Summary
Patients with Chagas’ disease may develop dysfunctions of oesophageal and colonic motility resulting from the degeneration or loss of the myenteric neurons of the enteric nervous system. Studies have shown that the use of aspirin, also known as acetylsalicylic acid (ASA), influences the pathogenesis of the disease. However, this remains controversial. The aim of this study was to evaluate the consequences of treatment with low doses of aspirin during the chronic phase of Chagas’ disease on oesophageal function. Twenty male Swiss mice, 60 days of age, were used. The animals were infected with Y strain of Trypanosoma cruzi, injected intraperitoneally. Aspirin was given at a dose of 50 mg/kg to some of the infected animals, from the 55th to 63rd day after inoculation on consecutive days, and from the 65th to 75th day on alternate days. We investigated food passage of time, wall structure and nitrergic neuronal population of the distal oesophagus. Our data revealed that the use of low doses of aspirin in chronic Chagas’ disease caused an increase in the number of nitrergic neurons and partially prevented hypertrophy of the oesophagus. In addition, the aspirin administration impeded Chagas' diseases associated changes in intestinal transit time. Thus treatment with aspirin in the chronic phase of Chagas’ disease changes the natural history of the disease and raises the possibility of using it as a new therapeutic approach to the treatment of this aspect of Chagas' disease pathology.
Keywords: acetylsalicylic acid, achalasia, American trypanosomiasis, enteric nervous system, myenteric neurons
Chagas’ disease (CD) is an infection caused by the protozoan Trypanosoma cruzi, of endemic nature and essentially chronic clinical evolution which is found all over the world (World Health Organization 2015).
In chronic Chagas’ disease (CCD), although the majority of individuals do not manifest any symptoms, about 30% are affected by cardiac problems and 10% have digestive disturbances, especially megacolon and megaoesophagus (Gascona et al. 2010; World Health Organization 2015). Patients with mega‐oesophagus show compromised deglutition and peristalsis (Nascimento et al. 2013) caused by alterations in the enteric nervous system (ENS) which is part of the autonomic nervous system (Adad et al. 1991).
The ENS is a complex network of several neuronal types and glial cells contained inside the wall of the gastrointestinal tract, present from the oesophagus to the rectum (Greggio et al. 2010; Foong et al. 2014). The destruction of these neurons leads to loss of peristaltic movement, muscle hypertrophy and enlargement of the lumen of the organ (Nascimento et al. 2013).
Enteric neurons can be identified as motor neurons, interneurons and intrinsic primary afferent neurons. The first can be divided into excitatory and inhibitory, both present in the myenteric plexus. Inhibitory motor neurons, such as the nitrergic subpopulation, are nerve cells where the transmissions are mediated by nitric oxide (NO) and have an important relaxing effect on the control of the gastrointestinal tract (Furness et al. 2014).
Due to the difficulty in early diagnosis, the most common form of treatment for chronic CD is symptomatic, in which the aim is to alleviate the clinical manifestations of the disease (Brazil 2010). Non‐steroidal anti‐inflammatory drugs (NSAIDs), such as aspirin, are widely used for the control of pain, fever and inflammation (Chan & Graham 2004). This drug impedes the conversion of arachidonic acid (AA) into eicosanoids, such as prostaglandins and thromboxanes, by irreversibly blocking cyclooxygenase‐1 and cyclooxygenase‐2 (FitzGerald & Patrono 2001). In CD, especially in experimental models, low doses (Mukherjee et al. 2011; Molina‐Berríos et al. 2013) have been used as supplementary treatment and have a protective effect.
The effects of treatment with low‐dose aspirin in the acute phase on the oesophagus of mice with CD were studied recently by our group and it was noted that treatment protects the oesophageal myenteric neurons from the atrophy caused by the T. cruzi infection (Massocatto et al. 2016); however, there are no studies in the literature examining treatment in the chronic phase. Thus this study aimed to determine the effect of using low doses of aspirin, administered during the chronic phase, on the structure and innervation of this organ in mice with CD.
Methods
Ethical aspects
This study was approved by the Committee for Ethical Conduct in the Use of Animals in Research, of the State University of Maringá (No 057/2013).
Animals and infection
Twenty male Swiss mice (Mus musculus) were used, which were 60 days old (2 months) and obtained from the animal house of the State University of Londrina. They were randomly distributed into four groups (n = 5): CG – control group, not infected and treated with phosphate‐buffered saline (0.01 M, pH 7.4; PBS); CGA – control group, not infected and treated with aspirin; IG – infected group treated with PBS; and IGA – infected group treated with aspirin. The mice were kept in a controlled environment of 21–23°C, with a 12/12‐h light–dark cycle.
