Abstract
Background
Our country is in the midst of an opioid epidemic. Although the problem is multifactorial, one issue is the presence of excess prescription opioid medications circulating in our communities. Our objective was to evaluate if the dissemination of an educational brochure would improve the disposal of unused opioids after surgery.
Study Design
Eligible surgery patients from an upper extremity/peripheral nerve clinic were enrolled into this prospective before and after study between February-September 2017. Patients who reported opioid use preoperatively were excluded from this study. The same survey was administered to the group of patients who did not receive the intervention and to those who did receive the intervention. Our primary endpoint was the proportion of patients who disposed of unused opioid medications.
Results
A total of 334 patients were studied: 164 who did not receive the brochure and 170 who received the brochure. Seventy-six patients were excluded for preoperative opioid use. After the dissemination of the brochure, there was a significant increase in the proportion of patients who disposed of their unused opioids (11% vs. 22%, p=0.02). Of those who disposed of their opioids, there was no significant difference in the proportion of patients from each group who disposed in a manner that was recommended by the brochure (43% vs. 64%, p=0.19).
Conclusions
Dissemination of the educational brochure improved disposal of unused opioids after surgery. This low-cost, easily implemented intervention can improve disposal of unused opioids and ultimately, decrease the amount of excess opioids circulating in our communities.
Keywords: Opioid, disposal, surgery, education, patient safety
Introduction
The opioid epidemic in the United States is a major public health crisis, accounting for alarming rates of morbidity and mortality. Drug overdose deaths have tripled from 1999 to 2014. In 2015, opioid-related drug overdoses accounted for over 33,000 deaths in the U.S. (1). In addition to the significant mortality associated with the epidemic, hospital admissions and associated healthcare costs have increased substantially in tandem over the last decade (2).
While opioid medications are effective in managing acute pain following surgery, even short-term use of opioids can lead to long-term dependence (3–7). Moreover, numerous studies have concluded that opioids following various types of surgical procedures are often overprescribed (8–11). Because many patients receive their first opioid prescription following a surgical procedure, educating and intervening on this patient population is especially critical. Additionally, patients are rarely counseled in proper disposal of unused opioid medications, (12, 13) and frequently retain the majority of leftover medication (9, 14). The excess opioid medications are then allowed to circulate in our communities for illegal sale or unintended consumption. For example, nearly 70% of people surveyed by the Substance Abuse and Mental Health Administration obtained their most recently used pain-reliever or sedative divergently from a friend or relative (15).
Given the problem of opioid prescribing leading to excess unused opioid medications and the lack of patient education regarding proper opioid disposal, the objective of the current study was to evaluate the effectiveness of a low-cost educational brochure designed to instruct and empower patients on proper disposal of excess opioid medication after surgery.
Methods
Pilot Study
Prior to launching the intervention study (i.e. dissemination of the educational brochure), we performed a small prospective pilot study approved by our Institutional Review Board between April– December 2016. The main goals of this pilot study were to determine if patients after surgery had unused opioids (i.e. they were overprescribed) and to assess their knowledge about disposal methods of excess medications. For this pilot study, we analyzed data from 30 respondents who were 18 years or older, were able to read and comprehend our survey, and who did not take opioids preoperatively.
Intervention Study
Study Population
Eligible patients were recruited into our intervention study from an upper extremity/peripheral nerve surgery clinic at Washington University between February– September 2017. All patients 18 years or older who underwent surgery at least two weeks prior and had the ability to read and comprehend the survey in English were eligible for enrollment into the study. We excluded any patient who self-reported using opioids preoperatively. The goal of this prospective before and after study was to determine the effectiveness of an educational brochure on increasing disposal of unused opioids after surgery. This study was approved and deemed exempt by the Institutional Review Board at Washington University.
Study Groups
Assignment to the two different groups (i.e. no brochure versus brochure) was determined based on the date of the patient’s first visit in our outpatient upper extremity/peripheral nerve surgery clinic date. Patients who presented to their first outpatient clinic visit between February 21– March 21, 2017 were enrolled in the control group (i.e. did not receive the educational brochure), while those who presented between March 22– September 15, 2017 were assigned to the intervention group and received the educational brochure. Except for the receipt of the brochure, the patients in both groups received similar surgical, anesthesia, and postoperative management. Additionally, clinic staff were instructed to leave practices in opioid-related education otherwise unchanged for the duration of this study.
