between 2 and 12 years. This finding needs to be interpreted cautiously, as it was driven by statistical methods, and thus may be related to the low sample size of only 59 patients <2 years in our cohort. However, it is conceivable that graft T cell doses, data we lacked, are higher in this group, which received the highest total nucleated cell dose doses at transplant.(24, 25) Further, larger studies will be needed to determine if young age is an independent risk factor for developing GVHD.
There was an impressive decrease in the rates of aGVHD observed over time. This is consistent with results of a CIBMTR study of unrelated donor HCT in children with leukemia.(26) In that study, including transplants performed between 1990 and 2003, risk of aGVHD was reduced after 1999. In our study, more recent time periods included a higher proportion of patients with AML, receiving non-TBI conditioning, and an increasing use of tacrolimus-based prophylaxis. The increased use of tacrolimus over time did not explain this finding, as we observed a similar effect for time period in a subgroup analysis of patients receiving cyclosporine prophylaxis. Time period was not a surrogate for above mentioned changes in practice, as excluding time period from the analysis did not bring forth any other significant factors. It is possible that other unaccounted for practice changes, such as more vigilant monitoring of trough levels, could explain the drop in risk.
In summary, this study identified a subgroup of children receiving MSD HCT for leukemia who are at low risk for GVHD, but these results should be validated before they can be employed in a clinical trial. Subsequent studies could build on these results by investigating the use of attenuated GVHD prophylaxis in this group and assessing impact on relapse through potentially promoting a GVL effect. Such an approach should be applied within the confines of a clinical trial, and could be combined with predictive biomarker parameters (27) to identify patients at higher risk of developing aGVHD and TRM.
Highlights.
Relapse remains the major cause of mortality of hematopoietic cell transplantation for pediatric acute leukemia
The intensity of immunosuppression may affect the risk of relapse
Acute graft versus host disease rates have decreased significantly over time among matched sibling donor transplants
Children 2–12 years are at very low risk for acute and chronic graft versus host disease thus may be good candidates for trials of reduced prophylaxis.
These findings should be validated before clinical application
Acknowledgments
The CIBMTR is supported primarily by Public Health Service Grant/Cooperative Agreement 5U24-CA076518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); a Grant/Cooperative Agreement 5U10HL069294 from NHLBI and NCI; a contract HHSH250201200016C with Health Resources and Services Administration (HRSA/DHHS); two Grants N00014-15-1-0848 and N00014-16-1-2020 from the Office of Naval Research; and grants from * Actinium Pharmaceuticals, Inc.; Alexion; * Amgen, Inc.; Anonymous donation to the Medical College of Wisconsin; Astellas Pharma US; AstraZeneca; Atara Biotherapeutics, Inc.; Be the Match Foundation; * Bluebird Bio, Inc.; * Bristol Myers Squibb Oncology; * Celgene Corporation; Cellular Dynamics International, Inc.; Cerus Corporation; * Chimerix, Inc.; Fred Hutchinson Cancer Research Center; Gamida Cell Ltd.; Genentech, Inc.; Genzyme Corporation; Gilead Sciences, Inc.; Health Research, Inc. Roswell Park Cancer Institute; HistoGenetics, Inc.; Incyte Corporation; Janssen Scientific Affairs, LLC; * Jazz Pharmaceuticals, Inc.; Jeff Gordon Children’s Foundation; The Leukemia & Lymphoma Society; Medac, GmbH; MedImmune; The Medical College of Wisconsin; * Merck & Co, Inc.; * Mesoblast; MesoScale Diagnostics, Inc.; * Miltenyi Biotec, Inc.; National Marrow Donor Program; Neovii Biotech NA, Inc.; Novartis Pharmaceuticals Corporation; Onyx Pharmaceuticals; Optum Healthcare Solutions, Inc.; Otsuka America Pharmaceutical, Inc.; Otsuka Pharmaceutical Co, Ltd. – Japan; PCORI; Perkin Elmer, Inc.; Pfizer, Inc; * Sanofi US; * Seattle Genetics; * Spectrum Pharmaceuticals, Inc.; St. Baldrick’s Foundation; * Sunesis Pharmaceuticals, Inc.; Swedish Orphan Biovitrum, Inc.; Takeda Oncology; Telomere Diagnostics, Inc.; University of Minnesota; and * Wellpoint, Inc. This work is supported in part by the National Center for Advancing Translational Sciences of the National Institutes of Health UL1TR000454 and KL2TR000455 (MQ)