Mice were infected with an inoculum of 1300 blood trypomastigote forms of the Y strain of T. cruzi, injected intraperitoneally (ip). The chronic phase was induced by administering six doses of 100 mg/kg benznidazole on the 11th, 13th, 15th, 25th, 29th and 48th days postinfection (dpi), by oral gavage.
Treatment with aspirin
The quantity of aspirin (acetylsalicylic acid – A2093, Sigma‐Aldrich; St. Louis, MO, USA) needed to prepare a 50 mg/kg solution was calculated taking into consideration the weight of the animals. This NSAID was prepared daily, where it was dissolved in 100 μl of dimethyl sulfoxide (DMSO). Next, 900 μl of PBS (pH 7.4) was added to the tube, which was again vortexed.
Treatment with aspirin was carried out on consecutive days from the 55th to 63rd dpi and alternate days on the 65th, 67th, 69th, 71st, 73rd and 75th dpi. Each animal of the groups CGA and IGA received 100 μl of 50 mg/kg aspirin stock solution i.p., at 8 am. For the groups CG and IG, 100 μl of PBS was administered i.p. to the animals.
Evolution of the infection
Parasitaemia was evaluated from the 4th to 15th dpi and on alternate days from the 15th to 20th dpi. The parasitaemia curve was drawn using the mean parasite count of the inoculated animals in the IG and IGA groups, showing the following features: the mean time between the day of experimental inoculation and the day on which the blood sample was positive for parasites (prepatent period); the mean number of days where the blood sample showed parasitaemia (patent period); and the day with the highest number of parasites observed (parasite peak).
Determination of food passage time
Food passage time was evaluated by the administration of a non‐absorbable marker (3% carmine red; 0.5% ethylcellulose), via gavage. Mice were placed in individual cages, with the availability of food and water ad libitum. Food passage time was determined by the appearance of the first red pellet.
Euthanasia and collection of the oesophagus
The animals were euthanized by deep anaesthesia with halothane gas (Tanohalo®, Cristália Produtos Químicos Farmacêuticos LTDA, Itapira, São Paulo, Brazil) at 81 dpi for the groups IG and IGA, which corresponded to 141 days of age for all mice. After thoracotomy and vertical laparotomy, the oesophagus was removed and washed with PBS.
NADPH‐dp method
The oesophagus was subjected to the NADPH‐dp method, where it was filled with PBS, forming a bladder, immersed and fixed in 4% paraformaldehyde in PBS for 30 min, and it was then placed in a container with 0.3% Triton X‐100 in PBS for 10 min and washed ten times (10 min each) in PBS. To detect the nitrergic neurons, the tissue was immersed in incubation medium consisting of 200 ml of Tris–HCl buffer, 0.05 g NBT (Sigma‐Aldrich®), 0.1 g β‐NADPH (Sigma‐Aldrich®) and 0.6 ml of Triton X‐100, for 120 min. The reaction was stopped, and the oesophagus was opened and placed in 4% paraformaldehyde (pH 7.4).
The oesophagus was microdissected under a stereomicroscope with transillumination, and the tunica mucosa and tela submucosa were removed. The total preparation containing the oesophageal musculature was dehydrated in alcohols (ascending series), cleared in xylol and mounted on a slide which was then coverslipped. The distal portion of the oesophagus was used in this study.
Quantitative and morphometric analysis of NADPH‐dp‐reactive neurons
A light microscope (Motic®), with a 40× objective, was used to quantify the neurons present in 100 microscopic fields distributed through the distal oesophagus (totalling 24.88 mm2).
Area (μm2) of the cell body and of the nucleus of 100 neurons of the myenteric plexus of the distal oesophagus was measured by capturing images using a digital camera (Pro Series 3CCD camera) coupled to a light microscope (Olympus BX50, Japan) and Image Pro Plus software (Media Cybernetics, USA).
Histologic and morphometric analysis of the oesophageal wall
The distal oesophagus was fixed in Bouin's solution and embedded in paraffin to obtain four 4‐μm‐thick cross‐sections in semi‐series, for each animal, totalling 20 sections per group.
Images captured with a 10× objective were used to measure the total thickness of the wall, tunica muscularis and circular and longitudinal muscle layers. Sixty‐four measurements were taken for each structure, evenly distributed in the whole circumference of the oesophagus of each mouse. We used sections stained with haematoxylin and eosin, and photographs were taken with a digital camera (Pro series 3CCD camera) coupled to a light microscope (Olympus BX50, Japan).
For qualitative histopathology, images captured with a 10× objective were evaluated for the presence of inflammatory infiltration and amastigote nest.
