Abstract
Moderate to severe chronic graft-versus-host disease (GVHD) is treated with potent immunosuppressive therapy (IST) to modulate the allo-immune response, control symptoms and prevent further organ damage. We sought to understand the types of treatments used in clinical practice and the likelihood of successful treatment associated with each. A chart review was performed for 250 adult patients at Fred Hutchinson Cancer Research Center enrolled in a prospective observational study. After a median follow-up of 5.6 years for survivors, approximately one third were still on IST (of whom half were on 4th or greater line of therapy), one third were alive and off IST, and one third had relapsed or died. Approximately half of survivors stopped all IST at least once, although half of these restarted IST after a median of 3.4 months off therapy (IQR 2.3-8.0 months). Successful discontinuation of IST for at least 9 months was associated with myeloblative conditioning (p=0.04), more years since transplant (p=0.009) and lack of oral (p<0.001) and skin (p=0.049) involvement compared to people who had to restart IST. We conclude that patients with chronic GVHD usually receive multiple lines and years of IST, with only a third off IST, alive and free of malignancy at 5 years after chronic GVHD diagnosis. Patients stopping IST should be cautioned to self-monitor and continue close medical follow up especially for 3-6 months after stopping IST.
Keywords: chronic graft-versus-host disease, allogeneic hematopoietic cell transplantation, immunosuppressive treatment
Introduction
Patients who receive allogeneic hematopoietic cell transplantation (HCT) typically require administration of immunosuppressive therapy (IST) for at least 6 months in order to prevent graft-versus-host disease (GVHD). With standard pharmacologic prophylaxis, significant acute GVHD occurs in 20-50% of patients.1 and chronic GVHD occurs in 30-50% of patients.2,3 If patients develop GVHD, treatment doses of IST are administered until GVHD is controlled, then IST is slowly tapered with the goal of eventually discontinuing IST permanently. Effective prophylaxis and treatment of GVHD are critical to reduce mortality and morbidity associated with GVHD, but IST may increase risks of infection, cumulative organ toxicities, subsequent neoplasms and recurrent malignancy.4
Unlike solid organ transplantation where life-long IST is usually required,5 many patients who have undergone allogeneic HCT can achieve sufficient “functional” tolerance even after development of GVHD and can eventually stop all IST permanently without any active manifestations of GVHD. Current management of GVHD often involves repeated attempts to taper and stop IST since there is no reliable way to predict when IST can be successfully withdrawn.6 Each taper attempt risks a GVHD flare with subsequent resumption of high-dose IST. Unsuccessful discontinuation of IST may increase rates of patient morbidity and mortality because resumption of IST may fail to control GVHD resulting in irreversible organ damage or IST complications. Biomarker studies are in progress to try to identify biologic predictors for successful IST discontinuation but actionable results have not yet been reported.
Previous studies have identified clinical factors associated with the need for prolonged IST in patients with chronic GVHD, including hyperbilirubinemia and multiple sites affected by chronic GVHD at the onset of disease.7,8 However, no studies have specifically described the treatment courses of patients with chronic GVHD in usual practice. With the increased availability of new agents and clinical trials testing novel approaches to chronic GVHD treatment, the current study aimed to examine the patterns of how providers change chronic GVHD therapies and the timing and outcome of those treatment decisions. We also sought to explore the clinical characteristics related to successful IST discontinuation and to understand the reasons, timing and outcome of resumption of IST.
Methods
Patients and Data Collection
The study cohort included 250 adult patients who had previously undergone allogeneic transplantation and subsequently received systemic treatment for chronic GVHD at the Fred Hutchinson Cancer Research Center (FHCRC)/Seattle Cancer Care Alliance (SCCA).9 Patients were enrolled in a prospective multicenter observational study between 2007 and 2012, and were serially evaluated every 3-6 months throughout the follow up period in order to observe chronic GVHD response to IST. Patients were eligible regardless of graft source, donor type, or GVHD prophylaxis. To prevent enrollment of patients with very long-standing chronic GVHD, patients had to be enrolled within 3 months of chronic GVHD diagnosis, or if prevalent cases, within 3 years of transplant. Only patients enrolled at FHCRC are included in this analysis because the chart review required was more detailed than designed for the primary study. All participants gave written consent allowing the use of medical records for research in accordance with the Declaration of Helsinki. The Institutional Review Board of the FHCRC approved the study.
Patient medical records, including records from our outpatient clinic and local clinics responsible for primary care, were reviewed through July 2015. Information about prevalent cases, who were enrolled more than 3 months after chronic GVHD diagnosis, was collected retrospectively for the time period prior to enrollment. Data regarding organ involvement, types of initial and subsequent systemic treatment for chronic GVHD, as well as reasons for starting and stopping treatment were collected.
Definitions
Chronic GVHD was diagnosed per 2005 NIH consensus criteria.10 Systemic treatment was defined as any medication or intervention that had intended systemic effects, including extracorporeal photopheresis. Treatment change was defined as the addition of systemic therapy to a patient’s chronic GVHD regimen, regardless of prior lines of therapy or prior treatment with the agent(s). Because temporary increases in medications doses are often necessary during treatment of chronic GVHD, any increase in dose when patients were already being treated with an agent was not considered a treatment change. Treatments that were stopped and restarted within 30 days were considered continuous treatment. A line of therapy was defined as one or more treatments recommended at the same time. For example, if a provider recommended prednisone and extracorporeal photopheresis (ECP) for treatment of sclerosis but the ECP could not be started until a month after the prednisone because of insurance approval and line placement, the prednisone and ECP were still considered the same line of therapy. Topical therapies and fluticasone/azithromycin/montelukast were not considered systemic treatments.
Recurrent malignancy was defined by hematologic, molecular, cytogenetic, flow cytometry or radiographic criteria, or any intervention to treat recurrent malignancy.
