Summary
Sepsis is associated with high mortality. Both critically ill humans and animal models of sepsis exhibit changes in their glucose homeostasis, that is, hypoglycaemia, with the progression of infection. However, the relationship between basal glycaemia, glucose tolerance and insulin sensitivity is not well understood. Thus, we aimed to evaluate this glucose homeostasis triad at the late stage of sepsis (24 h after surgery) in male Swiss mice subjected to lethal and sublethal sepsis by the caecal ligation and puncture (CLP) model. The percentage of survival 24 h after CLP procedure in the Lethal and Sublethal groups was around 66% and 100% respectively. Both Lethal and Sublethal groups became hypoglycaemic in fasting and fed states 24 h after surgery. The pronounced fed hypoglycaemia in the Lethal group was not due to worsening anorexic behaviour or hepatic inability to deliver glucose in relation to the Sublethal group. Reduction in insulin sensitivity in CLP mice occurred in a lethality‐dependent manner and was not associated with glucose intolerance. Analysis of oral and intraperitoneal glucose tolerance tests, as well as the gastrointestinal motility data, indicated that CLP mice had reduced intestinal glucose absorption. Altogether, we suggest cessation of appetite and intestinal glucose malabsorption are key contributors to the hypoglycaemic state observed during experimental severe sepsis.
Keywords: appetite, caecal ligation and puncture, glucose tolerance, insulin sensitivity, metabolism, sepsis
Sepsis is associated with organ dysfunction induced by a dysregulation of host defence against an infection (Singer et al. 2016). This complex clinical syndrome has been challenging both scientists and clinicians for many decades (Rittirsch et al. 2009; Dejager et al. 2011). Despite the advance of modern medicine, the mortality of septic patients continues to be very high (around 25% for sepsis and 46% for septic shock) and the pathophysiologic mechanisms associated with the development and outcome of this disease are poorly understood (Besen et al. 2016). Therefore, the understanding of the processes involved in the complex pathology of sepsis is important to refine the therapeutic approach (Dejager et al. 2011).
The gold standard preclinical model for studying sepsis‐related mechanisms is the caecum ligation and puncture (CLP) model (Rittirsch et al. 2009; Dejager et al. 2011). CLP model combines tissue trauma due to laparotomy, necrosis induced by caecum ligation and an infection caused by leakage of enteric microorganisms into the peritoneum, and in more severe degrees, this infection leads to a systemic inflammatory response, tissue injury, shock and death (Rittirsch et al. 2009; Dejager et al. 2011). All of these characteristics observed in CLP model are found in septic patients, making this a suitable model for studying the sepsis process (Deitch 1998).
The early cardiometabolic dysfunctions observed after CLP procedure include increased cardiac output, reduced peripheral insulin sensitivity and hyperglycaemia, a stage termed as hyperdynamic and hypermetabolic (Yelich 1990; Hahn et al. 1995; Tang et al. 1998; Maitra et al. 2000). Hypermetabolic sepsis stage involves increased circulating levels of catecholamines (Hahn et al. 1995), corticosterone (Tang et al. 1998) and glucagon (Yelich 1990) leading to enhanced gluconeogenesis and hyperglycaemia. This initial stage is followed by a hypodynamic and hypometabolic stage, which is related to decreased tissue perfusion, increased total peripheral vascular resistance and hypoglycaemia (Yelich 1990; Hahn et al. 1995; Tang et al. 1998; Maitra et al. 2000; Heuer et al. 2004; Sordi et al. 2011; Singamsetty et al. 2016; Yamashita et al. 2017). With the progression of sepsis installation in rodents (from hypermetabolic to hypometabolic stages), that varies between 16 and 24 h post‐CLP procedure, blood glucose may be found slightly increased (Igarashi et al. 1992), unchanged (Kelleher et al. 1982) or reduced (Maitra et al. 2000; Heuer et al. 2004; Sordi et al. 2011; Singamsetty et al. 2016; Yamashita et al. 2017). The understanding of glucose homeostasis in the hypometabolic stage is important to verify how the organism adapts to such septic context and to support the adequate therapeutic manoeuvres at this critical moment. For instance, both critically ill humans and CLP mice exhibit intestinal impairments that result in reduced glucose absorption (Deane et al. 2014). Thus, the appropriate sepsis stage for introducing and combining enteral or parenteral nutrition in intensive care patients to achieve the best glycemic control is under continuous debate (Wernerman 2012; Hoffer & Bistrian 2016; Oliveira Filho et al. 2016).
Conflicting data and the lack of any previous studies that focus specifically on the triad glycemic homeostasis – blood glucose, glucose tolerance and insulin sensitivity – in the hypometabolic stage of sepsis motivated us to investigate the main glucose homeostasis parameters (i.e., fasting and fed blood glucose, glucose tolerance and insulin sensitivity among other parameters). These parameters were evaluated in the hypometabolic stage of sepsis (24 h after CLP) in sublethal and lethal sepsis. We hypothesize that CLP mice at the hypometabolic stage develop glucose intolerance, insulin resistance and hypoglycaemia associated with poor outcomes, in a lethality‐dependent manner.
Methods
Materials
Regular human recombinant insulin (Humulin®) was purchased from Lilly (Indianapolis, IN, USA). The reagents used in the glucose tolerance test, hepatic glycogen content and gastrointestinal motilities protocols were from LabSynth (Diadema, SP, Brazil) and Sigma (St. Louis, MO, USA). Xylazine (2% xylazine hydrochloride) and ketamine (10% ketamine hydrochloride) were acquired from Syntec (Santana de Parnaíba, SP, Brazil). Plasma insulin was quantified by AlphaLISA® technology (Perkin Elmer, Waltham, MA, USA), TNF‐α was quantified by enzyme‐linked immunosorbent assay (ELISA) (R&D Systems, Minneapolis, MN, USA) and triacylglycerol by colorimetric (Labtest, Lagoa Santa, MG, Brazil).
Animals
Experiments were performed on male Swiss mice. The postweaned mice were obtained from the Federal University of Santa Catarina Animal Breeding Center and were kept at 21 ± 2°C on a 12‐h light–dark cycle (lights on at 06:00, lights off at 18:00). Mice (20–25 g) had access to food (commercial standard chow for rats, BIOBASE® 9301; Águas Frias, SC, Brazil) and water ad libitum for the entire period of housing acclimation lasting 3 days before caecum ligation and puncture (CLP). We used outbred mice to preserve the heterogeneity presented by septic patients.
Sepsis model
The mice were randomly distributed into four groups: control non‐operated (Naive), control operated (Sham), sublethal CLP (Sublethal) and lethal CLP (Lethal). Sepsis was induced by CLP based on previous descriptions (Wichterman et al. 1980; Spiller et al. 2011) between 08:00 and 09:00. Briefly, fed mice were anaesthetized intraperitoneally (i.p.) with ketamine and xylazine [80/15 mg/kg body mass (b.m.)]. Under aseptic conditions, a 1‐cm midline laparotomy was performed to allow the exposure of the caecum. The caecum was ligated with a 4.0 silk suture at its base, below the ileocaecal valve, without causing bowel obstruction. The caecum was then punctured one single time (crossing both intestine walls) with a 26‐ or 23‐gauge needle to induced two degrees of lethality as sublethal and lethal respectively. Then, the caecum was squeezed, and a ‘controlled’ amount of caecal content was released through the punctures. The caecum was placed back in the abdominal cavity, and the peritoneal wall and skin incision were sutured. Sham‐operated mice underwent a similar procedure, but the caecum was not ligated or punctured. Immediately after surgery, all mice received 1 ml of sterile saline subcutaneously. Chow pellets were kept inside the cage after CLP procedure for the entire period of the experiment (except when fasting was required). No antibiotics or anti‐inflammatory was administered to avoid confounding factors on the parameters evaluated. In order to monitor the health condition of experimental mice, we use the following clinical score to evaluate the symptoms reflecting murine sepsis: piloerection, periorbital exudates, diarrhoea, social isolation, respiratory distress and lethargy. Mice included in the survival curve measurements were evaluated each 6–12 h, and those with 3 or more of the previous moribund signs for more than 24 h were euthanized to avoid excessive suffering (Burkholder et al. 2012). Due to the variability in the mortality, the number of animals varied in each set of experiments.