Statistical analysis
Random‐effects models were considered for responses of normal measurements (cell body area, nuclear area, cytoplasmic area, wall, total muscle, circular muscle and longitudinal muscle), or y ij = μij + εij, with μij = μ0j + γij, γ: random effects of the ith (i = 1, 2,…, n) individual, so that , μ0j: the mean of the jth treatment group (j = 1, 2, 3 and 4, respectively, groups: CG, CGA, IG and IGA) and ε: the error associated with each observation, so that A normal model without random effects was considered for response time (Table 2).
Table 2.
Food passage time, nitrergic neuronal density and morphometry of myenteric neurons of the oesophagus distal portion of Swiss male mice, with 141 days of age
| Variable | Groups | |||
|---|---|---|---|---|
| CG | CGA | IG | IGA | |
| Food passage time (minutes) | 136.90b ± 3.93 | 183.40a ± 18.45 | 169.40a ± 15.14 | 129.30b ± 12.66 |
| Number of neurons NADPH‐dp | 1.48b ± 0.27 | 1.41bc ± 0.31 | 1.61abc ± 0.06 | 1.61a ± 0.23 |
| Cell body area (μm2) | 244.80a ± 4.56 | 249.90a ± 5.33 | 219.60b ± 3.95 | 191.60c ± 3.37 |
| Nuclear area (μm2) | 69.62a ± 1.14 | 68.98ab ± 1.56 | 65.90b ± 1.15 | 57.15c ± 1.11 |
| Cytoplasmic area (μm2) | 172.10a ± 3.94 | 178.90a ± 4.67 | 151.20b ± 3.31 | 131.50c ± 2.77 |
Uninfected control group treated with PBS (CG), uninfected control group treated with ASA (CGA), infected group treated with PBS (IG) and infected group treated with ASA (IGA). Infection with Y strain of Trypanosoma cruzi.
Data (expressed as posterior mean ± standard error) followed by the different letters in the same line are significantly different (P < 0.05; Bayesian contrasts).
For the number of NADPH‐dp‐positive neurons, a zero‐inflated Poisson distribution with random effects (Lambert 1992), that is y ij ~ ZiPoisson(α0j wij), where α0j is the mean of the jth treatment group and w is the random effect of the ith individual.
The whole modelling was performed with a Bayesian focus, considering a priori non‐informative distributions of all parameters of the models considered, that is μ0 ~ N(0, 10−6), γ ~ N(0, τγ), for τγ, τε, λ and α0 a Gamma (10−3, 10−3), such that the standard deviation is given by , according to OpenBugs parametrization, a program that allows the simulation of distributions a posteriori using MCMC (Monte Carlo Markov chain) algorithms by means of the BRugs package of the R program (R Development Core Team, Vienna, Austria, 2015). Thus, after 110,000 generated values, it was given a discard sampling period of 10,000 initial values. Avoiding autocorrelations, the final sample obtained contains 10,000 values generated considering jumps of equal size to 10. The convergence of the chains was verified by the coda package of R program, using the e criterion of Heidelberger and Welch (1983). Finally, comparisons were made a posteriori between distributions of the means of the groups considered. Groups whose 95% confidence intervals for the mean differences did not include zero were considered different at the 5% level of significance (P < 0.05).
Results
Evolution of the infection
The groups IG and IGA showed positive parasitaemia around the 6th dpi, with a parasite peak on the 8th dpi and patent period of about 6 days (Table 1, Figure 1).
Table 1.
Parasitological parameters evaluated in Swiss male mice, after 60 days of age, infected with Y strain of Trypanosoma cruzi. Infected group treated with PBS (IG), infected group treated with ASA (IGA)
| Groups | Prepatent period (days) | Patent period (days) | Parasite peak (8 days) (trypomastigotes/ml × 103) | Total parasitaemia (trypomastigotes/ml × 103) |
|---|---|---|---|---|
| IG | 7.0 ± 0.84 | 5.6 ± 0.74 | 1.68 ± 12.86 | 5.02 ± 22.59 |
| IGA | 6.5 ± 0.81 | 5.8 ± 0.77 | 1.76 ± 13.38 | 5.34 ± 23.43 |
Data (expressed as posterior mean ± standard error).
Figure 1.
