Abstract
In the present investigation, pentacyclic triterpenoids were extracted from different parts of Swertia chirata by solid–liquid reflux extraction methods. The total pentacyclic triterpenoids (UA, OA, and BA) in extracted samples were determined by HPTLC method. Preliminary studies showed that stem part contains the maximum pentacyclic triterpenoid and was chosen for further studies. Response surface methodology (RSM) has been employed successfully by solid–liquid reflux extraction methods for the optimization of different extraction variables viz., temperature (X1 35–70 °C), extraction time (X2 30–60 min), solvent composition (X3 20–80%), solvent-to-solid ratio (X4 30–60 mlg−1), and particle size (X5 3–6 mm) on maximum recovery of triterpenoid from stem parts of Swertia chirata. A Plackett–Burman design has been used initially to screen out the three extraction factors viz., particle size, temperature, and solvent composition on yield of triterpenoid. Moreover, central composite design (CCD) was implemented to optimize the significant extraction parameters for maximum triterpenoid yield. Three extraction parameters viz., mean particle size (3 mm), temperature (65 °C), and methanol–ethyl acetate solvent composition (45%) can be considered as significant for the better yield of triterpenoid A second-order polynomial model satisfactorily fitted the experimental data with the R2 values of 0.98 for the triterpenoid yield (p < 0.001), implying good agreement between the experimental triterpenoid yield (3.71%) to the predicted value (3.79%).
Keywords: Swertia chirata, Pentacyclic triterpenoids, RSM, Solid–liquid extraction, HPTLC
Introduction
Swertia chirata (Family: Gentianaceae) is mainly present at the higher altitudes of tropical Asia, America, Africa, and Europe regions, extensively used in traditional medicine for curing various ailments (Negi et al. 2011). Swertia is one of the most prized herbal drugs and its medical practice is also stated in Indian, UK, and US pharmacopoeias (Anonymous 1976). Swertia chirata is widely used for blood purification, skin diseases, malarial fever, dropsy, digestive, cough medicine, stimulating the heart, inhibiting cancer, scanty urine, laxative, febrifuge, dyspepsia anthelmintic, carminative, antidiarrhoeic, antiperiodic, asthma, etc. (Kumar et al. 2010; Negi et al. 2011; Khanal et al. 2014; Kumar and Staden 2015; Mahendran and Bai 2014). The medicinal value of Swertia chirata is due to the presence of some important bio-active compounds, such as secoiridoids, xanthonoids, triterpenoids etc. Among them, amarogentin and swertiamarin (Bhandari et al. 2006; Gupta et al. 2011; Samaddar et al. 2013), mangiferin (Pandey et al. 2012), oleanolic acid, and ursolic acid (Gao et al. 2015 ; Kshirsagar et al. 2015) are the most important bio-active compounds that have been considered as significant phytochemical markers and leading keys for the authentication of Swertia chirata.
Nowadays, pentacyclic triterpenoids viz., ursolic acid (UA), oleanolic acid (OA) and betulinic acid (BA) are in great demands due to its application in food and pharmaceutical industries. Recently, Silva et al. (2016) reported OA, UA, and BA as food supplements and pharmaceutical agents to cure Diabetes type 2. Besides potent drugs to cure diabetes, oleanolic acid (OA) exhibits anti-microbial (Jesus et al. 2015), anti-tumor (Soica et al. 2014) anti-inflammatory, and antioxidant activities (Liu 1995). Moreover, ursolic acid (UA) exhibits anti-microbial (Jesus et al. 2015), anti-tumor (Bonaccorsi et al. 2008; Soica et al. 2014) activities and betulinic acid (BA) shows anti-HIV, anti-malarial, and anti-cancer activities (Yogeeswari and Sriram 2005; Gheorgheosu et al. 2014). UA and OA are also known to inhibit prostate carcinoma, hepatocellular carcinoma, cervical carcinoma, lung carcinoma, and enhancing cellular immune response (Liu 1995; Yang et al. 2013).
Thus, pentacyclic triterpenoids viz., UA, OA, and BA are industrially important plant-derived compounds, so efficient extraction is the first major step in the recovery, and refinement of these bio-active compounds. Extraction of these compounds was carried out using different conventional solid–liquid extraction techniques like soxhlet method (Gopal et al. 2014), reflux method (Gao et al. 2015), and cold extraction (Kshirsagar et al. 2015). Moreover, quality of herbal medicines has been affected by various extraction parameters such as temperature, extraction time, type of solvent used, composition of different solvents, number of extraction steps, solid-to-liquid ratio, and mean particle size (Gad et al. 2013). In Swertia and other medicinal plants, different solvents such as methanol (Kshirsagar et al. 2015; Gao et al. 2015; Gopal et al. 2014; Gupta et al. 2011; Li et al. 2011) and ethanol (Verma et al. 2013) have been used for the extraction of UA, OA, and BA.
Optimization of extraction parameter by one factor at a time is time consuming, laborious, and inaccurate due to ignorance of the interactive effect of extraction factors on extraction yield. It is, therefore, absolutely needed to optimize the solid–liquid extraction technique recovering better yield of triterpenoids. Response surface methodology (RSM) is a statistical experimental protocol used in mathematical modeling for optimization of extraction and other bioprocess, where multiple variables or responses are present in the experimental design. In the last few years. Response surface methodology (RSM) has been extensively applied in the standardization of various extraction and bioprocesses techniques (Bai et al. 2010; Cheok et al. 2012; Liang et al. 2013; Sheng et al. 2013; Wang et al. 2014; Alberti et al. 2014; Ilaiyaraja et al. 2015; Jacob et al. 2016; Ameer et al. 2017). Therefore, RSM has been evidenced as an efficient modern tool for the screening of significant multiple variables at a time and to check the interactions between independent variables.
