Abstract
Double-hit lymphomas (DHL) and double-expressor lymphomas (DEL) are associated with resistance to frontline and salvage immunochemotherapy, as well as autologous stem cell transplantation (SCT). We hypothesized that allogeneic SCT (alloSCT) could overcome the chemoresistance associated with DEL/DHL. We retrospectively studied the impact of DEL/DHL status in a multicenter cohort of patients who underwent alloSCT for relapsed/refractory (rel/ref) aggressive B-cell non-Hodgkin lymphoma (B-NHL). 78 patients transplanted at 3 centers in whom tumor tissue was available for immunohistochemistry and FISH were enrolled; 47% had DEL and 13% had DHL. There were no significant differences in 4-year progression-free (PFS) or overall survival (OS) between patients with DEL compared to patients without DEL (PFS 30% v 39%, p=0.24; OS 31% v 49%, p=0.17) or between patients with DHL compared to patients without DHL (PFS 40% v 34%, p=0.62; OS 50% v 38%, p=0.46). The lack of association between DEL or DHL and outcome was confirmed in multivariable models, though limited sample size may have limited our ability to detect significant differences. In our cohort, alloSCT produced durable remissions in patients with rel/ref aggressive B-NHL irrespective of DEL and DHL status, justifying its consideration in the treatment of patients with rel/ref DEL/DHL.
Keywords: Double-hit lymphoma, double-expressor lymphoma, diffuse large B-cell lymphoma, MYC, allogeneic
Introduction
Double-hit lymphomas (DHL) with rearrangement of MYC and BCL2 and/or BCL6, and double-expressor lymphomas (DEL) with co-expression of MYC and BCL2 by immunohistochemistry, are subsets of aggressive B-cell non-Hodgkin lymphoma (B-NHL) associated with chemoresistance. Patients with DEL or DHL have poor outcomes after standard chemoimmunotherapy.1-18 Patients with relapsed or refractory (rel/ref) DEL or DHL have poor outcomes after salvage chemoimmunotherapy,12, 19, 20 and even patients with chemosensitive rel/ref DEL or DHL have inferior outcomes after autologous stem cell transplantation (autoSCT) compared to patients without either abnormality.21 Allogeneic stem cell transplantation (alloSCT) is an immune-based therapy that produces durable remissions in a subset of patients with rel/ref aggressive B-NHL even after failure of autoSCT.22, 23 We hypothesized that alloSCT, through its reliance on a graft-versus-lymphoma effect, could potentially abrogate the negative prognostic impact of DEL and DHL.
Methods
We performed a retrospective, multicenter study of adult patients with rel/ref aggressive B-NHL including diffuse large B-cell lymphoma (DLBCL), transformed indolent lymphoma (TIL), or high-grade B-cell lymphoma unclassified (BCLU, based on the 2008 WHO Classification) with available tumor tissue who underwent alloSCT at the Dana-Farber Cancer Institute/Brigham and Women’s Hospital, Massachusetts General Hospital, and City of Hope. Patients were transplanted between 1/2000 and 5/2014. Patients with Richter transformation of chronic lymphocytic leukemia or primary mediastinal B-cell lymphoma were excluded. Patients with a history of secondary central nervous system (CNS) involvement by lymphoma were eligible, but patients with primary CNS lymphoma were excluded. Patients who underwent a planned tandem transplantation or consolidative alloSCT in 1st response were excluded. Patients underwent alloSCT according to institutional practices.
Confirmation of the histologic diagnosis and review of immunohistochemistry was performed by two hematopathologists from the institutions (J.Y.S, Y.K., S.J.R, G.K.G); discrepancies were resolved at a multi-headed microscope. In all cases, even if previously evaluated in the course of a patient’s care, immunohistochemistry for MYC and BCL2 and fluorescence in situ hybridization (FISH) for MYC, BCL2, and BCL6 were performed as detailed previously.21 DEL was defined as MYC expression in ≥ 40% of tumor cells and BCL2 expression in ≥ 50% of tumor cells. DHL was defined as concurrent rearrangements of MYC and BCL2 and/or BCL6. “Atypical DHL” was defined as MYC without rearrangement but with at least 3+ copy gain (CG) along with one or more of the following: BCL2 3+CG, BCL6 3+CG, BCL2 or BCL6 rearrangement; or MYC rearrangement without BCL2 or BCL6 rearrangement, but with BCL2 3+ CG and/or BCL6 3+ CG. In all cases, the most recent available tumor specimen prior to alloSCT was analyzed.
Baseline characteristics were treated descriptively, and groups were compared as previously described.21 Survival analyses, incidences of non-relapse mortality (NRM), relapse/progression (CIR), and acute and chronic graft-versus-host disease (GVHD) as well as univariable and multivariable modeling were performed as described previously.24 In patients with TIL, relapse was considered a PFS event regardless of aggressive or indolent relapse histology. P-values were two-sided with a significance level of 0.05. All data was analyzed using SAS version 9.3 (Cary, NC) and R 3.1.2 (R Foundation for Statistical Computing, Vienna Austria). The study was approved by the Institutional Review Boards at all centers.
