Abstract
Aims
Patients hospitalized for heart failure (HF) are at high risk for 30-day readmission. This study sought to examine the timings and causes of readmission within 30 days of an HF hospitalization.
Methods and results
Timing and cause of readmission in the ASCEND-HF (Acute Study of Clinical Effectiveness of Nesiritide and Decompensated Heart Failure) trial were assessed. Early and late readmissions were defined as admissions occurring within 0–7 days and 8–30 days post-discharge, respectively. Patients who died in hospital or remained hospitalized at day 30 post-randomization were excluded. Patients were compared by timing and cause of readmission. Logistic and Cox proportional hazards regression analyses were used to identify independent risk factors for early vs. late readmission and associations with 180-day outcomes. Of the 6584 patients (92%) in the ASCEND-HF population included in this analysis, 751 patients (11%) were readmitted within 30 days for any cause. Overall, 54% of readmissions were for non-HF causes. The median time to rehospitalization was 11 days (interquartile range: 6–18 days) and 33% of rehospitalizations occurred by day 7. Rehospitalization within 30 days was independently associated with increased risk for 180-day all-cause death [hazard ratio (HR) 2.38, 95% confidence interval (CI) 1.93–2.94; P < 0.001]. Risk for 180-day all-cause death did not differ according to early vs. late readmission (HR 0.99, 95% CI 0.67–1.45; P = 0.94).
Conclusions
In this hospitalized HF trial population, a significant majority of 30-day readmissions were for non-HF causes and one-third of readmissions occurred in the first 7 days. Early and late readmissions within the 30-day timeframe were associated with similarly increased risk for death. Continued efforts to optimize multidisciplinary transitional care are warranted to improve rates of early readmission.
Keywords: Acute heart failure, Timing of readmission, Cause of readmission
Introduction
Acute heart failure (HF) hospitalization is a global problem; over 1 million such hospitalizations occur annually in the USA and a similar number is reported in Europe.1 Heart failure is one of the most common diagnoses for readmission in developed countries and accounts for 1–3% of all admissions and roughly a quarter of all 30-day readmissions in patients aged >65 years.2,3 Although countries worldwide have instituted different readmission policies, 30 days is the common benchmark for readmission.4 As previously demonstrated, the risk for death appears to increase with each subsequent readmission for HF.5,6 However, despite a rapid increase in the allocation of resources targeting the prevention of readmission, readmission rates following HF hospitalization remain persistently high,7 and interventions to reduce readmissions vary widely among institutions and often lack scientific rigour.8
Current guidelines strongly suggest the multidisciplinary management of patients admitted for HF, but place heavy emphasis on HF-specific modifiers such as volume management, and the initiation of guideline-directed medical therapy (GDMT).9,10 Although prior studies have suggested that patients are readmitted disproportionately early and in about half of cases for causes other than HF, no study has rigorously explored relationships between the precise timing of readmission within the 30-day timeframe, the cause of readmission, and associations with subsequent clinical outcomes.11–15 No study has investigated whether early readmission is related to worse outcome.
Thus, the objectives of this secondary analysis of the global ASCEND-HF (Acute Study of Clinical Effectiveness of Nesiritide and Decompensated Heart Failure) trial were to: (i) describe causes of readmission after hospitalization for HF; (ii) characterize the timing of readmission within the 30-day timeframe; (iii) determine the comparative prognostic values of early vs. late readmission within the 30-day timeframe, and (iv) identify patient or regional characteristics that may predict timing of readmission.
Methods
Overview
The study design16 and primary results17 of the ASCEND-HF trial have been previously published. Briefly, ASCEND-HF was a global, prospective, randomized, double-blind, placebo-controlled trial designed to examine the short- and long-term efficacy and safety of nesiritide, a recombinant natriuretic peptide. A total of 7141 patients hospitalized for HF were randomized to nesiritide or placebo, in addition to standard therapy, within 24 h of the first i.v. HF-related treatment. Pertinent exclusion criteria included a high likelihood of hospital discharge in ≤24 h and a comorbid condition with an associated life expectancy of <6 months. The ASCEND-HF trial was conducted in line with the Declaration of Helsinki; its protocol was approved by the institutional review board or ethics committee at each participating centre, and written consent was obtained from all participants.
Study definitions and endpoints
The primary outcomes of interest were 30-day all-cause and cause-specific hospitalization. Unlike previous ASCEND studies, which looked at hospitalization within 30 days of randomization, the present study used data directly derived from case report forms (CRFs) to calculate time to hospitalization from patient discharge date. Hospitalization for HF was defined as first readmission for worsening signs or symptoms of HF resulting in the new administration of i.v. therapies, mechanical or surgical intervention, or initiation of ultrafiltration, haemofiltration or dialysis. Only patients who remained alive at the day 30 post-randomization follow-up visit were included in the analysis. Data for patients who died after discharge but prior to the 30-day post-randomization follow-up visit, without readmission, were omitted from the endpoint analysis. The study authors then took all post-discharge readmission data reported on ‘30-day’ follow-up visit CRFs and calculated the readmission rate up to 30 days after the discharge date. Adjudicated and institutional data were used to identify and confirm patients readmitted within 30 days of discharge. For the purposes of this analysis, early and late rehospitalizations were defined as readmissions within 0–7 days and 8–30 days post-discharge, respectively. To assess the impact of hospitalization on longer-term outcomes, 180-day all-cause mortality was prespecified as a secondary outcome for the present analysis.
Statistical analysis
Clinical characteristics at randomization were used as representative of baseline characteristics because the data collected at discharge were less complete and referred to a smaller number of prespecified collected variables. Histograms were used to display the distribution of time to first hospitalization from discharge. Time to rehospitalization was stratified by non-HF vs. HF causes, as well as reduced vs. preserved left ventricular ejection fraction (LVEF) (40% cut-off). Non-parametric Wilcoxon rank sum tests were conducted to determine differences between the distributions of non-HF and HF time to readmission.
Multivariable generalized logistic regression analysis was used to determine risk factors for both any-cause rehospitalization and HF-related rehospitalization, categorized as no rehospitalization, early rehospitalization and late rehospitalization. Considering all baseline characteristics listed in Table 1 (excluding BNP and estimated glomerular filtration rate) as candidate variables, stepwise selection was used to determine final models of independent risk factors for both any-cause and HF-related rehospitalization, respectively, using as a selection criterion a P-value of <0.10 for inclusion in the final model. After the final multivariable model had been created, additional adjustment variables were forced into the model; these represent characteristics found to be significant predictors for early vs. late readmission in the subset of patients who actually experienced readmission. For any-cause rehospitalization, forced adjustment covariates included digoxin use, orthopnoea, nitrate use and baseline weight. For HF-related rehospitalization, forced adjustment covariates included digoxin use and history of ischaemic heart disease. Levels of missing data among candidate variables ranged from 0% to 8%. Assuming that these data were missing at random, multiple imputation was utilized to account for missingness. Results were reported as odds ratios (ORs) for early vs. no, late vs. no, and early vs. late rehospitalization. In addition, a sensitivity analysis was conducted to determine risk factors for any-cause rehospitalization and HF-related rehospitalization in patients for whom data on LVEF were available. The sensitivity analyses included an LVEF of <40% as an additional risk factor in the final multivariable model.
Table 1.