Intervention
An educational brochure (eFigure1) was designed based on previously published guidelines for educating patients on safe disposal of unused medications (16). The brochure was designed by a team consisting of attending surgeons, a clinical fellow, a nurse practitioner, a research nurse coordinator, the Supervisor of Patient Service Operations, and the Education Director. The main elements of the brochure included the following: (1) statistics about the opioid epidemic; (2) results from our pilot study indicating overprescribing and inadequate education about disposal methods among our own patient population; (3) step-by-step instructions for proper disposal of unused opioids; (4) website addresses to direct patients to opioid take-back locations. Patients in the intervention group (i.e. brochure group) were given the brochure preoperatively at the time of surgery scheduling and postoperatively at the time of hospital discharge.
Questionnaire
Because there are no validated surveys in the current literature on this topic, the survey tool was designed and trialed by the study team. The questionnaire (eFigure2) administered via paper/pencil consisted of eight questions and utilized logic to eliminate unnecessary questions when appropriate. In addition to collecting demographic factors such as birth year and gender, we asked patients to report the approximate time since surgery (2 weeks, >2 weeks but <1 month, >1 month but < 3 months, >3 months). Patients were also asked: (1) whether they received a brochure, (2) opioid use prior to surgery, (3) use of all prescribed opioids after surgery, (4) whether excess unused opioids were kept, and (5) what was done with excess opioids that were not kept. The cover page detailed the purpose and voluntary nature of the study, and continuation of the survey served as implied consent. Consenting patients completed the survey in the waiting room, prior to seeing providers for their regularly scheduled postoperative follow-up visits. We did not collect any identifying information from patients. Therefore, it was not possible to determine which patients completed the survey or to attempt follow-up with non-respondents.
Statistical Analysis
Previous studies have demonstrated a 10% difference in opioid disposal as clinically significant (17, 18). Based on a 10% difference between groups and a baseline rate of 5.5% of participants (5 of the first 90 respondents) properly disposing of excess medications, a power calculation (β = 0.2; α = 0.05) was used to determine an appropriate sample size. Categorical data were analyzed with Chi-Squared tests and numerical data were analyzed with Mann-Whitney U Tests, all with significance level α <0.05. All data were entered into an Excel spreadsheet and analyses were performed in IBM SPSS Statistics 23.0, Armonk NY.
Results
Pilot Study Results
Of the 30 patients included in the pilot study, 19 (63%) were female and the average age was 56 years old (range 20–85). The average number of opioid pills prescribed per patient was 36 (range 10–60), with the majority of patients (n=23, 77%) being prescribed oxycodone or oxycodone-acetaminophen. Interestingly, the average total number of opioid pills taken per person was 12 (range 0–50) out of 36, leaving an average excess of opioids at 24 pills (or 66% of the entire prescription) per person.
With regards to usage patterns among this cohort, the majority of patients (n=19, 63%) had kept their excess opioid medications after surgery. Unfortunately, most patients (n=17, 57%) reported not receiving any education on disposal of unused opioid medications. In total, there were 564 unused opioid pills sitting in the homes of these 30 respondents.
Intervention study
A total of 334 patients were included in the analysis. Of these, 164 did not receive the brochure while 170 did. We excluded a total of 76 patients for preoperative opioid use. Demographic data from our two groups are presented in Table 1. There was no significant difference between our two study groups with respect to age distribution, gender, time since surgery, or preoperative opioid use.
Table 1.
