Abstract
Signals from growth factors or mechanical stimuli converge to promote vascular smooth muscle cell (VSMC) migration and proliferation, key events in the pathogenesis of intimal hyperplasia upon vascular injury. Spry1, a regulator of receptor tyrosine kinases (RTK), plays a role in maintaining the contractile phenotype of VSMC. The aim of the current study was to determine the role of Spry1 in VSMC proliferation in vitro and injury induced neointimal hyperplasia in vivo. VSMC proliferation and neointima formation were evaluated in cultured human aortic SMC (hAoSMC) and ligation-induced injury of mouse carotid arteries from Spry1 gene targeted mice, and their corresponding wild type littermates. Human Spry1 or non-targeting control lentiviral shRNAs were used to knock down Spry1 in hAoSMC. Time course cell cycle analysis showed a reduced fraction of S-phase cells at 12 and 24 hours after growth medium stimulation in Spry1 shRNA transduced hAoSMC. Consistent with reduced S-phase entry, the induction of cyclinD1 and the levels of pRbS807/S811, pH3Ser10, and pCdc2 were also reduced, while the cell cycle inhibitor p27Kip1 was maintained in Spry1 knockdown hAoSMC. In vivo, loss of Spry1 attenuated carotid artery ligation-induced neointima formation in mice, and this effect was accompanied by a decrease in cell proliferation similar to the in vitro results. Our findings demonstrate that loss of Spry1 attenuates mitogen-induced VSMC proliferation, and thus injury-induced neointimal hyperplasia likely via insufficient activation of Akt signaling causing decreased cyclinD1 and increased p27Kip1 and a subsequent decrease in Rb and cdc2 phosphorylation.
Keywords: Spry1, restenosis, cell cycle, cyclinD1, p27kip1, PI3K/Akt, Rb, vascular smooth muscle cell
Vascular smooth muscle cell (VSMC) proliferation underlies the development of post-angioplasty restenosis, venous graft failure, and transplant arteriosclerosis [Newby and Zaltsman, 2000]. Numerous growth factors and cytokines as well as mechanical injury triggers VSMC migration and proliferation, resulting in neointima formation and vessel narrowing. Understanding the regulation of VSMC proliferation is of importance to the development of therapeutic strategies in treating the aforementioned cardiovascular diseases.
Cell proliferation is controlled by several checkpoints in the cell cycle. The critical cellular components that drive cell cycle progression are the cyclin-dependent kinases (CDKs), a family of multifunctional enzymes that phosphorylate protein substrates involved in cell cycle progression, particularly the G1/S transition restricting protein; retinoblastoma protein (Rb). CyclinD1, a positive cell cycle regulator binds to and activates CDK4/6, is highly inducible and plays a critical role in driving G1/S transition of cell cycle. Growth factors and other mitogenic stimuli induce cyclinD1 expression through multiple signaling pathways including MAPK/ERK, PI3K/Akt, p38MAPK and JNK [Lavoie et al., 1996; Liang and Slingerland, 2003; Sheng et al., 2003]. The activity of CDKs is also subjected to a negative regulation by cyclin-dependent kinases inhibitors (CKIs). P27Kip1 is a key member of the Cip/Kip family of CKIs that bind to and negatively regulate cyclin-CDK holoenzymes such as cyclinE-CDK2 complexes in the nucleus, resulting in cell cycle arrest in late G1. The importance of p27Kip1 in cell-cycle regulation is underscored by the phenotypes of p27Kip1 null mice, which exhibit overall increased body size and multiple organ hyperplasia [Fero et al., 1996]. The level of p27Kip1 is usually high in quiescent cells and declines upon mitogenic stimulation via an SCFSkp2 mediated ubiquitination and proteasome degradation. Anti-proliferative signals such as TGF-β, rapamycin and cAMP can up-regulate the expression of p27Kip1 [Kato et al., 1994; Nourse et al., 1994; Polyak et al., 1994].
Activation of receptor tyrosine kinases (RTK) by factors released from VSMC, macrophages, endothelium and platelets provokes downstream signaling pathways, mainly MAPK/ERK and PI3K/Akt signaling, that result in VSMC migration and proliferation [Gennaro et al., 2003; Hu et al., 1997; Kenagy et al., 1997; Lindner and Reidy, 1991; Yu et al., 2007]. Sprouty (Spry) proteins were discovered as feedback inhibitors of RTK mediated MAPK/ERK signaling, and have been shown to regulate cell proliferation, migration and differentiation, critical events involved in development of multiple organs as well as pathogenesis of several diseases [Basson et al., 2005; Lee et al., 2001; Minowada et al., 1999; Shaw et al., 2007; Yang et al., 2011]. Overexpression of Spry2 decreases injury-induced neointima formation in rat by inhibiting VSMC proliferation and migration [Zhang et al., 2005]. However, we recently showed that Spry1 is essential to maintaining the contractile phenotype of hAoSMC, and loss of Spry1 results in loss of expression of VSMC marker genes and unexpectedly attenuates hAoSMC migration and proliferation in part by decreasing PI3K/Akt signaling pathway [Yang et al., 2013]. Thus, Spry1 has a unique role in VSMC in vitro, albeit its role in vivo on vascular remodeling is unknown.
In this study, we aimed to understand the underlying mechanism by which endogenous Spry1 regulates VSMC cell cycle progression, and to investigate the role of Spry1 in injury-induced neointimal hyperplasia in vivo. We demonstrate that Spry1 is constitutively expressed in quiescent hAoSMC in vitro and in normal VSMC in mice. Loss of Spry1 in hAoSMC decreased the fraction of cells in S-phase in response to growth medium stimulation within 24 hours. Consistent will a reduced number of cells in S-phase, the level of pFoxO1/3, cyclinD1, pRbS807/S811, pH3, and pCdc2 was reduced, while the p27Kip1 protein was maintained in Spry1 knockdown hAoSMC. Our data show that carotid artery ligation-induced injury transiently down-regulated Spry expression that was accompanied by decreased VSMC marker genes expression in mice. Gene targeting of Spry1 in mice resulted in decreased injury-induced neointima formation and VSMC proliferation in arterial lesions. In summary, we demonstrate a unique function of Spry1 in maintaining the mitogenic sensitivity of VSMC to growth factors in response to growth factor enriched environment by ensuring full Akt/cyclinD1/Rb signaling.
Methods and Material
Cell Culture and Manipulation
Human aortic smooth muscle cells (hAoSMC) from at least three individual donors were obtained from Lonza and Invitrogen and cultured in SmGM-2 complete medium (Lonza). Cells were used at passage 5–7 for all experiments. For knockdown studies, hAoSMC were plated into 6-well plates (Falcon) at ~40 % confluence and transduced with human Spry1 or non-targeting control (NT) shRNA lentiviruses (Open Biosystems) for ~8 h. For analysis of cell cycle progress and mitogenic signaling pathways, cells were deprived of serum and growth factors for 24 h in smooth muscle cell basal medium (SmBM, Lonza), then stimulated with SmGM-2 medium with or without treatments as indicated for each experiment.
Cell cycle analysis
HAoSMC were plated into 6-well plates, transduced with Spry1 or NT shRNA lentiviruses for 8 h, with fresh SmGM-2 added for overnight incubation, and then starved in SmBM for 24 h and then stimulated with SmGM-2 for 12, 24 and 36 h. Cells were collected and fixed in 70% ETOH, stained with propidium iodide (PI) and subjected for flow cytometry analysis using a MACSQuant Analyzer (Miltenyi). FlowJo software was used for data collection and analysis.
Immunoblotting and Antibodies
For immunoblotting, cells were lysed in HNTG (50 mM HEPES, pH 7.4, 150 mM NaCl, 1% Triton X-100, 5 mM EGTA) buffer containing phosphatase inhibitors (1 mM sodium orthovanadate and 1 mM NaF) and proteinase inhibitor cocktail (Roche). Cell lysates were subjected to immunoblotting using antibodies to tubulin (Santa Cruz), pRbS780, pRbS807/811, Rb, cyclin D1, pH3(S10), pAkt(S473), Akt, pFoxO1/3a, pp38MAPK, p38MAPK, p21Cip1, p27Kip1, pCdc2, Skp2, Spry1, p42/44MAPK (Cell Signaling Technology), dpERK (Sigma). For immunostaining carotid artery sections were subjected for antigen retrieval in citrate buffer (pH=5.5, 10mM), and stained with antibodies against PCNA, Spry1, Spry2, Spry4, SMTN-B (Santa Cruz), Ki67 (Cell Marque).
