Abstract
Our aim was to examine the clinical relevance of white matter hyperintensities (WMH) in HIV. We used an automated approach to quantify WMH volume in HIV seropositive (HIV+; n = 65) and HIV seronegative (HIV−; n = 29) adults over age 60. We compared WMH volumes between HIV+ and HIV− groups in cross-sectional and multiple time-point analyses. We also assessed correlations between WMH volumes and cardiovascular, HIV severity, cognitive scores, and diffusion tensor imaging variables. Serostatus groups did not differ in WMH volume, but HIV+ participants had less cerebral white matter (mean: 470.95 [43.24] vs. 497.63 [49.42] mL, p = 0.010). The distribution of WMH volume was skewed in HIV+ with a high proportion (23%) falling above the 95th percentile of WMH volume defined by the HIV− group. Serostatus groups had similar amount of WMH volume growth over time. Total WMH volume directly correlated with measures of hypertension and inversely correlated with measures of global cognition, particularly in executive functioning, and psychomotor speed. Greater WMH volume was associated with poorer brain integrity measured from diffusion tensor imaging (DTI) in the corpus callosum and sagittal stratum. In this group of HIV+ individuals over 60, WMH burden was associated with cardiovascular risk and both worse diffusion MRI and cognition. The median total burden did not differ by serostatus; however, a subset of HIV+ individuals had high WMH burden.
Keywords: White matter, Leukoaraiosis, HIV, Aging, Cerebrovascular, Diffusion tensor imaging
Introduction
White matter hyperintensities (WMH) become more common with age in HIV-uninfected (HIV−) adults and are thought to relate to vascular, inflammatory, or blood brain barrier changes (Maniega et al. 2015; Shoamanesh et al. 2015). Small WMHs can be found on MRIs of otherwise healthy older adults (Boone et al. 1992; Ylikoski et al. 1995); but they are also associated with cerebrovascular disease, dementia, and multiple sclerosis (Debette and Markus 2010; Fern et al. 2014).
White matter changes were a common feature of HIV-associated dementia (HAD) before antiretroviral treatment was widely used. They have also been described in less severe HIV-related cognitive disorders (Pomara et al. 2001). An early study of HIV+ participants completed before the widespread use of combination antiretroviral therapy (cART) noted incidental white matter changes (McArthur et al. 1990). A recent study found that middle-aged HIV+ men had a higher WMH burden that correlated with cognitive deficits, cardiovascular risk factors, and duration of suppressed CD4 T-lymphocyte counts (Su et al. 2016). Prior studies detected no association of WMH burden with HIV disease severity biomarkers such as duration of disease or CD4 T-lymphocyte counts (Bornstein et al. 1992; Haddow et al. 2014). Instead, white matter abnormalities in HIV have been associated with other medical conditions (e.g., hepatitis C), use of cART, demographic variables (e.g., ethnicity), cardiovascular factors (e.g., hypertension and diabetes), and advancing age (Haddow et al. 2014; Morgello et al. 2014; Soontornniyomkij et al. 2014).
People living with HIV have a greater relative risk for cardiovascular disease and other medical comorbidities, and this frequency increases with age (Islam et al. 2013; Kendall et al. 2014). HIV+ individuals with cerebrovascular risk have increased cognitive difficulties (Foley et al. 2010; Nakamoto et al. 2011). These cognitive difficulties range from mild motor and information-processing abnormalities to severe dysfunction. Cognitive abnormalities tend to impact routine activities of daily living (Morgan et al. 2012). HIV infection most prominently affects the domains of motor functioning, attention, processing speed, executive functioning, and memory (Heaton et al. 1995, 2004).
There is some suggestion that, in general, WMH are related to global cognitive decline, as well as more specific decline in processing speed and executive functioning (Debette and Markus 2010). However, limited data exists to inform the clinical relevance and determinants of WMH in older HIV+ patients. To date, studies that considered the cognitive impact of WMH included subjects with only mild WMH burden (Soontornniyomkij et al. 2014) or a younger sample (Su et al. 2016), in contrast to the burden found in many subjects over age 60, as presented in the current work.
Studies have noted that WMH changes may precede brain atrophy and more distal white matter changes (Debette and Markus 2010; Maillard et al. 2014; Maniega et al. 2015). Indeed, reduced white matter integrity has been found in older HIV+ individuals (Nir et al. 2014). However, an association between WMH and white matter integrity changes has not been explored in HIV. Here we aimed to characterize WMH and its impact on cognitive function in older HIV+ adults compared to healthy HIV− age and sex-matched controls, and investigate associations between WMH and other white matter integrity metrics. We hypothesized that WMH burden would correlate with cardiovascular variables but not measures of HIV severity, and that WMH burden would associate with worse neuropsychological test performance, particularly on tasks that rely on frontostriatal function. In a subset of individuals, we explored the change in WMH volume over time by serostatus to test whether HIV potentiates the speed of change in WMH burden.
Methods
Participants
HIV+ participants over age 60 (n = 65) were recruited from the community. Exclusion criteria were neurological conditions known to impact cognition including stroke, opportunistic brain infection, loss of consciousness for greater than 30 min, or current illicit drug use. Participants also had to be able to speak English and identify a proxy to inform their daily function as it related to cognition. HIV+ participants were recruited regardless of cognitive symptoms. Demographically matched HIV− controls (n = 29) were selected from a healthy aging cohort at UCSF who underwent similar neuropsychological testing and were imaged with the same MRI protocol on the same scanner. All HIV− participants were between 60 and 70 years old. All participants signed UCSF IRB-approved consent forms. This study conforms to the standards set forth by the World Medical Association’s Code of Ethics in the Declaration of Helsinki.
