Abstract
Melanoma is one of the most aggressive types of cancer and the incidence has increased rapidly in the past few decades. In this study, we investigated a novel treatment approach using a low-intensity-ultrasound (2.3W/cm2 at 1MHz) mediated Optison microbubble (MB) destruction (UMMD) in the treatment of melanoma in a flank tumor model. The effect of UMMD was first evaluated in a melanoma cell line, B16F10 (B16) in vitro, and then in mice inoculated with B16 cells. MB+B16 were exposed to US in vitro resulting in significant cell death proportionally to duty cycle (R2=0.74); approximately 30, 50, 80 and 80 % cell death at 10, 30, 50 and 100% DC respectively. Tumors treated with direct implantation of MB followed by sonication resulted in retarded tumor growth along with improved survival (p=0.0106). Immunohistochemical analyses confirmed the significant changes in cell proliferation marker expression, Ki67 (p=0.037) and a microtubule-associated protein 2 (p=0.048) following US+MB treatment. These results suggest that UMMD could be used as a possible treatment approach in isolated melanoma and has the potential to translate to clinical trials.
Keywords: Low intensity ultrasound, Microbubbles, Ultrasound mediated microbubble destruction (UMMD), Melanoma, Optison microbubbles
Introduction
Cutaneous melanoma is the sixth most common cancer in the United States.(Miller, et al. 2016, Siegel, et al. 2016) The incidence of cutaneous melanoma has increased over the past decades and is associated with ultraviolet radiation exposure that may result in genetic mutation in the melanocytes in the basal layer of the epidermis.(Lazovich, et al. 2010, Parkin, et al. 2011) Melanoma can be treated by surgical excision, immunotherapy and chemotherapy; however, there are potential morbidity and adverse events associated with these approaches.(Lee, et al. 1995, Rosenberg, et al. 1994, Weber, et al. 2012) Although wide surgical excision when possible is currently used to treat isolated melanoma lesions, it would be advantageous to have alternative or additional therapeutic approaches to effectively eradicate or limit the tumor progression.
Low intensity ultrasound (US), typically less than 5W/cm2 (Wood and Sehgal 2015, Xin, et al. 2016), has been used as a diagnostic and therapeutic modality and when coupled with an infusion of US contrast agent biocompatible gas-filled microbubbles (MB) can increase image contrast in lesion detection or as a method to enhance drug or gene delivery when coupled with therapeutic or focused ultrasound.(Blomley, et al. 2001, Miwa, et al. 2012, Timbie, et al. 2015) The acoustic response of MBs is highly dependent on the level of US pressure as well as the size, stability, diffusion, and surface tension of MBs.(Chen, et al. 2013, Emmer, et al. 2009) Recent studies have demonstrated that the mechanical effects of dynamic interactions between US and MBs can be utilized for tumor treatment.(Hernot and Klibanov 2008, Liu, et al. 2014, Pu, et al. 2014) MBs oscillate linearly by changing their size and shape in a manner that is inversely proportional to the US pressure amplitude when exposed to low peak negative pressure (PNP) (i.e., mechanical index (MI) < 0.05). MBs exposed to high PNP, undergo inertial oscillating non-linearly that can lead to complete destruction. MB fragmentation occurs secondary to inertial cavitation that produces mechanical shock waves, elevates local microenvironment temperature, and can induce free radical formation.(Chomas, et al. 2001, Chomas, et al. 2000, de Jong, et al. 2009, Hernot and Klibanov 2008) For these reasons, the behavior of non-linear MB oscillation has been extensively investigated for the tumor treatment.(Carson, et al. 2012, Chen and Hwang 2013, Feril, et al. 2003, Hassan, et al. 2009) However, few studies have investigated the effect of directly injected MBs into tumors for the targeted drug delivery with applied US mediated MB destruction (UMMD) on the tumor treatment.(Sonoda, et al. 2007, Watanabe, et al. 2008) In addition, the direct injection of MBs alone into tumors with the intent of increasing cell kill following US exposure has not been thoroughly evaluated.
The purpose of this study was to test the hypothesis that UMMD in the melanoma flank tumors would suppress the growth. We evaluated the effects of duty cycle (DC)-modulated US on the MB destruction and their effects inducing tumor cell death in vitro and evaluated the efficacy of UMMD in the treatment of melanoma in a mouse model.
Materials and Methods
Low intensity ultrasound system
Versatile sweep function generator (BK Precision, Yorba Linda, CA, US) was used to generate 1MHz sinusoidal continuous or pulsed (pulse repetition frequency (PRF) fixed at 1KHz) waveforms. Input pulses (pulse length = 1μsec) with the modulation of DC at 0, 1, 10, 30, 50 and 100% (total on time was 0, 10, 100, 300, 500 and 1,000 msec/sec respectively) were generated by adjusting a number of cycles per US burst (total number of cycles was 0, 1×104, 1×105, 3×105, 5×105, 1×106 cycles respectively). DC with 0% and 100% are equivalent to no-US serving as control and continuous US respectively. The pulses were amplified by radio frequency power amplifier (Electronic Navigation Industries Inc., Rochester, NY) and transferred to an ultrasonic transducer (unfocused linear, 3cm in outer diameter and center frequency at 1MHz) (Ultrasonic S-Lab, Concord, CA). The output power of US was determined by radiation force balance (RFB) technique and it was regarded as spatial averaged and temporal averaged (SATA).(Preston 1986, Preston 1986, Zeqiri and Bickley 2000) US intensity at 0, 10, 50 and 100% DC was determined as 0, 0.03, 0.15 and 0.27 W/cm2, respectively.