RT-PCR and Quantitative real-time PCR
Total RNA was extracted from hAoSMC using RNeasy Plus (Qiagen). The purity and concentration of total RNA were measured with NanoDrop Spectrophotometer (NanoDrop Technologies) at 260nm/280nm. The ratios of 260nm/280nm of all samples were between 1.8 and 2.0. ProtoScript M-MuLV First Strand cDNA Synthesis kit (New England Biolabs) was used to generate cDNA. Quantitative real-time PCR (qPCR) of target genes was performed using SYBR Green (SABiosciences) on an IQ5 Multicolor Real-Time PCR Detection System (BioRad) according to the manufacturer’s instructions. GAPDH was used as an internal reference in each reaction. Melting curve analyses using the program run in the step acquisition mode was used to verify the presence of a single amplification production. Primers for qPCR are: hSpry1: AGGGCTATCTTCCTAGCA (forward), GTGAGAAGCATGGGGT (reverse); hp27Kip1: TCTGGTGATCTCCCAAGCTATC (forward), GAGTGATCACCATTCTGCTGAG (reverse); hp21Cip1: CTTTCCCTTCAGTACCCTCTCA (forward), AGGTAGAACTAGGGTGCCCTTC (reverse); hGADPH: TGCACCACCAACTGCTTAGC (forward), GGCATGGACTGTGGTCATGAG (reverse); mSpry1: ATGGATTCCCCAAGTCAGCAT (forward), CCTGTCATAGTCTAACCTCTGCC (reverse); mSpry2: ATAATCCGAGTGCAGCCTAAATC (forward), CGCAGTCCTCACACCTGTAG (reverse); mSpry4: CGACCAGAGGCTCCTAGATCA (forward), CAGCGGCTTACAGTGAACCA (reverse); mMHY11: TGGACACCATGTCAGGGAAA (forward), ATGGACACA AGTGCTAAGCAGTCT (reverse); mGADPH: ACACATTGGGGGTAGGAACA (forward), AACTTTGGCATTGTGGAAGG (reverse).
Animals, Carotid artery ligation injury and immunostaining
All procedures involving animals were approved by the Maine Medical Center Institutional Animal Care and Use Committee (IACUC), and conducted in compliance with ethical and safe research practices. Spry1lacz (Spry1−/−) mice on an FVB background were from the Mouse Mutant Regional Resource Center (UC, Davis) [Thum et al., 2008]. Spry1VSMC−/− mice were generated by cross Spry1f/f (C57BL6J background) [Basson et al., 2005] with SM22a-Cre (Jackson Laboratory, Tg(Tagln-cre)1Her/J). Two-month old male Spry1−/− or Spry1VSMC−/− and their littermates were subjected for ligation of the left carotid artery [Lindner et al., 1993]. At the end of experiment, mice were euthanized and carotid arteries were collected, fixed and processed for histology. Paraffin or O.C.T embedded arterial specimens were sectioned at 5μM and immunostained with Spry1, Spry2 or Spry4, SMTN-B antibodies or Ki67 (Cell Marque), pERK (Cell Signaling Technology), PCNA, (Santa Cruz) followed by color development using DAB peroxidase substrate (Vector Laboratories).
Statistics
Immunoblot and RT-qPCR results are expressed as means of at least three independent experiments. Error bars represent the standard deviation. Comparisons between two groups were performed by Student’s t test. For multiple comparisons, Student’s t test in conjunction with ANOVA analysis was carried out. P values ≤ 0.05 were considered statistically significant.
Results
Spry1 deficiency impairs growth medium mediated hAoSMC cell cycle progress associated with decreased cyclinD1 induction and Rb phosphorylation
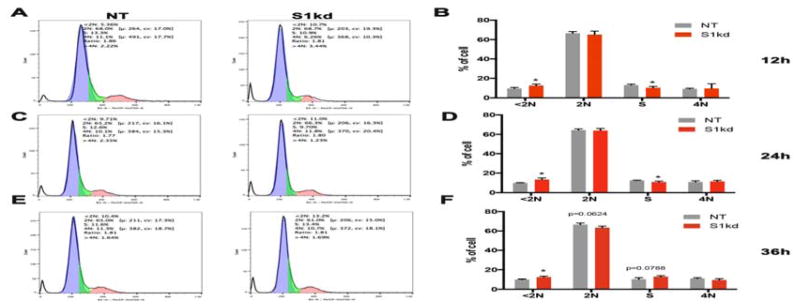
We previously showed that shRNA mediated knock down of Spry1 (S1kd) in hAoSMC showed slower growth than non-targeting shRNA (NT) control hAoSMC maintained in SmGM-2 [Yang et al., 2013]. To investigate the mechanism of this slowed growth rate, we performed a time course cell cycle analysis of NT and S1kd hAoSMC (Figure 1). In agreement with our previous report showing a reduction in growth of S1kd hAoSMC, the fraction of S1kd hAoSMC in S-phase was decreased compared to that of NT control cells after 12 and 24 h of SmGM-2 stimulation (Figure 1A–D). Interestingly, at 36 h the fraction of S-phase of S1kd hAoSMC was slightly increased, and the fraction of G0/G1 (=2N) cell slightly decreased compared to those of NT control hAoSMC (Figure 1E, F). These results suggest that knockdown of Spry1 impairs hAoSMC G1/S transition in response to growth medium stimulation. We also noticed more cellular debris (DNA content <2N) in S1kd hAoSMC than in NT hAoSMC (Figure 1A–F), suggesting that knockdown of Spry1 may impair hAoSMC survival.
Figure 1. Knockdown of Spry1 attenuates entry into S-phase of hAoSMC in response to growth medium stimulation.
Time course analysis of cell cycle progression using propidium iodide staining followed by flow cytometry. A) Representative cell cycle distribution histograms show a decrease in the fraction of S1kd hAoSMC in S-phase, and an increase of debris in these cells compared to NT control hAoSMC at 12 hours post-stimulation. B) Quantification of all phases of the cell cycle from triplicate experiments at 12 hours post-stimulation. C) Representative cell cycle distribution histograms shown for S1kd hAoSMC compared to NT control at 24 hours post-stimulation. D) Quantification of all phases of cell cycle from a triplicate experiments at 24 hours post-stimulation. E) Representative cell cycle distribution histograms of S1kd hAoSMC compared to NT control at 36 hours post-stimulation. F) Quantification of all phases of cell cycle from a triplicate experiments at 36 h post-stimulation.
