Abstract
Background
There is increasing demand for musculoskeletal ultrasound (MSKUS) to detect hemophilic joint bleeding, but there is uncertainty regarding blood detection concentration thresholds or if magnetic resonance imaging (MRI) is more accurate.
Aims
Compare the sensitivity of blood detection by MSKUS and MRI.
Methods
Increasing blood concentrations in plasma were imaged with MSKUS and MRI 1–2 hours, 3–4 days and 7 days after blood withdrawal in vitro, and after injection into cadaveric pig joints. Additionally, effusions in the joints of two patients with hemophilia joints were imaged, followed by aspiration. MSKUS was performed using an 8–18 MHz linear transducer; MRI was performed at 3T using T1-weighted and T2-weighted fat-suppressed sequences. Images were reviewed by a hematologist certified in MSKUS and a musculoskeletal radiologist.
Results
MSKUS permitted the detection of blood in vitro and in pig joint spaces at concentrations as low as 5%, demonstrated by the presence of echogenic signals that were absent with plasma alone. In contrast, no differences between fluids were discernible on the T1-weighted or T2-weighted MRI images. Results were confirmed in the two patients with hemophilia. Blood clots demonstrated varying and dynamic echogenicity patterns over time, and, using MRI, were visualized best with T2 sequences.
Conclusion
MSKUS is extremely sensitive in detecting low concentrations of intra-articular blood and in discriminating between bloody and non-bloody fluid, whereas conventional MRI is not. These observations demonstrate the advantages of MSKUS over MRI in detecting intra-articular blood, and show that MSKUS is ideal for rapid bleed detection in the clinic.
Keywords: Blood Clot, Hemophilia, Hemarthrosis, Magnetic Resonance Imaging, Musculoskeletal Ultrasound
Introduction
Hemophilia patients require lifelong clotting factor replacement therapy to mitigate spontaneous joint bleeding and other life threatening bleeding. However, clotting factor replacement therapy is costly and imposes a high financial burden on individuals, healthcare systems and society in general [1].
Therefore, timely objective detection of acute or persistent joint bleeding in hemophilia patients has become increasingly important. [2–5].
Magnetic resonance imaging (MRI) is considered the “gold standard” to detect various abnormalities in hemophilic arthropathy [6]. However, in the past few years, musculoskeletal ultrasound (MSKUS) has emerged as a point-of-care (POC) imaging tool to assess the extent of arthropathic changes [6–9], thus opening new avenues for the management of hemophilic arthropathy [2–5, 10] and also rapid joint bleed detection [2–4, 10]. Recent advances in technology, accessibility, and training have made POC MSKUS an attractive alternative to MRI [11] in instances where imaging is desired. MSKUS is faster, more economical, and without the need of sedation for claustrophobic subjects or children. In addition, MSKUS does not require intravenous contrast to distinguish synovial proliferation from fluid and can also be used to assess synovial vascularity [12–14].
MSKUS appears very adept in detecting joint effusions based on the ability of dynamic maneuvers during scanning. For hemophilia, this feature appears particularly valuable for the detection and management of hemarthrosis, where precise diagnosis of presence or absence of (bloody) effusions can complement patient or physician perception, thereby optimizing targeted treatment options [2–4, 10]. It allows visualization of shifting fluid in communicating spaces as well as sonopalpation. Sonopalpation assesses compressibility and displacement of echogenic intra-articular material. Effusions can be separated into simple versus complex. Complex fluid accumulations are characterized by mixed echogenicity and displaceable speckles, indicating the presence of particulate matter such as proteins or blood products, while simple effusions appear anechoic with clear and serous fluid upon aspiration [15–18]. Thus, MSKUS not only documents the presence of an effusion, but also distinguishes between bloody versus non-bloody effusions based on echogenicity (echogenic versus anechoic) and presence of displaceable echogenic reflectors. In the context of hemophilia, complex effusions with echogenic reflectors can be assumed to represent blood products based on previous documentation of the great accuracy of this approach as documented by joint aspiration [4, 9]. MSKUS algorithms to detect hemarthrosis are therefore well defined, and can be performed quickly as part of the daily clinic routine, thereby fulfilling POC criteria [19]. Moreover, MSKUS enables guided aspiration and fluid analysis as clinically indicated.
In this context, it is noteworthy that radiological MRI criteria to assess blood contents in the joint are less well defined, and mainly derived from previous neurological studies [20, 21]. A preliminary study 30 years ago suggested that MRI may not have the same usefulness for distinguishing between bloody and non-bloody effusions in joints [22]. However, formal studies employing modern imaging technology are lacking, and clinical imaging interpretation algorithms more commonly use inference rather than evidence. Moreover, in daily clinical practice, joint effusions on MRI automatically may be deemed as bloody if arising in the context of hemophilia.
The recent increasing demand to use MSKUS to detect hemophilic joint bleeding in the clinic is paired with the necessity to improve diagnostic accuracy for many new and evolving strategies for the management of hemophilic arthropathy [23]. Together, this provided the incentive to study the appearance of blood products and blood detection concentration thresholds with MSKUS in vitro and in a cadaver model, and determine applicability to human hemophilic joints. We compared MSKUS imaging results with conventional MRI, which is, despite absence of solid evidence, currently considered to be the “gold standard” for blood detection.
Materials and Methods
Blood Collection and Sample Preparation
Whole blood (termed “Blood”) was withdrawn from healthy donors into a syringe without anticoagulant and diluted into normal saline (NS) or into normal pooled plasma (NP) enriched with 2mg/cc high molecular weight hyaluronic acid (Fisher Scientific Company, LLC, Pittsburgh, PA, USA). The following dilutions were prepared: 0, 5, 10, 25, 50, 75, and 100% blood into a final volume of 3–5cc. The diluted blood samples were either contained in 3–6cc syringes or injected into the metatarsophalangeal (MTP) joints of cadaveric pig feet for imaging. Between imaging sessions, the syringes and cadaveric pig feet containing blood dilutions were stored up to 1 week at ≈24°C and ≈4°C, respectively. Approximately 5 repetitions of most dilutions and time points were performed for the in vitro portion of the study. For cadaveric pig feet imaging 2 metatarsal joints per foot (n ≈ 25 feet; 50 joints) were injected to afford 1–2 repetitions of most dilutions and time points.