Demographic Characteristics of Patients by Study Group (N=334)
| Variable | No brochure (N=164, 49%) | Brochure (N=170, 51%) | p Value |
|---|---|---|---|
| Age, y, mean ± SD | 51±15.1 | 52±14.6 | 0.4 |
| Sex, female, n (%) | 104 (63.4) | 109 (64.1) | 0.91 |
| Time since operation, n (%) | 0.59 | ||
| 2 weeks | 21 (12.8) | 25 (14.7) | |
| >2 weeks, <1 month | 34 (20.7) | 37 (21.8) | |
| >1 month, <3 months | 68 (41.5) | 76 (44.7) | |
| >3 months | 41 (25.0) | 32 (18.8) | |
| Preoperative opioid use, n (%) | 36 (22) | 40 (23.5) | 0.79 |
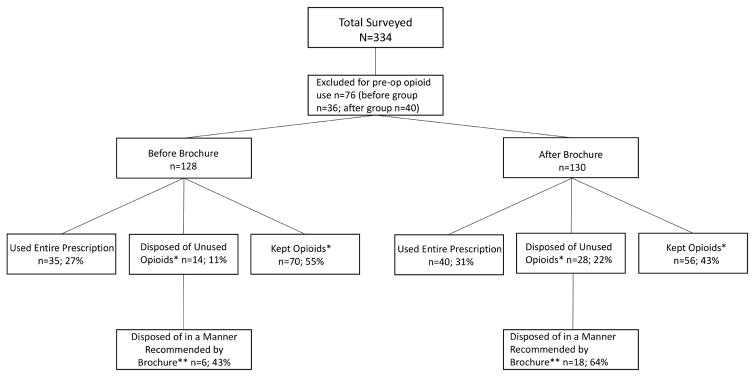
Between the two groups, a total of 75 patients (29%) reported using their entire prescription, 126 (49%) had kept their unused opioid pills, and 15 (6%) declined to answer what they did with their excess medication. Forty-two (16.3%) disposed of unused opioids; another 24 (9.3%) did so in a manner recommended by the brochure. Options for disposal outlined in the brochure included disposal by: (1) returning to a pharmacy (n=6; 14.3% of those who disposed); (2) returning to a police station (n=8; 19.1% of those who disposed); or (3) mixing with an unpalatable substance and disposing of the mixture in household trash (n=10; 23.8% of those who disposed). Figure 1 outlines the study population and breakdown of opioid medication disposal in the two study groups.
Figure 1.
Study Flow Diagram. *9 in before group and 6 in after group declined to answer what they did with excess medication; **Brochure recommended methods of disposal include throwing away in the trash with an unpalatable substance or returning to police or pharmacy
After education with the brochure, there was a 10.6% increase in proportion of patients who disposed of their opioids (Table 2), representing a 100% relative increase in disposal. This was significant (p=0.02, 95% CI [1.6%, 19.6%]), and greater than the 10% change desired to prove clinical significance. A 11.6% decrease in keeping unused medications was also observed (p=0.06, 95% CI [−0.5%, 23.7%]). Of those who disposed of their opioids, there was a 21.4% increase in usage of recommended disposal methods in the group who received the brochure (p=0.19, 95% CI [−10.1%, 52.7%]) though this was not significant between the two groups. Due to the small number of persons disposing of their medications, our study was not adequately powered to declare the difference in disposal methods to be significant.
Table 2.
Opioid Usage Patterns by Study Group
| Variable | No Brochure (N=128, 48%)* | Brochure (N=130, 52%)* | p Value |
|---|---|---|---|
| Used entire opioid prescription, n (%) | 35 (27) | 40 (31) | 0.55 |
| Kept excess opioid medications, n (%)† | 70 (55) | 56 (43) | 0.06 |
| Disposed of excess opioid medications, n (%)† | 14 (11) | 28 (22) | 0.02 |
Total patients in each group after excluding patients with preoperative opioid use.
Nine patients in the “No Brochure” group and six patients in the “Brochure” group declined to answer what they did with excess opioid medication.
We also compiled the comments from patients who kept their unused opioids. Some representative comments included: “Saved it for a rainy day for arthritis”; “Keeping it for future surgery”; “Pharmacy would not take them!”; “Insurance for if I ever have pain again” and “I’ll return to pharmacy on given day to recycle drugs.”
Discussion
Our pilot study confirmed our hypothesis that we overprescribe opioids following surgery in that patients on average consumed only 33% (12 of 36 pills) of their entire opioid prescriptions. Majority of patients also kept their unused opioid medications. Given the alarming findings from this pilot study, we sought to develop an intervention that would improve patient education on the opioid epidemic and to introduce methods for proper disposal of unused opioids. Our findings suggest that disseminating an educational brochure outlining safe opioid disposal methods is effective in increasing the disposal of unused excess opioid medications following surgery.