Survival, leucocytes migration in peritoneal lavage fluid and leucogram
After CLP procedure, the survival was monitored for six consecutive days in each group. Differences in survival were analysed using a log‐rank test (Prism 5 software, Graph Pad Software, La Jolla, CA, USA). Separate groups were euthanized (cervical dislocation) 24 h after CLP procedure and used for determination of leucocytes in peritoneal lavage fluid and leucogram. Briefly, 3 ml of sterile saline was administered into the peritoneal cavity, and the peritoneal fluid was then collected. The trunk blood was collected to leucogram. Total leucocytes count was performed using an automated cell counter (ADVIA 60 Hematology System, Bayer Corporation, USA).
Respiratory frequency
The respiratory frequency was measured 24 h after CLP procedure in a separate group of unanaesthetized mice. Briefly, mice were acclimatized for 6 min and posteriorly the respiratory frequency was performed using the whole‐body plethysmography method (Moraes et al. 2011). It was measured as the airflow was suspended for short periods (2 min), and the pressure oscillations caused by breathing were captured by a pressure differential transducer connected to a signal amplifier (ML Spirometer, PowerLab; ADInstruments; Colorado Springs, CO, USA). The signals were then captured by an acquisition system (PowerLab; ADInstruments), and data analysis was performed in LabChart 7 Pro (ADInstruments). The respiratory frequency was defined as the number of breathing per minute (bpm).
Metabolic measurements
Body weight and food intake were measured from one day before the CLP procedure and for the two following days (from the same group of mice used for survival curve). Twenty‐four hours after CLP, separate groups of fasted (12 h) and non‐fasted mice had blood collected from the tail tip to measure blood glucose levels with a glucometer (Accu‐Check® Performa; Roche Diagnostics GmbH, Mannheim, Germany). Then, mice were euthanized as previously mentioned and the trunk blood was collected in EDTA‐NaF‐containing tubes (Glistab – Labtest; Lagoa Santa, MG, Brazil) to obtain the plasma. The plasma, obtained after blood centrifugation (600 × g), was stored at −80°C to measure plasma insulin, TNF‐α and triacylglycerol levels with the kits and methods described according to the manufacturer's instructions.
Time course of blood glucose
The time course of glycemic values were performed in a separate group of fed mice. Blood samples were collected by snipping the tail tip. The first drop was discarded, and the second drop was used for the determination of glycaemia (time 0) using a glucometer as described before. Then, ketamine and xylazine 80/15 mg/Kg b.m. were immediately administered, and blood samples were collected from the tail tip at 1, 2, 4, 6, 12 and 24 h for blood glucose measurements. During the first 12 h, chow pellets were offered inside the cage and were weighted between each interval to determine the food consumption. Twenty‐four hours after CLP, fasted mice (12 h) were submitted to the procedures of gastric emptying and intestinal transit that are described thereafter.
Liver glycogen measurement
Determination of hepatic glycogen content was performed according to a previous publication (Giozzet et al. 2008) in the same groups of mice used for determination of fasting blood glucose. Briefly, liver samples were transferred to test tubes containing 30% KOH and boiled for 1 h until complete digestion. Na2SO4 was then added, and the glycogen was precipitated with ethanol. The samples were centrifuged at 800 × g for 10 min, the supernatants were discarded and the glycogen dissolved in hot distilled water. Ethanol was added and the pellets obtained after a second centrifugation were dissolved in distilled water in a final volume of 20 ml. Glycogen content was measured by treating a fixed volume of sample with phenol reagent and H2SO4, and the absorbance was then read at 490 nm with a spectrophotometer.
Intraperitoneal insulin tolerance test (ipITT)
The insulin tolerance test was performed 24 h after CLP procedure in a separate group of fed mice that had their tail tip cut for blood collection. The first drop was discarded, and the second drop was used for the determination of glycaemia (time 0) using a glucometer as described before. An insulin solution, prewarmed at 36°C (1 IU/Kg, b.m., i.p.), was immediately administered, and blood samples were collected from the tail tip at 5, 10 and 15 min for blood glucose measurements The constant rate of glucose disappearance (K ITT) was calculated from the slope of the regression line obtained with log‐transformed glucose values between 0 and 15 min after insulin administration (Protzek et al. 2014).
Oral (oGTT) and intraperitoneal (ipGTT) glucose tolerance tests
The glucose tolerance test was performed 24 h after CLP procedure in separated groups of mice. In one set of experiments, fasted (12 h) mice had blood collected and glycaemia measured (time 0) as for ITT. Then, a 50% glucose solution, prewarmed at 36°C (2 g/kg, b.m., i.p.), was immediately administered, and blood samples were collected from the tail tip at 15, 30 and 120 min for blood glucose measurements. In another set of experiments, the same procedure was performed, but glucose solution was administered orally. The area under glucose curve (AUC) was calculated as described previously (Rafacho et al. 2013; Protzek et al. 2014).
Gastric emptying and intestinal transit
The gastric emptying and intestinal transit were evaluated using a method previously reported (Anderson et al. 2007; Fergani et al. 2007). Briefly, mice received 0.1 ml of dye solution (methylene blue dissolved in 10% dextrose solution) via oral gavage in a separate group of fasted (12 h) mice. After 30 min, mice were euthanized, and the stomach was clamped above the oesophageal sphincter and below the pylorus. Then, the stomachs were immediately stored in −70°C for posterior determination of the amount of dye remained in the stomach according to previous publications (Anderson et al. 2007; Fergani et al. 2007). Higher the final absorbance, lower the gastric emptying. For determination of intestinal transit, the small intestine was carefully removed. The intestinal transit was determined as the most distal point of methylene blue in the intestine and expressed as a percentage of the total length of the intestine (Fergani et al. 2007).
Statistical analysis
The results are expressed as the mean ± SEM of the indicated number (n) of animals. All graphs were plotted and analysed using Prism 5 for Windows (GraphPad Software). The symmetry of the data was tested by Kolmogorov–Smirnov and Shapiro–Wilk normality tests. Analysis of variance (anova) (one‐way anova) for unpaired groups followed by Tukey's post hoc test was utilized for multiple comparisons of parametric data or Kruskal–Wallis followed by Dunn's post hoc when variables achieved asymmetric distribution. Extreme studentized deviate method was applied to determinate whether one of the values reached significant outlier (Grubb's test from online available GraphPad QuickCalcs). When indicated, parametric Student's t‐test or nonparametric Mann–Whitney and Wilcoxon tests, for unpaired or paired groups, were also applied. Significance was set at P < 0.05.
Ethical approval statement
The experiments with mice were approved by the Federal University of Santa Catarina Committee for Ethics in Animal Experimentation (approval ID number: PP00782) that is in accordance with the National Council for Animal Experimentation Control (CONCEA).