Image acquisition
All MRIs were collected on the same Siemens Magnetom TrioTim 3T scanner, with the following settings: a whole-brain 3D–T1 magnetization prepared rapid acquisition gradient-echo (MPRAGE (T1)) sequence (voxel size =1.0 × 1.0 × 1.0 mm3, repetition time (RT) =2300 ms, echo time (TE) =2.98 ms, flip angle (FA) =9°) and fluid-attenuated inversion recovery (FLAIR, voxel size =1.0 × 1.0 × 1.0 mm3, RT =6000 ms, TE =389 ms, FA =120°). Diffusion sequences were collected for diffusion tensor imaging (DTI) analysis (100 × 100 matrix; FOV =220 mm; 55 slices; voxel size =2.2 × 2.2 × 2.2 mm3; TR =8000 ms; TE =109 ms). Sixty-five separate images were acquired for each subject’s scan: one T2-weighted image with no diffusion sensitization (b0 image) and 64 distinct diffusion-weighted images (DWI; b = 2000 s/mm2).
Image processing
FLAIR images were processed using the FMRIB software library (FSL, http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/) FAST utility to normalize the field bias gradient. The T1 MPRAGE structural images were analyzed using the FreeSurfer image analysis software (http://surfer.nmr.mgh.harvard.edu). To produce skull-stripped FLAIR images, we used the T1-weighted images and processed them through the first six stages of FreeSurfer (recon-all—autorecon1). All skull-stripped T1 images were visually inspected for accuracy with manual corrections when necessary. T1 images were nonlinearly warped to a minimum deformation template using a constrained cubic spline interpolation. The T1 warp parameters were then applied to the FLAIR images to produce warped, skull-stripped FLAIR images (Schwarz et al. 2009).
We quantified WMH using a previously described method (Schwarz et al. 2009). In brief, warped, skull-stripped FLAIR images were processed through a Bayesian Markov Random Field approach to output a volumetrically quantifiable binary WMH mask. WMH volumes and counts per lobe were obtained using inclusive regional masks. After initial automated segmentation through FreeSurfer version 5.1, each subject’s image went through individual quality checks and was manually corrected with a built-in editing software. Cerebral white matter volumes were extracted using the FreeSurfer regions of interest (ROI).
Diffusion images were pre-processed with FSL to create tensor-derived measures of fractional anisotropy (FA), and mean, axial, and radial diffusivity. The FA maps were then registered using the Advance Normalization Tools (ANTs) software package with a mutual information cost function (Tustison et al. 2014) to the ENIGMA-DTI template, and further processed with the ENIGMA-DTI protocols (http://enigma.usc.edu/protocols/dti-protocols/) (Jahanshad et al. 2013; Kochunov et al. 2014) for quality control and to extract regional summary measures on the skeletonized image (Smith et al. 2006). A priori ROIs were determined based on the likelihood that they may be linked to cognitive abilities of interest as follows: corpus callosum (CC), sagittal stratum (SS), anterior limb of the internal capsule (ALIC), posterior limb of the internal capsule (PLIC), and external capsule (EC).
Cardiovascular disease (CVD) risk factors
Diabetes, hypertension, and hypercholesterolemia variables were collected by self-report, medication, and medical chart review, and augmented by clinical data captured during the research visit. A 12-h fasting serum was captured within 3 months of neuroimaging. Diabetes was defined as a fasting glucose level greater than 125 mg/dL or current clinical diagnosis from medical history. Hypertension (HTN) was defined as having a systolic blood pressure >140 mmHg or diastolic >90 mmHg; and hypercholesterolemia as having a fasting cholesterol level >200 mg/dL or current clinical diagnosis from medical history. Body mass index (BMI) was calculated as ((weight (pounds)/height (inches) squared)) ×703). Smoking history was defined as total tobacco use of more than 100 cigarettes in a lifetime with separate analyses examining ever vs. never smokers.
HIV variables
Clinical HIV variables were gathered during a structured physician interview including self-report of HIV duration and nadir HIV CD4+ T-lymphocyte count. CD4+ T-lymphocyte subsets and plasma HIV RNA levels were measured in local laboratories if not captured in clinical care within 3 months of the evaluation. Laboratory values were not available for five HIV+ participants.
Neuropsychological assessment
Participants completed a 90-min neuropsychological test battery that included the national Alzheimer’s Disease Research Center’s (ADRC) uniform dataset, a UCSF Memory and Aging Center Bedside Screen (Delis et al. 2000; Heaton 2004; Kramer et al. 2003; Weintraub et al. 2009), and tests of psychomotor and motor speed, as previously published (Chiao et al. 2013). Raw neuropsychological scores were transformed into z-scores using published or local (grooved pegboard test) age and education stratified normative data. Summary neuropsychological scores (NPZ) for cognitive domains (global, psychomotor speed, attention, memory, visuospatial, and executive functioning) were defined by averaging individual z-scores within each of the domains.
Statistical methods
We compared demographic characteristics of the HIV+ and HIV− groups by chi-squared analysis for categorical variables, one-way analysis of variance for parametric continuous variables and the Kruskal-Wallis test for non-parametric variables. To examine associations between predictors of interest and WMH burden, we employed Pearson correlation coefficients and chi-squared analyses. In general, we used one-way analysis of variance and the Kruskal-Wallis tests initially to examine differences between the HIV+ and HIV− groups; we then used general linear models to assess differences between groups, examine interactions between predictors, and control for predictors that were significantly associated with our outcome variable (p < 0.05) but were not predictors of interest. The raw values for WMH volume were positively skewed, so a square root transformation was applied to the data before use in general linear models. To analyze WMH volume change over time, we used mixed models controlling for the duration between scan acquisition dates. Analyses were two-sided with on overall p value of 0.05 (corrected for multiple comparisons, for multiple domains and other tests, as necessary), using the false discovery rate (Benjamini 1995). Statistical analyses were performed in Stata v 14.1 and SPSS v 22.2.