Footnotes
Corporate Members
Poster presentation at the American Society of Hematology annual meeting, 2016
Disclaimer: The views expressed in this article do not reflect the official policy or position of the National Institute of Health, the Department of the Navy, the Department of Defense, Health Resources and Services Administration (HRSA) or any other agency of the U.S. Government.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Horan JT, Alonzo TA, Lyman GH, Gerbing RB, Lange BJ, Ravindranath Y, et al. Impact of disease risk on efficacy of matched related bone marrow transplantation for pediatric acute myeloid leukemia: the Children’s Oncology Group. J Clin Oncol. 2008 Dec 10;26(35):5797–801. doi: 10.1200/JCO.2007.13.5244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Horan JT, Logan BR, Agovi-Johnson MA, Lazarus HM, Bacigalupo AA, Ballen KK, et al. Reducing the risk for transplantation-related mortality after allogeneic hematopoietic cell transplantation: how much progress has been made? J Clin Oncol. 2011 Mar 1;29(7):805–13. doi: 10.1200/JCO.2010.32.5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pulsipher MA, Langholz B, Wall DA, Schultz KR, Bunin N, Carroll WL, et al. The addition of sirolimus to tacrolimus/methotrexate GVHD prophylaxis in children with ALL: a phase 3 Children’s Oncology Group/Pediatric Blood and Marrow Transplant Consortium trial. Blood. 2014 Mar 27;123(13):2017–25. doi: 10.1182/blood-2013-10-534297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bacigalupo A, Lamparelli T, Gualandi F, Bregante S, Raiola A, di Grazia C, et al. Increased risk of leukemia relapse with high dose cyclosporine after allogeneic marrow transplantation for acute leukemia: 10 year follow-up of a randomized study. Blood. 2001;98(10):3174. doi: 10.1182/blood.v98.10.3174. [DOI] [PubMed] [Google Scholar]
- 5.Bacigalupo A, Van Lint MT, Occhini D, Gualandi F, Lamparelli T, Sogno G, et al. Increased risk of leukemia relapse with high-dose cyclosporine A after allogeneic marrow transplantation for acute leukemia. Blood. 1991 Apr 1;77(7):1423–8. [PubMed] [Google Scholar]
- 6.Locatelli F, Zecca M, Rondelli R, Bonetti F, Dini G, Prete A, et al. Graft versus host disease prophylaxis with low-dose cyclosporine-A reduces the risk of relapse in children with acute leukemia given HLA-identical sibling bone marrow transplantation: results of a randomized trial. Blood. 2000 Mar 1;95(5):1572–9. [PubMed] [Google Scholar]
- 7.Storb R, Deeg HJ, Pepe M, Appelbaum F, Anasetti C, Beatty P, et al. Methotrexate and cyclosporine versus cyclosporine alone for prophylaxis of graft-versus-host disease in patients given HLA-identical marrow grafts for leukemia: long-term follow-up of a controlled trial. Blood. 1989 May 1;73(6):1729–34. [PubMed] [Google Scholar]
- 8.Nash RA, Pepe MS, Storb R, Longton G, Pettinger M, Anasetti C, et al. Acute graft-versus-host disease: analysis of risk factors after allogeneic marrow transplantation and prophylaxis with cyclosporine and methotrexate. Blood. 1992 Oct 01;80(7):1838–45. [PubMed] [Google Scholar]
- 9.Weisdorf D, Hakke R, Blazar B, Miller W, McGlave P, Ramsay N, et al. Risk factors for acute graft-versus-host disease in histocompatible donor bone marrow transplantation. Transplantation. 1991 Jun;51(6):1197–203. doi: 10.1097/00007890-199106000-00010. [DOI] [PubMed] [Google Scholar]
- 10.Eisner MD, August CS. Impact of donor and recipient characteristics on the development of acute and chronic graft-versus-host disease following pediatric bone marrow transplantation. Bone marrow transplantation. 1995 May;15(5):663–8. [PubMed] [Google Scholar]
- 11.Zecca M, Prete A, Rondelli R, Lanino E, Balduzzi A, Messina C, et al. Chronic graft-versus-host disease in children: incidence, risk factors, and impact on outcome. Blood. 2002 Aug 15;100(4):1192–200. doi: 10.1182/blood-2001-11-0059. [DOI] [PubMed] [Google Scholar]
- 12.Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone marrow transplantation. 1995 Jun;15(6):825–8. [PubMed] [Google Scholar]
- 13.Shulman HM, Sullivan KM, Weiden PL, McDonald GB, Striker GE, Sale GE, et al. Chronic graft-versus-host syndrome in man. A long-term clinicopathologic study of 20 Seattle patients. Am J Med. 1980 Aug;69(2):204–17. doi: 10.1016/0002-9343(80)90380-0. [DOI] [PubMed] [Google Scholar]