Sample preparation prior to imaging
The syringes were carefully shaken to obtain homogeneous bloody fluid while not disrupting blood clots. The cadaveric pig feet were removed from the refrigerator and equilibrated to room temperature.
Patients
Patients with hemophilia, age 21 years and older, received joint evaluation with MSKUS during clinic visits as part of their routine care. MSKUS-guided aspiration for effusions was performed if clinically indicated after procedural written consent. Some of the patients participated in an observational study, which permitted additional evaluations with timely correlated MRI. All patients provided written consent for collection of imaging results, clinical patient data, and laboratory results. Patient confidentiality and data acquisition methods were reviewed and approved by the Human Research Protection Program at the University of California, San Diego.
Imaging Protocols
MSKUS
A GE LOGIQ S8 US-module (GE LOGIQ S8, General Electrics, Fairfield, CT, USA) with real-time spatial compound imaging and speckle reduction capabilities, equipped with high frequency 8–18 or 6–15 MHz linear transducers, was employed.
For in vitro experiments, ultrasound imaging of blood dilutions was performed with the transducer aligned in parallel with the syringes in vertical position (Fig 1A). To visualize fluid in the syringes, brightness adjustments required optimization beyond the manufacturer’s recommendations based on imaging fluid in syringes rather than within tissue with gain settings up to 90%. Ultrasound examinations of the cadaveric pig MTP joints were performed in longitudinal and transverse axes. Sonopalpation was used to evaluate compressibility and displacement of the intra-articular material. Imaging was performed shortly after blood collection, at day 1, day 3–4, and day 7 at room temperature. In vitro and cadaveric ultrasound imaging was performed by a medicine resident (SN), trained in the Continuing Medical Education-accredited course “Musculoskeletal Ultrasound in Hemophilia” at the Hemophilia and Thrombosis Treatment Center at University of California, San Diego, with additional 50 hours of practice prior to the initiation of the experiments. All ultrasound studies were supervised by a hematologist (AvD, with 5 years of musculoskeletal US experience) who was formally trained and certified in MSKUS through the American Registry for Diagnostic Medical Sonography (ARDMS).
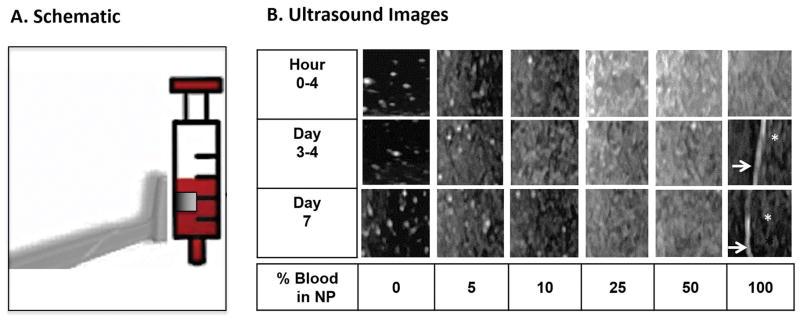
Figure 1. Time course of ultrasound appearance of blood dilutions and clotted blood in vitro.
NP, enriched with 2mg/mL hyaluronic acid, was diluted with increasing concentrations of freshly drawn human blood (3–6cc syringes) and subjected to serial ultrasound imaging at the indicated time points. A. Schematic of the transducer position relative to the syringe. The transducer was aligned in parallel with the syringes in vertical position. The boxed square represents the cropped image used for panel B. B. Ultrasound images. Arrows: Interface between blood clot and serosanguinous fluid. Asterisk: Blood clot abutted by serosanguineous fluid. NP was anechoic, while dilutions with as low as 5% blood generated echogenic signals. Echogenicity increased nonlinearly with increasing blood concentration. Blood clots appeared hypoechoic. As time proceeded, echogenicity of the blood solutions remained relatively unchanged. Hyperechoic speckles visible at 0–10% blood dilutions were generated by non-dissolved hyaluronic acid particles. NP, Normal Pooled Plasma.
For patient imaging, gray scale examinations were performed according to standardized imaging protocols for each joint area as previously described [7]. Transducer positions for the knee comprised 5 distinct views to visualize the suprapatellar recess, femoral trochlear cartilage (sunrise view), medial and lateral patellofemoral gutters, and medial femorotibial space. MSKUS presets as recommended by the manufacturer were used. Echogenicity of tissues was judged by conventional ultrasound criteria as predominantly anechoic, hypo- or hyperechoic in relation to echogenicity of fatty tissue [24]. Sonopalpation was used to evaluate compressibility and displacement of intra-articular material. MSKUS-guided arthrocentesis was performed for effusions. Informed consent was obtained for each procedure. Human knee joint evaluations and aspirations were performed by the trained and certified hematologist (AvD).
MRI
All MR imaging was performed on a 3T scanner (MR750, GE Healthcare, Milwaukee, WI) using dedicated extremity coils. Similarly to MSKUS, experiments included the following time points: shortly after blood collection, at day 1, day 3–4, and day 7 at room temperature.
Included in the field of view (FOV) of all images was a syringe containing pig muscle. The MRI protocol included fast spin-echo (FSE) T1-weighted (repetition time/echo time [TR/TE], 500–750 ms/7–9 ms) and T2-weighted fat-suppressed (TR/TE, 3064–7847 ms/68–70 ms) sequences. For patient imaging, conventional MRI protocols were employed, including axial FSE T1-weighted (TR/TE, 500 ms/9 ms), sagittal FSE T1-weighted (TR/TE, 758 ms/12 ms), and FSE T2-weighted fat-suppressed (TR/TE, 2650-5310 ms/66-77 ms) sequences.