For the purposes of multivariate logistic modeling, successful discontinuation of IST was defined as no exposure to systemic IST for at least 9 months without relapse or death, while unsuccessful discontinuation was defined as resumption of IST within 9 months of discontinuation. This cutoff was based on an evaluation of the data to capture most patients restarting therapy without excluding too many because of an extended follow up requirement. Patients who were less than 9 months since IST discontinuation at the time of chart review or who relapsed or died within 9 months of IST discontinuation were excluded from the multivariate analysis.
Clinical Management of Chronic GVHD
At FHCRC, chronic GVHD is usually treated initially by adding or increasing the dose of prednisone. Standard initial prednisone dosing is 0.5-1 mg/kg/day for 2 weeks, followed by a dose taper over the next 4-6 weeks to 0.5-1 mg/kg every other day as allowed by improvement in GVHD manifestations. Attending physicians may deviate from this regimen according to clinical judgment, often by either starting at a lower dose, tapering more rapidly, or tapering in an alternative fashion (for example, a daily rather than alternating day regimen). Continued improvement of reversible chronic GVHD manifestations or GVHD resolution prompts IST tapering by gradual and sequential withdrawal of all systemic treatment. However, decisions to taper systemic treatment or to initiate subsequent lines of treatment were at the discretion of the attending physician and physician behavior was not standardized because there are no treatment guidelines once a patient is beyond initial therapy. FHCRC is a group practice and multiple physicians could see and manage individual patients. We did not track which attending made decisions to start, stop or change immunosuppression.
Patients on systemic IST receive anti-viral, anti-bacterial and anti-pneumocystis prophylaxis. Anti-fungal prophylaxis is not generally given outside the context of high-dose steroid therapy or a history of prior invasive mold infections. Monitoring for cytomegalovirus (CMV) with PCR is resumed for patients at risk of late reactivation. Additional monitoring includes annual DEXA scans for patients on steroids, pulmonary function tests every 3-6 months, dental evaluations every 6 months, and annual ophthalmologic and gynecologic exams.
Statistical Methods
Descriptive statistics are provided for patient, transplant and chronic GVHD characteristics and use of IST. For each treatment added for first, second or third line therapy, “% failure-free survival (FFS)” is defined as no relapse or death within 6 months, and no addition of another chronic GVHD systemic agent during the observation period. Patients who relapsed, died or started a new systemic treatment within 6 months are considered treatment failures. Patients had to have a minimum of 6 months observation time to be considered to have FFS. The contribution of specific organ manifestations to the decisions to start initial and subsequent therapy is reported descriptively. Both incident and prevalent cases are included in these analyses.
Multivariable logistic regression was used to identify variables associated with successful IST discontinuation for ≥ 9 months among patients stopping IST. Patients who relapsed or died before 9 months or who had less than 9 months of observation time off IST were excluded from this analysis. Potential explanatory variables included patient age and sex, incident vs. prevalent case at enrollment, IST agent and line of therapy stopped, duration of chronic treatment since onset/last attempt at discontinuation, years since transplant, donor type, graft source, conditioning regimen intensity, prior acute grade II-IV GVHD, and organ involvement. A stepwise forward analysis was performed with entry criteria = 0.05 and retention criteria = 0.10.
A subset analysis was conducted for incident cases, since they were enrolled at the time of chronic GVHD onset so their entire history of treatments occurred during the study observation period, preventing bias attributable to the survival duration prior to enrollment. Cumulative incidence curves were generated for lines of therapy and resumption of IST after first or second discontinuation. In these analyses, relapse and death were considered competing risks. The median time to IST discontinuation was calculated by the Kaplan-Meier method, defining IST discontinuation as stopping IST and no resumption of IST, and censoring for relapse, death and last contact date. To calculate the chance that initial therapy will be successful, the Kaplan-Meier method was again used considering resumption of IST as an event, and censoring for relapse, death and last contact date.
Results
Characteristics of the Study Cohort
Patient characteristics are summarized in Table 1. The median age of patients at enrollment was 53 years. One-hundred forty-eight patients (59.2%) were incident cases who enrolled within three months of their chronic GVHD diagnosis, while 102 patients (40.8%) were prevalent cases who enrolled more than three months since diagnosis. At study entry, 16 (6.4%) had a mild NIH global score or were asymptomatic, 132 (52.8%) had a moderate score, and 102 (40.8%) had a severe score. There were no haploidentical related donor recipients and no patients received in vivo or ex vivo T cell depletion. Three (1%) cord blood recipients were included. The median follow-up duration since enrollment for the 177 surviving patients was 5.6 years (IQR, 4.5-6.8 years).
Table 1.