Results
Survival, respiratory frequency and leucocyte migration
To characterize the lethality of sepsis, we followed the survival rate during 6 days after CLP procedure. The percentage of survival 24 h after CLP procedure in the Lethal and Sublethal groups was about 66% and 100% respectively (Figure 1a). A hundred percentage of mortality was observed in the Lethal group 5 days post‐CLP procedure, while 48% of the Sublethal mice survived at the end of the observed period (n = 6–12, P < 0.05). All Sham mice survived at the end of observed period. The clinical score, based on five different parameters, reinforced the more pronounced lethality in the Lethal group (Figure 1b) (n = 12–16, P < 0.05). To indirectly verify the metabolic status of mice, we quantified the respiratory frequency. As shown in Figure 1c, the respiratory frequency was significantly lower in both groups of mice subjected to CLP procedure (P < 0.05). The median values were 273, 306, 205 and 210 bpm for Naive, Sham, Sublethal and Lethal groups, respectively (n = 6–9), which means a reduction of ~30% in the respiratory frequency for both CLP groups when compared to Sham mice (Figure 1d). To analyse whether the CLP procedure caused activation of the immune system, we counted the blood and peritoneal leucocytes 24 h after surgery (Figure 1e, f). A progressive reduction in total blood leucocytes with an increase in leucocytes in the peritoneal cavity was observed in Sublethal and Lethal groups (n = 7–19, P < 0.05). No significant differences were observed between Naive and Sham group (Figure 1f). These data demonstrated that CLP model can lead to different degrees of sepsis lethality, characterized by reduced metabolic state and increased leucocytes migration to the peritoneal cavity 24 h after surgery.
Figure 1.
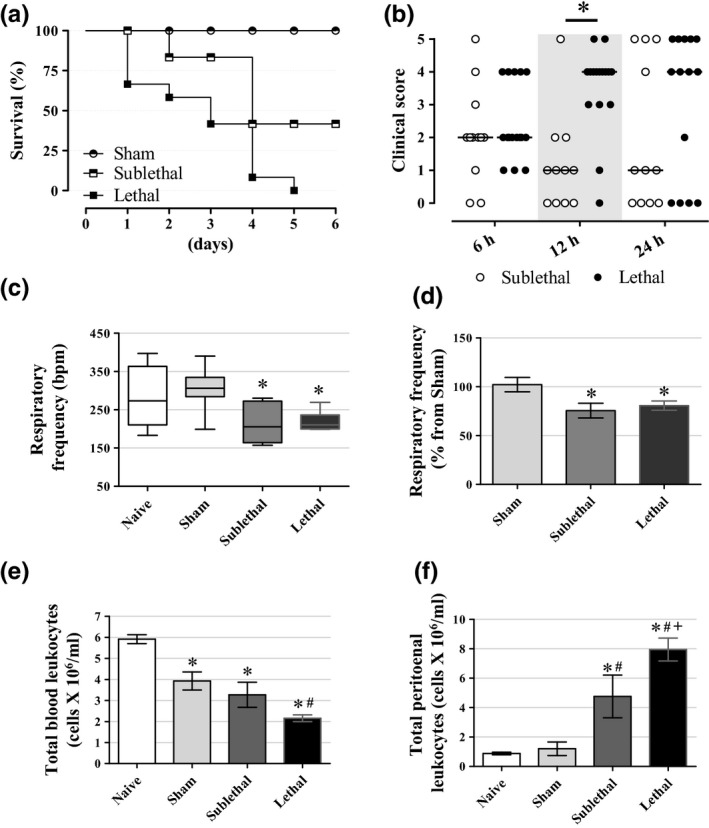
Survival and leucocyte migration (a) Survival during the progression of sepsis induced by CLP. The sepsis increased mortality according to CLP severity. Results are expressed as per cent survival using log‐rank test (n = 6 for Sham, 7 for Sublethal and 12 for Lethal groups). (b) Clinical score at 6, 12 and 24 h after CLP procedure (i.e., piloerection, periorbital exudates, diarrhoea, social isolation, respiratory distress) (n = 12 for Sublethal and 16 for Lethal groups). (c, d) The respiratory frequency was significantly reduced in CLP groups in relation to the Naive and Sham groups 24 h after surgery (n = 9 for Naive, 9 for Sham, 6 for Sublethal and 6 for Lethal groups). (e) Total blood leucocytes 24 h after CLP. We can see a significant decrease in blood leucocytes in the CLP groups, more pronounced in the Lethal group (n = 18 for Naive, 14 for Sham, 10 for Sublethal and 12 for Lethal groups). (f) Total peritoneal leucocytes. We can note the lethality dependence of leucocytes number inside the peritoneal cavity (n = 17 for Naive, 10 for Sham, 9 for Sublethal and 14 for Lethal groups). Results are expressed as mean ± SEM in ‘d, e, f’ graphs and as median and interquartiles in ‘c’ graph. *significantly different vs. Sublethal group in ‘b’ graph and vs. Naive group in ‘c, d, e, f’ graphs, #significantly different vs. Sham group and +significantly different vs. Sublethal group using anova with Tukey's post hoc test in ‘d, e, f’ data or Kruskal–Wallis with Dunn's post hoc test in ‘b, c’ data. P < 0.05.
Body mass, food intake and basal glycemic status
There were no differences in body mass and food intake among the groups before the CLP or Sham procedures (Figure 2a, b). Both groups subjected to CLP procedure exhibited ~10% and ~20% of body weight reduction in 24 h and 48 h after surgery respectively (n = 5–9, P < 0.05). This body mass reduction was accompanied by a sharp reduction in food intake for the next 2 days post‐CLP in both septic groups (P < 0.05). Sham‐operated mice had a similar evolution in body mass, and food intake as the Naive group did. Fasting blood glucose levels 24 h after sepsis induction were significantly lower in both the CLP groups when compared to the Naive mice (Figure 2c). The blood glucose values were also lower in the CLP groups during the fed state 24 h after sepsis induction, when compared with the Naive and Sham groups, being more pronounced in the Lethal group. The fed glycaemic values were higher in all groups in relation to their fasting levels except in the Lethal group that exhibited no increment of blood glucose levels compared to their fasting blood glucose levels (60 ± 3 mg/dl for fasting and 61 ± 6 mg/dl for fed state) (n = 8–12, P < 0.05). These results demonstrate that sepsis induces body mass loss together with hypophagia and hypoglycaemia. Triacylglycerolaemia was similar among all groups of mice (data not shown). To verify the hepatic glycogen stores, we measured it in fasted (~12 h) mice 24 h after CLP procedure (Figure 2d, e). The hepatic glycogen content was reduced in all operated mice (Sham and CLP) (n = 8–12, P < 0.05). Normalized values of hepatic glycogen in relation to the Naive group revealed a significant decrease in this parameter in both the CLP mice groups in relation to the Sham group (P < 0.05) with no differences between the Sublethal and Lethal groups (Figure 2e). These data demonstrated that sepsis negatively impacted basal glucose homeostasis and revealed that the pronounced reduction in blood glucose levels in the Lethal group, compared with the Sublethal group, was not related to an exacerbated anorexic behaviour or hepatic inability to delivers glucose.
Figure 2.