Baseline patient characteristics by all-cause rehospitalization status (n = 6584)
| Characteristic | Any-cause rehospitalization status
|
P-valuea | ||
|---|---|---|---|---|
| No readmission (n =5833) | Early readmission (n =248) | Late readmission (n =503) | ||
| Demographics | ||||
| Age, years, mean ± SD | 65 ± 14.0 | 67 ± 14.6 | 66 ± 14.7 | 0.033 |
| Female gender, n (%) | 1997 (34.2%) | 83 (33.5%) | 175 (34.8%) | 0.936 |
| Race, n (%) | <0.001 | |||
| White | 3224 (55.3%) | 150 (60.5%) | 302 (60.0%) | |
| Black or African American | 849 (14.6%) | 54 (21.8%) | 115 (22.9%) | |
| Asian | 1494 (25.6%) | 31 (12.5%) | 75 (14.9%) | |
| Other | 263 (4.5%) | 13 (5.2%) | 11 (2.2%) | |
| Baseline weight, kg, median (IQR) | 78 (64–95) | 83 (69–102) | 83 (68–97) | <0.001 |
| Region, n (%) | <0.001 | |||
| Asia-Pacific | 1491 (25.6%) | 31 (12.5%) | 74 (14.7%) | |
| Central Europe | 875 (15.0%) | 5 (2.0%) | 20 (4.0%) | |
| Latin America | 557 (9.6%) | 18 (7.3%) | 35 (7.0%) | |
| North America | 2511 (43.1%) | 184 (74.2%) | 350 (69.6%) | |
| Western Europe | 397 (6.8%) | 10 (4.0%) | 24 (4.8%) | |
| Medical history | ||||
| NYHA classification, n (%) | 0.041 | |||
| NYHA class not assessed | 1014 (17.4%) | 61 (24.6%) | 89 (17.7%) | |
| NYHA class I | 231 (4.0%) | 4 (1.6%) | 13 (2.6%) | |
| NYHA class II | 914 (15.7%) | 31 (12.5%) | 80 (15.9%) | |
| NYHA class III | 2338 (40.1%) | 105 (42.3%) | 207 (41.2%) | |
| NYHA class IV | 1336 (22.9%) | 47 (19.0%) | 114 (22.7%) | |
| Ischaemic heart disease, n (%) | 3493 (59.9%) | 163 (65.7%) | 310 (61.6%) | 0.150 |
| HF hospitalization past year, n (%) | 2116 (36.3%) | 134 (54.0%) | 283 (56.3%) | <0.001 |
| LVEF in previous 12 months, %, mean ± SD | 30 ± 12.6 | 33 ± 15.3 | 30 ± 14.0 | 0.187 |
| LVEF <40% past year, n (%) | 3529 (81.0%) | 141 (68.8%) | 319 (76.7%) | <0.001 |
| History of hypertension, n (%) | 4191 (71.9%) | 202 (81.5%) | 387 (76.9%) | <0.001 |
| History of diabetes mellitus, n (%) | 2428 (41.6%) | 124 (50.0%) | 255 (50.7%) | <0.001 |
| History of coronary artery disease, n (%) | 3151 (54.0%) | 158 (63.7%) | 296 (59.0%) | 0.002 |
| History of cerebrovascular disease, n (%) | 643 (11.0%) | 38 (15.3%) | 94 (18.7%) | <0.001 |
| History of peripheral arterial vascular disease | 589 (10.1%) | 41 (16.5%) | 68 (13.5%) | <0.001 |
| Baseline chronic respiratory disease, n (%) | 899 (15.4%) | 69 (27.8%) | 122 (24.3%) | <0.001 |
| History of atrial fibrillation/flutter, n (%) | 2133 (36.6%) | 118 (47.6%) | 217 (43.1%) | <0.001 |
| History of ICD/CRT, n (%) | 456 (7.8%) | 38 (15.3%) | 89 (17.7%) | <0.001 |
| Current smoker, n (%) | 799 (13.7%) | 31 (12.5%) | 71 (14.1%) | <0.001 |
| Laboratory values at baseline, median (IQR) | ||||
| Systolic BP, mmHg, | 124 (110–140) | 122 (110–138) | 120 (110–137) | 0.008 |
| Diastolic BP, mmHg, | 75 (67–84) | 72 (64–83) | 71 (64–80) | <0.001 |
| Heart rate, b.p.m. | 82 (72–95) | 80 (70–94) | 82 (70–94) | 0.110 |
| Respiratory rate, breaths/min | 23 (21–26) | 22 (20–24) | 22 (20–25) | 0.058 |
| Sodium, mmol/L | 139 (136–141) | 138 (136–141) | 139 (136–141) | 0.090 |
| BUN, mg/dL | 25 (18–37) | 28 (19–41) | 28 (20–40) | <0.001 |
| Creatinine, mg/dL | 1.2 (1.0–1.5) | 1.4 (1.1–1.8) | 1.3 (1.1–1.7) | <0.001 |
| Haemoglobin, g/dL | 13 (11–14) | 12 (11–13) | 12 (11–14) | <0.001 |
| NT-proBNP, pg/mL | 4242 (1982–8668) | 6002 (2991–12 197) | 5545 (2768–12 280) | <0.001 |
| BNP, pg/mL | 957 (523–1801) | 1019 (602–1906) | 1221 (671–2087) | <0.001 |
| GFR | 60 (45–76) | 54 (38–68) | 53 (40–70) | <0.001 |
| Medication at/before baseline, n (%) | ||||
| ACEIs or ARBs | 3549 (60.9%) | 161 (64.9%) | 321 (63.8%) | 0.207 |
| Beta-blockers | 3346 (57.4%) | 164 (66.1%) | 353 (70.2%) | <0.001 |
| Aldosterone antagonists | 1611 (27.6%) | 67 (27.0%) | 154 (30.6%) | 0.342 |
| Chronic use of loop diuretics | 3578 (61.4%) | 195 (78.6%) | 408 (81.1%) | <0.001 |
| Nitrates | 1344 (23.0%) | 77 (31.0%) | 123 (24.5%) | 0.012 |
| Hydralazine | 368 (6.3%) | 42 (16.9%) | 69 (13.7%) | <0.001 |
| Digoxin | 1510 (25.9%) | 57 (23.0%) | 162 (32.2%) | 0.004 |
| Oral anticoagulants | 1332 (22.8%) | 82 (33.1%) | 175 (34.8%) | <0.001 |
| Aspirin | 2819 (48.3%) | 144 (58.1%) | 287 (57.1%) | <0.001 |
| Clinical profile | ||||
| Baseline BMI, kg/m2, median (IQR) | 27.4 (23.7–32.6) | 29.0 (25.0–33.8) | 28.8 (24.7–33.1) | <0.001 |
| Orthopnoea, n (%) | 4451 (76.4%) | 212 (85.5%) | 396 (78.9%) | 0.002 |
| Rales >1/3 lung fields, n (%) | 3070 (52.6%) | 116 (46.8%) | 255 (50.7%) | 0.022 |
| JVD, n (%) | 3204 (55.0%) | 158 (63.7%) | 330 (65.6%) | <0.001 |
| Peripheral oedema, n (%) | 4317 (74.0%) | 190 (76.6%) | 394 (78.3%) | 0.079 |
| Clinical course | ||||
| Actual treatment group, n (%) | 0.977 | |||
| Placebo | 2863 (49.7%) | 123 (50.0%) | 247 (50.2%) | |
| Nesiritide | 2895 (50.3%) | 123 (50.0%) | 245 (49.8%) | |
ACEI, angiotensin converting-enzyme inhibitor; ARB, angiotensin receptor blocker; BMI, body mass index; BNP, B-type natriuretic peptide; BP, blood pressure; BUN, blood urea nitrogen; CRT, cardiac resynchronization therapy; GFR, glomerular filtration rate; HF, heart failure; ICD, implantable cardioverter defibrillator; IQR, interquartile range; JVD, jugular venous distension; LVEF, left ventricular ejection fraction; NT-proBNP, N-terminal pro B-type natriuretic peptide; NYHA, New York Heart Association; SD, standard deviation.
P-value from comparison for no readmission vs. early readmission vs. late readmission.