Patient characteristics for the total population (N=250) and separated into incident cases (enrollment less than 3 months since chronic GVHD diagnosis) and prevalent (>3 months since chronic GVHD diagnosis)
| Total | Incident | Prevalent | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| n | % | n | % | n | % | |
|
| ||||||
| Median age (yrs), IQR, [range] | 250 | 53 IQR 43-61 [19-79] |
148 | 53 IQR 42-62 [19-79] |
102 | 52 IQR 44-60 [22-74] |
|
| ||||||
| Gender | ||||||
| Males | 140 | 56.0 | 77 | 52.0 | 63 | 61.8 |
| Females | 110 | 44.0 | 71 | 48.0 | 39 | 38.2 |
|
| ||||||
| Type of transplant | ||||||
| Myeloablative | 114 | 45.6 | 65 | 43.9 | 49 | 48.0 |
| Not myeloablative | 136 | 54.4 | 83 | 56.1 | 53 | 52.0 |
|
| ||||||
| Conditioning regimens | ||||||
| Myeloablative | 134 | 53.6 | 79 | 53.4 | 55 | 53.9 |
| Busulfan/cyclophosphamide | 57 | 22.8 | 34 | 23.0 | 23 | 22.6 |
| Cyclophosphamide/TBI | 42 | 16.8 | 21 | 14.2 | 21 | 20.6 |
| Treosulfan/fludarabine +/− TBI | 26 | 10.4 | 18 | 12.2 | 8 | 7.8 |
| Other myeloablative regimen | 9 | 3.6 | 6 | 4.0 | 3 | 2.9 |
| Not myeloablative | 116 | 46.4 | 69 | 46.6 | 47 | 46.1 |
| Busulfan/fludarabine | 2 | 0.8 | 1 | 0.7 | 1 | 1.0 |
| Fludarabine/TBI | 101 | 40.4 | 60 | 40.5 | 41 | 40.2 |
| Other not myeloablative regimen | 13 | 5.2 | 8 | 5.4 | 5 | 4.9 |
|
| ||||||
| Type of donor | ||||||
| Unrelated | 150 | 60.0 | 93 | 62.8 | 57 | 55.9 |
| Related | 97 | 38.8 | 52 | 35.1 | 45 | 44.1 |
| Cord blood | 3 | 1.2 | 3 | 2.0 | 0 | – |
|
| ||||||
| Graft type | ||||||
| Peripheral blood | 227 | 90.8 | 134 | 90.5 | 93 | 91.2 |
| Bone marrow | 20 | 8.0 | 11 | 7.4 | 9 | 8.8 |
| Cord blood | 3 | 1.2 | 3 | 2.0 | 0 | – |
|
| ||||||
| Type of case | ||||||
| Incident | 148 | 59.2 | – | – | ||
| Prevalent | 102 | 40.8 | ||||
|
| ||||||
| NIH severity at enrollment | ||||||
| Mild/None | 16 | 6.4 | 10 | 6.8 | 6 | 5.9 |
| Moderate | 132 | 52.8 | 69 | 46.6 | 63 | 61.8 |
| Severe | 102 | 40.8 | 69 | 46.6 | 33 | 32.4 |
|
| ||||||
| Median time from chronic GVHD diagnosis to enrollment (months), IQR [range] | 250 | 1.7 IQR 0-7.8 [-0.1 - 26.6] |
148 | 0 IQR 0-0.9, [-0.1 - 2.8] |
102 | 8.7 IQR 5.3-14.7, [3.1-26.6] |
|
| ||||||
| Median time from transplant to last follow-up (years), IQR [range] | 250 | 5.8 IQR 4.0-7.1, [0.4-29] |
148 | 5.3 IQR 4.0-6.7 [0.4-9.8] |
102 | 6.7 IQR 4.1-7.9, [1.1-29] |
|
| ||||||
| Median follow up (years), IQR [range] | ||||||
| All patients | 250 | 4.9 IQR 3.5-6.5, [0.04-9.1] |
148 | 4.6 IQR 3.3-5.9, [0.04-7.9] |
102 | 5.8 IQR 3.7-7.2 [0.6-9.1] |
| Survivors | 177 | 5.6 IQR 4.5-6.8, [1.0-9.1] |
106 | 5.1 IQR 4.2-6.4, [1.0-7.9] |
71 | 6.4 IQR 5.0-7.6, [1.3-9.1] |
Abbreviations: IQR, intra-quartile range; TBI, total body irradiation
Treatment characteristics for chronic GVHD are summarized in Table 2. At diagnosis of chronic GVHD, 68.8% started a new agent, while the others had current IST dose increases (14.4%) or no change of systemic therapy (16.8%). During the observation period, the number of therapy lines distributed between the 250 study participants was: 1 (26.0%), 2 (19.2%), 3 (19.2%), 4-5 (25.2%) and 6 or more (10.4%). Of patients enrolled as prevalent cases more than 3 months after chronic GVHD diagnosis, patients were on first (n=52, 51.0%), second (n=30, 29.4%), third (n=16, 15.7%) or later (n=4, 3.9%) line of treatment and were a median of 8.7 (IQR 5.3-14.7) months since chronic GVHD diagnosis.
Table 2.