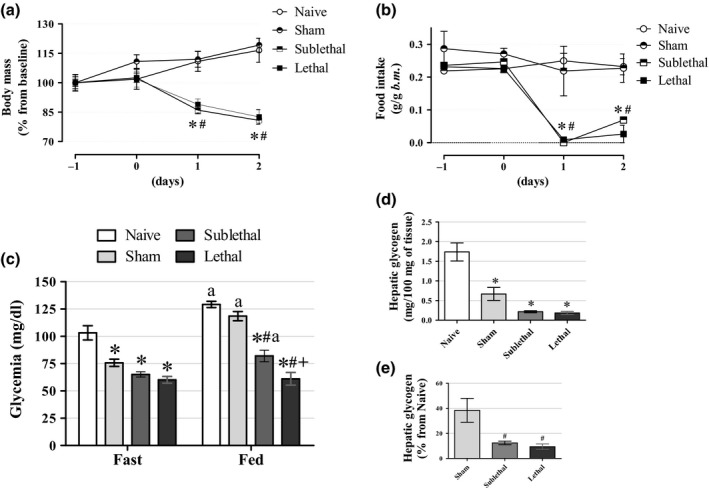
Body mass, food intake and blood glucose levels (a) The average body mass values during the progression of sepsis induced by CLP. We can note the significant reduction in body mass in the CLP groups after 24 and 48 h of surgery (n = 5 for Naive, 5 for Sham, 9 for Sublethal and 9 for Lethal groups). (b) The average food intake values during the progression of sepsis induced by CLP. We can observe the significant reduction in food intake after 24 and 48 h of surgery in the CLP groups (n = 5 for Naive, 5 for Sham, 9 for Sublethal and 9 for Lethal groups). (c) Fasting and fed blood glucose values 24 h after CLP. Fasting blood glucose values were significantly decreased in Sham and CLP mice, which remained reduced only in the CLP mice in their fed state (n = 12 for Naive, 12 for Sham, 8–11 for Sublethal and 9–11 for Lethal groups). (d, e) Hepatic glycogen content 24 h after CLP in 12‐h fasted mice. We can observe the pronounced reduction in hepatic glycogen content in both CLP groups (n = 8 for Naive, 8 for Sham, 9 for Sublethal and 7 for Lethal groups). Results are expressed as mean ± SEM. *significantly different vs. Naive group, #significantly different vs. Sham group and +significantly different vs. Sublethal group using anova with Tukey's post hoc test. P < 0.05.
Insulin sensitivity and serum insulin and TNF‐α levels
To verify whether the peripheral insulin sensitivity is altered in septic mice, we performed an ipITT in mice with no chow restriction 24 h after CLP procedure (Figure 3a). The ipITT data revealed a significant reduction in insulin sensitivity in both the CLP groups compared with the Naive mice as judged by the constant rate of glucose disappearance (K ITT; n = 8–13, P < 0.05; Figure 3b). Only the Lethal group had lower K ITT values in relation to the Sham group (2.1 ± 0.4%·min−1 and 5.9 ± 0.2%·min−1 for Lethal and Sham mice respectively). Accordingly, fasting plasma insulin values were higher in the CLP groups, being more pronounced in the Lethal group, when compared to the Naive mice (n = 4–8, P < 0.05; Figure 3c). The median values were 13.4 pg/ml, 42.8 pg/ml, 81.1 pg/ml and 101.3 pg/ml for Naive, Sham, Sublethal and Lethal groups, respectively. Plasma TNF‐α levels, a marker of an inflammatory stage, were increased in both the CLP groups, being more pronounced in the Sublethal group (n = 5–6, P < 0.05; Figure 3d). No differences in circulating TNF‐α levels were observed between the CLP groups or between the Sham and Naive groups. Altogether, these data indicated that insulin insensitivity occurs in a lethality‐dependent manner even in a context of hypoglycaemia. This could be an alternative route for glucose metabolic response which would facilitate shifting blood glucose from peripheral uptake to increase central nervous system availability.
Figure 3.
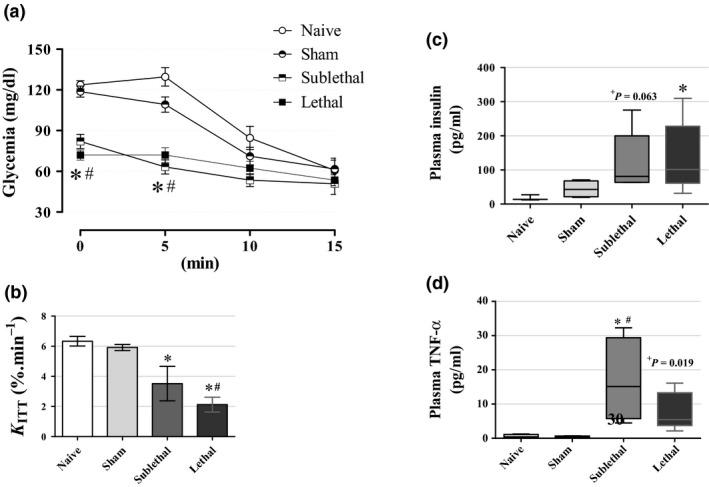
Insulin sensitivity and circulating insulin levels (a) Intraperitoneal insulin tolerance test (ipITT) and (b) the constant rate for glucose disappearance during ipITT (K ITT) (n = 13 for Naive, 12 for Sham, 8 for Sublethal and 8 for Lethal groups). We can observe the reduction in insulin sensitivity according to lethality degree in the CLP groups 24 h after surgery. (c) Fasting insulin values were significantly higher in the Lethal group with a tendency to increase in the Sublethal group (n = 3 for Naive, 5 for Sham, 8 for Sublethal and 8 for Lethal groups). (d) Circulating TNF‐α levels in fasted mice were significantly higher in the Sublethal group with a tendency to also increase in the Lethal group 24 h after CLP (n = 5 for all groups). Results are expressed as mean ± SEM in ‘a, b’ graphs and as median and interquartiles in ‘c, d’ graphs. *significantly different vs. Naive group and #significantly different vs. Sham group using anova with Tukey's post hoc test for ‘a, b’ data or Kruskal–Wallis with Dunn's post hoc test for ‘c, d’ data. P < 0.05. +means that unpaired Mann–Whitney test was applied for comparison of the Sublethal in ‘c’ data and the Lethal in ‘d’ data with the Sham group respectively.
Glucose tolerance
Considering the CLP mice were hypoglycaemic, but insulin insensitive, we aimed to determine whether they also present any glucose tolerance disturbance. Using the oral protocol for the glucose tolerance test, which preserves the incretin effect, we observed lower blood glucose values at min 15 and 30 postglucose load in both the CLP groups, when compared with the Naive and Sham groups (Figure 4a–c). Such reduced glycemic values mean that septic mice were more glucose tolerant as observed by AUC data (Figure 4d; n = 6–14, P < 0.05). No differences in glucose tolerance were observed between the Naive and Sham groups. By intraperitoneal glucose tolerance tests (n = 5–8; Figure 4e, f), the CLP mice were normotolerant in relation to both controls (Naive and Sham groups) as observed by the blood glucose curve as well as the AUC data respectively. The median AUC values were 8052 mg/dl.120 min−1, 5561 mg/dl.120 min−1, 6642 mg/dl.120 min−1 and 5805 mg/dl.120 min−1 for the Naive, Sham, Sublethal and lethal groups respectively. These results showed that CLP animals exhibited no glucose tolerance deficiency.
Figure 4.