Although physician prescribing patterns have been thoroughly studied, overprescribing is just one tier of the issue. Additionally, patients are not being educated on how to properly dispose of their unused excess opioids. Fortunately, patient education initiatives on proper disposal of excess medications—not only of opioids, have been shown to be effective (12, 17–20). For example, a cross-sectional survey showed that counseling patients on proper disposal of unused or expired medications was significantly associated with returning unused medications to a pharmacy (12). This study however, was not specifically focused on return of opioid medications, which may carry additional barriers. Moreover, McCauley and colleagues showed that a web-based educational intervention not only improved knowledge regarding safe disposal and the risk of keeping excess opioids, but also reduced self-reported misuse behavior. While this intervention was successful, participants were asked to participate in a 15-minute interactive online intervention, which requires a more substantial commitment from patients than our proposed intervention (17). In a more recent study, a personalized education program about safe use, storage, and disposal of opioids was administered to cancer patients. Interestingly, while awareness of proper disposal techniques improved, over one-third of patients still kept their excess opioid medications (19). This may reflect a desire to retain opioids for future use as chronic pain is common among cancer patients. Furthermore, Rose and colleagues designed a study similar to ours, but among lower limb arthroplasty patients in Canada. After dissemination of an educational pamphlet containing five sentences on safe opioid disposal, they found that there was a significant increase of proper opioid disposal by 22% (18).
For those who are willing to dispose of excess opioids, guidelines from different governing bodies are conflicting and may be difficult to follow. For example, the Food and Drug Administration (FDA) states that if a take-back program is unavailable, certain opioids can be safely flushed in the toilet, while the Environmental Protection Agency (EPA) discourages flushing of any medication due to its negative effects on our water system and ecosystem (21). The Drug Enforcement Agency (DEA)’s first line of opioid disposal are to either return them at a take-back program or to a collector registered by the DEA, which may include hospital or clinic pharmacies or law enforcement locations. These options may be challenging for patients not only to locate, but also to travel to. Written-in comments from our surveys highlighted some of the difficulties with these disposal options for patients. Some respondents stated that their pharmacy was unable to accept their medications, likely reflecting that these pharmacies were not registered collectors with the DEA. For example, our own hospital pharmacy dispenses these opioids to postoperative patients but are not able to take back unused medications. Additionally, some reported having to wait until a specified day to return their unused medications. Lack of knowledge and difficulty returning medication to these designated collection agencies may result in patients hoarding unused opioid medications, leaving them available for divergent use.
Moreover, if take-back programs are unavailable, all agencies—the FDA, EPA, and DEA recommend mixing unused medications with an unpalatable substance and disposing of the mixture in the trash, like what we advocated for in our educational brochure. This option may be more accessible for patients who may not be able/willing to locate or travel to a designated take-back location, and improve the compliance of safe opioid disposal after surgery. Future investigations are underway testing the efficacy of an improved brochure outlining one disposal method using soap or dish detergent, items that are commonly found in most households (eFigure3). We hypothesize that this simplified brochure and disposal technique will eliminate more barriers for patients and will further increase opioid disposal beyond the 22% of patients we observed with our first educational brochure.
In addition to discussing proper disposal of opioids, it is imperative that we provide comprehensive pre-operative counseling regarding the risks and expected benefits of these powerful medications. During this counseling session, patients should be educated and encouraged to use non-opioid adjunct medications such as acetaminophen and NSAIDs, when appropriate. In our clinic, we have also established a “one prescriber rule,” in which we have patients who are chronically on opioid medications prior to surgery sign a contract that affirms that they will only receiving opioid medications after surgery from one provider. Not only does this improve the coordination of care between our staff and the patient’s primary pain medication provider, but it also reduces the risk of overprescribing opioids and excess pills to circulate in our communities. For patients who report that they are opioid naïve prior to surgery, it is important to screen these individuals for their risk of opioid misuse or long-term opioid dependence following surgery. There are many screening tools that may be used that identify risk factors such as personal or family history of substance abuse, tobacco use, history of depression or anxiety, history of childhood trauma or abuse, among others (22–24). As surgical providers, we must view and treat opioid misuse and long-term opioid dependence as serious postoperative complications just as we do for other complications. By doing so, we can do our part in tackling the opioid epidemic and improve care of our patients.
We acknowledge the limitations of our study. First, we used a before and after study design, which can be affected by secular trends and sudden changes that make it difficult to attribute observed changes to the intervention alone. However, we believe that opioid use and improper disposal have been longstanding, widespread problems over the past decade, which have not been susceptible to secular trends or sudden changes. Second, there is a possibility of duplicate responses based on our study design. Due to the restrictions put forth by our Institutional Review Board, we were unable to identify respondents to ensure that no person received and completed more than one survey. To minimize duplicate responses, we distributed the survey to all postoperative patients who were at least two weeks out from surgery and instructed them verbally and in written form to not complete the survey if they had done so in the past. Third, our study may have been affected by recall and/or social desirability bias. To limit recall bias, we designed questions to be dichotomous yes/no responses eliminating the need for detail. Additionally, social desirability bias would be expected to bias our results toward the null, and anonymity and clinic staff blindness to participation were employed to attempt to reduce this; surveys were distributed and collected at the front desk prior to patient entering clinic or interacting with patient care staff. Fourth, while our study was adequately powered to detect an improvement in opioid disposal, it was underpowered to evaluate to detect differences in the method of disposal between the two groups. Finally, this study was performed at a large urban academic institution, potentially limiting its generalizability.