In vitro MB destruction by US
Two hundred microliter of culture medium containing 10% (v/v) biologically safe MBs (Optison, GE Healthcare, Princeton, NJ) was prepared and was exposed to DC-modulated US at 0, 1, 10, 30, 50 and 100% for 10sec. Entire volume of cells/MBs suspension was exposed to US that traveled through degassed water. Immediately after US exposure, the number of remaining MBs in suspension was counted using hemocytometer and light microscope.
In vitro cytotoxicity by UMMD
The melanoma cell line, B16F10 (B16) was purchased (ATCC, Manassas, VA) and cultured in 90% Dulbecco’s Modified Eagle’s Medium (DMEM) (Gibco, Waltham, MA) supplemented with 10% Fetal Bovine Serum (FBS) (Atlanta Biologicals, Lawrenceville, GA, US), 10mM (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid) (HEPES) (Gibco), 50μg/mL Gentamycin (Cellgro, Manassas, VA), 1mM GlutaMAX (Gibco), and 1mM sodium pyruvate (Gibco) at 37°C and 5% CO2.(Overwijk and Restifo 2001) For in vitro cytotoxic evaluation of UMMD, intermediate cells/MBs suspension (0.2 × 106 cells/mL containing 10% (v/v) Optison MBs) was prepared by mixing Optison MBs with B16 cells by gently pipetting up and down several times. The 200ul of cells/MBs suspension was then transferred to into a PCR tube (BR781326, Sigma) and the tube was placed at the bottom of a sterile specimen container (Medsupply partners, GA) filled with degassed water. During the placement of a PCR tube from upright to a horizontal position, there was no cells/MBs suspension movement due to surface tension suggesting that there was no interference of ultrasound propagation between air bubbles and cells/MBs suspension. A 1MHz un-focused water-immersible transducer with 30mm outer diameter was placed at 1cm in distance in near field zone and the effective area of the transducer was greater enough to cover the entire volume of cells/MBs. The cells/MBs were exposed to DC-modulated US for 10sec using (n=3–9 per group). This procedure was performed as quickly as possible to maximize the interactions among ultrasound, MBs and cells due to the fact that MBs tend to float up and cells tend to sink down in the tubes. Following sonication cells in suspension were labeled with 1μM Calcein-AM, a live cell indicator (Invitrogen Life Technologies, Waltham, MA), and 1μM ethidium homodimer-2 (EthD-2), a dead cell indicator (Invitrogen Life Technologies), and were counted by a hemocytometer using a fluorescence microscope (BX60, Olympus, Center Valley, PA, US). Cell viability was determined as the percentage of live cells over total cells.
In vivo UMMD treatment
All animal experimental procedures were approved and performed according to the guidelines established by the Institutional Animal Care and Use Committee (IACUC) at the University of Iowa. Eight-week-old female C57BL/6J mice (n=30) were purchased from Harlan Laboratories (Indianapolis, IN, US) and housed with free access to food and water. Total of 3 cohort studies was performed and B16 cells (0.1 × 106 cells suspended in 100μL of PBS) were subcutaneously injected into flank in mice at day 0. Mice were divided into 4 groups: SHAM (saline infusion on days on 11, 14 and 16), 1X UMMD (Optison MB infusion followed by US at days on 11, 14 and 16), SHAM (saline infusion on days on 7, 8, 9, 11 and 13) and 2X UMMD (Optison MB infusion followed by US at days on 7, 8, 9, 11 and 13) (n=5–13 per groups). Mice were anesthetized with intraperitoneal injection of 100μL of ketamine (17.5mg/mL)/xylazine (2.5mg/mL) dissolved in saline and either 75μL Optison MBs (Optison vial contains 5–8 × 108 MBs/ml (1X concentration) and 2X concentrated MBs were obtained by removing half volume of MB suspending liquid in the Optison vial) or saline was directly injected into the tumor. Following direct intra-tumor injection, the US transducer was coupled to the skin with ultrasonic coupling gel and sonicated at 1MHz; 100% DC, 2.3W/cm2 for 10 sec. UMMD treatments (MB infusion followed by sonication) were performed on days 7, 8, 9, 11 and 13 and the tumor growth was monitored and measured everyday using a digital caliper (See S3 Figure). Mice were euthanized when flank tumors reached 20 mm × 10 mm (width and height) according to the IACUC guidelines and we decided to follow tumor burden for mice up to 30 days of post B16 cell inoculation.
Histological Analysis
Mice (n=3 per group of 2X UMMD and SHAM group) were euthanized at day 15 and the tumors were harvested and fixed using 10% (w/v) neutral buffered formalin buffer. For histologic examination, the tumors were embedded with paraffin and tissues sections (10μm) were prepared. Tissue sections were stained with hematoxylin and eosin (H&E) in order to evaluate tumor morphology and were scanned using a bright field scanner (Aperio SC2, Leica Biosystem Inc.). For fluorescent immunohistochemical (fIHC) analyses, paraffin embedded tissue sections (10μm) were permeabilized with 0.1% Tween-20 in 1X PBS (TPBS) following an antigen retrieval with proteinase-K (Life Technologies, Frederick, MD). The tissues were then rinsed with TPBS, blocked using SuperBlock (ThermoFisher Scientific), incubated with a primary antibody using anti-ki67 and anti-microtubule-associated protein 2 (MAP2) (10 μg/mL) (Abcam, Cambridge, MA, US) for 1 hour at room temperature, conjugated by DyLight Fluor (488 nm) conjugated Goat anti-mouse IgG secondary antibody (10 μg/mL), and incubated for 1 hour at room temperature.(Burks, et al. 2015) The slides were then coverslipped with a DAPI-containing mounting medium and tissue sections were scanned using an immunofluorescence slide scanner (Aperio FL, Leica Biosystem Inc., Buffalo Grove, IL, US).