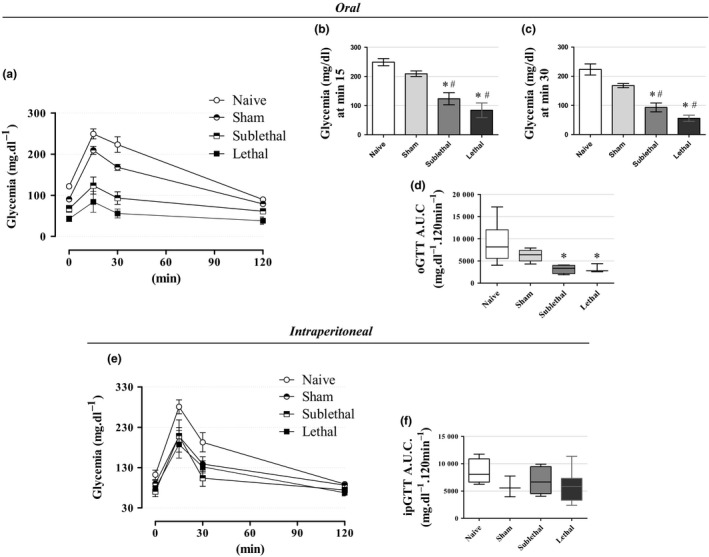
Glucose tolerance (a‐c) The average blood glucose values during an oral glucose tolerance test (oGTT) and (d) the respective area under curve (A.U.C.) for blood glucose values in fasted mice. The oGTT values revealed an increase in glucose tolerance in the CLP groups 24 h after surgery (n = 14 for Naive, 10 for Sham, 6 for Sublethal and 6 for Lethal groups). (e) The average blood glucose values during an intraperitoneal glucose tolerance test (ipGTT) and (f) the respective A.U.C. for blood glucose values. The ipGTT values revealed no alterations in glucose tolerance in the CLP groups 24 h after surgery (n = 5 for Naive, 5 for Sham, 7 for Sublethal and 8 for Lethal groups). Results are expressed as mean ± SEM in ‘a, b, c, e’ graphs, and as median and interquartiles in ‘d, f’ graphs. *significantly different vs. Naive group and #significantly different vs. Sham group using anova with Tukey's post hoc test for ‘a, b, c, e’ data or Kruskal–Wallis with Dunn's post hoc test for ‘d, f’ data. P < 0.05.
Time‐course blood glucose and gastrointestinal motility
To verify the course of blood glucose levels after the CLP procedure, we accompanied the glycemic values during 24 h postsurgery period. All groups exhibited a marked increase in blood glucose values during the first and second hours as a result of anaesthesia effect (see Naive group results) (n = 5–16; Figure 5a, b). However, the glucose decay between 1–6 h was higher in both CLP groups (P < 0.05; Figure 5d), which occurred together with the abolishment of any significative food intake in these CLP groups (Figure 5c). This hypoglycaemic state was maintained at 12 h (both CLP groups) and 24 h (especially in the Lethal group; P < 0.05) after the CLP surgery (Figure 5a, b). The blood glucose values at 24 h were as follows: 78 ± 1.7, 72 ± 4.0, 66 ± 3.3 and 45 ± 5.4 mg/dl for Naive, Sham, Sublethal and Lethal groups respectively. To verify the possible involvement of gastrointestinal motility in this septic context, we next determined the gastric emptying and intestinal transit. The gastric emptying was significantly lower in the Lethal group (n = 5–10, P < 0.05; Figure 5e) when compared with the Naive mice. Moreover, a lower intestinal transit in both the CLP groups was observed when compared to the control groups (n = 5–10, P < 0.05), which may corroborate the reduced glucose absorption as discussed thereafter. Altogether, these data indicated that hypoglycaemia is involved with the disruption of glucose homeostasis and reduced gastrointestinal motility may suggest a reduction in glucose absorption.
Figure 5.
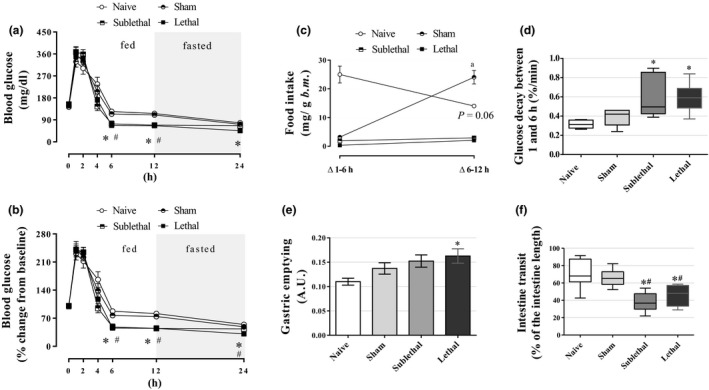
Time course of blood glucose and gastrointestinal motility (a, b) The average blood glucose values during the course of 24 h after CLP procedure (n = 5 for Naive, 7 for Sham, 11 for Sublethal and 16 for Lethal groups). (c) Food intake during the course of 12 h after the CLP procedure (n = 5 for Naive, 7 for Sham, 11 for Sublethal and 16 for Lethal groups). (d) Glucose decay between 1 and 6 h after the CLP procedure (n = 5 for Naive, 7 for Sham, 11 for Sublethal and 16 for Lethal groups). (e) The gastric emptying determined as the remained dye in the stomach [higher the arbitrary unit (A.U.), lower was the gastric emptying] (n = 6 for Naive, 6 for Sham, 8 for Sublethal and 8 for Lethal groups). (f) The intestinal transit measured by the percentage of the intestine length (n = 8 for all groups). Results are expressed as mean ± SEM in ‘a, b, c, e’ graphs, and as median and interquartiles in ‘d, f’ graphs. *significantly different vs. Naive group and #significantly different vs. Sham group using anova with Tukey's post hoc test for ‘a, b, e’ data or Kruskal–Wallis with Dunn's post hoc test for ‘d, f’ data. Wilcoxon paired test was applied to ‘c’ data where the letter ‘a’ and p‐value means difference from Δ1‐6 h. P < 0.05. In ‘b’ graph, only the Lethal group was different from the Naive and Sham groups at 24 h.
Discussion
Our study confirms the hypometabolic stage during sepsis, especially hypoglycaemia, which occurred in a lethality‐dependent manner in our outbred Swiss mice. The pronounced hypoglycaemia found in the Lethal group was paralleled with (i) bradypnea, (ii) loss of body mass, (iii) marked hypophagia, (iv) depletion of hepatic glycogen stores, (v) hyperinsulinemia with reduced insulin sensitivity and (vi) normal glucose tolerance associated with reduced gastrointestinal motility.
The lethality of CLP procedure was based on the percentage of deaths observed after surgery. The mortality rate found in our work was in accordance with previous studies with mice submitted to a similar CLP protocol (Rittirsch et al. 2009; Rattmann et al. 2013). These authors demonstrated that death rate 24 h after CLP was around 12% and 30% in C57BL/6 male mice submitted to a mid‐ and high‐grade CLP model respectively (Rittirsch et al. 2009). The difference of survival curve observed in Sublethal mice, between the present work and other works (Godshall et al. 2002; Spiller et al. 2011, 2012), may be explained by several factors, such as age, sex, the genetic background and strain of mice, interlaboratory standardization of surgery, particularly the amount of faeces released from the caecum after puncture and the intestinal microbiota (Dejager et al. 2011; Lobo et al. 2016). These two lethality degrees achieved by our study were associated with reduced blood leucocytes, an increase in leucocytes in the peritoneal cavity and an increase in TNF‐α levels (Figures 1e,f and 3d, respectively), which are in accordance with the pathophysiologic changes observed in sepsis (Wiersinga et al. 2014). Our Sham mice also exhibited a reduction in the blood leucocytes with no increase in leucocyte in the peritoneal cavity. This reduction in leucocytes was mainly due to a decrease in the blood granulocytes (data not shown). This blood granulocytes reduction may be explained, at least in part, by the expected neutrophils infiltration within the wound healing after the skin incision as based on previous evidence (Wang et al. 2016).