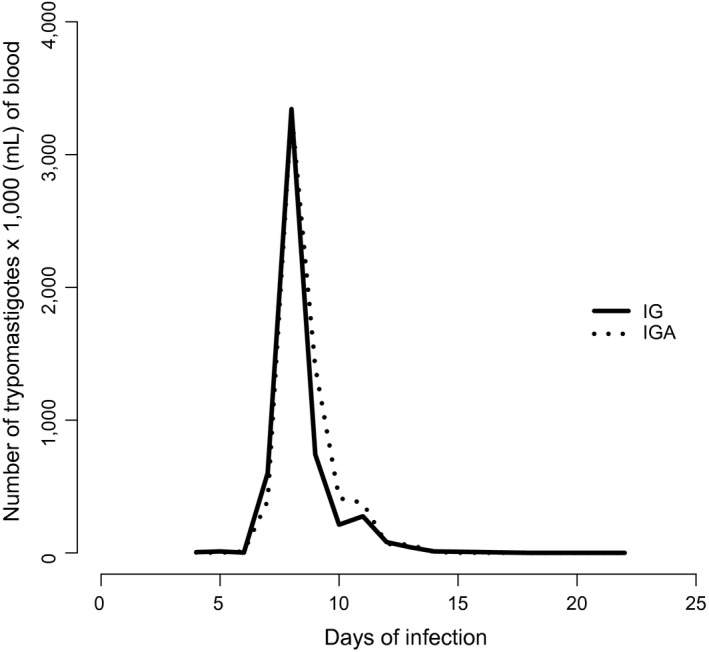
Curve of mean parasitaemia of Swiss male mice after 60 days of age, infected with Y strain of Trypanosoma cruzi; untreated (IG) and acetylsalicylic acid‐treated (IGA).
Food passage time
Both aspirin (CGA) and CD (IG) showed reduced speed of food traffic. In animals infected and treated with aspirin, the use of the NSAID appeared to have prevented this alteration, as the group IGA showed a similar traffic time as CG (Table 2).
Quantitative analysis of NADPH‐dp‐positive myenteric neurons
CD did not alter the number of nitrergic neurons found in the distal oesophageus of the infected animals (IG) significantly in comparison with the controls (CG). However, when infected animals were treated with aspirin, there was a significant increase (P < 0.05) in this neuronal subpopulation in comparison with the control group (Table 2, Figure 2).
Figure 2.
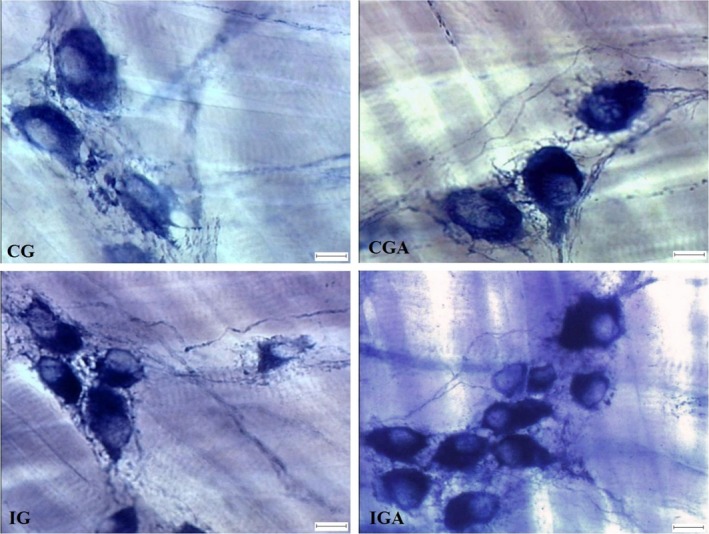
Microphotograph showing nitrergic myenteric plexus neurons in the oesophagus of Swiss male mice, at 141 days of age, stained with the NADPH‐dp technique. Uninfected control group treated with PBS (CG), uninfected control group treated with ASA (CGA), infected group treated with PBS (IG), infected group treated with ASA (IGA). Infection Y strain of Trypanosoma cruzi. Photonic microscope (Olympus BX50), 400×. Bar: 10 μm. [Colour figure can be viewed at wileyonlinelibrary.com].
Morphometry of myenteric NADPH‐dp‐positive neurons
Infection by T. cruzi led to atrophy of 10.29% of the cell body area of nitrergic myenteric neurons in the IG in relation to the GC. This alteration occurred due to a 5.34% decrease in nuclear area and 12.14% decrease in cytoplasmic area. The use of aspirin intensified this morphometric alteration, where animals in IGA showed a 12.75% reduction in the cell body area, with 13.28% nuclear and 13.03% cytoplasmic atrophy (IG × IGA) (Table 2, Figure 2).