Statistical analysis
All data are presented as the mean ± standard deviation. One-way analysis of variance (ANOVA) with the Tukey post hoc was performed to test all possible pairwise comparisons. Gehan-Breslow-Wilcoxon test was performed for the in vivo survival analysis between groups. The level of statistical significance was set at p < 0.05. Data analyses and data presentation were performed using Prism6 (GraphPad Software, Inc., La Jolla, CA).
Results
In this study, the major findings are as follows: (i) Low intensity ultrasound exposure with varying DC resulted in decrease in the percentage of MB; (ii) Sonication of melanoma cells mixed with MB in vitro resulted in DC-dependent cell death; and (iii) Tumor growth suppression was achieved in a B16 melanoma tumor model in vivo by combining low intensity US and intra-tumor implanted MBs.
MB destruction by DC-modulated US
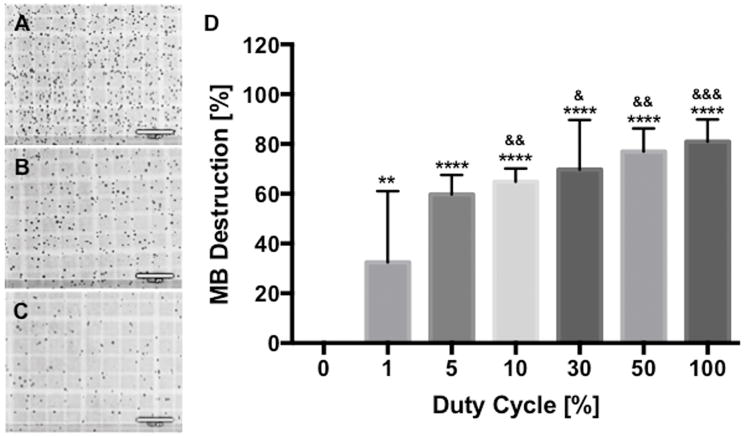
US exposure at 1MHz using a 100% DC was determined to have a calculated intensity spatially and temporally averaged (ISATA) of 0.27 W/cm2 based on radiation force balance analysis (see S2 Figure.). Sonication of Optison (10% v/v) MB mixed with culture media with a PRF of 1kHz and various duty cycles resulted in significant MB destruction of approximately 32% at 1% DC (p<0.01) that incrementally increased to ~80% at 100% DC (p<0.001) (60% at 5% DC, 65% at 10% DC, 70% at 30% DC, 77% at 50 DC) based on direct visualization and MB counts by hemocytometer (Figure. 1). There was statistical significance (p<0.05 by ANOVA) of MB destruction between groups when compared to 1% DC. Based on these results we decided to evaluate the US at various DC to determine the effects of MB mixed melanoma on cell lysis.
Figure 1.
The higher duty cycle (DC) destroys the more microbubbles (MBs). MB suspension was exposed to DC-modulated ultrasound (US) and the number of MBs was counted using a hemocytometer. (A, B and C) Light microscopy shows that the greater number of MBs was destroyed by the sonication with the longer DC (0.27 W/cm2 for 10sec at 100% DC); (A) 0%, (B) 10% and (C) 100%. (D) Normalized percentage of remaining MBs after US exposure revealed that approximately 30% of MBs was destroyed at 1% DC and the percentage gradually increased when increasing DC. Data are presented as mean ± SD (n=4–10) and asterisks represent statistical significance compared with 0% DC and ampersands represent statistical significance compared with 1% DC (& p<0.05, ** and && p<0.01, &&& p<0.001 and ****p<0.0001). Bars = 100μm.
US MB destruction of B16 melanoma in vitro
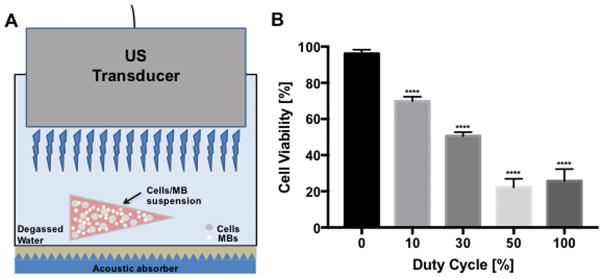
B16 cells (0.2 × 106 cells/mL) mixed with MB suspension (10% Optison (v/v)) to determine the effect of US exposure at various DC based on hemocytometer counts of cell viability using live (Calcein-AM) and dead (ethidium homodimer-2) cell indicator uptake. The viability of B16 cell + MB mixture (n=3–9 per group) without sonication (0% DC, sham control) was determined to be 95%. When B16 cells mixed with MB were sonicated with increasing DC we observed significant decreases in viability to 70% at 10% DC, 51% at 30% DC, 22% at 50% DC and 26% at 100% DC (p<0.0001) (Figure. 2). We observed also linear correlation (R2 = 0.7417, slope = 0.69) between the percentage of MB destruction and cell death (Figure. 3) indicating that there was a correlation between MB fragmentation and tumor cell death.
Figure 2.
Cell viability in the presence of MBs decreases when increasing DC in an in vitro setup. (A) US transducer was immersed in a sterile specimen container containing culture medium and US was applied to cell suspension in a PCR tube containing MBs (10% (v/v) Optison) with approximately 1 cm in distance. Acoustic absorber (Precision Acoustics, UK) was placed at the bottom at all times. (B) Duty cycle (DC)-modulated US exposure to cells/MBs suspension resulted in controlled cytotoxicity; cell viability was gradually decreased with increasing DC. Data are presented as mean ± SD (n=3–9) Asterisk (*) represents statistical significance compared with control (0% DC) (****p<0.0001).