Cox regression models were used to determine the relationship between the presence (vs. absence) of 30-day readmission and the timing of 30-day readmission (i.e. early vs. late) with 180-day mortality. This analysis set a landmark at 30 days after discharge, and only patients who survived to that point were included in this analysis. Rates of 180-day mortality were calculated from randomization. Adjustment variables consisted of the risk factors from the final multivariable models previously identified above. All analyses were performed using SAS Version 9.4 (SAS Institute, Inc., Cary, NC, USA). A two-tailed P-value of <0.05 was considered to indicate differences of statistical significance.
Funding and manuscript preparation
Financial and material support for the ASCEND-HF trial was provided by Scios, Inc. (Sunnyvale, CA, USA), since acquired by Johnson & Johnson (New Brunswick, NJ, USA). Database management and statistical analysis were performed by the Duke Clinical Research Institute. The present authors take responsibility for the manuscript’s integrity and had complete control and authority over its preparation and the decision to publish.
Results
Study population
In total, 6584 patients were included in the analysis. The mean ± standard deviation (SD) age of study participants was 65 ± 14 years; 66% were male, and 44% self-identified as non-White. Ischaemic heart disease was reported in 60% of patients, and the mean ± SD LVEF was 30 ± 12.8%. Prevalences of cardiac and non-cardiac comorbidities were high, and the majority of patients were treated with GDMT on admission [in patients with LVEF <40%, levels of use of angiotensin-converting enzyme inhibitors (ACEIs) or angiotensin receptor blockers (ARBs), and beta-blockers, amounted to 62% and 58%, respectively]. Table 1 shows the baseline characteristics of patients who were readmitted either early or late, or were not readmitted during the first 30 days after discharge.
A total of 751 patients (11% of the total population) were readmitted within 30 days after discharge for any cause. Compared with patients who were not readmitted, patients who were readmitted for any cause within 30 days were more likely to be older, African American and enrolled at a site in North America, compared with patients not readmitted. Further, patients who were readmitted for any cause tended to have a higher body mass index (BMI), to carry higher numbers of cardiac and non-cardiac comorbidities, to have a higher rate of prior HF hospitalization, and to be receiving more cardiovascular medications at baseline. These patients also exhibited worse baseline renal function and more severe congestion on physical examination and by laboratory markers. Baseline characteristics by cause of rehospitalization are displayed in the supplementary material online, Table S1. Average time from randomization to discharge (i.e. length of stay) was 5 days, regardless of cause of readmission (supplementary material online, Table S2).
Cause of readmission
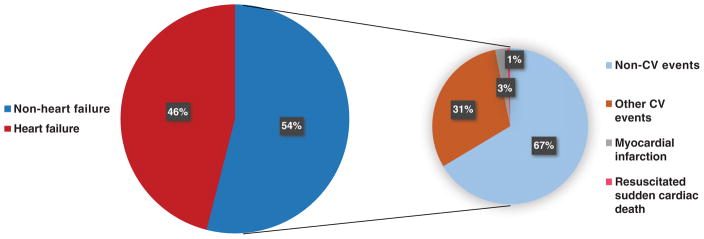
Among patients who were rehospitalized, 345 patients (46% of the readmitted population and 5% of the total cohort) were readmitted within 30 days after discharge for HF, and 406 patients (54% of the readmitted population and 6% of the total cohort) were readmitted for non-HF causes. Non-HF causes included myocardial infarction (2.5%), resuscitated sudden cardiac death (0.6%), other cardiovascular events (30.5%) and other non-cardiovascular events (66.5%) (Figure 1). Thus, the total rate of readmission for non-cardiovascular causes was 36%. Non-cardiovascular causes of readmission included respiratory disease, infections and renal disorders.
Figure 1.
Adjudicated and investigator-reported causes of rehospitalization after heart failure (HF)-related hospitalization stratified by HF and non-HF causes. Non-HF causes are further stratified by cardiovascular (CV) and non-CV causes.
Timing of readmission following heart failure hospitalization
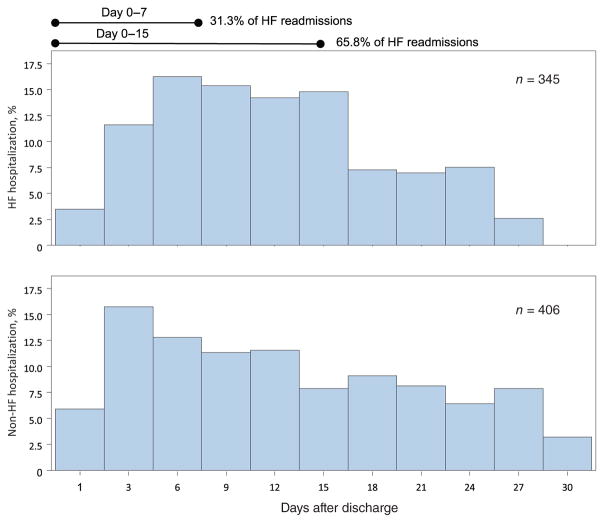
Among all patients readmitted within 30 days of discharge, the median time of readmission was day 11. By day 7, 33% of patients had been readmitted, and by day 15, 67% had been rehospitalized. Heart failure-related readmissions within the 30-day mark occurred at a median of 11 days. By day 7, 31% of patients had been readmitted, and by day 15, 66% had been rehospitalized (Figure 2). Non-HF-related readmissions occurred at a median of 12 days. By day 7, 35% of patients had been readmitted, and by day 15, 63% had been rehospitalized. There was no significant difference in time to readmission between HF- vs. non-HF-related readmission (P = 0.453).
Figure 2.
Timing of rehospitalization for heart failure (HF) and non-HF-related rehospitalization.
Early vs. late readmission
Compared with late readmission, early readmission occurred more frequently at North American sites and in patients with hypertension and coronary artery disease. On discharge, patients readmitted early had a higher weight and worse renal function (creatinine and blood urea nitrogen). Further, patients readmitted early were more likely to be on GDMT with a comparable rate of diuretic use at discharge. Average lengths of stay were 5 days for patients readmitted either early or late, irrespective of HF or non-HF causes (Table S2).
Independent predictors of all-cause and HF rehospitalization are shown in Tables 2 and 3. When the no-readmission group was used as the reference group, baseline chronic respiratory disease was associated with an increased likelihood of early all-cause readmission [OR 1.51, 95% confidence interval (CI) 1.11–2.07], whereas history of cerebrovascular disease and history of cardiac resynchronization therapy (CRT) were associated with increased likelihoods of late readmission [OR 1.52 (95% CI 1.18–1.96) and OR 1.39 (95% CI 1.07–1.82), respectively]. Chronic loop diuretics use on admission was associated with increased risk for late HF rehospitalization (OR 2.37, 95% CI 1.56–3.61), and baseline use of ACEI or ARB and systolic blood pressure were associated with decreased likelihoods of late HF readmission [OR 0.66 (95% CI 0.49–0.89) and OR 0.91 (95% CI 0.85–0.97), respectively] in comparison with no HF readmission. Nesiritide use was not associated with either early or late readmission in a comparison between the early and late all-cause readmission groups (OR 1.04, 95% CI 0.76–1.42) or no rehospitalization (early vs. no rehospitalization: OR 1.04, 95% CI 0.79–1.36; late vs. no rehospitalization: OR 1.00, 95% CI 0.83–1.21). Odds ratios for direct comparisons between early and late readmission are presented in Tables 2 and 3.
Table 2.