Results
At the participating centers, 318 patients with aggressive B-NHL underwent alloSCT during the study period and 220 patients met the eligibility criteria. Tumor tissue was available in 103 patients and complete immunohistochemistry, FISH, and clinical data were available in 78 patients, who comprised the study cohort. Immunohistochemistry, FISH, and baseline characteristics in the cohort are summarized in Table 1. There were 50 patients with de novo DLBCL, 25 patients with TIL, and 3 patients with BCLU. The median number of lines of prior therapy was 4 (range, 2-9), 58% of patients had prior autologous stem cell transplantation, and 49% of patients had primary refractory disease with initial therapy. Reduced intensity conditioning was utilized in 77% of patients, 36% of patients had a matched sibling donor, 42% a had fully HLA-matched (8/8) unrelated donor, and 22% had HLA-mismatched, haploidentical or umbilical cord donors. Most patients (58%) had tacrolimus and sirolimus based GVHD prophylaxis, and the use of peri-transplantation rituximab or anti-thymocyte globulin and other T-cell depleting strategies was infrequent (< 15% of the cohort) with no statistically significant imbalance in their use among the study groups (Supplemental Table 1). In order to evaluate for a possible selection bias, we compared the outcomes of these 78 patients with those of 142 patients with available relapse and survival information who were not included due to lack of tissue or incomplete data. The outcomes of the 2 groups were similar: 3-year PFS was 39% (95CI, 28-50%) in the cohort versus 38% (95CI, 30-47%) in the other patients (p=0.5), and 3-year OS was 44% (95CI, 32-55%) compared to 47% (95CI, 38-56%, p=0.7).
Table 1.
Summary of Immunohistochemistry and FISH Results and Comparison of Clinical Characteristics Between Patients with DEL, DHL, and Neither DEL nor DHL
| Variable | All N (%)a | Non-DHL/Non-DEL N (%)a | DEL (non-DHL) N (%)a | DHL N (%)a | p valueb |
|---|---|---|---|---|---|
|
| |||||
| Total | 78 | 37 | 31 | 10 | |
|
| |||||
| IHC | |||||
| MYC median (range) | 40% (0-90) | ||||
| BCL2 median (range) | 90% (0-100) | ||||
| DEL | 37 (47) | ||||
| FISH | |||||
| MYC not rearranged | 57 (73) | 33 (89) | 24 (77) | 0 (0) | |
| MYC rearranged | 21 (27) | 4 (11) | 7 (23) | 10 (100) | |
| MYC only | 11 (14) | 4 (11) | 7 (23) | 0 (0) | |
| DHL | 10 (13) | 0 (0) | 0 (0) | 10 (100) | |
| Atypical DHL | 17 (22) | 9 (24) | 8 (26) | 0 (0) | |
|
| |||||
| Age median (range) | 54 (24-69) | 55 (24-69) | 54 (32-69) | 47 (34-62) | 0.4 |
|
| |||||
| Gender | |||||
| Male | 53 (68) | 28 (76) | 21 (68) | 4 (40) | |
| Female | 25 (32) | 9 (24) | 10 (32) | 6 (60) | |
|
| |||||
| Histology | 0.089 | ||||
| DLBCL/BCLU | 53 (68) | 25 (68) | 24 (77) | 4 (40) | |
| TIL | 25 (32) | 12 (32) | 7 (23) | 6 (60) | |
|
| |||||
| Prior lines of therapy (median, range) | 4 (2-9) | 4 (2-8) | 4 (2-6) | 5 (2-9) | 0.16 |
|
| |||||
| Prior autoSCT | 45 (58) | 21 (57) | 17 (55) | 7 (70) | 0.8 |
|
| |||||
| Disease status at SCT | 0.8 | ||||
| Remission | 58 (74) | 27 (73) | 23 (74) | 8 (80) | |
| CR | 33 (42) | 18 (49) | 11 (35) | 4 (40) | |
| PR | 25 (32) | 9 (24) | 12 (39) | 4 (40) | |
| Not in remission | 19 (24) | 10 (27) | 7 (23) | 2 (20) | |
| SD | 10 (13) | 5 (14) | 4 (13) | 1 (10) | |
| PD | 9 (12) | 5 (14) | 3 (10) | 1 (10) | |
| Unknown | 1 (1) | 0 (0) | 1 (3) | 0 (0) | |
|
| |||||
| Primary refractory disease | 38 (49) | 15 (41) | 17 (55) | 6 (60) | 0.4 |
|
| |||||
| GVHD prophylaxis | 0.3 | ||||
| CNI/MTX | 13 (18) | 7 (19) | 6 (19) | 0 (0) | |
| CNI/MMF +/- MTX | 10 (13) | 4 (11) | 3 (10) | 3 (30) | |
| CNI/SIRO +/- MTX | 45 (58) | 22 (60) | 16 (52) | 7 (70) | |
| Other | 10 (13) | 4 (11) | 6 (19) | 0 (0) | |
|
| |||||
| Donor | 0.2 | ||||
| MSD | 28 (36) | 17 (46) | 10 (32) | 1 (10) | |
| MUD | 33 (42) | 14 (38) | 13 (42) | 6 (60) | |
| MMUD | 8 (10) | 4 (11) | 3 (10) | 1 (10) | |
| Haplo | 3 (4) | 0 (0) | 3 (10) | 0 (0) | |
| Cord | 6 (8) | 2 (5) | 2 (6) | 2 (20) | |
|
| |||||
| Graft source | 0.14 | ||||
| PBSC | 69 (88) | 35 (95) | 26 (84) | 8 (80) | |
| BM | 3 (4) | 0 (0) | 3 (10) | 0 (0) | |