Figure 3.
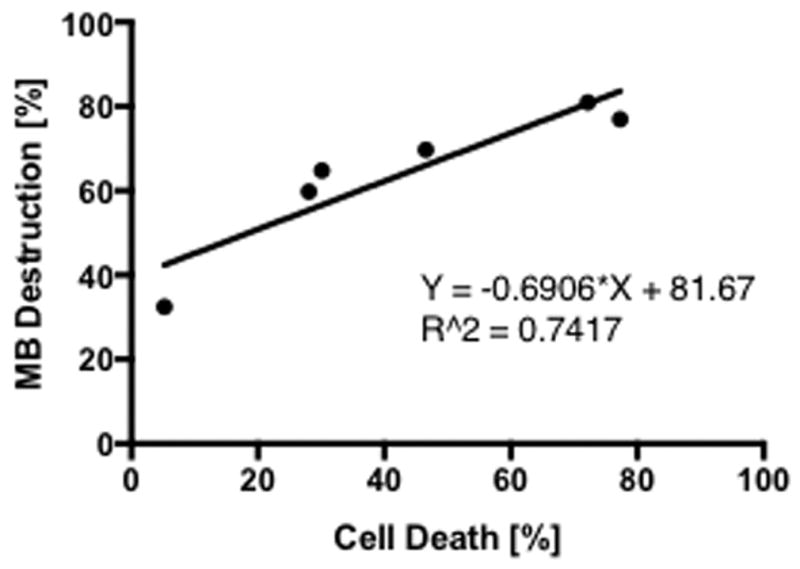
The percentage of cell death linearly correlates with the percentage of MB destruction. Under US exposure linearly correlated relationship was observed between the percentage of MB destruction and cell death when fitting a linear regression; R2 = 0.7417. (n=3–9).
In vivo tumor treatment by UMMD
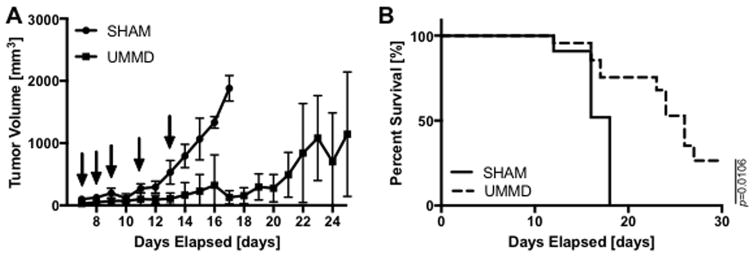
Seven days after B16 melanoma cell implantation in mice flank, PBS (control group) or 1X or 2X concentrated MBs (75μL Optison) was directly injected into tumor immediately followed by sonication (2.3W/cm2 at 100% DC for 10sec). We initially performed an experiment to determine the timing of US in combination with the concentration of MB injected into tumors would suppress tumor growth. To determine if the concentration of injected MB and the time of injection would alter flank tumor growth, tumors with and without Optison were sonicated on days 11, 14 and 16. Little effect was observed in the combination of US+MB (50% v/v) on the alteration of tumor growth compared to animals that only received US out to 16 days (see S1 Figure.). Based on our initial results, we altered the in vivo experiment by increasing both the amount of MB (75μL of 2X concentrated (two-fold greater number of MB inoculated)) and frequency of MB injection/sonications to the tumors (i.e., days 7, 8, 9, 11 and 13) and compared the results with those in animals receiving PBS (75μL) to the B16 flank tumors. In PBS injected control group, the tumors continued to increase in volume with rapid tumor growth starting at day 12 until they reached criteria (20mm × 10mm in size) for euthanasia at 18 days. Mice treated with multiple courses of UMMD showed delayed tumor growth to 21days post inoculation and had significantly prolong survival (p<0.0106) compared to control animals (Figure. 4) The mean survival for PBS group was 15.3±2.1 and 24.4±4.3 days for US+MB group.
Figure 4.
UMMD treatment suppresses the melanoma growth and prolongs survival. (A–B) The volume of melanoma tumors was gradually increased in PBS injected control mice until day 12 and started to increase exponentially from day 12. However, UMMD treatment performed at days 7, 8, 9, 11 and 13 significantly suppressed the melanoma growth resulting in delayed euthanizing criteria. Arrows indicate the days of treatment, either PBS or UMMD. Statistical significance was set at p < 0.05 and error bars represent sample variations (n=7–13).