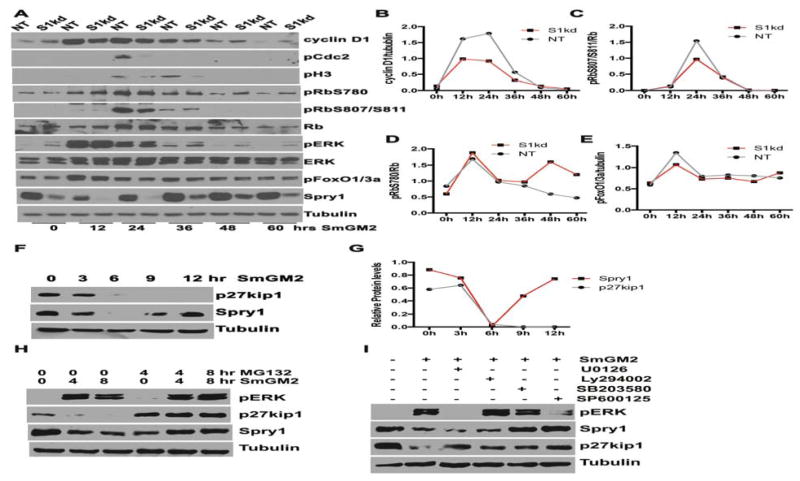
Mitogenic stimuli triggered multiple signaling pathways such as MAPK/ERK and PI3K/Akt that converge to induce expression of cyclins and the subsequent phosphorylation and inactivation of Rb proteins to drive the cell cycle progression through the restriction point R during G1 phase; beyond this point cell cycle progression becomes mitogen-independent [Dick and Rubin, 2013; Giacinti and Giordano, 2006; Zhang and Liu, 2002]. Therefore, we performed time course immunoblotting experiments to compare the status of ERK and FoxO1/3a phosphorylation, an indicator of Akt signaling, cyclinD1 expression and Rb phosphorylation, as well as pCdc2 (pCdk1) and pH3Ser10 of S1kd and NT hAoSMC in response to SmGM-2 stimulation. In agreement with the attenuated S-phase entry (Figure 1), knockdown of Spry1 decreased the extent of phosphorylation of Cdc2 and H3Ser10 (Figure 2A), which are critical to entry into mitosis. The results show that SmGM-2 stimulation induced robust ERK phosphorylation in S1kd hAoSMC similar to that in control cells within the first 24h. However, the activation of ERK in S1kd hAoSMC at 36, 48 and 60 h after stimulation declined to pre-stimulation levels but was higher than that in NT hAoSMC (Figure 2A). Interestingly, SmGM-2 induced cyclinD1 expression is lower in S1kd hAoSMC (at 12, 24 and 36 h) than in NT hAoSMC, while basal cyclinD1 expression is higher in S1kd hAoSMC than in NT hAoSMC (Figure 2A, B). SmGM-2 stimulation of hAoSMC resulted in increased expression of Rb protein and robust phosphorylation at Ser807 and Ser811 after 24h in NT hAoSMC. However, knockdown of Spry1 decreased RbS807/811 phosphorylation of Rb relative to NT hAoSMC (Figure 2A, C), and this effect coincided with lower induction of cyclinD1 expression. We also observed basal phosphorylation at S780 of Rb, and SmGM-2 stimulation further increased phosphorylation of RbS780 at 12 h of stimulation in both NT and S1kd hAoSMC. However, the level of pRbS780 in NT hAoSMC gradually declined over time to basal levels, whereas the level of pRbS780 in S1kd hAoSMC remained elevated at 48 and 60 h time points relative to NT hAoSMC (Figure 2A, D). This difference coincided with higher pERK and cyclinD1 levels in S1kd hAoSMC relative to NT hAoSMC at these time points. These observations suggest additional signaling pathway(s) other than MAPK/ERK are involved in reduced cyclinD1 induction and pRbS807/811 phosphorylation. PI3K/Akt signaling has been shown to promote cell proliferation and survival by phosphorylation of glycogen synthase kinase 3β on serine 9 that inhibit its kinase activity for phosphorylation of Thr286 on cyclinD1 resulting in proteasomal degradation of cyclinD1 [Diehl et al., 1998; Shimura et al., 2012]. Previously, we have reported that knockdown of Spry1 in hAoSMC have decreased pAkt signaling when grown in SmGM-2 [Yang et al., 2013], therefore we examined the levels of pFoxO1/3a, a downstream target of PI3K/Akt signaling. Knockdown of Spry1 reduced SmGM-2-stimulated expression of pFoxO1/3a at 12, 24 h (Figure 2A, E) coincident with the time points that have reduced cyclinD1 and pRbS807/811 levels.
Figure 2. Knockdown of Spry1 decreases growth medium induced hAoSMC cell cycle progression in association with reduced cyclinD1 expression and phosphorylation of pRbS807/811 and pFoxO1/3a.
A) Representative immunoblots showing the status of regulators involving cell cycle progression. The results show that knockdown of Spry1 in hAoSMC decreases expression of cyclinD1, phosphorylation of pRb807/811, pCdc2 and pH3 compared to NT hAoSMC controls at 12 and 24 hours post-stimulation without affecting activation of pERK. However, basal pERK is increased at 36, 48 and 60 h post-stimulation. B, C, D, E) Quantification of the relative levels of cyclinD1 (B), pRbS807/811 (C), pRbS780 (D) and pFoxO1/3a (E) from A.
SmGM-2 stimulation transiently down regulates Spry1 expression
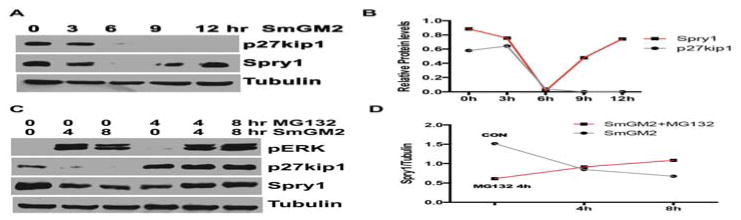
SmGM-2 stimulation markedly down-regulated Spry1 protein in hAoSMC with a nadir around 6h by time course analysis; thereafter the expression of Spry1 was partially restored (Fig. 3A, B). As expected, SmGM-2 stimulation also resulted in down regulation of the cell cycle inhibitor p27Kip1 with time, and is consistent with these cells traversing the cell cycle (Fig. 3A, B). Treatment with the proteasome inhibitor MG132 prevented SmGM-2 induced down regulation of Spry1 and p27Kip1 protein levels (Figure 3C). SmGM-2 stimulated ERK activation was unaffected by MG132 treatment.
Figure 3. Mitogenic stimulation down-regulates Spry1 expression via proteasome degradation in hAoSMC.
A) Immunoblotting shows that growth medium stimulation results in a rapid down-regulation of Spry1 with nadir at 6 h post-stimulation, in contrast to the continuous degradation of p27Kip1 in hAoSMC. B) Quantification of bands from A to show the difference in protein levels of p27Kip1 and Spry1 in response to mitogenic stimulation. C) Immunoblotting shows that the proteasome inhibitor MG132 alone slightly down-regulates Spry1, however it does rescued the expression of p27Kip1 and Spry1 after SmGM-2 stimulation. D) Quantification of Spry1 in panel C to show the changes in Spry1 levels in response to SmGM-2 stimulation and MG132 treatment.
Knock down of Spry1 increases p27kip1 expression in hAoSMC
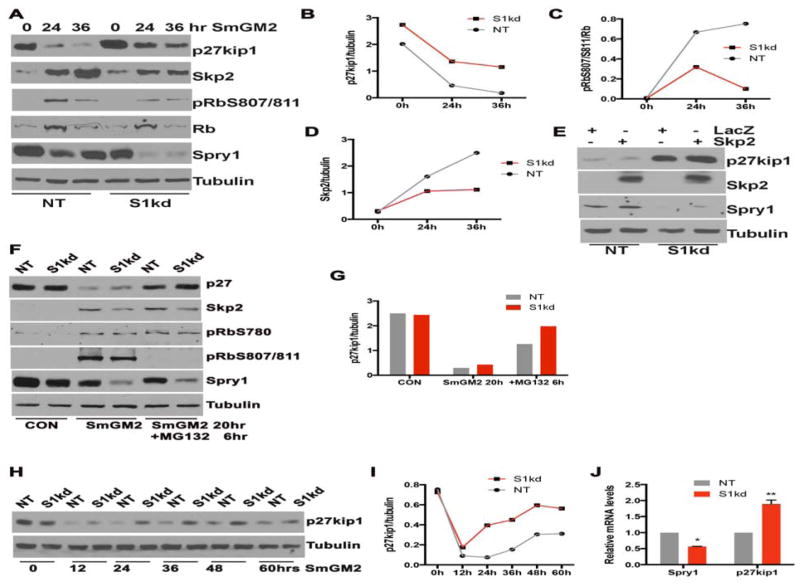
The activity of CDK:cyclin complexes are subject to negative regulation by the cyclin-dependent kinase inhibitors (CKIs) such as Cip/Kip family (p21Cip1, p27Kip1 and p57Kip2) [Denicourt and Dowdy, 2004; Sherr and Roberts, 1999]. Degradation of p27Kip1 is prerequisite for S phase entry into the cell cycle, and Spry1 and p27Kip1 are down regulated by SmGM-2 with similar kinetics (Figure 3A). Therefore we examined whether knockdown of Spry1 alters p27Kip1 levels in hAoSMC. As expected, p27Kip1 expression was high in quiescent NT and S1kd hAoSMC, and it was markedly down-regulated in response to mitogenic stimulation in NT hAoSMC, however in S1kd hAoSMC a substantial amount of p27Kip1 remained after 24h compared to NT hAoSMC (Figure 4A, B). The high level of p27Kip1 in S1kd hAoSMC coincided with lower levels of pRbS807/811 (Fig. 4A, C). Knock down of Spry1 in hAoSMC also attenuated SmGM-2 induced up regulation of Skp2 protein, a specific component of SCFSkp2 E3 ubiquitin ligase that controls late G1/S phase degradation of p27Kip1 (Figure 4A, D). We hypothesized that higher levels of p27Kip1 in S1kd hAoSMC was attributable to decreased induction of Skp2. To test this idea, we introduced exogenous Skp2 by adenoviral transduction into S1kd and NT hAoSMC and examine p27Kip1 expression by immunoblotting. Overexpression of Skp2 has no effect on p27Kip1 protein levels in quiescent hAoSMC (data not shown), however, NT hAoSMC cultured in SmGM-2 for 24h had minimal p27Kip1 expression, while S1kd hAoSMC had a higher amount of p27Kip1 and overexpression of Skp2 had little effect on p27Kip1 protein levels in S1kd hAoSMC suggesting that Spry1 affects p27Kip1 levels independent of induction of Skp2 expression (Figure 4E).