Image Analysis
On MSKUS, the echogenicity of fluid in the syringes, in MTP joints of cadaveric pig feet, and in human joints was qualitatively recorded as anechoic or hypo-/hyper-echoic. Compressibility of spaces upon sonopalpation in human joints and cadaveric pig MTP joints was diagnostic for the presence of fluid. The in vitro images of blood dilutions shortly after blood withdrawal were analyzed on 8-bit grey scale with ImageJ software (National Institutes of Health, Bethesda, MD, USA) to quantify the number of pixels within grey areas corresponding to echogenicity. All images were set in identical windows and subjected to post-processing modifications to ensure congruency of brightness and contrast.
MR images were evaluated by a musculoskeletal radiologist (EYC). The signal intensity of fluid in the syringes, MTP joints of cadaveric pig feet, and human joints were qualitatively recorded as iso-, hypo-, or hyper-intense relative to muscle.
With regard to comparing interpretations of MSKUS and MRI in human joints, the musculoskeletal radiologist (EYC) was blinded to the MSKUS results and independently recorded and interpreted the appearance of intra-articular joint fluid on MRI. Interpretations of in vitro and cadaveric pig feet images obtained with MSKUS were performed by consensus after randomization (EYC, AVD, SN) while blinded to individual results, whereas interpretation of the corresponding randomized MR images was performed by the musculoskeletal radiologist (EYC).
Results
Time course of ultrasound appearance of blood dilutions and clotted blood in vitro
First, we established that normal saline, NP, NP with hyaluronic acid, or joint fluid, when contained in syringes, appeared anechoic on ultrasound. The fluids were indistinguishable from each other. Non-dissolved particles of hyaluronic acid in NP and bubbles in aspirated joint fluid generated coarse hyperechoic speckles, which were artefactual and could be clearly distinguished from homogeneously speckled, hypoechoic blood (Supplemental Fig 1). A schematic of transducer probe orientation performed during US imaging is depicted in Fig 1A.
To determine the concentration threshold of blood detection and the appearance of aging blood products and clot formation over time in vitro, NP was diluted with increasing concentrations of freshly drawn human blood. The samples were imaged immediately, as well as over the time course of one week. To mimic the composition of synovial fluid, the blood dilutions were enriched with high molecular weight hyaluronic acid at concentrations of 2 mg/cc. This concentration was chosen based on previous measurements yielding concentrations of 1–3 mg/cc hyaluronic in synovial fluids of normal volunteers and patients with arthritic conditions [25]. Hyaluronic acid is an abundant protein found in joint fluid and is thought to contribute to viscosity and joint lubrication [26], as well as influencing fibrin clot formation in the joint space [27]. A mixture of NP and hyaluronic acid was used in lieu of joint fluid because the procurement of large amounts of normal joint fluid is practically difficult and NP is a major constituent of hemarthrosis. Furthermore, the identical anechoic appearance of joint fluid and NP on ultrasound provided assurance of relevant and interpretable results (Supplemental Fig 1).
Ultrasound imaging of increasing dilutions of blood in NS or in NP with hyaluronic demonstrated that NS or NP with hyaluronic were anechoic. In contrast, with blood dilutions, a concentration as low as 5% generated echogenic signals (Fig 1; Supplemental Fig 2). Visual inspection did not permit blood quantification based on echo intensity, although lower concentrations of blood (5 or 10%) appeared less echogenic than higher concentrations of blood (25–100%). When the appearance of blood serially diluted into NS was quantified by objective grey tone analysis it appeared that the percentage of grey signals increased proportionally with increasing concentrations of blood (Supplemental Fig 2B). Altogether, these findings indicated that ultrasound could easily distinguish between bloody and non-bloody fluid in vitro, and that blood detection was feasible at very low blood concentrations. However, blood concentrations were not linearly associated with the magnitude of generated echogenicity.
Blood clot formation could be observed in non-diluted blood, as well as in partially diluted blood (down to 25–75%). Blood clot appearance in saline differed from plasma. Blood clots formed in plasma appeared hypoechoic with a line demarcating the separation from hypoechoic serosanguinous plasma (Fig 1B). In saline, blood clots appeared more hyperechoic when compared to the surrounding serosanginous fluid (Supplemental Fig 2A).
Time course of MRI appearance of blood dilutions and clotted blood in vitro
Increasing dilutions of blood in normal plasma enriched with 2 mg/cc hyaluronic acid were prepared for MRI, analogous to previous ultrasound imaging. Standard T1-weighted and T2-weighted fat-suppressed sequences were employed. In contrast to ultrasound imaging, conventional MRI could not qualitatively discriminate between the presence or absence of blood, nor distinguish between different blood concentrations. Increasing blood concentrations did not change signal intensity qualitatively in T1-weighted and T2-weighted FS images (Fig 2). However, blood clots were clearly visible on the T2- weighted FS sequences as hypointense signal areas relative to their surrounding serosanguinous fluid (Fig 2B). On the T1-weighted images, albeit much less clearly demarcated, faintly hyperintense signal areas were appreciated in 50–100% blood solutions at the 3–7 day time point (Fig 2A).
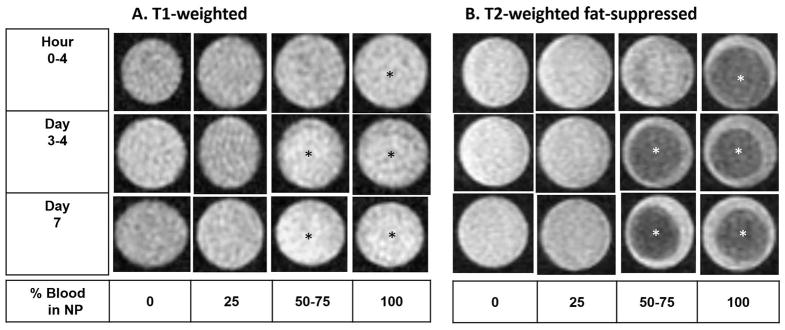
Figure 2. Time course of MRI appearance of blood dilutions and clotted blood in vitro.