Histologic Analyses
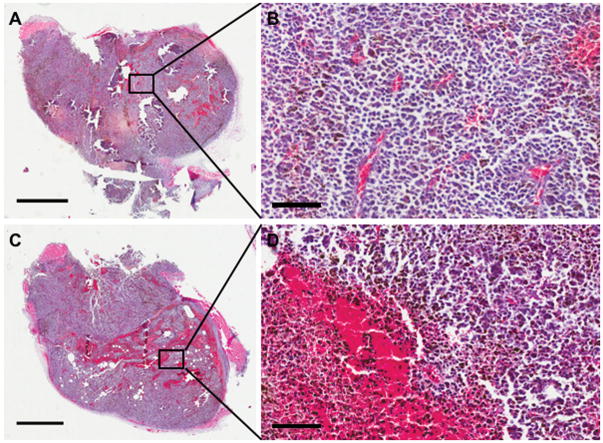
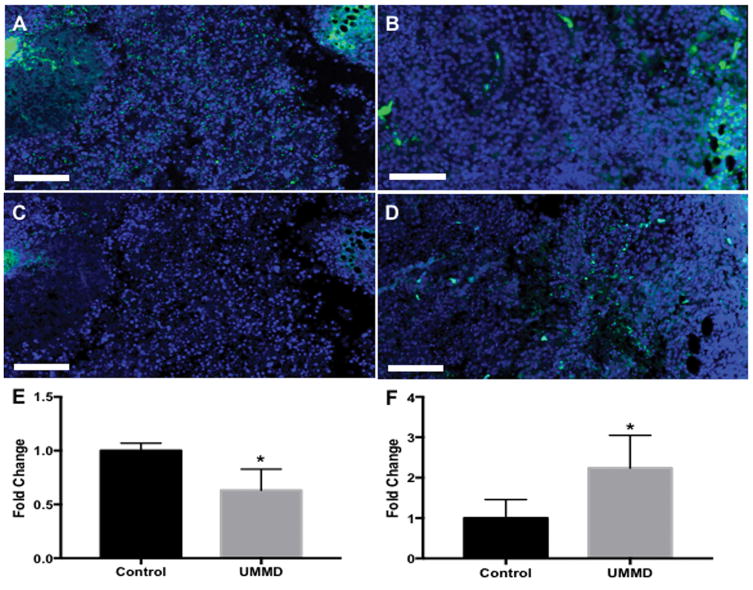
Animals from each cohort were randomly selected (n=3/group) for histological examination of the tumors on day 15 post tumor implantations. H&E staining revealed significantly greater regions with hemorrhages in UMMD treated melanoma compared to control group (Figure. 5). Fluorescence IHC analysis was performed to compared the expression of cell proliferation markers, Ki-67 and Microtubule-associated protein-2 (MAP2). UMMD treatment of flank tumors had significantly decreased Ki-67 expression (p=0.037 by unpaired t-test) when compared to untreated control (Figure. 6A and B). B16 cell damage was clearly evident on MAP2 fIHC staining (p=0.048 by unpaired t-test) in mice treated with UMMD compared to control animals (Figure. 6C and D).
Figure 5.
Gross histologic examination by H&E staining shows the greater amount of microhemorrhage in UMMD treated melanoma. (A and B) Tumors were harvested at day 15 and were stained with H&E for gross morphological examination. Compared with the PBS-treated control group (A and B), UMMD treatment resulted in the appearance of significantly greater region of microhemorrhage (C and D). C and D show a higher magnification of A and C, respectively. Bars = 2mm (A and C) and = 100μm (B and D).
Figure 6.
Fluorescent IHC analysis reveals that UMMD treatment reduces Ki-67 expression and increases MAP2 expression. (A and B) Ki-67 staining shows that UMMD treatment (B) significantly reduced the expression of Ki-67 compared to untreated control (A) and the difference was statistically significant (E). (C and D) The treatment of UMMD significantly enhanced MAP2 expression (D), whereas little was found in the untreated control (C) and the difference was statistically significant (F). Bars = 100 μm. Asterisk (*) represents statistical significance compared with control (*p<0.05 by unpaired T test).
Discussion and Conclusion
The main purpose of this study was to determine if intra-tumor injection of MB coupled with sonication would result in greater cell kill in vitro and prolong survival in mice with implanted B16 melanoma flank tumors. In order to confirm the production of cytotoxicity by UMMD, we first performed in vitro experiment to investigate the interactions of US on MB destruction and evaluated the effect of duty cycle on tumor cell death. It has previously been reported(Feril, et al. 2003) that there was a relationship between ultrasound intensity mediated microbubble destruction in the presence of tumor cells and cytotoxicity. Acoustic response of Optison has been widely investigated that they exhibit linear oscillation or undergoing destruction dependent on US parameters.(Feril, et al. 2003, Hernot and Klibanov 2008) Thus, in the current study, Optison ultrasound contrast agent was chosen and mixed with B16 melanoma in culture media was sonicated at 1MHz for 10sec at various DC resulting in a linear correlation between the amount of MB destruction and cell death in vitro. Although it could be possible that different behavior of MB destruction by US may occur that is dependent on the total exposure time, such as longer US duration with shorter DC or vice versa, we chose for the short sonication duration for in vivo application. These results suggest that tumor cell kill in vitro was presumably due to mechanical shock waves generated from interaction of the US pressure and MB inducing inertial cavitation and altering survival in nearby B16 cells.(Kodama, et al. 2006, Zhang, et al. 2015) It is clear that greater tumor cell death occurs at higher DC US exposure in the presence of MB.