Figure 4. Knockdown of Spry1 maintains p27Kip1 levels by increasing its transcription.
A) Immunoblotting shows that S1kd hAoSMC have more p27Kip1 but less Skp2 and pRbS807/811. B, C, D) Quantification of bands in panel A show the changes in p27Kip1, pRbS807/811 and Skp2 in response to mitogenic stimulation. E) Immunoblotting shows that exogenous Skp2 has no effect on p27Kip1 level in S1kd hAoSMC. F) Immunoblotting shows that inhibition of proteasome degradation pathway by MG132 inhibits growth medium induced p27Kip1 degradation similarly in NT and S1kd hAoSMC. G) Quantification of bands from panel F shows that knockdown of Spry1 had little effect on p27Kip1 degradation. H) Immunoblotting of a time course experiment verifies the increased p27Kip1 in S1kd hAoSMC compared to NT control hAoSMC. I) Quantification of bands from panel H shows the difference in p27Kip1 levels in S1kd and NT control hAoSMC. J) RT-qPCR shows that knockdown of Spry1 increases p27Kip1 mRNA.
We use the proteasome inhibitor MG132 to tested if knock down of Spry1 alters the degradation of p27Kip1, and found that 6 hours of MG132 treatment prior to collecting cells markedly restore the p27Kip1 expression both in NT and S1kd hAoSMC (Figure 4F, G). MG132 treatment had little effect on the level of Skp2 protein or phosphorylation of pRbS780 (Figure 4F). Interestingly, we observed a complete block of phosphorylation of pRb807/811 (Figure 4F) by addition of MG132 both in NT and S1kd hAoSMC, suggesting that different regulatory mechanisms exist in terms of pRbS780 and pRbS807/S811 protein phosphorylation.
We further verified that knock down of Spry1 resulted in maintenance of p27kip1 protein by immunoblotting over an extended time course (Figure 4H, I). Results show that p27kip1 levels are similar between S1kd and NT hAoSMC prior to SmGM-2 stimulation, and those levels decline to similar levels 12h post addition of SmGM-2. However, 24h post stimulation, p27kip1 levels were higher in S1kd hAoSMC than in NT hAoSMC, and this trend continued out to 60h.
Next, we examined whether knocking down of Spry1 affected p27kip1 transcript levels. By RT-qPCR analyses we show that knock down of Spry1 increased p27kip1 mRNA in S1kd hAoSMC relative to NT hAoSMC (Figure 4J). Additionally, we also observed an increase of p21Cip1 and p57Kip2 mRNA levels (data not shown), suggesting promoting p27Kip1 transcription contributed to the increase of p27Kip1 expression by knock down of Spry1.
Loss of Spry1 attenuates injury-induced neointima formation in vivo
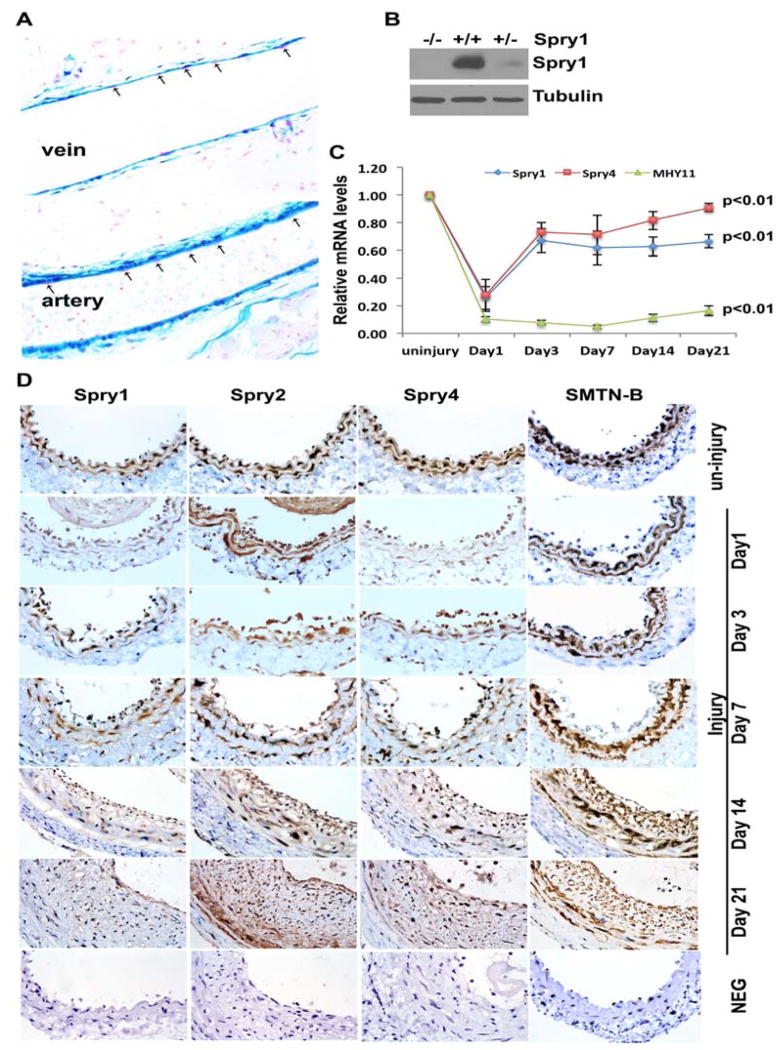
VSMC proliferation is a major contributor to restenosis after angioplasty. Therefore we sought to test whether Spry1 also plays a role in vivo particularly in the response to vascular injury. Beta-galactosidase staining of veins and arteries of Spry1lacZ mice shows that Spry1 is expressed predominantly in VSMC of the vessel wall and confirmed previous immunostaining results (Figure 5A) [Yang et al., 2013]. Immunoblotting of artery lysates confirmed the absence of Spry1 protein in Spry1−/− mice (Figure 5B). Using a carotid artery ligation (CAL) injury model [Lindner et al., 1993], RT-qPCR shows that Spry1 mRNA is transiently down-regulated 24 h after injury, followed by a gradual increase in expression that did not achieve pre-injury levels even after 21 days (Figure 5C). A similar transient decline in Spry4 mRNA was also observed, however, expression of Spry4 returned to near pre-injury levels by 21 days (Figure 5C). We also assessed the time course of injured arteries by immunohistochemistry (IHC). In agreement with injury induced dedifferentiation of VSMC, the expression of smoothelin B (SMTN-B) and myosin heavy chain 11 (MHY11) was abruptly down-regulated at 24 h after CAL, and this down-regulation persisted until day 21 (Figure 5C, D). We observed that Spry1, Spry2 and Spry4 were all expressed in VSMC of the medial layer of uninjured carotid arteries, however, consistent with RT-qPCR results, Spry1 and Spry4 proteins were down-regulated one day after ligation, whereas Spry2 expression was relatively unchanged (Figure 5D). On day 3 the expression of Spry1 and Spry4 in injured SMC began to increase, and thereafter Spry4 expression gradually approached the level in uninjured arteries, while Spry1 expression remained at a relatively low level for 21 days (Figure 5D).
