Abstract
Inflammatory bowel disease (IBD) causes inflammation to the gastrointestinal tract. Local administration of Anti-inflammatory drugs such as 5-aminosalysilic acid (5-ASA) can alleviate the symptoms of IBD. The application of nanoparticles for IBD treatment in direct rectal administration showed high drug availability and treatment efficacy. However, relying on size-dependent adsorption of smaller particles is not sufficient for making the formulation capable of targeting. Intestinal organoids can improve the functionality of the nanoparticles due to their ability to adsorb small nanoparticle inside the lumen and attach to the damaged area. In this study, intestinal organoids were used as carriers of 5-ASA-loaded PLGA nanoparticles. The nanoparticle sizes, confirmed by scanning electron microscopy, were 200–300 nm and the zeta potential were negative. The nanoparticles did not have any noticeable pernicious effect on organoid growth and viability. After mixing the nanoparticles with Matrigel and organoids, Rhodamine B loaded inside the nanoparticles was highly detected inside the organoid’s lumen after 3 days by confocal fluorescent microscopy and no longer detected in the lumen after day 4. It may be attributed to the ability of the lumen to digest particles. Thus, the organoid Trojan horse system is a possible approach for delivering drugs to inflamed areas.
Keywords: Inflammatory bowel disease, PLGA, nanoparticles, organoids, intestinal stem cells
Introduction
The gastrointestinal (GI) tract is a complex organ composed of different parts with diverse functions, including ingestion, digestion, nutrient absorption, and excretion of waste.1 Because of the broad functionality of the intestinal tissue, defects in its structure may affect the operation of the entire body. Severe diseases may negatively impact the intestine, with one such example being inflammatory bowel disease (IBD), a group of diseases that result in inflammation in any part of the GI tract.2 IBD affects approximately 1.6 million people in the U.S.,3 and the number of people affected has increased by approximately 200,000 from 2011 to 2014, resulting in an unmet clinical need requiring of innovative and effective treatment.3 To date, no permanent cure for IBD exists. Anti-inflammatory drugs such as 5-Aminosalicylic acid (5-ASA) or Mesalamine and immunosuppressive agents are prescribed, but their curative ability is highly dependent on the degree and intensity of the disease.4,5 5-ASA can ameliorate the symptoms of IBD by locally affecting the inflamed area.6,7 Drug targeting has become a potential means to improve the local effectiveness of the prescribed drugs by inhibiting the major disadvantages of the systematic delivery including the nonselective distribution of the drugs throughout the body, wasted drugs in normal tissues, and negative side effects of the drug molecules.8
Different delivery strategies for targeting have been proposed, such as bioadhesion, nanoparticles, liposomal drug carriers, and bacteria-based drug delivery.4,9 Nanoparticle- and microparticle-based delivery is regarded as one of the most promising methods of IBD treatment that can provide sustained drug release at the desired location with decreased side effects. In mice models, orally administered dexamethasone-loaded poly(DL-lactic acid) (PDLLA) microspheres have been shown to preferentially distribute in inflamed regions of colon affected by ulcerative colitis, a chronic and relapsing type of IBD, by targeting immune-regulating cells such as macrophages. Compared to the models treated with free dexamethasone, no signs of side effects were observed.10 Microspheres resulted in lessened inhibitory effects on epithelium regeneration and inflammation.11 In 2001, Lamprecht demonstrated that the smaller the particle size, the higher the accumulation of particles in both inflamed and healthy regions of the colon.12 However, the deposition of smaller particles was noticeably increased in ulcerated regions and was correlated with increased penetration of the smaller particles into the mucus membrane rather than internalization by phagocytic cells.12 Nanoparticles have since become one of the most prevalent research topics in IBD drug delivery.
Polymers have been widely used in biomedicine for drug delivery and regenerative medicine13,14 and different types of polymer have been used to make nanoparticles for IBD treatment.15 Poly(lactic-co-glycolic acid) (PLGA) nanoparticles demonstrated ideal characteristics, including high deposition on the inflamed region, enough biodegradability to prevent polymer deposition in inflamed tissue, and avoidance of adverse effects because of the negligible amount of drug release in other tissues. These proper characteristics make them a promising carrier system for IBD treatment.16 Scientists have been conducting research on drug-related strategies and approaches to improve their effectiveness through rectal, oral, or parenteral delivery routes.3 Despite the superior properties of orally delivered nanoparticles, rectally delivered nanoparticles showed desirable therapeutic efficiency, they have been shown to help drug absorption through Peyer’s patch and lessened the drug’s enzymatic degradation.17 The direct transport and delivery of a drug to the inflamed area is still a great obstacle.18 In addition, human administration of micro and nanoparticles by colonoscopy showed that size-dependent adsorption is not the most reliable and applicable method for colonic drug delivery.19 A more advanced drug delivery technology should accompany particulate delivery in order to achieve better rectal delivery properties.
Cell-mediated drug delivery brings numerous advantages, including targeted drug delivery to the inflamed, injured, or tumor sites, controlled drug release, and lowered or no drug immunogenicity or toxicity owing to the homing properties of cell carriers.20 However, there are also some disadvantages in these systems, such as low drug loading, lack of complete drug detachment from cell carriers, earlier release of loaded drug, and harmful effects of the drug on the cell carrier.20 Many of these drawbacks can be mitigated using cell carriers containing drug-loaded nanocarriers.20 Trojan horse systems are those in which a drug is entrapped or concealed into the host cells. Passive diffusion, exocytosis, and efflux are regarded as the three major phenomena of drug release from cell carriers in Trojan horse systems.21
Cheng and Leblond first proposed the existence of intestinal stem cells (ISCs) at the base of the crypts and introduced the term crypt-based columnar (CBC) stem cells.22 The high turnover rate of the intestinal epithelium (~3–4 days) in comparison with other mucous layers encouraged researchers to consider the existence of a source of stem cells with an ability to differentiate into all types of intestinal epithelial cells.23,24 CBC cells are at the origin of the differentiating process. Although it is theoretically applicable to use CBCs as an appropriate source of cells for drug delivery, it is generally agreed that conventional long-term culture of adult tissues is not feasible.25
To solve this problem, in 2009, Sato succeeded in developing a culture system to maintain viable cells for over 1.5 years.26 With the use of a 3D culture system with Matrigel instead of 2D systems27 to achieve previous memory of the cells28–30 and employing necessary elements to prepare the natural biology of the intestine26, a single LGR5+ stem cell can be used to generate intestinal epithelium called an organoid structure that would mimic normal intestinal epithelium. The resulting organoid structure consists of several crypts, presented as buds around a hollow cyst, and villus-like epithelium located between the crypts.25
The hollow cyst of the organoids seems to be ideal to encapsulate the particles due to the organoids numerous characteristic. First, it can be loaded with nanoparticles. Previous work has reported the successful encapsulation of small nanoparticles without therapeutic agents inside the lumen of the organoids.21 In 2015, Wang’s research group worked on organoid Trojan horses loaded with DNA-functionalized gold nanoparticles (GNPs).21 The ultimate goal was to evaluate the possibility of loading gold nanoparticles within the lumen of the organoids and to assess the toxicity of nanoparticles to the host cells. It was assumed that because of the high positive charge of the inflamed tissue, DNA-functionalized GNPs can easily attach to the inflamed mucosa owing to their high negative charge.21 Moreover, it was shown that transplanted organoids can preferentially target the inflamed area.31 Thus, a Trojan horse system of ISCs can be attained by loading particles inside the hollow cyst of organoids with the aim of targeting the inflamed region. In addition, the anti-inflammatory effect of drugs, which will be loaded inside the particles, as well as the organoids regeneration into the intact epithelium31,32 can facilitate the tissue healing.