The present study was undertaken to optimize the level of various extraction parameters viz., extraction time, temperature, particle size, solvent composition, and solvent–solid ratio to maximize the recovery of pentacyclic triterpenoids from S. chirata stem.
Materials and methods
Plant material and chemicals
The whole plant material of S. chirata (vegetative phase) was collected from Chakrata district (Uttrakhand). Plants were authenticated by a taxonomist expert. The plant material was washed with tap water and shade dried and stored at room temperature. Before extraction, the plant materials were separated into roots, stem and leaves and were ground separately in an electric grinder.
Reagents, i.e., Hexane, Ethyl Acetate, Acetone, Ethanol, Sulphuric acid, chloroform etc., were of HPLC grade that have been procured from Sigma-Aldrich company (USA). The authentic marker compounds, i.e., UA, OA, and BA (> 98% purity), were also obtained from Sigma-Aldrich (USA). The methanol used for extraction is of AR grade (Analytical grade). The stock solutions of each marker were stored at 4 °C after prepared in methanol solvent (1 mg/ml).
Extraction of triterpenoid
Preliminary experiment was conducted for screening of potent part containing triterpenoids. The dried plant part, i.e., root, stem, and leaves 1 g each, were extracted using 50 ml of methanol solvent by heat reflux method to screen the potent part containing pentacyclic triterpenoids. To assess the effect of solid–liquid extraction on the yield of triterpenoid, experiments were done using different extraction variables viz., temperature (X1 35–70 °C), extraction time (X2 30–60 min), solvent composition (X3 20–80%), solvent-to-solid ratio (X4 30–60 ml g−1), and particle size (X5 3–6 mm). Dry stem powder (1.0 g) was placed in a 50 ml round bottom flask and extraction was done by reflux method in temperature controlled hot water bath under different extraction conditions (temperature, different solvent mixtures, mean particle size, and solid–liquid ratio).
All samples were concentrated and dried under vacuum and finally kept at 4 °C in refrigerator for further analysis. Stock solutions of 10 mg OA, UA, and BA were standardized and prepared in 10 mL of methanol solvent.
HPTLC method for estimation of triterpenoid content
The amount of OA, UA, and BA was estimated by the modified method of Sethiya and Mishra (2015) using HPTLC. The HPTLC system comprised of a CAMAG (Muttenz, Switzerland) Linomat-5 automatic sample applicator and CATS software (version: 1.4.4.6337) CAMAG TLC scanner-3. The stationary phase comprised of 20 cm × 10 cm pre-coated silica gel 60 F254 TLC aluminium plates with 0.25 mm layer thickness (Merck). With Linomat-5, automatic sample applicator equipped with a 100 µl Hamilton syringe; samples were applied to the plates as 6 mm-wide bands (Delivery rate of Hamilton syringe was 150 nls−1). Methanol was used as the sample solvent type.
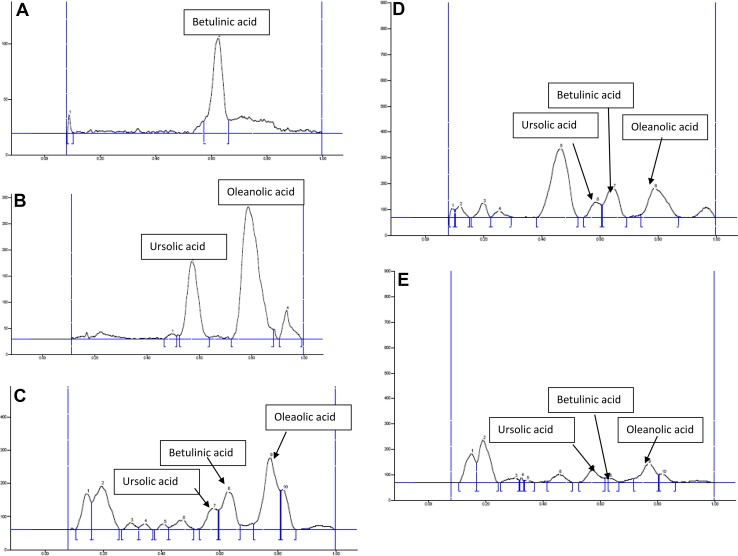
The plates were pre-derivatized with 1% iodine solution in chloroform and post-derivatized with 10% ethanolic (v/v) H2SO4. The mobile phase was Hexane–Ethyl Acetate–Acetone 8.2:1.8:0.1 (v/v) saturated in CAMAG twin trough glass chamber. Densitometric scanning was performed at 530 nm. OA, UA, and BA gave well-resolved spots at Rf 0.8, 0.62, and 0.58, respectively (Fig. 1).
Fig. 1.
a Chromatogram of betulinic acid (standard). b Chromatogram of ursolic acid and oleanolic acid (standard). c Chromatogram of Swertia chirata stem. d Chromatogram of Swertia chirata leaf. e Chromatogram of Swertia chirata root
Preparation of calibration curve of UA, OA, and BA
For preparation of calibration curves of UA, OA, and BA, different concentrations of working standard solution [2 μl (200 ng), 4 μl (400 ng), 6 μl (600 ng), 8 μl (800 ng), and 10 μl (1000 ng)] were applied to obtain Linearity Range of 200–1000 ng/spot. Densitometric scanning was performed at λ = 520 nm.
Validation of the developed method
Method validation was performed on the parameters such as linearity, limit of sensitivity, specificity, precision, accuracy, recovery, and robustness following the methods (Wojciak-Kosior (2007) with modifications.
Experimentation and statistical study
Experimentation has been conducted in two stages, first, Plackett–Burman design was used for the testing of important independent parameters, and then, central composite design was used to check the optimum level and possible interactions between significant parameters. Minitab statistical software package was used for experimental design.