Results
Demographics
Among the 94 participants enrolled, 65 were HIV+ (Table 1). The HIV+ participant mean age was slightly younger (1.5 years difference, p = 0.012) and they reported fewer years of formal education (1.5 years difference, p < 0.026) than those in the HIV− group. Three HIV+ participants were over the age of 70.
Table 1.
Participant demographics
| HIV+ (n = 61) | HIV− (n = 29) | P value | |
|---|---|---|---|
| Demographics | |||
| Age (years, median; range) | 63; 60–80 | 65; 61–69 | 0.012 |
| Sex (% male) | 90 | 94 | 0.672 |
| Education (mean [SD]) | 16.4 [2.3] | 17.9 [1.8] | 0.026 |
| Ethnicity (% Caucasian) | 92.3 | 96.5 | 0.266 |
| HIV variables | |||
| Duration of HIV (years) | 19.9 [6.6] | – | – |
| CD4 (count/mm3, (mean [SD])) | 534 [219] | – | – |
| Nadir CD4 (cells/mm3, (mean [SD])) | 196 [153] | – | – |
| Plasma HIV RNA (copies/mL) | |||
| ≤50 (n; mean) | 38; − | – | – |
| 51–500 (n; mean) | 15; 183 | – | – |
| ≥500 (n; mean) | 7; 55,736 | – | – |
| On cART (n, %) | 63 [97%] | – | – |
| Vascular factors | |||
| BMI (mean [SD]) | 25.3 [3.9] | 27.3 [4.2] | 0.032 |
| Current or lifetime smoker | 67% | 41% | 0.015 |
| High cholesterol | 63% | 31% | 0.007 |
| Hypertension | 63% | 48% | 0.257 |
| Diabetes mellitus | 11% | 0% | 0.098 |
WMH burden by HIV status
The median WMH volume within the HIV+ group did not differ from that of the HIV− control group (median [range] of 2.78 [0 to 22.34] mL vs. 3.18 [0 to 6.91] mL, respectively (p = 0.687; Fig. 1). This finding remained after controlling for age, education, and total cerebral white matter volume (CWMV) (p = 0.198). However, HIV+ participants had lower total CWMV compared to HIV− controls (mean difference =26.68 mL; p = 0.010), a finding independent of age (p = 0.117) or education (p = 0.459). In multivariable models, covariates that met our threshold for inclusion as WMH volume predictors (e.g., those with p < 0.05) were age, education, and CWMV (coefficients =.054, −.103, and .003; p = 0.027, 0.007, and 0.043, respectively), but not HIV serostatus (coefficients =0.224; p = 0.251). Excluding HIV+ participants over the age of 70 did not alter the outcomes of the analyses.
Fig. 1.
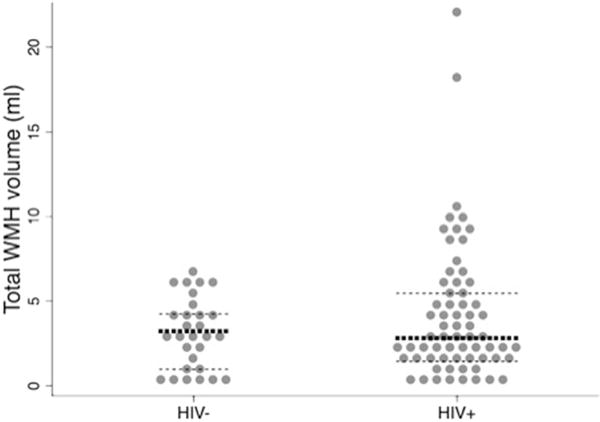
WMH burden by HIV serostatus 15/65 cases (23%) of HIV+ participants had a total WMH volume >95th percentile of controls (dark lines median, light lines =25th/75th percentile)
Given the skewed distribution of WMH (Fig. 1), we then examined the distribution by group noting that the number of cases at the upper extremes differed by HIV status with 24/65 (37%) of HIV+ participants having WMH volumes that were >75th percentile of controls and 15/65 (23%) having a burden greater than the 95th percentile of controls (p < 0.091). The distribution of the proportion of WMH volume per white matter volume in each lobe (e.g., frontal WMH/frontal CWMV) was highest in the occipital lobe (1.8%), then parietal (0.9%), frontal (0.7%), and lastly temporal (0.4%). We identified no difference in lobar volume of WMH or distribution of WMH volumes by serostatus in a paired analysis.
We examined correlations between WMH volume and WMH counts (i.e., number of lesions) noting strong associations for HIV+ (r = 0.897, p < 0.001) and HIV− control groups (r = 0.735, p < 0.001). Visually, we noted a variable pattern of WMH distribution among participants. Some had prominent periventricular hyperintensities that included capping (white matter signal abnormalities that abut the ventricles, and are particularly prominent around the ventricular horns) and some discrete and deep white matter lesions (distribution “typical” of cerebrovascular disease (CVD)). Others had only discrete deep white matter lesions with little or no periventricular capping (“atypical” of CVD, Fig. 2) (Yoshita et al. 2006). Roughly, 41% of HIV+ and 48% of HIV− participants had white matter lesion distributions classified as mostly “typical” of CVD, 32% of HIV+, and 21% of HIV− participants had mostly “atypical” white matter lesion distributions. The remaining participants had mixed distribution types. Groups did not significantly differ in the type of white matter lesion distribution. However, of the participants who had the largest WMH volumes (>75th percentile), HIV+ participants were more likely to have an “atypical” distribution (p = 0.038).
Fig. 2.