| UCB | 6 (8) | 2 (5) | 2 (6) | 2 (20) | |
|
| |||||
| Conditioning | 1.0 | ||||
| MAC | 18 (23) | 9 (24) | 7 (23) | 2 (20) | |
| RIC | 60 (77) | 28 (76) | 24 (77) | 8 (80) | |
|
| |||||
| Months from Dx to AlloSCT | 0.220 | ||||
| Median | 33.2 | 30.4 | 30.5 | 63.5 41.3, 92.2 | |
| Interquartile range | 12.4, 58.3 | 10.7, 55.7 | 13.3, 45.8 | ||
| Range | (0.2-161.9) | (1.8-153.0) | (4.3-161.9) | (0.2-128.6) | |
IHC indicates immunohistochemistry, DEL indicates double-expressor lymphoma, FISH indicates fluorescence in situ hybridization, DHL indicates double-hit lymphoma, DLBCL indicates diffuse large B-cell lymphoma, BCLU indicates B-cell lymphoma unclassified, TIL indicates transformed indolent lymphoma, autoSCT indicates autologous stem cell transplantation, SCT indicates stem cell transplantation, CR indicates complete response, PR indicates partial response, SD indicates stable disease, PD indicates progressive disease, GVHD indicates graft-versus-host disease, CNI indicates calcineurin inhibitor, MTX indicates methotrexate, MMF indicates mycophenolate mofetil, SIRO indicates sirolimus, MSD indicates matched sibling donor, MUD indicates matched unrelated donor, MMUD indicates mismatched unrelated donor, Haplo indicates haploidentical donor, Cord indicates umbilical cord blood donor, PBSC indicates peripheral blood stem cells, BM indicates bone marrow, UCB indicates umbilical cord blood, MAC indicates myeloablative conditioning, RIC indicates reduced intensity conditioning, Dx indicates diagnosis
In the study cohort, the median follow-up for survivors was 46 months (range, 18-147). The overall cumulative incidences of GVHD were 22% grade II-IV acute GVHD at day +100, 10% grade III-IV acute GVHD at day +100, and 37% chronic GVHD at 1 year. The PFS, OS, CIR, and NRM at 4 years were 35% (95CI, 24-46%), 40% (95CI, 29-51%), 43% (95CI, 32-54%) and 22% (95CI, 13-32%), respectively.
Among the 78 patients, 47% had DEL and 13% had DHL. There were no significant differences in clinical characteristics (Table 1) or outcome when patients with DHL or DEL were compared to patients with neither DHL nor DEL. The 4-year PFS in patients with DEL compared to patients without MYC/BCL2 coexpression was 30% (95CI, 18-51%) versus 39% (95CI, 26-58%, p=0.24); the corresponding 4-year OS was 31% (95CI, 18-52%) versus 49% (95CI, 35-68%, p=0.17); 4-year CIR was 50% (95CI, 35-70%) versus 38% (95CI, 25-57%, p=0.20); and 4-year NRM was 20% (95CI, 10-40%) versus 23% (95CI, 13-42%, p=0.75, Figure 1A and 1B). The 4-year PFS in patients with DHL compared to patients who did not have DHL was 40% (95CI, 19-85%) versus 34% (95CI, 24-48%, p=0.62); 4-year OS 50% (95CI, 27-93%) versus 38% (95CI, 27-53%, p=0.46); 4-year CIR 40% (95CI, 18-91%) versus 44% (95CI, 33-58%, p=0.90); and 4-year NRM 20% (95CI, 5-77%) versus 22% (95CI, 14-36%, p=0.81, Figure 2A and 2B) As DHL and DEL status can overlap, we also compared mutually exclusive subgroups: no significant differences in PFS were observed when patients with DHL were compared to patients with DEL/non-DHL, and to patients with neither DEL nor DHL (4-year PFS 40% versus 29%, versus 38%, respectively, p=0.56, Figure 2C). Among 45 patients who had previously undergone autoSCT, there was no significant difference in PFS between patients with DHL, DEL/non-DHL, and neither DEL nor DHL (data not shown, p=0.4). Of note, there was no imbalance in cause of death among these subgroups (Supplemental Table 3). When the analyses were limited to patients with DLBCL/BCLU or separately to patients with TIL, there were no significant differences in PFS between patients with DHL, DEL/non-DHL, and neither DEL nor DHL (data not shown, DLBCL/BCLU subgroup, p=0.4; TIL subgroup, p=0.6). Lastly, 22% of patients had atypical DHL, which was not associated with a significant difference in outcome: 4-year PFS of 34% compared to 36% in patients who did not have atypical DHL (p=0.9), 4-year OS of 46% compared to 38% (p=0.9).
Figure 1.
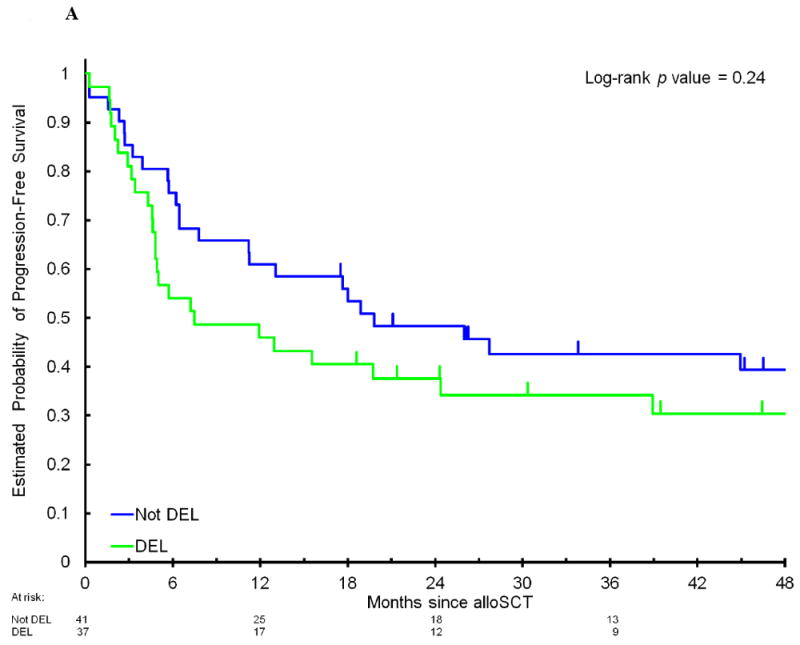

Progression-free survival (A) and overall survival (B) after allogeneic stem cell transplantation in patients with DEL compared to non-DEL patients.