Plackett–Burman model
For optimization of triterpenoid extraction from Swertia chirata, Plackett–Burman model has been used to evaluate the substantial parameters. Plackett–Burman design is based on the first-order model:
| 1 |
where Y is the expected target function, β0 is the scaling constant, and βi is the regression coefficients. The effect of variables (i.e., mean particle size, temperature, solvent composition, time, and solid–solvent ratio on triterpenoid extraction were tested. Experiment was conducted at two levels in which (+) means maximum value and (−) means minimum value (depicted in Table 1). All variables mentioned above were tested in duplicates by conducting 12 experiments (scheme depicted in Table 2). The significant parameters have been tested from regression analyses at 5% level (P < 0.05), as shown in Tables 3 and 4.
Table 1.
Different extraction parameters used for triterpenoid extraction from Swertia chirata
| Variable code | Variables | High level (+) | Low level (−) |
|---|---|---|---|
| X 1 | Temperature | 70 °C | 35 °C |
| X 2 | Time | 60 min. | 30 min. |
| X 3 | Solvent composition (methanol and ethyl acetate ratio) | 80% (v/v) | 20% (v/v) |
| X 4 | Solvent:solid ratio | 60:1 (ml/g) | 30:1 (ml/g) |
| X 5 | Particle size | 6 mm | 3 mm |
Table 2.
Yield of total triterpenoid from Swertia chirata using different extraction variables
| Run order | Temperature | Time | Solvent composition | Solid–solvent ratio | Mean particle size | % triterpenoid |
|---|---|---|---|---|---|---|
| 1 | − | + | − | − | − | 3.12 |
| 2 | + | − | + | − | − | 3.89 |
| 3 | − | − | + | + | + | 2.75 |
| 4 | + | − | − | − | + | 2.87 |
| 5 | − | + | + | + | − | 3.11 |
| 6 | + | + | − | + | − | 3.21 |
| 7 | + | − | + | + | − | 3.98 |
| 8 | − | − | − | − | − | 2.65 |
| 9 | − | − | − | + | + | 2.43 |
| 10 | + | + | − | + | + | 2.65 |
| 11 | + | + | + | − | + | 2.76 |
| 12 | − | + | + | − | + | 2.65 |
Table 3.
Regression analysis for % triterpenoid extraction from Swertia chirata using Plackett–Burman design criterion
| Term | Effect | Coefficient | SE coefficient | T | P |
|---|---|---|---|---|---|
| Constant | − | 3.0058 | 0.06731 | 44.65 | 0.000 |
| Temperature | 0.4417 | 0.2208 | 0.06731 | 3.28 | 0.017 |
| Time | − 0.1783 | − 0.0892 | 0.06731 | − 1.32 | 0.234 |
| Solvent composition | 0.3683 | 0.1842 | 0.06731 | 2.74 | 0.034 |
| Solid–solvent ratio | 0.0317 | 0.0158 | 0.06731 | 0.24 | 0.822 |
| Particle size | − 0.6417 | − 0.3208 | 0.06731 | − 4.77 | 0.003 |
Table 4.
Analysis of variance for % triterpenoid
| Source | df | Seq SS | Adj SS | Adj MS | F | P |
|---|---|---|---|---|---|---|
| Main effects | 5 | 2.3258 | 2.3258 | 0.46517 | 8.55 | 0.011 |
| Residual error | 6 | 0.3263 | 0.3263 | 0.05438 | ||
| Total | 11 | 2.6521 |
Central composite model
To define the optimal level of important extraction factors already screened out by means of Placket–Burman design, a central composite design has been employed. Optimum levels of three significant variables, i.e., temperature, solvent composition, and mean particle size on triterpenoid extraction, were studied (Table 5). In this design, five variable levels were tested, i.e., −a, −1, 0, +1, +a (a = 2n/4), where n is the sum of variables and 0 relates to the central point (as shown in Table 5). This number of variable levels was selected for our experimental preliminary work.
Table 5.