So far, ISCs have been successfully cultured as carriers of cell delivery26 and organoid structures capable of particle encapsulation have been formed.26 Furthermore, organoids have been used as carriers for small nanoparticles.21 Despite the remarkable improvements in making organoid Trojan horses, the system is still lacking a therapeutic agent that can be applicable in IBD treatment. Furthermore, the effectiveness of the system as well as its adverse effects have not been evaluated.
Therefore, it is hypothesized that the addition of encapsulated nanoparticles with therapeutic agents to the organoid Trojan horse structure will be a great step in IBD treatment because of its direct action on the inflamed area and low immunogenic properties. The aim of this study is to investigate the effect of PLGA nanoparticle loaded with 5-ASA as a model drug on the organoid structure and assessing the organoid ability to entrap the nanoparticles.
Materials and Methods
Materials
Matrigel was purchased from Corning Inc (NY, USA). Epidermal growth factor (EGF), noggin, and R-spondin-1 (RS-1) were purchased from PeproTech Inc (Rocky Hill, NJ, USA). Poly(lactic-co-glycolic acid) (PLGA) was purchased from Evonik Industries (Essen, Germany) (5050 DLG 2A). 5-Aminosalicylic acid (5-ASA), alginic acid sodium salt, dichloromethane (DCM), latex beads, and green and red fluorescent nucleic acids were purchased from Sigma-Aldrich (St. Louis, MO, USA). Rhodamine B was purchased from Acros (Geel, Belgium). All other materials were purchased from Life Technologies (Carlsbad, CA, USA).
Nanoparticle preparation
PLGA nanoparticles loaded with 5-ASA or Rhodamine B were prepared by a double-emulsion (water/oil/water)-based solvent evaporation/extraction method.33 To achieve different drug and dye concentrations, four different 5-ASA-to-polymer ratios (0%, 2.5%, 5%, and 7.5% (w/w)) and four different Rhodamine B-to-polymer ratios (0%, 1%, 2.5%, and 5% (w/w)) were prepared. The weighed drug (or dye) and PLGA were dissolved in 0.5 mL of nano-pure water and 5.5 mL of DCM, respectively. The drug (or dye) solutions were added to the polymer solutions and sonicated for 30 cycles (1 s each with a duty cycle of 80%). To obtain the negative charge required for the attachment of nanoparticles to the inflamed area, alginate, a negatively charged natural biopolymer34,35, was chosen as the surfactant to coat the drug molecules. The sonicated PLGA/5-ASA and PLGA/Rhodamine B primary emulsions were quickly poured into 25 mL of 1% (w/v) alginate solution and sonicated for 30 cycles (1 s each with a duty cycle of 80%). The double emulsions were poured into 150 mL of 0.2% (w/v) alginate solution and the mixture was stirred for 2h at room temperature. Afterwards, the suspensions were centrifuged at 10,000 rpm at 4°C and resuspended three times. The final suspensions of nanoparticles were analyzed to evaluate the properties before being loaded inside the organoid units.
Stem cell isolation and culture
Organoids were extracted based on previously reported methods.21,36 Briefly, primary ISCs were extracted from C57BL/6 (B6) mice donated by the College of Veterinary Medicine of Iowa State University. All animal procedures were conducted with the approval of the Iowa State University Institutional Animal Care and Use Committee. All methods and procedures in the experiments were performed in full compliance with the committee’s guidelines and regulations. The small intestine of the mice was cut horizontally and vertically into small pieces. The small pieces were washed with PBS several times. After washing, the upper solution was removed and 30 mL of Ethylenediaminetetraacetic acid (EDTA) was added to the small intestine pieces. The tube containing the pieces was placed on ice and after 30 min of shaking, the EDTA solution was sucked out. To remove the remaining EDTA, the pieces were washed with PBS. Thereafter, PBS was added to the pieces and by pipetting up and down, the final solution was achieved. The final solution was filtered and after centrifugation for 5 min at 1400 rpm at 4°C, the final crypts will appear.
Extracted cells will be suspended with Matrigel, a soluble extract of basement membrane,37 and applied on a pre-warmed 24-well plate. Culture medium was prepared by adding 0.5 mL of N2, 50 μL of RS-1, 1 mL of B27, 50 μL of N-acetyl-l-cysteine (NALC), 50 μL of EGF, and 50 μL of noggin to 50 mL of basal medium and was added after gel formation in the wells.
Nanoparticle characterization
In order to use the nanoparticles, it is necessary to analyze the different characteristics of the nanoparticles before loading them inside the organoids. The encapsulation efficiency (EE) of the Rhodamine B loaded PLGA nanoparticles was calculated using the following equation (λmax of 555).
The size of the particles was determined using the Zetasizer (Nano-Zs 90). To confirm the results of the Zetasizer, the particle size was also evaluated by scanning electron microscopy (SEM, FEI Quanta 250 FE-SEM). The zeta potential of the particles was investigated by Zetasizer (Nano-Zs 90).
Microscopy
In order to track the particles, 40 μL or 400 μL of the nanoparticle suspensions (2 mg/ml) containing 0%, 1%, 2.5%, and 5% Rhodamine B were added directly to the cell culture medium after culturing the organoids, and fresh culture medium was added to the system every two days. A control organoid culture without nanoparticles was also prepared. The images were recorded by a Leica DMi1 inverted microscope to show the rate of organoid growth. The organoids were chosen from the middle of each well. To track the presence of nanoparticles inside the lumen, images were taken by confocal fluorescent microscopy (Olympus IX2). To evaluate the effect of chemical structures on the nanoparticle diffusion, latex beads were added to the system.
The second method of adding nanoparticles to the system was to mix the nanoparticle suspension with the cell suspension and subsequently adding obtained suspension to the Matrigel in the passage step of organoid culture. After resuspension, the resultant mixture was applied onto the plate and culture medium was added after gel formation. To test this method, 10 μL of 2.5% Rhodamine B nanoparticles (2 mg/ml) was tested. Moreover, 200-nm latex beads, 200-nm and 400-nm polyanhydride (PA) nanoparticles (donated by the Narasimhan group) (2 mg/ml) were also tested to check the effect of chemical structure on the nanoparticle absorption inside the organoid.