Hypoglycaemia at the advanced period (approximately 24 h after CLP in mice models) was previously explained by a reduced peripheral response to catecholamines, that is, in the liver (Tang et al. 1998), even though circulating catecholamines are found elevated during this period (Hahn et al. 1995). Other counterregulatory hormones including corticosterone and glucagon are also elevated in this later stage of sepsis induced by CLP in rats (Maitra et al. 2000). However, these last authors demonstrated a reduced mRNA abundance of glucose‐6‐phosphatase (G‐6‐Pase) and unaltered G‐6‐Pase hepatic activity 20 h after CLP compared to the presepsis stage pointing to a failure of the compensatory mechanisms that might corroborate to the hypoglycaemia state. Accordingly, there is evidence that hypoglycaemia observed after lipopolysaccharide administration or caecal incision in rats is associated with reduced hepatic phosphoenolpyruvate carboxykinase (PEPCK) expression due to high levels of circulating TNF‐α (Chang et al. 1996), which plays a role against the gluconeogenesis. In the same perspective, there is also evidence that TNF‐α promotes an attenuation of G‐6‐Pase mRNA abundance in hepatocytes (H4IIE), an effect mediated by nuclear factor‐κ B, an intracellular pathway responsive to TNF‐α (Grempler et al. 2004). These data are in accordance with our observations that TNF‐α levels are increased concomitant with hypoglycaemia in the CLP mice (Figure 3d). We suggest that hypoglycaemia may also be a sum of an interruption of food intake caused by the marked anorexigenic effects of proinflammatory cytokines (McCarthy 2000) and a depletion of hepatic glycogen stores. Parenteral glucose administration is among the ways to answer this question in CLP models, but hyperglycaemia (sometimes fulminant) can be a side effect (Watanabe et al. 2013; Singamsetty et al. 2016). Although the absence of any therapeutic manipulation (i.e., antibiotics, parenteral nutrition) impedes a more reliable translation of our data into a clinical context, we aimed to evaluate glucose homeostasis during the disease progression without therapeutic manipulation to avoid any confounding factors to study the disease in a more reliable pathophysiological context. By evaluating our data with the Sham group in relation to the Naive mice, we observed that the Sham group also exhibited a reduction in the blood glucose and in the hepatic content of glycogen under fasting condition. This reduced hepatic glycogen is partially due to reduced food intake over the 6 h after the surgical procedure (Figure 5c). However, mice from the Sham group, but not the CLP groups, can recover the feeding behaviour over the next 12 h after the surgical procedure (Figures 2b and 5c). It is important to highlight that during the fed condition, only the Naive and Sham group were normoglycemic, whereas both the CLP groups remained hypoglycaemic, which was more pronounced in the Lethal group. This evaluation is important because it implicates a combination of both severe hypophagia with depletion of hepatic glycogen content and gastrointestinal hypomotility as being among the main culprits for such hypoglycaemic states, and reinforces the need for a combination of enteral and parenteral nutrition.
The CLP groups showed hyperinsulinemia in a lethality‐dependent manner in the hypometabolic stage (24 h after CLP surgery; Figure 3c), which is in accordance with previous studies using CLP model in rats and mice (Igarashi et al. 1992; Maitra et al. 2000; Heuer et al. 2004; Watanabe et al. 2013). However, it seems to be not the factor behind hypoglycaemia, as CLP groups also showed a significant lethality‐dependent reduction in insulin sensitivity that reduces glucose disposal, as demonstrated by clamp studies in a similar murine CLP model at 22 h after surgery (Singamsetty et al. 2016). Downregulation of the insulin signalling pathway is demonstrated in adipocytes (Igarashi et al. 1992), skeletal muscle and in liver (Matsuda et al. 2009) of CLP animals that are paralleled with increased proinflammatory cytokines (Heuer et al. 2004; Matsuda et al. 2009; Singamsetty et al. 2016). Although our ITT data revealed a reduction in the peripheral insulin sensitivity, we did not evaluate which tissue is involved in the reduction in the insulin response. Hyperinsulinemia may also be a direct result of reduced insulin clearance. Insulin clearance occurs predominantly in the liver (Duckworth et al. 1998), and insulin degradation is mainly processed by insulin‐degrading enzyme (IDE), a 110‐kDa zinc metalloproteinase that is ubiquitously expressed (Duckworth et al. 1998). There is evidence that TNF‐α decreases IDE protein content in hepatoma cell line (1c1c7 cells) (Wei et al. 2014), which would be expected in our CLP groups that exhibited elevated circulating TNF‐α levels (Figure 3d).
Considering that insulin resistance context parallels with glucose intolerance (Weir & Bonner‐Weir 2004), we explored it by conducting two glucose tolerance tests – the oral (oGTT) and intraperitoneal (ipGTT). The former preserves the incretin axis, and the latter excludes it. Mice submitted to the CLP procedure exhibited reduced blood glucose levels at 15 and 30 min after oral glucose load in a lethality‐dependent manner (Figure 4a–c). This culminated with an increased glucose tolerance, as shown by the AUC data (Figure 4d). The increased glucose tolerance was intriguing, and sparked our interest in the gastric emptying and intestinal transit studies, as this would allow us to develop a broader overview of about the intestinal glucose absorption. The data with gastric emptying and intestinal transit presented herein provided evidence that gastrointestinal motility was reduced in both the CLP groups with a more pronounced effect in the lethal group (Figure 5e, f). Reduction in the gastric emptying implies in a negative nutritional aspect per se, and although reduced intestinal transit favours optimal absorption, the glucose transporters could be downregulated in this context. In this perspective, a previous study showed that mediators in mesenteric lymph nodes, possibly cytokines, may be responsible for the inhibition of gastric motility during peritonitis or sepsis (Glatzle et al. 2004). Also corroborating this hypothesis, a study demonstrated that mRNA abundance of sodium‐dependent glucose transporter‐1 (SGLT‐1) and glucose transporter‐2 (GLUT‐2) (luminal and basolateral transporters of glucose into intestinal epithelial cells, respectively) was remarkably reduced in the duodenum and jejunum of critically ill humans and the CLP model in mice respectively (Deane et al. 2014). This molecular evidence was accompanied by a reduced glucose absorption in both clinical and preclinical contexts (Deane et al. 2014). Furthermore, some murine studies with CLP models observed impaired intestinal morphology and function 24 h after surgery (i.e., reduction in the villus length, epithelial cells apoptosis, mucosa inflammation) (Yang et al. 2013; Gao et al. 2015; Zhang et al. 2015). Although blood glucose was not measured in these studies, they demonstrated that animal survival was significantly improved by reducing the intestinal injury in CLP septic animals by the treatment with heparin‐binding epidermal growth factor‐like growth factor, carbon monoxide‐releasing molecule‐2 or pioglitazone. These evidence corroborate with the hypothesis that intestinal glucose absorption is reduced in CLP mice. In fact, our ipGTT data did not reveal any alteration in the glucose tolerance (Figure 4e, f). The absence of glucose intolerance, revealed by the ipGTT test, indicates that the catabolic state of CLP mice is ravenous for glucose even in the presence of insulin resistance. Accordingly, there is evidence for an increased glycolytic flux during shock‐mediated hypotension (Levy et al. 2008). In addition, infiltration of inflammatory cells into peritoneal cavity probably deviates part of this glucose mass to be used in their own metabolism as these cells oxidize glucose (El Kasmi & Stenmark 2015).