Treatment characteristics
| n | % | |
|---|---|---|
|
| ||
| Initial treatment of chronic GVHD (N=250) | ||
| New treatment added | 172 | 68.8 |
| Steroid dose increased | 20 | 8.0 |
| Other non-steroid dose increased | 16 | 6.4 |
| No treatment change | 42 | 16.8 |
|
| ||
| Total number of lines of therapy, regardless of recurrent malignancy (N=250) | ||
| One | 65 | 26.0 |
| Two | 48 | 19.2 |
| Three | 48 | 19.2 |
| Four | 42 | 16.8 |
| Five | 21 | 8.4 |
| Six or more | 26 | 10.4 |
|
| ||
| Reasons for starting new treatments | ||
| Progressed/lack of improvement | 446 | 86.1 |
| Toxicity | 23 | 4.4 |
| Steroid-sparing | 43 | 8.3 |
| Insurance/compliance | 5 | 1.0 |
| Requirement for enrollment in clinical trial | 1 | 0.2 |
|
| ||
| Median duration of time between lines of IST, for patients starting a new line of therapy, months [IQR] | ||
| Between first and second line therapy (n=185) | 7.3 | [2.7-18.6] |
| Between second and third line therapy (n=137) | 6.6 | [2.9-18.4] |
| Between third and fourth line therapy (n=89) | 8.0 | [3.8-19.1] |
| Between fourth and fifth line therapy (n=47) | 5.7 | [1.9-11.2] |
| Between fifth and sixth line therapy (n=26) | 4.4 | [1.8-7.5] |
|
| ||
| Current status (N=250) | ||
| Disease-free, still on IST | 76 | 30.4 |
| Alive, off IST | 91 | 36.4 |
| Relapse or death | 83 | 33.2 |
|
| ||
| Still on IST (N=76) | ||
| First treatment | 5 | 6.6 |
| Second treatment | 15 | 19.7 |
| Third treatment | 16 | 21.1 |
| Fourth treatment | 19 | 25.0 |
| Fifth or higher treatment | 21 | 27.6 |
|
| ||
| Number of times all IST stopped (N=250) | ||
| None, on IST currently or until death/relapse | 122 | 48.8 |
| One | 94 | 37.6 |
| Two | 29 | 11.6 |
| Three | 4 | 1.6 |
| Four | 1 | 0.4 |
|
| ||
| Reasons for stopping individual IST meds | ||
| GVHD improved/stable | 422 | 50.2 |
| GVHD progressed | 115 | 13.7 |
| Toxicity | 155 | 18.4 |
| Insurance or compliance | 30 | 3.6 |
| Induce graft-versus-leukemia effect | 35 | 4.2 |
| Planned course of treatment completed | 50 | 6.0 |
| Other | 26 | 3.1 |
| Unknown | 8 | 1.0 |
|
| ||
| Previous grade II-IV acute GVHD (N=250) | 168 | 67.2 |
|
| ||
| Median months off IST for first time restart (n=59), [IQR] | 3.4 | [2.3-8.0] |
|
| ||
| Median months off IST for all restarts (n=72), [IQR] | 3.4 | [1.9-8.0] |
Abbreviations: GVHD, graft-versus-host disease; IST, immunosuppressive therapy, IQR, intra-quartile range
After a median follow-up of 5.6 years for all survivors, 76 (30.4%) were still on IST on initial (n=5, 6.6%), second (n=15, 19.7%), third (n=16, 21.1%) or later courses of IST (n=40, 52.6%), regardless of previous attempts to discontinue IST. Ninety-one patients (36.4%) are alive and off IST. Eighty-three (33.2%) have relapsed or died.
Success rate of specific agents
The most common agents used in first and second line treatments were prednisone, calcineurin-inhibitors, mycophenolate mofetil and sirolimus. (Table 3). The percentages who did not require additional systemic therapy or relapse or die within the next 6 months are also shown for each agent, and are generally 10-30%, indicating that most currently available agents provide suboptimal chronic GVHD disease control. Approximately 50% or more received treatment with steroids, calcineurin inhibitors, or sirolimus. Also common were mycophenolate mofetil (41%), extracorporeal photopheresis (18%), rituximab (16%) and methotrexate (12%). The other agents were used in fewer than 10% of patients but included some very rarely used approaches for the most refractory patients. Table 4 shows the most common organs precipitating initial therapy or treatment changes by line of therapy. The need to treat skin and oral manifestations was the most frequent reason for adding initial and subsequent treatment, although fascia, eye, liver and gastrointestinal tract also prompted treatment changes. Sclerotic skin changes and lung GVHD were more frequent reasons for fourth line therapy and beyond compared with third line or earlier therapy.
Table 3.
New treatments started and the failure-free survival rates for 1-3 lines of therapy
| New treatment added | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1st Line (continuing agents plus new agents) n (%) | 1st Line | % FFS* | 2nd Line | % FFS* | 3rd Line | % FFS* | ≥4th Line | Ever received | |
| Prednisone | 203 (81.2) | 141 (56.4) | 49 (24.1) | 83 | 18 (22.5) | 38 | 13 (36.1) | 31 | 240 (96.0) |
| Tacrolimus | 136 (54.4) | 36 (14.4) | 35 (25.7) | 31 | 3 (10.0) | 15 | 4 (26.7) | 24 | 166 (66.4) |
| Cyclosporine | 49 (19.6) | 9 (3.6) | 6 (12.2) | 9 | 2 (22.2) | 3 | 0 (0) | 3 | 56 (22.4) |
| Sirolimus | 34 (13.6) | 31 (12.4) | 9 (26.5) | 37 | 11 (29.7) | 38 | 10 (31.2) | 30 | 123 (49.2) |
| Mycophenolate mofetil | 52 (20.8) | 21 (8.4) | 15 (28.8) | 26 | 5 (19.2) | 23 | 6 (27.3) | 22 | 102 (40.8) |
| Methotrexate | 5 (2.0) | 5 (2.0) | 3 (60.0) | 7 | 1 (14.3) | 8 | 2 (28.6)) | 10 | 29 (11.6) |
| Extra-corporeal photopheresis | 5 (2.0) | 3 (1.2) | 0 | 9 | 3 (33.3) | 11 | 1 (9.1) | 23 | 46 (18.4) |
| Imatinib | 0 | – | 0 (0) | 2 | 0 (0) | 4 | 2 (50.0) | 11 | 17 (6.8) |
| Rituximab | 2 (0.8) | 2 (0.8) | 2 (100) | 16 | 3 (18.8) | 9 | 1 (11.1) | 17 | 39 (15.6) |
| Azathioprine | 0 | – | 0 | 0 | – | 3 | 1 (33.3) | 12 | 13 (5.2) |
| Thalidomide | 0 | – | 0 | 1 | 0 (0) | 0 | – | 3 | 4 (1.6) |
| Nilotinib | 0 | – | – | 2 | 0(0) | 0 | – | 2 | 4 (1.6) |
| Bortezomib | 0 | – | – | 0 | – | 1 | 0 (0) | 3 | 4 (1.6) |
| Interleukin-2 | 0 | – | – | 0 | – | 0 | – | 3 | 3 (1.2) |
| Total lymphoid irradiation | 0 | – | – | 0 | – | 0 | – | 1 | 1 (0.4) |
| Lidocaine | 0 | – | – | 0 | – | 0 | – | 3 | 3 (1.2) |
| Anti-thymocyte globulin | 0 | – | – | 0 | – | 0 | – | 1 | 1 (0.4) |
| Alemtuzumab | 0 | – | – | 0 | – | 0 | – | 1 | 1 (0.4) |
| Hydroxychloroquine | 0 | – | – | 0 | – | 1 | 0/1 (0) | 0 | 1 (0.4) |
% FFS” is defined as no relapse or death within 6 months, and no addition of another chronic GVHD systemic agent during the observation period. Patients who relapse, die or start a new systemic treatment within 6 months are considered treatment failures
Table 4.