Figure 5. Spry1 is constitutively expressed in normal arterial VSMC and transiently down regulated by injury.
A) X-gal staining of vascular tissues from Spry1lacZ mice, that have LacZ reporter under the control of Spry1 promoter shows staining is predominantly in VSMC and adventia but not in endothelial cells (arrows). B) Immunoblotting of the aorta lysates from Spry1+/+ or Spry1−/− mice confirms the expression of Spry1 protein Spry1+/+ vessels and Spry1−/− vessels. C) RT-qPCR analysis of a time course LCL experiment to show injury-mediated changes in Spry1, Spry4 and MHY11 gene expression. D) Immunohistochemistry (IHC) on a time course LCL experiment to show changes in expression of Spry1, Spry2, Spry4 and SMTN-B proteins.
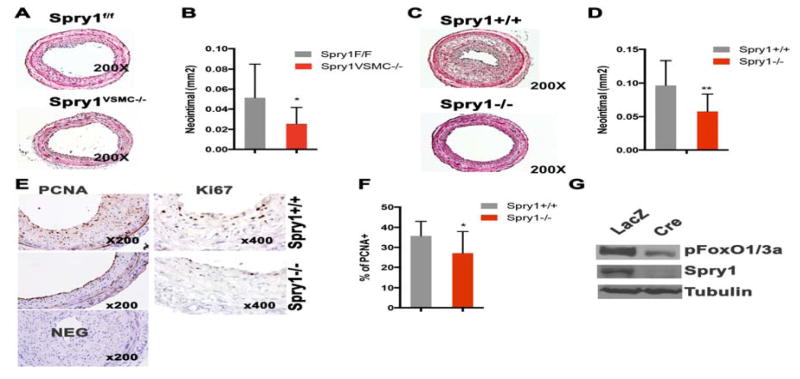
Next, we performed CAL on mice with loss of Spry1 function. We used the loxP/Cre system to conditionally delete Spry1 in VSMC by mating Spry1f/f (C57Bl6/J background) to SM22α-Cre mice. Mice that genotyped for both Spry1f/f and Cre are hereafter called Spry1VSMC−/−. Spry1VSMC−/− mice appear normal with no apparent vascular abnormalities (data not shown). Two-month-old male Spry1VSMC−/− and their Cre negative Spry1f/f littermate controls were subjected to CAL. The results show that mice with conditional deletion of Spry1 in VSMC had decreased injury-induced neointima formation compared to littermate controls (Figure 6A, B). We also performed CAL on mice with global knockout of Spry1 (FVB background) and observed a decrease in intimal thickening compared to wild type controls (Figure 6C, D).
Figure 6. Loss of Spry1 attenuates injury induced neointima formation by decreasing VSMC proliferation in vivo.
A) Representative H & E stained images of ligated carotid artery sections from VSMC condition Spry1 null mice (Spry1VSMC−/−) and controls (Spry1f/f). B) Quantification of neointima thickness 21 days after injury from experiment in panel A. C) Representative H & E staining images of ligated carotid artery sections from global Spry1 null and wild type controls. D) Quantification of neointima thickness 21 days after injury from the experiment in panel C. E) Immunostaining for PCNA and Ki67 on ligated carotid artery sections shows a decrease of PCNA and Ki67 stained cells in Spry1−/− samples relative to wild type injured arteries. F) Quantification shows loss of Spry1 significantly decreased the percentage of PCNA positive cells in the neointima. G) Immunoblotting of primary VSMC lysates from Spry1f/f mice treated with adenoviral-LacZ (control) or Adeno-Cre shows loss of Spry1 decreases pFoxO1/3 expression.
Because VSMC proliferation is a primary factor of injury-induced neointima formation, and knockdown of Spry1 attenuates hAoSMC proliferation in vitro Figure 1 and [Yang et al., 2013], we performed immunostaining for PCNA and Ki67, two markers of proliferation, on sections from injured arteries taken at day 21. Figure 6E and F show that loss of Spry1 decreased the number of PCNA and Ki67 positive cells. To test if Akt/pFoxO signaling has any contribution to the observed in vivo phenotype, we isolated primary VSMC from Spry1f/f mice and transduced them with Cre expressing adenovirus to delete Spry1 in vitro. The result shows that deletion of Spry1 in Spry1f/f VSMC decreased pFoxO1/3 expression in cells grown in DMEM supplied with 10% FBS (Figure 6G). Cell death may also influence injury-induced neointima formation, therefore we examined cell apoptosis by TUNEL staining on ligated and control carotid artery sections. However, we did not observe significant apoptotic cells in the neointima of either wild type or Spry1 manipulated mice (data not shown). Thus, loss of Spry1 in VSMC in vivo attenuates injury induced intimal hyperplasia in part due to decreased VSMC proliferation.
Discussion
Spry proteins were discovered as feedback inhibitors of RTK/ERK signaling pathways and inhibit the pathways that induced their expression, thus limiting the intensity and duration of MAPK signaling [Basson et al., 2005; Lee et al., 2001; Minowada et al., 1999; Shaw et al., 2007; Yang et al., 2011]. The inhibitory effect of Sprys on RTK/ERK signaling varies by cell type and the RTK pathways they interact with as demonstrated by the fact that Sprys may also stabilize RTKs thus resulting in sustained RTK/MAPK signaling [Wong et al., 2002; Yang et al., 2008]. We have reported non-redundant functions for Spry1 and Spry4 in hAoSMC as well as in breast cancer cells [He et al., 2016; Yang et al., 2013]. In addition to MAPK/ERK signaling, Sprys also modify other signaling pathways such as PI3K/Akt [Gao et al., 2012]. Thus, the functions of Sprys are complex, and are cellular context- and receptor-dependent, and specific to individual Spry family members. In the present study, we found that Spry1 is constitutively expressed in quiescent VSMC and in normal vessel wall smooth muscle cells, and it is rapidly down-regulated by growth-factor containing medium in vitro and mechanical injury in vivo. The down regulation of Spry1 is accompanied by a decrease in the expression of SMC contractile genes, and is consistent with our previous report that Spry1 is essential to maintaining the VSMC contractile phenotype under homeostatic conditions [Yang et al., 2013]. We demonstrate that down-regulation of Spry1 by mitogenic stimulation is due in part to proteasome degradation. It has been documented that Spry1 both negatively and positively regulates RTK/ERK signaling pathway [Hanafusa et al., 2002; Schaaf et al., 2010; Yang et al., 2011; Yang et al., 2008]. Our time course analyses show that knockdown of Spry1 in hAoSMC has no effect on the acute activation of ERK elicited by growth medium stimulation (at 12 and 24h). However, knockdown of Spry1 elevated basal ERK activation relative to control cells, and prolonged the activation of ERK after growth medium stimulation for 36, 48 and 6 0h. This prolonged ERK activation is correlated with slight increase in the fraction of cells in S-phase at 36 h, suggesting that constitutive expression of Spry1 may suppress basal ERK activation and VSMC entry into cell cycle thus promoting vessel wall homeostasis.
Hypo-phosphorylated Rb protein plays a fundamental role in restraining the G1/S transition by binding to and inhibiting the activity of the E2F family transcription factors in quiescent mammalian cells [Chellappan et al., 1991; Dyson, 1998; Nevins, 1998; Weinberg, 1995]. Mitogenic signals triggering exit from G0 and entry into the cell cycle largely relies on induction of cyclinD proteins to form cyclinD/CDK4/6 complexes and initiating CDK4/6 activity, which in turn phosphorylates Rb proteins and releases E2F allowing for transcription of E2F-responsive genes required for cell cycle progression [Dick and Rubin, 2013; Giacinti and Giordano, 2006].