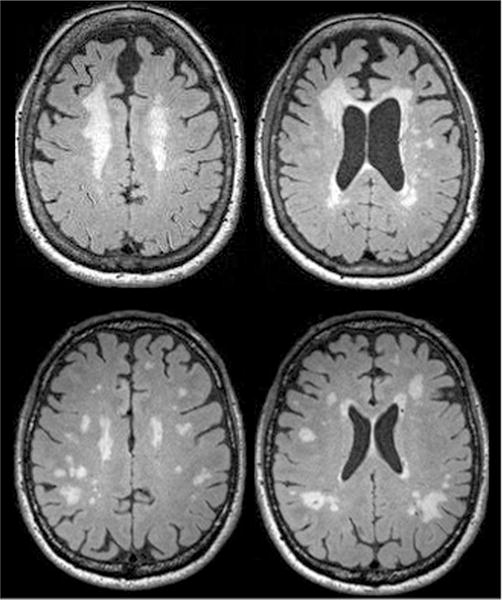
Representative examples of distributions thought to be “typical” (top) and atypical (bottom) of cerebrovascular disease
Cognitive measures and WMH volumes
The HIV+ group performed worse than controls on cognitive measures: global cognition (difference =−0.752, p < 0.001), memory (difference =−0.942, p < 0.001), visuospatial (difference =−0.628, p < 0.001), executive functioning (difference = −0.665, p < 0.001) and attention (difference =−0.144, p < 0.001), and psychomotor speed (difference =−0.497, p = 0.012). After controlling for CWMV, total WMH volumes inversely correlated to global cognition (coefficient =−0.172, p = 0.035), executive functioning (coefficient =−0.328, p = 0.001), and psychomotor speed (coefficient =−0.331, p = 0.009). HIV serostatus did not modify the effect of WMH volume on cognitive measures. Only executive functioning was significantly associated with CWMV (coefficient =0.003, p = 0.042).
Associations between CVD risk factors, HIV severity variables, and WMH volume
We identified a greater burden of total WMH volume in participants with hypertension in a model with both groups combined (median total WMH volume: hypertension =3.52 mL, without hypertension =2.30 mL, p = 0.021). There was no detectable interaction of hypertension and HIV serostatus on total WMH volume (p > 0.2). No other cardiovascular risk factors (BMI, hypercholesterolemia, smoking history, current blood pressure, or diabetes) were associated with WMH volumes or HIV serostatus interactions. In the HIV+ group, we did not identify significant correlations between total WMH volumes and key HIV variables including plasma HIV RNA (p = 0.330), proximal CD4 T-lymphocyte count (p = 0.849), nadir CD4 T-lymphocyte count (p = 0.580), or estimated duration of HIV infection (p = 0.800). Adding use of a protease inhibitor (current, past, or never) to these models did not change the results. However, CWMV was significantly related to estimated duration of HIV infection (coefficient =1.83, p = 0.032) and current CD4 count (coefficient =0.06, p = 0.016). There were no other significant relationships between CWMV and HIV virus variables or cardiovascular risk factors.
WMH volumes and DTI
We identified correlations between WMH volume and white matter integrity by DTI. When age, HIV status, and total WMH volumes were entered into a model to predict regional fractional anisotropy, total WMH volumes were significant (p = 0.005), whereas age and education were not. Total WMH volume predicted FA in two priori chosen regions of interest: corpus callosum (coefficient =−0.010, p = 0.012) and sagittal stratum ((SS): coefficient =−0.016, p = 0.001). Total WMH volumes were marginally associated with fractional anisotropy in the external capsule (EC, p = 0.057), but were unrelated to the anterior limb of the internal capsule (p = 0.112) or posterior limb of the internal capsule (PLIC, p = 0.745).
Longitudinal evaluation of WMH
Among individuals with more than one MRI (HIV−: n = 22; HIV+: n = 28), the duration between scans was similar for HIV+ and HIV− participants (mean [SD] = 2.13 [.189] vs. 1.68 [.164] years; p = 0.09). Total brain WMH volumes increased with time [median change (range) =0.90 [−2.52–9.05] mL] as did lobar WMH volumes (frontal: median change (range) =0.163 (−0.491–1.884) mL, temporal: median change (range) =0.079 (−0.43–0.68) mL, parietal: median change (range) =0.087 (−1.03–3.35) mL, occipital: median change (range) =0.14 (−0.63–1.99) mL; all p’s ≤ 0.001). Using a mixed model that evaluated WMH volume change over time, initial WMH volume (p < 0.001) and age (p = 0.036) were significant covariates; hypertension and HIV serostatus were not significant covariates (p’s > .14).
Discussion
In this study, we found similar median WMH volumes across serostatus groups despite smaller total CWMV in the HIV+ group, which suggests a greater ratio of abnormal to normal white matter in HIV+ adults over 60 years old. We identified a subset of HIV+ individuals over age 60 with a substantially high burden of WMH. Our analyses and careful inspection of individual cases would suggest that the pathophysiology for cases above the 75th percentile was similar to other cases and not differing by serostatus; but, it remains possible that there is a unique contribution to the underlying pathophysiology for the HIV+ group in these extreme cases. Notably, this was not driven by confluent white matter changes by visual inspection, but, instead by the sum of smaller discrete lesions, since those with greatest burden were more commonly noted to have an “atypical” distribution. A larger sample would be needed to delineate this accurately.