Organs and other manifestations causing need for additional systemic immunosuppressive therapy
| Ever a cause for adding treatment | Present at 1st Line, n (%) | Impetus* for adding 2nd line, n (%) | Impetus* for adding 3rd line, n (%) | Impetus* for adding 4th line, n (%) | |
|---|---|---|---|---|---|
| Number of patients | N=250 (%) | N=250 (%) | N=185 (%) | N=137 (%) | N=89 (%) |
| Oral | 227 (90.8) | 218 (87.2) | 69 (37.3) | 43 (31.4) | 29 (32.6) |
| Skin | 202 (80.8) | 176 (70.4) | 79 (42.7) | 33 (24.1) | 23 (25.8) |
| Eye | 117 (46.8) | 70 (28.0) | 41 (22.2) | 26 (19.0) | 13 (14.6) |
| Liver | 113 (45.2) | 80 (32.0) | 35 (18.9) | 17 (12.4) | 12 (13.5) |
| Sclerosis | 70 (28.0) | 13 (5.2) | 29 (15.7) | 29 (21.2) | 31 (34.8) |
| Fascia | 58 (23.2) | 19 (7.6) | 22 (11.9) | 19 (13.9) | 12 (13.5) |
| Joint | 48 (19.2) | 22 (8.8) | 18 (9.7) | 11 (8.0) | 5 (5.6) |
| Upper gastrointestinal | 63 (25.2) | 39 (15.6) | 20 (10.8) | 11 (8.0) | 4 (4.5) |
| Lower gastrointestinal | 50 (20.0) | 30 (12.0) | 17 (9.2) | 12 (8.8) | 3 (3.4) |
| Gastrointestinal, location not specified | 35 (14.0) | 29 (11.6) | 5 (2.7) | 0 | 1 (1.1) |
| Genitalia | 40 (16.0) | 27 (10.8) | 7 (3.8) | 10 (7.3) | 2 (2.2) |
| Lung | 33 (13.2) | 15 (6.0) | 5 (2.7) | 5 (3.6) | 9 (10.1) |
| Esophagus | 12 (4.8) | 4 (1.6) | 5 (2.7) | 1 (0.7) | 1 (1.1) |
| Eosinophils | 68 (27.2) | 45 (18.0) | 16 (8.6) | 9 (6.6) | 2 (2.2) |
| Other** | 46 (18.4) | 15 (6.0) | 19 (10.3) | 11 (8.0) | 8 (9.0) |
defined as reason listed in medical records for adding therapy
Other (may be >100% due to multiple manifestations per patients): serositis (n=9); myalgias/arthralgias (n=7), myopathy (n=5), renal (n=3), hematologic (n=3), hair (n=2), miscellaneous (n=6), not listed (n=2)
Individual IST medications were stopped because of overall GVHD improvement/stability in half of cases, chronic GVHD progression in 13.7%, and toxicity in 18.4% of cases. Stopping an agent for toxicity did not always result in starting a new treatment to take its place if GVHD symptoms were under control. There were too few cases (n=30) where treatments were stopped and the same agent restarted for a flare after more than a month off therapy to compare the success of this strategy versus starting a new agent.
Discontinuation of IST
The rate of attempted IST discontinuation was the same between incident and prevalent cases (p=0.47). One hundred twenty-eight (51%) patients were able to stop IST at least one time, although half, 59 (46%), of these restarted IST after a median of 3.4 months off therapy (IQR 2.3-8.0). (Table 2) Of patients stopping IST for the first time, 76 (59%) were on initial therapy, 23 (18%) were on second line therapy, and 29 (23%) were on third line or later therapy. The last agent to be discontinued was prednisone (n=36, 28%), calcineurin inhibitors (n=55, 43%), mycophenolate mofetil (n=13, 10%), and sirolimus (n=24, 19%) after a median of 626 days (range 19-2100) since the last treatment was started and 20.6 months (IQR 12.0-31.1) since chronic GVHD treatment started. Of the 69 who did not restart IST after initial discontinuation of IST, 3 (4.3%) had recurrent malignancy, 1 (1.4%) died without recurrent malignancy, and 65 (97.0%) were alive and off IST at last followup. (Figure 1)
Figure 1.