Conclusions
Interventions to curb the morbidity and mortality resulting from the opioid epidemic are urgently needed. Moreover, as many patients receive their first opioid prescription following a surgical procedure, educating postoperative patients on proper opioid disposal presents a critical opportunity for surgical providers to intervene. It is our hope that our findings will encourage widespread dissemination and implementation of this intervention in other surgical clinics to increase disposal of excess opioids and ultimately, decrease the risk of divergent use of these medications.
Supplementary Material
Acknowledgments
Support: Dr Katherine B Santosa was supported in part from the NIH National Institute of Neurological Disorders and Stroke (NINDS) (F32NS098561).
This study was supported in part by the Foundation for Barnes-Jewish Hospital.
Abbreviations
- FDA
Food and Drug Administration
- EPA
Environmental Protection Agency
- DEA
Drug Enforcement Agency
Footnotes
Disclosure Information: Nothing to disclose.
Disclaimer: The content is solely the responsibility of the authors and does not necessarily reflect the official view of the NIH. These funding sources had no involvement in the study design, data collection or analysis, interpretation of the data, in writing the report, or in the decision to submit the findings for publication.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rudd RA, Aleshire N, Zibbell JE, Gladden RM. Increases in Drug and Opioid Overdose Deaths--United States, 2000–2014. MMWR Morbidity and mortality weekly report. 2016 Jan 01;64(50–51):1378–82. doi: 10.15585/mmwr.mm6450a3. [DOI] [PubMed] [Google Scholar]
- 2.Hsu DJ, McCarthy EP, Stevens JP, Mukamal KJ. Hospitalizations, costs and outcomes associated with heroin and prescription opioid overdoses in the United States 2001–12. Addiction (Abingdon, England) 2017 Sep;112(9):1558–64. doi: 10.1111/add.13795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brummett CM, Waljee JF, Goesling J, et al. New Persistent Opioid Use After Minor and Major Surgical Procedures in US Adults. JAMA surgery. 2017 Jun 21;152(6):e170504. doi: 10.1001/jamasurg.2017.0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Waljee JF, Zhong L, Hou H, et al. The Use of Opioid Analgesics following Common Upper Extremity Surgical Procedures: A National, Population-Based Study. Plast Reconstr Surg. 2016 Feb;137(2):355e–64e. doi: 10.1097/01.prs.0000475788.52446.7b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shah AS, Blackwell RH, Kuo PC, Gupta GN. Rates and Risk Factors for Opioid Dependence and Overdose after Urological Surgery. J Urol. 2017 May 12; doi: 10.1016/j.juro.2017.05.037. [DOI] [PubMed] [Google Scholar]
- 6.Connolly J, 3rd, Javed Z, Raji MA, et al. Predictors of Long-term Opioid Use Following Lumbar Fusion Surgery. Spine. 2017 Sep 15;42(18):1405–11. doi: 10.1097/BRS.0000000000002133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson SP, Chung KC, Zhong L, et al. Risk of Prolonged Opioid Use Among Opioid-Naive Patients Following Common Hand Surgery Procedures. The Journal of hand surgery. 2016 Oct;41(10):947–57. e3. doi: 10.1016/j.jhsa.2016.07.113. [DOI] [PubMed] [Google Scholar]
- 8.Rodgers J, Cunningham K, Fitzgerald K, Finnerty E. Opioid consumption following outpatient upper extremity surgery. The Journal of hand surgery. 2012 Apr;37(4):645–50. doi: 10.1016/j.jhsa.2012.01.035. [DOI] [PubMed] [Google Scholar]
- 9.Bates C, Laciak R, Southwick A, Bishoff J. Overprescription of postoperative narcotics: a look at postoperative pain medication delivery, consumption and disposal in urological practice. J Urol. 2011 Feb;185(2):551–5. doi: 10.1016/j.juro.2010.09.088. [DOI] [PubMed] [Google Scholar]