Figure 2.
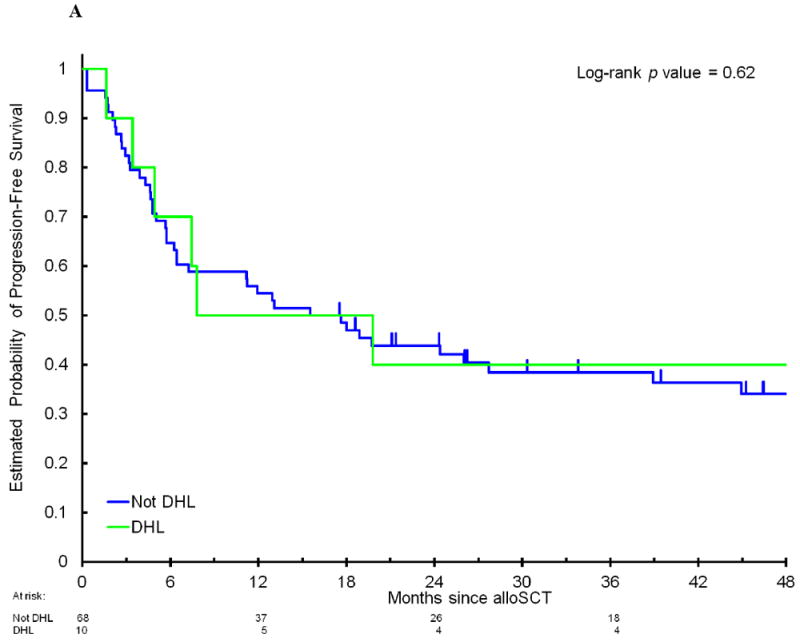
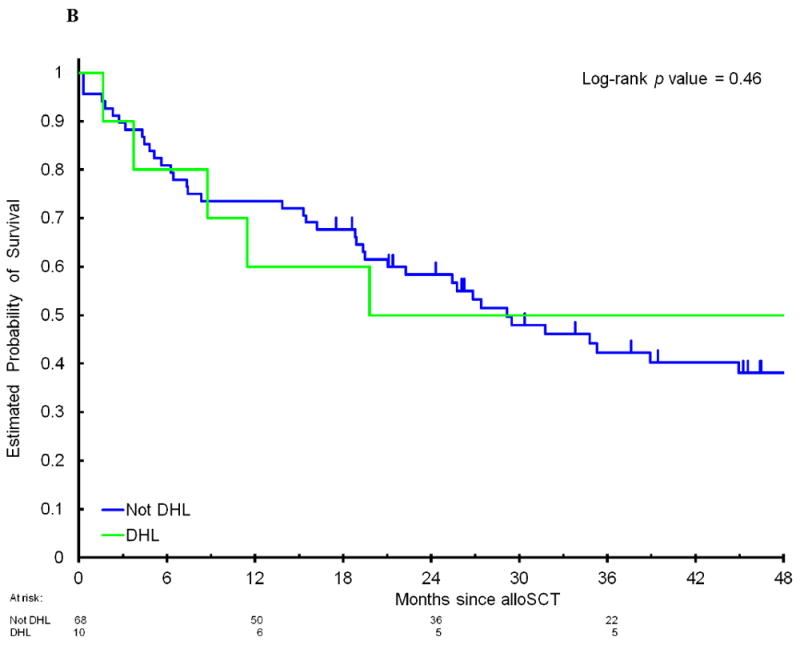
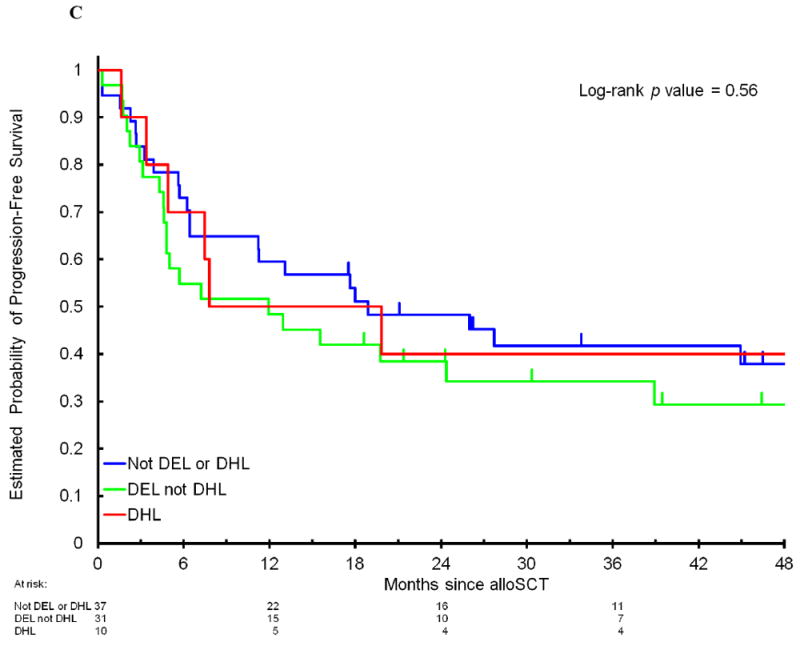
Progression-free survival (A) and overall survival (B) after allogeneic stem cell transplantation in patients with DHL compared to non-DHL patients. (C) Progression-free survival in patients with DHL compared to patients with DEL without DHL and patients with neither DEL nor DHL. DEL indicates double-expressor lymphoma. DHL indicates double-hit lymphoma.