The glucose time course (Figure 5a, b) showed that hyperglycaemia is an adverse effect of ketamine anaesthesia, dissociating the acute hyperglycaemia from the inflammatory response. It is important to highlight that insulin hypersecretion, which probably was induced by the elevation of blood glucose levels, may be a bias in the interpretation of the glycemic values after CLP procedure. However, the CLP mice seem not to be able to preserve the normal counterregulatory process during the hypometabolic stage. This can be attributed to impaired catecholamine response or gluconeogenic enzymes activities as discussed before (Chang et al. 1996; Tang et al. 1998; Maitra et al. 2000; Grempler et al. 2004), a fact that occurs together with the abolished feeding behaviour (Figures 2b and 5c). However, it is important to highlight that the insulin sensitivity and the peripheral glucose disposal seem not to be affected during the first 6 h as demonstrated by the rate of glucose decay between 1 and 6 h after surgery (Figure 5a, b, d). This observation suggests that the reduction in the insulin sensitivity is a later event.
In summary, we confirmed that the hypometabolic stage occurs in mice 24 h after sepsis, supported by the hypoglycaemia, bradypnea, hypophagia, gastrointestinal hypomotility and depletion of hepatic glycogen content. Also, we revealed that the insulin resistance is associated with a reduced glucose absorption and high mortality in sepsis. Therefore, cessation of appetite and intestinal glucose malabsorption reinforces the need to combine enteral and parenteral nutrition during septic progression.
Conflict of interest
The authors declare no conflict of interest.
Funding source
A.R. was provided with laboratory mice and commercial rodent chow from the Federal University of Santa Catarina – UFSC. Almost all reagents and materials were provided by personal A.R. earnings.
Authorship contributions
A.R. and F.S participated in research design. F.B.D.F., F.S., C.S. and M.A.B conducted experiments. A.R. and E.A.N contributed with analytic tools and data analysis. A.R., F.B.D.F., C.S. and M.A.B performed data collection and interpretation. A.R and E.A.N provided reagents/materials. A.R wrote the manuscript. A.R. and F.S contributed to discussion. All authors read and approved manuscript final format.
Acknowledgements
The authors appreciate the technical assistance during experimental procedures of Laboratory of Investigation in Chronic Diseases – LIDoC's staff, especially to Luiz M. Gonçalves‐Neto. We also thank Dr. Daniel B. Zoccal for technical support with respiratory frequency experiments and Jeffrey M. Christian, who reviewed the English in the manuscript. These data were partially presented in the final undergraduate conclusion work by F.B.D.F. C.S. received a fellowship from National Council for Scientific and Technological Development (PIBIC/CNPq), and M.A.B received a fellowship from Coordination for the Improvement of Higher Education Personnel (CAPES) during the period of study conduction. A.R. is sponsored by CNPq (302261/2014‐1).
References
- Anderson G., Noorian A.R., Taylor G. et al (2007) Loss of enteric dopaminergic neurons and associated changes in colon motility in an MPTP mouse model of Parkinson's disease. Exp. Neurol. 207, 4–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besen B.A., Romano T.G., Nassar A.P. Jr et al (2016) Sepsis‐3 definitions predict ICU mortality in a low‐middle‐income country. Ann. Intensive Care 6, 107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkholder T., Foltz C., Karlsson E., Linton C.G. & Smith J.M. (2012) Health evaluation of experimental laboratory mice. Curr. Protoc. Mouse Biol. 2, 145–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C.K., Gatan M. & Schumer W. (1996) Efficacy of anti‐tumor necrosis factor polyclonal antibody on phosphoenolpyruvate carboxykinase expression in septic and endotoxemic rats. Shock 6, 57–60. [DOI] [PubMed] [Google Scholar]
- Deane A.M., Rayner C.K., Keeshan A. et al (2014) The effects of critical illness on intestinal glucose sensing, transporters, and absorption. Crit. Care Med. 42, 57–65. [DOI] [PubMed] [Google Scholar]
- Deitch E.A. (1998) Animal models of sepsis and shock: a review and lessons learned. Shock 9, 1–11. [DOI] [PubMed] [Google Scholar]
- Dejager L., Pinheiro I., Dejonckheere E. & Libert C. (2011) Cecal ligation and puncture: the gold standard model for polymicrobial sepsis? Trends Microbiol. 19, 198–208. [DOI] [PubMed] [Google Scholar]
- Duckworth W.C., Bennett R.G. & Hamel F.G. (1998) Insulin degradation: progress and potential. Endocr. Rev. 19, 608–624. [DOI] [PubMed] [Google Scholar]
- El Kasmi K.C. & Stenmark K.R. (2015) Contribution of metabolic reprogramming to macrophage plasticity and function. Semin. Immunol. 27, 267–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fergani A., Oudart H., Gonzalez De Aguilar J.L. et al (2007) Increased peripheral lipid clearance in an animal model of amyotrophic lateral sclerosis. J. Lipid Res. 48, 1571–1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao M., Jiang Y., Xiao X., Peng Y., Xiao X. & Yang M. (2015) Protective effect of pioglitazone on sepsis‐induced intestinal injury in a rodent model. J. Surg. Res. 195, 550–558. [DOI] [PubMed] [Google Scholar]
- Giozzet V.A., Rafacho A., Boschero A.C., Carneiro E.M. & Bosqueiro J.R. (2008) Dexamethasone treatment in vivo counteracts the functional pancreatic islet alterations caused by malnourishment in rats. Metabolism 57, 617–624. [DOI] [PubMed] [Google Scholar]
- Glatzle J., Leutenegger C.M., Mueller M.H., Kreis M.E., Raybould H.E. & Zittel T.T. (2004) Mesenteric lymph collected during peritonitis or sepsis potently inhibits gastric motility in rats. J. Gastrointest. Surg. 8, 645–652. [DOI] [PubMed] [Google Scholar]
- Godshall C.J., Scott M.J., Peyton J.C., Gardner S.A. & Cheadle W.G. (2002) Genetic background determines susceptibility during murine septic peritonitis. J. Surg. Res. 102, 45–49. [DOI] [PubMed] [Google Scholar]
- Grempler R., Kienitz A., Werner T. et al (2004) Tumour necrosis factor alpha decreases glucose‐6‐phosphatase gene expression by activation of nuclear factor kappaB. Biochem. J. 382, 471–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn P.Y., Wang P., Tait S.M., Ba Z.F., Reich S.S. & Chaudry I.H. (1995) Sustained elevation in circulating catecholamine levels during polymicrobial sepsis. Shock 4, 269–273. [DOI] [PubMed] [Google Scholar]
- Heuer J.G., Bailey D.L., Sharma G.R. et al (2004) Cecal ligation and puncture with total parenteral nutrition: a clinically relevant model of the metabolic, hormonal, and inflammatory dysfunction associated with critical illness. J. Surg. Res. 121, 178–186. [DOI] [PubMed] [Google Scholar]