- 10.Chiu AS, Healy JM, DeWane MP, et al. Trainees as Agents of Change in the Opioid Epidemic: Optimizing the Opioid Prescription Practices of Surgical Residents. J Surg Educ. 2017 Jul 10; doi: 10.1016/j.jsurg.2017.06.020. [DOI] [PubMed] [Google Scholar]
- 11.Kim N, Matzon JL, Abboudi J, et al. A Prospective Evaluation of Opioid Utilization After Upper-Extremity Surgical Procedures: Identifying Consumption Patterns and Determining Prescribing Guidelines. The Journal of bone and joint surgery American volume. 2016 Oct 19;98(20):e89. doi: 10.2106/JBJS.15.00614. [DOI] [PubMed] [Google Scholar]
- 12.Seehusen DA, Edwards J. Patient practices and beliefs concerning disposal of medications. Journal of the American Board of Family Medicine: JABFM. 2006 Nov-Dec;19(6):542–7. doi: 10.3122/jabfm.19.6.542. [DOI] [PubMed] [Google Scholar]
- 13.Kumar K, Gulotta LV, Dines JS, et al. Unused Opioid Pills After Outpatient Shoulder Surgeries Given Current Perioperative Prescribing Habits. Am J Sports Med. 2017 Mar;45(3):636–41. doi: 10.1177/0363546517693665. [DOI] [PubMed] [Google Scholar]
- 14.Bicket MC, Long JJ, Pronovost PJ, et al. Prescription Opioid Analgesics Commonly Unused After Surgery: A Systematic Review. JAMA surgery. 2017 Aug 02; doi: 10.1001/jamasurg.2017.0831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES. [Accessed October 23, 2017];Results from the 2012 National Survey on Drug Use and Health: Summary of National Findings. Available at: https://www.samhsa.gov/data/sites/default/files/NSDUHresults2012/NSDUHresults2012.pdf.
- 16.Gandhi T, Best K. Educate patients about proper disposal of unused Rx medications-for their safety. Current Psychiatry. 2015;14(4):60–7. [Google Scholar]
- 17.McCauley JL, Back SE, Brady KT. Pilot of a brief, web-based educational intervention targeting safe storage and disposal of prescription opioids. Addictive behaviors. 2013 Jun;38(6):2230–5. doi: 10.1016/j.addbeh.2013.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rose P, Sakai J, Argue R, et al. Opioid information pamphlet increases postoperative opioid disposal rates: a before versus after quality improvement study. Can J Anaesth. 2016 Jan;63(1):31–7. doi: 10.1007/s12630-015-0502-0. [DOI] [PubMed] [Google Scholar]
- 19.de la Cruz M, Reddy A, Balankari V, et al. The Impact of an Educational Program on Patient Practices for Safe Use, Storage, and Disposal of Opioids at a Comprehensive Cancer Center. Oncologist. 2017 Jan;22(1):115–21. doi: 10.1634/theoncologist.2016-0266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McCarthy DM, Wolf MS, McConnell R, et al. Improving patient knowledge and safe use of opioids: a randomized controlled trial. Acad Emerg Med. 2015 Mar;22(3):331–9. doi: 10.1111/acem.12600. [DOI] [PubMed] [Google Scholar]
- 21.Khan U, Bloom RA, Nicell JA, Laurenson JP. Risks associated with the environmental release of pharmaceuticals on the U.S. Food and Drug Administration “flush list”. The Science of the total environment. 2017 Dec 31;609:1023–40. doi: 10.1016/j.scitotenv.2017.05.269. [DOI] [PubMed] [Google Scholar]
- 22.Ciesielski T, Iyengar R, Bothra A, et al. A Tool to Assess Risk of De Novo Opioid Abuse or Dependence. Am J Med. 2016 Jul;129(7):699–705. e4. doi: 10.1016/j.amjmed.2016.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rice JB, White AG, Birnbaum HG, et al. A model to identify patients at risk for prescription opioid abuse, dependence, and misuse. Pain medicine (Malden, Mass) 2012 Sep;13(9):1162–73. doi: 10.1111/j.1526-4637.2012.01450.x. [DOI] [PubMed] [Google Scholar]
- 24.Webster LR, Webster RM. Predicting aberrant behaviors in opioid-treated patients: preliminary validation of the Opioid Risk Tool. Pain medicine (Malden, Mass) 2005 Nov-Dec;6(6):432–42. doi: 10.1111/j.1526-4637.2005.00072.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.