Live-dead cytotoxicity test
After 7 days, the medium of the cells was removed and replaced with Hank’s Balanced Salt Solution (HBSS) for 2–3 min. Green (SYTO® 10) and red (DEAD REDTM) florescent nucleic acid stain (1 μL) was added to 500 μL of HBSS and the covered HBSS layer of the cells was replaced with the new solution. The cells were incubated in the dark for over 15 min. Then, the dye solution was replaced with fresh HBSS. The next step was fixation with 4% paraformaldehyde for 1 h, and the organoids were then observed with confocal fluorescent microscopy.
Results
Size and zeta potential of the nanoparticles
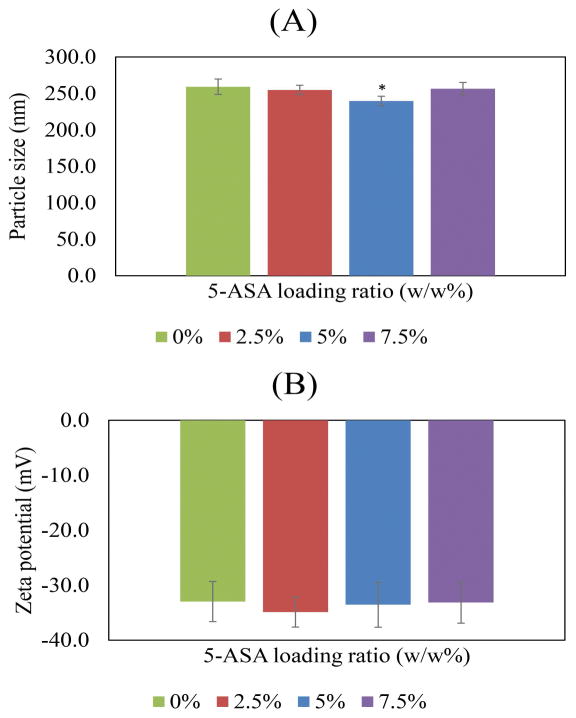
The EEs for 1%, 2.5% and 5% Rhodamine B loaded nanoparticles were 32.8%±11.1, 45.5%±7.1 and 44.7%±9.6, respectively. The recorded sizes of the particles were approximately 259 ± 10.58, 255 ± 6.4, 240 ± 6.5, and 256 ± 8.6 nm for 5-ASA/PLGA ratios of 0%, 2.5%, 5%, and 7.5% (w/w), respectively, as shown in Figure 1A. The zeta potential of the particles were −33 ± 3.65, −34.9 ± 2.73, −33.6 ± 4.09, and −33.2 ± 3.74 mV for 5-ASA/PLGA ratios of 0%, 2.5%, 5%, and 7.5% (w/w), respectively, as shown in Figure 1B. The SEM images are shown in Figure 2. Particles were approximately 250 nm in the SEM images, which verifies the results from the Zetasizer.
Figure 1.
(A) The trend of nanoparticle size changes with different 5-ASA loading ratios (B) The trend of nanoparticle zeta-potential changes with increasing the 5-ASA loading ratios. * significant at p<0.05.
Figure 2.
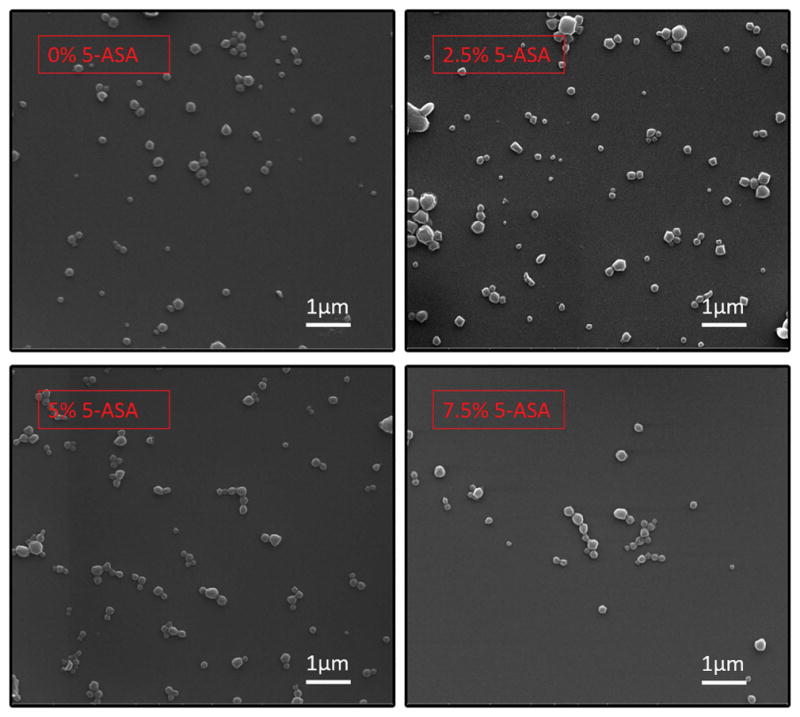
SEM of the nanoparticles with different 5-ASA loading ratios.