To assess the cytotoxic effect of UMMD in vivo, we directly injected Optison into B16 flank tumors followed by an US exposure at 1MHz and 2.3 W/cm2 for 10sec using a fixed DC at 100% in order to maximize the MB destruction. No other DC-modulated sonications were evaluated in this study because the MB dynamics and microenvironment of MBs in vitro and in vivo setup were probably different(Brujan and Vogel 2006). In addition, there was a potential loss of MBs with an in vivo application into the systemic circulation during intra-tumoral injection with an in vivo application. Nonetheless, we surmised that the most of MBs were retained inside of the tumor because US was started immediately after injection. All control animals that were treated with direct injection of PBS into tumors reached euthanasia criteria by day 18 whereas mice that received the combination of intra-tumor injection of MB+US exposure had met the delayed euthanizing criteria. We observed that suppression of tumor growth was both dependent on the initial concentration of MB injected into tumor and the timing post implantation of the tumor in the flanks of mice when US was administered. Although we observed with delayed euthanizing criteria in the US+MB cohort of mice, there was clear evidence on histological examination of residual viable tumor cells in the UMMD cohort. We chose to analyze at day 15 since it was the most proliferative phase of tumor growth in this study. UMMD treated tumors exhibited greater amount of hemorrhage and fewer Ki-67 positive cells with more MAP2 positive staining consistent with greater cell death when compared to control animals. The decrease in Ki-67 and MAP2 staining in the UMMD cohort would suggest that there was suppression of tumor cell proliferation(Henrique, et al. 2000, Ladstein, et al. 2010, Soltani, et al. 2005, Song, et al. 2010, Straume, et al. 2000) compared to animals receiving intra-tumor injection of PBS alone. It is clear however that once the combination of MB+US treatment in the flank tumors was stopped on day 13 post-inoculation, there was a time delay in the progression of tumor growth accompanied by a significant improvement in mean survival (i.e., reaching euthanasia criteria). Although US-mediated IV-infused MB destruction also retards the tumor growth via inhibition of angiogenesis(Huang, et al. 2013), MB supply through IV is highly dependent on vascularization of tumors. Thus, we suggest that direct MB injection could be considered as one of the clinical treatment options based on tumor vascularization.
There are several limitations of this study that need to be discussed. This study was performed in easily to reach single flank tumors that allowed for direct injection of MB followed by direct ultrasound exposure resulting in only partial cell death. Isolated melanoma tumors would normally be surgically excised in the clinic removing all visible tumor deep to the dermal layers if needed and would include surgical resection of local lymph nodes for evaluation of metastatic disease.(Morton, et al. 1992, Mun 2012) In this study, all sonicated tumors were superficial and it is unclear how effective the UMMB approach would be for isolated metastasis deep in the body. The inability of UMMD to only retard the growth of B16 tumor cells is also a limitation and would indicate that multiple treatments would be required to continue the suppression of tumor growth. Future studies should consider the combination of using US hyperthermia or ablation therapy followed by US+MB exposure to determine if that would result in greater cell kill to micrometastases and prolong time to tumor recurrence. Moreover, we did not evaluate if the UMMD approach to treating the B16 tumor resulted in increased metastases to other organs. In summary, the results of this study would indicate that it is possible to suppress tumor growth and improve survival in a flank tumor model when combining intra-tumor injection of clinically approved ultrasound contrast agent with high duty cycle short duration low intensity ultrasound. Further research would be needed to determine if this technique could be applied for treating micrometastatic satellite tumors following tumor resection or ablation in improving recurrent disease.
Supplementary Material
S1 Figure. The tumor suppressive effects of UMMD treatment on mice bearing with melanoma. Non-US treated control mice received PBS directly into the tumor at day 11, 14 and 16. Tumor growth showed gradual increase in volume until at day 12 and started growing exponentially from day 13. The tumor growth was temporarily suppressed after first delivery of UMMD at day 11. However, the tumor growth returned back to exponential growth and additional UMMD treatment at days 14 and 16 had no effect with respect to the tumor growth.
S2 Figure. Radiation force balance measurement. Ultrasonic intensity was measured and calculated based on radiation force balance technique. The ultrasonic intensity was linearly correlated with US DC.
S3 Figure. Skin morphology following 5 days of UMMD treatment. There was no apparent morphologic difference between sham control (A) and UMMD treatment (B).
Acknowledgments
This work was supported by the Department of Orthopaedics and Rehabilitation and Pharmaceutics and Translation Therapeutics at the University of Iowa, the Intramural Research Program of the National Institutes of Health Clinical Center and the National Institute of Biomedical Imaging and Bioengineering. The authors thank Barbara J. Laughlin and John F. Bierman for providing experimental resources; and Dr. Nick Solokhin in Ultrasonic S-Lab for indispensable advice.
Footnotes
Additional Information