Morphometric and histopathologic analysis of the oesophagus
Aspirin increased the thickness of the total wall of the oesophagus by 11.82% in non‐infected animals (CG × CGA). This increase was even greater in animals infected with T. cruzi, which showed an increase in total wall thickness of 20.37% and in the total tunica muscularis of 18.46% (CG × IG). On comparing the infected groups, IG and IGA, the treatment with aspirin caused a 6.46% decrease in the thickness of the total wall with reductions of 4.33% for the total tunica muscularis and 11.80% for the circular muscular and an increase of the longitudinal muscle by 6.80% (P < 0.05). No amastigote nests and inflammatory foci were found in the oesophagus of the mice of the groups IG and IGA (Table 3, Figure 3).
Table 3.
Wall morphometric analysis of Swiss male mice oesophagus, with 141 days of age
| Variable (μm) | Groups | |||
|---|---|---|---|---|
| CG | CGA | IG | IGA | |
| Wall | 168.40c ± 1.43 | 188.30b ± 3.41 | 202.70a ± 2.12 | 189.60b ± 1.89 |
| Musc Total | 120.80c ± 1.25 | 125.40c ± 2.33 | 143.10a ± 1.74 | 136.90b ± 1.61 |
| Musc Circ | 64.36c ± 1.18 | 67.31c ± 1.29 | 82.59a ± 1.21 | 72.84b ± 1.27 |
| Musc Long | 55.67ab ± 1.07 | 55.68ab ± 1.38 | 54.54b ± 0.88 | 58.25a ± 0.79 |
Data (expressed as posterior mean ± standard error) followed by the different letters in the same line are significantly different (P < 0.05; Bayesian contrasts).
Uninfected control group treated with PBS (CG), uninfected control group treated with ASA (CGA), infected group treated with PBS (IG) and infected group treated with ASA (IGA). Infection with Y strain of Trypanosoma cruzi.
Figure 3.
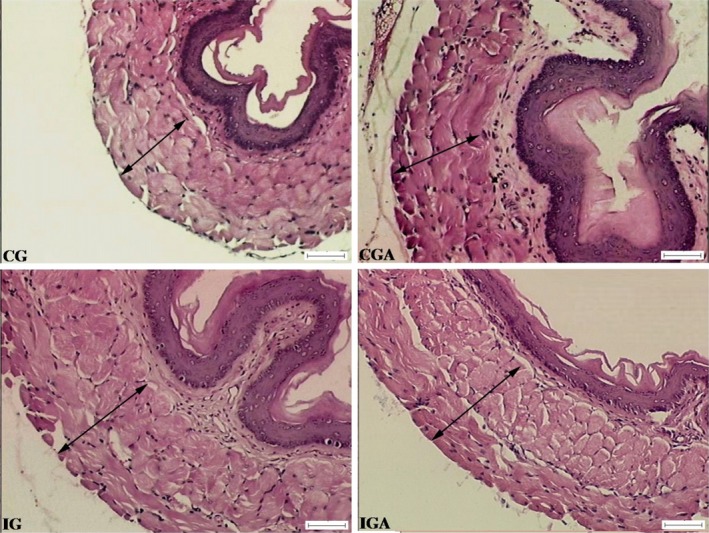
Microphotograph showing the overall muscular layer (arrow) in the oesophageal wall of Swiss male mice, with 141 days of age. Uninfected control group treated with PBS (CGC), uninfected control group treated with ASA (CGA), infected group treated with PBS (IG), infected group treated with ASA (IGA). Infection with 1300 blood trypomastigotes of Y strain of Trypanosoma cruzi. H. E. Photonic microscope (Olympus BX50), 100×. Bar: 50 μm. [Colour figure can be viewed at wileyonlinelibrary.com].
Discussion
Infected animals showed a characteristic profile of infection by the Y strain of T. cruzi. The parasitological parameters confirmed the disease and the beginning of the chronic phase indicated by the disappearance of the trypomastigote form from the bloodstream.
Despite the consensus in the literature that CD leads to oesophageal denervation (Nascimento et al. 2013; Adad et al. 2014), the present study demonstrated that T. cruzi infection did not lead to a reduction in the nitrergic neuron population, similar to our findings in an earlier study, in which we found a greater number of neurons compared with the present results (Massocatto et al. 2016). We attribute the atrophy of the oesophageal wall found in those animals to be reflected in a greater concentration of nitrergic neurons (Massocatto et al. 2016). In this study, however, the oesophageal wall showed hypertrophy, leading to a possible dispersion of the neurons and resulting in an apparent absence of a significant increase. It should be pointed out that our study analysed the nitrergic neurons, which in many disease situations appear to be protected from death compared with other neuronal subpopulations (Phillips et al. 2003; Hermes‐Uliana et al. 2011).