Treatment variables for optimization of triterpenoid extraction from Swertia chirata using central composite design
| Variables | Levels of variables | ||||||
|---|---|---|---|---|---|---|---|
| Codes | − 1.68 | − 1 | 0 | + 1 | + 1.68 | ||
| Temperature (°C) | X 1 | 20 | 35 | 50 | 65 | 80 | |
| Solvent composition | X 3 | 15 | 30 | 45 | 60 | 75 | |
| Particle size (mm) | X 5 | 0.5 | 2.0 | 3.5 | 4.0 | 5.5 | |
Optimal level for each significant variable has been calculated with equation, as given by Paul et al. (1992):
| 2 |
The designed experimentation scheme is given in Table 6. Total triterpenoid content has been evaluated by applying second-order polynomial equation, as given below:
| 3 |
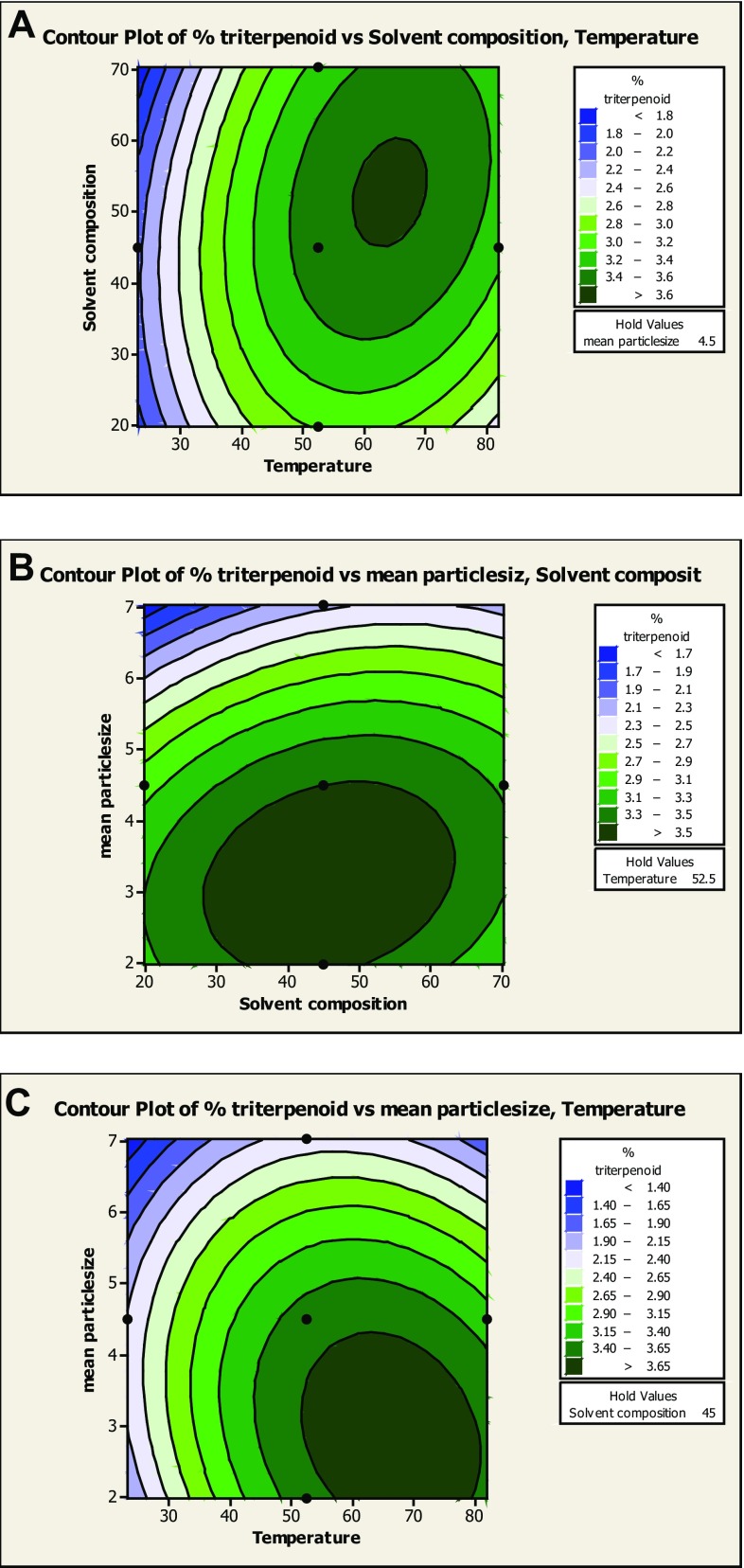
in which Y shows the predicted response, β0 is a scaling constant; X1, X3, and X5 show the levels of the extraction factors; β1, β3, and β5 are linear coefficients; β11, β33, and β55 are quadratic coefficients; and β13, β15, β31, β35, β51, and β53 are the interactive coefficients. Regression coefficients and analysis of variance (ANOVA) have been assessed for total triterpenoid content from Swertia chirata. Contour plots of % triterpenoids for each interactive coefficient have been drawn with the help of Minitab statistical software package (Fig. 2).
Table 6.
Central composite design criteria of significant extraction variables with total % triterpenoid content (experimentation as well as prediction value)
| Run order | Treated variables % triterpenoid | ||||
|---|---|---|---|---|---|
| Temperature (X1) | Solvent composition (X3) | Particle size (X5) | Experimental | Predicted | |
| 1 | 35.0000 | 30.0000 | 3.00000 | 2.87 | 2.916 |
| 2 | 70.0000 | 30.0000 | 3.00000 | 3.48 | 3.601 |
| 3 | 35.0000 | 60.0000 | 3.00000 | 2.65 | 2.743 |
| 4 | 70.0000 | 60.0000 | 3.00000 | 3.71 | 3.787 |
| 5 | 35.0000 | 30.0000 | 6.00000 | 2.17 | 2.229 |
| 6 | 70.0000 | 30.0000 | 6.00000 | 2.45 | 2.493 |
| 7 | 35.0000 | 60.0000 | 6.00000 | 2.32 | 2.336 |
| 8 | 70.000 | 60.0000 | 6.00000 | 2.87 | 2.960 |
| 9 | 23.0686 | 45.0000 | 4.50000 | 2.23 | 2.168 |
| 10 | 81.9314 | 45.0000 | 4.50000 | 3.43 | 3.269 |
| 11 | 52.5000 | 19.7731 | 4.50000 | 3.11 | 3.015 |
| 12 | 52.5000 | 70.2269 | 4.50000 | 3.36 | 3.262 |
| 13 | 52.5000 | 45.0000 | 1.97731 | 3.67 | 3.502 |
| 14 | 52.5000 | 45.0000 | 7.02269 | 2.32 | 2.262 |
| 15 | 52.5000 | 45.0000 | 4.50000 | 3.51 | 3.502 |
| 16 | 52.5000 | 45.0000 | 4.50000 | 3.56 | 3.502 |
| 17 | 52.5000 | 45.0000 | 4.50000 | 3.46 | 3.502 |
| 18 | 52.5000 | 45.0000 | 4.50000 | 3.41 | 3.502 |
| 19 | 52.5000 | 45.0000 | 4.50000 | 3.46 | 3.502 |
| 20 | 52.5000 | 45.0000 | 4.50000 | 3.58 | 3.502 |
Fig. 2.
a Contour plot for triterpenoids extraction at varying level of temperature and solvent composition (% methanol in methanol–ethyl acetate mixture). b Contour plot for triterpenoid extraction at varying level of particle size and solvent composition (% methanol in methanol–ethyl acetate mixture). c Contour plot for triterpenoid extraction at varying level of particle size and temperature
Results and discussion
In this study, quantitative estimation of triterpenoids in different extracts was quantified by HPTLC methods. HPTLC fingerprinting (Fig. 1c–e) on different parts of Swertia chirata showed that triterpenoids were present highest in stem parts (3.41%) followed by leaf (2.42%) and root (2.11%) of plants. Table 9 shows the analytical characteristics of validation of triterpenoids, i.e., UA, BA, and OA.