Competing financial interests: The authors declare no competing financial interests.
References
- Blomley MJ, Cooke JC, Unger EC, Monaghan MJ, Cosgrove DO. Microbubble contrast agents: a new era in ultrasound. BMJ. 2001;322:1222–5. doi: 10.1136/bmj.322.7296.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brujan EA, Vogel A. Stress wave emission and cavitation bubble dynamics by nanosecond optical breakdown in a tissue phantom. J Fluid Mech. 2006;558:281–308. [Google Scholar]
- Burks SR, Nguyen BA, Tebebi PA, Kim SJ, Bresler MN, Ziadloo A, Street JM, Yuen PST, Star RA, Frank JA. Pulsed Focused Ultrasound Pretreatment Improves Mesenchymal Stromal Cell Efficacy in Preventing and Rescuing Established Acute Kidney Injury in Mice. Stem Cells. 2015;33:1241–53. doi: 10.1002/stem.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson AR, McTiernan CF, Lavery L, Grata M, Leng X, Wang J, Chen X, Villanueva FS. Ultrasound-targeted microbubble destruction to deliver siRNA cancer therapy. Cancer Res. 2012;72:6191–9. doi: 10.1158/0008-5472.CAN-11-4079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CC, Wu SY, Finan JD, Morrison B, Konofagou EE. An Experimental Study on the Stiffness of Size-Isolated Microbubbles Using Atomic Force Microscopy. Ieee Transactions on Ultrasonics Ferroelectrics and Frequency Control. 2013;60:524–34. doi: 10.1109/TUFFC.2013.2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Hwang JH. Ultrasound-targeted microbubble destruction for chemotherapeutic drug delivery to solid tumors. J Ther Ultrasound. 2013;1:10. doi: 10.1186/2050-5736-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomas JE, Dayton P, Allen J, Morgan K, Ferrara KW. Mechanisms of contrast agent destruction. IEEE Trans Ultrason Ferroelectr Freq Control. 2001;48:232–48. doi: 10.1109/58.896136. [DOI] [PubMed] [Google Scholar]
- Chomas JE, Dayton PA, May D, Allen J, Klibanov A, Ferrara K. Optical observation of contrast agent destruction. Applied Physics Letters. 2000;77:1056–58. [Google Scholar]
- de Jong N, Emmer M, van Wamel A, Versluis M. Ultrasonic characterization of ultrasound contrast agents. Med Biol Eng Comput. 2009;47:861–73. doi: 10.1007/s11517-009-0497-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmer M, Vos HJ, Goertz DE, van Wamel A, Versluis M, de Jong N. Pressure-Dependent Attenuation and Scattering of Phospholipid-Coated Microbubbles at Low Acoustic Pressures. Ultrasound in Medicine and Biology. 2009;35:102–11. doi: 10.1016/j.ultrasmedbio.2008.07.005. [DOI] [PubMed] [Google Scholar]
- Feril LB, Kondo T, Zhao QL, Ogawa R, Tachibana K, Kudo N, Fujimoto S, Nakamura S. Enhancement of ultrasound-induced apoptosis and cell lysis by echo-contrast agents. Ultrasound in Medicine and Biology. 2003;29:331–37. doi: 10.1016/s0301-5629(02)00700-7. [DOI] [PubMed] [Google Scholar]
- Hassan MA, Feril LB, Jr, Suzuki K, Kudo N, Tachibana K, Kondo T. Evaluation and comparison of three novel microbubbles: enhancement of ultrasound-induced cell death and free radicals production. Ultrason Sonochem. 2009;16:372–8. doi: 10.1016/j.ultsonch.2008.10.003. [DOI] [PubMed] [Google Scholar]
- Henrique R, Azevedo R, Bento MJ, Domingues JC, Silva C, Jeronimo C. Prognostic value of Ki-67 expression in localized cutaneous malignant melanoma. Journal of the American Academy of Dermatology. 2000;43:991–1000. doi: 10.1067/mjd.2000.109282. [DOI] [PubMed] [Google Scholar]
- Hernot S, Klibanov AL. Microbubbles in ultrasound-triggered drug and gene delivery. Adv Drug Deliv Rev. 2008;60:1153–66. doi: 10.1016/j.addr.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang PT, You XD, Pan MQ, Li SY, Zhang Y, Zhao YZ, Wang MH, Hong YR, Pu ZX, Chen LR, Yang GG, Guo YM. A novel therapeutic strategy using ultrasound mediated microbubbles destruction to treat colon cancer in a mouse model. Cancer Lett. 2013;335:183–90. doi: 10.1016/j.canlet.2013.02.011. [DOI] [PubMed] [Google Scholar]
- Kodama T, Tomita Y, Koshiyama KI, Blomley MJK. Transfection effect of microbubbles on cells in superposed ultrasound waves and behavior of cavitation bubble. Ultrasound in Medicine and Biology. 2006;32:905–14. doi: 10.1016/j.ultrasmedbio.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Ladstein RG, Bachmann IM, Straume O, Akslen LA. Ki-67 expression is superior to mitotic count and novel proliferation markers PHH3, MCM4 and mitosin as a prognostic factor in thick cutaneous melanoma. Bmc Cancer. 2010:10. doi: 10.1186/1471-2407-10-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazovich D, Vogel RI, Berwick M, Weinstock MA, Anderson KE, Warshaw EM. Indoor Tanning and Risk of Melanoma: A Case-Control Study in a Highly Exposed Population. Cancer Epidem Biomar. 2010;19:1557–68. doi: 10.1158/1055-9965.EPI-09-1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SM, Betticher DC, Thatcher N. Melanoma - Chemotherapy. Brit Med Bull. 1995;51:609–30. doi: 10.1093/oxfordjournals.bmb.a072982. [DOI] [PubMed] [Google Scholar]
- Liu H, Chang S, Sun J, Zhu S, Pu C, Zhu Y, Wang Z, Xu RX. Ultrasound-mediated destruction of LHRHa-targeted and paclitaxel-loaded lipid microbubbles induces proliferation inhibition and apoptosis in ovarian cancer cells. Mol Pharm. 2014;11:40–8. doi: 10.1021/mp4005244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller KD, Siegel RL, Lin CC, Mariotto AB, Kramer JL, Rowland JH, Stein KD, Alteri R, Jemal A. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016;66:271–89. doi: 10.3322/caac.21349. [DOI] [PubMed] [Google Scholar]
- Miwa H, Numata K, Tanabe T, Koh R, Kaneko T, Sugimori K, Tanaka K, Maeda S. Differential Diagnosis of Solid Pancreatic Lesions by Using Three-Dimensional Contrast Enhanced Ultrasonography With High Mechanical Index Mode. Gastroenterology. 2012;142:S617–S17. [Google Scholar]