Microscopy
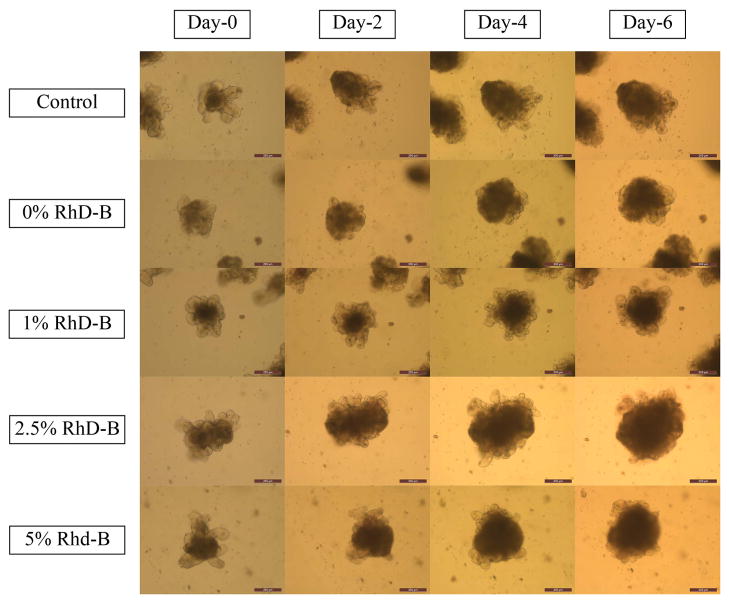
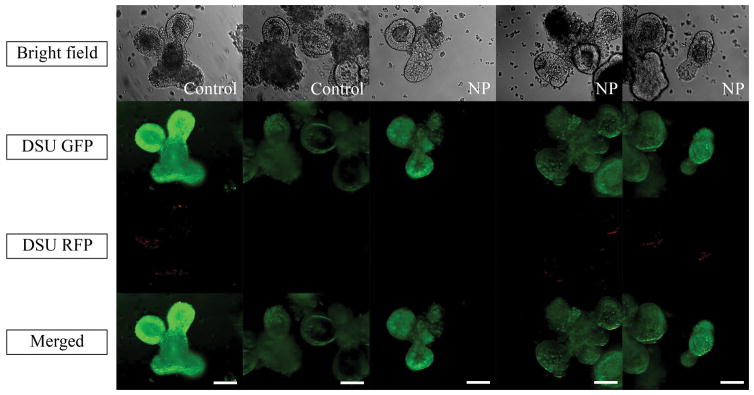
Figure 3 shows the growth of the crypts within a 6-day timescale. Much like the control sample, none of the samples containing nanoparticles showed unusual growth behavior. As a confirmation, Supplementary Figure 1 shows that the size of organoids in all samples increased after 6 days. Moreover, Figure 4 shows the effect of nanoparticles on organoids after 7 days. The green fluorescent nucleic acid stain is a membrane-permeable dye and a marker of live and dead cells. The red fluorescent nucleic acid stain only labels dead cells. As shown in Figure 4, none of the control samples showed red dots in the organoid area. Samples containing nanoparticles showed only two or three dead cells inside the organoid, the organoid structure was maintained and the organoid was still healthy.
Figure 3.
Organoid’s growth in 6-day time scale using inverted microscope. The magnification is 10X. Scale bars represent 200 μm.
Figure 4.
Live (SYTO® 10) /dead (DEAD REDTM) cytotoxicity test recorded by confocal fluorescent microscope. Green fluorescent nucleic acid labels all live and dead cells. Red fluorescent nucleic acid labels the dead cells with compromised membrane. The magnification is 20X. Scale bars represent 100 μm. NP stands for nanoparticle.
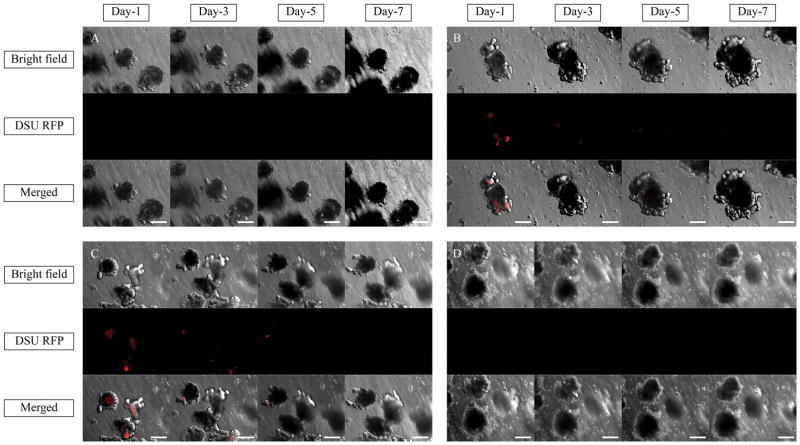
Figure 5 shows the absorption of 40 μL of nanoparticle solution containing 0%, 1%, 2.5%, and 5% the tracer dye Rhodamine B inside the organoid’s lumen in a 7-day experiment. Red fluorescence is an indicator of the presence of Rhodamine B in the system. The test was performed on the border of Matrigel for the sample containing 5% dyed nanoparticles and is shown in Figure 6. To evaluate the effect of larger amounts of nanoparticles on the diffusion, 400 μL of nanoparticle solution containing 2.5% Rhodamine B was added to the cell culture medium. Thus, the volume of the particle solution was 10 times higher than that in the first experiment. The picture of the system in z-direction is shown in Supplementary Figure 2. To assess the effect of chemistry on the diffusion, polystyrene (PS) nanoparticles with the same size as PLGA nanoparticles were tested. The fluorescent pictures are shown in Supplementary Figure 3A–C.
Figure 5.
Bright field, DSU RFP and merged pictures of the organoids exposing 40 μl of (A) 0% Rhodamine B (B) 1% Rhodamine B (C) 2.5% Rhodamine B (D) 5% Rhodamine B loaded PLGA nanoparticles in the cell culture medium using confocal fluorescent microscope within one week of experiments. Red areas are the indicators of the Rhodamine B loaded inside the PLGA nanoparticles.. The magnification is 10X.
Figure 6.
Bright field, DSU RFP and merged pictures of the organoids exposing 40 μl of 5% Rhodamine B loaded PLGA nanoparticles suspension at the border of Matrigel using confocal fluorescent microscope within one week of the experiments. Red areas are the indicators of the Rhodamine B loaded inside the PLGA nanoparticles. The magnification is 10X. Scale bars represent 200 μm.