Baseline predictors of all-cause rehospitalization after discharge
| Variable | OR (95% CI)
|
Wald | P-value | ||
|---|---|---|---|---|---|
| Early vs. No readmission | Late vs. No readmission | Early vs. Late readmission | |||
| History of cerebrovascular disease | 1.11 (0.76–1.64) | 1.52 (1.18–1.96) | 0.73 (0.47–1.14) | 10.302 | 0.006 |
| Chronic respiratory disease | 1.51 (1.11–2.07) | 1.25 (0.99–1.58) | 1.21 (0.84–1.76) | 9.254 | 0.010 |
| Prior CRT (with or without ICD) | 1.01 (0.67–1.52) | 1.39 (1.07–1.82) | 0.73 (0.46–1.15) | 5.951 | 0.051 |
| Current smoker vs. never smoked | 0.94 (0.60–1.47) | 1.13 (0.84–1.53) | 0.83 (0.49–1.40) | 0.746 | 0.689 |
| Past smoker vs. never smoked | 1.27 (0.94–1.71) | 1.18 (0.95–1.46) | 1.07 (0.75–1.53) | 4.343 | 0.114 |
| Hospitalization in last year | 1.63 (1.22–2.17) | 1.70 (1.38–2.08) | 0.96 (0.68–1.35) | 34.446 | <0.001 |
| Asia Pacific vs. North America | 0.49 (0.31–0.78) | 0.52 (0.38–0.71) | 0.94 (0.54–1.64) | 23.980 | <0.001 |
| Central Europe vs. North America | 0.11 (0.05–0.28) | 0.19 (0.12–0.30) | 0.60 (0.22–1.70) | 68.157 | <0.001 |
| Latin America vs. North America | 0.47 (0.26–0.85) | 0.60 (0.41–0.87) | 0.78 (0.39–1.56) | 12.625 | 0.002 |
| Western Europe vs. North America | 0.45 (0.24–0.88) | 0.53 (0.34–0.82) | 0.85 (0.40–1.85) | 12.928 | 0.002 |
| Chronic loop diuretic use | 1.46 (1.02–2.09) | 1.94 (1.49–2.52) | 0.76 (0.49–1.17) | 27.453 | <0.001 |
| Hydralazine use | 1.56 (1.03–2.34) | 1.38 (1.01–1.90) | 1.12 (0.69–1.83) | 7.636 | 0.022 |
| Digoxin use | 0.73 (0.52–1.01) | 1.18 (0.96–1.46) | 0.61 (0.42–0.90) | 6.495 | 0.039 |
| Orthopnoea | 1.49 (1.01–2.19) | 0.94 (0.74–1.19) | 1.59 (1.02–2.46) | 4.509 | 0.105 |
| Nitrate use | 1.15 (0.83–1.58) | 0.78 (0.62–1.00) | 1.46 (1.00–2.15) | 4.947 | 0.084 |
| Baseline heart rate, per 5 b.p.m. | 1.00 (0.96–1.05) | 1.03 (1.00–1.06) | 0.98 (0.93–1.03) | 3.497 | 0.174 |
| Baseline weight < 85 kg, per 5 kg | 1.02 (0.95–1.20) | 1.04 (0.99–1.09) | 0.98 (0.90–1.07) | 2.762 | 0.251 |
| Baseline weight ≥85 kg, per 5 kg | 1.01 (0.97–1.04) | 0.97 (0.94–1.00) | 1.04 (0.99–1.09) | 4.368 | 0.113 |
| Baseline sodium, per 5 mmol/L | 0.89 (0.76–1.05) | 0.87 (0.78–0.97) | 1.03 (0.85–1.24) | 7.541 | 0.023 |
CI, confidence interval; CRT, cardiac resynchronization therapy; ICD, implantable cardioverter defibrillator; OR, odds ratio.
Table 3.
Baseline predictors of heart failure-related rehospitalization after discharge
| Variable | OR (95% CI)
|
Wald | P-value | ||
|---|---|---|---|---|---|
| Early vs. No readmission | Late vs. No readmission | Early vs. Late readmission | |||
| History of cerebrovascular disease | 0.87 (0.48–1.59) | 1.56 (1.10–2.22) | 0.56 (0.28–1.10) | 6.615 | 0.037 |
| History of ischaemic heart disease | 1.84 (1.14–2.96) | 0.83 (0.63–1.10) | 2.22 (1.29–3.81) | 8.240 | 0.016 |
| Hospitalization in last year | 2.01 (1.30–3.10) | 1.85 (1.39–2.46) | 1.08 (0.65–1.80) | 27.064 | <0.001 |
| Latin America vs. North America | 0.41 (0.16–1.03) | 0.69 (0.42–1.14) | 0.59 (0.21–1.68) | 5.599 | 0.061 |
| Western Europe vs. North America | 0.42 (0.16–1.06) | 0.28 (0.13–0.62) | 1.47 (0.44–4.88) | 13.176 | 0.001 |
| ACEI or ARB use | 1.00 (0.62–1.60) | 0.66 (0.49–0.89) | 1.51 (0.88–2.60) | 7.613 | 0.022 |
| Chronic loop diuretic use | 1.65 (0.91–2.96) | 2.37 (1.56–3.61) | 0.69 (0.34–1.42) | 18.744 | <0.001 |
| Digoxin use | 0.81 (0.50–1.32) | 1.32 (0.98–1.76) | 0.62 (0.35–1.08) | 4.239 | 0.120 |
| Log of creatinine (mg), per doubling | 1.40 (0.94–2.08) | 1.56 (1.21–2.02) | 0.90 (0.56–1.42) | 13.887 | 0.001 |
| Respiratory rate < 20, per 5 breaths/min | 0.57 (0.22–1.51) | 0.51 (0.27–0.95) | 1.13 (0.37–3.43) | 5.406 | 0.067 |
| Respiratory rate ≥20, per 5 breaths/min | 1.34 (1.03–1.76) | 1.17 (0.96–1.42) | 1.15 (0.83–1.59) | 6.778 | 0.034 |
| Systolic BP < 130 mmHg, per 5 mmHg | 0.95 (0.85–1.06) | 0.91 (0.85–0.97) | 1.04 (0.92–1.18) | 8.067 | 0.018 |
| Systolic BP ≥130 mmHg, per 5 mmHg | 1.00 (0.91–1.11) | 1.04 (0.98–1.11) | 0.96 (0.86–1.08) | 1.690 | 0.430 |
ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; BP, blood pressure; CI, confidence interval; OR, odds ratio.
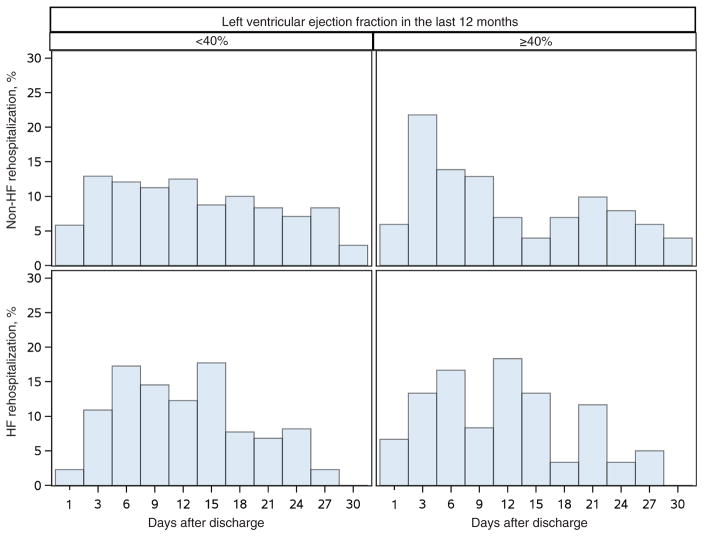
In patients for whom data on LVEF were available (n = 4887), LVEF was found to be an independent predictor of all-cause readmission (P = 0.005). Specifically, LVEF <40% was associated with a decreased likelihood of early all-cause readmission (OR 0.59, 95% CI 0.42–0.83) in comparison with no readmission; however, LVEF was not associated with HF-specific rehospitalization. The relationship between LVEF and the timing of readmission is displayed in Figure 3.
Figure 3.
Time to rehospitalization by left ventricular ejection fraction (<40% vs. ≥40%) and heart failure (HF) vs. non-HF-related rehospitalization.