We confirmed our findings in multivariable Cox models stratified by conditioning regimen. In those models, factors associated with a lower incidence of relapse and improved PFS and OS were age > 55, CR/PR prior to alloSCT, and TIL histology (for PFS only). No variable was significantly associated with NRM. DEL and DHL were not significantly associated with PFS, OS, CIR or NRM in the models (Tables 2 and 3). Due to the unexpected association between older age and improved PFS and OS, we assessed whether there were differences in the HCT-CI scores of older versus younger patients, as well as between the DHL, DEL, and non-DEL/non-DHL subgroups, but no differences in HCT-CI were observed (Supplemental Table 2). In addition, older age remained associated with favorable PFS and OS when only patients who underwent RIC alloSCT (n = 60) were analyzed (data not shown). Patients with DEL appeared to have a lower incidence of chronic GVHD (24% versus 49%, p=0.039) as compared with patients who did not have DEL but no difference in acute GVHD was observed.
Table 2.
Multivariable Cox regression model for Progression-Free and Overall Survival
| PFS | OS | ||||
|---|---|---|---|---|---|
| Variable | N | HR (95%CI) | Wald test P value* | HR (95%CI) | Wald test P value* |
| Age, years | |||||
| ≤55 | 41 | Reference | Reference | ||
| >55 | 37 | 0.43 (0.24 to 0.78)* | 0.005* | 0.46 (0.25 to 0.85)* | 0.013* |
| Disease status at transplant | |||||
| Remission (CR or PR) | 58 | Reference | Reference | ||
| Not in remission or unknown | 20 | 2.46 (1.28 to 4.70)* | 0.007* | 2.52 (1.28 to 4.97)* | 0.007* |
| Histology | |||||
| DLBCL/BCL-u | 53 | Reference | Reference | ||
| Transformed indolent B-NHL | 25 | 0.48 (0.25 to 0.91)* | 0.024* | 0.48 (0.24 to 0.93)* | 0.030* |
| DEL | |||||
| No | 41 | Reference | Reference | ||
| Yes | 37 | 1.24 (0.71 to 2.17)§ | 0.45§ | 1.52 (0.85 to 2.73)§ | 0.16§ |
| DHL | |||||
| No | 68 | Reference | Reference | ||
| Yes | 10 | 0.88 (0.37 to 2.11) § | 0.78§ | 0.86 (0.32 to 2.27)§ | 0.76§ |
Based on the Cox proportional hazards regression model including only three top variables in the table and stratified by conditioning regimen.
Based on the Cox proportional hazards regression model adjusting three top variables in the table and stratified by conditioning regimen.
Table 3.
Multivariable Cox regression model with competing risks for Relapse and Non-Relapse Mortality
| Relapse | NRM | |||
|---|---|---|---|---|
| Variable | HR (95%CI) | Wald test P value* | HR (95%CI) | Wald test P value* |
| Age, years | ||||
| ≤55 | Reference | |||
| >55 | 0.37 (0.18 to 0.78) | 0.008 | 0.87 (0.33 to 2.25) | 0.77 |
| Disease status at transplant | ||||
| Remission (CR or PR) | ||||
| Not in remission or unknown | 3.99 (1.83 to 8.71) | <0.001 | 0.41 (0.13 to 1.34) | 0.14 |
| Histology | ||||
| DLBCL/BCL-u | Reference | |||
| Transformed indolent B-NHL | 0.34 (0.15 to 0.80) | 0.013 | 1.51 (0.68 to 3.37) | 0.32 |
| DEL | ||||
| No | Reference | |||
| Yes | 1.28 (0.63 to 2.61) | 0.50 | 0.75 (0.31 to 1.84) | 0.53 |
| DHL | ||||
| No | Reference | |||
| Yes | 1.17 (0.39 to 3.50) | 0.79 | 0.85 (0.27 to 2.65) | 0.77 |
Based on the proportional subdistribution hazards model for competing risks including only three top variables in the table and stratified by conditioning regimen.
Based on the Cox proportional hazards regression model with competing risks adjusting three top variables in the table and stratified by conditioning regimen.
Discussion
In our multicenter cohort study evaluating the prognostic impact of DEL and DHL in a real-world population of patients with rel/ref aggressive B-NHL who underwent alloSCT, patients with DEL or DHL did not have a significantly inferior outcome compared to patients without either abnormality. In addition, patients with cytogenetic abnormalities of MYC and BCL2 and/or BCL6 other than concurrent rearrangement did not have a significantly different outcome than patients who did not have atypical DHL. Because of the small number of patients included, our ability to detect meaningful differences between the groups may have been hampered.
As a retrospective study, our analysis is subject to inherent limitations and biases. For example, although the incidence of chronic GVHD in our cohort is consistent with what was observed in prior studies of allo SCT for DLBCL, the incidence of grade 2-4 acute GVHD was lower than expected in our cohort. This was not explained by the use of peri-transplantation rituximab, ATG, or other T-cell depleting strategies, which occurred in < 15% of patients. We attempted to minimize these potential biases by including all patients who had tissue available for testing among consecutively transplanted patients during the specified period. In addition, there was no significant difference in outcome between patients included in the study and patients who were not included due to lack of tissue or incomplete data. Our study only evaluated patients who were able to undergo alloSCT, and thus does not answer the question of whether patients with rel/ref DHL or DEL are less likely to eventually receive alloSCT, which is plausible given the difficulty of obtaining remission with salvage therapy. It is possible that there was a selection bias in the choice of transplant strategy for these patients. However, there were virtually no patients in whom DEL or DHL status was considered in the transplant decision-making, as most of the time the testing to identify DEL/DHL was performed only for this study, after the alloHSCT.