The infection was studied for 81 days, of which about 60 days, were during the CCD. In spite of the chronicity of the disease and of the short lifespan of the mouse, we believe that neuronal changes could be in progress, as functional changes in the human may take many years (Fiocruz 2013). A study by Köberle (1968) suggests that the loss of neurons in CCD is a slow and continuous process, occurring over decades, and that functional disturbances preceded macroscopic changes. In the CD megacolon atonic dilatation is the principal macroscopic characteristic and it can be expected that the first and/or main cause of this change is the loss of neurons, mostly excitatory neurons (Jabari et al. 2011).
NO is the principal inhibitory neurotransmitter responsible for relaxation of the lower oesophageal sphincter (LES), where guanylate cyclase is the enzyme target (GC‐NO) (Groneberg et al. 2015), which results in the synthesis of cyclic guanosine monophosphate (cGMP) (Friebe & Koesling 2003) in various target cells. The expression of GC‐NO was identified in various parts of the gastrointestinal tract, including smooth muscle cells (SMC) and interstitial cells of Cajal (ICC) (Lino et al. 2008; Groneberg et al. 2011). In humans, it has been reported in the literature that there is a dual mechanism of relaxation for the LES by NO through the SMC and ICC (Groneberg et al. 2015). In this study, we saw the maintenance of nitrergic neurons, but we were unaware of possible alterations that could have occurred in the ICC.
Treatment with aspirin in CCD increased the population density of nitrergic neurons in IGA when compared with CG. Oda et al. (2017) related the ASA treatment to a protective effect on colonic myenteric neurons, including nitrergic, of T. cruzi infected mice. The nitrergic neurons are those that use NO, whose synthesis is catalysed by nNOS. This gas, which is highly lipophilic, has a substantial antitrypanosomal action (Borges et al. 2009) and is linked to factors that facilitate the inflammatory process by having an effect on blood flow and vascular permeability (Liu et al. 2002). Moreover, the literature suggests that the use of NSAIDs promotes, indirectly, a decrease in plasma NO levels and an increase in parasite load (Tatakihara et al. 2008). Thus, the growth of the nitrergic population could be a compensatory mechanism to the use of aspirin, taking into account that increased NO synthesis maintains oesophageal motility and contains the development of the infection.
The infection led to atrophy of 10.29% of the cell body area of NADPH‐dp‐positive neurons, where there was a 5.34% decrease in the nuclear area and 12.14% decrease in the cytoplasmic area in the infected group in relation to the control (CG × IG). The infected animals treated with aspirin showed neurons with more marked atrophy in relation to infected animals not treated with aspirin. The decrease in neuronal area can indicate a reduction in function (Araújo et al. 2009), and consequently a lower production of NO.
The digestive manifestations of CD in the oesophagus are related to food retention and dilatation of the organ, with or without thickening of the oesophageal wall. In this study, treatment with aspirin as well as infection led to hypertrophy of the wall of the organ, where CD caused a greater thickening compared to the use of the NSAID. However, with the simultaneous presence of these two factors, in the group of animals infected and treated with aspirin (IGA), this hypertrophy was prevented so that there was only a 6.46% increase in total wall thickness, with a decrease in thickness of the total tunica muscularis by 4.33% and that of the circular muscle by 11.80%, on comparing IGA with the group infected but not treated (IG × IGA). Although IGA showed a more evident hypertrophy of the wall in relation to CGA and IG, when compared to CG, there was a 12.59% increase in wall thickness. In this case, the use of aspirin during chronic CD appeared to have exerted partial protection against hypertrophy of the organ under study.
The literature relates the pathogenesis of CD to the persistence of T. cruzi parasites in the affected organ, where there is a chronic inflammatory process (Tarleton 2001). No amastigote nests or inflammatory process could have contributed to the preservation of myenteric neurons of the oesophagus wall in infected mice.
Aspirin as well infection by T. cruzi induced an increase in intestinal transit time in chronic CD, which was similar to that found by Oliveira et al. (2008) in studying alterations in intestinal motility in Swiss mice infected with the Y strain of T. cruzi, during ACD, where the infected mice displayed a significant increase in time of defecation. Another study using aspirin reported delayed gastric emptying in humans (Rinetti et al. 1982). The animals of the infected group that were treated with aspirin exhibited an intestinal transit time equal to that of the control group, indicating that the use of NSAIDS prevented this alteration. Although we did find changes at the microscopic level in the oesophagus of the animals studied, the small intestine and other organs of the gastrointestinal tract must also be considered, as they often undergo changes due to CD. Thus, we cannot say that the differences in intestinal transit times were a result only of the changes described here in the oesophagus.