Relationship between readmission and mortality
Any-cause [univariable hazard ratio (HR) 2.81, 95% CI 2.30–3.43 (P < 0.001); multivariable HR 2.38, 95% CI 1.93–2.94 (P < 0.001)] or HF-specific [univariable HR 2.83, 95% CI 2.18–3.67 (P < 0.001); multivariable HR 2.04, 95% CI 1.56–2.67 (P < 0.001)] readmission within 30 days of discharge were associated with increased 180-day all-cause mortality. However, timing of readmission for any cause, whether it was early or late, was not predictive of 180-day all-cause mortality using a univariable model (HR 0.90, 95% CI 0.61–1.31; P = 0.57). This was confirmed in the multivariable model (HR 0.99, 95% CI 0.67–1.45; P = 0.94). The lack of association between timing and 180-day all-cause mortality remained for patients readmitted for HF-related (univariable HR 1.04, 95% CI 0.1–1.75; P = 0.9) or non-HF-related (univariable HR 0.79, 95% CI 0.45–1.34; P = 0.39) causes. The full model for all-cause and HF-related readmission in relation to 180-day all-cause mortality can be found in Tables 4 and 5.
Table 4.
All-cause rehospitalization vs. no rehospitalization in relation to 180-day all-cause death
| Risk factor | Multivariable
|
||
|---|---|---|---|
| HR (95% CI) | SE | P-value | |
| Any rehospitalization vs. no rehospitalization | 2.38 (1.93–2.94) | 0.108 | <0.001 |
| History of cerebrovascular disease | 1.51 (1.20–1.90) | 0.118 | 0.001 |
| Chronic respiratory disease | 1.19 (0.95–1.49) | 0.115 | 0.130 |
| Prior CRT (with or without ICD) | 0.83 (0.61–1.12) | 0.153 | 0.212 |
| Baseline heart rate, per 5 b.p.m. | 1.00 (0.98–1.03) | 0.003 | 0.861 |
| Current smoker vs. never smoked | 0.79 (0.59–1.07) | 0.153 | 0.123 |
| Past smoker vs. never smoked | 0.96 (0.80–1.17) | 0.098 | 0.706 |
| Chronic loop diuretic use | 1.93 (1.53–2.43) | 0.118 | <0.001 |
| Hospitalization in last year | 1.23 (1.02–1.48) | 0.095 | 0.027 |
| Asia Pacific vs. North America | 0.74 (0.57–0.97) | 0.138 | 0.029 |
| Central Europe vs. North America | 0.68 (0.48–0.95) | 0.173 | 0.024 |
| Latin America vs. North America | 1.22 (0.90–1.65) | 0.153 | 0.198 |
| Western Europe vs. North America | 1.18 (0.84–1.66) | 0.174 | 0.337 |
| Hydralazine use | 0.89 (0.64–1.25) | 0.173 | 0.504 |
| Baseline sodium, per 5 mmol/L | 0.81 (0.73–0.88) | 0.010 | <0.001 |
| Digoxin use | 1.15 (0.95–1.39) | 0.097 | 0.156 |
| Orthopnoea | 0.84 (0.69–1.03) | 0.104 | 0.093 |
| Oral/topical nitrate use | 1.11 (0.90–1.36) | 0.104 | 0.325 |
| Baseline weight < 85 kg, per 5 kg | 0.92 (0.88–0.96) | 0.004 | <0.001 |
| Baseline weight ≥85 kg, per 5 kg | 0.96 (0.92–0.99) | 0.004 | 0.015 |
CI, confidence interval; CRT, cardiac resynchronization therapy; HR, hazard ratio; ICD, implantable cardioverter defibrillator; SE, standard error.
Table 5.
Heart failure-related rehospitalization vs. no rehospitalization in relation to 180-day all-cause death
| Risk factor | Multivariable
|
||
|---|---|---|---|
| HR (95% CI) | SE | P-value | |
| HF rehospitalization vs. no HF rehospitalization | 2.04 (1.56–2.67) | 0.137 | <0.001 |
| ACEI or ARB use | 0.68 (0.56–0.82) | 0.097 | 0.001 |
| Respiratory rate < 20, per 5 breaths/min | 1.10 (0.96–1.25) | 0.014 | 0.174 |
| Respiratory rate ≥20, per 5 breaths/min | 1.20 (0.88–1.64) | 0.032 | 0.249 |
| Systolic BP <130 mmHg, per 5 mmHg | 0.94 (0.90–0.98) | 0.005 | 0.003 |
| Systolic BP ≥130 mmHg, per 5 mmHg | 0.99 (0.95–1.04) | 0.005 | 0.801 |
| Chronic loop diuretic use | 1.66 (1.31–2.09) | 0.119 | <0.001 |
| Hospitalization in last year | 1.22 (1.02–1.46) | 0.093 | 0.032 |
| Log of creatinine (mg), per doubling | 1.45 (1.22–1.72) | 0.087 | <0.001 |
| Asia Pacific vs. North America | 1.12 (0.89–1.41) | 0.118 | 0.339 |
| Central Europe vs. North America | 0.67 (0.49–0.96) | 0.169 | 0.026 |
| Latin America vs. North America | 1.55 (1.16–2.07) | 0.148 | 0.003 |
| Western Europe vs. North America | 1.23 (0.88–1.73) | 0.172 | 0.221 |
| History of cerebrovascular disease | 1.50 (1.19–1.89) | 0.118 | 0.001 |
| Digoxin use | 1.24 (1.03–1.50) | 0.097 | 0.027 |
| History of ischaemic heart disease | 1.19 (0.99–1.44) | 0.096 | 0.067 |
ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; BP, blood pressure; CI, confidence interval; HF, heart failure; HR, hazard ratio; SE, standard error.
Discussion
Outcomes at 30 days after discharge from HF-related hospitalization are used by the Centers for Medicaid and Medicare Services (CMS) as a health care quality metric. The present analysis from a large trial in patients with acute HF found that one-third of patients readmitted after an initial HF hospitalization were readmitted within 7 days and two-thirds were rehospitalized within 15 days, which suggests that all-cause and HF-related readmissions are skewed and occur disproportionately more frequently in the first 2 weeks after discharge. A large portion of readmissions are driven by non-HF-related (54%) or even non-cardiovascular (36%) causes. Although readmission within 30 days by itself was associated with increased all-cause mortality, there was no differential association between early (0–7 days) or late (8–30 days) readmission, regardless of cause, with outcomes.
Patients who are discharged from HF-related hospitalization enter what is called the ‘vulnerable phase’. Although it is not clearly defined, the vulnerable phase includes the period immediately after discharge until 2–3 months later18,19 and is marked by high rates of mortality and readmission. The present findings regarding timing and cause of readmission are in agreement with previous analyses of Medicare populations,14 State Inpatient Databases,12 and smaller randomized HF trials.15 In fact, most descriptive characteristics, such as median day of readmission, percentage of patients readmitted within 7 days and the proportion of HF-related and non-HF-related readmissions are strikingly similar, despite differences in study cohorts (national registries vs. randomized controlled trials), age groups (mean ± SD ages are 80.3 ± 7.9 years in the Medicare patient cohort,14 74.7 ± 14.1 years in the State Inpatient Database cohort,12 and 65.0 ± 14.1 years in the ASCEND-HF trial cohort), and time of sampling (Medicare: 2004–2006; State Inpatient Databases: 2007–2011; ASCEND-HF: 2007–2010). Notably, geographic site appears to play a role: in the present study, the likelihoods of early and late all-cause readmission were higher at North American trial sites than at sites in all other continents. Both early and late HF-specific readmissions were more likely to occur in North America in comparison with Asian-Pacific or Central European countries, and late readmission was more likely at North American sites than in Western European and Latin American countries.
Similarly, patients were at increased risk for early and late all-cause readmission if they were enrolled at a trial site in North America, had a history of prior hospitalization in the past year, or were on chronic loop diuretics at admission. Interestingly, although an LVEF of ≤35% in the ASCEND-HF population was associated with worse outcomes,20 LVEF did not predict the timing of HF-related readmission, which may suggest that the physiology leading to recurrent decompensation is similar in both HF with preserved EF and HF with reduced EF patients.