- Morton DL, Wen DR, Wong JH, Economou JS, Cagle LA, Storm FK, Foshag LJ, Cochran AJ. Technical Details of Intraoperative Lymphatic Mapping for Early Stage Melanoma. Arch Surg-Chicago. 1992;127:392–99. doi: 10.1001/archsurg.1992.01420040034005. [DOI] [PubMed] [Google Scholar]
- Mun GH. Management of malignant melanoma. Arch Plast Surg. 2012;39:565–74. doi: 10.5999/aps.2012.39.5.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overwijk WW, Restifo NP. B16 as a mouse model for human melanoma. Curr Protoc Immunol. 2001;Chapter 20(Unit 20):1. doi: 10.1002/0471142735.im2001s39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkin DM, Mesher D, Sasieni P. Cancers attributable to solar (ultraviolet) radiation exposure in the UK in 2010. Brit J Cancer. 2011;105:S66–S69. doi: 10.1038/bjc.2011.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston RC. Measurement and characterisation of the acoustic output of medical ultrasonic equipment. Part 1 Med Biol Eng Comput. 1986;24:113–20. doi: 10.1007/BF02443923. [DOI] [PubMed] [Google Scholar]
- Preston RC. Measurement and characterisation of the acoustic output of medical ultrasonic equipment. Part 2 Med Biol Eng Comput. 1986;24:225–34. doi: 10.1007/BF02441617. [DOI] [PubMed] [Google Scholar]
- Pu C, Chang S, Sun J, Zhu S, Liu H, Zhu Y, Wang Z, Xu RX. Ultrasound-mediated destruction of LHRHa-targeted and paclitaxel-loaded lipid microbubbles for the treatment of intraperitoneal ovarian cancer xenografts. Mol Pharm. 2014;11:49–58. doi: 10.1021/mp400523h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg SA, Yang JC, Topalian SL, Schwartzentruber DJ, Weber JS, Parkinson DR, Seipp CA, Einhorn JH, White DE. Treatment of 283 Consecutive Patients with Metastatic Melanoma or Renal-Cell Cancer Using High-Dose Bolus Interleukin-2. Jama-J Am Med Assoc. 1994;271:907–13. [PubMed] [Google Scholar]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- Soltani MH, Pichardo R, Song ZQ, Sangha N, Camacho F, Satyamoorthy K, Sangueza OP, Setaluri V. Microtubule-associated protein 2, a marker of neuronal differentiation, induces mitotic defects, inhibits growth of melanoma cells, and predicts metastatic potential of cutaneous melanoma. American Journal of Pathology. 2005;166:1841–50. doi: 10.1016/S0002-9440(10)62493-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song ZQ, He CD, Sun CK, Xu YN, Jin X, Zhang Y, Xiao T, Wang YK, Lu P, Jiang Y, Wei HC, Chen HD. Increased expression of MAP2 inhibits melanoma cell proliferation, invasion and tumor growth in vitro and in vivo. Experimental Dermatology. 2010;19:958–64. doi: 10.1111/j.1600-0625.2009.01020.x. [DOI] [PubMed] [Google Scholar]
- Sonoda S, Tachibana K, Uchino E, Yamashita T, Sakoda K, Sonoda KH, Hisatomi T, Izumi Y, Sakamoto T. Inhibition of melanoma by ultrasound-microbubble-aided drug delivery suggests membrane permeabilization. Cancer Biol Ther. 2007;6:1276–83. doi: 10.4161/cbt.6.8.4485. [DOI] [PubMed] [Google Scholar]
- Straume O, Sviland L, Akslen LA. Loss of nuclear p16 protein expression correlates with increased tumor cell proliferation (Ki-67) and poor prognosis in patients with vertical growth phase melanoma. Clinical Cancer Research. 2000;6:1845–53. [PubMed] [Google Scholar]
- Timbie KF, Mead BP, Price RJ. Drug and gene delivery across the blood-brain barrier with focused ultrasound. J Control Release. 2015;219:61–75. doi: 10.1016/j.jconrel.2015.08.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y, Aoi A, Horie S, Tomita N, Mori S, Morikawa H, Matsumura Y, Vassaux G, Kodama T. Low-intensity ultrasound and microbubbles enhance the antitumor effect of cisplatin. Cancer Sci. 2008;99:2525–31. doi: 10.1111/j.1349-7006.2008.00989.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber JS, Kahler KC, Hauschild A. Management of Immune-Related Adverse Events and Kinetics of Response With Ipilimumab. J Clin Oncol. 2012;30:2691–97. doi: 10.1200/JCO.2012.41.6750. [DOI] [PubMed] [Google Scholar]
- Wood AK, Sehgal CM. A review of low-intensity ultrasound for cancer therapy. Ultrasound Med Biol. 2015;41:905–28. doi: 10.1016/j.ultrasmedbio.2014.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin Z, Lin G, Lei H, Lue TF, Guo Y. Clinical applications of low-intensity pulsed ultrasound and its potential role in urology. Transl Androl Urol. 2016;5:255–66. doi: 10.21037/tau.2016.02.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeqiri B, Bickley CJ. A new anechoic material for medical ultrasonic applications. Ultrasound Med Biol. 2000;26:481–5. doi: 10.1016/s0301-5629(99)00147-7. [DOI] [PubMed] [Google Scholar]
- Zhang B, Hou YR, Chen T, Hu B. Microscopic study of ultrasound-mediated microbubble destruction effects on vascular smooth muscle cells. Asian Pac J Trop Med. 2015;8:325–9. doi: 10.1016/S1995-7645(14)60339-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
S1 Figure. The tumor suppressive effects of UMMD treatment on mice bearing with melanoma. Non-US treated control mice received PBS directly into the tumor at day 11, 14 and 16. Tumor growth showed gradual increase in volume until at day 12 and started growing exponentially from day 13. The tumor growth was temporarily suppressed after first delivery of UMMD at day 11. However, the tumor growth returned back to exponential growth and additional UMMD treatment at days 14 and 16 had no effect with respect to the tumor growth.
S2 Figure. Radiation force balance measurement. Ultrasonic intensity was measured and calculated based on radiation force balance technique. The ultrasonic intensity was linearly correlated with US DC.
S3 Figure. Skin morphology following 5 days of UMMD treatment. There was no apparent morphologic difference between sham control (A) and UMMD treatment (B).