NP, enriched with 2mg/mL hyaluronic acid, was diluted with increasing concentrations of freshly drawn human blood (3–6cc syringes) and subjected to serial conventional MRI at the indicated time points. A. T1-weighted MR images with identical window level and width B. T2-weighted fat-suppressed MR images with identical window level and width. Asterisk: Blood clots. Conventional MRI did not discriminate between the presence and absence of blood. Increasing blood concentrations did not change signal intensity qualitatively in T1- and T2-weighted fat suppressed images. Blood clots were most easily distinguishable on the T2-weighted fat-suppressed images since they were hypointense relative to surrounding serosanguinous fluid, but were also faintly appreciable as hyperechoic on the T1-weighted images after day 3. NP, Normal Pooled Plasma; MRI, Magnetic Resonance Imaging.
Time course of ultrasound appearance of blood dilutions and clotted blood in the MTP joints of cadaveric pig feet
To investigate the ultrasound appearance of increasing blood dilutions against the background of musculoskeletal structures, blood diluted in NS or in NP was injected into cadaveric pig MTP joints for imaging at different time points (Fig 3 and Supplemental Fig 3). Hyaluronic acid was omitted in experiments employing tissue imaging to avoid confusion in terms of delineating soft tissue from fluid speckle artefacts generated by non-dissolving hyaluronic crystals as observed earlier in the in vitro experiments (Supplemental Fig 1, Fig 1). Real time sonopalpation was performed during scanning sessions to help distinguish between fully compressible fluid versus partially- to non-compressible soft tissues or clots.
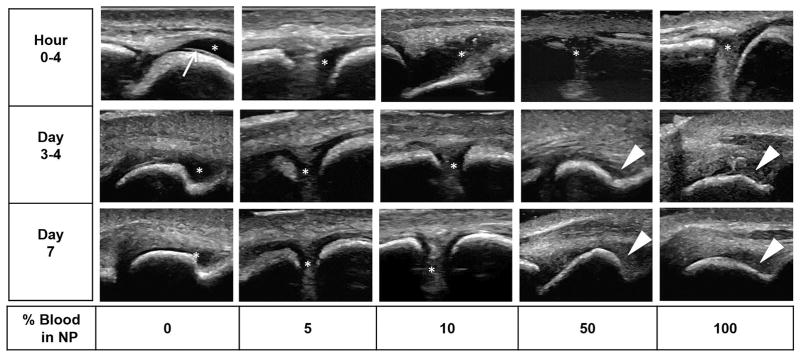
Figure 3. Time course of ultrasound appearance of blood dilutions and clotted blood in the MTP joints of cadaveric pig feet.
NP, diluted with increasing concentrations of freshly drawn human blood (3–5 mL) were injected into the MTP joint space. Serial ultrasound and sonopalpation was performed to identify fluid versus soft tissues at the indicated time points. Arrow: Interface between anechoic articular cartilage and fluid. Asterisk: Fluid-filled compressible areas in the joint space. Triangle : Partially or non- compressible clotted and aging blood products. Non-bloody fluid was anechoic. Echogenic signals could be appreciated starting as low as 5–10% blood dilution. Aging blood products/clots appeared hypoechoic and granular relative to the surrounding soft tissue. NP, Normal Pooled Plasma; MTP, Metatarsophalangeal.
Similar to the in vitro experiment, NS and NP appeared anechoic after injection into the MTP joint space and did not change appearance over time. Fresh blood could be detected by hypoechoic speckled appearance in concentrations as low as 5–10% (Fig 3 and Supplemental Fig 3). However, increasing concentrations of blood were not easily discernable by increasing echogenicity. As time proceeded to day 3–4 and onward, internal echoes were visible even at blood concentrations as low as 2.5–5% (Supplemental Fig 3). This observation potentially could be explained by sedimentation and concentration of blood cells at the bottom of the joint space, abutting neighboring soft tissues.
Blood clot formation was observed at higher concentrations of blood (≥ 50% in our dilution series), and their appearance was hypoechoic in relation to surrounding soft tissue. Clot formation was distinguishable from fluid based on the lack of full compressibility (only partially compressible) with real-time sonopalpation and their stationary properties, in contrast to fully compressible and displaceable fluids (Fig 3 and Supplemental Fig 3).
Time course of MRI appearance of blood dilutions and clotted blood in the MTP joints of cadaveric pig feet
Analogous to ultrasound imaging, increasing blood dilutions were prepared by admixing freshly drawn blood with increasing concentrations of normal saline or NP, prior to injection into cadaveric pig MTP joints for MRI. Similar to the in vitro results, MRI imaging was unable to distinguish between the presence of non-bloody and bloody fluid on conventional T1- and T2-weighted fat-suppressed images. Figure 4 depicts the appearance on MRI of MTP joints injected with either NP or 100% blood. Both injectables appeared to be isointense and hyperintense on the T1-weighted and T2-weighted FS sequences, respectively, and did not change appearance during the one-week time course.
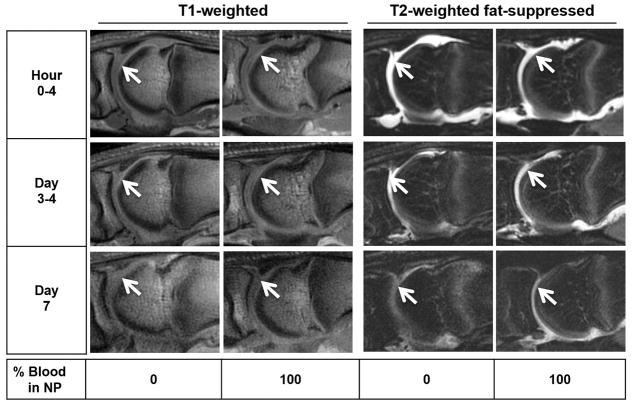
Figure 4. Time course of MRI appearance of blood dilutions and clotted blood in the MTP joints of cadaveric pig feet.