We also sought to understand the clinical relevance of these WMH. As expected, our HIV+ group performed worse than the HIV− group on global cognitive measures and in specific domains including memory, visuospatial skills, executive functioning, attention, and psychomotor speed. WMH volumes were inversely related to global cognition, psychomotor speed, and executive functioning; and these relationships were similar across serostatus groups. This is consistent with published work revealing associations between white matter changes and cognitive difficulties (McMurtray et al. 2007; Ovbiagele and Saver 2006). As WMH volumes were greatest in the frontal lobes, our findings are consistent with the well accepted associations between frontostriatal white matter abnormalities (Ipser et al. 2015; Pfefferbaum et al. 2009) and associated frontal-executive cognitive deficits in HIV+ individuals (Plessis et al. 2014).
Among the correlates, we aimed to investigate pathological mechanisms; only hypertension reached a threshold of significance. Other factors (e.g., diabetes mellitus, body mass index, smoking history, and hypercholesterolemia) were not associated with WMH volume in this sample. We may have lacked power for these investigations given the relatively good health of our sample (e.g., only 11% of HIV+ participants had diabetes), a smaller control sample, and a lack of African American participants, a population where these associations have been reported (Haddow et al. 2014; Nyquist et al. 2014). Previous investigations have revealed relationships between white matter signal abnormality and both older age and cerebrovascular risk factors (McMurtray et al. 2008). In these other studies, like ours, no associations were identified with HIV disease biomarkers (McMurtray et al. 2007). The sum of the literature would thus suggest that typical cerebrovascular factors rather than HIV factors are more important, a finding that has important clinical implications related to prevention.
To extend these findings to brain architecture, we examined the relationship between WMH burden and white matter integrity by DTI, assessing brain regions on DTI that did not have overt white matter lesions. Independent of age and HIV status, greater WMH volumes were related to lower measures of white matter integrity in the corpus callosum and sagittal stratum, and marginally related to the external capsule. Thus, the WMH burden may be an extreme manifestation of broader and more subtle brain injury as measured through DTI. Other recent studies report this phenomenon as well, and suggest that WMH are mechanistically associated with more diffuse white matter degradation (Maniega et al. 2015). These findings are consistent with a model where white matter changes occur along a continuum in which WMH are the most advanced form, or, at the very least, that there are shared pathologies (Maillard et al. 2014). However, what remains to be answered is if several small discrete lesions have the same cognitive impact as one or two large confluent lesions, and what are the common and distinct mechanisms that underlie the two types of lesions.
In a separate study of these same study participants, we found pervasive white matter microstructural defects in HIV+ older adults compared to controls and inverse associations with neuropsychological testing performance (Nir et al. 2014). Others have reported that CD4 count, viral load, and other blood and CSF markers were associated with reduced white matter integrity (Wright et al. 2015), indicating that, unlike our study of WMH, alternative HIV-related pathways may underlie white matter changes on DTI. We found associations between CMWV, CD4 count, and duration of HIV infection, which is similar to past studies of abnormal white matter in HIV (Jernigan et al. 2011). This suggests that there are distinct or parallel biological processes that impact cerebral white matter integrity and white matter volume from those that influence WMH volume.
Unexpectedly, we found a positive relationship between CWMV and duration of HIV infection, independent of age. Increased white matter volume due to white matter edema has been reported in patients with CADASIL (De Guio et al. 2015); a similar but less severe process may also be present in older adults who have lived with the HIV virus for longer periods of time. Alternatively, this may represent a survival bias with those living for more than two decades with HIV having resilience features. Future studies that examine the relationship between duration of HIV infection and gray matter volume and change over time may help to clarify this finding—the absence of which is a limitation of this study.
Recent studies imply that older age may increase WMH burden, but our study was not designed to address this issue as we had a limited age range, enrolling only participants over age 60. Others identify age effects; although it is less clear if age itself or the duration of risk factors (e.g., hypertension) is most important (Seider et al. 2015). The main contributing factor is unlikely to be HIV disease duration as we found no association with HIV disease duration and total WMH volume. Additionally, our HIV− group was 1.5 years older than our HIV+ group, which may increase the chance of a type II error with respect to an interaction between age and HIV effects. Arterial remodeling is a pathological process that occurs along a continuum and may be a candidate for a progressive pathological state that may accumulate damage over time (Gutierrez et al. 2015).
Our study did not identify a synergistic impact of HIV on other variables related to brain WMHs, or a reciprocal relationship between HIV and associated variables (such as cerebrovascular risks or cART medication effects). We examined if current, past, or never use of a protease inhibitor had an impact on our findings, based on a recent report linking cerebrovascular disease to protease inhibitor use (Soontornniyomkij et al. 2014). However, we have limited ability to examine in more depth the role of individual antiretrovirals or class of antiretroviral medications, given that the WMH observed likely developed over years and detailed antiretroviral therapy histories are not available in the sample. Instead, we found that initial WMH volume and age were the most important contributors to WMH volume change.
In ad hoc analyses, we found an inverse relationship between WMH volumes and education (r = −0.28, p = 0.006). There is limited and conflicting evidence on the impact of education on WMH burden (Mortamais et al. 2014), and our coincidental finding points to the need for further exploration on the impact of cognitive reserve, and mediation analyses with life style factors that are related to education and impact on white matter, such as diet and exercise.
The strengths of this paper include a quantitative approach to study WMH volumes, which provides better sensitivity and reliability than qualitative approaches (Schwarz et al. 2009). Additionally, our study included two points to detect change over time. We also showed a relationship with cognition, which increases the clinical significance of WMH in HIV. Finally, our study includes individuals over age 60, while prior research had limited sampling of individuals in this somewhat older range.
In summary, our HIV+ group had greater WMH burden (ratio of WMH volume to CWMV) overall than the HIV− group despite similar total WMH volume. WMH were inversely related to global cognitive performance, and measures of executive function and psychomotor speed. Hypertension was the only significant cardiovascular factor to associate with WMH volume; HIV severity variables were not detectably associated. WMH volumes inversely correlated with FA in white matter areas not directly impacted by white matter lesions, suggesting more distal effects. WMH volumes changed significantly and similarly over time for both HIV+ and HIV− groups. Age and initial WMH volume were significant predictors of change in WMH volume over time.