There are limitations in multivariable modeling of cohorts with small sample sizes. Here we performed multivariable analyses only to exclude a strong confounding effect by other known prognostic variables. As expected, refractory disease at alloSCT was associated with poorer outcome. A history of prior indolent lymphoma was associated with favorable outcome, a finding which has been previously reported25 and requires validation in a larger cohort. The result that older patients had a favorable outcome is unexpected. That older patients had improved outcomes was not explained by an unexpected imbalance in HCT-CI scores, nor could it be explained by differences in conditioning regimen intensity, since older patients had more favorable outcome even in the subgroup of patients who underwent RIC alloSCT. The implication of this finding is unclear, and we feel that they are likely an artifact of our small study cohort. The observed association between DEL and a lower incidence of chronic GVHD may have been explained by the relatively higher proportion of patients in the DEL group (19%) who received ATG or other T-cell depleting strategies (post-transplant cyclophosphamide) compared to other groups (3% in non-DEL/non-DHL patients and 0% in DHL patients) though the difference in strategies among groups was not quite significant (p=0.052).
In conclusion, our results confirm that alloSCT produces durable remissions in a subset of patients with rel/ref aggressive B-NHL. More importantly, the outcome of alloSCT did not appear to differ based on either DEL or DHL status. This stands in contrast to previous studies showing that DHL and DEL are associated with adverse outcome after standard front-line chemoimmunotherapy,5, 8-14, 17 salvage therapy,19, 20 and autoSCT.21 Biologically, this suggests that while DEL and DHL confer chemoresistance, they are not necessarily associated with immune resistance, and thus can potentially be targeted with the evolving weaponry of immunotherapy. Nevertheless, the high incidence of post-alloSCT disease progression we observed across the disease subgroups demonstrates that improvement of alloSCT outcomes in patients with aggressive B-NHL remains a tremendous unmet need. Novel approaches to decreasing post-alloSCT disease relapse are desperately needed and the incorporation of targeted agents (e.g. ibrutinib in activated B-cell subtype DLBCL) or immunotherapies (e.g. PD-1 inhibitors or chimeric antigen receptor modified T-cells) prior to, during, and following conditioning (e.g. maintenance) should be studied. Clinically, the main implication of our study is for patients with rel/ref DEL or DHL who are able to attain remission. Since salvage treatment is often ineffective in these patients and autoSCT yields poor outcomes, alloSCT should be considered when remission can be achieved. Our intriguing findings merit further study of this question in a larger cohort and ideally should be confirmed in a prospective study.
Supplementary Material
Highlights.
We retrospectively studied the prognostic impact of double-expressor (DEL) and double-hit lymphoma (DHL) in a multicenter alloSCT cohort.
In patients with relapsed or refractory aggressive B-cell non-Hodgkin lymphoma, DEL and DHL status did not impact alloSCT outcome.
The study findings were confirmed in multivariable analyses
Acknowledgments
A.F.H was supported by a Conquer Cancer Foundation/ASCO Young Investigator Award, and the National Cancer Institute of the National Institutes of Health under award numbers NIH 2K12CA001727-21 and P50CA107399. Research reported in this publication included work performed in the COH Pathology Core supported by the National Cancer Institute of the National Institutes of Health under award number P30CA033572. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. A.F.H., S.J.R., D.M.W., and P.A. were supported by a Dana-Farber Cancer Institute Award Fund for Collaborative Research Initiatives in Hematologic Oncology award for this work. D.M.W. is a Leukemia and Lymphoma Society Scholar. This work was also generously supported by the Harold and Virginia Lash/David Lash Fund for Lymphoma Research.