NP, diluted with increasing concentrations of freshly drawn human blood (3–5 mL) were injected into the MTP joint space. Serial MRI was performed using T1-weighted and T2-weighted fat- suppressed sequences at the indicated time points.. Arrows: Fluid-filled areas in the joint space. There was no appreciable difference in signal intensity between plasma and blood solutions on T1- and T2-weighted fat-suppressed sequences. NP, Normal Pooled Plasma; MRI, Magnetic Resonance Imaging; MTP, Metatarsophalangeal.
Timely correlated MSKUS and MR images in two hemophilic patients with simple and bloody effusions
To demonstrate that the ultrasonographic and MRI appearance of non-bloody and bloody fluid obtained by in vitro and cadaveric imaging would apply to in vivo imaging, effusions were evaluated by timely correlated MSKUS and MRI in patients with hemophilia. Among 30 patients who obtained MSKUS and conventional MR imaging studies for the assessment of hemophilic arthropathy, two patients who presented with joint pain were studied within several hours followed by joint aspiration. Their results presented in Figure 5 demonstrate that conventional MRI was unable to discern bloody from non-bloody fluid, while MSKUS could do so based on differences in echogenicity of compressible spaces.
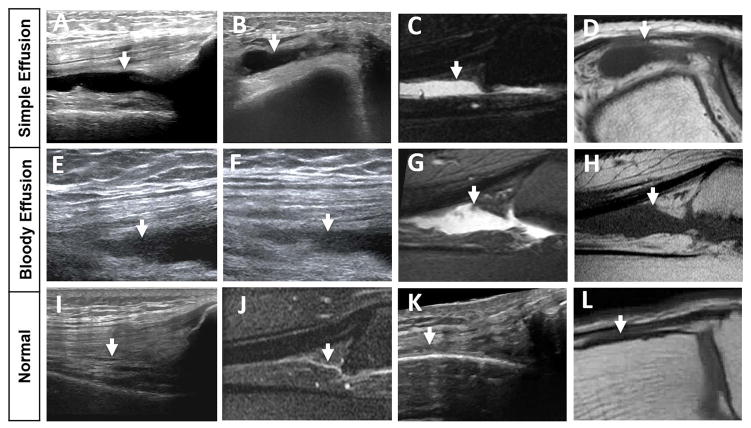
Figure 5. Timely correlated MSKUS and MR images in 2 hemophilic patients with simple and bloody effusions in relation to normal anatomy.
A/B: a 70-year-old-man with a simple effusion on MSKUS: Longitudinal image through the suprapatellar recess and short axis image through the lateral recess show anechoic joint fluid (arrows) consistent with simple effusion, confirmed through joint aspiration. E/F: a 57-year-old man with a bloody effusion on MSKUS: Longitudinal images through the suprapatellar recess applying different degrees of real-time compression show joint fluid with echogenic reflectors (arrows), consistent with bloody effusion, confirmed through joint aspiration. C/D and G/H: Corresponding MR images to A/B and E/F. Sagittal T2-weighted fat-suppressed and axial/sagittal T1-weighted images confirm joint effusions (arrows) in both cases, but are unable to distinguish between simple and bloody effusion. Simple fluid in C and D appears qualitatively similar to the bloody fluid in G and H. I/K and J/L represent normal sonoanatomy and MR anatomy of the suprapatellar and lateral recess, respectively. The normal recesses, which are entirely collapsed, are annotated with arrows. MSKUS, Musculoskeletal Ultrasound; MR, Magnetic Resonance.
The first patient was a 70-year-old male with moderate Hemophilia A, who presented with several weeks of fluctuating joint pains and swelling in a knee that had undergone arthroscopic meniscal repair several years previously. The second patient was a 57-year-old male with moderate Hemophilia A, who presented with persistent knee pain and swelling one week after a fall.
On MSKUS, imaging through the suprapatellar recesses in long axis and the lateral recesses in short axis, employing real-time sonopalpation, revealed compressible spaces with anechoic signals for the first patient (Fig 5A and B), and granular and speckled signals with mixed echogenicity for the second patient (Fig 5E and F). These observations were consistent with the ultrasonographic appearance of simple versus complex effusions, indicating either serous or bloody contents, as confirmed by aspiration in both patients. In contrast, corresponding images of the same joint locations on MRI showed no differences in signal intensities between the patients. Sagittal T2-weighted FS and axial/sagittal T1-weighted sequences depicted the effusions as equally hyperintense and hypointense, respectively (Fig 5C/D and Fig 5G/H). The qualitative signal intensity on MRI was the same, irrespective of fluid content and/or characteristics. Altogether, these findings align with the in vitro observations and during cadaveric imaging, demonstrating that conventional MRI cannot easily distinguish bloody from non-bloody effusions. The MRI observations reported here may not pertain to the appearance of (partially) clotted blood or blood sediments in the joint, which to our knowledge, have not yet been formally studied in vivo.
Discussion
Rapid and accurate detection of acute or persistent bleeding in the joints of patients with hemophilia has evolved as a critical shortcoming during the past several years and resulted in the development of POC MSKUS for improved and rapid bleed diagnosis [2–4].
Findings from our study demonstrate that MRI cannot easily distinguish between bloody and non-bloody joint effusions, while MSKUS can. This has important clinical implications for hemophilia care since the prevailing opinion assumes MRI as “gold standard” for blood detection in the joint, despite a lack of supporting evidence. In contrast, MSKUS was highly sensitive to detect blood at rather low concentrations (5–10% blood) in low volumes (3–5cc). This is noteworthy considering that normal joint fluid volumes are described to be only a few cc in larger joints [28, 29]. While our results stem from a series of in vitro and cadaveric pig studies, relevance to human pathology was provided by simultaneous imaging of hemophilic joints with conventional MRI and MSKUS, followed by definitive proof through joint aspiration.