In conclusion, the results obtained in this study demonstrated for the first time in the literature that treatment with aspirin in chronic CD was capable of increasing the number of nitrergic neurons in the distal oesophageal portion, partially preventing hypertrophy of the oesophagus caused by the infection and impeding the change in intestinal transit time. Moreover, the use of the NSAID intensified atrophy of the myenteric nitrergic neurons observed in the animals infected with T. cruzi.
Authorship
I, Cristina Lorena Massocatto, confirm that all listed authors meet ICMJE authorship criteria and that nobody who qualifies for authorship has been excluded.
References
- Adad S.J., Andrade D.C., Lopes E.R. & Chapadeiro E. (1991) Pathological anatomy of chagasic megaesophagus. Rev. Inst. Med. Trop. 33, 443–450. [PubMed] [Google Scholar]
- Adad S.J., Etchebehere R.M. & Jammal A.A. (2014) Blood vessels in ganglia in human esophagus might explain the higher frequency of megaesophagus compared with megacolon. Rev. Inst. Med. Trop. 56, 529–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araújo E.J.A, Hermes C., Miranda N.M.H., Almeida E.C., Sant'Ana D.M.G. (2009) Atrophy of the nitrergic myenteric neurons in the descending colon rats submitted to protein and vitamin deficiency. Int. J. Morphol. 27, 939–945. [Google Scholar]
- Borges C.R., Rodrigues V. Jr, dos Reis M.A. et al (2009) Role of nitric oxide in the development of cardiac lesions during the acute phase of experimental infection with Trypanosoma cruzi . Rev. Soc. Bras. Med. Trop. 42, 170–174. [DOI] [PubMed] [Google Scholar]
- Brasil. Ministério da Saúde . (2010) Secretaria de Vigilância em Saúde. Departamento de Vigilância Epidemiológica. Doenças infecciosas e parasitárias: guia de bolso/Ministério da Saúde, Brasília: Ministério da Saúde. 444 p.
- Chan F.K. & Graham D.Y. (2004) Review article: prevention of non‐steroidal anti‐inflammatory drug gastrointestinal complications – review and recommendations based on risk assessment. Aliment. Pharmacol. Ther. 19, 1051–1061. [DOI] [PubMed] [Google Scholar]
- Fiocruz . Fundação Oswaldo Cruz (2013). Available from URL: http://www.agencia.fiocruz.br/doen%C3%A7a-de-chagas (Accessed February 5, 2016).
- FitzGerald G.A. & Patrono C.P. (2001) The coxibs, selective inhibitors of cyclooxygenase‐2. N. Engl. J. Med. 345, 433–442. [DOI] [PubMed] [Google Scholar]
- Foong J.P., Tough I.R., Cox H.M. & Bornstein J.C. (2014) Properties of cholinergic and non‐cholinergic submucosal neurons along the mouse colon. J. Physiol. 15, 777–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friebe A. & Koesling D. (2003) Regulation of nitric oxide‐sensitive guanylyl cyclase. Circ. Res. 93, 96–105. [DOI] [PubMed] [Google Scholar]
- Furness J.B., Callaghan B.P., Rivera L.R. & Cho H.J. (2014) The Enteric Nervous System and Gastrointestinal Innervation: Integrated Local and Central Control. Microbial Endocrinology: the Microbiota‐Gut‐Brain Axis in Health and Disease. Lyte M., Cryan J.F. Adv. Exp. Med. Biol. 817, 39–71. [DOI] [PubMed] [Google Scholar]
- Gascona J., Bern C. & Pinazo M.J. (2010) Chagas disease in Spain, the United States and other non‐endemic countries. Acta Trop. 115, 22–27. [DOI] [PubMed] [Google Scholar]
- Greggio F.M., Fontes R.B.V., Maifrino L.B., Castelucci P., Souza R.R. & de Liberti E.A. (2010) Effects of perinatal protein deprivation and recovery on esophageal myenteric plexus. World J. Gastroenterol. 16(5), 563–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groneberg D., Konig P., Koesling D. & Friebe A. (2011) Nitric oxide‐sensitive guanylyl cyclase is dispensable for nitrergic signaling and gut motility in mouse intestinal smooth muscle. Gastroenterology 140, 1608–1617. [DOI] [PubMed] [Google Scholar]
- Groneberg D., Zizer E., Lies B. et al (2015) Dominant role of interstitial cells of Cajal in nitrergic relaxation of murine lower oesophageal sphincter. J. Physiol. 593, 403–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidelberger P. & Welch P. (1983) Simulation run length control in the presence of an initial transient. J. Oper. Res. 31, 1109–1144. [Google Scholar]