Table 9.
Analytical characteristics of the method validation
| S. no | Parameters | Ursolic acid (UA) | Oleanolic acid | Betulinic acid |
|---|---|---|---|---|
| 1 | Linearity range (ng/spot; n = 12a) | 200–3000 | 200–3000 | 200–3000 |
| 2 | Correlation coefficient (r2) | 0.991 | 0.997 | 0.982 |
| 3 | Regression equation | Y = − 126.87.0 + 11.05X | Y = − 1111.13 + 10.0914X | Y = − 1003.11 + 6.0766X |
| 4 | Calculated SD value (CATS software) | 2.59 | 3.26 | 6.72 |
| 5 | bLimit of detection (LOD) (ng) [3 × SD/S] | 30 | 27 | 25 |
| 6 | bLimit of quantitation (LOQ) (ng) [10 × SD/S] | 90 | 81 | 75 |
| 7 | R f | 0.58 | 0.8 | 0.62 |
| Precision and accuracy | ||||
| 8 | Intra-day RSD (%), n = 5 | 0.357 | 0.487 | 0.317 |
| 9 | Inter-day RSD (%), n = 5 (day-1/day-2/day-3) | 0.353/0.425/0.532 | 0.484/0.498/0.514 | 0.315/0.376/0.412 |
| Recovery | ||||
| 10 | Amount of standard in stem samples (μg mg−1) containing highest triterpenoid | 8.1 | 19.0 | 7.0 |
| 11 | Amount of standard added in stem sample (μg mg−1) | 2/4/6 | 2/4/6 | 2/4/6 |
| 12 | Amount of standard found (μg) | 10.1/12.05/14.11 | 20.89/22.97/24.89 | 8.97/10.96/12.97 |
| 13 | Recovery (%) | 100/99.59/99.92 | 99.48/99.87/99.56 | 99.66/99.64/99.24 |
aFour concentration levels in triplicate
bSD is the standard deviation of the blank response and S is the slope of the calibration plot
Screening out significant extraction variables
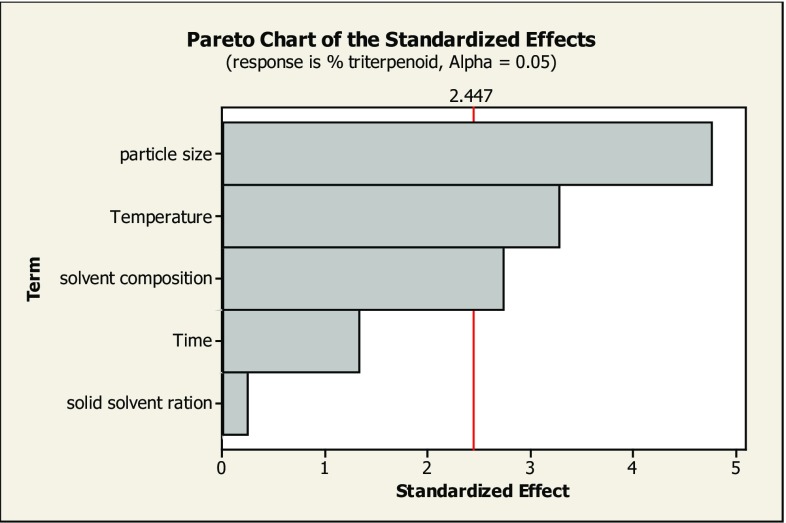
Plackett–Burman design criterion has been used to evaluate the impact of five different independent variables on triterpenoid yield (Table 1). In this scheme, selected designed matrix and the resultant total % triterpenoid content obtained from Swertia chirata stem are given in Table 2. The effect of extraction parameters on triterpenoid extraction was screened by means of regression analysis (Table 3). Only three parameters, i.e., temperature, solvent composition, and mean particle size, had shown significant effect on triterpenoid extraction as revealed by their P values at 5% level (P < 0.05 values shown in Table 3) (Fig. 3). In our experiment, triterpenoid content as attained by Plackett–Burman design has indicated disparity up to 2.65–3.98%.
Fig. 3.
Comparative effect of temperature, time, solvent composition, solid–solvent ratio, and particle size on triterpenoidal yield
Effect of extraction variables on triterpenoidal yield
In this scheme, total 20 experiments have been conducted by means of Central Composite design. Table 6 evidently shows experimental values along with predicted values that have been attained by the model equation. The quadratic model contribution was significant (0.0001) and R2 and R2 adj values of 0.98 and 0.96, respectively, and lack of fit was insignificant (0.051), confirming the model adequacy. Multiple regression analysis has been applied on the investigational data, which gives the second-order polynomial equation as follows:
| 4 |
The effects of temperature (X1), solvent composition (X3), and mean particle size (X5) on triterpenoid extraction are reported in Table 7. Regression coefficients obtained from the investigation data had revealed the significant positive linear effects of temperature and solvent composition, whereas particle size has shown negative linear impact on triterpenoid content (Table 7). Out of three parameters, temperature has shown maximum impact on triterpenoid yield that was assumed by means of their maximum linear coefficient value (0.327) followed by solvent composition (0.073) and mean particle size (− 0.378). Table 7 indicates that interactive effect of temperature and solvent composition (X13) significantly affects the triterpenoid yield. However, the interactive effect of temperature and particle size (X15) had significant effect on triterpenoid extraction, while solvent composition and particle size (X25) had not found to be significant on pentacyclic triterpenoid yield. Therefore, only interaction between temperature and solvent composition (X13) and temperature and particle size (X15) were shown in the above said model regression [Eq. (4)]. The quadratic effect of variables was found to be significant for all the responses such as temperature (), solvent composition() and mean particle size (). In Swertia chirata, analysis of variance for the triterpenoid yield from our designed criterion is given in Table 8.