Knock down of Spry1 increased basal ERK phosphorylation and cyclinD1 expression under quiescent conditions, however, mitogen-triggered cyclinD1 expression and S-phase entry was reduced without affecting acute ERK activation. The reduction in cyclinD1 expression is further supported by a similar reduction in pRbS807/811. Our findings suggest that additional signaling other than MAPK/ERK is involved in the reduction of mitogen-induced cyclinD1, pRbS807/811 expression and cell cycle progression by knocking down of Spry1. Furthermore, we demonstrated that knockdown of Spry1 reduces growth medium induced phosphorylation of FoxO1/3a, a down-stream target of PI3K/Akt signaling in hAoSMC. We also observed reduced pFoxO1/3a in primary VSMC isolated from Spry1 deficient mice. Immunostaining showed reduced PCNA and Ki67 positive VSMC in sections from injured arteries of Spry1−/− mice compared to wild type control mice. Akt signaling has been shown to increase c-Myc expression, a strong promoter of cell cycle progression, causing cells to exit G0 both by inducing the expression of D-type cyclins and suppressing the expression of multiple negative cell cycle regulators such as p21Cip1, p27Kip1, and p15 [Gera et al., 2004]. Akt also controls the stability of c-Myc and cyclinD1 indirectly via its downstream substrate, GSK-3β that phosphorylates c-Myc at Thr58, and cyclinD1 at Thr286 [Diehl et al., 1998]. In vessels, Akt has been shown to promote VSMC survival and proliferation [Allard et al., 2008; Stabile et al., 2003]. We and others have shown that activation of Akt promotes the VSMC differentiation phenotype [Martin et al., 2007; Yang et al., 2013]. Thus, our findings suggest that in pathological conditions such as carotid artery ligation-induced injury, loss of Spry1 attenuates mitogen-induced VSMC proliferation and intimal hyperplasia, and this effect may be due at least in part to reduced Akt/FoxO/cyclinD1/Rb signaling.
P27Kip1 is a key member of the Cip/Kip family of CKIs that negatively regulate cyclin-Cdk holoenzymes such as cyclinE-Cdk2 complexes in the nucleus, resulting in cell cycle arrest at the G1/S transition. Thus, p27Kip1 must be degraded for cell cycle progression to proceed. Skp2 plays an important role in promoting the destruction of p27Kip1 and driving the cell cycle through the G1/S transition. We observed that Spry1 knockdown in hAoSMC attenuated mitogen-induced loss of p27Kip1, while also resulting in less Skp2 expression. This suggests that knock down of Spry1 may impair the proteasome degradation pathway. However, introducing exogenous Skp2 could not counteract the maintenance of p27Kip1 levels by knock down of Spry1 suggesting alternative mechanisms. It has been proposed that an E2F binding site on Skp2 promoter results in a pRb/Skp2 auto-induction loop for cell cycle progression [Yung et al., 2007], in which mitogen-induced cyclin D expression activates Cdk, resulting in phosphorylation and inactivation of Rb thus releasing E2F transcription factors to promote expression of downstream targets including Skp2. The expression of Skp2 promotes the degradation of p27Kip1 and eliminates the inhibition of p27Kip1 on cyclin/Cdk and again leads Rb phosphorylation and inactivation, thus forming a pRb/Skp2 auto-induction loop participating in cell cycle progression after the R checkpoint [Harbour and Dean, 2000; Weinberg, 1995]. We suggest that decreased Skp2 expression as a result of knock down of Spry1 is likely a secondary effect due to alterations in Rb phosphorylation relative to control cells. Further study with Rb mutants is needed to confirm this possibility.
Our RT-qPCR analyses show that knockdown of Spry1 increases p27Kip1 mRNA expression. FoxO has been shown to enhance transcription of p27Kip1 leading to increased levels of p27Kip1 protein [Medema et al., 2000; Stahl et al., 2002]. Although knock down of Spry1 in hAoSMC had little effect on mitogen-induced phosphorylation of FoxO relative to NT control hAoSMC, VSMC from Spry1−/− mice has reduced FoxO phosphorylation relative to VSMC from wild type mice. Together with changes in Rb phosphorylation, Spry1 deficiency alters normal mitogen-induced Akt/FoxO/p27kip1/Rb signaling leading to impaired cell cycle progression.
Our cell cycle analyses showed increased cellular debris in S1kd hAoSMC suggesting reduced cell survival or increased apoptosis. Sustained ERK activation has been shown to play a role in promoting several forms of cell death in response to numerous stress stimuli [Cagnol and Chambard, 2010]. It was demonstrated that in PC-12 cells transient Ras/Raf signaling induces cell proliferation whereas sustained activation causes these cells to differentiate and slowly withdraw from the cell cycle [Kimmelman et al., 2002]. In addition, Akt has been shown to promote VSMC survival and proliferation [Allard et al., 2008]. Thus the increase in cellular debris and starvation-induced caspase 3 cleavage by knock down of Spry1 (data not shown) may be due to prolonged ERK activation and decrease of pFoxO1/3a expression in VSMC in vitro. However, we did not observe any apoptotic cells in injury-induced neointima both in wild type and Spry1−/− mice (data not shown), indicating that apoptosis is not involved in the attenuation of neointimal hyperplasia in Spry1−/− mice.
Although our study elucidates an essential role of Spry1 in maintaining VSMC homeostasis in quiescent conditions and in promoting VSMC proliferation in response to mitogenic stimulation both in vitro and in vivo, critical issues remain. While knockdown of Spry1 impairs Akt activation and increases p27Kip1 transcription, the mechanisms remain unclear. We observed that different regulatory mechanisms likely exist in the phosphorylation of Rb protein at S780 and at S807/811 due to knock down of Spry1. Elucidation the function and the regulatory mechanisms of pRbS780 and pRbS807/811 in the context of Spry1 will have significance in targeting pathological VSMC hyperplasia.
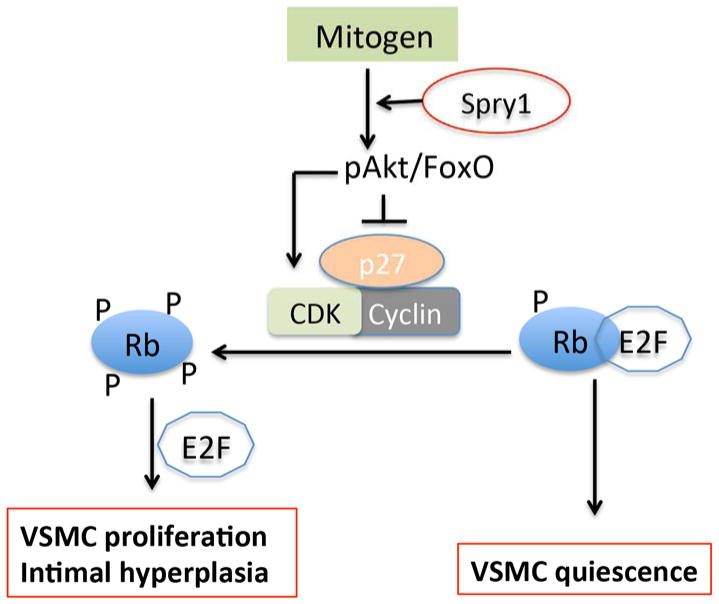
In conclusion, we have demonstrated that Spry1 has a role in supporting mitogen-induced vascular hyperplasia, at least in part by promoting Akt/Rb signaling and increasing mitogen-induced cyclinD1 expression and decreasing p27Kip1 expression (Figure 7).
Figure 7. Model for regulation of VSMC phenotype by Spry1.
Spry1 facilitates mitogen induced Akt/FoxO signaling, which promotes cyclinD1 and inhibits p27Kip expression to regulate VSMC proliferation and intimal hyperplasia.