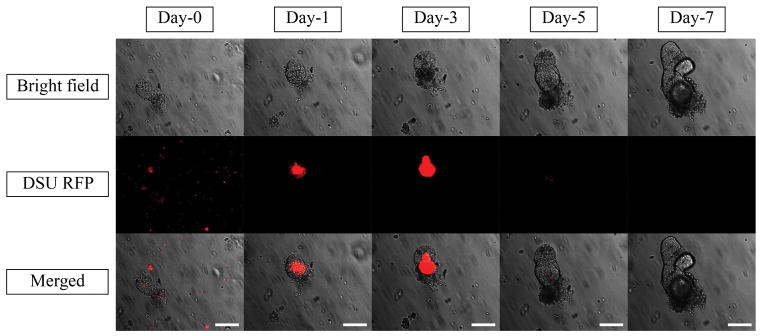
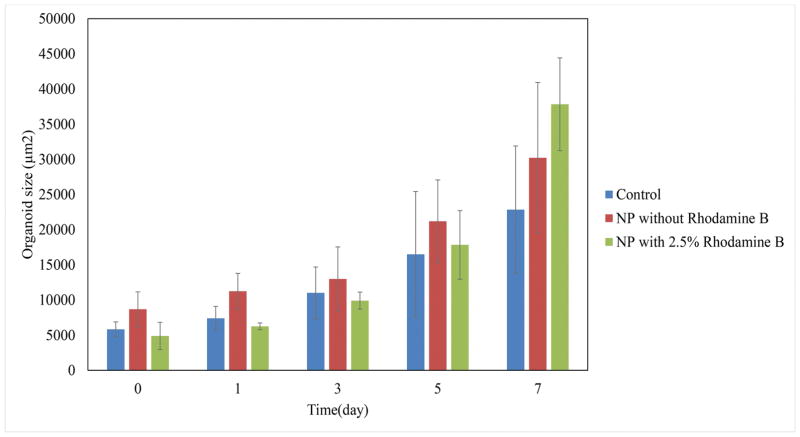
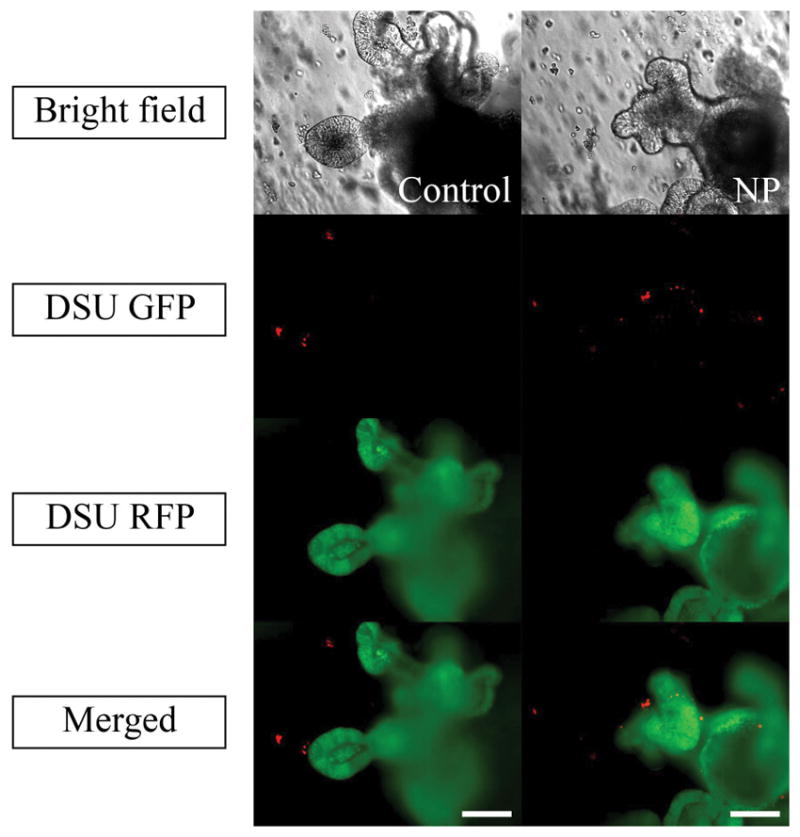
To assess the second method, Figure 7 shows the effect of mixing PLGA nanoparticles containing 2.5% Rhodamine B with the organoids and Matrigel suspension on the diffusion of particles. The live/dead cytotoxicity test was performed without Rhodamine B to observe the harmful effect of nanoparticles on the viability of the organoids (Figure 8). To quantify the effect of organoid growth rate, the rate of size increase was quantified, as shown in Figure 9.
Figure 7.
Bright field, DSU RFP and merged pictures of the mixture of 10 μl of 2.5% Rhodamine B loaded PLGA nanoparticles suspension, Matrigel and Organoids using confocal fluorescent microscope within one week of experiments. Red areas are the indicators of the Rhodamine B loaded inside the PLGA nanoparticles. The magnification is 20X. Scale bars represent 100 μm.
Figure 8.
Live (SYTO® 10)/ dead (DEAD REDTM) cytotoxicity test of the mixture of 10 μl 0% Rhodamine B loaded nanoparticles, Matrigel and Organoids recorded by confocal fluorescent microscope. Green fluorescent nucleic acid labels all live and dead cells. Red fluorescent nucleic acid labels the dead cells with compromised membrane. The magnification is 20X. Scale bars represent 100 μm.
Figure 9.
Comparison of organoids sizes without nanoparticle, with 0% Rhodamine B and with 2.5% Rhodamine B loaded nanoparticles in a one week experiment.
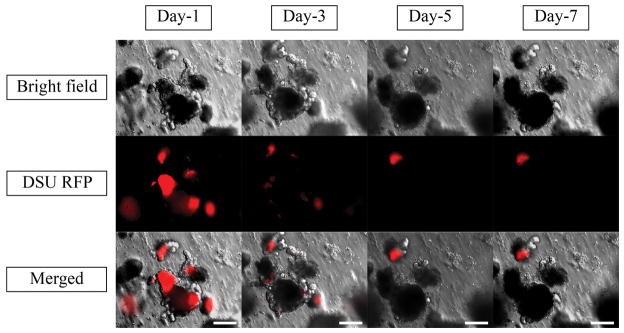
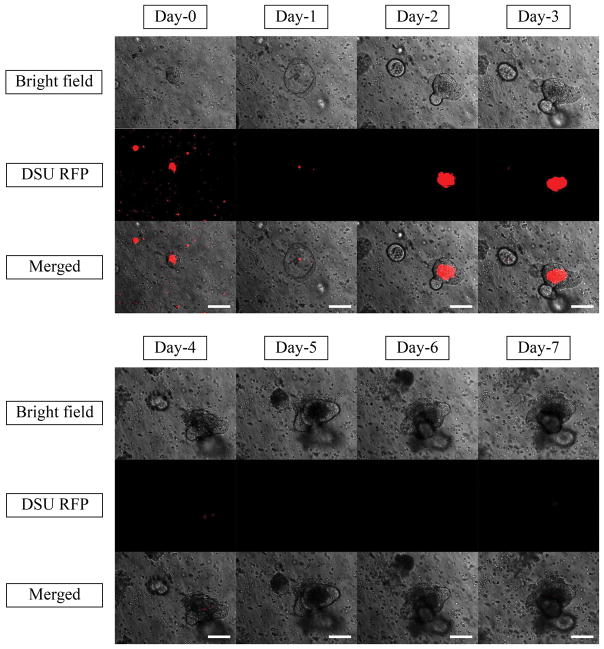
To find out the exact time of nanoparticle disappearance, the daily trend of nanoparticle tracking is shown in Figure 10. Moreover, the chemical structure and the size of the nanoparticles are of great importance in the organoid absorption. Thus, 200-nm latex beads loaded with FITC and 200-nm and 400-nm PA nanoparticles loaded with Rhodamine B were tested to check the effect of size and chemical structure. The daily trends of tracking these nanoparticles in a 1-week time scale are shown in Supplementary Figure 4–6.
Figure 10.
Bright field, DSU RFP and merged pictures of the mixture of 10 μl 2.5% Rhodamine B loaded nanoparticles, Matrigel and Organoids using confocal fluorescent microscope within one week of experiments. Red areas are the indicators of the Rhodamine B loaded inside the PLGA nanoparticles. The magnification is 20X. Scale bars represent 100 μm.