Disclosure of Conflicts of Interest
Alex F. Herrera
Consulting or Advisory Role: Pharmacyclics, Genentech, Merck; Research Funding: Seattle Genetics (Inst), Pharmacyclics (Inst), Genentech (Inst), Immune Design (Inst), Sequenta (Inst), Merck (Inst), Bristol-Myers Squibb (Inst), KiTE Pharma (Inst), Rhizen Pharmaceuticals S.A. (Inst)
Scott J. Rodig
Honoraria: Perkin Elmer, Bristol-Myers Squibb, Consulting or Advisory Role: AstraZeneca, Perkin Elmer, Research Funding: Bristol-Myers Squibb
Joo Y. Song
Consulting or Advisory Role: Seattle Genetics
Young Kim
Research Funding: Seattle Genetics
Gabriel K. Griffin: No relationship to disclose
Dongyun Yang: No relationship to disclose
Liana Nikolaenko: No relationship to disclose
Matthew Mei: No relationship to disclose
Victoria Bedell: No relationship to disclose
Paola Dal Cin: No relationship to disclose
Christine Pak: No relationship to disclose
Edwin P. Alyea: No relationship to disclose
Lihua E. Budde: No relationship to disclose
Robert Chen
Consulting or Advisory Role: Seattle Genetics, Merck, Genentech; Speakers’ Bureau: Seattle Genetics, Genentech, Millennium Pharmaceuticals; Research Funding: Seattle Genetics, Pharmacyclics, Merck, Millennium Pharmaceuticals
Yi-Bin Chen: No relationship to disclose
Wing C. Chan: No relationship to disclose
Corey S. Cutler: No relationship to disclose
Vincent T. Ho: No relationship to disclose
John Koreth: No relationship to disclose
Amrita Krishnan
Stock or Other Ownership: Celgene (I), Infinity Pharmaceuticals (I); Consulting or Advisory Role: Celgene, Spectrum Pharmaceuticals, Onyx, Janssen Oncology, Takeda; Speakers’ Bureau: Celgene, Millennium Pharmaceuticals, Onyx, Janssen Oncology; Research Funding: Celgene (Inst), Takeda (Inst)
Joyce Murata-Collins: No relationship to disclose
Sarah Nikiforow: No relationship to disclose
Joycelynne M. Palmer: No relationship to disclose
German A. Pihan: No relationship to disclose
Raju Pillai
Research Funding: Trillium Therapeutics
Leslie Popplewell
Honoraria: Cardinal Health
Steven T. Rosen
Honoraria: Celgene, Genentech, Seattle Genetics; Consulting or Advisory Role: Celgene, Genentech Health Practices Consulting, Seattle Genetics; Speakers’ Bureau: Celgene, Seattle Genetics
Tanya Siddiqi
Speakers’ Bureau: Pharmacyclics, Seattle Genetics; Research Funding: Pharmacyclics (Inst), Juno Therapeutics (Inst), Kite Pharma (Inst), Acerta Pharma (Inst), MedImmune (Inst), Genentech (Inst), TG Therapeutics (Inst), Merck (Inst), Boehringer Ingelheim (Inst), Karyopharm Therapeutics (Inst)
Aliyah R. Sohani
Research Funding: Sysmex America
Jasmine Zain
Honoraria: Seattle Genetics, Celgene, Spectrum Pharmaceuticals; Consulting or Advisory Role: Seattle Genetics, Celgene, Spectrum
Pharmaceuticals
Speakers’ Bureau: Seattle Genetics, Celgene, Spectrum Pharmaceuticals
Larry W. Kwak
Stock or Other Ownership: XEME BioPharma, Antigenics; Consulting or Advisory Role: XEME BioPharma, Celltrion, Sella Life Sciences
Dennis D. Weisenburger
Honoraria: Seattle Genetics; Consulting or Advisory Role: Seattle Genetics; Speakers’ Bureau: Seattle Genetics
David M. Weinstock
Honoraria: Seattle Genetics, Infinity Pharmaceuticals, Genentech; Consulting or Advisory Role: Novartis, Pangaea Biotech; Research Funding: Novartis; Expert Testimony: Monsanto
Robert J. Soiffer: No relationship to disclose
Joseph H. Antin: No relationship to disclose
Stephen J. Forman: No relationship to disclose
Auayporn P. Nademanee
Consulting or Advisory Role: Seattle Genetics, Gilead Sciences; Speakers’ Bureau: Seattle Genetics
Research Funding: Seattle Genetics
Philippe Armand
Consulting or Advisory Role: Bristol-Myers Squibb, Merck Sharp & Dohme, Pfizer; Research Funding: Merck Sharp & Dohme (Inst), Bristol-Myers Squibb (Inst), Sequenta (Inst), Tensha Therapeutics (Inst), Sigma-Tau (Inst), Otsuka (Inst), Affimed Therapeutics (Inst)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pedersen MO, Gang AO, Poulsen TS, et al. MYC translocation partner gene determines survival of patients with large B-cell lymphoma with MYC- or double-hit MYC/BCL2 translocations. European journal of haematology. 2014;92:42–48. doi: 10.1111/ejh.12212. [DOI] [PubMed] [Google Scholar]
- 2.Tzankov A, Xu-Monette ZY, Gerhard M, et al. Rearrangements of MYC gene facilitate risk stratification in diffuse large B-cell lymphoma patients treated with rituximab-CHOP. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2014;27:958–971. doi: 10.1038/modpathol.2013.214. [DOI] [PubMed] [Google Scholar]
- 3.Pillai RK, Sathanoori M, Van Oss SB, Swerdlow SH. Double-hit B-cell lymphomas with BCL6 and MYC translocations are aggressive, frequently extranodal lymphomas distinct from BCL2 double-hit B-cell lymphomas. The American journal of surgical pathology. 2013;37:323–332. doi: 10.1097/PAS.0b013e31826cebad. [DOI] [PubMed] [Google Scholar]
- 4.Copie-Bergman C, Cuilliere-Dartigues P, Baia M, et al. MYC-IG rearrangements are negative predictors of survival in DLBCL patients treated with immunochemotherapy: a GELA/LYSA study. Blood. 2015;126:2466–2474. doi: 10.1182/blood-2015-05-647602. [DOI] [PubMed] [Google Scholar]