Although the present analysis is the first to evaluate the association of early vs. late readmission after an initial hospitalization for HF with subsequent mortality, similar analyses have been performed for in-hospital worsening HF. Analyses using data from ASCEND-HF21 and PROTECT22 stratified in-hospital worsening HF by early (before hospital day 4) or late (from day 5 until discharge) events and did not find an association between timing and outcomes. However, in an analysis of ADHERE data,23 a different definition of early (HF worsening on day 1) and late (HF worsening after day 1) worsening yielded different results. Early HF compared with late in-hospital worsening HF was associated with a lower rate of all-cause mortality, but similar rates of HF-related and all-cause rehospitalization at 30 days and 1 year. It is possible that, in the present analysis, alternative choices for the early vs. late cut-off would have changed the results. Although the choice of 1 week as a cut-off is arbitrary, it has been used in prior analyses11,12,14 and hospitals that conduct follow-up appointments within 1 week appear to have lower rates of 30-day readmission.24 Further, the 1-week cut-off has been the target of quality improvement programmes25 and, most importantly, is clinically relevant by virtue of being suggested as a target follow-up date by the current American College of Cardiology/American Heart Association (ACC/AHA) guidelines.9
Current guidelines and financial penalties place a strong emphasis on minimizing 30-day rehospitalization with a particular focus on HF-specific measures. However, the present analysis complements prior retrospective studies by showing that a third of patients readmitted within the 30-day window may present to hospital within 1 week, and a majority of readmissions will be for causes other than HF. Registry and trial data indicate that comorbidities such as chronic obstructive lung disease and diabetes mellitus are present in more than 30% of patients with HF,26–28 and the presence of comorbidities in acute HF patients is associated with added morbidity and mortality.27,29 Additionally, the number of non-cardiac chronic conditions increases the risk for hospitalization.30 Current strategies to reduce hospitalization in patients with HF place strong emphasis on weight and vitals monitoring (telemonitoring),31,32 as well as invasive pressure monitoring.33,34 So far, strategies to reduce hospitalization rates have shown mixed results. Given that the majority of readmissions may occur for reasons other than HF, inadequate diuresis35 and short-term worsening of haemodynamics36 are not to be viewed as solely responsible for the high rate of rehospitalization. The wide range of acute conditions precipitating readmission exposes the heightened vulnerability of patients with HF and particularly those admitted for an acute decompensation. Hospitalized patients frequently develop a new impairment, suffer a loss of mobility and strength,37,38 and become nutritionally deficient.39 Whereas the ASCEND-HF trial did not test the effectiveness of any specific post-discharge interventions, a multidisciplinary approach40 might address a greater number of potential causes of readmission in comparison with a single intervention.41 Finally, current ACC/AHA guidelines recommend a follow-up visit at 7–14 days9 and early telephone follow-up within 3 days, and the European Society of Cardiology (ESC) guidelines recommend a first visit within 7 days.10 The present findings, in aggregate with existing work, support added emphasis on early follow-up appointment and increased preventive efforts during the discharge phase.
Limitations
Firstly, this study was designed post hoc and thus is subject to the potential biases intrinsic to secondary analyses of randomized clinical trials, including unmeasured or residual confounding. However, unlike prior retrospective analyses, by virtue of its use of a clinical trial database with comprehensive data capture and adjudicated outcomes, the present analysis of the ASCEND-HF trial allows for a greater degree of detail. Secondly, despite the large size of the original trial, the current analysis remains limited by low 30-day readmission rates in comparison with those seen in real-world practice, as suggested by CMS data.7 The discrepancy in readmission rates can potentially be explained by the fact that the present study used randomized trial data with specific inclusion and exclusion criteria, a more definitive diagnosis of HF and improved follow-up compared with those in the general population. Thirdly, despite the strength of the adjudication process and investigator-reported outcomes in ASCEND-HF, readmission events were attributed to only a limited number of diagnoses and about a third of non-cardiovascular events were not attributed to a specific diagnosis. Fourthly, post-randomization and/or discharge patient characteristics would be preferable covariates for an analysis of 30-day outcomes. The present group chose to use patient characteristics at randomization in view of the superior quality and quantity of the data in ASCEND-HF at that time-point. Finally, the endpoint analysis using 30-day readmission data was limited by the exclusion of patients who died before the landmark analysis. The characteristics of all excluded patients can be found in the supplementary material online, Table S3.
Conclusions
In this analysis of a large randomized clinical trial of patients with acute HF, 54% of 30-day readmissions were attributable to non-HF-related causes and a third of readmissions occurred within the first 7 days. Early and late readmissions within the 30-day timeframe, regardless of cause of readmission, were associated with similar increases in risk for death. The present analysis underscores the fact that given the high burden of early and non-HF-related readmissions, there is a need for a multidisciplinary approach to patients admitted for HF.
Supplementary Material
Table S1. Baseline characteristics by cause of rehospitalization group.
Table S2. Discharge characteristics by timing and cause of 30-day rehospitalization.
Table S3. Baseline patient characteristics by exclusion status.
Acknowledgments
Funding
The ASCEND-HF study was supported by Scios, Inc.
Footnotes
Additional Supporting Information may be found in the online version of this article:
Conflict of interest: M.F. has received remuneration from Axon Therapies, Coridea, Cibiem and GE Healthcare, and is supported by an American Heart Association grant (17MCPRP33460225) and a National Heart, Lung and Blood Institute (NHLBI) T32 post-doctoral training grant (5T32HL007101-42). C.M.O’C. has received remuneration from Amgen, Astellas, GE Health-care, Gilead, Novella, Otsuka, Roche Diagnostics and Resmed. P.W.A. has received remuneration from Merck, MAST Therapeutics, AstraZeneca, Bayer, Merck and Sanofi-Aventis. J.A.E. has received remuneration from Abbott Labs, Amgen, Johnson & Johnson, Pfizer and Servier. S.J.G. is supported by an NHLBI T32 post-doctoral training grant (5T32HL069749-14). M.M. has received remuneration from Bayer, Novartis and Servier. R.C.S. has received remuneration from BioControl, Biotronik, Cardiomems, Medtronic, Novartis, the National Institutes of Health, OnoPharma and Thoratec. A.A.V. has received remuneration from Amgen, Bayer, Boehringer Ingelheim, Merck, Novartis and Servier. A.F.H. has received remuneration from Sanofi, Johnson & Johnson, AstraZeneca and Corthera. G.M.F. has received remuneration from Amgen, Bristol Myers Squibb, GSK, Medtronic, MyoKardia, Novartis, Stealth, Trevena, Amgen, Otsuka and Roche Diagnostics. R.J.M. has received remuneration from Amgen, AstraZeneca, Bristol Myers Squibb, Gilead, GlaxoSmithKline, Novartis, Otsuka, ResMed and Thoratec. All other authors report no disclosures.