Bloody fluid appeared homogenously echogenic on ultrasound, compressible with displaceable echogenic reflectors upon sonopalpation. Bloody fluid could be easily distinguished from anechoic serous fluid in vitro, in cadaveric pig joints, and in live human joints. Echogenic properties of diluted blood remained relatively unchanged during the time course of observation, although blood components presumably decayed to some extent. These observations highlight that red cells and blood components have no unique features on ultrasound, and may resemble other particulate or proteinaceous aggregates. Clinical scenarios such as joint infections may have similar ultrasound appearances. Also it may be difficult to distinguish fresh bloody effusions (intact erythrocytes) from aging, serosanginous effusions (erythrocyte remnants). Therefore, additional evaluations such as aspiration with joint fluid analysis are warranted in the appropriate clinical context.
Blood clots tended to form once blood concentration was increased to 50–75%. The clots were best visible on T2- weighted FS sequences on MRI, with easier discrimination from surrounding fluid and soft tissue compared to ultrasound. Especially in vitro, this observation is consistent with previous studies [30, 31]. On MSKUS, blood clots appeared partially compressible, hypoechoic, variable in appearance, with little structural detail. Previous ultrasound studies imaging clot formation in vitro, or, investigating composition of explanted blood clots, noted that echogenicity of clots can vary based on parameters such as red cell and/or platelet contents, extent of clot retraction or compression, and fibrin mesh composition [31–33].
This study has several limitations. First, the cadaveric imaging studies were performed in relatively small joints. While the size of a joint should not affect blood concentration detection, we acknowledge that larger joints have more capacity to accumulate larger effusions, which may alter imaging physics slightly. In clinical practice, the need for higher tissue penetration with larger effusions or more surrounding soft tissue is addressed by adjustments of penetration depth, grey scale (B-mode settings), transducer frequency and positioning, in part as per manufacturers’ pre-sets for different joints. Therefore, the type or size of joints may influence the threshold of blood concentration recognition in relation to volume on ultrasound slightly, but the trend and overall conclusions should remain the same. Second, the formation of blood clots in our study was artificial, lacking correct proportions and/or continued supply of blood and joint fluid constituents that regulate clot formation and fibrinolysis. The described appearance may be contrived and not apply in vivo. Echogenicity changes of aging blood products over time, especially in hemophilic joints, will require targeted investigations to develop appropriate diagnostic algorithms for POC MSKUS evaluation of intra-articular blood clot formation and resolution. Third, although MRI criteria for fluid assessment are well defined, this study relies on MRI readings provided by one, although very experienced, musculoskeletal radiologist. Forth, while our observations provided proof-of-principle regarding the applicability of ultrasound appearances of serous and bloody joint effusions, continued validation in prospective studies is necessary.
Our study has several important clinical implications. While MRI has excellent sensitivity for the detection of acute intracranial hemorrhage and vascular diseases [34–36], our findings do not support that conventional MRI can reliably discriminate the complexity of intra-articular fluid in the absence of blood clots or aging blood products. Our findings regarding a potential limitation to detect intra-articular blood are corroborated by the pilot study from Beltran et al., which claimed the similarity of signal intensities in MRI sequences of intra-articular injection of normal saline and fresh blood into healthy immature swine knees [22].
In contrast, MSKUS appears very sensitive and appropriate for rapid bleed detection in hemophilic joints. Aspiration of an effusion may be required to determine red cell content or hematocrit if clinically important since blood concentration quantification based on differences in echogenicity appeared difficult with current usual ultrasound settings. However, in current clinical practice, visual inspection appears sufficient to determine the presence or absence of blood in the POC setting.
In conclusion, MSKUS is extremely sensitive to detect low concentrations of intra-articular blood at low volumes, and to discriminate between bloody and non-bloody fluid, whereas conventional MRI is not. These observations demonstrate the advantages of MSKUS over MRI to detect intra-articular blood, and establish POC MSKUS for rapid bleed detection as a major advancement for the management of hemophilic arthropathy.
Supplementary Material
Essentials.
The best imaging modality for joint blood detection in hemophilia is unknown.
Blood appearance and detection thresholds were studied with ultrasound and conventional MRI.
Ultrasound is sensitive to low volume and concentration of blood, whereas conventional MRI is not.
The findings establish the validity of ultrasound for rapid bleed detection in hemophilia care.
Acknowledgments
This work was supported by Bioverativ and HRSA service grant H30MC24045 (A. von Drygalski), MSU CHM Oncology Related Scholarship (S. Nguyen), VA Clinical Science R&D Service (I01CX001388) (E. Y. Chang), and the NIH (R01AR062581 and 1R01 AR068987) (E. Y.C hang. and J. Du).
Footnotes
Addendum
S. Nguyen performed ultrasound studies, contributed to data collection, data analysis, data interpretation and manuscript drafting. X. Lu contributed to data collection and data analysis. Y. Ma contributed to data collection. J. Du contributed to data collection and provided project oversight for MRI findings. E. Y. Chang performed MRI, interpreted MRI and MSKUS findings and contributed to manuscript drafting. A. von Drygalski designed concept and research, performed and supervised ultrasound studies, provided project oversight and analysis guidance, and contributed to manuscript drafting. All authors critically reviewed the manuscript and approved its final version.