- 5.Hu S, Xu-Monette ZY, Tzankov A, et al. MYC/BCL2 protein coexpression contributes to the inferior survival of activated B-cell subtype of diffuse large B-cell lymphoma and demonstrates high-risk gene expression signatures: a report from The International DLBCL Rituximab-CHOP Consortium Program. Blood. 2013;121:4021–4031. doi: 10.1182/blood-2012-10-460063. quiz 4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Valera A, Lopez-Guillermo A, Cardesa-Salzmann T, et al. MYC protein expression and genetic alterations have prognostic impact in patients with diffuse large B-cell lymphoma treated with immunochemotherapy. Haematologica. 2013;98:1554–1562. doi: 10.3324/haematol.2013.086173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Visco C, Tzankov A, Xu-Monette ZY, et al. Patients with diffuse large B-cell lymphoma of germinal center origin with BCL2 translocations have poor outcome, irrespective of MYC status: a report from an International DLBCL rituximab-CHOP Consortium Program Study. Haematologica. 2013;98:255–263. doi: 10.3324/haematol.2012.066209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Green TM, Young KH, Visco C, et al. Immunohistochemical double-hit score is a strong predictor of outcome in patients with diffuse large B-cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30:3460–3467. doi: 10.1200/JCO.2011.41.4342. [DOI] [PubMed] [Google Scholar]
- 9.Johnson NA, Slack GW, Savage KJ, et al. Concurrent expression of MYC and BCL2 in diffuse large B-cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30:3452–3459. doi: 10.1200/JCO.2011.41.0985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Snuderl M, Kolman OK, Chen YB, et al. B-cell lymphomas with concurrent IGH-BCL2 and MYC rearrangements are aggressive neoplasms with clinical and pathologic features distinct from Burkitt lymphoma and diffuse large B-cell lymphoma. The American journal of surgical pathology. 2010;34:327–340. doi: 10.1097/PAS.0b013e3181cd3aeb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oki Y, Noorani M, Lin P, et al. Double hit lymphoma: the MD Anderson Cancer Center clinical experience. British journal of haematology. 2014;166:891–901. doi: 10.1111/bjh.12982. [DOI] [PubMed] [Google Scholar]
- 12.Petrich AM, Gandhi M, Jovanovic B, et al. Impact of induction regimen and stem cell transplantation on outcomes in double-hit lymphoma: a multicenter retrospective analysis. Blood. 2014;124:2354–2361. doi: 10.1182/blood-2014-05-578963. [DOI] [PubMed] [Google Scholar]
- 13.Savage KJ, Johnson NA, Ben-Neriah S, et al. MYC gene rearrangements are associated with a poor prognosis in diffuse large B-cell lymphoma patients treated with R-CHOP chemotherapy. Blood. 2009;114:3533–3537. doi: 10.1182/blood-2009-05-220095. [DOI] [PubMed] [Google Scholar]
- 14.Johnson NA, Savage KJ, Ludkovski O, et al. Lymphomas with concurrent BCL2 and MYC translocations: the critical factors associated with survival. Blood. 2009;114:2273–2279. doi: 10.1182/blood-2009-03-212191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barrans S, Crouch S, Smith A, et al. Rearrangement of MYC is associated with poor prognosis in patients with diffuse large B-cell lymphoma treated in the era of rituximab. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28:3360–3365. doi: 10.1200/JCO.2009.26.3947. [DOI] [PubMed] [Google Scholar]
- 16.Cohen JB, Geyer SM, Lozanski G, et al. Complete response to induction therapy in patients with Myc-positive and double-hit non-Hodgkin lymphoma is associated with prolonged progression-free survival. Cancer. 2014;120:1677–1685. doi: 10.1002/cncr.28642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Horn H, Ziepert M, Becher C, et al. MYC status in concert with BCL2 and BCL6 expression predicts outcome in diffuse large B-cell lymphoma. Blood. 2013;121:2253–2263. doi: 10.1182/blood-2012-06-435842. [DOI] [PubMed] [Google Scholar]
- 18.Perry AM, Alvarado-Bernal Y, Laurini JA, et al. MYC and BCL2 protein expression predicts survival in patients with diffuse large B-cell lymphoma treated with rituximab. British journal of haematology. 2014;165:382–391. doi: 10.1111/bjh.12763. [DOI] [PubMed] [Google Scholar]
- 19.Allen GA, Ruano Mendez AL, Rybicki, et al. Outcomes of patients with relapsed/refractory diffuse large B-cell lymphoma treated with R-ICE based on dual expression of MYC and BCL2J. Clin Oncol. 2016;34(suppl) Abstract e19043. [Google Scholar]
- 20.Miura K, Takahashi H, Nakagawa M, et al. Clinical significance of co-expression of MYC and BCL2 protein in aggressive B-cell lymphomas treated with a second line immunochemotherapy. Leukemia & lymphoma. 2016;57:1335–1341. doi: 10.3109/10428194.2015.1096352. [DOI] [PubMed] [Google Scholar]
- 21.Herrera AF, Mei M, Low L, et al. Relapsed or Refractory Double-Expressor and Double-Hit Lymphomas Have Inferior Progression-Free Survival After Autologous Stem-Cell Transplantation. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2017;35:24–31. doi: 10.1200/JCO.2016.68.2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bacher U, Klyuchnikov E, Le-Rademacher J, et al. Conditioning regimens for allotransplants for diffuse large B-cell lymphoma: myeloablative or reduced intensity? Blood. 2012;120:4256–4262. doi: 10.1182/blood-2012-06-436725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fenske TS, Ahn KW, Graff TM, et al. Allogeneic transplantation provides durable remission in a subset of DLBCL patients relapsing after autologous transplantation. British journal of haematology. 2016;174:235–248. doi: 10.1111/bjh.14046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Armand P, Gannamaneni S, Kim HT, et al. Improved survival in lymphoma patients receiving sirolimus for graft-versus-host disease prophylaxis after allogeneic hematopoietic stem-cell transplantation with reduced-intensity conditioning. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26:5767–5774. doi: 10.1200/JCO.2008.17.7279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tada K, Kim SW, Asakura Y, et al. Comparison of outcomes after allogeneic hematopoietic stem cell transplantation in patients with follicular lymphoma, diffuse large B-cell lymphoma associated with follicular lymphoma, or de novo diffuse large B-cell lymphoma. American journal of hematology. 2012;87:770–775. doi: 10.1002/ajh.23246. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