References
- 1.Ambrosy AP, Fonarow GC, Butler J, Chioncel O, Greene SJ, Vaduganathan M, Nodari S, Lam CS, Sato N, Shah AN, Gheorghiade M. The global health and economic burden of hospitalizations for heart failure: lessons learned from hospitalized heart failure registries. J Am Coll Cardiol. 2014;63:1123–1133. doi: 10.1016/j.jacc.2013.11.053. [DOI] [PubMed] [Google Scholar]
- 2.Hines A, Barrett M, Jiang H, Steiner C. Healthcare Cost and Utilization Project (HCUP) Statistical Brief 172. Rockville, MD: Agency for Healthcare Research and Quality; 2014. Conditions with the largest number of adult hospital readmissions by payer, 2011. [PubMed] [Google Scholar]
- 3.Cowie MR, Anker SD, Cleland JGF, Felker GM, Filippatos G, Jaarsma T, Jourdain P, Knight E, Massie B, Ponikowski P, López-Sendón J. Improving care for patients with acute heart failure: before, during and after hospitalization. ESC Heart Fail. 2014;1:110–145. doi: 10.1002/ehf2.12021. [DOI] [PubMed] [Google Scholar]
- 4.Kristensen SR, Bech M, Quentin W. A roadmap for comparing readmission policies with application to Denmark, England, Germany and the United States. Health Policy. 2015;119:264–273. doi: 10.1016/j.healthpol.2014.12.009. [DOI] [PubMed] [Google Scholar]
- 5.Arundel C, Lam PH, Khosla R, Blackman MR, Fonarow GC, Morgan C, Zeng Q, Fletcher RD, Butler J, Wu WC, Deedwania P, Love TE, White M, Aronow WS, Anker SD, Allman RM, Ahmed A. Association of 30-day all-cause readmission with long-term outcomes in hospitalized older Medicare beneficiaries with heart failure. Am J Med. 2016;129:1178–1184. doi: 10.1016/j.amjmed.2016.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Setoguchi S, Stevenson LW, Schneeweiss S. Repeated hospitalizations predict mortality in the community population with heart failure. Am Heart J. 2007;154:260–266. doi: 10.1016/j.ahj.2007.01.041. [DOI] [PubMed] [Google Scholar]
- 7.Krumholz HM, Normand SL, Wang Y. Trends in hospitalizations and outcomes for acute cardiovascular disease and stroke, 1999–2011. Circulation. 2014;130:966–975. doi: 10.1161/CIRCULATIONAHA.113.007787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bradley EH, Curry L, Horwitz LI, Sipsma H, Thompson JW, Elma M, Walsh MN, Krumholz HM. Contemporary evidence about hospital strategies for reducing 30-day readmissions: a national study. J Am Coll Cardiol. 2012;60:607–614. doi: 10.1016/j.jacc.2012.03.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:e240–e327. doi: 10.1161/CIR.0b013e31829e8776. [DOI] [PubMed] [Google Scholar]
- 10.Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, Gonzalez-Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2016;18:891–975. doi: 10.1002/ejhf.592. [DOI] [PubMed] [Google Scholar]
- 11.Eastwood CA, Howlett JG, King-Shier KM, McAlister FA, Ezekowitz JA, Quan H. Determinants of early readmission after hospitalization for heart failure. Can J Cardiol. 2014;30:612–618. doi: 10.1016/j.cjca.2014.02.017. [DOI] [PubMed] [Google Scholar]
- 12.Davis JD, Olsen MA, Bommarito K, LaRue SJ, Saeed M, Rich MW, Vader JM. All-payer analysis of heart failure hospitalization 30-day readmission: comorbidities matter. Am J Med. 2017;130:93e9–93.e28. doi: 10.1016/j.amjmed.2016.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Desai AS, Claggett B, Pfeffer MA, Bello N, Finn PV, Granger CB, McMurray JJ, Pocock S, Swedberg K, Yusuf S, Solomon SD. Influence of hospitalization for cardiovascular versus noncardiovascular reasons on subsequent mortality in patients with chronic heart failure across the spectrum of ejection fraction. Circ Heart Fail. 2014;7:895–902. doi: 10.1161/CIRCHEARTFAILURE.114.001567. [DOI] [PubMed] [Google Scholar]
- 14.Dharmarajan K, Hsieh AF, Lin Z, Bueno H, Ross JS, Horwitz LI, Barreto-Filho JA, Kim N, Bernheim SM, Suter LG, Drye EE, Krumholz HM. Diagnoses and timing of 30-day readmissions after hospitalization for heart failure, acute myocardial infarction, or pneumonia. JAMA. 2013;309:355–363. doi: 10.1001/jama.2012.216476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vader JM, LaRue SJ, Stevens SR, Mentz RJ, DeVore AD, Lala A, Groarke JD, AbouEzzeddine OF, Dunlay SM, Grodin JL, Davila-Roman VG, de Las Fuentes L. Timing and causes of readmission after acute heart failure hospitalization – insights from the Heart Failure Network Trials. J Card Fail. 2016;22:875–883. doi: 10.1016/j.cardfail.2016.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hernandez AF, O’Connor CM, Starling RC, Reist CJ, Armstrong PW, Dickstein K, Lorenz TJ, Gibler WB, Hasselblad V, Komajda M, Massie B, McMurray JJ, Nieminen M, Rouleau JL, Swedberg K, Califf RM. Rationale and design of the Acute Study of Clinical Effectiveness of Nesiritide in Decompensated Heart Failure Trial (ASCEND-HF) Am Heart J. 2009;157:271–277. doi: 10.1016/j.ahj.2008.07.031. [DOI] [PubMed] [Google Scholar]
- 17.O’Connor CM, Starling RC, Hernandez AF, Armstrong PW, Dickstein K, Hasselblad V, Heizer GM, Komajda M, Massie BM, McMurray JJ, Nieminen MS, Reist CJ, Rouleau JL, Swedberg K, Adams KF, Jr, Anker SD, Atar D, Battler A, Botero R, Bohidar NR, Butler J, Clausell N, Corbalan R, Costanzo MR, Dahlstrom U, Deckelbaum LI, Diaz R, Dunlap ME, Ezekowitz JA, Feldman D, Felker GM, Fonarow GC, Gennevois D, Gottlieb SS, Hill JA, Hollander JE, Howlett JG, Hudson MP, Kociol RD, Krum H, Laucevicius A, Levy WC, Mendez GF, Metra M, Mittal S, Oh BH, Pereira NL, Ponikowski P, Tang WH, Tanomsup S, Teerlink JR, Triposkiadis F, Troughton RW, Voors AA, Whellan DJ, Zannad F, Califf RM. Effect of nesiritide in patients with acute decompensated heart failure. N Engl J Med. 2011;365:32–43. doi: 10.1056/NEJMoa1100171. [DOI] [PubMed] [Google Scholar]
- 18.Desai AS, Stevenson LW. Rehospitalization for heart failure: predict or prevent? Circulation. 2012;126:501–506. doi: 10.1161/CIRCULATIONAHA.112.125435. [DOI] [PubMed] [Google Scholar]
- 19.Chun S, Tu JV, Wijeysundera HC, Austin PC, Wang X, Levy D, Lee DS. Lifetime analysis of hospitalizations and survival of patients newly admitted with heart failure. Circ Heart Fail. 2012;5:414–421. doi: 10.1161/CIRCHEARTFAILURE.111.964791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Toma M, Ezekowitz JA, Bakal JA, O’Connor CM, Hernandez AF, Sardar MR, Zolty R, Massie BM, Swedberg K, Armstrong PW, Starling RC. The relationship between left ventricular ejection fraction and mortality in patients with acute heart failure: insights from the ASCEND-HF Trial. Eur J Heart Fail. 2014;16:334–341. doi: 10.1002/ejhf.19. [DOI] [PubMed] [Google Scholar]