Discussion
The nanoparticle sizes are close to those found in the literature for PLGA nanoparticles with alginate as a surfactant.33 As it is shown in Figure 1A, the size of nanoparticles are all in range. The zeta potential of the nanoparticles prepared by the double-emulsion method is similar to those of the nanoparticles previously attained by solvent diffusion.38 These numbers show that the zeta potential is stable with changing 5-ASA ratios and that the overall charge of the particles is negative. Thus, the particles may easily attach to the positively charged inflamed region and release the drug there.
As seen in Figure 3, the normal growth of the organoids in samples containing nanoparticles compared to the control sample can be an indicator of the harmless characteristics of PLGA nanoparticles on the growth of organoids. This test can be verified as shown in Supplementary Figure 1, which quantifies the growth rate. All samples showed a progressive growth rate after 6 days. Live/dead cytotoxicity testing (Figure 4) confirmed this conclusion by showing the small number of dead cells inside the organoid area, which is comparable to that in the control sample without nanoparticles. As it is shown in Figure 4, the nanoparticles did not cause considerable cell death compared to the control sample and the major cell death in both samples occurred in the well plates.
The EEs for 1%, 2.5% and 5% Rhodamine B loaded nanoparticles were 32.8%, 45.5% and 44.7%, respectively. Due to the higher loaded Rhodamine B inside the nanoparticles by increasing the loading ratio, higher red color is expected in the lumen of the organoids. By increasing the amount of Rhodamine B in the nanoparticle formulation, the red dye was more pronounced in the lumen of organoids (Figure 5A–C). However, this trend was not confirmed by 5% Rhodamine B (Figure 5D). One possibility was the presence of nanoparticles at the border of the Matrigel and the lack of permeation inside the Matrigel. Figure 6 confirms the higher amount of Rhodamine B in the lumen in response to the higher EE and shows a strong red color at the border of the Matrigel and the culture media. This figure confirms that the dyed particles did not enter the Matrigel.
The red color in Figure 5A–C can be explained as either single dye molecules, which were present in the nanoparticle solution individually, or dye molecules that were already released from the nanoparticles. Three possible reasons can be considered in this case. First, the volume of nanoparticle solution added to the system was too small and hence, the amount of nanoparticles present in the solution was too small to penetrate the gel. Second, the chemical structure of PLGA does not allow the particles to penetrate the Matrigel. The third explanation may relate to the larger size of particles. As shown in a previous study, gold nanoparticles with a size of around 13 nm easily diffused inside the Matrigel.21
The first explanation is rejected based on Supplementary Figure 2, where the volume of nanoparticles added to the system was 10 times higher than that used previously. The dyed nanoparticles accumulated on the upper curved side of the Matrigel and could not penetrate the gel. It is interesting that the organoids present in the Matrigel were also dyed, which is a confirmation of the presence of single Rhodamine B molecules inside the gel. Evidently, there are no sharp red dots inside the entire Matrigel. Furthermore, the second explanation is not acceptable. As observed in Supplementary Figure 3A–C, the PS nanoparticles were all populated at the border of the Matrigel and could not penetrate the gel. Thus, the chemical structure is not the obstacle at the present stage of project but may need to be evaluated further.
The only remaining explanation that needs to be investigated is the particle size. To correct for the failure of the nanoparticles to penetrate the Matrigel, a condition should be prepared in which organoids can be contacted without the diffusion requirement. As mentioned before, in the passage step of organoid culture, the nanoparticle suspension was mixed with the cell solution and subsequently with the Matrigel. After resuspension, the resultant mixture was applied onto the plate. Therefore, the Matrigel was seeded with crypts and nanoparticles that were located around the crypts. When the organoids grew, they were in close contact with the nanoparticles and absorbed them. As a result, there was no need for nanoparticle penetration into the Matrigel. It is shown in Figure 7 that the nanoparticles were all absorbed into the lumen after 3 days. The red dots representing PLGA nanoparticles loaded with 2.5% Rhodamine B at day 0 are seen all around the lumen. The red color inside the lumen at day 0 can be attributed to the free Rhodamine B in the nanoparticle suspension. At day 3, there were no nanoparticles around the organoid, but they were all absorbed into the lumen. The sharp red color is an indicator that supports this theory. High EE of 2.5% loaded Rhodamine B results in the high amount of Rhodamine B entrapped inside the lumen. In contrast to the method of adding nanoparticle suspension to the culture medium, high EE of 2.5% loaded Rhodamine B nanoparticles is completely sensible in the lumen of the organoids. Figure 8 shows that after 7 days of adding nanoparticles, no considerable harmful effect was observed compared to that in the control sample and the organoid growth rate was comparable to that of the control sample (Figure 9). However, the main challenge is the phenomenon that was observed after day 3 in Figure 7, when no Rhodamine B was detected. To explain this phenomenon, tracking was performed every day for 7 days.
Figure 10 shows the same phenomenon. No red indicator was observed after day 3, which can be correlated to the ability of the organoids to digest the particles. Furthermore, no red indicator was observed in the lumen after day 3. Another possibility is the degradation of the particles due to the enzymatic activity of the organoids, which results in the absorption of free Rhodamine B inside the lumen and its desorption out of the lumen after day 4. To test this hypothesis, Rhodamine B-loaded PA nanoparticles were tested. As Supplementary Figure 5 and 6 show, the Rhodamine B-loaded PA nanoparticles showed the same trend. For larger nanoparticles, fewer nanoparticles can be absorbed inside the lumen, but for both samples, the Rhodamine B was not active after day 4, confirming the conclusion from Figure 10. As the absorption mechanism of organoids is not fully understood, it can be implied that the loaded organoids are mostly applicable in day 3. In day 3, the drug in fully absorbed inside the lumen and the organoid is ready to release the drug into the surrounding tissue. By complete release of the drug from organoid to the tissue, the drug molecule can locally affect the inflamed region. Latex bead nanoparticles could not diffuse inside the lumen and the FITC remained around the organoid for 7 days (Supplementary Figure 4). Latex beads are more stable, and less free FITC can be released from the nanoparticles. Moreover, the rate of degradation was lower in latex beads, which can confirm the second hypothesis that the released Rhodamine B diffused inside the lumen.
Conclusion
IBD is a group of diseases that result in inflammation in any part of the GI tract. No permanent cure for this disease exists. The high number of people suffering from this disease all over the world leads to the design and production of different drug formulations to alleviate the symptoms. Trojan horse systems using ISCs seems to have promising potential in the pharmaceutical field. In this research, cell delivery treatments using 5-ASA-loaded PLGA nanoparticles have been proposed. It was hypothesized that loading nanoparticles containing drugs inside the lumen of intestinal organoids have highly appropriate characteristics that would be helpful in IBD treatment.
PLGA nanoparticles were made by a double-emulsion (water/oil/water) method and tested to determine the characteristics. The size of nanoparticles containing 0, 2.5%, 5%, and 7.5% (w/w) 5-ASA was in the range of 200–300 nm, and the data was confirmed with SEM images. The zeta potentials of the samples were all approximately −30 to −40 mV, which makes the particles completely ideal for attaching to the inflamed area.