Acknowledgments
National Institutes of Health grants: K23 AG032872 (VV), P30 AG010129 (CD), K01 AG030514 (OC); The Larry L. Hillblom Foundation; the University of California, San Francisco/Gladstone Institute of Virology and Immunology Center For Acquired Immune Deficiency Syndrome Research; the University of California, San Francisco Acquired Immune Deficiency Syndrome Research Institute, K24MH098759 (VV) and P50-AG023501 (BM). Additional support from the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through the University of California, San Francisco-Clinical and Translational Science Institute Grant No. UL1 RR024131.
Footnotes
Compliance with ethical standards
Disclosures Dr. Valcour has served as a consultant for ViiV Healthcare and Merck related to aging and HIV. The authors declare that they have no conflict of interest.
References
- Benjamini YHY. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc. 1995;57:289–300. [Google Scholar]
- Boone KB, Miller BL, Lesser IM, Mehringer CM, Hill-Gutierrez E, Goldberg MA, Berman NG. Neuropsychological correlates of white-matter lesions in healthy elderly subjects. A threshold effect. Arch Neurol. 1992;49:549–554. doi: 10.1001/archneur.1992.00530290141024. [DOI] [PubMed] [Google Scholar]
- Bornstein RA, Chakeres D, Brogan M, Nasrallah HA, Fass RJ, Para M, Whitacre C. Magnetic resonance imaging of white matter lesions in HIV infection. J Neuropsychiatry Clin Neurosci. 1992;4:174–178. doi: 10.1176/jnp.4.2.174. [DOI] [PubMed] [Google Scholar]
- Chiao S, Rosen HJ, Nicolas K, Wendelken LA, Alcantar O, Rankin KP, Miller B, Valcour V. Deficits in self-awareness impact the diagnosis of asymptomatic neurocognitive impairment in HIV. AIDS Res Hum Retrovir. 2013;29:949–956. doi: 10.1089/aid.2012.0229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Guio F, Mangin JF, Duering M, Ropele S, Chabriat H, Jouvent E. White matter edema at the early stage of cerebral autosomal-dominant arteriopathy with subcortical infarcts and leukoencephalopathy. Stroke. 2015;46:258–261. doi: 10.1161/STROKEAHA.114.007018. [DOI] [PubMed] [Google Scholar]
- Debette S, Markus HS. The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: systematic review and meta-analysis. BMJ. 2010;341:c3666. doi: 10.1136/bmj.c3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test—second edition. Manual Psychological Corporation; San Antonio: 2000. Adult version. [Google Scholar]
- Fern RF, Matute C, Stys PK. White matter injury: ischemic and nonischemic. Glia. 2014;62:1780–1789. doi: 10.1002/glia.22722. [DOI] [PubMed] [Google Scholar]
- Foley J, Ettenhofer M, Wright MJ, Siddiqi I, Choi M, Thames AD, Mason K, Castellon S, Hinkin CH. Neurocognitive functioning in HIV-1 infection: effects of cerebrovascular risk factors and age. Clin Neuropsychol. 2010;24:265–285. doi: 10.1080/13854040903482830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez J, Goldman J, Dwork AJ, Elkind MSV, Marshall RS, Morgello S. Brain arterial remodeling contribution to nonembolic brain infarcts in patients with HIV. Neurology. 2015;85:1139–1145. doi: 10.1212/WNL.0000000000001976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddow LJ, Dudau C, Chandrashekar H, Cartledge JD, Hyare H, Miller RF, Jager HR. Cross-sectional study of unexplained white matter lesions in HIV positive individuals undergoing brain magnetic resonance imaging. AIDS Patient Care STDs. 2014;28:341–349. doi: 10.1089/apc.2013.0230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK. Revised comprehensive norms for an expanded Halstead-Reitan battery: demographically adjusted neuropsychological norms for African American and Caucasian adults, professional manual. Psychological Assessment Resources; Lutz: 2004. [Google Scholar]
- Heaton RK, Grant I, Butters N, White DA, Kirson D, Atkinson JH, McCutchan JA, Taylor MJ, Kelly MD, Ellis RJ, et al. The HNRC 500—neuropsychology of HIV infection at different disease stages. HIV Neurobehavioral Research Center J Int Neuropsychol Soc. 1995;1:231–251. doi: 10.1017/s1355617700000230. [DOI] [PubMed] [Google Scholar]
- Heaton RK, Marcotte TD, Mindt MR, Sadek J, Moore DJ, Bentley H, McCutchan JA, Reicks C, Grant I, Group H The impact of HIV-associated neuropsychological impairment on everyday functioning. J Int Neuropsychol Soc. 2004;10:317–331. doi: 10.1017/S1355617704102130. [DOI] [PubMed] [Google Scholar]
- Ipser JC, Brown GG, Bischoff-Grethe A, Connolly CG, Ellis RJ, Heaton RK, Grant I, Translational Methamphetamine ARCG HIV infection is associated with attenuated frontostriatal intrinsic connectivity: a preliminary study. J Int Neuropsychol Soc. 2015;21:203–213. doi: 10.1017/S1355617715000156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam MM, Topp L, Conigrave KM, Haber PS, White A, Day CA. Sexually transmitted infections, sexual risk behaviours and perceived barriers to safe sex among drug users. Aust N Z J Public Health. 2013;37:311–315. doi: 10.1111/1753-6405.12077. [DOI] [PubMed] [Google Scholar]