Acknowledgments
We thank MMCRI Histology Core Armie Mangoba, Katrina Abramo and Dr. Volkhard Lindner for assistance with histology. We also thank MMCRI Virus Core Nancy Chandler-Conrey and Dr. Jeong Kyo Yoon for amplification of adenoviruses and lentiviruses.
Source of funding
This work was supported by NIH grants P30 GM103392 (transgenic mouse and small animal imaging core, histology core) to RF, P30 GM106391 (cell phenotyping, progenitor cell analysis and histology cores) to D.M. Wojchowski, PI, and R01 HL109652 to LL, and generous support form the Maine Medical Center Research Institute. Yan Gong was the recipient of an American Heart Association predoctoral fellowship.
Footnotes
Disclosure: none
References
- Allard D, Figg N, Bennett MR, Littlewood TD. Akt regulates the survival of vascular smooth muscle cells via inhibition of FoxO3a and GSK3. J Biol Chem. 2008;283:19739–47. doi: 10.1074/jbc.M710098200. [DOI] [PubMed] [Google Scholar]
- Basson MA, Akbulut S, Watson-Johnson J, Simon R, Carroll TJ, Shakya R, Gross I, Martin GR, Lufkin T, McMahon AP, Wilson PD, Costantini FD, Mason IJ, Licht JD. Sprouty1 Is a Critical Regulator of GDNF/RET-Mediated Kidney Induction. Dev Cell. 2005;8:229–239. doi: 10.1016/j.devcel.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Cagnol S, Chambard JC. ERK and cell death: mechanisms of ERK-induced cell death--apoptosis, autophagy and senescence. FEBS J. 2010;277:2–21. doi: 10.1111/j.1742-4658.2009.07366.x. [DOI] [PubMed] [Google Scholar]
- Chellappan SP, Hiebert S, Mudryj M, Horowitz JM, Nevins JR. The E2F transcription factor is a cellular target for the RB protein. Cell. 1991;65:1053–61. doi: 10.1016/0092-8674(91)90557-f. [DOI] [PubMed] [Google Scholar]
- Denicourt C, Dowdy SF. Cip/Kip proteins: more than just CDKs inhibitors. Genes Dev. 2004;18:851–5. doi: 10.1101/gad.1205304. [DOI] [PubMed] [Google Scholar]
- Dick FA, Rubin SM. Molecular mechanisms underlying RB protein function. Nat Rev Mol Cell Biol. 2013;14:297–306. doi: 10.1038/nrm3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diehl JA, Cheng M, Roussel MF, Sherr CJ. Glycogen synthase kinase-3beta regulates cyclin D1 proteolysis and subcellular localization. Genes Dev. 1998;12:3499–511. doi: 10.1101/gad.12.22.3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyson N. The regulation of E2F by pRB-family proteins. Genes Dev. 1998;12:2245–62. doi: 10.1101/gad.12.15.2245. [DOI] [PubMed] [Google Scholar]
- Fero ML, Rivkin M, Tasch M, Porter P, Carow CE, Firpo E, Polyak K, Tsai LH, Broudy V, Perlmutter RM, Kaushansky K, Roberts JM. A syndrome of multiorgan hyperplasia with features of gigantism, tumorigenesis, and female sterility in p27(Kip1)-deficient mice. Cell. 1996;85:733–44. doi: 10.1016/s0092-8674(00)81239-8. [DOI] [PubMed] [Google Scholar]
- Gao M, Patel R, Ahmad I, Fleming J, Edwards J, McCracken S, Sahadevan K, Seywright M, Norman J, Sansom O, Leung HY. SPRY2 loss enhances ErbB trafficking and PI3K/AKT signalling to drive human and mouse prostate carcinogenesis. EMBO Mol Med. 2012;4:776–90. doi: 10.1002/emmm.201100944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gennaro G, Menard C, Giasson E, Michaud SE, Palasis M, Meloche S, Rivard A. Role of p44/p42 MAP kinase in the age-dependent increase in vascular smooth muscle cell proliferation and neointimal formation. Arterioscler Thromb Vasc Biol. 2003;23:204–10. doi: 10.1161/01.atv.0000053182.58636.be. [DOI] [PubMed] [Google Scholar]
- Gera JF, Mellinghoff IK, Shi Y, Rettig MB, Tran C, Hsu JH, Sawyers CL, Lichtenstein AK. AKT activity determines sensitivity to mammalian target of rapamycin (mTOR) inhibitors by regulating cyclin D1 and c-myc expression. J Biol Chem. 2004;279:2737–46. doi: 10.1074/jbc.M309999200. [DOI] [PubMed] [Google Scholar]
- Giacinti C, Giordano A. RB and cell cycle progression. Oncogene. 2006;25:5220–7. doi: 10.1038/sj.onc.1209615. [DOI] [PubMed] [Google Scholar]
- Hanafusa H, Torii S, Yasunaga T, Nishida E. Sprouty1 and Sprouty2 provide a control mechanism for the Ras/MAPK signalling pathway. Nat Cell Biol. 2002;4:850–8. doi: 10.1038/ncb867. [DOI] [PubMed] [Google Scholar]
- Harbour JW, Dean DC. The Rb/E2F pathway: expanding roles and emerging paradigms. Genes Dev. 2000;14:2393–409. doi: 10.1101/gad.813200. [DOI] [PubMed] [Google Scholar]
- He Q, Jing H, Liaw L, Gower L, Vary C, Hua S, Yang X. Suppression of Spry1 inhibits triple-negative breast cancer malignancy by decreasing EGF/EGFR mediated mesenchymal phenotype. Sci Rep. 2016;6:23216. doi: 10.1038/srep23216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Cheng L, Hochleitner BW, Xu Q. Activation of mitogen-activated protein kinases (ERK/JNK) and AP-1 transcription factor in rat carotid arteries after balloon injury. Arterioscler Thromb Vasc Biol. 1997;17:2808–16. doi: 10.1161/01.atv.17.11.2808. [DOI] [PubMed] [Google Scholar]
- Kato JY, Matsuoka M, Polyak K, Massague J, Sherr CJ. Cyclic AMP-induced G1 phase arrest mediated by an inhibitor (p27Kip1) of cyclin-dependent kinase 4 activation. Cell. 1994;79:487–96. doi: 10.1016/0092-8674(94)90257-7. [DOI] [PubMed] [Google Scholar]
- Kenagy RD, Hart CE, Stetler-Stevenson WG, Clowes AW. Primate smooth muscle cell migration from aortic explants is mediated by endogenous platelet-derived growth factor and basic fibroblast growth factor acting through matrix metalloproteinases 2 and 9. Circulation. 1997;96:3555–60. doi: 10.1161/01.cir.96.10.3555. [DOI] [PubMed] [Google Scholar]
- Kimmelman AC, Nunez Rodriguez N, Chan AM. R-Ras3/M-Ras induces neuronal differentiation of PC12 cells through cell-type-specific activation of the mitogen-activated protein kinase cascade. Mol Cell Biol. 2002;22:5946–61. doi: 10.1128/MCB.22.16.5946-5961.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavoie JN, L’Allemain G, Brunet A, Muller R, Pouyssegur J. Cyclin D1 expression is regulated positively by the p42/p44MAPK and negatively by the p38/HOGMAPK pathway. J Biol Chem. 1996;271:20608–16. doi: 10.1074/jbc.271.34.20608. [DOI] [PubMed] [Google Scholar]
- Lee SH, Schloss DJ, Jarvis L, Krasnow MA, Swain JL. Inhibition of angiogenesis by a mouse sprouty protein. J Biol Chem. 2001;276:4128–33. doi: 10.1074/jbc.M006922200. [DOI] [PubMed] [Google Scholar]
- Liang J, Slingerland JM. Multiple roles of the PI3K/PKB (Akt) pathway in cell cycle progression. Cell Cycle. 2003;2:339–45. [PubMed] [Google Scholar]
- Lindner V, Fingerle J, Reidy MA. Mouse model of arterial injury. Circ Res. 1993;73:792–6. doi: 10.1161/01.res.73.5.792. [DOI] [PubMed] [Google Scholar]