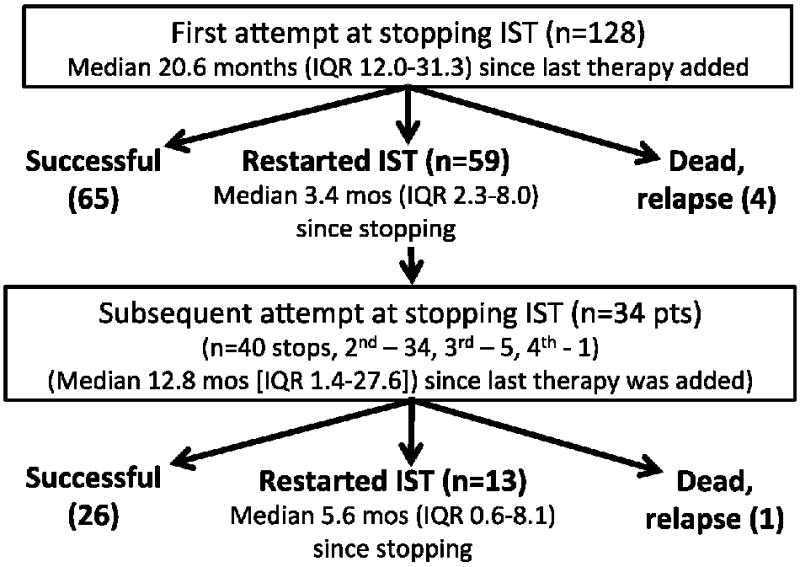
Population disposition
Thirty-four (14%) patients were able to stop IST one or more times after previously having to resume IST (n=40 attempts). Twelve (35%) were on secondary therapy, 9 (27%) were on tertiary therapy, and the rest were on fourth line or later therapy at the time of attempted discontinuation. Time to second discontinuation was a median of 12.8 months (range 1.4-27.6) since the last therapy was added. Thirteen (33%) restarted IST after a median of 5.6 months (IQR 0.6-8.1) for chronic GVHD recurrence, and 1 of these subsequently relapsed or died. There was no difference in the success of stopping IST the first time compared to the second time, with most who are going to restart doing so by 9 months. (Figure 2) During our observation period, the longest duration between stopping IST and restarting was 4.5 years with 34% restarting more than 6 months after discontinuation.
Figure 2.
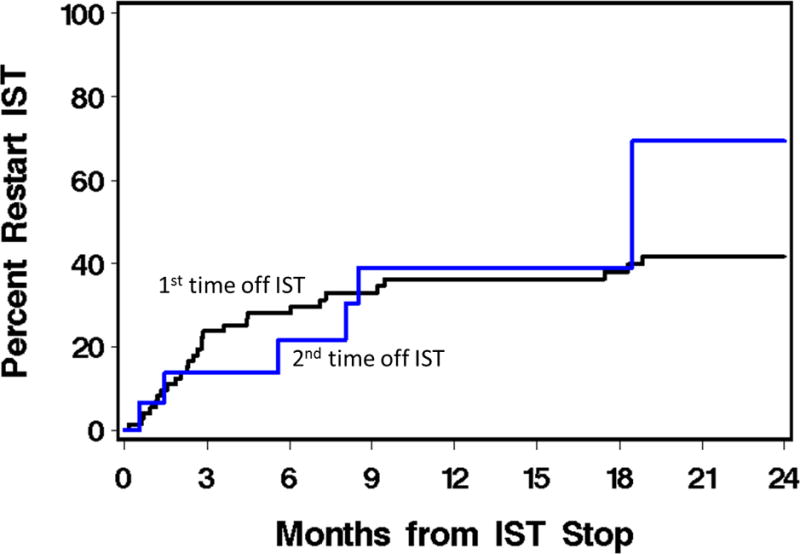
Cumulative incidence of restarting immunosuppression after discontinuation
In univariate analysis limited to those who did not relapse or die within 9 months of stopping IST (n=112), successfully stopping IST for ≥9 months was associated with longer time since transplant, myeloablative conditioning, longer time from start to discontinuation of IST, and lack of skin, oral or eye involvement. In multivariable logistic regression analysis, successfully stopping IST for ≥9 months was associated with more years since transplant (OR=1.66, 95% CI=1.14-2.42, p=0.009). Oral (OR=0.05, 95% CI=0.01-0.16, p<0.001) and skin (OR=0.25, 95% CI=0.07-0.86, p=0.03) involvement were associated with the need to restart IST. (Table 5) When case type (incident vs. prevalent) was forced into the model, it was not significant (OR 1.21, 95% CI 0.42-3.51, p=0.72).
Table 5.
Multivariable logistic regression results for successful discontinuation of IST for >=9 months using stepwise selection (N=112)
| Parameter | OR | 95% CI | p-value | |
|---|---|---|---|---|
| Years since transplant | 1.66 | 1.14 | 2.42 | 0.009 |
| Skin involvement for last line of therapy prior to discontinuing IST | 0.25 | 0.07 | 0.86 | 0.03 |
| Oral involvement for last line of therapy prior to discontinuing IST | 0.05 | 0.01 | 0.16 | <0.001 |
Subset analysis: Incident cases of chronic GVHD
Although we did not detect differences between incident and prevalent cases, we performed a subset analysis of incident cases due to concern about potential bias due to different duration of observation before enrollment. When the subset of patients enrolled within 3 months of diagnosis (n=148) were analyzed separately, Figure 3 shows the pattern of adding new lines of therapy, starting with initial therapy. The chance that a patient starting initial therapy for chronic GVHD will never need additional treatment is only 21.3%. Patients starting initial therapy received a median of 3 lines of therapy. At 5 years after diagnosis, approximately a third of patients were malignancy-free, alive and off IST, one third had died, and one third were still on IST. (Figure 3). The median time to IST permanent discontinuation was 69 months.
Figure 3.
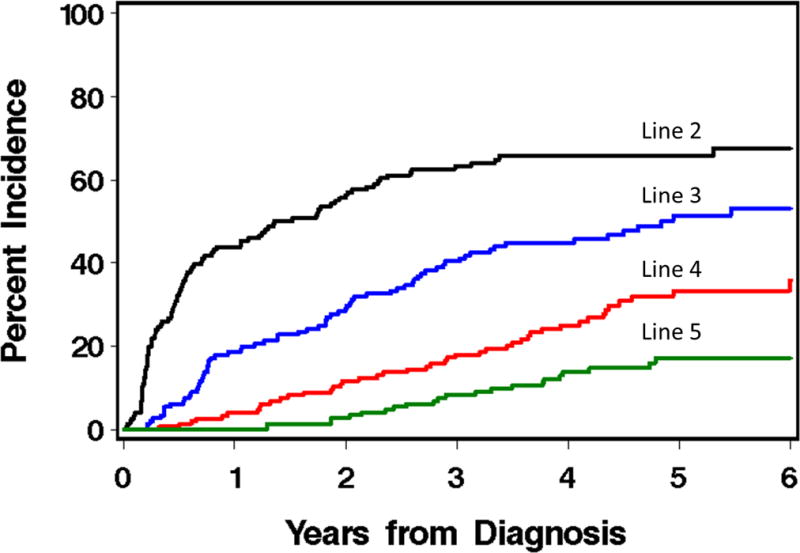
Cumulative incidence of starting lines of therapy for chronic GVHD, among patients enrolled as incident cases
In multivariable logistic regression analysis limited to incident cases who stopped IST at least once (n=60), oral (OR=0.03, 95% CI=0.005-0.16, p<0.001) and skin (OR=0.12, 95% CI=0.02-0.80, p=0.03) involvement predicted the need to restart therapy.