- 14.Lee SJ, Klein JP, Barrett AJ, Ringden O, Antin JH, Cahn JY, et al. Severity of chronic graft-versus-host disease: association with treatment-related mortality and relapse. Blood. 2002 Jul 15;100(2):406–14. doi: 10.1182/blood.v100.2.406. [DOI] [PubMed] [Google Scholar]
- 15.Schrauder A, von Stackelberg A, Schrappe M, Cornish J, Peters C, Group A-BS, et al. Allogeneic hematopoietic SCT in children with ALL: current concepts of ongoing prospective SCT trials. Bone marrow transplantation. 2008 Jun;41( Suppl 2):S71–4. doi: 10.1038/bmt.2008.58. [DOI] [PubMed] [Google Scholar]
- 16.Ringden O, Horowitz MM, Sondel P, Gale RP, Biggs JC, Champlin RE, et al. Methotrexate, cyclosporine, or both to prevent graft-versus-host disease after HLA-identical sibling bone marrow transplants for early leukemia? Blood. 1993 Feb 15;81(4):1094–101. [PubMed] [Google Scholar]
- 17.Inamoto Y, Flowers ME, Lee SJ, Carpenter PA, Warren EH, Deeg HJ, et al. Influence of immunosuppressive treatment on risk of recurrent malignancy after allogeneic hematopoietic cell transplantation. Blood. 2011 Jul 14;118(2):456–63. doi: 10.1182/blood-2011-01-330217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bleyzac N, Cuzzubbo D, Renard C, Garnier N, Dubois V, Domenech C, et al. Improved outcome of children transplanted for high-risk leukemia by using a new strategy of cyclosporine-based GVHD prophylaxis. Bone marrow transplantation. 2016 May;51(5):698–704. doi: 10.1038/bmt.2015.350. [DOI] [PubMed] [Google Scholar]
- 19.Saber W, Opie S, Rizzo JD, Zhang M-J, Horowitz MM, Schriber J. Outcomes after matched unrelated donor versus identical sibling hematopoietic cell transplantation in adults with acute myelogenous leukemia. Blood. 2012;119(17):3908–16. doi: 10.1182/blood-2011-09-381699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Horowitz MM, Gale RP, Sondel PM, Goldman JM, Kersey J, Kolb HJ, et al. Graft-versus-leukemia reactions after bone marrow transplantation. Blood. 1990 Feb 01;75(3):555–62. [PubMed] [Google Scholar]
- 21.Hahn T, McCarthy PL, Jr, Zhang MJ, Wang D, Arora M, Frangoul H, et al. Risk factors for acute graft-versus-host disease after human leukocyte antigen-identical sibling transplants for adults with leukemia. J Clin Oncol. 2008 Dec 10;26(35):5728–34. doi: 10.1200/JCO.2008.17.6545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dobbelstein C, Ahn KW, Haagenson M, Hale GA, van Rood JJ, Miklos D, et al. Birth Order and Transplantation Outcome in HLA-Identical Sibling Stem Cell Transplantation: An Analysis on Behalf of the Center for International Blood and Marrow Transplantation. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2013 Feb 1; doi: 10.1016/j.bbmt.2013.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bucher C, Stern M, Buser A, Heim D, Paulussen M, Halter J, et al. Role of primacy of birth in HLA-identical sibling transplantation. Blood. 2007 Jul 1;110(1):468–9. doi: 10.1182/blood-2007-02-076257. [DOI] [PubMed] [Google Scholar]
- 24.Gaziev J, Isgro A, Marziali M, Daniele N, Gallucci C, Sodani P, et al. Higher CD3(+) and CD34(+) cell doses in the graft increase the incidence of acute GVHD in children receiving BMT for thalassemia. Bone Marrow Transplant. 2012 Jan;47(1):107–14. doi: 10.1038/bmt.2011.3. [DOI] [PubMed] [Google Scholar]
- 25.Remberger M, Mattsson J, Hassan Z, Karlsson N, LeBlanc K, Omazic B, et al. Risk factors for acute graft-versus-host disease grades II-IV after reduced intensity conditioning allogeneic stem cell transplantation with unrelated donors: a single centre study. Bone marrow transplantation. 2008 Feb;41(4):399–405. doi: 10.1038/sj.bmt.1705913. [DOI] [PubMed] [Google Scholar]
- 26.Davies SM, Wang D, Wang T, Arora M, Ringden O, Anasetti C, et al. Recent decrease in acute graft-versus-host disease in children with leukemia receiving unrelated donor bone marrow transplants. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2009 Mar;15(3):360–6. doi: 10.1016/j.bbmt.2008.12.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hartwell MJ, Ozbek U, Holler E, Renteria AS, Major-Monfried H, Reddy P, et al. An early-biomarker algorithm predicts lethal graft-versus-host disease and survival. JCI Insight. 2017 Feb 09;2(3):e89798. doi: 10.1172/jci.insight.89798. [DOI] [PMC free article] [PubMed] [Google Scholar]