- Lindner V, Reidy MA. Proliferation of smooth muscle cells after vascular injury is inhibited by an antibody against basic fibroblast growth factor. Proc Natl Acad Sci U S A. 1991;88:3739–43. doi: 10.1073/pnas.88.9.3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin KA, Merenick BL, Ding M, Fetalvero KM, Rzucidlo EM, Kozul CD, Brown DJ, Chiu HY, Shyu M, Drapeau BL, Wagner RJ, Powell RJ. Rapamycin promotes vascular smooth muscle cell differentiation through insulin receptor substrate-1/phosphatidylinositol 3-kinase/Akt2 feedback signaling. J Biol Chem. 2007;282:36112–20. doi: 10.1074/jbc.M703914200. [DOI] [PubMed] [Google Scholar]
- Medema RH, Kops GJ, Bos JL, Burgering BM. AFX-like Forkhead transcription factors mediate cell-cycle regulation by Ras and PKB through p27kip1. Nature. 2000;404:782–7. doi: 10.1038/35008115. [DOI] [PubMed] [Google Scholar]
- Minowada G, Jarvis LA, Chi CL, Neubuser A, Sun X, Hacohen N, Krasnow MA, Martin GR. Vertebrate Sprouty genes are induced by FGF signaling and can cause chondrodysplasia when overexpressed. Development. 1999;126:4465–75. doi: 10.1242/dev.126.20.4465. [DOI] [PubMed] [Google Scholar]
- Nevins JR. Toward an understanding of the functional complexity of the E2F and retinoblastoma families. Cell Growth Differ. 1998;9:585–93. [PubMed] [Google Scholar]
- Newby AC, Zaltsman AB. Molecular mechanisms in intimal hyperplasia. J Pathol. 2000;190:300–9. doi: 10.1002/(SICI)1096-9896(200002)190:3<300::AID-PATH596>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Nourse J, Firpo E, Flanagan WM, Coats S, Polyak K, Lee MH, Massague J, Crabtree GR, Roberts JM. Interleukin-2-mediated elimination of the p27Kip1 cyclin-dependent kinase inhibitor prevented by rapamycin. Nature. 1994;372:570–3. doi: 10.1038/372570a0. [DOI] [PubMed] [Google Scholar]
- Polyak K, Kato JY, Solomon MJ, Sherr CJ, Massague J, Roberts JM, Koff A. p27Kip1, a cyclin-Cdk inhibitor, links transforming growth factor-beta and contact inhibition to cell cycle arrest. Genes Dev. 1994;8:9–22. doi: 10.1101/gad.8.1.9. [DOI] [PubMed] [Google Scholar]
- Schaaf G, Hamdi M, Zwijnenburg D, Lakeman A, Geerts D, Versteeg R, Kool M. Silencing of SPRY1 triggers complete regression of rhabdomyosarcoma tumors carrying a mutated RAS gene. Cancer Res. 2010;70:762–71. doi: 10.1158/0008-5472.CAN-09-2532. [DOI] [PubMed] [Google Scholar]
- Shaw AT, Meissner A, Dowdle JA, Crowley D, Magendantz M, Ouyang C, Parisi T, Rajagopal J, Blank LJ, Bronson RT, Stone JR, Tuveson DA, Jaenisch R, Jacks T. Sprouty-2 regulates oncogenic K-ras in lung development and tumorigenesis. Genes Dev. 2007;21:694–707. doi: 10.1101/gad.1526207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng H, Shao J, Townsend CM, Jr, Evers BM. Phosphatidylinositol 3-kinase mediates proliferative signals in intestinal epithelial cells. Gut. 2003;52:1472–8. doi: 10.1136/gut.52.10.1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherr CJ, Roberts JM. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 1999;13:1501–12. doi: 10.1101/gad.13.12.1501. [DOI] [PubMed] [Google Scholar]
- Shimura T, Noma N, Oikawa T, Ochiai Y, Kakuda S, Kuwahara Y, Takai Y, Takahashi A, Fukumoto M. Activation of the AKT/cyclin D1/Cdk4 survival signaling pathway in radioresistant cancer stem cells. Oncogenesis. 2012;1:e12. doi: 10.1038/oncsis.2012.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stabile E, Zhou YF, Saji M, Castagna M, Shou M, Kinnaird TD, Baffour R, Ringel MD, Epstein SE, Fuchs S. Akt controls vascular smooth muscle cell proliferation in vitro and in vivo by delaying G1/S exit. Circ Res. 2003;93:1059–65. doi: 10.1161/01.RES.0000105086.31909.1B. [DOI] [PubMed] [Google Scholar]
- Stahl M, Dijkers PF, Kops GJ, Lens SM, Coffer PJ, Burgering BM, Medema RH. The forkhead transcription factor FoxO regulates transcription of p27Kip1 and Bim in response to IL-2. J Immunol. 2002;168:5024–31. doi: 10.4049/jimmunol.168.10.5024. [DOI] [PubMed] [Google Scholar]
- Thum T, Gross C, Fiedler J, Fischer T, Kissler S, Bussen M, Galuppo P, Just S, Rottbauer W, Frantz S, Castoldi M, Soutschek J, Koteliansky V, Rosenwald A, Basson MA, Licht JD, Pena JT, Rouhanifard SH, Muckenthaler MU, Tuschl T, Martin GR, Bauersachs J, Engelhardt S. MicroRNA-21 contributes to myocardial disease by stimulating MAP kinase signalling in fibroblasts. Nature. 2008;456:980–4. doi: 10.1038/nature07511. [DOI] [PubMed] [Google Scholar]
- Weinberg RA. The retinoblastoma protein and cell cycle control. Cell. 1995;81:323–30. doi: 10.1016/0092-8674(95)90385-2. [DOI] [PubMed] [Google Scholar]
- Wong ES, Fong CW, Lim J, Yusoff P, Low BC, Langdon WY, Guy GR. Sprouty2 attenuates epidermal growth factor receptor ubiquitylation and endocytosis, and consequently enhances Ras/ERK signalling. Embo J. 2002;21:4796–4808. doi: 10.1093/emboj/cdf493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Gong Y, Friesel R. Spry1 is expressed in hemangioblasts and negatively regulates primitive hematopoiesis and endothelial cell function. PLoS One. 2011;6:e18374. doi: 10.1371/journal.pone.0018374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Gong Y, Tang Y, Li H, He Q, Gower L, Liaw L, Friesel RE. Spry1 and Spry4 differentially regulate human aortic smooth muscle cell phenotype via Akt/FoxO/myocardin signaling. PLoS One. 2013;8:e58746. doi: 10.1371/journal.pone.0058746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Harkins LK, Zubanova O, Harrington A, Kovalenko D, Nadeau RJ, Chen PY, Toher JL, Lindner V, Liaw L, Friesel R. Overexpression of Spry1 in chondrocytes causes attenuated FGFR ubiquitination and sustained ERK activation resulting in chondrodysplasia. Dev Biol. 2008;321:64–76. doi: 10.1016/j.ydbio.2008.05.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu PJ, Ferrari G, Pirelli L, Gulkarov I, Galloway AC, Mignatti P, Pintucci G. Vascular injury and modulation of MAPKs: a targeted approach to therapy of restenosis. Cell Signal. 2007;19:1359–71. doi: 10.1016/j.cellsig.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Yung Y, Walker JL, Roberts JM, Assoian RK. A Skp2 autoinduction loop and restriction point control. J Cell Biol. 2007;178:741–7. doi: 10.1083/jcb.200703034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Chaturvedi D, Jaggar L, Magnuson D, Lee JM, Patel TB. Regulation of Vascular Smooth Muscle Cell Proliferation and Migration by Human Sprouty 2. Arterioscler Thromb Vasc Biol. 2005;25:533–538. doi: 10.1161/01.ATV.0000155461.50450.5a. [DOI] [PubMed] [Google Scholar]
- Zhang W, Liu HT. MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res. 2002;12:9–18. doi: 10.1038/sj.cr.7290105. [DOI] [PubMed] [Google Scholar]