Discussion
Patients with chronic GVHD require prolonged IST to control symptoms and prevent organ damage so that function and quality of life can be maintained. Most receive multiple lines of treatment. In our analysis, approximately half of patients stopping IST for the first time had to restart therapy a median of 3 months later. Approximately a third of people attempting a second IST discontinuation restarted therapy a median of 5.6 months later. Thus, patients stopping IST should be cautioned to monitor themselves and continue close medical follow up especially in the first 3-6 months after IST discontinuation, given the common situation of having to restart treatment. In our cohort of 250 patients who were enrolled on a chronic GVHD observational trial, only 91 (36%) are alive, malignancy-free, and off IST with a median follow up of survivors of 5.6 years. These observations highlight that prolonged IST treatment is the reality of the experience for patients with chronic GVHD and that practice patterns, even within a single practice, are very heterogeneous.
Most patients received multiple agents during their chronic GVHD treatment and there are no treatment guidelines once patients are beyond initial therapy. Almost 40 agents have reported activity in chronic GVHD. In the unbiased subset of participants enrolled at the time of diagnosis, a median of 3 lines of therapy and 69 months to permanent discontinuation of IST was observed. Many clinicians struggle with choosing which treatments to try next when a patient requires additional therapy, anecdotally basing their recommendations on considerations of prior therapies, organ manifestations and co-morbidities. Although we provide “success” rates for different agents and the frequency that different organ manifestations prompt additional treatment, we were unable to fully capture from medical records the rationale for treatment decisions in this observational cohort. We considered trying to quantify “aggressiveness” of the regimens, but could not develop standardized definitions given the heterogeneity of clinical situations. Also, although patient-reported outcomes were collected as part of the parent study, the timing of administration was not dependent on current treatment status.
Clinical trials testing new chronic GVHD treatments face challenges in study design because the field lacks specific benchmarks.11 Prior publications from our institution reported a failure-free survival (FFS, duration of survival without relapse, death or starting a subsequent IST) of 68% at six months for initial therapy12 and 56% for secondary therapy.13 A previous report from the Chronic GVHD Consortium reported an aggregate 63% FFS at 6 months, and included patients in the present analysis.14 An updated analysis showed that less than 20% had a complete or partial remission at one year after starting chronic GVHD treatment without addition of another agent, relapse or death.11 Another study from Fred Hutchinson showed that treatment change was a predictor of higher nonrelapse mortality and decreased survival among patients with classic chronic GVHD.15 Our findings complement these prior reports by providing more detail about the specific agents used, their sequencing, and the outcome of treatment lines beyond second line therapy. However, certain subsets in our cohorts are very small and preclude comment. These include recipients of cord blood transplants, haploidentical donors with post-transplant cyclophosphamide, and recipients who received in vivo or ex vivo T cell depletion. Our cohort also predated the use of some recently reported treatments, such as ibrutinib and ruxolitinib.16,17 Finally, we did not capture whether agents were given in the context of a clinical trial. The increased monitoring and response assessment could have altered the behavior of treating physicians from what they would do in usual, non-trial, practice.
It is unclear if any currently available GVHD treatments or approaches promote development of tolerance, thus fostering the ability to permanently discontinue IST. Since many IST are well-tolerated at low doses, our findings raise the question of whether aggressive attempts to permanently discontinue IST are wise.18 If chronic GVHD is indeed a chronic condition, similar to chronic rheumatologic and other autoimmune diseases, maybe the treatment goal should be to use the lowest dose of medication that controls GVHD rather than trying to stop all IST, only to see symptomatic flares or progression to more severe GVHD manifestations (e.g., sclerosis, fasciitis, lung involvement) in a third to half of patients. Alternatively, ongoing biomarker and clinical studies may help identify patients who can successfully stop IST. We did not collect information about steroid dosing, infections and IST complications although these considerations are important when assessing the risk-benefit profiles of specific IST medications and the duration of IST treatment.
In summary, for patients who have chronic GVHD and start initial systemic treatment, there is only a 32% chance that they will be alive, in remission, and off IST by 5 years. More likely, they will require treatment with multiple agents for several years. If they do reach a point of stopping all IST, they have a 50% chance of needing to restart therapy. More effective treatments for chronic GVHD are urgently needed.
Figure 4.