- Jahanshad N, Kochunov PV, Sprooten E, Mandl RC, Nichols TE, Almasy L, Blangero J, Brouwer RM, Curran JE, de Zubicaray GI, Duggirala R, Fox PT, Hong LE, Landman BA, Martin NG, McMahon KL, Medland SE, Mitchell BD, Olvera RL, Peterson CP, Starr JM, Sussmann JE, Toga AW, Wardlaw JM, Wright MJ, Hulshoff Pol HE, Bastin ME, McIntosh AM, Deary IJ, Thompson PM, Glahn DC. Multi-site genetic analysis of diffusion images and voxelwise heritability analysis: a pilot project of the ENIGMA-DTI working group. NeuroImage. 2013;81:455–469. doi: 10.1016/j.neuroimage.2013.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jernigan TL, Archibald SL, Fennema-Notestine C, Taylor MJ, Theilmann RJ, Julaton MD, Notestine RJ, Wolfson T, Letendre SL, Ellis RJ, Heaton RK, Gamst AC, Franklin DR, Jr, Clifford DB, Collier AC, Gelman BB, Marra C, McArthur JC, McCutchan JA, Morgello S, Simpson DM, Grant I, Group C Clinical factors related to brain structure in HIV: the CHARTER study. J Neurovirol. 2011;17:248–257. doi: 10.1007/s13365-011-0032-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendall CE, Wong J, Taljaard M, Glazier RH, Hogg W, Younger J, Manuel DG. A cross-sectional, population-based study measuring comorbidity among people living with HIV in Ontario. BMC Public Health. 2014;14:161. doi: 10.1186/1471-2458-14-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochunov P, Jahanshad N, Sprooten E, Nichols TE, Mandl RC, Almasy L, Booth T, Brouwer RM, Curran JE, de Zubicaray GI, Dimitrova R, Duggirala R, Fox PT, Hong LE, Landman BA, Lemaitre H, Lopez LM, Martin NG, McMahon KL, Mitchell BD, Olvera RL, Peterson CP, Starr JM, Sussmann JE, Toga AW, Wardlaw JM, Wright MJ, Wright SN, Bastin ME, McIntosh AM, Boomsma DI, Kahn RS, den Braber A, de Geus EJ, Deary IJ, Hulshoff Pol HE, Williamson DE, Blangero J, van’t Ent D, Thompson PM, Glahn DC. Multisite study of additive genetic effects on fractional anisotropy of cerebral white matter: comparing meta and megaanalytical approaches for data pooling. NeuroImage. 2014;95:136–150. doi: 10.1016/j.neuroimage.2014.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer JH, Jurik J, Sha SJ, Rankin KP, Rosen HJ, Johnson JK, Miller BL. Distinctive neuropsychological patterns in frontotemporal dementia, semantic dementia, and Alzheimer disease. Cogn Behav Neurol. 2003;16:211–218. doi: 10.1097/00146965-200312000-00002. [DOI] [PubMed] [Google Scholar]
- Maillard P, Fletcher E, Lockhart SN, Roach AE, Reed B, Mungas D, DeCarli C, Carmichael OT. White matter hyperintensities and their penumbra lie along a continuum of injury in the aging brain. Stroke. 2014;45:1721–1726. doi: 10.1161/STROKEAHA.113.004084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniega SM, Valdes Hernandez MC, Clayden JD, Royle NA, Murray C, Morris Z, Aribisala BS, Gow AJ, Starr JM, Bastin ME, Deary IJ, Wardlaw JM. White matter hyperintensities and normal-appearing white matter integrity in the aging brain. Neurobiol Aging. 2015;36:909–918. doi: 10.1016/j.neurobiolaging.2014.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McArthur JC, Kumar AJ, Johnson DW, Selnes OA, Becker JT, Herman C, Cohen BA, Saah A. Incidental white matter hyperintensities on magnetic resonance imaging in HIV-1 infection. Multicenter AIDS Cohort Study J Acquir Immune Defic Syndr. 1990;3:252–259. [PubMed] [Google Scholar]
- McMurtray A, Nakamoto B, Shikuma C, Valcour V. Small-vessel vascular disease in human immunodeficiency virus infection: the Hawaii aging with HIV cohort study. Cerebrovasc Dis. 2007;24:236–241. doi: 10.1159/000104484. [DOI] [PubMed] [Google Scholar]
- McMurtray A, Nakamoto B, Shikuma C, Valcour V. Cortical atrophy and white matter hyperintensities in HIV: the Hawaii Aging with HIV Cohort Study. J Stroke Cerebrovasc Dis. 2008;17:212–217. doi: 10.1016/j.jstrokecerebrovasdis.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan EE, Woods SP, Grant I, Group HIVNRP Intra-individual neurocognitive variability confers risk of dependence in activities of daily living among HIV-seropositive individuals without HIV-associated neurocognitive disorders. Arch Clin Neuropsychol. 2012;27:293–303. doi: 10.1093/arclin/acs003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgello S, Murray J, Van Der Elst S, Byrd D. HCV, but not HIV, is a risk factor for cerebral small vessel disease. Neurol Neuroimmunol Neuroinflamm. 2014;1:e27. doi: 10.1212/NXI.0000000000000027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortamais M, Portet F, Brickman AM, Provenzano FA, Muraskin J, Akbaraly TN, Berr C, Touchon J, Bonafe A, le Bars E, Menjot de Champfleur N, Maller JJ, Meslin C, Sabatier R, Ritchie K, Artero S. Education modulates the impact of white matter lesions on the risk of mild cognitive impairment and dementia. Am J Geriatr Psychiatry. 2014;22:1336–1345. doi: 10.1016/j.jagp.2013.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamoto BK, Valcour VG, Kallianpur K, Liang CY, McMurtray A, Chow D, Kappenburg E, Shikuma CM. Impact of cerebrovascular disease on cognitive function in HIV-infected patients. J Acquir Immune Defic Syndr. 