- 21.Kelly JP, Mentz RJ, Hasselblad V, Ezekowitz JA, Armstrong PW, Zannad F, Felker GM, Califf RM, O’Connor CM, Hernandez AF. Worsening heart failure during hospitalization for acute heart failure: insights from the Acute Study of Clinical Effectiveness of Nesiritide in Decompensated Heart Failure (ASCEND-HF) Am Heart J. 2015;170:298–305. doi: 10.1016/j.ahj.2015.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mentz RJ, Metra M, Cotter G, Milo O, McKendry C, Chiswell K, Davison BA, Cleland JG, Bloomfield DM, Dittrich HC, Fiuzat M, Ponikowski P, Givertz MM, Voors AA, Teerlink JR, O’Connor CM. Early vs. late worsening heart failure during acute heart failure hospitalization: insights from the PROTECT trial. Eur J Heart Fail. 2015;17:697–706. doi: 10.1002/ejhf.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.DeVore AD, Hammill BG, Sharma PP, Qualls LG, Mentz RJ, Waltman Johnson K, Fonarow GC, Curtis LH, Hernandez AF. In-hospital worsening heart failure and associations with mortality, readmission, and healthcare utilization. J Am Heart Assoc. 2014;3:e001088. doi: 10.1161/JAHA.114.001088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hernandez AF, Greiner MA, Fonarow GC, Hammill BG, Heidenreich PA, Yancy CW, Peterson ED, Curtis LH. Relationship between early physician follow-up and 30-day readmission among Medicare beneficiaries hospitalized for heart failure. JAMA. 2010;303:1716–1722. doi: 10.1001/jama.2010.533. [DOI] [PubMed] [Google Scholar]
- 25.Baker H, Oliver-McNeil S, Deng L, Hummel SL. Regional hospital collaboration and outcomes in Medicare heart failure patients: see you in 7. JACC Heart Fail. 2015;3:765–773. doi: 10.1016/j.jchf.2015.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Adams KF, Jr, Fonarow GC, Emerman CL, LeJemtel TH, Costanzo MR, Abraham WT, Berkowitz RL, Galvao M, Horton DP ADHERE Scientific Advisory Committee and Investigators. Characteristics and outcomes of patients hospitalized for heart failure in the United States: rationale, design, and preliminary observations from the first 100,000 cases in the Acute Decompensated Heart Failure National Registry (ADHERE) Am Heart J. 2005;149:209–216. doi: 10.1016/j.ahj.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 27.O’Connor CM, Abraham WT, Albert NM, Clare R, Gattis Stough W, Gheorghiade M, Greenberg BH, Yancy CW, Young JB, Fonarow GC. Predictors of mortality after discharge in patients hospitalized with heart failure: an analysis from the Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF) Am Heart J. 2008;156:662–673. doi: 10.1016/j.ahj.2008.04.030. [DOI] [PubMed] [Google Scholar]
- 28.Konstam MA, Gheorghiade M, Burnett JC, Jr, Grinfeld L, Maggioni AP, Swedberg K, Udelson JE, Zannad F, Cook T, Ouyang J, Zimmer C, Orlandi C Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study with Tolvaptan (EVER-EST) Investigators. Effects of oral tolvaptan in patients hospitalized for worsening heart failure: the EVEREST Outcome Trial. JAMA. 2007;297:1319–1331. doi: 10.1001/jama.297.12.1319. [DOI] [PubMed] [Google Scholar]
- 29.O’Connor CM, Mentz RJ, Cotter G, Metra M, Cleland JG, Davison BA, Givertz MM, Mansoor GA, Ponikowski P, Teerlink JR, Voors AA, Fiuzat M, Wojdyla D, Chiswell K, Massie BM. The PROTECT in-hospital risk model: 7-day outcome in patients hospitalized with acute heart failure and renal dysfunction. Eur J Heart Fail. 2012;14:605–612. doi: 10.1093/eurjhf/hfs029. [DOI] [PubMed] [Google Scholar]
- 30.Braunstein JB, Anderson GF, Gerstenblith G, Weller W, Niefeld M, Herbert R, Wu AW. Noncardiac comorbidity increases preventable hospitalizations and mortality among Medicare beneficiaries with chronic heart failure. J An Coll Cardiol. 2003;42:1226–1233. doi: 10.1016/s0735-1097(03)00947-1. [DOI] [PubMed] [Google Scholar]
- 31.Pandor A, Thokala P, Gomersall T, Baalbaki H, Stevens JW, Wang J, Wong R, Brennan A, Fitzgerald P. Home telemonitoring or structured telephone support programmes after recent discharge in patients with heart failure: systematic review and economic evaluation. Health Technol Assess. 2013;17:1–207. v–vi. doi: 10.3310/hta17320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ong MK, Romano PS, Edgington S, Aronow HU, Auerbach AD, Black JT, De Marco T, Escarce JJ, Evangelista LS, Hanna B, Ganiats TG, Greenberg BH, Greenfield S, Kaplan SH, Kimchi A, Liu H, Lombardo D, Mangione CM, Sadeghi B, Sadeghi B, Sarrafzadeh M, Tong K, Fonarow GC Better Effectiveness After Transition – Heart Failure Research Group. Effectiveness of remote patient monitoring after discharge of hospitalized patients with heart failure: the Better Effectiveness After Transition – Heart Failure (BEAT-HF) randomized clinical trial. JAMA Intern Med. 2016;176:310–318. doi: 10.1001/jamainternmed.2015.7712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bourge RC, Abraham WT, Adamson PB, Aaron MF, Aranda JM, Jr, Magalski A, Zile MR, Smith AL, Smart FW, O’Shaughnessy MA, Jessup ML, Sparks B, Naftel DL, Stevenson LW COMPASS-HF Study Group. Randomized controlled trial of an implantable continuous hemodynamic monitor in patients with advanced heart failure: the COMPASS-HF study. J Am Coll Cardiol. 2008;51:1073–1079. doi: 10.1016/j.jacc.2007.10.061. [DOI] [PubMed] [Google Scholar]
- 34.Abraham WT, Stevenson LW, Bourge RC, Lindenfeld JA, Bauman JG, Adamson PB CHAMPION Trial Study Group. Sustained efficacy of pulmonary artery pressure to guide adjustment of chronic heart failure therapy: complete follow-up results from the CHAMPION randomised trial. Lancet. 2016;387:453–461. doi: 10.1016/S0140-6736(15)00723-0. [DOI] [PubMed] [Google Scholar]
- 35.Felker GM, Mentz RJ. Diuretics and ultrafiltration in acute decompensated heart failure. J Am Coll Cardiol. 2012;59:2145–2153. doi: 10.1016/j.jacc.2011.10.910. [DOI] [PubMed] [Google Scholar]
- 36.Zile MR, Bennett TD, St John Sutton M, Cho YK, Adamson PB, Aaron MF, Aranda JM, Jr, Abraham WT, Smart FW, Stevenson LW, Kueffer FJ, Bourge RC. Transition from chronic compensated to acute decompensated heart failure: pathophysiological insights obtained from continuous monitoring of intracardiac pressures. Circulation. 2008;118:1433–1441. doi: 10.1161/CIRCULATIONAHA.108.783910. [DOI] [PubMed] [Google Scholar]
- 37.Krumholz HM. Post-hospital syndrome – an acquired, transient condition of generalized risk. N Engl J Med. 2013;368:100–102. doi: 10.1056/NEJMp1212324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kortebein P, Ferrando A, Lombeida J, Wolfe R, Evans WJ. Effect of 10 days of bed rest on skeletal muscle in healthy older adults. JAMA. 2007;297:1772–1774. doi: 10.1001/jama.297.16.1772-b. [DOI] [PubMed] [Google Scholar]
- 39.Sullivan DH, Sun S, Walls RC. Protein-energy undernutrition among elderly hospitalized patients: a prospective study. JAMA. 1999;281:2013–2019. doi: 10.1001/jama.281.21.2013. [DOI] [PubMed] [Google Scholar]
- 40.Rich MW, Beckham V, Wittenberg C, Leven CL, Freedland KE, Carney RM. A multidisciplinary intervention to prevent the readmission of elderly patients with congestive heart failure. N Engl J Med. 1995;333:1190–1195. doi: 10.1056/NEJM199511023331806. [DOI] [PubMed] [Google Scholar]
- 41.Hansen LO, Young RS, Hinami K, Leung A, Williams MV. Interventions to reduce 30-day rehospitalization: a systematic review. Ann Intern Med. 2011;155:520–528. doi: 10.7326/0003-4819-155-8-201110180-00008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Baseline characteristics by cause of rehospitalization group.
Table S2. Discharge characteristics by timing and cause of 30-day rehospitalization.
Table S3. Baseline patient characteristics by exclusion status.