- Hoffer L.J., Bistrian B.R. (2016) Nutrition in critical illness: a current conundrum. F1000Res. 5, 2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igarashi M., Yamatani K., Fukase N. et al (1992) Sepsis inhibits insulin‐stimulated glucose transport in isolated rat adipocytes. Diabetes Res. Clin. Pract. 15, 213–218. [DOI] [PubMed] [Google Scholar]
- Kelleher D.L., Puinno P.A., Fong B.C. & Spitzer J.A. (1982) Glucose and lactate kinetics in septic rats. Metabolism 31, 252–257. [DOI] [PubMed] [Google Scholar]
- Levy B., Desebbe O., Montemont C. & Gibot S. (2008) Increased aerobic glycolysis through beta2 stimulation is a common mechanism involved in lactate formation during shock states. Shock 30, 417–421. [DOI] [PubMed] [Google Scholar]
- Lobo L.A., Benjamim C.F. & Oliveira A.C. (2016) The interplay between microbiota and inflammation: lessons from peritonitis and sepsis. Clin. Transl. Immunology 5, e90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maitra S.R., Wang S., Brathwaite C.E. & El‐Maghrabi M.R. (2000) Alterations in glucose‐6‐phosphatase gene expression in sepsis. J. Trauma 49, 38–42. [DOI] [PubMed] [Google Scholar]
- Matsuda N., Yamamoto S., Yokoo H., Tobe K. & Hattori Y. (2009) Nuclear factor‐kappaB decoy oligodeoxynucleotides ameliorate impaired glucose tolerance and insulin resistance in mice with cecal ligation and puncture‐induced sepsis. Crit. Care Med. 37, 2791–2799. [DOI] [PubMed] [Google Scholar]
- McCarthy D.O. (2000) Tumor necrosis factor alpha and interleukin‐6 have differential effects on food intake and gastric emptying in fasted rats. Res. Nurs. Health 23, 222–228. [DOI] [PubMed] [Google Scholar]
- Moraes D.J., Bonagamba L.G., Zoccal D.B. & Machado B.H. (2011) Modulation of respiratory responses to chemoreflex activation by L‐glutamate and ATP in the rostral ventrolateral medulla of awake rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 300, R1476–R1486. [DOI] [PubMed] [Google Scholar]
- Oliveira Filho R.S., Ribeiro L.M., Caruso L., Lima P.A., Damasceno N.R. & García Soriano F. (2016) Quality indicators for enteral and parenteral nutrition therapy: application in critically ill patients “at nutritional risk”. Nutr. Hosp. 33, 563. [DOI] [PubMed] [Google Scholar]
- Protzek A.O., Costa‐Júnior J.M., Rezende L.F. et al (2014) Augmented β‐cell function and mass in glucocorticoid‐treated rodents are associated with increased Islet Ir‐β/AKT/mTOR and decreased AMPK/ACC and AS160 signaling. Int. J. Endocrinol. 2014, 983453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafacho A., Gonçalves‐Neto L.M., Ferreira F.B. et al (2013) Glucose homoeostasis in rats exposed to acute intermittent hypoxia. Acta Physiol. (Oxf) 209, 77–89. [DOI] [PubMed] [Google Scholar]
- Rattmann Y.D., Malquevicz‐Paiva S.M., Iacomini M. & Cordeiro L.M. (2013) Galactofuranose‐rich polysaccharides from Trebouxia sp. induce inflammation and exacerbate lethality by sepsis in mice. Phytochemistry 94, 206–210. [DOI] [PubMed] [Google Scholar]
- Rittirsch D., Huber‐Lang M.S., Flierl M.A. & Ward P.A. (2009) Immunodesign of experimental sepsis by cecal ligation and puncture. Nat. Protoc. 4, 31–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singamsetty S., Shah F.A., Guo L. et al (2016) Early initiation of low‐level parenteral dextrose induces an accelerated diabetic phenotype in septic C57BL/6J mice. Appl. Physiol. Nutr. Metab. 41, 12–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer M., Deutschman C.S., Seymour C.W. et al (2016) The third international consensus definitions for sepsis and septic shock (Sepsis‐3). JAMA 315, 801–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sordi R., Fernandes D., Heckert B.T. & Assreuy J. (2011) Early potassium channel blockade improves sepsis‐induced organ damage and cardiovascular dysfunction. Br. J. Pharmacol. 163, 1289–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiller F., Costa C., Souto F.O. et al (2011) Inhibition of neutrophil migration by hemopexin leads to increased mortality due to sepsis in mice. Am. J. Respir. Crit. Care Med. 183, 922–931. [DOI] [PubMed] [Google Scholar]
- Spiller F., Carlos D., Souto F.O. et al (2012) α1‐Acid glycoprotein decreases neutrophil migration and increases susceptibility to sepsis in diabetic mice. Diabetes 61, 1584–1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang C., Yang J. & Liu M.S. (1998) Progressive internalization of beta‐adrenoceptors in the rat liver during different phases of sepsis. Biochim. Biophys. Acta 1407, 225–233. [DOI] [PubMed] [Google Scholar]
- Wang L.L., Zhao R., Liu C.S. et al (2016) A fundamental study on the dynamics of multiple biomarkers in mouse excisional wounds for wound age estimation. J. Forensic Leg. Med. 39, 138–146. [DOI] [PubMed] [Google Scholar]
- Watanabe Y., Singamsetty S., Zou B. et al (2013) Exogenous glucose administration impairs glucose tolerance and pancreatic insulin secretion during acute sepsis in non‐diabetic mice. PLoS ONE 8, e67716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei X., Ke B., Zhao Z., Ye X., Gao Z. & Ye J. (2014) Regulation of insulin degrading enzyme activity by obesity‐associated factors and pioglitazone in liver of diet‐induced obese mice. PLoS ONE 9, e95399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir G.C. & Bonner‐Weir S. (2004) Five stages of evolving beta‐cell dysfunction during progression to diabetes. Diabetes 53(Suppl 3), S16–S21. [DOI] [PubMed] [Google Scholar]
- Wernerman J. (2012) Combined enteral and parenteral nutrition. Curr. Opin. Clin. Nutr. Metab. Care 15, 161–165. [DOI] [PubMed] [Google Scholar]
- Wichterman K.A., Baue A.E. & Chaudry I.H. (1980) Sepsis and septic shock–a review of laboratory models and a proposal. J. Surg. Res. 29, 189–201. [DOI] [PubMed] [Google Scholar]
- Wiersinga W.J., Leopold S.J., Cranendonk D.R. & van der Poll T. (2014) Host innate immune responses to sepsis. Virulence 5, 36–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita H., Ishikawa M., Inoue T., Usami M., Usami Y. & Kotani J. (2017) Interleukin‐18 reduces blood glucose and modulates plasma corticosterone in a septic mouse model. Shock 47, 455–462. [DOI] [PubMed] [Google Scholar]
- Yang J., Radulescu A., Chen C.L., Zhang H.Y., James I.O. & Besner G.E. (2013) Heparin‐binding epidermal growth factor‐like growth factor improves intestinal barrier function and reduces mortality in a murine model of peritonitis. Surgery 153, 52–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yelich M.R. (1990) Glucoregulatory, hormonal, and metabolic responses to endotoxicosis or cecal ligation and puncture sepsis in the rat: a direct comparison. Circ. Shock 31, 351–363. [PubMed] [Google Scholar]
- Zhang S., Zheng S., Wang X. et al (2015) Carbon monoxide‐releasing molecule‐2 reduces intestinal epithelial tight‐junction damage and mortality in septic rats. PLoS ONE 10, e0145988. [DOI] [PMC free article] [PubMed] [Google Scholar]