Table 7.
Estimated regression coefficients for % triterpenoid extraction from Swertia chirata using central composite design (coded units)
| Model parameters | Regression coefficient | S.E. coefficient | T | P |
|---|---|---|---|---|
| Constant | 3.50218 | 0.04609 | 75.992 | 0.000 |
| Temperature | 0.32714 | 0.03058 | 10.699 | 0.000 |
| Solvent composition | 0.07326 | 0.03058 | 2.396 | 0.038 |
| Particle size | − 0.37860 | 0.03058 | − 12.382 | 0.000 |
| Temperature2 | − 0.27704 | 0.02977 | − 9.307 | 0.000 |
| Solvent composition2 | − 0.12855 | 0.02977 | − 4.319 | 0.002 |
| Particle size2 | − 0.21341 | 0.02977 | − 7.169 | 0.000 |
| Temperature × solvent composition | 0.09000 | 0.03995 | 2.253 | 0.048 |
| Temperature × particle size | − 0.10500 | 0.03995 | − 2.628 | 0.025 |
| Solvent composition × particle size | 0.07000 | 0.03995 | 1.752 | 0.110 |
Table 8.
Analysis of variance for triterpenoids extraction from Swertia chirata using central composite design criterion
| Source | df | Seq SS | Adj SS | Adj MS | F | P |
|---|---|---|---|---|---|---|
| Regression | 9 | 5.39781 | 5.39781 | 0.59976 | 46.97 | 0.000 |
| Linear | 3 | 3.49235 | 3.49235 | 1.16412 | 91.17 | 0.000 |
| Square | 3 | 1.71326 | 1.71326 | 0.57109 | 44.73 | 0.000 |
| Interaction | 3 | 0.19220 | 0.19220 | 0.06407 | 5.02 | 0.022 |
| Residual error | 10 | 0.12769 | 0.12769 | |||
| Lack of fit | 5 | 0.10635 | 0.01277 | 0.02127 | 4.99 | 0.051 |
| Pure error | 5 | 0.02133 | 0.10635 | 0.00427 | ||
| Total | 19 | 5.52550 | 0.02133 |
R-Sq = 97.69% R-Sq(pred) = 84.79% R-Sq(adj) = 95.61%
Contour plot maps of different levels of temperature, solvent composition, and particle size on the triterpenoid yield are displayed in Fig. 3a–c. As shown in Fig. 3a, the maximum triterpenoid yield was obtained in keeping the extraction temperature 65 °C and solvent composition of 50% methanol. This reflects that both temperature and solvent composition have strong effect on the triterpenoid yield, as there is increase in the temperature the viscosity of the solvent decreased which leads to increase in the wetting of the matrix and solubilization of the solutes. Moreover, due to increase in the temperature more energy breaks, the analyte–matrix bond thus increase diffusion of these analytes in the solvents. This increase in extraction yield of triterpenoids was also observed by Fang et al. (2010). Increase in polarity of solvent composition (% methanol–ethyl acetate) leads to better yield of triterpenoids, but further increase in polarity of solvent did not affect the solubility of the analyte in the solvent. Figure 3b shows the evolution of triterpenoid yield according to mean particle size and solvent composition (% methanol in methanol–ethyl acetate mixture). Here, the increase in % triterpenoid yield at 45% methanol–ethyl acetate mixture and particle size 3 mm. A further decrease in the particle size (3 mm) and increase in 45% methanol–ethyl acetate composition had not increased the yield of triterpenoid. Figure 3c shows the evolution of triterpenoid yield according to extraction temperature and a mean particle size. Maximum extraction of triterpenoid occurs at 3 mm mean particle size and 65% methanol–ethyl acetate solvents. Banik and Pandey (2008) found the similar types of results on extraction of triterpenoids in which temperature, mean particle size, and solvent composition play a vital role (Table 9).
Validation of the model
The experimental data were fitted into the model equation (4) and the optimum values were found to be: extraction temperature (65 °C), mean particle size (3 mm), and solvent composition (45% methanol in methanol–ethyl acetate mixture). At these optimum levels of extraction parameters, total triterpenoid extracted from Swertia chirata stem was 3.71%, which is very close to the predicted value of 3.79%. The experimental data of triterpenoid extraction were accurately developed to the mathematical model.
Conclusion
Due to the increase economic relevance of triterpenoids, this study was conducted to optimize the extraction parameters for maximum triterpenoid yield. The total pentacyclic tripertenoid (OA, UA, and BA) were determined in stem, leaf, and root parts of Swertia chirata. HPTLC fingerprinting showed that stem part was the potent part containing pentacyclic triterpenoids followed by leaf and root. The five variables were tested using Plackett–Burman design and three variables exerted significant effects on triterpenoid yield from Swertia chirata stem. To optimize the triterpenoid yield from Swertia chirata stem, RSM has been efficaciously applied using the screened extraction variables. The optimum levels were found to be: extraction temperature (65 °C), mean particle size (3 mm) and solvent composition (45% methanol in methanol–ethyl acetate mixture). At these optimum levels of extraction parameters, total triterpenoid extracted from Swertia chirata stem was 3.71%, which is very close to the predicted value of 3.79%. The experimental data of triterpenoid extraction were accurately developed to the mathematical model. This is the first study report of the optimization of triterpenoid extraction parameters from Swertia chirata stem.
Acknowledgements
The authors are grateful to the IPLS-DBT Project (Project No. BT/PR-4548/INF/22/146/2012) sanctioned to Punjabi University, Patiala for providing the facilities to carry out the present work.
Compliance with ethical standards
Conflict of interest
We declare that we have no conflict of interest.