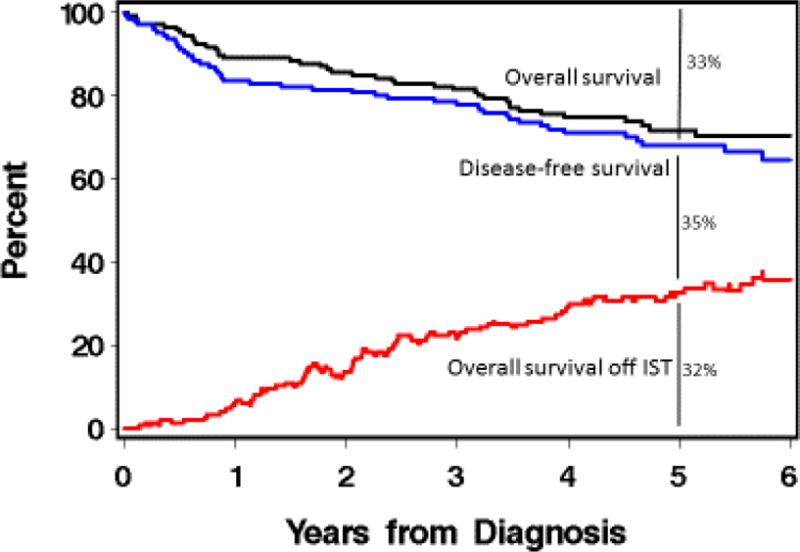
Prevalence of being alive, disease-free and off immunosuppression for incident cases
Highlights.
After stopping therapy for cGVHD, half restarted after a median of 3.4 months
After 5.6 years, 1/3 each were still on IST, alive off IST, or dead/relapsed
Acknowledgments
This work was supported by CA163438, CA118953 and CA18029.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors do not have any conflicts of interest.
Author contributions: S.J. Lee designed research and analyzed data. T.D. Nguyen, M. Rodriguez and A.M. Hall collected data. L. Onstad and B. Storer conducted analyses. All authors interpreted results and wrote the paper.
References
- 1.Champlin RE, Schmitz N, Horowitz MM, et al. Blood stem cells compared with bone marrow as a source of hematopoietic cells for allogeneic transplantation. IBMTR Histocompatibility and Stem Cell Sources Working Committee and the European Group for Blood and Marrow Transplantation (EBMT) Blood. 2000;95(12):3702–3709. [PubMed] [Google Scholar]
- 2.Arora M, Cutler CS, Jagasia MH, et al. Late Acute and Chronic Graft-versus-Host Disease after Allogeneic Hematopoietic Cell Transplantation. Biol Blood Marrow Transplant. 2016;22(3):449–455. doi: 10.1016/j.bbmt.2015.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee SJ, Vogelsang G, Flowers ME. Chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2003;9(4):215–233. doi: 10.1053/bbmt.2003.50026. [DOI] [PubMed] [Google Scholar]
- 4.Inamoto Y, Flowers ME, Lee SJ, et al. Influence of immunosuppressive treatment on risk of recurrent malignancy after allogeneic hematopoietic cell transplantation. Blood. 2011;118(2):456–463. doi: 10.1182/blood-2011-01-330217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feng S, Ekong UD, Lobritto SJ, et al. Complete immunosuppression withdrawal and subsequent allograft function among pediatric recipients of parental living donor liver transplants. JAMA. 2012;307(3):283–293. doi: 10.1001/jama.2011.2014. [DOI] [PubMed] [Google Scholar]
- 6.Pidala J, Lee SJ, Quinn G, Jim H, Kim J, Anasetti C. Variation in management of immune suppression after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2011;17(10):1528–1536. doi: 10.1016/j.bbmt.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stewart BL, Storer B, Storek J, et al. Duration of immunosuppressive treatment for chronic graft-versus-host disease. Blood. 2004;104(12):3501–3506. doi: 10.1182/blood-2004-01-0200. [DOI] [PubMed] [Google Scholar]
- 8.Vigorito AC, Campregher PV, Storer BE, et al. Evaluation of NIH consensus criteria for classification of late acute and chronic GVHD. Blood. 2009;114(3):702–708. doi: 10.1182/blood-2009-03-208983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chronic GVHD Consortium. Rationale and design of the chronic GVHD cohort study: improving outcomes assessment in chronic GVHD. Biol Blood Marrow Transplant. 2011;17(8):1114–1120. doi: 10.1016/j.bbmt.2011.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Filipovich AH, Weisdorf D, Pavletic S, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11(12):945–956. doi: 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 11.Martin PJ, Storer BE, Inamoto Y, et al. An endpoint associated with clinical benefit after initial treatment of chronic graft-versus-host disease. Blood. 2017;130(3):360–367. doi: 10.1182/blood-2017-03-775767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Inamoto Y, Flowers ME, Sandmaier BM, et al. Failure-free survival after initial systemic treatment of chronic graft-versus-host disease. Blood. 2014;124(8):1363–1371. doi: 10.1182/blood-2014-03-563544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Inamoto Y, Storer BE, Lee SJ, et al. Failure-free survival after second-line systemic treatment of chronic graft-versus-host disease. Blood. 2013;121(12):2340–2346. doi: 10.1182/blood-2012-11-465583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Palmer J, Chai X, Martin PJ, et al. Failure-free survival in a prospective cohort of patients with chronic graft-versus-host disease. Haematologica. 2015;100(5):690–695. doi: 10.3324/haematol.2014.117283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flowers ME, Storer B, Carpenter P, et al. Treatment change as a predictor of outcome among patients with classic chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2008;14(12):1380–1384. doi: 10.1016/j.bbmt.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zeiser R, Burchert A, Lengerke C, et al. Ruxolitinib in corticosteroid-refractory graft-versus-host disease after allogeneic stem cell transplantation: a multicenter survey. Leukemia. 2015;29(10):2062–2068. doi: 10.1038/leu.2015.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hurabielle C, Sicre de Fontbrune F, Moins-Teisserenc H, et al. Efficacy and tolerance of ruxolitinib in refractory sclerodermatous chronic Graft-Versus-Host Disease. Br J Dermatol. 2017 doi: 10.1111/bjd.15593. [DOI] [PubMed] [Google Scholar]
- 18.Lee SJ. Classification systems for chronic graft-versus-host disease. Blood. 2017;129(1):30–37. doi: 10.1182/blood-2016-07-686642. [DOI] [PMC free article] [PubMed] [Google Scholar]