As observed, the nanoparticles did not have a considerable harmful effect on the viability of the organoids, and they exhibited normal growth with or without the presence PLGA nanoparticles. It was also concluded that because of the large size of the nanoparticles, they could not diffuse into the Matrigel as they were. As the nanoparticles could not cross the border of the Matrigel, a new technique was proposed to improve the loading efficiency of the organoids. Nanoparticle suspension was mixed with the organoid suspension and Matrigel, and finally applied on the well. It was demonstrated that the nanoparticles were absorbed into the lumen and digested after 3 days. Furthermore, the PLGA nanoparticles mixed with organoids and Matrigel did not exhibit any negative effect on the organoids after 7 days, and the organoid growth rate was not significantly different compared to that of the control sample. It was interesting that the same trend of penetration was observed even after changing the nanoparticles to PA or changing the size of the nanoparticles from 200 nm to 400 nm. Therefore, this technique affirms the penetration of the nanoparticles inside the lumen without considerable negative effect on organoid growth.
Despite the great development of nanoparticle-loaded organoids, much needs to be done to make the system more applicable. The insertion of a delivery system inside the colon and the assurance of its accuracy and operation in mice are necessary goals for this field. Thus, immunohistochemistry is of great help in determining the functionality and effectiveness of the system. Moreover, a method of checking the functionality of the system in mice (in vivo) must be designed. However, for the periodic evaluation, some external factors such as weight loss, food and water uptake, and the color of excrement can be monitored. It is worth noting that these tests are not generally precise because of the influence of various factors, including stress or infections.39 Colonoscopy of mice is useful in achieving this goal. Based on the advances made,40 it can be helpful to apply the delivery system to the colon and monitor the colon constantly. Future goals will be to determine the effect of the treatment on mouse health.
Supplementary Material
Acknowledgments
Dr. Wang is grateful for the support from Crohn’s & Colitis Foundation of America (CCFA) Career Award (No. 348137), PhRMA Foundation Research Starter Award (No. RSGTMT17), and McGee-Wagner Interdisciplinary Research Foundation. The research was also supported (TAB) by NIH (2RO1 DK095662) and VA Merit (1I01CX001353) grants.
References
- 1.Barker N, Oudenaarden A, Van Clevers H. Identifying the Stem Cell of the Intestinal Crypt: Strategies and Pitfalls. Cell Stem Cell. 2012;11:452–60. doi: 10.1016/j.stem.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 2.Michalčíková RB, Dryahina K, Španěl P. SIFT-MS quantification of several breath biomarkers of inflammatory bowel disease, IBD: a detailed study of the ion chemistry. Int J Mass Spectrom. 2016;396:35–41. [Google Scholar]
- 3.Crohn’s & Colitis Foundation of America. The Facts About Inflammatory Bowel Diseases. 2014. [Google Scholar]
- 4.Lautenschläger C, Schmidt C, Fischer D, Stallmach A. Drug delivery strategies in the therapy of inflammatory bowel disease. Adv Drug Deliv Rev. 2014;71:58–76. doi: 10.1016/j.addr.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 5.Kriegel C, Amiji M. Oral TNF- α gene silencing using a polymeric microsphere-based delivery system for the treatment of inflammatory bowel disease. J Control Release. 2011;150:77–86. doi: 10.1016/j.jconrel.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dallegri F, Ottonello L, Ballestrero A, Bogliolo F, Ferrando F, Patrone F. Cytoprotection against neutrophil derived hypochlorous acid: a potential mechanism for the therapeutic action of 5-aminosalicylic acid in ulcerative colitis. Gut. 1990;31:184–6. doi: 10.1136/gut.31.2.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gisbert JP, Gomollon F, Mate J, Pajares JM. Role of 5-Aminosalicylic Acid ( 5-ASA ) in Treatment of Inflammatory Bowel Disease. Dig Dis Sci. 2002;47:471–88. doi: 10.1023/a:1017987229718. [DOI] [PubMed] [Google Scholar]
- 8.Torchilin VP. Drug targeting. Eur J Pharm Sci. 2000;11:81–91. doi: 10.1016/s0928-0987(00)00166-4. [DOI] [PubMed] [Google Scholar]
- 9.Ali H, Weigmann B, Collnot E, Khan SA, Windbergs M, Lehr C. Budesonide Loaded PLGA Nanoparticles for Targeting the Inflamed Intestinal Mucosa — Pharmaceutical Characterization and Fluorescence Imaging. Pharm Res. 2015;33:1085–92. doi: 10.1007/s11095-015-1852-6. [DOI] [PubMed] [Google Scholar]
- 10.Nakase H, Okazaki K, Tabata Y, Uose S, Ohana M, Uchida K. Development of an Oral Drug Delivery System Targeting Immune-Regulating Cells in Experimental Inflammatory Bowel Disease: A New Therapeutic Strategy 1. J Pharmacol Exp Ther. 2000;292:15–21. [PubMed] [Google Scholar]
- 11.Nakase H, Okazaki K, Tabata Y, Uose S, Ohana M, Uchida K, Nishi T, Debreceni A, Itoh T, Kawanami C, Iwano M, Ikada Y, Chiba T. An oral drug delivery system targeting immune-regulating cells ameliorates mucosal injury in trinitrobenzene sulfonic acid-induced colitis. J Pharmacol Exp Ther. 2001;297:1122–8. [PubMed] [Google Scholar]
- 12.Lamprecht A, Schäfer U, Lehr CM. Size-dependent bioadhesion of micro- and nanoparticulate carriers to the inflamed colonic mucosa. Pharm Res. 2001;18:788–93. doi: 10.1023/a:1011032328064. [DOI] [PubMed] [Google Scholar]
- 13.Hu X, Tang Y, Wang Q, Li Y, Yang J, Du Y, Kennedy JF. Rheological behaviour of chitin in NaOH / urea aqueous solution. Carbohydr Polym. 2011;83:1128–33. [Google Scholar]
- 14.Wang Q, Du Y, Fan L-H, Liu H, Wang X. Structures and Properties of Chitosan-Starch-Sodium Benzoate Blend Films. J Wuhan Univ (Natural Sci Ed. 2003;49:725–30. [Google Scholar]