2011;57:e66–e68. doi: 10.1097/QAI.0b013e31821ff8bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nir TM, Jahanshad N, Busovaca E, Wendelken L, Nicolas K, Thompson PM, Valcour VG. Mapping white matter integrity in elderly people with HIV. Hum Brain Mapp. 2014;35:975–992. doi: 10.1002/hbm.22228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyquist PA, Bilgel MS, Gottesman R, Yanek LR, Moy TF, Becker LC, Cuzzocreo J, Prince J, Yousem DM, Becker DM, Kral BG, Vaidya D. Extreme deep white matter hyperintensity volumes are associated with African American race. Cerebrovasc Dis. 2014;37:244–250. doi: 10.1159/000358117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovbiagele B, Saver JL. Cerebral white matter hyperintensities on MRI: current concepts and therapeutic implications. Cerebrovasc Dis. 2006;22:83–90. doi: 10.1159/000093235. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Rosenbloom MJ, Rohlfing T, Kemper CA, Deresinski S, Sullivan EV. Frontostriatal fiber bundle compromise in HIV infection without dementia. AIDS. 2009;23:1977–1985. doi: 10.1097/QAD.0b013e32832e77fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plessis SD, Vink M, Joska JA, Koutsilieri E, Stein DJ, Emsley R. HIV infection and the fronto-striatal system: a systematic review and meta-analysis of fMRI studies. AIDS. 2014;28:803–811. doi: 10.1097/QAD.0000000000000151. [DOI] [PubMed] [Google Scholar]
- Pomara N, Crandall DT, Choi SJ, Johnson G, Lim KO. White matter abnormalities in HIV-1 infection: a diffusion tensor imaging study. Psychiatry Res. 2001;106:15–24. doi: 10.1016/s0925-4927(00)00082-2. [DOI] [PubMed] [Google Scholar]
- Schwarz C, Fletcher E, DeCarli C, Carmichael O. Fully-automated white matter hyperintensity detection with anatomical prior knowledge and without FLAIR. Inf Process Med Imaging. 2009;21:239–251. doi: 10.1007/978-3-642-02498-6_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seider TR, Gongvatana A, Woods AJ, Chen H, Porges EC, Cummings T, Correia S, Tashima K, Cohen RA. Age exacerbates HIV-associated white matter abnormalities. J Neurovirol. 2015 doi: 10.1007/s13365-015-0386-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoamanesh A, Preis SR, Beiser AS, Vasan RS, Benjamin EJ, Kase CS, Wolf PA, DeCarli C, Romero JR, Seshadri S. Inflammatory biomarkers, cerebral microbleeds, and small vessel disease: Framingham Heart Study. Neurology. 2015;84:825–832. doi: 10.1212/WNL.0000000000001279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, Watkins KE, Ciccarelli O, Cader MZ, Matthews PM, Behrens TE. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. NeuroImage. 2006;31:1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Soontornniyomkij V, Umlauf A, Chung SA, Cochran ML, Soontornniyomkij B, Gouaux B, Toperoff W, Moore DJ, Masliah E, Ellis RJ, Grant I, Achim CL. HIV protease inhibitor exposure predicts cerebral small vessel disease. AIDS. 2014;28:1297–1306. doi: 10.1097/QAD.0000000000000262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su T, Wit FW, Caan MW, Schouten J, Prins M, Geurtsen GJ, Cole JH, Sharp DJ, Richard E, Reneman L. White matter hyperintensities in relation to cognition in HIV-infected men with sustained suppressed viral load on cART. AIDS. 2016 doi: 10.1097/QAD.0000000000001133. [DOI] [PubMed] [Google Scholar]
- Tustison NJ, Cook PA, Klein A, Song G, Das SR, Duda JT, Kandel BM, van Strien N, Stone JR, Gee JC, Avants BB. Large-scale evaluation of ANTs and FreeSurfer cortical thickness measurements. NeuroImage. 2014;99:166–179. doi: 10.1016/j.neuroimage.2014.05.044. [DOI] [PubMed] [Google Scholar]
- Weintraub S, Salmon D, Mercaldo N, Ferris S, Graff-Radford NR, Chui H, Cummings J, DeCarli C, Foster NL, Galasko D, Peskind E, Dietrich W, Beekly DL, Kukull WA, Morris JC. The Alzheimer’s disease centers’ uniform data set (UDS): the neuropsychologic test battery. Alzheimer Dis Assoc Disord. 2009;23:91–101. doi: 10.1097/WAD.0b013e318191c7dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright PW, Vaida FF, Fernandez RJ, Rutlin J, Price RW, Lee E, Peterson J, Fuchs D, Shimony JS, Robertson KR, Walter R, Meyerhoff DJ, Spudich S, Ances BM. Cerebral white matter integrity during primary HIV infection. AIDS. 2015;29:433–442. doi: 10.1097/QAD.0000000000000560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ylikoski A, Erkinjuntti T, Raininko R, Sarna S, Sulkava R, Tilvis R. White matter hyperintensities on MRI in the neurologically nondiseased elderly. Analysis of cohorts of consecutive subjects aged 55 to 85 years living at home. Stroke. 1995;26:1171–1177. doi: 10.1161/01.str.26.7.1171. [DOI] [PubMed] [Google Scholar]
- Yoshita M, Fletcher E, Harvey D, Ortega M, Martinez O, Mungas DM, Reed BR, DeCarli CS. Extent and distribution of white matter hyperintensities in normal aging, MCI, and AD. Neurology. 2006;67:2192–2198. doi: 10.1212/01.wnl.0000249119.95747.1f. [DOI] [PMC free article] [PubMed] [Google Scholar]