References
- Alberti A, Zielinski AA, Zardo DM, Demiate IM, Nogueira A, Mafra LI. Optimisation of the extraction of phenolic compounds from apples using response surface methodology. Food Chem. 2014;149:151–158. doi: 10.1016/j.foodchem.2013.10.086. [DOI] [PubMed] [Google Scholar]
- Ameer K, Bae SW, Jo Y, Lee HG, Ameer A, Kwon JH. Optimization of microwave-assisted extraction of total extract, stevioside and rebaudioside-A from Stevia rebaudiana (Bertoni) leaves, using response surface methodology (RSM) and artificial neural network (ANN) modelling. Food Chem. 2017;229:198–207. doi: 10.1016/j.foodchem.2017.01.121. [DOI] [PubMed] [Google Scholar]
- Anonymous (1976) The wealth of India—a dictionary of Indian raw materials and industrial products. Raw materials. New Delhi: Publications and Information Directorate, Council of Scientific and Industrial Research. 10 (Sp–W), pp78–81
- Bai XL, Yue TL, Yuan YH, Zhang HW. Optimization of microwave-assisted extraction of polyphenols from apple pomace using response surface methodology and HPLC analysis. J Sep Sci. 2010;33(23–24):3751–3758. doi: 10.1002/jssc.201000430. [DOI] [PubMed] [Google Scholar]
- Banik RM, Pandey DK. Optimizing conditions for oleanolic acid extraction from Lantana camara roots using response surface methodology. Ind Crops Prod. 2008;27(3):241–248. doi: 10.1016/j.indcrop.2007.09.004. [DOI] [Google Scholar]
- Bhandari P, Gupta A, Singh B, Kaul V. HPTLC determination of swertiamarin and amarogentin in Swertia species from the Western Himalayas. J Planar Chromatogr. 2006;19(109):212–215. doi: 10.1556/JPC.19.2006.3.8. [DOI] [Google Scholar]
- Bonaccorsi I, Altieri F, Sciamanna I, Oricchio E, Grillo C, Contartese G, Galati EM. Endogenous reverse transcriptase as a mediator of ursolic acid’s anti-proliferative and differentiating effects in human cancer cell lines. Cancer Lett. 2008;263(1):130–139. doi: 10.1016/j.canlet.2007.12.026. [DOI] [PubMed] [Google Scholar]
- Cheok CY, Chin NL, Yusof YA, Talib RA, Law CL. Optimization of total phenolic content extracted from Garcinia mangostana Linn. hull using response surface methodology versus artificial neural network. Ind Crops Prod. 2012;40:247–253. doi: 10.1016/j.indcrop.2012.03.019. [DOI] [Google Scholar]
- Fang XJ, Wang XY, Zhang G, Zhao J. Optimization of microwave-assisted extraction followed by RP-HPLC for the simultaneous determination of oleanolic acid and ursolic acid in the fruits of Chaenomeles sinensis. J Sep Sci. 2010;33:1147–1155. doi: 10.1002/jssc.200900726. [DOI] [PubMed] [Google Scholar]
- Gad HA, El-Ahmady SH, Abou-Shoer MI, Al-Azizi MM. Application of chemometrics in authentication of herbal medicines: a review. Phytochem Anal. 2013;24(1):1–24. doi: 10.1002/pca.2378. [DOI] [PubMed] [Google Scholar]
- Gao R, Wang L, Yang Y, Ni J, Zhao L, Dong S, Guo M. Simultaneous determination of oleanolic acid, ursolic acid, quercetin and apigenin in Swertia mussotii Franch by capillary zone electrophoresis with running buffer modifier. Biomed Chromatogr. 2015;29(3):402–409. doi: 10.1002/bmc.3290. [DOI] [PubMed] [Google Scholar]
- Gheorgheosu D, Duicu O, Dehelean C, Soica C, Muntean D. Betulinic acid as a potent and complex antitumor phytochemical: a minireview. Anticancer Agents Med Chem. 2014;14(7):936–945. doi: 10.2174/1871520614666140223192148. [DOI] [PubMed] [Google Scholar]
- Gopal V, Mandal V, Mandal SC. HPTLC evaluation of oleanolic acid and ursolic acid from the methanol extract of Wattakaka volubilis. J Acute Dis. 2014;3(1):59–61. doi: 10.1016/S2221-6189(14)60013-5. [DOI] [Google Scholar]
- Gupta M, Bisht D, Khatoon S, Srivastava S, Rawat AK. Determination of ursolic acid a biomarker in different Swertia species through high performance thin layer chromatography. Chin Med. 2011;2(04):121. doi: 10.4236/cm.2011.24020. [DOI] [Google Scholar]
- Ilaiyaraja N, Likhith KR, Babu GS, Khanum F. Optimisation of extraction of bioactive compounds from Feronia limonia (wood apple) fruit using response surface methodology (RSM) Food Chem. 2015;173:348–354. doi: 10.1016/j.foodchem.2014.10.035. [DOI] [PubMed] [Google Scholar]
- Jacob S, Banerjee R. Modeling and optimization of anaerobic codigestion of potato waste and aquatic weed by response surface methodology and artificial neural network coupled genetic algorithm. Bioresource Technol. 2016;214:386–395. doi: 10.1016/j.biortech.2016.04.068. [DOI] [PubMed] [Google Scholar]
- Jesus JA, Lago JH, Laurenti MD, Yamamoto ES, Passero LF (2015) Antimicrobial activity of oleanolic and ursolic acids: an update. Evid Based Complement Alternat Med 2015 [DOI] [PMC free article] [PubMed]
- Khanal S, Shakya N, Nepal N, Pant D. Swertia chirayita: the himalayan herb. Int J Appl Sci Biotechnol. 2014;2(4):389–392. [Google Scholar]
- Kshirsagar PR, Pai SR, Nimbalkar MS, Gaikwad NB. Quantitative determination of three pentacyclic triterpenes from five Swertia L. species endemic to Western Ghats, India, using RP-HPLC analysis. Nat Prod Res. 2015;29(19):1783–1788. doi: 10.1080/14786419.2015.1004174. [DOI] [PubMed] [Google Scholar]