References
- 1.Zhou ZY, Koerper MA, Johnson KA, Riske B, Baker JR, Ullman M, Curtis RG, Poon JL, Lou M, Nichol MB. Burden of illness: direct and indirect costs among persons with hemophilia A in the United States. J Med Econ. 2015;18:457–65. doi: 10.3111/13696998.2015.1016228. [DOI] [PubMed] [Google Scholar]
- 2.Aznar JA, Abad-Franch L, Perez-Alenda S, Haya S, Cid AR, Querol F. Ultrasonography in the monitoring of management of haemarthrosis. Haemophilia. 2011;17:826–8. doi: 10.1111/j.1365-2516.2011.02538.x. [DOI] [PubMed] [Google Scholar]
- 3.Aznar JA, Perez-Alenda S, Jaca M, Garcia-Dasi M, Vila C, Moret A, Querol F, Bonanad S. Home-delivered ultrasound monitoring for home treatment of haemarthrosis in haemophilia A. Haemophilia. 2015;21:e147–50. doi: 10.1111/hae.12622. [DOI] [PubMed] [Google Scholar]
- 4.Ceponis A, Wong-Sefidan I, Glass CS, von Drygalski A. Rapid musculoskeletal ultrasound for painful episodes in adult haemophilia patients. Haemophilia. 2013;19:790–8. doi: 10.1111/hae.12175. [DOI] [PubMed] [Google Scholar]
- 5.Kidder W, Nguyen S, Larios J, Bergstrom J, Ceponis A, von Drygalski A. Point-of-care musculoskeletal ultrasound is critical for the diagnosis of hemarthroses, inflammation and soft tissue abnormalities in adult patients with painful haemophilic arthropathy. Haemophilia. 2015 doi: 10.1111/hae.12637. [DOI] [PubMed] [Google Scholar]
- 6.Doria AS, Keshava SN, Mohanta A, Jarrin J, Blanchette V, Srivastava A, Moineddin R, Kavitha ML, Hilliard P, Poonnoose P, Gibikote S. Diagnostic accuracy of ultrasound for assessment of hemophilic arthropathy: MRI correlation. AJR Am J Roentgenol. 2015;204:W336–47. doi: 10.2214/AJR.14.12501. [DOI] [PubMed] [Google Scholar]
- 7.Martinoli C, Della Casa Alberighi O, Di Minno G, Graziano E, Molinari AC, Pasta G, Russo G, Santagostino E, Tagliaferri A, Tagliafico A, Morfini M. Development and definition of a simplified scanning procedure and scoring method for Haemophilia Early Arthropathy Detection with Ultrasound (HEAD-US) Thromb Haemost. 2013;109:1170–9. doi: 10.1160/TH12-11-0874. [DOI] [PubMed] [Google Scholar]
- 8.Muca-Perja M, Riva S, Grochowska B, Mangiafico L, Mago D, Gringeri A. Ultrasonography of haemophilic arthropathy. Haemophilia. 2012;18:364–8. doi: 10.1111/j.1365-2516.2011.02672.x. [DOI] [PubMed] [Google Scholar]
- 9.Melchiorre D, Linari S, Innocenti M, Biscoglio I, Toigo M, Cerinic MM, Morfini M. Ultrasound detects joint damage and bleeding in haemophilic arthropathy: a proposal of a score. Haemophilia. 2011;17:112–7. doi: 10.1111/j.1365-2516.2010.02380.x. [DOI] [PubMed] [Google Scholar]
- 10.Querol F, Cortina V, Cid AR, Haya S, Aznar JA. Clinical and echographical control protocol of haemarthrosis in haemophilia patients with inhibitors: evaluation of the efficacy of recombinant factor VIIa in the evolution process (EFFISEVEN protocol) Haemophilia. 2008;14(Suppl 6):36–44. doi: 10.1111/j.1365-2516.2008.01888.x. [DOI] [PubMed] [Google Scholar]
- 11.Lesniak BP, Loveland D, Jose J, Selley R, Jacobson JA, Bedi A. Use of ultrasonography as a diagnostic and therapeutic tool in sports medicine. Arthroscopy. 2014;30:260–70. doi: 10.1016/j.arthro.2013.10.011. [DOI] [PubMed] [Google Scholar]
- 12.Loeuille D, Rat AC, Goebel JC, Champigneulle J, Blum A, Netter P, Gillet P, Chary-Valckenaere I. Magnetic resonance imaging in osteoarthritis: which method best reflects synovial membrane inflammation? Correlations with clinical, macroscopic and microscopic features. Osteoarthritis Cartilage. 2009;17:1186–92. doi: 10.1016/j.joca.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 13.Roemer FW, Kassim Javaid M, Guermazi A, Thomas M, Kiran A, Keen R, King L, Arden NK. Anatomical distribution of synovitis in knee osteoarthritis and its association with joint effusion assessed on non-enhanced and contrast-enhanced MRI. Osteoarthritis Cartilage. 2010;18:1269–74. doi: 10.1016/j.joca.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 14.Ohrndorf S, Backhaus M. Advances in sonographic scoring of rheumatoid arthritis. Ann Rheum Dis. 2013;72(Suppl 2):ii69–75. doi: 10.1136/annrheumdis-2012-202197. [DOI] [PubMed] [Google Scholar]
- 15.JAJ . Fundamentals of Musculoskeletal Ultrasound. Philadelphia: Elsevier Inc; 2013. [Google Scholar]
- 16.Mandl P, Brossard M, Aegerter P, Backhaus M, Bruyn GA, Chary-Valckenaere I, Iagnocco A, Filippucci E, Freeston J, Gandjbakhch F, Jousse-Joulin S, Moller I, Naredo E, Schmidt WA, Szkudlarek M, Terslev L, Wakefield RJ, Zayat A, D’Agostino MA, Balint PV. Ultrasound evaluation of fluid in knee recesses at varying degrees of flexion. Arthritis Care Res (Hoboken) 2012;64:773–9. doi: 10.1002/acr.21598. [DOI] [PubMed] [Google Scholar]