- 15.Pertuit D, Moulari B, Betz T, Nadaradjane A, Neumann D, Ismaïli L, Refouvelet B, Pellequer Y, Lamprecht A. 5-amino salicylic acid bound nanoparticles for the therapy of inflammatory bowel disease. J Control Release. 2007;123:211–8. doi: 10.1016/j.jconrel.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 16.Lamprecht ALF, Ubrich N, Yamamoto H, Afer USCH, Takeuchi H. Biodegradable Nanoparticles for Targeted Drug Delivery in Treatment of Inflammatory Bowel Disease. J Pharmacol Exp Ther. 2001;299:775–81. [PubMed] [Google Scholar]
- 17.Lamprecht A, Yamamoto H, Takeuchi H, Kawashima Y. Nanoparticles Enhance Therapeutic Efficiency by Selectively Increased Local Drug Dose in Experimental Colitis in Rats. J Pharmacol Exp Ther. 2005;315:196–202. doi: 10.1124/jpet.105.088146. [DOI] [PubMed] [Google Scholar]
- 18.Klotz U, Schwab M. Topical delivery of therapeutic agents in the treatment of inflammatory bowel disease. Adv Drug Deliv Rev. 2005;57:267–79. doi: 10.1016/j.addr.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 19.Schmidt C, Lautenschlaeger C, Collnot E, Schumann M, Bojarski C, Schulzke J, Lehr C, Stallmach A. Nano- and microscaled particles for drug targeting to inflamed intestinal mucosa — A first in vivo study in human patients. J Control Release. 2013;165:139–45. doi: 10.1016/j.jconrel.2012.10.019. [DOI] [PubMed] [Google Scholar]
- 20.Batrakova EV, Gendelman HE, Kabanov AV. Cell-Mediated Drugs Delivery. Expert Opin Drug Deliv. 2011;8:415–33. doi: 10.1517/17425247.2011.559457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peng H, Wang C, Xu X, Yu C, Wang Q. An intestinal Trojan horse for gene delivery. Nanoscale. 2015;7:4354–60. doi: 10.1039/c4nr06377e. [DOI] [PubMed] [Google Scholar]
- 22.Sato T, Clevers H. Growing self-organizing mini-guts from a single intestinal stem cell: mechanism and applications. Science. 2013;340:1190–4. doi: 10.1126/science.1234852. [DOI] [PubMed] [Google Scholar]
- 23.Leedham SJ, Brittan M, Mcdonald SAC, Wright NA. Intestinal stem cells. J Cell Mol Med. 2005;9:11–24. doi: 10.1111/j.1582-4934.2005.tb00333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Asfaha S. Intestinal stem cells and inflammation. Curr Opin Pharmacol. 2015;25:62–6. doi: 10.1016/j.coph.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 25.Leushacke M, Barker N. Ex vivo culture of the intestinal epithelium: strategies and applications. Gut. 2014;63:1345–54. doi: 10.1136/gutjnl-2014-307204. [DOI] [PubMed] [Google Scholar]
- 26.Sato T, Vries RG, Snippert HJ, Van De Wetering M, Barker N, Stange DE, Van Es JH, Abo A, Kujala P, Peters PJ, Clevers H. Single Lgr5 stem cells build crypt – villus structures in vitro without a mesenchymal niche. Nature. 2009;459:262–6. doi: 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- 27.Li BL, Setyawati MI, Chen L, Xie J, Ariga K, Lim C, Garaj S, Leong DT. Directing Assembly and Disassembly of 2D MoS2 Nanosheets with DNA for Drug Delivery. ACS Appl Mater Interfaces. 2017;9:15286–96. doi: 10.1021/acsami.7b02529. [DOI] [PubMed] [Google Scholar]
- 28.Tay CY, Muthu MS, Chia SL, Nguyen KT, Feng S, Leong DT. Reality Check for Nanomaterial-Mediated Therapy with 3D Biomimetic Culture Systems. Adv Funct Mater. 2016;26:4046–65. [Google Scholar]
- 29.Chia SL, Tay CY, Setyawati MI, Leong DT. Biomimicry 3D Gastrointestinal Spheroid Platform for the Assessment of Toxicity and Inflammatory Effects of Zinc Oxide Nanoparticles. Small. 2015;11:702–12. doi: 10.1002/smll.201401915. [DOI] [PubMed] [Google Scholar]
- 30.Qian H, Tay CY, Setyawati MI, Chia SL, Lee DS, Leong DT. Protecting microRNAs from RNase degradation with steric DNA nanostructures. Chem Sci. 2017;8:1062–7. doi: 10.1039/c6sc01829g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yui S, Nakamura T, Sato T, Nemoto Y, Mizutani T, Zheng X, Ichinose S, Nagaishi T, Okamoto R, Tsuchiya K, Clevers H, Watanabe M. Functional engraftment of colon epithelium expanded in vitro from a single adult Lgr5+ stem cell. Nat Med. 2012;18:618–23. doi: 10.1038/nm.2695. [DOI] [PubMed] [Google Scholar]
- 32.Barker N. Adult intestinal stem cells: critical drivers of epithelial homeostasis and regeneration. Nat Rev Mol CELL Biol. 2013;15:19–33. doi: 10.1038/nrm3721. [DOI] [PubMed] [Google Scholar]
- 33.Gu Z, Aimetti AA, Wang Q, Dang TT, Zhang Y, Veiseh O, Cheng H, Langer R, Anderson D. Injectable Nano-Network for Glucose-Mediated Insulin Delivery. ACS Nano. 2013;7:4194–201. doi: 10.1021/nn400630x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee KY, Mooney DJ. Alginate: Properties and biomedical applications. Prog Polym Sci. 2012;37:106–26. doi: 10.1016/j.progpolymsci.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gombotz WR, Wee SF. Protein release from alginate matrixes. Adv Drug Deliv Rev. 1998;31:267–85. doi: 10.1016/s0169-409x(97)00124-5. [DOI] [PubMed] [Google Scholar]
- 36.Peng H, Poovaiah N, Forrester M, Cochran E, Wang Q. Ex Vivo Culture of Primary Intestinal Stem Cells in Collagen Gels and Foams. ACS Biomater Sci Eng. 2015;1:37–42. doi: 10.1021/ab500041d. [DOI] [PubMed] [Google Scholar]
- 37.Kleinman HK, Martin GR. Matrigel: Basement membrane matrix with biological activity. Semin Cancer Biol. 2005;15:378–86. doi: 10.1016/j.semcancer.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 38.Büyüktimkin B, Wang Q, Kiptoo P, Stewart JM, Berkland C, Siahaan TJ. Vaccine-like controlled-release delivery of an immunomodulating peptide to treat experimental autoimmune encephalomyelitis. Mol Pharm. 2012;9:979–85. doi: 10.1021/mp200614q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Becker C, Fantini MC, Neurath MF. High resolution colonoscopy in live mice. Nat Protoc. 2006;1:2900–4. doi: 10.1038/nprot.2006.446. [DOI] [PubMed] [Google Scholar]
- 40.Kodani T, Rodriguez-palacios A, Corridoni D, Lopetuso L, Di Martino L, Marks B, Pizarro J, Pizarro T, Chak A, Cominelli F. Flexible Colonoscopy in Mice to Evaluate the Severity of Colitis and Colorectal Tumors Using a Validated Endoscopic Scoring System. J Vis Exp. 2013;80:1–16. doi: 10.3791/50843. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.