- Kumar V, Van Staden J. A review of Swertia chirayita (Gentianaceae) as a traditional medicinal plant. Front Pharmacol. 2015;6:308. doi: 10.3389/fphar.2015.00206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar KS, Bhowmik D, Chandira M. Swertia chirayita: a traditional herb and its medicinal uses. J Chem Pharm Res. 2010;2(1):262–266. [Google Scholar]
- Li G, Zhang X, You J, Song C, Sun Z, Xia L, Suo Y. Highly sensitive and selective pre-column derivatization high-performance liquid chromatography approach for rapid determination of triterpenes oleanolic and ursolic acids and application to Swertia species: optimization of triterpenic acids extraction and pre-column derivatization using response surface methodology. Anal Chim Acta. 2011;688(2):208–218. doi: 10.1016/j.aca.2011.01.010. [DOI] [PubMed] [Google Scholar]
- Liang J, Ito Y, Zhang X, He J, Sun W. Rapid preparative separation of six bioactive compounds from Gentiana crassicaulis Duthie ex Burk. using microwave-assisted extraction coupled with high-speed counter-current chromatography. J Sep Sci. 2013;36(24):3934–3940. doi: 10.1002/jssc.201300897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J. Pharmacology of oleanolic acid and ursolic acid. J Ethnopharmacol. 1995;49(2):57–68. doi: 10.1016/0378-8741(95)90032-2. [DOI] [PubMed] [Google Scholar]
- Mahendran G, Bai VN. Micropropagation, antioxidant properties and phytochemical assessment of Swertia corymbosa (Griseb.) Wight ex CB Clarke: a medicinal plant. Acta Physiol Plant. 2014;36(3):589–603. doi: 10.1007/s11738-013-1435-2. [DOI] [Google Scholar]
- Negi JS, Singh P, Rawat B. Chemical constituents and biological importance of Swertia: a review. Curr Res Chem. 2011;3(1):1–15. doi: 10.3923/crc.2011.1.15. [DOI] [Google Scholar]
- Pandey DK, Basu S, Jha TB. Screening of different East Himalayan species and populations of Swertia L. based on exomorphology and mangiferin content. Asian Pac J Tropical Biomed. 2012;2(3):S1450–S1456. doi: 10.1016/S2221-1691(12)60436-5. [DOI] [Google Scholar]
- Paul GC, Kent CA, Thomas CR. Quantitative characterization of vacuolization in Penicillium chrysogenum using automatic image analysis. Trans Inst Chem Eng C. 1992;70:13–20. [Google Scholar]
- Samaddar T, Chaubey B, Jha S, Jha TB. Determination of swertiamarin and amarogentin content and evaluation of antibacterial activity in Eastern Himalayan species of Swertia L. Pharmacogn. 2013;3(4):64–70. [Google Scholar]
- Sethiya NK, Mishra S. Simultaneous HPTLC analysis of ursolic acid, betulinic acid, stigmasterol and lupeol for the identification of four medicinal plants commonly available in the indian market as Shankhpushpi. J Chromatogr Sci. 2015;53(5):816–823. doi: 10.1093/chromsci/bmu111. [DOI] [PubMed] [Google Scholar]
- Sheng ZL, Wan PF, Dong CL, Li YH. Optimization of total flavonoids content extracted from Flos Populi using response surface methodology. Ind Crops Prod. 2013;43:778–786. doi: 10.1016/j.indcrop.2012.08.020. [DOI] [Google Scholar]
- Silva FSG, Oliveira PJ, Duarte MF. Oleanolic, ursolic, and betulinic acids as food supplements or pharmaceutical agents for type 2 diabetes: promise or Illusion? J Agric Food Chem. 2016;64(15):2991–3008. doi: 10.1021/acs.jafc.5b06021. [DOI] [PubMed] [Google Scholar]
- Soica C, Oprean C, Borcan F, Danciu C, Trandafirescu C, Coricovac D, Crăiniceanu Z, Dehelean CA, Munteanu M. The synergistic biologic activity of oleanolic and ursolic acids in complex with hydroxypropyl-γ-cyclodextrin. Molecules. 2014;19(4):4924–4940. doi: 10.3390/molecules19044924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma SC, Jain CL, Nigam S, Padhi MM. Rapid extraction, isolation, and quantification of oleanolic acid from Lantana camara L. Roots using microwave and HPLC-PDA techniques. Acta Chromatogr. 2013;25(1):181–199. doi: 10.1556/AChrom.25.2013.1.12. [DOI] [Google Scholar]
- Wang C, Wang Y, Zhang J, Wang Z. Optimization for the extraction of polysaccharides from Gentiana scabra Bunge and their antioxidant in vitro and anti-tumor activity in vivo. J Taiwan Inst Chem Eng. 2014;45(4):1126–1132. doi: 10.1016/j.jtice.2013.12.004. [DOI] [Google Scholar]
- Wójciak-Kosior M. Separation and determination of closely related triterpenic acids by high performance thin-layer chromatography after iodine derivatization. J Pharmaceut Biomed Anal. 2007;45(2):337–340. doi: 10.1016/j.jpba.2007.05.011. [DOI] [PubMed] [Google Scholar]
- Yang YC, Wei MC, Hong SJ, Huang TC, Lee SZ. Development/optimization of a green procedure with ultrasound-assisted improved supercritical carbon dioxide to produce extracts enriched in oleanolic acid and ursolic acid from Scutellaria barbata D. Don. Ind Crops Prod. 2013;49:542–553. doi: 10.1016/j.indcrop.2013.05.013. [DOI] [Google Scholar]
- Yogeeswari P, Sriram D. Betulinic acid and its derivatives: a review on their biological properties. Curr Med Chem. 2005;12(6):657–666. doi: 10.2174/0929867053202214. [DOI] [PubMed] [Google Scholar]