- 17.Fessell DP, Jacobson JA, Craig J, Habra G, Prasad A, Radliff A, van Holsbeeck MT. Using sonography to reveal and aspirate joint effusions. AJR Am J Roentgenol. 2000;174:1353–62. doi: 10.2214/ajr.174.5.1741353. [DOI] [PubMed] [Google Scholar]
- 18.Backhaus M, Burmester GR, Gerber T, Grassi W, Machold KP, Swen WA, Wakefield RJ, Manger B Trials WGfMUitESCoICSiT. Guidelines for musculoskeletal ultrasound in rheumatology. Ann Rheum Dis. 2001;60:641–9. doi: 10.1136/ard.60.7.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lawson W, Uy M, Strike K, Iorio A, Stein N, Koziol L, Chan A. Point of care ultrasound in haemophilia: Building a strong foundation for clinical implementation. Haemophilia. 2017 doi: 10.1111/hae.13269. [DOI] [PubMed] [Google Scholar]
- 20.Bradley WG., Jr MR appearance of hemorrhage in the brain. Radiology. 1993;189:15–26. doi: 10.1148/radiology.189.1.8372185. [DOI] [PubMed] [Google Scholar]
- 21.Clark RA, Watanabe AT, Bradley WG, Roberts JD. Acute hematomas: effects of deoxygenation, hematocrit, and fibrin-clot formation and retraction on T2 shortening. Radiology. 1990;175:201–6. doi: 10.1148/radiology.175.1.2315481. [DOI] [PubMed] [Google Scholar]
- 22.Beltran J, Noto AM, Herman LJ, Mosure JC, Burk JM, Christoforidis AJ. Joint effusions: MR imaging. Radiology. 1986;158:133–7. doi: 10.1148/radiology.158.1.3940370. [DOI] [PubMed] [Google Scholar]
- 23.Monahan PE. Emerging genetic and pharmacologic therapies for controlling hemostasis: beyond recombinant clotting factors. Hematology Am Soc Hematol Educ Program. 2015;2015:33–40. doi: 10.1182/asheducation-2015.1.33. [DOI] [PubMed] [Google Scholar]
- 24.Wakefield RJ, Balint PV, Szkudlarek M, Filippucci E, Backhaus M, D’Agostino MA, Sanchez EN, Iagnocco A, Schmidt WA, Bruyn GA, Kane D, O’Connor PJ, Manger B, Joshua F, Koski J, Grassi W, Lassere MN, Swen N, Kainberger F, Klauser A, Ostergaard M, Brown AK, Machold KP, Conaghan PG, Group OSI. Musculoskeletal ultrasound including definitions for ultrasonographic pathology. J Rheumatol. 2005;32:2485–7. [PubMed] [Google Scholar]
- 25.DECKER B, McGUCKIN WF, McKENZIE BF, SLOCUMB CH. Concentration of hyaluronic acid in synovial fluid. Clin Chem. 1959;5:465–9. [PubMed] [Google Scholar]
- 26.Greene GW, Banquy X, Lee DW, Lowrey DD, Yu J, Israelachvili JN. Adaptive mechanically controlled lubrication mechanism found in articular joints. Proc Natl Acad Sci U S A. 2011;108:5255–9. doi: 10.1073/pnas.1101002108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weigel PH, Frost SJ, McGary CT, LeBoeuf RD. The role of hyaluronic acid in inflammation and wound healing. Int J Tissue React. 1988;10:355–65. [PubMed] [Google Scholar]
- 28.Kraus VB, Stabler TV, Kong SY, Varju G, McDaniel G. Measurement of synovial fluid volume using urea. Osteoarthritis Cartilage. 2007;15:1217–20. doi: 10.1016/j.joca.2007.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moss SG, Schweitzer ME, Jacobson JA, Brossmann J, Lombardi JV, Dellose SM, Coralnick JR, Standiford KN, Resnick D. Hip joint fluid: detection and distribution at MR imaging and US with cadaveric correlation. Radiology. 1998;208:43–8. doi: 10.1148/radiology.208.1.9646791. [DOI] [PubMed] [Google Scholar]
- 30.Jeong J, Park S, Jeong E, Kim N, Kim M, Jung Y, Cho Y, Lee K. Time-dependent low-field MRI characteristics of canine blood: an in vitro study. J Vet Sci. 2016;17:103–9. doi: 10.4142/jvs.2016.17.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tratar G, Blinc A, Podbregar M, Kralj E, Balazic J, Sabovic M, Sersa I. Characterization of pulmonary emboli ex vivo by magnetic resonance imaging and ultrasound. Thromb Res. 2007;120:763–71. doi: 10.1016/j.thromres.2006.12.024. [DOI] [PubMed] [Google Scholar]
- 32.Vidmar J, Sersa I, Kralj E, Tratar G, Blinc A. Discrimination between red blood cell and platelet components of blood clots by MR microscopy. Eur Biophys J. 2008;37:1235–40. doi: 10.1007/s00249-008-0336-6. [DOI] [PubMed] [Google Scholar]
- 33.Sigel B, Feleppa EJ, Swami V, Justin J, Consigny M, Machi J, Kikuchi T, Lizzi FL, Kurohiji T, Hui J. Ultrasonic tissue characterization of blood clots. Surg Clin North Am. 1990;70:13–29. doi: 10.1016/s0039-6109(16)45030-9. [DOI] [PubMed] [Google Scholar]
- 34.Kilburg C, Scott McNally J, de Havenon A, Taussky P, Kalani MY, Park MS. Advanced imaging in acute ischemic stroke. Neurosurg Focus. 2017;42:E10. doi: 10.3171/2017.1.FOCUS16503. [DOI] [PubMed] [Google Scholar]
- 35.Vick GW., 3rd The gold standard for noninvasive imaging in coronary heart disease: magnetic resonance imaging. Curr Opin Cardiol. 2009;24:567–79. doi: 10.1097/HCO.0b013e3283315553. [DOI] [PubMed] [Google Scholar]
- 36.Viereck J, Ruberg FL, Qiao Y, Perez AS, Detwiller K, Johnstone M, Hamilton JA. MRI of atherothrombosis associated with plaque rupture. Arterioscler Thromb Vasc Biol. 2005;25:240–5. doi: 10.1161/01.ATV.0000149673.00564.0a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.