- Hermes‐Uliana C., Pereira‐Severi L.S., Luerdes R.B. et al (2011) Chronic infection with Toxoplasma gondii causes myenteric neuroplasticity of the jejunum in rats. Auton. Neurosci. 160, 3–8. [DOI] [PubMed] [Google Scholar]
- Jabari S., da Silveira A.B.M., de Oliveira E.C. et al (2011) Partial, selective survival of nitrergic neurons in chagasic megacolon. Histochem. Cell Biol. 135, 47–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köberle F. (1968) Chagas’ disease and Chagas’ syndromes: the pathology of American trypanosomiasis. Adv. Parasitol. 6, 63–116. [DOI] [PubMed] [Google Scholar]
- Lambert D. (1992) Zero‐inflated Poisson regression, with an application to defects in manufacturing. Technometrics 34, 1–14. [Google Scholar]
- Lino S., Horiguchi K. & Nojyo Y. (2008) Interstitial cells of Cajal are innervated by nitrergic nerves and express nitric oxide‐sensitive guanylate cyclase in the guinea‐pig gastrointestinal tract. Neuroscience 152, 437–448. [DOI] [PubMed] [Google Scholar]
- Liu B., Liu X., Tang C., Liu J., Wang H. & Xie P. (2002) Esophageal dysmotility and the change of synthesis of nitric oxide in a feline esophagitis model. Dis. Esophagus 15, 193–198. [DOI] [PubMed] [Google Scholar]
- Massocatto C.L., Moreira N.M., Muniz E. et al (2016) Aspirin prevents atrophy of esophageal nitrergic myenteric neurons in a mouse model of chronic Chagas disease. Dis. Esophagus 30, 1–8. [DOI] [PubMed] [Google Scholar]
- Molina‐Berríos A., Campos‐Estrada C., Henriquez N. et al (2013) Protective role of acetylsalicylic acid in experimental Trypanosoma cruzi infection: evidence of a 15‐epi‐lipoxin A₄‐mediated effect. PLoS Negl. Trop Dis. 7(4), e2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee S., Machado F.S., Huang H. et al (2011) Aspirin Treatment of Mice Infected with Trypanosoma cruzi and Implications for the Pathogenesis of Chagas Disease. PLoS ONE 6, e16959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nascimento R.D., Martins P.R., Lisboa A.S., Adad S.J., da Silveira A.B.M. & Reis D. (2013) An imbalance between substance P and vasoactive intestinal polypeptide might contribute to the immunopathology of megaesophagus after Trypanosoma cruzi infection. Hum. Pathol. 44, 269–276. [DOI] [PubMed] [Google Scholar]
- Oda J.Y., Belém M.O., Carlos T.M. et al (2017) Myenteric neuroprotective role of aspirin in acute and chronic experimental infections with Trypanosoma cruzi . Neurogastroenterol. Motil. 29(10), 1–13. [DOI] [PubMed] [Google Scholar]
- Oliveira G.M., Medeiros M.M., da Silva B.W., Santana R., Araújo‐Jorge T.C. & Souza A.P. (2008) Applicability of the use of charcoal for the evaluation of intestinal motility in a murine model of Trypanosoma cruzi infection. Parasitol. Res. 102, 747–750. [DOI] [PubMed] [Google Scholar]
- Phillips R.J., Kieffer E.J. & Powley T.L. (2003) Aging of the myenteric plexus: neuronal loss is specific to cholinergic neurons. Auton. Neurosci. 106, 69–83. [DOI] [PubMed] [Google Scholar]
- R Development Core Team . (2015). R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; ISBN3‐900051‐07‐0. Available from URL: http://www.R-project.org (Acessed August 1 2015). [Google Scholar]
- Rinetti M., Ugolotti G., Calbiani B., Colombi‐Zinelli L., Cisternino M. & Papa N. (1982) Anti‐inflammatory drugs and gastric emptying. A comparison between acetylsalicylic acid and carprofen. Arzneimittelforschung 32, 1561–1563. [PubMed] [Google Scholar]
- Tarleton R.L. (2001) Parasite persistence in the etiology of Chagas disease. Int. J. Parasitol. 31, 550–554. [DOI] [PubMed] [Google Scholar]
- Tatakihara V.L.H., Cecchini R., Borges C.L. et al (2008) Effects of cyclooxygenase inhibitors on parasite burden, anemia and oxidative stress in murine Trypanosoma cruzi infection. Immunol. Med. Microbiol. 52, 47–58. [DOI] [PubMed] [Google Scholar]
- World Health Organization . (2015). Chagas disease (American trypanosomiasis). Available from URL: http://www.who.int/mediacentre/factsheets/fs340/en/ (Accessed February 5, 2016).


